Abstract
Context
The antiaging protein Klotho is shed and released into the blood stream (soluble Klotho). Growth hormone (GH) is considered an active Klotho regulator, because growth retardation is described in Klotho-deficient mice. The origin of circulating Klotho is, however, not fully understood.
Objectives
Our objective was to analyze a possible role of the pituitary in regulating soluble Klotho in patients with pituitary adenomas.
Patients, Design, and Setting
We analyzed serum levels of soluble Klotho, GH, and insulin-like growth factor 1 (IGF-1) from 21 consecutive patients in our center with pituitary tumor, 7 with GH-producing adenomas (GHomas), and 14 with non–GH-producing pituitary adenomas (non-GHomas), before and after endoscopic transsphenoidal surgery (eTSS).
Main Outcome Measure
Soluble Klotho levels were determined by ELISA with antihuman Klotho antibodies.
Results
Baseline soluble Klotho levels in all patients, those with GHoma and those with non-GHoma, were 542 (median) (interquartile range: 403, 652), 1083 (425, 1213), and 525 (399, 590), respectively. A drastic reduction in Klotho levels was identified in those with GHoma, accompanied by decreases in GH and IGF-1 levels, after eTSS. Interestingly, patients with non-GHoma had significant declines in soluble Klotho without any significant changes in GH levels. Moreover, an oral glucose tolerance test revealed that soluble Klotho levels decreased, whereas a paradoxical GH peak was observed after glucose intake in a patient with GHoma.
Conclusions
Our data suggest that the pituitary may be a key organ that regulates circulating Klotho concentrations, implying that the pituitary possibly controls circulating Klotho through GH-dependent and/or GH-independent mechanisms.
Keywords: pituitary, klotho, growth hormone, glucose tolerance
The antiaging gene klotho, encoding a 130-kDa transmembrane glycoprotein, was first identified in 1997 in a transgenic mouse strain whose mutation resulted in a syndrome resembling premature aging, which included shortened lifespan, growth retardation, vascular calcification, genital atrophy, emphysema, and osteomalacia [1]. Abundant evidence has accumulated that supports the association between klotho and an extended lifespan [2, 3]. Klotho expression is decreased in aged brain white matter, indicating a role for klotho as a lifespan gene in the nervous system [4]. α-Klotho, lately known as one of the Klotho homologs, possesses aging-suppressing properties and has three primary isoforms: full-length transmembrane form (membrane Klotho), shed soluble form (soluble Klotho), and secreted truncated form that is produced by alternative splicing of klotho mRNA [5, 6]; this last form is considered the minor form of Klotho. Membrane Klotho is associated with fibroblast growth factor receptors [7, 8] to form coreceptors for the bone-derived phosphaturic hormone FGF23. Soluble Klotho is produced when the extracellular domain of membrane Klotho is shed from the cell surface into the blood, urine, and cerebrospinal fluid following proteolytic cleavage near the juxtamembrane region by the metalloproteinases ADAM10 and ADAM17 [9–13]. Following its release from the cell membrane, circulating soluble Klotho exerts its biological effects on distant organs or tissues. Interestingly, the atomic structure of the coreceptor complex that consists of shed Klotho, fibroblast growth factor receptor, and FGF23 has been revealed, indicating that shed Klotho is an enzyme-dependent active scaffold protein [14].
Gene and protein expression analyses indicate that Klotho expression in rodents and humans is relatively strong in the kidney, choroid plexus of the brain, pituitary, and parathyroid gland [15], whereas it is expressed to a lesser extent in areas such as the thyroid gland, pancreas, and sex organs [1, 16–18]. In contrast, β-Klotho, another Klotho homolog that modulates FGF21 [19], is overwhelmingly expressed in the liver, adipose tissue, and pancreas and acts as a critical targeting signal for FGF21 [20].
To be more specific regarding the antiaging property, the unique organ-specific expression and shedding process of Klotho may be the reason for human homeostasis, because Klotho plays a pivotal role in the lifespan. There is still the crucial issue of how soluble Klotho is regulated in the blood stream. The current study was carried out to focus on a possible key player, the pituitary, and to examine whether it is a candidate source of soluble Klotho. Our hypothesis was that pituitary cells including adenoma produce and release soluble Klotho into the blood stream. To address this intriguing issue clinically, we performed soluble Klotho measurements before and after endoscopic endonasal transsphenoidal surgery (eTSS) in patients with pituitary adenoma in our center to detect any postsurgical changes in soluble Klotho.
1. Subjects and Methods
The current study was a retrospective cohort study performed at Nagoya Daini Red Cross Hospital in Nagoya, Japan. The study design was in accordance with the ethical standards of the Declaration of Helsinki and approved by the regional institutional review board (Nagoya Daini Red Cross Hospital).
A. Patients
We collected data from 21 consecutive patients with pituitary tumors, 7 with growth hormone (GH)–producing adenoma (GHoma) and 14 with non–GH-producing pituitary adenoma (non-GHoma), who underwent eTSS at the Endoscopic Neurosurgery Center, Nagoya Daini Red Cross Hospital, between July 2016 and March 2017.
B. Blood Analysis
Routine blood biochemical analyses were performed at local laboratories according to current laboratory standards. Serum Klotho levels were determined by using a sandwich ELISA kit (Immuno-Biological Laboratories Co., Ltd., Gunma, Japan) using two antihuman monoclonal Klotho antibodies that recognize the extracellular domain of Klotho [21, 22].
C. Diagnosis
We reviewed pathological materials, including a full panel of pituitary hormone immunohistochemistry and MIB-1 (Ki-67), a cell proliferation marker, to confirm the diagnosis. Pituitary adenomas were then categorized as either GHomas or non-GHomas [prolactinomas, ACTH-producing adenomas, or adenomas without hormone expression (nonfunctioning pituitary adenomas)]. One patient with GHoma underwent an oral glucose tolerance test (OGTT) before eTSS to identify any changes in plasma glucose, GH, and soluble Klotho levels.
D. Statistics
Continuous data are expressed as the mean (SD) or median [interquartile range (IQR)] depending on the distribution of continuous variables. Nonparametric tests were used where necessary. Analyses were performed with SPSS for Windows version 13.0 (IBM, Chicago, IL). All statistical tests were two sided, and P values <0.05 were considered to indicate significance.
2. Results
A. Baseline Characteristics
Table 1 presents the baseline characteristics in our patients who underwent eTSS for pituitary adenomas. The overall mean (SD) age was 54.3 (14.4). Patients with GHoma (n = 7) had a larger physique than those with non-GHoma [n = 14, height: 1.71 (0.140) vs 1.60 (0.0790) and weight: 74.7 (17.3) vs 65.2 (14.8), *P < 0.01]. Two had prolactinomas, one had ACTH-producing adenoma, and the rest of the patients with non-GHoma had nonfunctioning pituitary adenomas. Notably, patients with GHoma had higher GH, IGF-1, and Klotho levels than those with non-GHoma [5.9 (IQR, 2.7 to 9.2) vs 0.31 (0.030 to 0.73), 488 (396 to 742) vs 90.0 (57.0 to 114), 1083 (425 to 1213) vs 525 (399 to 590), respectively, *P < 0.01]. No statistically significant difference was detected in estimated glomerular filtration rate between patients with GHoma and non-GHoma.
Table 1.
Baseline Characteristics
| Overall (n = 21) | GHoma (n = 7) | Non-GHoma (n = 14) | |
|---|---|---|---|
| Age | 54.3 (14.4) | 61.3 (7.78) | 50.8 (15.9) |
| Sex, n (male/female) | 9/12 | 5/2 | 4/10 |
| Height, m | 1.64 (0.111) | 1.71 (0.140) | 1.60 (0.0790)a |
| Weight, kg | 68.3 (15.9) | 74.7 (17.3) | 65.2 (14.8)a |
| ACTH, pg/mL | 22.5 (18.0, 28.5) | 25.9 (22.9, 31.0) | 21.5 (15.3, 25.0) |
| Cortisol, μg/dL | 10.9 (7.42, 16.1) | 10.7 (6.98, 15.3) | 11.1 (2.70, 16.2) |
| LH, mIU/mL | 1.7 (0.88, 5.4) | 3.6 (1.6, 7.2) | 1.4 (0.70, 3.5) |
| FSH, mIU/mL | 9.5 (3.6, 13) | 15 (10, 27) | 6.6 (3.2, 11) |
| TSH, μIU/mL | 0.70 (0.50, 1.4) | 0.66 (0.22, 0.71) | 1.1 (0.57, 2.1) |
| FT3, pg/mL | 2.7 (1.8, 3.0) | 2.7 (1.9, 3.1) | 2.3 (1.9, 2.8) |
| FT4, ng/dL | 1.2 (0.92, 1.3) | 1.3 (1.2, 1.5) | 1.0 (0.85, 1.3) |
| GH, ng/mL | 0.73 (0.13, 2.7) | 5.9 (2.7, 9.2)a | 0.31 (0.030, 0.73)a |
| IGF-1, ng/mL | 114 (76.8, 396) | 488 (396, 742)a | 90.0 (57.0, 114)a |
| PRL, ng/mL | 9.0 (4.9, 17) | 11 (4.8, 34) | 8.7 (5.5, 17) |
| Klotho, pg/mL | 542 (403, 652) | 1083 (425, 1213)a | 525 (399, 590)a |
| eGFR, mL/min/1.73 m2 | 80.5 (69.9, 92.8) | 91.0 (76.7, 117) | 80.4 (69.6, 86.5) |
Data are expressed as the number, mean (SD), or median (IQR), depending on the distribution of continuous variables. Groups were compared using the Mann-Whitney U test.
Abbreviation: eGFR, estimated glomerular filtration rate; FT3, free triiodothyronine; FT4, free thyroxine, PRL, prolactin.
P < 0.01.
B. Variables Before and After Endoscopic Pituitary Surgery
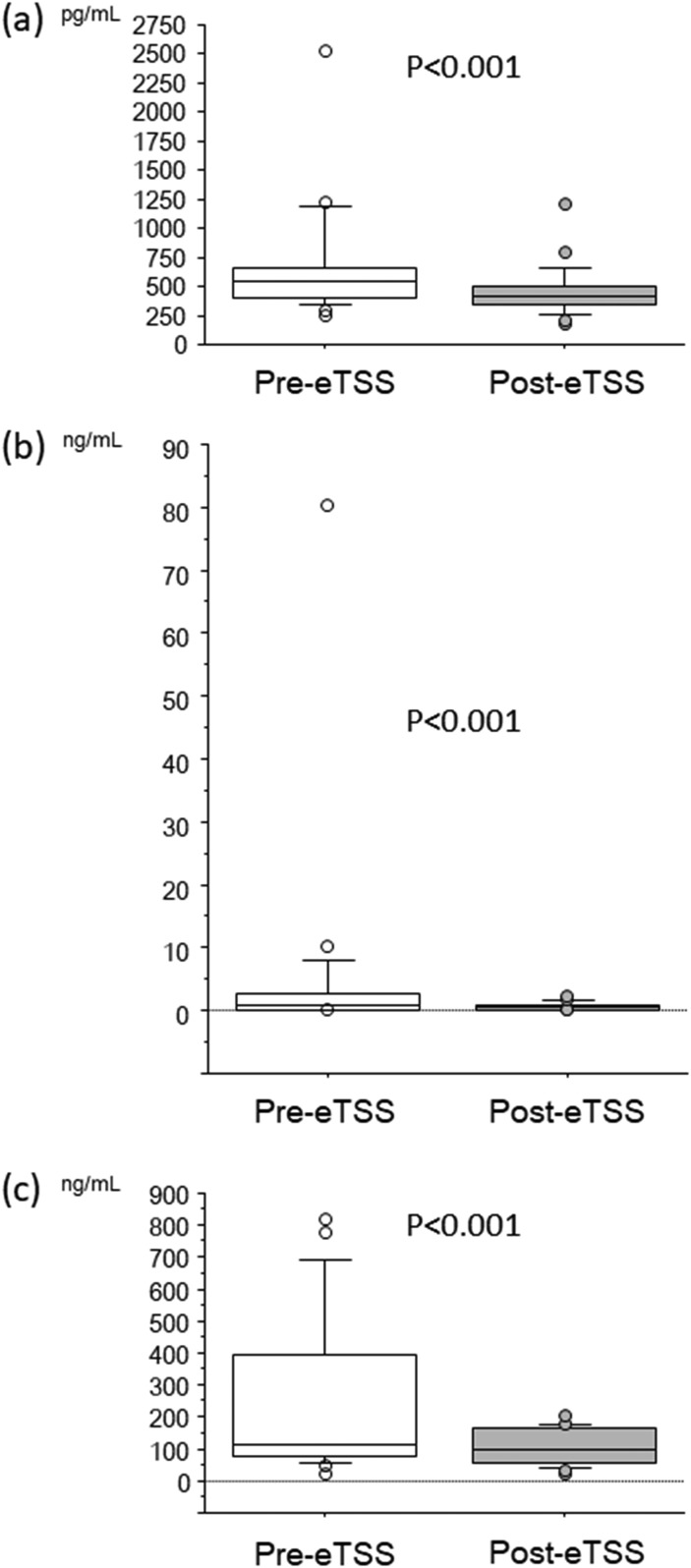
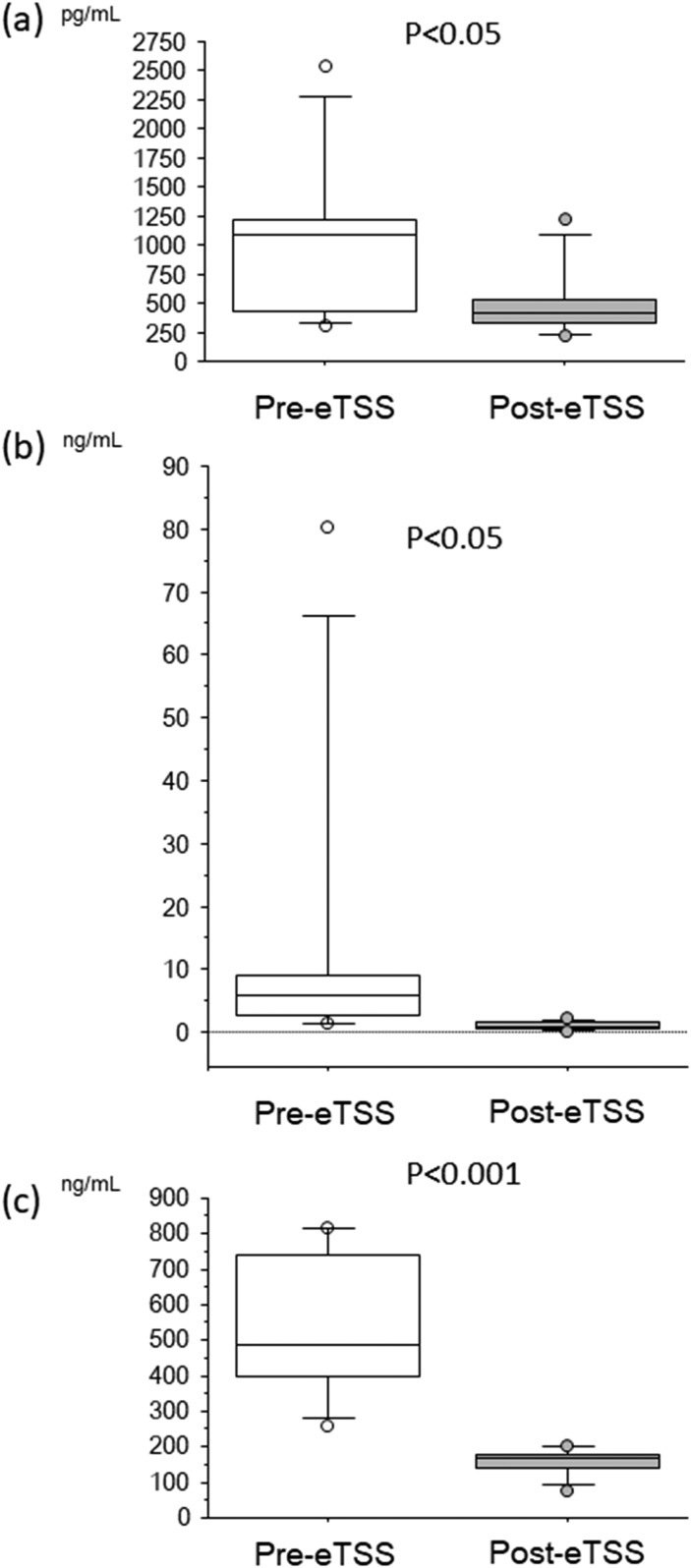
Serum Klotho levels decreased after eTSS in the overall patient sample [Fig. 1(a)]. GH and IGF-1 levels also decreased after eTSS [Fig. 1(b) and 1(c)]. Notably, upregulated circulating Klotho levels showed a drastic reduction after eTSS in patients with GHoma [Fig. 2(a)]. GH and IGF-1 levels showed a robust decline after surgical GHoma resection in those patients [Fig. 2(b) and 2(c)].
Figure 1.
Box and whisker plots before (Pre) and after (Post) eTSS: (a) soluble Klotho, (b) GH, and (c) IGF-1 in all patients. The Wilcoxon signed-rank test identified a significant reduction in soluble Klotho, GH, and IGF-1 after eTSS.
Figure 2.
Box and whisker plots before (Pre) and after (Post) eTSS: (a) soluble Klotho, (b) GH, and (c) IGF-1 in patients with GHoma showing a robust decline in soluble Klotho, GH, and IGF-1 after eTSS.
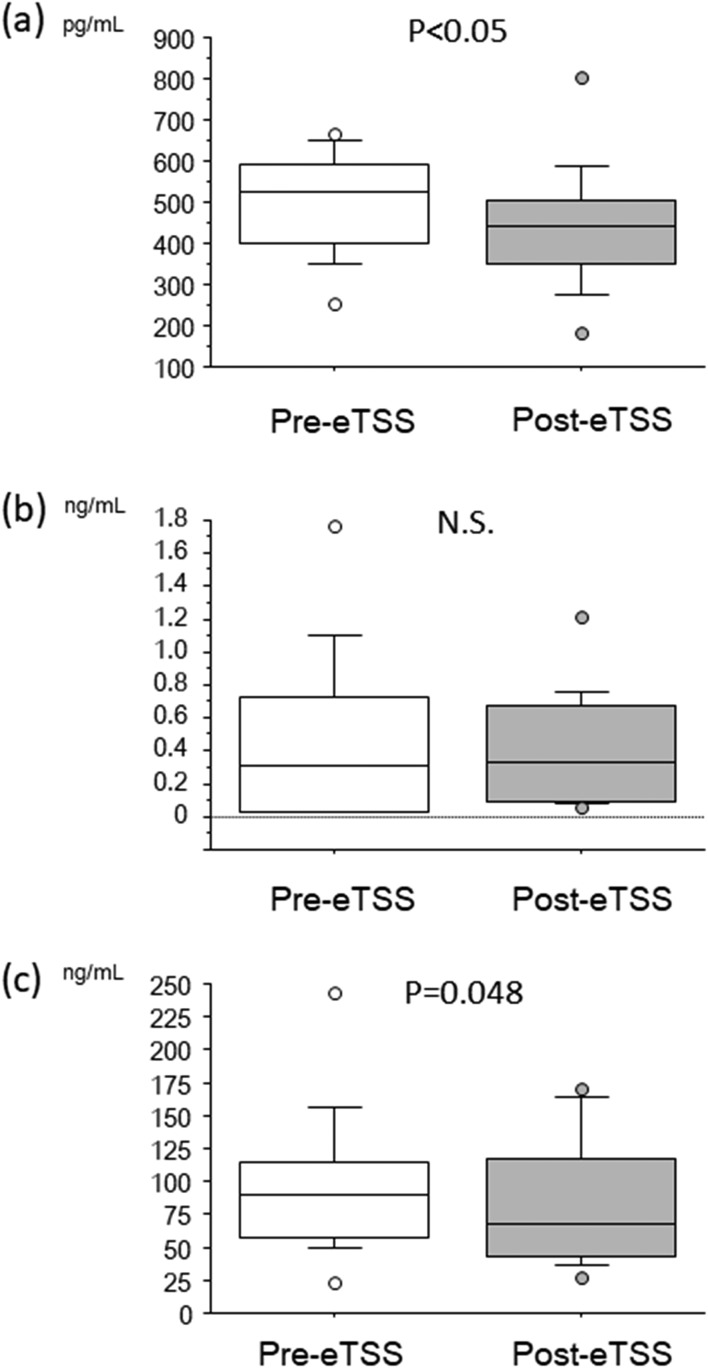
Interestingly, soluble Klotho levels in patients with non-GHomas showed a statistically significant reduction after resection of pituitary adenoma [Fig. 3(a)], although no remarkable difference in GH levels was observed before or after the surgery [Fig. 3(b)], suggesting that circulating Klotho levels are regulated through both GH-dependent and GH-independent mechanisms. IGF-1 levels showed a borderline reduction after eTSS [Fig. 3(c), P = 0.048].
Figure 3.
Box and whisker plots before (Pre) and after (Post) eTSS: (a) soluble Klotho, (b) GH, and (c) IGF-1 in patients with non-GHoma indicating a modest decrease in soluble Klotho and IGF-1 after eTSS, although no significant change in GH was observed. N.S., not significant.
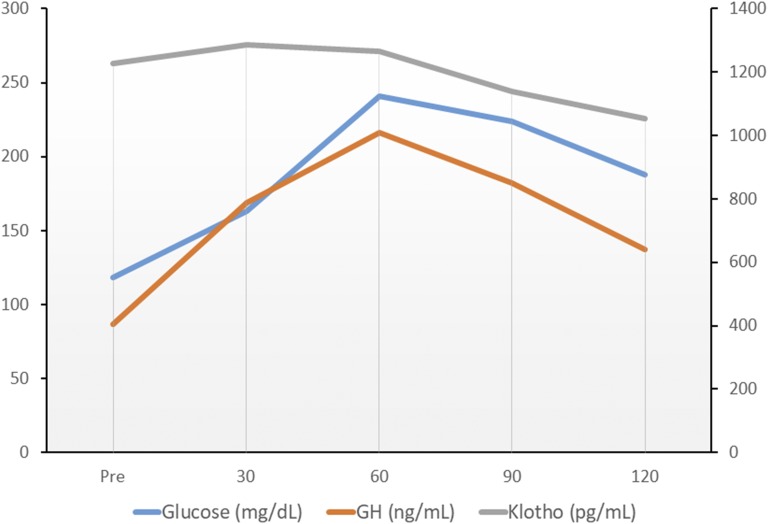
OGTT revealed a patient with GHoma had a markedly elevated fasting GH level. The GH levels throughout the test were not suppressed by glucose intake at all and were even increased after glucose intake, which was the absolute opposite of the effect that would normally be expected (Fig. 4). The fasting soluble Klotho level of the patient was quite elevated (1228 pg/mL), even compared with patients with GHomas. Circulating Klotho levels time-dependently decreased during glucose tolerance, although the serum Klotho level was maintained at a relatively high level 2 hours after glucose intake (1053 pg/mL).
Figure 4.
Result of a 75-g OGTT from a patient with GHoma before pituitary surgery. The paradoxical GH increase was remarkable, whereas impaired glucose tolerance was presented. Serum Klotho levels steadily decreased during the test.
3. Discussion
Circulating soluble Klotho has long been considered to be protective against kidney injury because recombinant Klotho administration restores kidney function [23]. However, a crucial issue is whether the kidney is the major organ that produces and secretes shed Klotho into the bloodstream. Regarding the secreted form, Klotho was not found to be related to kidney function and did not predict adverse renal outcomes in one cohort [24], but its relationship with kidney function has been reported in another study [25, 26]. The current study demonstrates that pituitary adenoma, whether it produces GH, is a possible source of the soluble form of Klotho because soluble Klotho levels declined after pituitary surgery, although a remarkable difference was observed between patients with GHomas and those with non-GHomas. Our data are consistent with the results that GH triggers endogenous soluble Klotho levels [27, 28]. Interestingly, GHomas as well as non-GHomas have been proven to show Klotho expression by immunohistochemistry [29], suggesting that non–GH-producing pituitary cells have an ability to produce Klotho. Strikingly, non-GHomas have stronger Klotho positivity than GHomas according to a semiquantitative analysis [29]. Our data from non-GHomas have indicated the clinical relevance of elevated soluble Klotho levels before eTSS because soluble Klotho levels were modestly reduced after the surgical resection of pituitary adenoma. These observations clearly show that the pituitary is related to soluble Klotho regulation. GH-producing cells are likely responsible for the upregulation of soluble Klotho levels because GHomas had markedly elevated circulating Klotho levels, which eventually showed a robust reduction after pituitary surgery. We encountered a patient with a GHoma who underwent an octreotide test before surgery. Serum Klotho and GH levels at baseline and 2, 4, 6, and 8 hours after a single injection of 50-µg octreotide were 1861, 1821, 1800, 1800, and 1950 pg/mL and 33.0, 6.84, 11.8, 14.6, and 15.0 ng/mL, respectively. Apparently, treatment with somatostatin analog promptly reduces GH levels but suppresses soluble Klotho levels modestly and gradually. Other mediators might regulate circulating Klotho levels. However, the next question is whether elevated GH levels predominantly upregulate the shed form of Klotho or whether there are any other crucial mechanisms that regulate soluble Klotho. Our OGTT data imply some possibilities regarding the relation of GH and Klotho in the bloodstream. Markedly upregulated soluble Klotho levels in a patient with a GHoma (Fig. 4) steadily decreased after oral glucose administration, whereas GH levels increased during OGTT, which was completely different from the normal response. OGTT paradoxically triggered the GH reaction and steadily reduced soluble Klotho levels. GH is likely linked to elevated soluble Klotho levels, whereas oral glucose intake may be another regulator of circulating Klotho levels.
Pituitary cells, particularly GH-producing adenoma cells, may increase soluble Klotho levels, presumably via a GH-related mechanism, whereas oral glucose intake may negatively regulate Klotho even in the presence of robustly elevated GH levels in patients with GHomas. Given all our data, we would like to emphasize that certain pituitary cells and functions may affect soluble Klotho production and/or secretion, where both GH-dependent and GH-independent mechanisms underlie this antiaging hormone regulation, which deserves more attention in endocrinology.
This study has some limitations. First, we could not demonstrate that normal pituitary cells express Klotho and secrete soluble Klotho. Whether GH directly enhances shed Klotho expression and whether other energy balance mechanisms, including oral glucose intake, influence clinically relevant Klotho levels are also crucial issues. Second, there is a lack of direct evidence regarding Klotho expression in pituitary adenomas. Our unpublished preliminary results, which were obtained through quantitative polymerase chain reaction, that the normal mouse pituitary gland has modest klotho expression are encouraging for further studies. Interestingly enough, Klotho-deficient mice have been found to possess hypopituitarism-resembling phenotypes, including growth retardation, hypogonadism, and hypoglycemia [1]. Experimental animal models are needed to clarify normal pituitary function regarding Klotho expression.
In conclusion, the pituitary is the organ responsible for the release of shed Klotho into the blood stream, and pituitary adenomas potentially produce clinically relevant levels of soluble Klotho. The somatotrophic axis may play a key role in the underlying mechanism, although a GH-independent pathway is another possible compensatory player in the regulation of circulating soluble Klotho.
The authors have provided supplementary data at the designated repository storage site [30].
Acknowledgments
We thank the medical assistants of Tokai University School of Medicine for their kind advice on Klotho data quality management.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- GH
growth hormone
- GHoma
GH-producing adenoma
- eTSS
endoscopic transsphenoidal surgery
- IGF-1
insulin-like growth factor 1
- IQR
interquartile range
- non-GHoma
non–GH-producing adenoma
- OGTT
oral glucose tolerance test
References and Notes
- 1. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. [DOI] [PubMed] [Google Scholar]
- 2. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280(45):38029–38034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duce JA, Podvin S, Hollander W, Kipling D, Rosene DL, Abraham CR. Gene profile analysis implicates Klotho as an important contributor to aging changes in brain white matter of the rhesus monkey. Glia. 2008;56(1):106–117. [DOI] [PubMed] [Google Scholar]
- 5. Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242(3):626–630. [DOI] [PubMed] [Google Scholar]
- 6. Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424(1-2):6–10. [DOI] [PubMed] [Google Scholar]
- 7. Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281(10):6120–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–774. [DOI] [PubMed] [Google Scholar]
- 9. Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104(50):19796–19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583(19):3221–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565(1-3):143–147. [DOI] [PubMed] [Google Scholar]
- 12. Akimoto T, Yoshizawa H, Watanabe Y, Numata A, Yamazaki T, Takeshima E, Iwazu K, Komada T, Otani N, Morishita Y, Ito C, Shiizaki K, Ando Y, Muto S, Kuro-o M, Kusano E. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol. 2012;13(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen CD, Tung TY, Liang J, Zeldich E, Tucker Zhou TB, Turk BE, Abraham CR. Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry. 2014;53(34):5579–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, Moe OW, Liang G, Li X, Mohammadi M. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553(7689):461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K, Tominaga Y, Otsuki N, Nibu K, Nakagawa K, Tsugawa N, Okano T, Kitazawa R, Fukagawa M, Kita T. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77(3):232–238. [DOI] [PubMed] [Google Scholar]
- 16. Krajisnik T, Olauson H, Mirza MA, Hellman P, Akerström G, Westin G, Larsson TE, Björklund P. Parathyroid Klotho and FGF-receptor 1 expression decline with renal function in hyperparathyroid patients with chronic kidney disease and kidney transplant recipients. Kidney Int. 2010;78(10):1024–1032. [DOI] [PubMed] [Google Scholar]
- 17. Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125(18):2243–2255. [DOI] [PubMed] [Google Scholar]
- 18. Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, James S, Wilkinson IB, Ting S, Hsiao LL, Hiemstra TF, Zehnder D. α-Klotho expression in human tissues. J Clin Endocrinol Metab. 2015;100(10):E1308–E1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282(37):26687–26695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee S, Choi J, Mohanty J, Sousa LP, Tome F, Pardon E, Steyaert J, Lemmon MA, Lax I, Schlessinger J. Structures of β-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature. 2018;553(7689):501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, Kitaoka T, Ozono K, Sakai T, Hataya H, Ichikawa S, Imel EA, Econs MJ, Nabeshima Y. Establishment of sandwich ELISA for soluble alpha-klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398(3):513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. IBL-America, Cat# 27998, RRID:AB_2750859.
- 23. Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78(12):1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seiler S, Wen M, Roth HJ, Fehrenz M, Flügge F, Herath E, Weihrauch A, Fliser D, Heine GH. Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int. 2013;83(1):121–128. [DOI] [PubMed] [Google Scholar]
- 25. Scholze A, Liu Y, Pedersen L, Xia S, Roth HJ, Hocher B, Rasmussen LM, Tepel M. Soluble α-klotho and its relation to kidney function and fibroblast growth factor-23. J Clin Endocrinol Metab. 2014;99(5):E855–E861. [DOI] [PubMed] [Google Scholar]
- 26. Fountoulakis N, Maltese G, Gnudi L, Karalliedde J. Reduced levels of anti-ageing hormone Klotho predict renal function decline in type 2 diabetes. J Clin Endocrinol Metab. 2018;103(5):2026–2032. [DOI] [PubMed] [Google Scholar]
- 27. Sze L, Bernays RL, Zwimpfer C, Wiesli P, Brändle M, Schmid C. Excessively high soluble Klotho in patients with acromegaly. J Intern Med. 2012;272(1):93–97. [DOI] [PubMed] [Google Scholar]
- 28. Wolf I, Shahmoon S, Ben Ami M, Levy-Shraga Y, Mazor-Aronovitch K, Pinhas-Hamiel O, Yeshayahu Y, Hemi R, Kanety H, Rubinek T, Modan-Moses D. Association between decreased klotho blood levels and organic growth hormone deficiency in children with growth impairment. PLoS One. 2014;9(9):e107174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neidert MC, Sze L, Zwimpfer C, Sarnthein J, Seifert B, Frei K, Leske H, Rushing EJ, Schmid C, Bernays RL. Soluble α-klotho: a novel serum biomarker for the activity of GH-producing pituitary adenomas. Eur J Endocrinol. 2013;168(4):575–583. [DOI] [PubMed] [Google Scholar]
- 30.Sato T, Komaba H, Nagatani T, Watanabe T, Kishida Y, Fukagawa M. Data from: Dataset of overall cohort before and after pituitary surgery, consisting of 7 GHomas and 14 non-GHomas in Nagoya Daini Red Cross Hospital. figshare 2017. Deposited 11 July 2018. www.figshare.com/s/feb9bc44fd1022cfcf61.