Abstract
Background and Aims
If two plant species share pollinators, it has been proposed that the interaction between them may range from competitive to facilitative, depending on the way in which they intermingle. In particular, the presence of a rewarding plant species may increase the rate of pollinator visitation to a less rewarding species in its vicinity, but the beneficial increase in visitation may be counteracted by a detrimental increase in heterospecific pollen transfer. We assessed this trade-off using bumble-bees foraging over a gradual spatial transition between two plant species in an indoor cage experiment.
Methods
We used two ‘species’ of artificial flowers – one more rewarding than the other – in arrays that varied in the degree of species intermingling. The flowers dispensed and received powdered food dyes serving as pollen analogues. Captive bumble-bees visited to collect sucrose solution. We quantified dye delivery to the adhesive-tape ‘stigmas’ in flowers by spectrophotometry.
Key Results
Across the spatial transition between species, the less attractive species received more dye (more bee visits) when in proximity to the more attractive species than it did when alone, but the larger dye loads were less pure (more heterospecific pollen transfer). The decline in purity cancelled out the gain in acquisition, so conspecific pollen receipt by the less attractive species was neutrally affected. The more attractive species received fewer visits when surrounded by the less attractive species, so the interaction between the two species was amensalism when considering conspecific pollen reception.
Conclusions
Pollinator-mediated interactions between plant species depend on pollination quantity and purity, both of which can depend on spatial intermingling.
Keywords: Amensalism, artificial flowers, Bombus, bumble-bee, competition, co-flowering, facilitation, magnet species effect, heterospecific pollen transfer, plant spatial distribution, pollen quality, pollen quantity
INTRODUCTION
Plant species often flower and share pollinators with others in communities, and this community context can alter plant reproduction (e.g. Brown et al., 2002; Moeller, 2004) and floral trait evolution (e.g. Arceo-Gómez and Ashman, 2014a; Muchhala et al., 2014). This is because the presence of other plant species can change the abundance or foraging behaviour of shared pollinators, leading to an increase or decrease in a plant’s pollination success (Rathcke, 1983; Mitchell et al., 2009). There are different mechanisms through which plants facilitate or compete for pollination service that influence the quantity (i.e. amount of pollen) or quality (i.e. type or purity of pollen) components of pollen transfer that contribute to pollination success (Thomson, 1982; Rathcke, 1983). First, pollinator-sharing neighbours can enhance (e.g. Johnson et al., 2003; Lopezaraiza-Mikel et al., 2007) or usurp (e.g. Brown et al., 2002) a focal plant’s pollinator visits. Second, pollinators may forage among the different species, causing heterospecific pollen transfer that can have negative effects on a focal plant’s reproduction (e.g. Waser, 1978; Morales and Traveset, 2008). These mechanisms that act on pollen quantity and quality can occur simultaneously, and the balance of their effects will determine the outcome of the interaction. In particular, the negative effects of heterospecific pollen transfer may negate benefits in pollinator visits, as suggested in some studies (e.g. Thomson, 1982; Lopezaraiza-Mikel et al., 2007; Sun et al., 2013). However, very few studies have examined both components simultaneously and how they may vary – and potentially trade-off with each other – over differing ecological conditions (but see de Waal et al., 2015; Bruckman and Campbell, 2016).
Interactions among plant species for pollination service can depend on plant spatial distribution (Thomson, 1982; Rathcke, 1983; Feldman et al., 2004). The direction and intensity of interactions for pollination service can vary with the abundance (e.g. Ghazoul, 2006; Flanagan et al., 2010; Yang et al., 2011), density (e.g. Seifan et al., 2014; Bruckman and Campbell, 2016) and distance (e.g. Cariveau and Norton, 2009; Jakobsson et al., 2015) of interacting plants. Such spatial dependence is often attributed to changes in pollinator visitation (pollen quantity), because pollinator foraging is often responsive to aspects of plant spatial distribution (Ghazoul, 2005). However, heterospecific pollen transfer (pollen quality) is likely to be particularly sensitive to plant spatial distribution, because the pollen transfer patterns of foraging pollinators may reflect the fine-scale distribution of co-occurring flowers. Although recent studies have concentrated on the species purity of pollen loads, setting impressive new standards for documenting the composition of stigma loads and the consequences for plant reproduction in nature (Fang and Huang, 2013; Arceo-Gómez and Ashman, 2014b; Briggs et al., 2015), it has been impractical to combine such laborious studies with those of other sources of variation.
The distribution of co-occurring plant species in patches will vary from highly intermixed to monospecific (e.g. Levin, 1972; Schemske, 1981). In an early attempt to couple quantitative and qualitative aspects of pollination service, Thomson (1982, fig. 2) proposed that the degree of spatial intermixing between two plant species could influence whether the interaction between them would be competitive or facilitative. The mechanism depends on a hypothesized trade-off between the quantity and species purity of pollen receipt, as follows. If two species occur in larger, spatially segregated patches, the primary interaction between them is likely to be competition for visitation, as pollinators choose one patch or another. When multiple smaller patches of the two species are intermingled to an intermediate extent, magnet species effects (e.g. Thomson, 1978; Laverty and Plowright, 1988; Johnson et al., 2003) might increase the visitation rates of one or both. However, the patchiness would limit the amount of impure pollination (e.g. de Waal et al., 2015) because most plant-to-plant moves, which tend to be near-neighbour moves (e.g. Pyke, 1978), would be conspecific. If the two species are thoroughly intermingled, flower constancy – when pollinators restrict their visits to only one of multiple available rewarding floral types (Waser, 1986) – may break down (Thomson, 1982; Chittka et al., 1997). The magnet effects on visitation may persist, but the negative effects of impure pollen loads may outweigh the beneficial effects of increased visitation. We focus on the ecologically relevant spatial scale of a few metres – a distance over which pollinator-sharing plant species can intermix and over which pollinators can cause a trade-off between pollen quantity and purity. The effect of fine-scale spatial arrangement on pollinator visitation has been investigated for rewardless species relative to a rewarding one (Internicola et al., 2006, 2007), although for rewardless plants the predictions are reversed. For more common rewarding plants, recent theoretical models continue to emphasize the probable importance of spatial intermingling (Hanoteaux et al., 2013), and field experiments by Seifan et al. (2014) demonstrate small-scale local effects on visitation, but the idea that a trade-off between pollen amount and purity should depend on the spatial arrangement of the interacting plant species has not been tested experimentally.
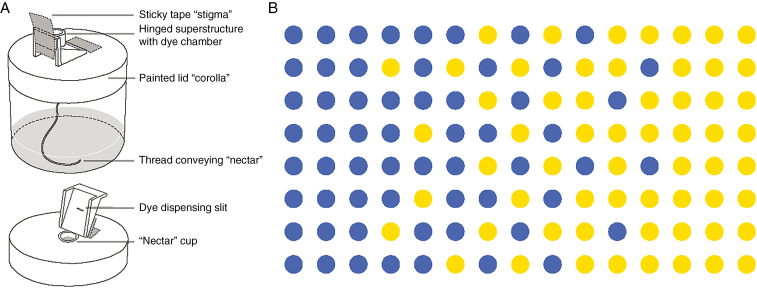
Clean experiments in a natural situation would require the ability to manipulate the amount of intermingling, to control the attractiveness of the plants (to produce consistent magnet effects), and to measure the amounts of both conspecific and heterospecific pollen received, while holding other ecological influences constant. Daunted by that prospect, we used a novel artificial experimental setup to investigate whether bumble-bees really act to produce a spatially sensitive trade-off between stigma-load size and purity. By having captive bees visit arrays of two ‘species’ of artificial flowers with different nectar rewards, we achieved complete control over spacing, context and reward. By devising the flowers to dispense and receive different colours of powdered food dyes serving as pollen analogues (Fig. 1A; see also Thomson et al., 2015), we could analyse ‘stigma loads’ colourimetrically to determine how much conspecific and heterospecific ‘pollen’ the flowers received.
Fig. 1.
(A) Illustrations of the cosexual artificial flowers used in the experiments. The flowers attract visits from bumble-bee workers by offering sucrose solution at a nectary that is continuously replenished by capillary action. ‘Sexual organs’ are housed in a plastic superstructure, formed by 3-D printing, that is positioned over the nectar cup by a piece of tape serving as a hinge. The flowers receive and transfer pollen analogue dye when a bee pushes under the hinged superstructure: the sticky tape stigma picks up dye and a slit in the dye chamber sifts dye on the back of the bee. (B) Spatial arrangement of artificial flowers designed to represent an idealized transition zone between two plant species. Blue and yellow flowers provide 1.4 m and 0.6 m sucrose, respectively.
We offered these flowers to free-foraging bees in a spatial arrangement that simulated a zone where two plant species intermix (Fig. 1B). Pure stands at each end of the array intergraded across a mixed transition zone in the middle. One species, with flowers painted blue, presented more concentrated nectar than the other, yellow species. We expected the blue end of the array to exert a magnet effect that would draw more nectar foragers to that end, therefore producing larger deposits of pollen analogue on stigmas at the blue end. If bees moved freely enough between the two colours, we expected that such a magnet effect would mean that yellow flowers at the blue end of the array, where they were minority stragglers, would receive more pollen analogue than would yellow flowers at the yellow end. However, the blue-end advantage in the quantity of analogue received by those yellows might be offset by a decline in species purity. Such a pattern would show that bumble-bees could plausibly produce the spatially dependent trade-offs proposed by Thomson (1982, fig. 2). It would also demonstrate the potential importance of very local spatial effects in determining the amounts of heterospecific pollen delivery.
MATERIALS AND METHODS
We used artificial flowers that were co-sexual (Fig. 1A; for further description and photographs, see Thomson et al., 2015), unlike an earlier ‘diclinous’ design with separate sexes (Thomson et al., 2012). Briefly, a squat screw-top jar serves as a reservoir for sucrose solution. By capillary action, a wick of silk sewing thread conveys this ‘nectar’ upward to a cup set into the jar’s lid, which serves as a nectary that bees learn to probe for sugar rewards. Lids are painted colours that signal the nectar concentration. To feed, a bee must push itself beneath a hinged plastic superstructure that covers the nectary hole. As it enters, it brushes against a ‘stigma’ of sticky tape that harvests some of the dye particles from its dorsum. After pushing past the stigma to feed, the bee moves under a slit in the roof of the superstructure that allows some dye to sift down onto the bee from a powder chamber above. The stigmas are held in place using Micro-Mark ‘Detail Tack’ adhesive, a liquid pressure-sensitive adhesive that dries clear but remains sticky. This allows us to replace the stigmas without re-applying adhesive. After bees have interacted with the flowers for an experimental period, we remove each stigma, dissolve the dye in a known volume of water and determine the amounts of each dye by spectrophotometry. The amounts of the two dye colours can be determined by mathematically decomposing the combined absorption spectra (details in Thomson et al., 2015).
We ran experiments from 20 May to 2 July, 2015, simultaneously using four Bombus impatiens colonies from Biobest (Leamington, Ontario, Canada). Experiments ran for 8 h in an indoor flight cage (dimensions 3.9 × 9.3 m) lit with four banks of four-tube overhead fluorescent tubes (two ‘daylight’, one ‘warm white’ and one ‘black light’), with the flowers placed on the grey-painted floor of the cage. Newly delivered bumble-bee colonies were placed inside the flight cage, one on each centre edge of the rectangular cage, and were fed pollen ad libitum and additional sucrose solution if the honey pots appeared empty.
Five-day training phase
To train the bees to forage in the flight cage, we set up a 4 × 5 array of 20 artificial feeders with an equal number of yellow and blue flowers ~40 cm apart in a checkerboard pattern. The training array used the same jars as our dye-dispensing flowers, but we replaced the nectar cup and wick with a cotton dental roll submerged in 1 m sucrose solution to provide unlimited reward. During the first 2 d of training, we used superstructures without dye and stigmas. On the third day, we introduced stigmas and bees foraged on the fully assembled flowers until the end of the fifth day of training. Experiments began following the 5-d training phase.
Experiment
Our experiment comprised an 8 × 15 array of 120 artificial flowers (Fig. 1B) with two flower types: blue flowers offered 1.4 m sucrose solution and yellow flowers offered 0.6 m sucrose solution. Blue flowers conveyed red food dye (FD&C red no. 40, allura red); yellow flowers conveyed yellow food dye (FD&C yellow no. 5, tartrazine). Within a row, flowers were arranged ~47.5 cm apart; within a column, flowers were ~35 cm apart.
After one pilot trial to gain practice with the procedures, we ran 14 trials in total. The first seven trials took place every 2–3 d, with the training array set out between trials to preserve the bees’ motivation to forage. To address the possible erosion of learned preferences between trials, we amended the protocol such that the next eight trials took place (for the most part) on consecutive days, without the interposition of the training array. During this second set of trials, bees were exposed to the experimental array only. We did not attempt to control or measure foraging intensity, but casual observations typically found five to ten bees foraging at any given time. Visitation rates declined over time, with the highest visitation rates during the first six trials.
Stigmas were replaced between trials. Following each trial, we collected the stigmas, dissolved their loads in 5 mL distilled water and measured the absorbance of each solution at 427 and 504 nm for yellow and red dye, respectively (Thomson et al., 2012). We used a standard mixing model to separate the absorbance values into their red and yellow dye components (see Thomson et al., 2015; and Supplementary Data S1).
Statistical analysis: trends of pollen delivery across the array.
The bees in the four colonies were free to forage at will, and we would expect total foraging activity to vary substantially over days depending on the numbers of workers and on the food demands from larvae. Therefore, we could not simply compare raw dye amounts across trials. To render data comparable across trials, we divided the mass of dye on each stigma by the total mass received by all stigmas in that trial. The resulting relative numbers indicate the spatial distribution of dye received at each position in the array within a single trial.
The array of eight rows and 15 columns was designed to represent a gradual transition along its longer axis, with a pure blue (rich nectar) stand at the left end grading into a pure yellow (poor nectar) stand at the right. Variation across the short axis (among the rows) was not of interest; multiple rows were needed only to allow the transition from yellow to blue to be gradual. We therefore chose to examine the patterns of dye receipt as column means. To determine whether a response variable varied along the long axis of the array, we used linear regression of log-transformed column means against column position from the yellow end to the blue end. Inspection of graphs suggested no case that warranted fitting a quadratic function instead of a linear one, so we used the slopes of the linear fits as our primary response variable. A positive slope indicates that a response variable increased from the yellow end of the array to the blue end. Each of the 14 trials yielded a single estimate of the slope for each response variable. We assessed the significance of these spatial trends by t-tests comparing the 14 slopes to the null expectation of zero. To account for the data coming from two groups of trials (1–6 and 7–14) with slightly different methodology, we used the pooled standard deviations to calculate t values as t = (mean of 14 trials/pooled s.d.) × (√14). This sacrifices a degree of freedom, so we calculate P values for 12 degrees of freedom. To account for multiple tests, we adopt a Bonferroni criterion that requires a P value to be smaller than 0.00455 (i.e. 0.05/11 variables tested) to be considered significant.
Three response variables bear most directly on our prediction that a species that benefits from higher visitation through a magnet-species effect may simultaneously suffer from increased heterospecific pollen deposition. If the less rewarding yellow species gets more visits because of the magnetic property of the more rewarding blue species, yellow flowers should receive more total dye at the blue end of the array, where they occur as scattered individuals surrounded by blues. If that magnet effect on visitation induces more heterospecific deposition, the purity of loads received by yellow flowers should decline at the blue end of the array. The net resolution of such a trade-off will be indicated by the pattern of conspecific dye received by yellow flowers.
RESULTS
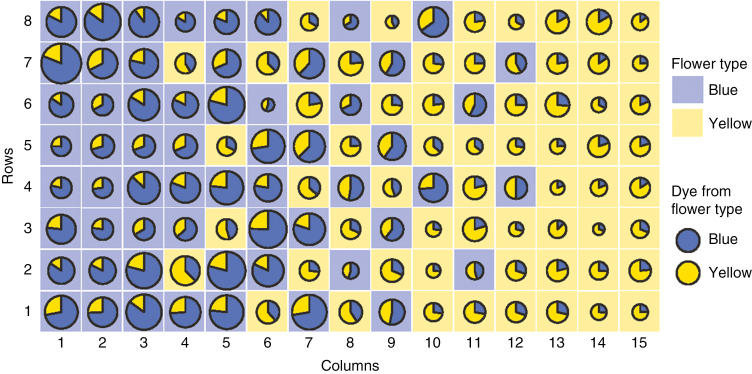
The pollen-analogue dyes percolated extensively through the array; both colours of dye reached flowers in all array positions (Fig. 2). Substantial amounts of dye were transported between the two colours of flowers. Dye receipt was clearly non-random: in every trial, flowers (considering both colours combined) at the blue end received more total dye from both flower colours (Table 1, variable 1; Fig. 2). Total dye receipt by yellows and blues alone (Table 1, variables 4 and 8) was also concentrated at the blue end, although these trends did not retain significance after Bonferroni correction. Unsurprisingly, flowers at the blue end received more dye from blue flowers (Table 1, variable 2) and less dye from yellow flowers (variable 3), but the overall trend was dominated by the dye from blue. Two factors could contribute to the greater receipt of dye at the rich-nectar blue end: bees may have made more visits to the end of the array where nectar was richer, or the flowers with richer nectar may have induced longer visits or visits with more stigma contacts. These two mechanisms could act simultaneously and additively; our data do not allow us to separate them, although casual observations of bees in the cage suggested that visitation rate contributed strongly. Regardless of the exact contributions of visit number and visit length, the result was a quantitative increase in dye delivery to the nectar-rich end of the array. That finding fulfils a necessary condition for a magnet effect by which pollination of the yellows is aided by proximity of the blues. Given the localization of the most pollination service at the rich end, it is plausible that the scattered yellows at that end might benefit.
Fig. 2.
Map of dye receipt by artificial flowers in the array locations depicted in Fig. 1B. The values represented are means of the 14 trials. Before averaging, dye amounts on each flower position are ‘relativized’ by dividing by the total amount of dye received at all positions in that trial. The yellow and blue sectors of the ‘pie diagrams’ indicate the relative quantities of dye received from yellow and blue flowers, respectively. The area of the pie is proportional to the fraction of all dye that was received at that position. The background colour of the grid square over which a pie floats indicates the ‘corolla colour’ of the artificial flower at that location (as also shown in Fig. 1B).
Table 1.
Slopes of linear regressions of amounts of pollen analogue received by stigmas of artificial flowers, regressed on the position of the column within the array
| Statistic | Dye received by all flowers | Dye received by yellow flowers | Dye received by blue flowers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Total dye | 2. Dye from blue flowers | 3. Dye from yellow flowers | 4. Total dye | 5. Dye from blue flowers | 6. Dye from yellow flowers | 7. Yellow purity (fraction of conspecific dye) | 8. Total dye | 9. Dye from blue flowers | 10. Dye from yellow flowers | 11. Blue purity (fraction of conspecific dye) | |
| Trials with positive slopes | 14/14 | 14/14 | 0/14 | 11/14 | 11/14 | 6/14 | 1/14 | 10/14 | 13/14 | 2/14 | 14/14 |
| Mean slope | 0.075 | 0.162 | −0.100 | 0.039 | 0.108 | −0.016 | −2.410 | 0.054 | 0.089 | −0.087 | 2.316 |
| s.d. | 0.035 | 0.047 | 0.068 | 0.057 | 0.095 | 0.081 | 2.473 | 0.077 | 0.092 | 0.079 | 1.699 |
| t | 8.085 | 15.694 | −11.222 | 2.607 | 6.409 | −0.813 | −6.055 | 2.628 | 3.542 | −4.965 | 4.913 |
| P | <0.0001 | <0.0001 | <0.0001 | 0.0115 | <0.0001 | 0.2159 | <0.0001 | 0.0110 | 0.0020 | 0.0002 | 0.0002 |
Positive values indicate that the variable increases from the less rewarding yellow end of the array to the more rewarding blue end. Within each trial, data for dye receipt by ‘all flowers’ have sample sizes of n = 192 stigma loads. Data for receipt by single colours are based on n = 60 stigma loads per trial. For data for the 14 separate trials see Supplementary Data Table S1. Values of t are for one-sample tests of the hypothesis that the slope = 0 (with 12 d.f., using pooled standard deviations as explained in the text). P values are given for each test considered alone. Those in bold type remain significant after Bonferroni correction for making 11 tests (0.05/11 = 0.00455).
The yellow flowers evidently did receive more pollination service at the blue-dominated end, in that they received significantly more dye from the blue flowers at that end of the array (Table 1, variable 5; Fig. 2). However, they did not receive more ‘conspecific’ dye (Table 1, variable 6): the extra pollen they received was primarily heterospecific pollen as a component of highly impure loads. Indeed, this variable was the only variable that did not show a suggestive trend, with six positive and eight negative slopes. The patterns of dye load purity are what would be expected if bees foraged without strong constancy to floral colour. Both flower types received significantly more pure dye loads at the end of the array where they were most numerous (Table 1, variables 7 and 11). The result is that any magnet-species benefit that the yellow flowers receive from blues in terms of visitation is cancelled out by the accompanying decline in purity of load; that is, the increase in dye receipt for yellow flowers is a result of heterospecific and not conspecific dye.
In contrast, blue flowers receive less conspecific dye and more heterospecific dye as they intergrade more into the yellow end of the array. Considering only the receipt of conspecific dye, therefore, the spatial arrangement we have modelled produces neither pure competition nor pure facilitation, but rather amensalism; proximity to the richer blue flowers has a neutral effect on the poorer yellows while proximity to the poorer yellows has a negative effect on the richer blues.
DISCUSSION
We show that the scale and extent of spatial intermingling can strongly influence interactions between co-flowering plant species. Even in our flight cage, pollination service was not uniform – both the quantity (visitation) and the quality (pollen-load purity) of pollination service were influenced by the local mix of flowers at a sub-metre scale. In particular, we documented a trade-off in visitation and purity that depended on plant species spatial intermixing. Many investigations into the community ecology of pollination – for example, the structure of pollination networks (e.g. Vázquez et al., 2009 and many others) or heterospecific pollen transfer (e.g. Fang and Huang, 2013 and others) – proceed without specific attention to the spatial patterning of the plants within study sites. In such studies, the list of local plant species is effectively considered to be interacting with the list of local flower visitors, and the resulting patterns are considered to apply to the entire community. This amounts to an implicit assumption reminiscent of the assumption of panmictic mating in simple formulations of population genetics. It is evident that such simplifications are useful for summarizing data and building theories, but we should bear in mind what distortions they might generate. Our physical simulation model with mechanical flowers is a step toward clarifying the effects of smaller-scale spatial heterogeneity for bumble-bees and the plants they pollinate.
Local influences on species interactions
Within our model of a two-species interface, free-foraging bumble-bees distributed pollen analogue in a manner consistent with several well-known component processes. First, dye transport is localized, as if bees prefer to make short flights between flowers. This is consistent with direct observations during our experiments and very many previous studies, of which Pyke’s (1978) was early and influential. Second, bees tend to concentrate their activity in areas where blue flowers prevail. Presumably the bees are responding to the higher nectar concentration, although but we cannot rule out possible colour preferences because colour and nectar concentration are confounded by design. However, in a previous study with the same flowers, we varied colour and nectar characteristics in a full factorial design. We found nectar effects but no effects of colour and no interaction between colour and nectar (Thomson et al., 2015). Responses to nectar are consistent with decades of study, again largely stimulated by Pyke’s (1978) emphasis on area-restricted foraging based on nectar volumes. Cnaani et al. (2006) and Thomson et al. (2012) later showed analogous preferences of bumble-bees for higher sugar concentration, as we used here. Third, the universal impurity of loads indicates that bees move rather freely between the two flower colours. Although a preference for blue flowers causes them to concentrate their activities at the blue end, they do not show much flower constancy, in the sense that constancy and preference are distinct components of non-random flower choice (Waser, 1986; Chittka et al., 1999). A low level of constancy is consistent with experiments demonstrating reduced constancy by Bombus impatiens workers when artificial flowers differed only in a single sensory modality, such as colour, compared to flowers varying in multiple traits (Gegear and Laverty, 2005). Alternatively, low constancy could have resulted from bees having initial flower preferences (e.g. Wilson and Stine, 1996) that eroded with experience as the experiments progressed.
Therefore, it is not surprising that the results are consistent with Thomson’s (1982) hypothesis of a spatially mediated trade-off between pollen purity and the increased visitation in a magnet-species relationship. Although some studies have documented that visitation (e.g. Ghazoul, 2006) or pollen purity (e.g. Bruckman and Campbell, 2016) can vary with aspects of spatial distribution, we believe this is the first experimental demonstration of the trade-off between the two components. By using artificial flowers and a pollen analogue, we have sacrificed reality for the sake of gaining manipulative control and simple response variables. Nevertheless, we feel that the principal consequences have plausible relevance to field situations. The most important consequence is that pollination intensity and purity are both likely to vary, based on proximity to neighbouring species. We found such effects even with many foragers confined to a small flight cage. Most field situations will offer larger flight distances (e.g. Elliott, 2009) and scales of resource patchiness (e.g. Kotliar and Wiens, 1990), giving foragers more scope to discover and exploit rewarding locations.
Recent investigations of the prevalence and importance of heterospecific pollen transfer in natural communities have tried to identify characteristics that influence the probability of plants receiving foreign pollen. Montgomery and Rathcke (2012), Fang and Huang (2013), and Arceo-Gómez et al. (2016) focused on floral morphological characters such as stigma area and corolla morphology, along with characteristics of the pollinator assemblages such as generalization. None of these studies could consider the spatial proximity of the sources of the heterospecific pollen, because the stigmas were collected without reference to maps of floral abundance. The first two studies sampled stigmas haphazardly, and the third sampled them along a few long line transects. Therefore, these studies necessarily characterized pollen receipt as community-wide means and variances, not as local maps or response surfaces. The results from our flight-cage experiments suggest that the local availability of heterospecific flowers is likely to contribute substantially to those variances, at least when bumble-bees are prominent among the flower visitors. Adding an explicitly spatial component to such studies would be desirable, although extremely laborious.
The quality–quantity trade-off
We found that yellow flowers received similar amounts of conspecific pollen analogue regardless of their position along the transition zone, but this does not mean that they received identical pollination service. We infer that yellow flowers surrounded by blues got more or longer visits than yellows surrounded by yellows, but that the dye loads delivered by that additional visitation were less pure. In our experiments, those opposing influences cancelled out neatly, but it is important to note that such cancelling-out should not be expected as a general result. Suppose, for example, that the yellow flowers at the blue end of the array occurred as pairs or triplets rather than singles. They might well receive the same boosted visitation through the magnetic attractiveness of the richer blues, but we would expect them to receive more conspecific dye than they did in our experiments because they would have yellow neighbours to serve as a source. The patch structure or ‘grain size’ of the spatial intermingling is likely to be a crucial determinant of pollination success, as argued by Thomson (1982, fig. 2). Another variable would be the amount of flower-species constancy displayed. Because our flowers differed in only a single character (colour), constancy in our simulation was probably lower than it would be in natural situations where plants vary in multiple floral characters (Gegear and Laverty, 2005). Although a magnet-species effect will inevitably set the stage for a possible quality–quantity trade-off, the net result will depend on the situation-specific balance between competitive and facilitative tendencies.
Other factors also impinge on the balance between costs and benefits. We demonstrated the trade-off by measuring the amount of conspecific dye received. Using that currency implicitly assumes that more conspecific pollen is always better, and that heterospecific pollen has no effect. In fact, heterospecific pollen may have detrimental effects, such as pollen allelopathy (Murphy and Aarsen, 1995; Murphy, 2000) or possibly stigma clogging (Galen and Gregory, 1989), as documented in a few specific cases (reviewed by Morales and Traveset, 2008). However, we know little about the consequences of naturally deposited heterospecific pollen on plant reproduction (but see Briggs et al., 2015). If such effects are important, the benefits of extra visitation through magnet effects will be further reduced. Those effects will depend on particular properties of the plants involved (Ashman and Arceo-Gómez, 2013). If we are asking evolutionary questions that require estimates of reproductive success, it would also be important to include male function. Here we can draw a general conclusion that the loss of pollen to heterospecific stigmas will always entail a potential loss of siring ability (reviewed by Muchhala and Thomson, 2012). Fang and Huang (2013) were able to estimate such effects, in the sense that they estimated a directed network of pollen transfers among a group of plant species. Our system of artificial flowers also has potential for directly quantifying losses of male function through heterospecific deposition, but experimental designs are limited because we can only distinguish a few colours of dye.
Other evidence for the importance of spatial intermingling comes from meta-analyses of the pollination-mediated interactions of co-flowering plants. Although Morales and Traveset (2009) reported that alien species tended to harm natives, that finding was not supported in a larger analysis by Charlebois and Sargent (2017). In fact, Charlebois and Sargent (2017) found that the only consistent factor to influence outcomes across 76 studies was the spatial arrangement of the interacting species. Extensive interspersion, such as checkerboard arrays, tended to reduce reproductive success, but not visitation rates. That pattern is consistent with the hypothesis that heterospecific pollen transfer may be harmful. Although checkerboard patterns represent an extreme level of interspersion that will maximize heterospecific pollen transfer – and are unlikely to be encountered in nature – they are a common experimental design used to examine interactions between plant species for pollination service (e.g. Waser, 1978; Brown et al., 2002; Yang et al., 2011).
Future studies of heterospecific pollen transfer would be improved by explicit attention to the spatial distributions of flowers, although the logistical challenges are severe. In particular, experiments should ideally incorporate both variation in spatial intermingling and floral density because pollinator visitation and interspecific pollen transfer can vary with both (Thomson, 1982; Rathcke, 1983). Photography by aerial drones, coupled with automated image analysis, might allow efficient capture of spatial data at the relevant scales. A more careful examination of both quality and quantity components would be fruitful, such as the contrasting contributions of male and female reproductive success to total fitness, and to aspects of pollination quality other than the species purity of loads, such as the genetic relatedness of pollen (Mitchell et al., 2009), especially in a spatial context.
SUPPLEMENTARY INFORMATION
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. S1: Standard mixing model calculations used to separate the ‘stigma’ spectrophotometry absorbance values into their red and yellow dye components. Table S1: Slopes of linear regressions of amounts of pollen analogue received by stigmas of artificial flowers, regressed on the position of the column within the array.
ACKNOWLEDGEMENTS
We thank Giorgia Bardini and especially Miruna Draguleasa David for conducting earlier trials that were essential in refining our methods and apparatus. Barbara A. Thomson and David F. Andrews provided statistical advice. We thank two anonymous reviewers and Jeffrey Karron for comments that improved the manuscript. This work was supported by Discovery Grants to JDT from Natural Sciences and Engineering Research Canada by the Faculty of Arts and Sciences, University of Toronto.
LITERATURE CITED
- Arceo-Gómez G, Ashman T-L. 2014. a Coflowering community context influences female fitness and alters the adaptive value of flower longevity in Mimulus guttatus. American Naturalist 183: E50–E63. [DOI] [PubMed] [Google Scholar]
- Arceo-Gómez G, Ashman T-L. 2014. b Patterns of pollen quantity and quality limitation of pre-zygotic reproduction in Mimulus guttatus vary with co-flowering context. Oikos 123: 1261–1269. [Google Scholar]
- Arceo‐Gómez G, Abdala‐Roberts L, Jankowiak A, et al. 2016. Patterns of among‐ and within‐species variation in heterospecific pollen receipt: the importance of ecological generalization. American Journal of Botany 103: 396–407. [DOI] [PubMed] [Google Scholar]
- Ashman TL, Arceo-Gómez G. 2013. Toward a predictive understanding of the fitness costs of heterospecific pollen receipt and its importance in coflowering communities. American Journal of Botany 100: 1061–1070. [DOI] [PubMed] [Google Scholar]
- Briggs HM, Anderson LM, Atalla LM, Delva AM, Dobbs EK, Brosi BJ. 2015. Heterospecific pollen deposition in Delphinium barbeyi: linking stigmatic pollen loads to reproductive output in the field. Annals of Botany 117: 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BJ, Mitchell RJ, Graham SA. 2002. Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology 83: 2328–2336. [Google Scholar]
- Bruckman D, Campbell DR. 2016. Pollination of a native plant changes with distance and density of invasive plants in a simulated biological invasion. American Journal of Botany 103: 1458–1465. [DOI] [PubMed] [Google Scholar]
- Cariveau DP, Norton AP. 2009. Spatially contingent interactions between an exotic and native plant mediated through flower visitors. Oikos 118: 107–114. [Google Scholar]
- Charlebois JA, Sargent RD. 2017. No consistent pollinator-mediated impacts of alien plants on natives. Ecology Letters 20: 1479–1490. [DOI] [PubMed] [Google Scholar]
- Chittka L, Gumbert A, Kunze J. 1997. Foraging dynamics of bumble bees: correlates of movements within and between plant species. Behavioral Ecology 8: 239–249. [Google Scholar]
- Chittka L, Thomson JD, Waser NM. 1999. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften 86: 361–377. [Google Scholar]
- Cnaani JC, Thomson JD, Papaj DR. 2006. The effect of reward properties on learning and choice in foraging bumblebees. Ethology 112: 278–285. [Google Scholar]
- De Waal C, Anderson B, Ellis AG. 2015. Relative density and dispersion pattern of two southern African Asteraceae affect fecundity through heterospecific interference and mate availability, not pollinator visitation rate. Journal of Ecology 103: 513–525. [Google Scholar]
- Elliott SE. 2009. Subalpine bumble bee foraging distances and densities in relation to flower availability. Environmental Entomology 38: 748–756. [DOI] [PubMed] [Google Scholar]
- Fang Q, Huang S-Q. 2013. A directed network analysis of heterospecific pollen transfer in a biodiverse community. Ecology 94: 1176–1185. [DOI] [PubMed] [Google Scholar]
- Feldman TS, Morris WF, Wilson WG. 2004. When can two plant species facilitate each other’s pollination?Oikos 105: 197–207. [Google Scholar]
- Flanagan RJ, Mitchell RJ, Karron JD. 2010. Increased relative abundance of an invasive competitor for pollination, Lythrum salicaria, reduces seed number in Mimulus ringens. Oecologia 164: 445–454. [DOI] [PubMed] [Google Scholar]
- Gegear RJ, Laverty TM. 2005. Flower constancy in bumblebees: a test of the trait variability hypothesis. Animal Behaviour 69: 939–949. [Google Scholar]
- Galen C, Gregory T. 1989. Interspecific pollen transfer as a mechanism of competition: consequences of foreign pollen contamination for seed set in the alpine wildflower, Polemonium viscosum. Oecologia 81: 120–123. [DOI] [PubMed] [Google Scholar]
- Ghazoul J. 2005. Pollen and seed dispersal among dispersed plants. Biological Reviews 80: 413–443. [DOI] [PubMed] [Google Scholar]
- Ghazoul J. 2006. Floral diversity and the facilitation of pollination. Journal of Ecology 94: 295–304. [Google Scholar]
- Hanoteaux S, Tielboerger K, Seifan M. 2013. Effects of spatial patterns on the pollination success of a less attractive species. Oikos 122: 867–880. [Google Scholar]
- Internicola AI, Juillet N, Smithson A, Gigord LDB. 2006. Experimental investigation of the effect of spatial aggregation on reproductive success in a rewardless orchid. Oecologia 150: 435–441. [DOI] [PubMed] [Google Scholar]
- Internicola AI, Page PA, Bernasconi G, Gigord LDB. 2007. Competition for pollinator visitation between deceptive and rewarding artificial inflorescences: an experimental test of the effects of floral colour similarity and spatial mingling. Functional Ecology 21: 864–872. [Google Scholar]
- Jakobsson A, Padrón B, Ågren J. 2015. Distance-dependent effects of invasive Lupinus polyphyllus on pollination and reproductive success of two native herbs. Basic and Applied Ecology 16: 120–127. [Google Scholar]
- Johnson SD, Peter CI, Nilsson LA, Ågren J. 2003. Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology 84: 2919–2927. [Google Scholar]
- Kotliar NB, Wiens JA. 1990. Multiple scales of patchiness and patch structure: a hierarchical framework for the study of heterogeneity. Oikos 59: 253–260. [Google Scholar]
- Laverty TM, Plowright RC. 1988. Fruit and seed set in mayapple (Podophyllum peltatum): influence of intraspecific factors and local enhancement near Pedicularis canadensis.Canadian Journal of Botany 66: 173–178. [Google Scholar]
- Levin DA. 1972. Pollen exchange as a function of species proximity in Phlox. Evolution 26: 251–258. [DOI] [PubMed] [Google Scholar]
- Lopezaraiza-Mikel ME, Hayes RB, Whalley MR, Memmott J. 2007. The impact of an alien plant on a native plant–pollinator network: an experimental approach. Ecology Letters 10: 539–550. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Flanagan RJ, Brown BJ, Waser NM, Karron JD. 2009. New frontiers in competition for pollination. Annals of Botany 103: 1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller DA. 2004. Facilitative interactions among plants via shared pollinators. Ecology 85: 3289–3301. [Google Scholar]
- Montgomery BR, Rathcke BJ. 2012. Effects of floral restrictiveness and stigma size on heterospecific pollen receipt in a prairie community. Oecologia 168: 449–458. [DOI] [PubMed] [Google Scholar]
- Morales C, Traveset A. 2008. Interspecific pollen transfer: magnitude, prevalence and consequences for plant fitness. Critical Reviews in Plant Sciences 27: 221–238. [Google Scholar]
- Morales C, Traveset A. 2009. A meta-analysis of impacts of alien vs. native plants on pollinator visitation and reproductive success of co-flowering native plants. Ecology Letters 12: 716–728. [DOI] [PubMed] [Google Scholar]
- Muchhala N, Thomson JD. 2012. Interspecific competition in pollination systems: costs to male fitness via pollen misplacement. Functional Ecology 26: 476–482. [Google Scholar]
- Muchhala N, Johnsen S, Smith SD. 2014. Competition for hummingbird pollination shapes flower color variation in Andean Solanaceae. Evolution 68: 2275–2286. [DOI] [PubMed] [Google Scholar]
- Murphy SD. 2000. Field testing for pollen allelopathy – A review. Chemical Ecology 26: 2155–2172. [Google Scholar]
- Murphy SD, Aarssen LW. 1995. Reduced seed set in Elytrigia repens caused by allelopathic pollen from Phleum pratense. Canadian Journal of Botany 73: 1417–1422. [Google Scholar]
- Pyke GH. 1978. Optimal foraging: movement patterns between inflorescences. Theoretical Population Biology 13: 72–98. [DOI] [PubMed] [Google Scholar]
- Rathcke B. 1983. Competition and facilitation among plants for pollination. In: Real LA, ed. Pollination biology. Orlando: Academic Press, 305–329. [Google Scholar]
- Schemske DW. 1981. Floral convergence and pollinator sharing in two bee-pollinated tropical herbs. Ecology 62: 946–954. [Google Scholar]
- Seifan M, Hoch E-M, Hanoteaux S, Tielbörger K. 2014. The outcome of shared pollination services is affected by the density and spatial pattern of an attractive neighbour. Journal of Ecology 102: 953–962. [Google Scholar]
- Sun S-G, Montgomery BR, Li B. 2013. Contrasting effects of plant invasion on pollination of two native species with similar morphologies. Biological Invasions 15: 2165–2177. [Google Scholar]
- Thomson JD. 1978. Effects of stand composition on insect visitation in two-species mixtures of Hieracium. American Midland Naturalist 100: 431–440. [Google Scholar]
- Thomson JD. 1982. Patterns of visitation by animal pollinators. Oikos 39: 241–250. [Google Scholar]
- Thomson JD, Ogilvie JE, Makino TT, et al. 2012. Estimating pollination success with novel artificial flowers: effects of nectar concentration. Journal of Pollination Ecology 9: 108–114. [Google Scholar]
- Thomson JD, Draguleasa M, Tan M. 2015. Flowers with caffeinated nectar receive better pollination. Arthropod-Plant Interactions 9: 1–7. [Google Scholar]
- Vázquez DP, Chacoff N, Cagnolo L. 2009. Evaluating multiple determinants of the structure of plant-animal mutualistic networks. Ecology 90: 2039–2046. [DOI] [PubMed] [Google Scholar]
- Waser NM. 1978. Competition for hummingbird pollination and sequential flowering in two Colorado wildflowers. Ecology 59: 934–944. [Google Scholar]
- Waser NM. 1986. Flower constancy: definition, cause, and measurement. American Naturalist 127: 593–603. [Google Scholar]
- Wilson P, Stine M. 1996. Floral constancy in bumble bees: handling efficiency or perceptual conditioning?Oecologia 106: 493–499. [DOI] [PubMed] [Google Scholar]
- Yang S, Ferrari MJ, Shea K. 2011. Pollinator behavior mediates negative interactions between two congeneric invasive plant species. American Naturalist 177: 110–118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.