Abstract
Chronic hyperinsulinemia, in vivo, increases the resistance of peripheral tissues to insulin by desensitizing insulin signaling. Insulin, in a heterologous manner, can also cause IGF-1 resistance. The aim of the current study was to investigate whether insulin-mediated insulin and IGF-1 resistance develops in pancreatic β-cells and whether this resistance results in β-cell decompensation. Chronic exposure of rat islets or INS1E β-cells to increasing concentrations of insulin decreased AktS473 phosphorylation in response to subsequent acute stimulation with 10 nM insulin or IGF-1. Prolonged exposure to high insulin levels not only inhibited AktS473 phosphorylation, but it also resulted in a significant inhibition of the phosphorylation of P70S6 kinase and Erk1/2 phosphorylation in response to the acute stimulation by glucose, insulin, or IGF-1. Decreased activation of Akt, P70S6K, and Erk1/2 was associated with decreased insulin receptor substrate 2 tyrosine phosphorylation and insulin receptor β-subunit abundance; neither IGF receptor β-subunit content nor its phosphorylation were affected. These signaling impairments were associated with decreased SERCA2 expression, perturbed plasma membrane calcium current and intracellular calcium handling, increased endoplasmic reticulum stress markers such as eIF2αS51 phosphorylation and Bip (GRP78) expression, and increased islet and β-cell apoptosis. We demonstrate that prolonged exposure to high insulin levels induces not only insulin resistance, but in a heterologous manner causes resistance to IGF-1 in rat islets and insulinoma cells resulting in decreased cell survival. These findings suggest the possibility that chronic exposure to hyperinsulinemia may negatively affect β-cell mass by increasing β-cell apoptosis.
Keywords: hyperinsulinemia, insulin/IGF-1 signaling, islet and β-cell apoptosis, ER-stress
The changes in modern lifestyles, that is, with abundant nutrients and physical inactivity, have resulted in an alarming rise in the rates of obesity and associated chronic diseases, such as type 2 diabetes, high blood pressure, and dyslipidemia. Obesity is characterized by insulin resistance in peripheral tissues, which leads to a rise in insulin demand and triggers β-cell adaptation by increasing both their mass and function to release sufficient insulin and therein maintain normoglycemia. This compensatory response results in insulin hypersecretion and the development of hyperinsulinemia; in a vicious cycle, insulin circulating at levels higher than normal participates in the metabolic dysregulations observed in obesity [1, 2]. Insulin resistance and hyperinsulinemia precede the development of hyperglycemia, with the latter developing only when β-cells fail to compensate for peripheral insulin resistance. A number of in vivo studies have demonstrated the important role of insulin/IGF-1 signaling pathways in maintaining peripheral insulin sensitivity as well as β-cell function and mass, suggesting that common molecular defects may underlie both abnormalities [3–6].
In peripheral tissues, persistent hyperinsulinemia induces insulin resistance by a variety of mechanisms, including decreasing insulin receptor (IR) expression and altering intracellular signaling cascades, inhibition of IR kinase activity [7, 8], and IR substrates 1 and 2 (IRS1/2) tyrosine phosphorylation, increasing IRS1/2 proteasome-mediated degradation [9, 10], phosphatase-mediated dephosphorylation, and kinase-mediated serine/threonine phosphorylations of IRS1/2 [11, 12]. Furthermore, although insulin and IGF-1 receptors are relatively specific for their respective ligands, homologous and heterologous desensitization occurs as hormone concentrations increase [13, 14]; these can also occur after chronic exposure to low hormone concentrations. Indeed, pretreatment of fibroblast cells with physiological concentrations of insulin, as low as 0.1 nM for 48 hours, rendered cells refractory to subsequent IGF-1 stimulation [15].
It is still unclear whether long-term hyperinsulinemia negatively impacts β-cell function and mass through desensitization of the insulin and/or IGF-1 signaling pathways. Of note, concentrations of insulin in the interstitial space surrounding β-cells in pancreatic islets are predicted to be >10-fold higher than plasma levels in conditions of increased insulin secretion and hyperinsulinemia [16]. Our hypothesis that chronic exposure to high concentrations of insulin induces signaling defects in β-cells gains credence based on data demonstrating the critical role of the insulin/IGF-1/IRS2 and Akt signaling pathway in maintaining normal β-cell physiology [17, 18]. For example, β-cell knockout of the IR or IGF-1 receptor leads to a progressive loss of glucose-stimulated first phase insulin secretion and glucose intolerance [19, 20]. IRS2−/− mice develop diabetes due to aberrant β-cell proliferation and reduced mass by apoptosis [21]. Local expression of IGF-1 in β-cells prevents β-cell apoptosis in streptozotocin-treated mice and in human islets [22, 23]. Three potential mechanisms were shown to underlie the protective effects of IGF-1: (i) an upregulation of antiapoptotic proteins (e.g., bcl-2 and bcl-x), (ii) a downregulation of apoptotic proteins (e.g., Bad and Bax), and (iii) an inhibition of caspase-9–mediated mitochondrial-dependent β-cell apoptosis; these effects appeared to be mediated via Akt [23, 24].
Moreover, recent studies have shown that low doses of insulin, by an autocrine feedback mechanism, activate antiapoptotic mechanisms in human and mouse islets as well as in β-cell lines; this occurs via the activation of the Akt/Pdx1 and the Raf-1/Erk1/2 signaling cascades of the insulin/IGF-1 signaling pathway [25, 26]. Additionally, prolonged exposure to high doses of insulin primes apoptosis and endoplasmic reticulum (ER) stress-inducing mechanisms and leads to a reduction in β-cell viability [27]. Taken together, these studies suggest that insulin itself, by autocrine feedback mechanisms, might play a role in regulating pancreatic islet and β-cell mass.
With that established, one can recognize that we are still far from having a clear understanding of how hyperinsulinemia alters islet and β-cell mass. In the present studies we tested (i) whether prolonged exposure of islets and INS1E β-cells to exogenous insulin desensitizes the insulin/IGF-1 signaling pathway, and (ii) whether such exposure has negative consequences on islet and β-cell survival.
1. Materials and Methods
A. Materials
Cell culture reagents were from Invitrogen (Carlsbad, CA). Polyvinylidene difluoride membranes were from Millipore (Bedford, MA). Polyclonal anti–phospho-AktS473 (sc-7985, RRID: AB_667741) [28], anti-total-Akt (sc-1619, RRID: AB_671713) [29], anti–phospho-P70S6K (sc- 8416, RRID: AB_2182256) [30], anti–total-P70S6K (sc-9027, RRID: AB_2182106) [31], anti–phospho-Erk1/2 (sc-16981, RRID: AB_668967) [32], anti-IRS2 (sc-8299, RRID: AB_2125783) [33], anti-IRβ (sc-711, RRID: AB_631835) [34], anti–IGF receptor (IGFR)β (sc-713, RRID: AB_671792) [35], and ECL reagents were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti–phospho-Tyr-100 (9411, RRID: AB_331228) [36], polyclonal anti–caspase-3 (9662, RRID: AB_331439) [37], anti-Bip (3183, RRID: AB_2119843) [38], anti-SERCA2 (9580, RRID: AB_10827913) [39], anti–total-Erk1/2 (4695, RRID: AB_390779) [40], and monoclonal anti-eIF2α (3398, RRID: AB_2096481) [41] were from Cell Signaling Technology (Beverly, MA). Mouse anti–β-actin (A9357, RRID: AB_1840685) [42], recombinant human insulin, IGF-1, and fura 2-AM were from Sigma-Aldrich (St. Louis, MO). N-acetyl–Asp-Glu-Val-Asp–p-nitroanilide (DEVD-pNA), Asp-Glu-Val-Asp–7-amino-trifluoromethyl coumarin (DEVD-AFC) caspase-3, N-acetyl–Leu-Glu-His-Asp–7-amino-4-trifluoromethyl coumarin (LEHD-AFC) caspase-9, Ac-Ala-Thr-Ala-Asp–7-amino-4-trifluoromethyl coumarin (ATAD-AFC) caspase-12 substrates were from Axxora (Farmingdale, NY). The annexin V Alexa Fluor 488 apoptosis kit was from Thermo Fisher (Waltham, MA; V13241, RRID: AB_2575162 [43]. Collagenase was from Crescent Chemical Company (Islandia, NY).
B. Animals and Islet Isolation and Culture
Wistar rats (250 to 300 g) (Charles River, Wilmington, MA) were anesthetized using a ketamine cocktail, then killed by exsanguination. Islets were isolated as previously described [44]. Briefly, after digestion with collagenase, islets were washed twice with Hanks balanced salt solution and spun at 1200 rpm for 5 minutes at 4°C and then hand-picked several times under a stereomicroscope to ensure 90% to 95% purity. Islets were then washed twice in sterile RPMI 1640 medium supplemented with 5% FBS/5% newborn calf serum and 1% penicillin/streptomycin and cultured in a humidified atmosphere at 5% CO2 and 37°C for 48 hours until the experiment. All animal experiments were performed in accordance with an Institutional Animal Care and Use Committee–approved protocol at Case Western Reserve University.
C. INS1E β-Cell Culture and Treatment
INS1E β-cells (a gift from Drs. P. Maechler and C. B. Wollheim, University of Geneva, Geneva, Switzerland) at passages 56 to 80 were cultured as previously described [45]. Cells were treated with or without insulin for 24 to 48 hours for study of signal transduction through the insulin/IGF-1 pathway and of calcium signaling, or for 24 and 72 hours with or without glucose for measurement of apoptosis.
D. Western Blotting
After pretreatment with insulin, islets or INS1E β-cells were serum starved in RPMI 1640 medium containing 0.5 mM glucose for 5 hours, then washed with Krebs-Ringer bicarbonate buffer containing 20 mM HEPES (pH 7.4), 0.2% BSA, and 0.5 mM glucose. After a 30-minute incubation, islets or INS1E β-cells were acutely stimulated with insulin (10 nM) or IGF-1 (10 nM) with or without glucose. Cells were lysed in RIPA buffer, proteins were measured, and Western blots were done as previously described [46, 47]. Lysates were probed for phospho-Akt, total-Akt, phospho-P70S6k, total-P70S6K, phospho-Erk1/2, total-Erk1/2, total-IRS2, IRβ, IGFRβ, total-Bip (GRP78), total-SERCA2, phospho-eIF2α, total-eIF2α, and β-actin. Levels of tyrosine phosphorylated IRS2 and IGFRβ were measured in immunoprecipitates from INS1E β-cells pretreated with or without 1 μM insulin for 24 hours. One milligram of protein lysate was immunoprecipitated with anti–phospho-tyrosine antibody (P-Tyr-100), separated on 7.5% SDS-PAGE, and membranes were probed for IRS2 and IGFRβ. For IRS2, IRβ, and IGFRβ abundance, total protein lysates were separated on 7.5% SDS-PAGE and the same polyvinylidene difluoride membranes were stripped and used for immunoblotting for IRS2, IRβ, and IGFRβ subunits. Proteins were detected by enhanced chemiluminescence after incubation of membranes with horseradish peroxidase–conjugated secondary antibodies. Signal intensities were quantified with Scion Image (National Institutes of Health, Bethesda, MD) after scanning unsaturated Kodak BioMax MR-1 films (Kodak, Rochester, NY).
E. Hoechst 33342 Staining
INS1E β-cells were plated on gelatin-coated coverslips and at 80% confluence were cultured with or without 1 μM insulin, 30 mM glucose, or insulin and glucose combined for 72 hours. Cells were washed with PBS, fixed with 4% paraformaldehyde, rinsed with PBS, and exposed to Hoechst 33342 stain (1 μg/mL) for 15 minutes at 22°C. Coverslips were washed and then mounted using antifade solution. Apoptotic cells were visualized and counted using a fluorescence microscope.
F. Caspase-3 and Caspase-9 Activities
For INS1E β-cells, caspase activity was assayed colorimetrically using DEVD-pNA as a caspase-3 substrate. After treatments, cells were washed with buffer A (150 mM NaCl, 20 mM Tris-HCl, and 1 mM EDTA (pH 7.5), and then lysed in buffer B (20 mM Tris-HCl, 150 mM NaCl, 10 mM dithiothreitol, 5 mM EDTA, 5 mM EGTA, and 1% Triton X-100 (pH 7.5)], incubated for 30 minutes at 4°C, and centrifuged at 16,000 × g for 20 minutes. Fifty microliters of cell supernatant (∼100 μg of protein) was combined with 50 μL of lysis buffer B containing 200 μM DEVD-pNA in a 96-well plate, incubated for 2 hours at 37°C, and absorbance was measured at 405 nm using an ELISA plate reader. For rat islets, fluorometric substrates were used to assess caspase-3 and caspase-9 activities (DEVD-AFC and LEHD-AFC, respectively). A group of 100 islets per condition were cultured in six-well plates and treated with either 500 nM insulin, 30 mM glucose, or the combination for 72 hours. Islets were then washed with ice-cold PBS, lysed in 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) buffer [50 mM HEPES, 10% sucrose, 0.1% CHAPS (pH 7.5), 0.5% Triton X-100, 1 mM EDTA, 2 mM phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 mM dithiothreitol] for 30 minutes on ice and 15 μg of the proteins was incubated with 50 μM of each substrate at 37°C for 1 hour. Samples were then read in a plate reader (excitation, 400 nm; emission, 505 nm). Data were expressed as picomoles of DEVD or AFC substrate hydrolyzed per minute. In some experiments, cleaved caspase-3 was also measured by immunoblotting using specific antibodies.
G. Detection of Apoptosis by Annexin V Immunostaining
For detection of phosphatidylserine externalization, a characteristic of early apoptosis, an annexin V Alexa Fluor 488 staining kit (Thermo Fisher) was used according to the manufacturer’s instructions. Briefly, after treatment, INS1E β-cells or isolated islets were dispersed into single-cell suspensions using 0.25% trypsin/EDTA solution. Cells were washed with PBS by centrifugation at 600 rpm for 5 minutes and resuspended in annexin V binding buffer at ∼106 cells/mL. A 100-μL cell suspension from each sample was used. Ten microliters of annexin V Alexa Fluor 488 conjugate and 2 μL of propidium iodide were added and cells were incubated for 15 minutes at room temperature. Cells were washed with annexin V binding buffer and mounted on glass slides with mounting medium. Cells were analyzed using an Olympus FSX 100 fluorescence microscope.
H. Caspase-12 Activity
Caspase-12 activity was assayed using the fluorometric substrate ATAD-AFC. After treatment with prolonged insulin, INS1E β-cells were washed with ice-cold PBS, lysed in CHAPS buffer [50 mM HEPES, 10% sucrose, 0.1% CHAPS (pH 7.5), 0.5% Triton X-100, 1 mM EDTA, 2 mM phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 mM dithiothreitol) for 30 minutes on ice and 15 μg of the proteins was incubated with 50 μM of substrate at 37°C for 1 hour. Samples were then read in a plate reader (excitation, 400 nm; emission, 505 nm). Data were expressed as picomoles of AFC substrate hydrolyzed per minute.
I. Intracellular Ca2+ Concentration Measurement
INS1E β-cells were cultured on round gelatin-coated glass coverslips and treated with or without insulin for different intervals of time. For intracellular Ca2+ concentration ([Ca2+]i) measurement, cells were loaded with fura-2–PE3 (2 µmol/L) (Molecular Probes, Eugene, OR). Pluronic acid (0.01%) was added to the medium and cells were incubated for 30 minutes at 37°C. After loading, cells were washed with RPMI 1640 medium to remove excess dye and incubated for 20 minutes for dye esterification. The Ca2+ concentration was measured by placing the coverslips in a small recording chamber mounted on the stage of an Olympus IX-50 inverted epifluorescence microscope (Olympus, Tokyo, Japan). Absolute [Ca2+]i was determined from the fluorescence ratio (R) of Ca2+-bound fura-2 (excited at 340 nm) to unbound fura-2 (excited at 380 nm). In some experiments, INS1E β-cells were subjected to glucose-stimulated calcium signaling by incubation with 5 or 15 mM glucose for 10 to 60 minutes and [Ca2+]i was measured using fura-2 as described above. Ca2+ levels were determined using a standard equation for calibration. Rmax and Rmin were obtained by adding ionomycin (10 µM) or EGTA (1 mM), respectively, to fura-2–loaded β-cells at the end of each experiment.
J. Patch-Clamp Recording of Calcium Current
Recordings were done at room temperature (22 to 24°C), and the standard (ruptured membrane patch) whole-cell patch-clamp configuration was used. INS1E β-cells were seeded in 3.5-cm culture dishes and used at 40% confluence. Pipettes (0.8 to 2 MΩ) were prepared from N51A glass, coated with Sylgard, and fire polished. Current leaks were subtracted with a P/4 protocol using the pClamp software package (Axon Instruments) using a Pentium 4 microcomputer in concert with an AxoPatch 200B amplifier. The currents were four-pole filtered at 3 kHz and digitized at 40 KHz. The external solution contained 150 mM tetraethylammonium chloride (TEA-Cl), 5 to 10 mM BaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES and 1 mM tetrodotoxin (pH 7.4) with 320 ± 5 mOsm/L. The pipette solution contained 90 mM N-methyl-d-glucamine-chloride, 30 mM CsCl, 10 mM TEA-Cl, 5 mM EGTA–N-methyl-d-glucamine, 10 mM HEPES, 4 mM MgCl2, 4 mM creatine phosphate, 4 mM ATP-Na+, leupetine, creatine kinase, and 0.2 mM GTP (pH 7.4 with CsOH) with osmolarity of 305 ± 5 mOsm/L. The substitution of NaCl for TEA-Cl in the external solution allows a 40% to 70% increase in the magnitude of the Ca2+ currents [48]. To minimize the contamination of Ca2+ currents by other currents, the experiments were conducted with Ba2+ as the permeating cation. The voltage protocols were designed in accordance with the known voltage characteristics of the different Ca2+ channels. Effects were determined at the peak of the current and at the steady-state.
K. Statistical Analysis
Statistical significance was determined by one-way ANOVA using Origin software, and in some experiments (as indicated) the Student t test for unpaired data was used. Data are expressed as mean ± SEM. A P value ≤0.05 was considered statistically significant.
2. Results
A. Effects of Prolonged Exposure to Insulin on the Insulin/IGF-1 Signaling Pathway
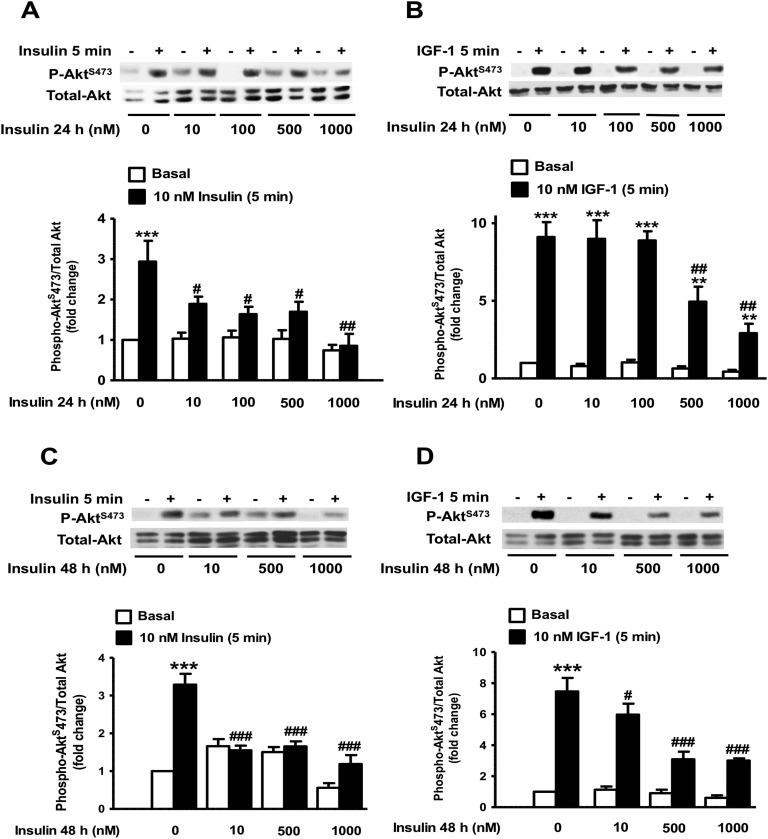
To investigate whether prolonged exposure to insulin induces insulin and IGF-1 resistance in β-cells, we used the INS1E β-cell line as well as freshly isolated rat islets to examine the activation of key signaling molecules in the insulin/IGF-1 signaling pathway. INS1E β-cells were pre-exposed to various concentrations of insulin for 24 hours, serum starved for 5 hours, and then acutely challenged with 10 nM insulin or 10 nM IGF-1. Figure 1 shows that exposure of INS1E β-cells to 10 and 100 nM insulin for 24 hours resulted in ∼50% and ∼70% decreases in AktS473 phosphorylation, respectively, in response to the acute challenge with 10 nM insulin, whereas stimulation of Akt phosphorylation was nearly completely abolished in cells pre-exposed to >500 nM doses of insulin (Fig. 1A). Exposure of INS1E β-cells to insulin concentrations up to 100 nM had no significant effect on the stimulation of AktS473 phosphorylation in response to 10 nM IGF-1 for 5 minutes (Fig. 1B). However, pre-exposure of INS1E β-cells to insulin concentrations >100 nM for 24 hours significantly inhibited the subsequent IGF-1–mediated AktS473 phosphorylation, which reached ∼70% inhibition in cells pre-exposed to 1 μM insulin for 24 hours (Fig. 1B). These studies were repeated following 48 hours of incubation with various concentrations of insulin (Fig. 1C and 1D). Similarly, we found that exposure to insulin leads to a dose-dependent decrease in Akt phosphorylation. At 48 hours of exposure, inhibition of Akt phosphorylation in response to the acute stimulation by 10 nM insulin was maximal in cells pre-exposed to 1 μM insulin (Fig. 1C). The response to an acute challenge with 10 nM IGF-1 was significantly diminished even in cells pre-exposed to lower concentrations of insulin (10 nM) for 48 hours (Fig. 1D).
Figure 1.
Effect of prolonged exposure to insulin on the acute insulin and IGF-1 phosphorylation of Akt in INS1E β-cells. (A–D) INS1E β-cells were treated with the indicated concentrations of insulin for 24 or 48 h, then exposed to 10 nM insulin (A and C) or 10 nM IGF-1 (B and D) for 5 min. Phospho-AktS473 was measured by Western blot using specific antibodies in whole-cell lysates and normalized to total Akt. Data are presented as fold change over the basal nontreated cells. Representative autoradiograms are shown. Means ± SEM are from four to nine independent experiments. **P < 0.005, ***P < 0.0005, comparing 10 nM insulin or 10 nM IGF-1 to basal; #P < 0.05, ##P < 0.005, ###P < 0.0005, comparing chronic insulin to control cells.
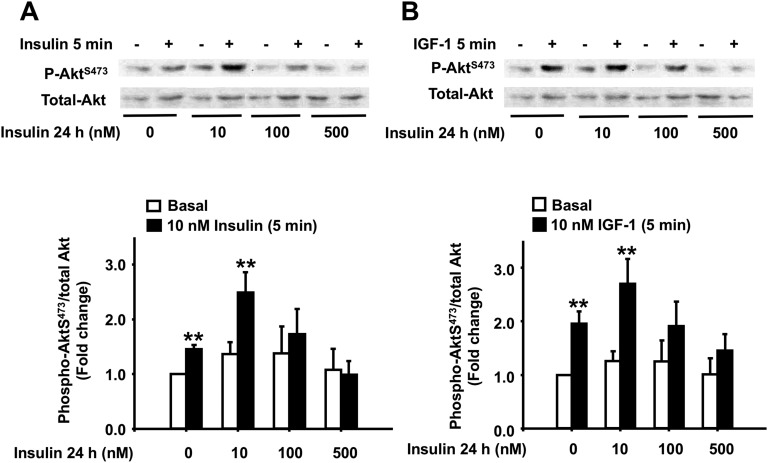
To validate our findings in INS1E β-cells, we next examined the effects of prolonged exposure to insulin on Akt phosphorylation in isolated rat pancreatic islets. Rat islets were pre-exposed to increasing concentrations of insulin (0 to 500 nM) for 24 hours, washed free of insulin, and then acutely challenged with 10 nM insulin or 10 nM IGF-1 for 5 minutes. We found that exposure to insulin concentrations >100 nM for 24 hours significantly decreased AktS473 phosphorylation in response to the acute challenge with insulin or IGF-1, with a maximum decrease that reached basal levels at 500 nM insulin (Fig. 2A and 2B). Interestingly, however, islets pre-exposed to 10 nM insulin showed an increase in AktS473 phosphorylation following the 5-minute challenge with 10 nM insulin or 10 nM IGF-1, perhaps indicating a positive feedback loop at physiological insulin concentrations as earlier studies have shown in human islets [25]. Total Akt was not affected by prolonged exposure to insulin in any of these experiments.
Figure 2.
Effect of prolonged exposure to insulin on the acute insulin and IGF-1 phosphorylation of Akt in rat pancreatic islets. (A and B) Cultured rat isolated islets were treated with the indicated concentrations of insulin for 24 h, then exposed to 10 nM insulin (A) or 10 nM IGF-1 (B) for 5 min. Phospho-Akt S473 was measured by Western blot using specific antibodies in whole-cell lysates and normalized to total Akt. Data are presented as fold change over the basal nontreated islets. Representative autoradiograms are shown. Means ± SEM are from four independent experiments. **P < 0.005.
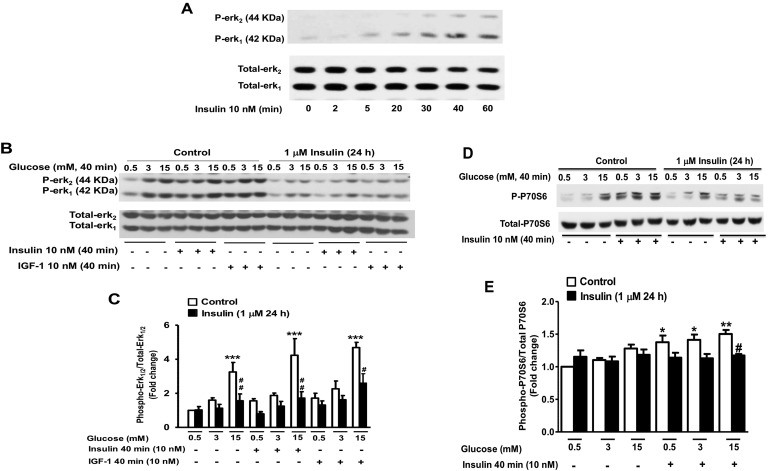
To investigate the impact of a nearly complete inhibition of the response to insulin or IGF-1 on islet and β-cell survival, in all subsequent experiments, INS1E β-cells or islets were pre-exposed to the highest insulin dose for 24 to 72 hours. Next, we investigated the effects of prolonged exposure to high insulin levels on other insulin/IGF-1 signaling molecules. Because earlier studies have demonstrated that the ras/raf/Erk1/2 signaling cascade downstream of the insulin/IGF-1 signaling pathway plays an important role in the antiapoptotic actions of low concentrations of insulin [49], we explored the effects of prolonged exposure to high insulin on Erk1/2 phosphorylation in response to the acute stimulation by insulin or IGF-1. At physiological concentrations, glucose was shown to stimulate Erk1/2 phosphorylation in pancreatic islets and in insulinoma cells in a Ca2+-dependent manner [50, 51] or through an autocrine action by secreted insulin, and to induce gene expression and cell proliferation [7]. We found that Erk1/2 phosphorylation in response to the acute stimulation by 10 nM insulin reached a robust stimulation at 40 minutes (Fig. 3A), which is in agreement with previously published studies on Erk1/2 activation in pancreatic β-cells [52]. Therefore, following the 24-hour exposure of INS1E β-cells to insulin, Erk1/2 phosphorylation was measured in response to 40 minutes of stimulation with 15 mM glucose alone or in combination with 10 nM insulin or 10 nM IGF-1 (Fig. 3B and 3C). We found that glucose at 3 and 15 mM increased Erk1/2 phosphorylation by 1.6- and 3.2-fold, respectively, in control INS1E β-cells. A 24-hour pre-exposure to high insulin levels diminished the stimulation of Erk1/2 phosphorylation by glucose. Insulin at 10 nM and in the presence of 0.5 mM (nonstimulatory) glucose concentration increased Erk1/2 phosphorylation by 1.7-fold, which was slightly enhanced at 3 mM and 15 mM glucose. In contrast, in cells pre-exposed to high insulin levels for 24 hours, Erk1/2 phosphorylation in response to 10 nM insulin or 10 nM IGF-1 was markedly reduced. In these studies, the abundance of Erk1/2 was not affected by prolonged exposure to insulin (Fig. 3B and 3C). We also explored the effects of prolonged exposure to high insulin levels on the phosphorylation of ribosomal S6 kinase, P70S6K, a downstream molecule in the insulin signaling pathway activated by the phosphorylation and interaction of Akt with the Ser/Thr protein kinase mammalian target of rapamycin [51]. Similar to Erk1/2 phosphorylation, it was shown that the kinase P70S6K is also activated by physiological concentrations of glucose in β-cells [51]. Figure 3D and 3E show that a 24-hour exposure of INS1E β-cells to high insulin levels led to a significant decrease in P70S6K phosphorylation in response to the 40-minute acute stimulation by either 10 nM insulin alone or in combination with 15 mM glucose.
Figure 3.
Effect of prolonged exposure to insulin on the acute glucose, insulin, and IGF-1 phosphorylation of Erk1/2 and P70S6K in INS1E β-cells. (A) Time course of Erk1/2 phosphorylation in response to the stimulation by 10 nM insulin. (B–E) INS1E β-cells were treated with 1 μM insulin for 24 h and then exposed to increasing concentrations of glucose (0.5, 3, and 15 mM) alone or in combination with 10 nM insulin or 10 nM IGF-1 for 40 min. Phospho-Erk1/2 (B and C) and phospho-P70S6K (D and E) were measured by Western blot using specific antibodies in whole-cell lysates and normalized to total Erk1/2 and total P70S6K. A representative autoradiogram of four to six independent experiments is shown. *P < 0.05, **P < 0.005, ***P < 0.0005, 15 mM glucose, 10 nM insulin, or IGF-1 compared with basal; #P < 0.05, ##P < 0.005, chronic insulin treatment compared with control nontreated.
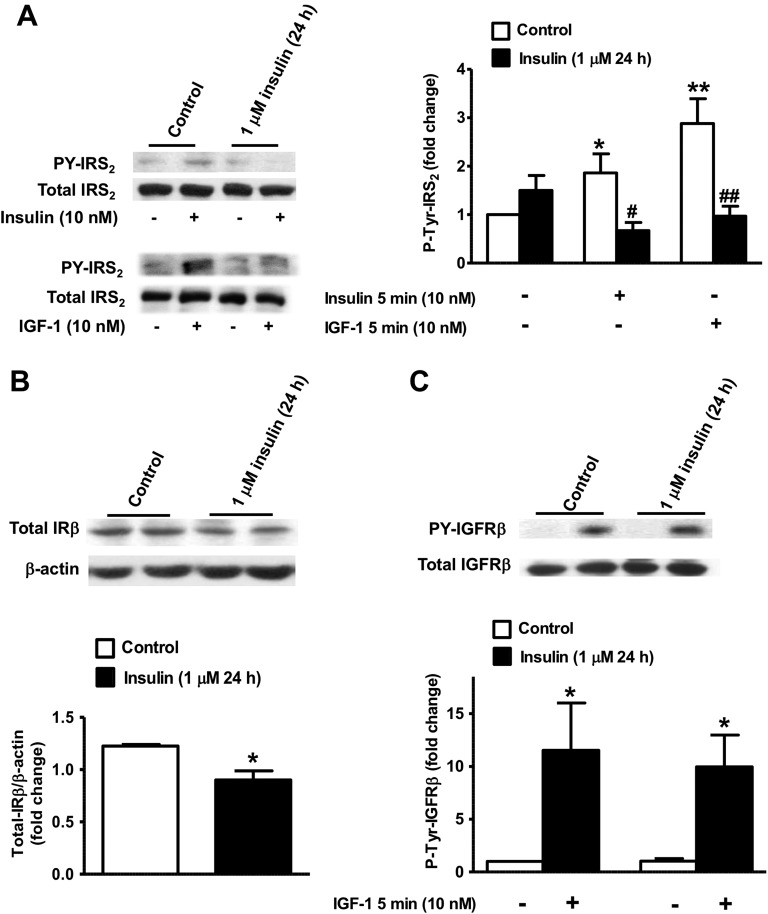
These effects of prolonged exposure to insulin on Akt, Erk1/2, and P70S6K indicate that upstream signaling molecules in the insulin/IGF-1 pathway might be affected. Next, we examined the effects of prolonged exposure to insulin on IRS2, IRβ, and IGFRβ tyrosine phosphorylation and abundance. Both insulin (10 nM) and IGF-1 (10 nM) significantly stimulated IRS2 tyrosine phosphorylation in control INS1E β-cells by 1.9- and 2.9-fold, respectively (Fig. 4A). A 24-hour exposure to high insulin levels slightly increased basal levels of tyrosine-phosphorylated IRS2 by 1.5-fold but did not show any stimulation of IRS2 phosphorylation following the 5-minute acute challenge with 10 nM insulin or 10 nM IGF-1 (Fig. 4A). We also found that the abundance of the IRβ subunit was decreased by ∼23% in INS1E β-cells pretreated with high insulin levels (Fig. 4B). In contrast, neither the content of IGFRβ nor its tyrosine phosphorylation in response to the 5-minute acute challenge with 10 nM IGF-1 was changed following chronic exposure to 1 μM insulin (Fig. 4C).
Figure 4.
Effect of prolonged exposure to insulin on the acute insulin and IGF-1 activation of IRS2, IRβ, and IGFRβ in INS1E β-cells. INS1-E β-cells were treated for 24 h with 1 μM insulin then exposed for 5 min to 10 nM insulin or 10 nM IGF-1. (A) Left: representative anti-IRS2 autoradiograms of phospho-tyrosine IRS2 measured in phospho-tyrosine immunoprecipitates and of total IRS2 in whole-cell lysates. Right: IRS2 tyrosine phosphorylation was measured by Western blot using an anti-IRS2–specific antibody in immunoprecipitates. Data are presented as fold change to basal nontreated cells. (B) Total IR content was analyzed in whole-cell lysates using an anti-IRβ antibody and intensity was normalized to β-actin. (C) Tyrosine phosphorylated IGFRβ was measured in phospho-tyrosine immunoprecipitates using anti-IGFRβ and presented as fold change to basal nontreated cells. Total IGFRβ was analyzed in whole-cell lysates. Means ± SEM are from three to five independent experiments. *P < 0.05, **P < 0.005, 10 nM insulin or 10 nM IGF-1 in control compared with basal nontreated cells; #P < 0.05, ##P < 0.005, chronic insulin compared with control nontreated.
B. Effects of Prolonged Exposure to Insulin on β-Cell Apoptosis
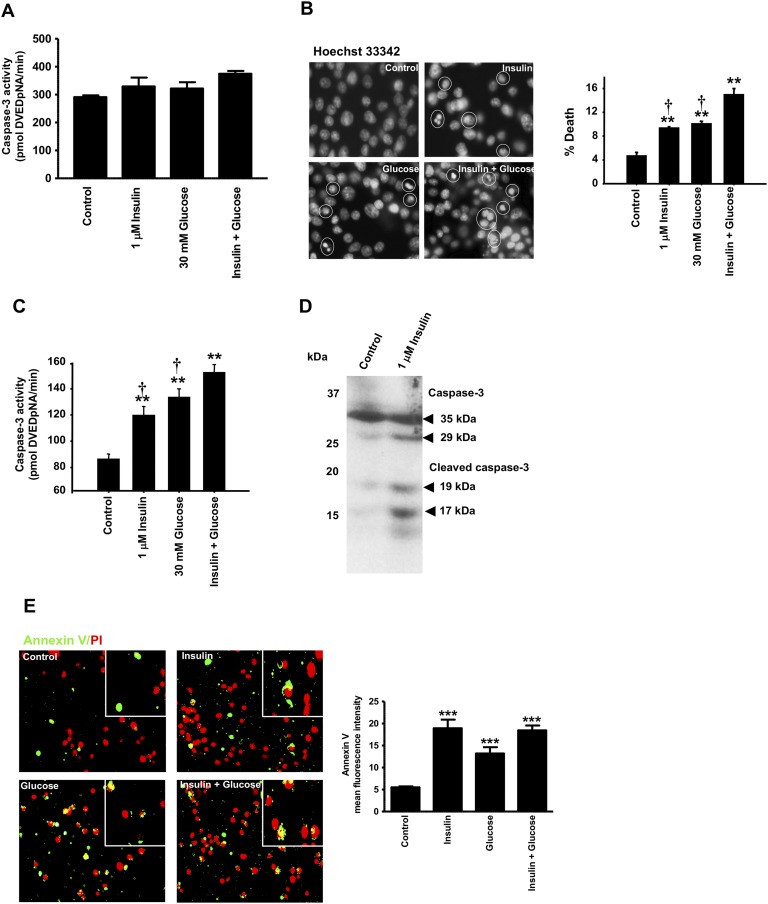
Because it is now well established that the insulin/IGF-1 signaling pathway, through activation of Akt and Erk1/2 proteins, plays an important role in the maintenance of β-cell mass, we asked whether inhibition of Akt and Erk1/2 phosphorylation by prolonged exposure to high insulin levels leads to decreased islet and β-cell survival. Isolated rat islets and INS1E β-cells were exposed to high insulin levels and/or high glucose levels and caspase activities and annexin V staining was measured (Figs. 5 and 6). We found that at 24 hours exposure to insulin, there were no measurable differences in caspase-3 activity between control-, insulin-, and glucose-treated INS1E β-cells (Fig. 5A). In contrast, after 72 hours of exposure of INS1E β-cells to 1 μM insulin, 30 mM glucose, or to both, we noted cytoplasmic shrinkage, nuclear fragmentation, and chromatin condensation as shown by Hoechst 33342 staining (Fig. 5B). Exposure of INS1E β-cells to 1 μM insulin or 30 mM glucose led to ∼1.4- and 1.6-fold increases, respectively, in caspase-3 activity (Fig. 5C). When combined together, insulin and glucose had a larger effect on caspase-3 activity, resulting in an ∼1.8-fold increase compared with control nontreated cells. Figure 5D shows that exposure of INS1E β-cells to 1 μM insulin for 72 hours increased the cleaved and active forms of caspase-3 (i.e., 17- and 19-kDa fragments) compared with control nontreated cells. During early apoptosis, phosphatidylserine, a phospholipid that is located on the inner surface of the plasma membrane, is translocated to the outer membrane. Annexin V has high affinity for phosphatidylserines and is used to stain early apoptotic cells. Using an annexin V Alexa Fluor conjugate, we measured annexin V staining of INS1E β-cells exposed to 1 μM insulin, 30 mM glucose, or to both. We found that the mean fluorescence of annexin V was significantly increased by prolonged exposure to insulin, glucose, or to their combination when compared with control untreated INS1E β-cells (Fig. 5E).
Figure 5.
Effect of prolonged exposure to insulin, glucose, or their combination on INS1E β-cell apoptosis. (A–E) INS1E β-cells were treated for 24 h (A) or 72 h (B–E) with 1 μM insulin, 30 mM glucose, or 1 μM insulin plus 30 mM glucose. (A) Caspase-3 activity assay using DEVD-pNA was measured. Results are expressed as picomoles DEVD-pNA/min. (B) DNA fragmentation analysis was determined by Hoechst 33342 staining at ×20 original magnification. Representative images and quantitative measure of cells with fragmented DNA or condensed chromatin are shown. (C) Caspase-3 activity assay using DEVD-pNA was measured after 72-h treatment with insulin, glucose, or their combination. Results are expressed as picomoles DEVD-pNA/min. (D) Immunoblotting for cleaved caspase-3 in control and insulin-treated cells. A representative autoradiogram of three independent experiments is shown. (E) INS1E β-cells in suspension were stained with annexin V and propidium iodide and visualized using a fluorescence microscope at ×20 original magnification. The mean fluorescence intensity was measured using ImageJ. Means ± SEM are from three to four independent experiments with each done in duplicates. **P < 0.005, ***P < 0.0005, chronic insulin, glucose, or their combination compared with control nontreated cells; †P < 0.05 compared with insulin plus glucose treated cells.
Figure 6.
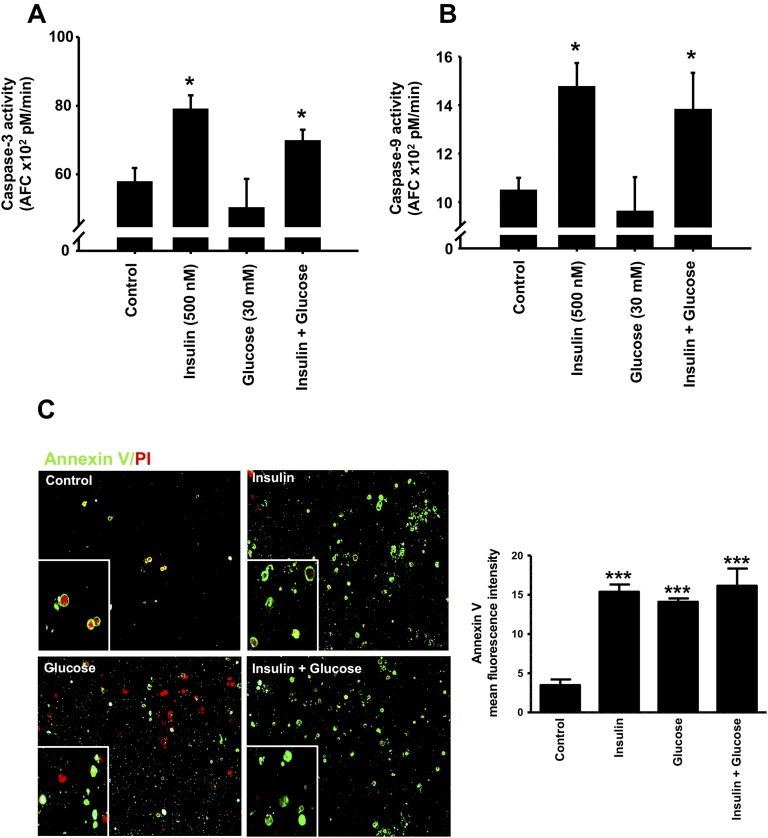
Effect of prolonged exposure to insulin on apoptosis in rat pancreatic islets. Islets were treated for 72 h with 500 nM insulin, 30 mM glucose, or insulin plus glucose, and caspase-3 (A) and caspase-9 (B) activities were measured using fluorometric substrates (DEVD-AFC and LEHD-AFC, respectively). Results are expressed as picomoles of FCA/min. (C) Annexin V staining of dispersed islet cells was measured using an annexin V Alexa Fluor 488 conjugate using a fluorescence microscope at ×20 original magnification and mean fluorescence intensity was measured using ImageJ. Means ± SEM are from four independent experiments with each done in duplicates. *P < 0.05, ***P < 0.0005, chronic insulin, glucose, or insulin plus glucose compared with control nontreated cells.
We extended these findings to isolated pancreatic rat islets exposed to 500 nM insulin, 30 mM glucose, or to their combination for 72 hours, and caspase-3 and caspase-9 activities as well as annexin V staining were measured. Exposure of islets to high concentrations of insulin resulted in an ∼40% increase in caspase-3 and caspase-9 activities, suggesting diminished islet cell survival (Fig. 6A and 6B). Exposure to 30 mM glucose did not affect caspase-3 and caspase-9 activities in islets, and the combination of insulin and glucose elicited the same response as that observed in presence of high insulin levels alone (Fig. 6A and 6B). Figure 6C shows that a 72-hour exposure of isolated islets to 500 nM insulin, 30 mM glucose, or to their combination leads to a significant increase in the mean fluorescence intensity of annexin V staining. Treatment of isolated islets with insulin and glucose together did not further increase annexin V staining compared with insulin or glucose alone (Fig. 6C).
C. Effects of Prolonged Exposure to Insulin on β-Cell Intracellular Ca2+ Signaling
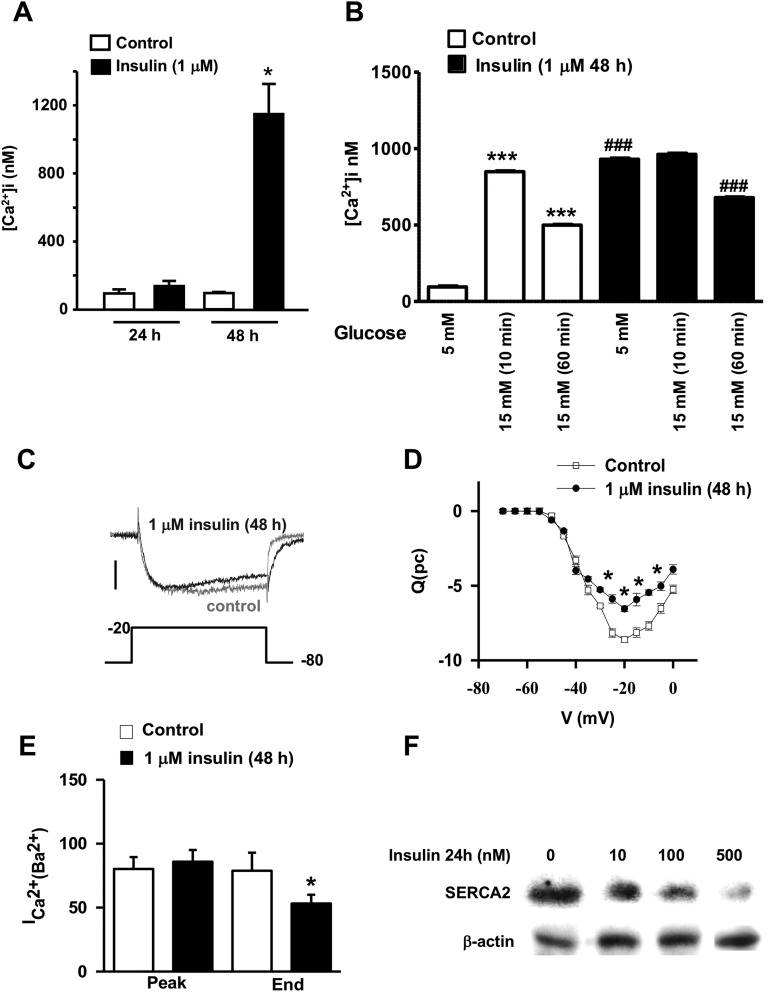
Studies have shown that obesity and diabetes are conditions in which intracellular Ca2+ homeostasis is impaired in many cell types, including pancreatic β-cells, and where the most common abnormality is increased intracellular Ca2+ [53, 54]. Calcium signaling plays a critical role in β-cell function and insulin secretion. Disruptions in cytosolic calcium handling, for example in response to cytokines, can impair insulin secretion and lead to cell death [55]. Additionally, recent studies have shown that an acute exposure to insulin affects intracellular Ca2+ signaling in β-cells of transplantable human islets [56]. Therefore, we investigated whether prolonged exposure of pancreatic β-cells to high insulin levels changes intracellular Ca2+ signals. We employed fura-2 dye and fluorescence microscopy to measure changes in [Ca2+]i. Figure 7A shows that at 24 hours, high insulin levels had no effect on INS1E β-cell [Ca2+]I; however, a 48-hour exposure to insulin led to a significant and robust increase in [Ca2+]i (Fig. 7A). To investigate the effects of prolonged exposure to high insulin levels on the glucose-stimulated Ca2+ response, INS1E β-cells where incubated with either 5 or 15 mM glucose for 10 to 60 minutes, and similarly [Ca2+]i was measured using fura-2 labeling. Figure 7B shows that in control cells, a 10-minute incubation with 15 mM glucose elicited a significant and robust increase in [Ca2+]i. A 60-minute incubation of INS1E β-cells with 15 mM glucose still increased [Ca2+]i compared with the basal 5 mM glucose concentration; however, the increase was less pronounced than that of the 10-minute incubation with 15 mM glucose. Chronic exposure to high insulin levels increased basal [Ca2+]i in INS1E β-cells, and 15 mM glucose had no further stimulation of the calcium response at a 10- or 60-minute incubation, indicating a disruption of INS1E β-cell Ca2+ signaling (Fig. 7B). To further explore the effects of prolonged exposure to high insulin levels on β-cell calcium signaling, we evaluated the magnitude and behavior of the plasma membrane calcium currents in control and insulin-pretreated INS1E β-cells by performing voltage-clamp studies under whole-cell patch configuration. We specifically designed conditions to eliminate contamination by other currents. Cells were stimulated using a 100 msec step depolarization from −80 mV to +20 mV with increments of 5 mV. Figure 7C shows representative tracings of the profile of the total calcium current from control cells and cells exposed to 1 μM insulin for 48 hours. As shown previously, the presence of small currents were observed at depolarizations as low as −60 mV; subsequently, the current increased to reach a peak at about −20 mV (Fig. 7D). Cells pretreated with 1 μM insulin for 48 hours exhibited an increase in the rate of inactivation of the total current with no change in the peak of the current as shown by the two traces superimposed in Fig. 7C and 7D. The changes in inactivation resulted in a statistically significant decrease in the influx of total charge during the step depolarization as shown by the integrated Ca2+ current vs voltage relationship in Fig. 7E.
Figure 7.
Effect of prolonged exposure to insulin on [Ca2+]i, total calcium current (ICa2+), and SERCA2 protein. (A and B) INS1E β-cells cultured on glass coverslips were loaded with fura-2–PE3 (2 µmol/L) and Mag–fluo-4-AM (1 μM), respectively (Molecular Probes, Eugene, OR). Pluronic acid (0.01%) was added to the medium and cells were incubated for 30 min at 37°C. [Ca2+]i was measured using an Olympus IX-50 inverted epifluorescence microscope (Olympus, Tokyo, Japan). Absolute [Ca2+]i was determined from R of Ca2+-bound fura-2 (excited at 340 nm) to unbound fura-2 (excited at 380 nM). The Ca2+ levels were determined using a standard equation for calibration. Rmax and Rmin were obtained by adding ionomycin (10 µM) or EGTA (1 mM), respectively, to fura-2–loaded β-cells at the end of each experiment. [Ca2+]i was obtained using an excitation wavelength of 488 nm and an emission band-pass filter of 515/15 nm. Means ± SEM are from four independent experiments. The ICa2+ (Ba2+) from control and insulin pretreated cells for 48 h were evoked by a 100 msec step depolarization under whole-cell voltage clamp configuration. *P < 0.05, chronic insulin (48 h) compared with chronic insulin (24 h); ***P < 0.0005, 15 mM glucose (10 and 60 min control) compared with 5 mM basal; ###P < 0.0005, chronic insulin compared with control nontreated cells. (C) Representative two typical traces at the maximal current (−20 mV) from control (gray) and insulin-pretreated cells (1 μM, 48 h) (black). (D) Average integrated current (C)–voltage (V) relationships. Data represent means ± SEM from control (□, n = 18) or insulin-treated cells (●, n=6). (E) Quantification of the magnitude of the peak of the calcium current and the end of the voltage step (95 msec) of the maximal current (determined by the correspondent Influx-Voltage relationship for each cell tested, usually about −20 mV). *P < 0.05, compared with control nontreated cells. (F) Islets were treated with the indicated concentrations of insulin for 24 h, and then the expression of the ER-Ca2+-ATPase SERCA2 was analyzed by Western blot using a specific antibody in islet whole-cell lysates. A representative autoradiogram of four independent experiments is shown.
Additionally, the ER is the largest reservoir of intracellular Ca2+. The Ca2+ gradient that exists between the cytosol and the ER is maintained by ER-specific Ca2+ ATPases (e.g., SERCA2) that pump the Ca2+ into the ER where it is buffered by Ca2+-binding proteins such as calcineurin and calreticulin [57]. We next asked whether exposure to high insulin levels affects SERCA2 expression. A 24-hour exposure of rat islets to increasing concentrations of insulin resulted in a dose-dependent decrease in SERCA2 protein expression (Fig. 7F). Taken together, the above findings suggest that dysfunction of the voltage-dependent calcium channels and/or of the ER-specific Ca2+ pump SERCA2 could partly explain the perturbed [Ca2+]i that is observed following prolonged exposure to high insulin levels.
D. Effects of Prolonged Exposure to Insulin on β-Cell ER Stress Responses and ER-Mediated Apoptosis
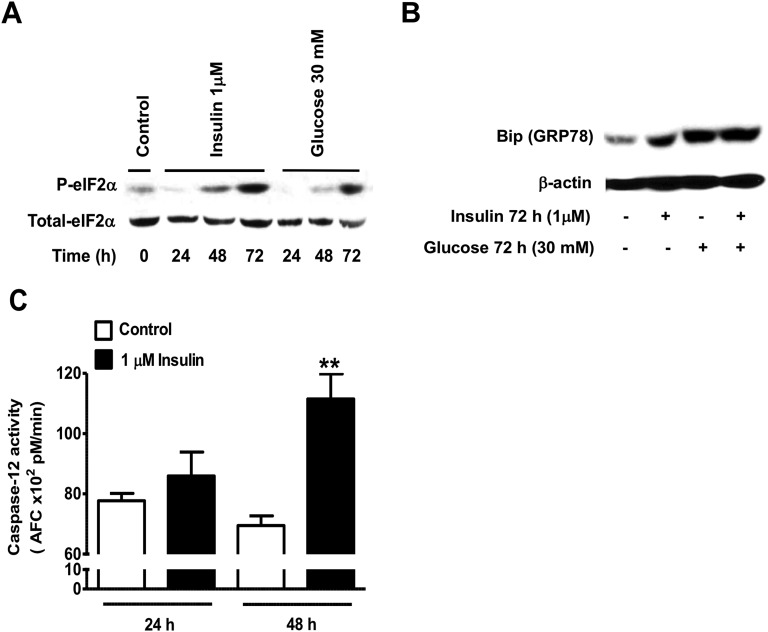
Because insults that disrupt intracellular Ca2+ homeostasis can trigger the activation of ER stress pathways such as the unfolded protein response, we next examined the effects of prolonged exposure to insulin on the expression and activation of ER stress markers. Chronic exposure of INS1E β-cells to 1 μM insulin led to a time-dependent increase in eIF2αS51 phosphorylation that was greater than the response observed following chronic exposure to 30 mM glucose (Fig. 8A). The abundance of Bip (GRP78) was also increased in INS1E β-cells exposed for 72 hours to 1 μM insulin, 30 mM glucose, or to insulin and glucose combined (Fig. 8B). Perturbations in intracellular Ca2+ signaling were also shown to trigger cell apoptosis via activation of calpains (calcium-sensitive proteases) [58]. Caspase-12 is the primary substrate of calpains and was shown to be involved in ER stress–mediated apoptosis in β-cells [59]. We then asked whether chronic exposure to high insulin levels activates ER stress apoptotic pathways through activation of caspase-12. We found that exposure to high insulin levels lead to the activation of caspase-12, an effect that was not detected at 24 hours but was significant at 48 hours and correlated with the increase in [Ca2+]i (Fig. 8C). Another indication that calpains might be involved in the high insulin–mediated β-cell apoptosis through the ER stress pathway is the increase in the 29-kDa band of caspase-3 observed in Fig. 5C, which was previously described as a calpain-mediated breakdown product in other cell types [60, 61]. Further studies are needed to explore this possibility.
Figure 8.
Effect of prolonged exposure to high insulin levels on ER stress markers. (A and B) INS1E β-cells were treated with the indicated concentrations of insulin or glucose for 24, 48, or 72 h and then ER stress markers such as eIF2α (A) and Bip (B) were measured by immunoblot in whole-cell lysates using specific antibodies. The activity of the ER stress–activated caspase-12 was also quantified using the fluorometric substrate LEHD-AFC and results are expressed as picomoles of FCA/min (C). Means ± SEM are from four independent experiments with each done in duplicates. *P < 0.05, compared with control nontreated cells.
3. Discussion
Hyperinsulinemia is a hallmark of insulin-resistant states such as obesity and early type 2 diabetes. Persistent hyperinsulinemia causes desensitization to insulin as well as to IGF-1 in peripheral tissues, an effect that may be part of a vicious cycle involved in the metabolic dysregulations observed in obesity and in the progression of the pathogenesis of type 2 diabetes [7, 8]. Hyperglycemia and frank diabetes only develop when pancreatic β-cells become dysfunctional and fail to adequately compensate for the peripheral insulin resistance [62, 63].
Insulin and IGF-1 signaling pathways play critical and positive roles in maintaining pancreatic β-cell function and mass. Several studies using insulin/IGF-1 signaling knockout models have suggested that common molecular defects in these signaling pathways may underlie both peripheral and pancreatic β-cell insulin resistance, thereby leading to β-cell decompensation [3–6, 64]. The interest in exploring the autocrine effects of insulin on pancreatic β-cells has increased in recent years. However, we are far from having a clear understanding of how insulin affects pancreatic β-cell mass; the molecular and signaling mechanisms involved are still not fully identified. In the current study we sought to investigate whether prolonged exposure to elevated levels of insulin, mimicking hyperinsulinemia, could lead to “insulin as well as IGF-1 resistance” in pancreatic islets and in INS1E β-cells. We demonstrate, to our knowledge for the first time, that persistent exposure of pancreatic islets and insulinoma cells to increasing concentrations of insulin leads to a desensitization to insulin and, in a heterologous manner, to IGF-1 signaling; this resulted in nearly complete inhibition of Akt phosphorylation at higher insulin concentrations. We also made the interesting observation that Akt phosphorylation in response to an acute stimulation with insulin or IGF-1 was enhanced when isolated islets were exposed to lower concentrations of insulin (10 nM) for 24 hours, an effect that was not observed in the insulinoma cells. It is now clear that Akt activation has antiapoptotic effects on islets [24, 65, 66], and enhanced Akt activation was suggested to be involved in the processes of pancreatic β-cell compensation for the insulin resistance in the early stages of type 2 diabetes [67]. We suspect that in the case of mild hyperinsulinemia, insulin might promote pancreatic β-cell survival. These autocrine and paracrine effects of insulin on Akt activation require islet integrity, as was demonstrated by Aikin et al. [49] in their study on human islets. Insulin had no effect on Akt phosphorylation when islets were dispersed to single cells [49]. In addition to the Akt pathway, earlier studies have also shown that the antiapoptotic actions of low doses of insulin are mediated via the activation of Raf-1/Erk1/2 signaling cascades of the insulin/IGF-1 signaling pathway [25]. Our data demonstrate that prolonged exposure to high insulin levels not only inhibits Akt phosphorylation, but it also results in a significant inhibition of Erk1/2 phosphorylation in response to the acute stimulation by glucose, insulin, or IGF-1. These findings suggest that common upstream signaling events in the insulin/IGF-1 signaling pathway might be involved in the inhibitions that are observed following prolonged exposure to high insulin levels in pancreatic β-cells.
Reduction of IR number and/or kinase activity by chronic exposure to insulin has been suggested to be involved in peripheral insulin resistance [7, 8]. We found that the abundance of IR was somewhat decreased in cells pre-exposed to insulin. Several studies have shown in other cell types, such as adipocytes and pancreatic acinar cells, that chronic exposure to insulin leads to IR dysfunction primarily by downregulating receptor level at the cell surface, perhaps through internalization and degradation [68, 69], or decreased receptor expression and translocation to the cell membrane [69]. Prolonged exposure to high insulin levels neither downregulated the abundance of IGF-1 receptor nor decreased tyrosine phosphorylation of the IGFR β-subunit in response to the acute challenge with IGF-1. Thus, it appears that unlike the insulin-signaling pathway, insulin-induced desensitization of IGF-1 signaling in β-cells occurs at postreceptor levels. The reduction in tyrosine phosphorylation of IRS2 in response to both insulin and IGF-1 could be of importance because IRS2 was shown to play a critical role in maintaining normal β-cell function and mass [17, 21]. Deletion of the IRS2 gene in mice induces diabetes due to defects in both insulin signaling in peripheral tissues and in pancreatic β-cell function [70], and β-cell–specific knockout of IRS2 resulted in inadequate β-cell compensatory response to insulin resistance [3, 71]. Overexpression of IRS2 in isolated rat or human islets increases β-cell proliferation, decreases glucose-induced apoptosis, and increases basal and glucose-stimulated insulin secretion [72].
As discussed earlier, given the importance of the IRS2/Akt and IRS2/Erk1/2 pathways to the maintenance of normal pancreatic β-cell physiology, our findings of decreased insulin and IGF-1–stimulated phosphorylation of IRS2, Akt, and ERk1/2 in pancreatic β-cells following prolonged exposure to high insulin levels raises the possibility that, in vivo, a persistent hyperinsulinemia through an autocrine action might have detrimental effects on pancreatic β-cell survival and mass. We provide evidence that prolonged exposure to high levels of insulin increases the activity of caspase-3 and caspase-9 and apoptosis in isolated rat islets and INS1E β-cells. This is in agreement with previous studies in mouse and human islets and in pancreatic β-cell lines following prolonged exposure to high concentrations of insulin [26, 27]. Several previous studies have suggested that persistent hyperglycemia, through glucotoxicity, leads to increased oxidative stress and β-cell death by apoptosis [73, 74]. We found that in INS1E β-cells, the number of apoptotic cells and caspase-3 activity were further increased when cells were incubated in the presence of high insulin levels plus high glucose levels. However, exposure to 30 mM glucose had no effect on caspase-3 and caspase-9 activities, but it increased annexin V staining in islets. The reasons for these discrepancies are not clear. Although a number of reports suggest that chronic exposure of β-cells to high glucose levels induce cell death by apoptosis through production of reactive oxygen species and activation of the mitochondrial apoptotic pathway [74, 75], other reports have shown opposite effects whereby glucose promotes β-cell survival and proliferation [76–78].
Additionally, disturbances in intracellular Ca2+ signaling were proposed as mechanisms involved in pancreatic β-cell death by apoptosis; these are known to be present in disorders of insulin resistance such as obesity and diabetes [53, 54, 79, 80]. Our data indicate that prolonged exposure of INS1E β-cells to high insulin levels results in disturbances in calcium signaling. Levels of intracellular Ca2+ are under tight control, and the ER plays an important role in regulating Ca2+ signaling. The movement of the intracellular Ca2+ is predominantly mediated through SERCA proteins, the major ER Ca2+ pumps that are implicated in the sequestration of cytosolic Ca2+ in the ER [81]. Among SERCA proteins that have been identified, SERCA2a, SERCA2b, and SERCA3 are expressed in pancreatic β-cells [82]. In the current study, we show that prolonged exposure to high insulin levels significantly decreased the expression of the major ER-Ca2+-ATPase SERCA2 protein in isolated rat islets, which could partly explain the observed increase in [Ca2+]i. Several studies have shown that insulin regulates the activity and expression of SERCA proteins through the IRS1/phosphatidylinositol 3-kinase signaling pathway [82]. An acute stimulation of isolated β-cells from wild-type IRS1+/+ mice with L783-281, an IR agonist, increased [Ca2+]i through an IRS1-mediated inhibition of SERCA activity and significantly increased insulin exocytosis [83]. The same agonist was unable to induce such effects in islets from IRS1−/− mice [83]. However, long-term exposure to insulin or L783-281 or chronic activation of insulin signaling by overexpression of IRS1 resulted in a sustained increase in [Ca2+]i [84].
Insults that disrupt intracellular Ca2+ homeostasis can trigger the activation of ER stress pathways such as the unfolded protein response. For example, exposure of pancreatic β-cells to cytokines or free fatty acids decreases SERCA expression, depletes ER-Ca2+, increases [Ca2+]i, and triggers ER stress–mediated β-cell apoptosis [85, 86]. Our data show that prolonged exposure of INS1E β-cells to high insulin levels increases ER stress markers, such as eIF2αS51 phosphorylation and Bip (GRP78) expression. Additionally, we show that exposure to high insulin levels lead to the activation of caspase-12, a substrate of the calcium-sensitive proteases (i.e., calpains), which are known to be involved in the ER stress–mediated apoptosis in β-cells [58, 59]. This is indicated by the increase in the 29-kDa band of caspase-3 observed in Fig. 5D, which was previously described in other cell types [60, 61]. Additional studies are necessary to further investigate the role of calpains in the effects of insulin on the ER-mediated β-cell apoptosis. Additionally, it is well established that [Ca2+]i is also fine-tuned by proapoptotic and antiapoptotic proteins located on the mitochondria and that increases in [Ca2+]i occur at early and late stages of apoptosis [87, 88]. Studies have also shown that because of the proximity of mitochondria to the ER or to the plasma membrane, increases in cytosolic Ca2+ concentration lead to a rise in mitochondrial calcium [89], which leads to organelle swelling and fragmentation and release of proapoptotic mediators [90]
Taken together, our findings suggest that chronic exposure to hyperinsulinemia might be detrimental to pancreatic β-cells via an induction of insulin and IGF-1 resistance and inhibition of the IRS2/Akt and IRS2/ERk1/2 signaling cascades (Fig. 9). Because these pathways play important roles in maintaining β-cell function and mass [5, 6], we think that hyperinsulinemia-induced dysregulations of these signaling pathways might result in increased β-cell death through both mitochondrial and ER stress–mediated apoptotic pathways (Fig. 9). Therefore, we think that in conditions of insulin resistance, such as obesity and prediabetes, an increased insulin secretion in the vicinity of β-cells, through an autocrine action, might participate in pancreatic β-cell decompensation and the progression of diabetes. Future studies are warranted to further explore this hypothesis and elucidate additional mechanisms.
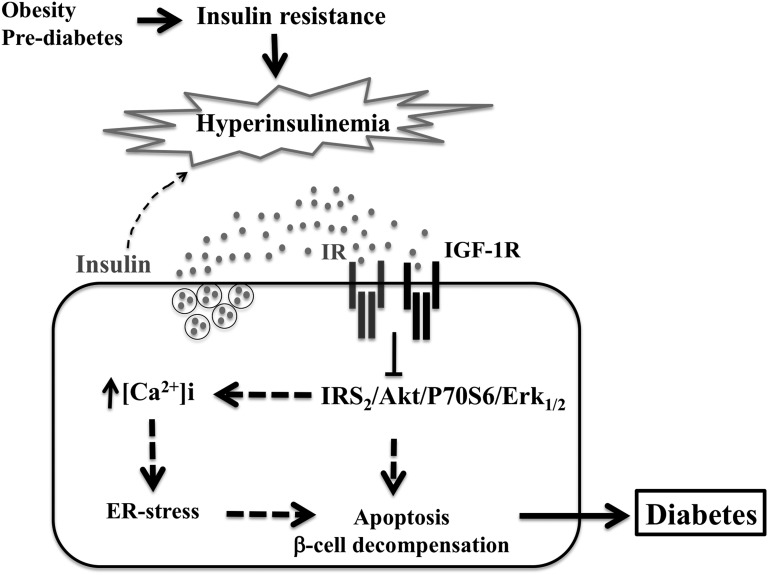
Figure 9.
Chronic exposure to hyperinsulinemia participates in pancreatic β-cell decompensation. In conditions of insulin resistance and increased insulin secretion in the vicinity of pancreatic β-cells, such as in obesity and prediabetes, chronic exposure of pancreatic β-cells to insulin might induce insulin and IGF-1 resistance and inhibition of IRS2/Akt/P70S6K and IRS2/ERk1/2 activation. Because these pathways play an important role in pancreatic β-cell survival, inhibition of Akt and Erk1/2 could result in increased islet and β-cell death through both mitochondrial and calcium-sensitive ER stress apoptotic pathways and therefore lead to pancreatic β-cell decompensation and the progression of diabetes.
Acknowledgments
We thank Drs. P. Maechler and C. B. Wollheim (University of Geneva, Geneva, Switzerland) for providing us with the INS1E β-cell line.
Financial Support: This work was supported by Diabetes Association of Greater Cleveland Grant-in-Aid 502-05 (to N.R.), Juvenile Diabetes Research Foundation International Innovative Award 5-2008-310 (to N.R.), and National Institutes of Health Grant DK 061994A1 (to F.I.-B.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ATAD-AFC
Ac-Ala-Thr-Ala-Asp–7-amino-4-trifluoromethyl coumarin
- [Ca2+]i
intracellular Ca2+ concentration
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- DEVE-AFC
Asp-Glu-Val-Asp–7-amino-trifluoromethyl coumarin
- DEVD-pNA
N-acetyl–Asp-Glu-Val-Asp–p-nitroanilide
- ER
endoplasmic reticulum
- IGFR
IGF receptor
- IR
insulin receptor
- IRS
IR substrate
- LEHD-AFC
Glu-His-Asp–7-amino-4-trifluoromethyl coumarin
- R
fluorescence ratio
- TEA-Cl
tetraethylammonium chloride
References and Notes
- 1. Chiasson JL, Rabasa-Lhoret R. Prevention of type 2 diabetes: insulin resistance and beta-cell function. Diabetes. 2004;53(Suppl 3):S34–S38. [DOI] [PubMed] [Google Scholar]
- 2. Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(Suppl 2):S262–S268. [DOI] [PubMed] [Google Scholar]
- 3. Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF. Dysregulation of insulin receptor substrate 2 in β cells and brain causes obesity and diabetes. J Clin Invest. 2004;114(7):908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Otani K, Kulkarni RN, Baldwin AC, Krutzfeldt J, Ueki K, Stoffel M, Kahn CR, Polonsky KS. Reduced β-cell mass and altered glucose sensing impair insulin-secretory function in βIRKO mice. Am J Physiol Endocrinol Metab. 2004;286(1):E41–E49. [DOI] [PubMed] [Google Scholar]
- 5. Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR. β-Cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter β-cell mass. Nat Genet. 2002;31(1):111–115. [DOI] [PubMed] [Google Scholar]
- 6. Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96(3):329–339. [DOI] [PubMed] [Google Scholar]
- 7. Gherzi R, Briata P. [Insulin receptors, mechanism of signal transduction. Examples of abnormalities in states of insulin resistance]. Journ Annu Diabetol Hotel Dieu. 1993:11–21. [PubMed] [Google Scholar]
- 8. Treadway JL, Whittaker J, Pessin JE. Regulation of the insulin receptor kinase by hyperinsulinism. J Biol Chem. 1989;264(25):15136–15143. [PubMed] [Google Scholar]
- 9. Rui L, Fisher TL, Thomas J, White MF. Regulation of insulin/insulin-like growth factor-1 signaling by proteasome-mediated degradation of insulin receptor substrate-2. J Biol Chem. 2001;276(43):40362–40367. [DOI] [PubMed] [Google Scholar]
- 10. Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes. 2001;50(1):24–31. [DOI] [PubMed] [Google Scholar]
- 11. Mothe I, Van Obberghen E. Phosphorylation of insulin receptor substrate-1 on multiple serine residues, 612, 632, 662, and 731, modulates insulin action. J Biol Chem. 1996;271(19):11222–11227. [DOI] [PubMed] [Google Scholar]
- 12. Scioscia M, Gumaa K, Kunjara S, Paine MA, Selvaggi LE, Rodeck CH, Rademacher TW. Insulin resistance in human preeclamptic placenta is mediated by serine phosphorylation of insulin receptor substrate-1 and -2. J Clin Endocrinol Metab. 2006;91(2):709–717. [DOI] [PubMed] [Google Scholar]
- 13. Rechler MM, Nissley SP. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47(1):425–442. [DOI] [PubMed] [Google Scholar]
- 14. Heaton JH, Krett NL, Alvarez JM, Gelehrter TD, Romanus JA, Rechler MM. Insulin regulation of insulin-like growth factor action in rat hepatoma cells. J Biol Chem. 1984;259(4):2396–2402. [PubMed] [Google Scholar]
- 15. Conover CA, Clarkson JT, Bale LK. Physiological concentrations of insulin induce cellular desensitization to the mitogenic effects of insulin-like growth factor I. Diabetes. 1994;43(9):1130–1137. [DOI] [PubMed] [Google Scholar]
- 16. Nakagawa A, Stagner JI, Samols E. In situ binding of islet hormones in the isolated perfused rat pancreas: evidence for local high concentrations of islet hormones via the islet-acinar axis. Diabetologia. 1995;38(3):262–268. [DOI] [PubMed] [Google Scholar]
- 17. Kubota N, Terauchi Y, Tobe K, Yano W, Suzuki R, Ueki K, Takamoto I, Satoh H, Maki T, Kubota T, Moroi M, Okada-Iwabu M, Ezaki O, Nagai R, Ueta Y, Kadowaki T, Noda T. Insulin receptor substrate 2 plays a crucial role in β cells and the hypothalamus. J Clin Invest. 2004;114(7):917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, Han Z, Polonsky KS, Permutt MA. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet β cells. J Clin Invest. 2004;114(7):928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Da Silva Xavier G, Qian Q, Cullen PJ, Rutter GA. Distinct roles for insulin and insulin-like growth factor-1 receptors in pancreatic beta-cell glucose sensing revealed by RNA silencing. Biochem J. 2004;377(Pt 1):149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hill DJ, Petrik J, Arany E, McDonald TJ, Delovitch TL. Insulin-like growth factors prevent cytokine-mediated cell death in isolated islets of Langerhans from pre-diabetic non-obese diabetic mice. J Endocrinol. 1999;161(1):153–165. [DOI] [PubMed] [Google Scholar]
- 21. Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated β-cell development and peripheral insulin signalling. Nat Genet. 1999;23(1):32–40. [DOI] [PubMed] [Google Scholar]
- 22. Giannoukakis N, Mi Z, Rudert WA, Gambotto A, Trucco M, Robbins P. Prevention of beta cell dysfunction and apoptosis activation in human islets by adenoviral gene transfer of the insulin-like growth factor I. Gene Ther. 2000;7(23):2015–2022. [DOI] [PubMed] [Google Scholar]
- 23. Chen W, Salojin KV, Mi QS, Grattan M, Meagher TC, Zucker P, Delovitch TL. Insulin-like growth factor (IGF)-I/IGF-binding protein-3 complex: therapeutic efficacy and mechanism of protection against type 1 diabetes. Endocrinology. 2004;145(2):627–638. [DOI] [PubMed] [Google Scholar]
- 24. Dickson LM, Rhodes CJ. Pancreatic β-cell growth and survival in the onset of type 2 diabetes: a role for protein kinase B in the Akt? Am J Physiol Endocrinol Metab. 2004;287(2):E192–E198. [DOI] [PubMed] [Google Scholar]
- 25. Alejandro EU, Kalynyak TB, Taghizadeh F, Gwiazda KS, Rawstron EK, Jacob KJ, Johnson JD. Acute insulin signaling in pancreatic beta-cells is mediated by multiple Raf-1 dependent pathways. Endocrinology. 2010;151(2):502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson JD, Bernal-Mizrachi E, Alejandro EU, Han Z, Kalynyak TB, Li H, Beith JL, Gross J, Warnock GL, Townsend RR, Permutt MA, Polonsky KS. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci USA. 2006;103(51):19575–19580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bucris E, Beck A, Boura-Halfon S, Isaac R, Vinik Y, Rosenzweig T, Sampson SR, Zick Y. Prolonged insulin treatment sensitizes apoptosis pathways in pancreatic β cells. J Endocrinol. 2016;230(3):291–307. [DOI] [PubMed] [Google Scholar]
- 28.RRID:AB_667741.
- 29.RRID:AB_671713.
- 30.RRID:AB_2182256.
- 31.RRID:AB_2182106.
- 32.RRID:AB_668967.
- 33.RRID:AB_2125783.
- 34.RRID:AB_631835.
- 35.RRID:AB_671792.
- 36.RRID:AB_331228.
- 37.RRID:AB_331439.
- 38.RRID:AB_2119843.
- 39.RRID:AB_10827913.
- 40.RRID:AB_390779.
- 41.RRID:AB_2096481.
- 42.RRID:AB_1840685.
- 43.RRID:AB_2575162.
- 44. Xia M, Laychock SG. Insulin secretion, myo-inositol transport, and Na+-K+-ATPase in glucose-desensitized rat islets. Diabetes. 1993;42(10):1392–1400. [DOI] [PubMed] [Google Scholar]
- 45. Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology. 2004;145(2):667–678. [DOI] [PubMed] [Google Scholar]
- 46. Rachdaoui N, Nagy LE. Endothelin-1-stimulated glucose uptake is desensitized by tumor necrosis factor-α in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2003;285(3):E545–E551. [DOI] [PubMed] [Google Scholar]
- 47. Brady-Kalnay SM, Rimm DL, Tonks NK. Receptor protein tyrosine phosphatase PTPmu associates with cadherins and catenins in vivo. J Cell Biol. 1995;130(4):977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Polo-Parada L, Korn SJ. Block of N-type calcium channels in chick sensory neurons by external sodium. J Gen Physiol. 1997;109(6):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aikin R, Hanley S, Maysinger D, Lipsett M, Castellarin M, Paraskevas S, Rosenberg L. Autocrine insulin action activates Akt and increases survival of isolated human islets. Diabetologia. 2006;49(12):2900–2909. [DOI] [PubMed] [Google Scholar]
- 50. Lawrence M, Shao C, Duan L, McGlynn K, Cobb MH. The protein kinases ERK1/2 and their roles in pancreatic beta cells. Acta Physiol (Oxf). 2008;192(1):11–17. [DOI] [PubMed] [Google Scholar]
- 51. Briaud I, Lingohr MK, Dickson LM, Wrede CE, Rhodes CJ. Differential activation mechanisms of Erk-1/2 and p70S6K by glucose in pancreatic β-cells. Diabetes. 2003;52(4):974–983. [DOI] [PubMed] [Google Scholar]
- 52. Dickson LM, Lingohr MK, McCuaig J, Hugl SR, Snow L, Kahn BB, Myers MG Jr, Rhodes CJ. Differential activation of protein kinase B and p70S6K by glucose and insulin-like growth factor 1 in pancreatic β-cells (INS-1). J Biol Chem. 2001;276(24):21110–21120. [DOI] [PubMed] [Google Scholar]
- 53. Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14(1):20–28. [DOI] [PubMed] [Google Scholar]
- 54. Efanova IB, Zaitsev SV, Zhivotovsky B, Köhler M, Efendić S, Orrenius S, Berggren PO. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. J Biol Chem. 1998;273(50):33501–33507. [DOI] [PubMed] [Google Scholar]
- 55. Ramadan JW, Steiner SR, O’Neill CM, Nunemaker CS. The central role of calcium in the effects of cytokines on beta-cell function: implications for type 1 and type 2 diabetes. Cell Calcium. 2011;50(6):481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Luciani DS, Johnson JD. Acute effects of insulin on beta-cells from transplantable human islets. Mol Cell Endocrinol. 2005;241(1-2):88–98. [DOI] [PubMed] [Google Scholar]
- 57. Meldolesi J, Pozzan T. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem Sci. 1998;23(1):10–14. [DOI] [PubMed] [Google Scholar]
- 58. Huang CJ, Gurlo T, Haataja L, Costes S, Daval M, Ryazantsev S, Wu X, Butler AE, Butler PC. Calcium-activated calpain-2 is a mediator of beta cell dysfunction and apoptosis in type 2 diabetes. J Biol Chem. 2010;285(1):339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Díaz-Horta O, Van Eylen F, Herchuelz A. Na/Ca exchanger overexpression induces endoplasmic reticulum stress, caspase-12 release, and apoptosis. Ann N Y Acad Sci. 2003;1010(1):430–432. [DOI] [PubMed] [Google Scholar]
- 60. Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathological apoptosis”? J Biol Chem. 2001;276(13):10191–10198. [DOI] [PubMed] [Google Scholar]
- 61. Lankiewicz S, Marc Luetjens C, Truc Bui N, Krohn AJ, Poppe M, Cole GM, Saido TC, Prehn JH. Activation of calpain I converts excitotoxic neuron death into a caspase-independent cell death. J Biol Chem. 2000;275(22):17064–17071. [DOI] [PubMed] [Google Scholar]
- 62. Leonardi O, Mints G, Hussain MA. Beta-cell apoptosis in the pathogenesis of human type 2 diabetes mellitus. Eur J Endocrinol. 2003;149(2):99–102. [DOI] [PubMed] [Google Scholar]
- 63. Steppel JH, Horton ES. Beta-cell failure in the pathogenesis of type 2 diabetes mellitus. Curr Diab Rep. 2004;4(3):169–175. [DOI] [PubMed] [Google Scholar]
- 64. Kulkarni RN, Winnay JN, Daniels M, Brüning JC, Flier SN, Hanahan D, Kahn CR. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured β-cell lines. J Clin Invest. 1999;104(12):R69–R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet β cell expression of constitutively active Akt1/PKBα induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001;108(11):1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rao P, Roccisana J, Takane KK, Bottino R, Zhao A, Trucco M, García-Ocaña A. Gene transfer of constitutively active Akt markedly improves human islet transplant outcomes in diabetic severe combined immunodeficient mice. Diabetes. 2005;54(6):1664–1675. [DOI] [PubMed] [Google Scholar]
- 67. Jetton TL, Lausier J, LaRock K, Trotman WE, Larmie B, Habibovic A, Peshavaria M, Leahy JL. Mechanisms of compensatory β-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes. 2005;54(8):2294–2304. [DOI] [PubMed] [Google Scholar]
- 68. Garvey WT, Olefsky JM, Marshall S. Insulin receptor down-regulation is linked to an insulin-induced postreceptor defect in the glucose transport system in rat adipocytes. J Clin Invest. 1985;76(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Okabayashi Y, Maddux BA, McDonald AR, Logsdon CD, Williams JA, Goldfine ID. Mechanisms of insulin-induced insulin-receptor downregulation. Decrease of receptor biosynthesis and mRNA levels. Diabetes. 1989;38(2):182–187. [DOI] [PubMed] [Google Scholar]
- 70. Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391(6670):900–904. [DOI] [PubMed] [Google Scholar]
- 71. Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI, Kadowaki T. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000;49(11):1880–1889. [DOI] [PubMed] [Google Scholar]
- 72. Mohanty S, Spinas GA, Maedler K, Zuellig RA, Lehmann R, Donath MY, Trüb T, Niessen M. Overexpression of IRS2 in isolated pancreatic islets causes proliferation and protects human β-cells from hyperglycemia-induced apoptosis. Exp Cell Res. 2005;303(1):68–78. [DOI] [PubMed] [Google Scholar]
- 73. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279(41):42351–42354. [DOI] [PubMed] [Google Scholar]
- 74. Kaiser N, Leibowitz G, Nesher R. Glucotoxicity and β-cell failure in type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2003;16(1):5–22. [DOI] [PubMed] [Google Scholar]
- 75. Kim WH, Lee JW, Suh YH, Hong SH, Choi JS, Lim JH, Song JH, Gao B, Jung MH. Exposure to chronic high glucose induces β-cell apoptosis through decreased interaction of glucokinase with mitochondria: downregulation of glucokinase in pancreatic β-cells. Diabetes. 2005;54(9):2602–2611. [DOI] [PubMed] [Google Scholar]
- 76. Kaung HC. Effect of glucose on beta cell proliferation and population size in organ culture of foetal and neonatal rat pancreases. J Embryol Exp Morphol. 1983;75:303–312. [PubMed] [Google Scholar]
- 77. Srinivasan S, Bernal-Mizrachi E, Ohsugi M, Permutt MA. Glucose promotes pancreatic islet β-cell survival through a PI 3-kinase/Akt-signaling pathway. Am J Physiol Endocrinol Metab. 2002;283(4):E784–E793. [DOI] [PubMed] [Google Scholar]
- 78. Hoorens A, Van de Casteele M, Klöppel G, Pipeleers D. Glucose promotes survival of rat pancreatic beta cells by activating synthesis of proteins which suppress a constitutive apoptotic program. J Clin Invest. 1996;98(7):1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Váradi A, Molnár E, Ostenson CG, Ashcroft SJ. Isoforms of endoplasmic reticulum Ca2+-ATPase are differentially expressed in normal and diabetic islets of Langerhans. Biochem J. 1996;319(Pt 2):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zarain-Herzberg A, García-Rivas G, Estrada-Avilés R. Regulation of SERCA pumps expression in diabetes. Cell Calcium. 2014;56(5):302–310. [DOI] [PubMed] [Google Scholar]
- 81. Beauvois MC, Merezak C, Jonas JC, Ravier MA, Henquin JC, Gilon P. Glucose-induced mixed [Ca2+]c oscillations in mouse β-cells are controlled by the membrane potential and the SERCA3 Ca2+-ATPase of the endoplasmic reticulum. Am J Physiol Cell Physiol. 2006;290(6):C1503–C1511. [DOI] [PubMed] [Google Scholar]
- 82. Kulkarni RN, Roper MG, Dahlgren G, Shih DQ, Kauri LM, Peters JL, Stoffel M, Kennedy RT. Islet secretory defect in insulin receptor substrate 1 null mice is linked with reduced calcium signaling and expression of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)-2b and -3. Diabetes. 2004;53(6):1517–1525. [DOI] [PubMed] [Google Scholar]
- 83. Roper MG, Qian WJ, Zhang BB, Kulkarni RN, Kahn CR, Kennedy RT. Effect of the insulin mimetic L-783,281 on intracellular Ca2+ and insulin secretion from pancreatic beta-cells. Diabetes. 2002;51(Suppl 1):S43–S49. [DOI] [PubMed] [Google Scholar]
- 84. Xu GG, Gao ZY, Borge PD Jr, Jegier PA, Young RA, Wolf BA. Insulin regulation of β-cell function involves a feedback loop on SERCA gene expression, Ca2+ homeostasis, and insulin expression and secretion. Biochemistry. 2000;39(48):14912–14919. [DOI] [PubMed] [Google Scholar]
- 85. Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes. 2005;54(2):452–461. [DOI] [PubMed] [Google Scholar]
- 86. Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in β-cells. Am J Physiol Endocrinol Metab. 2009;296(4):E690–E701. [DOI] [PubMed] [Google Scholar]
- 87. Kruman I, Guo Q, Mattson MP. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res. 1998;51(3):293–308. [DOI] [PubMed] [Google Scholar]
- 88. Tombal B, Denmeade SR, Isaacs JT. Assessment and validation of a microinjection method for kinetic analysis of [Ca2+]i in individual cells undergoing apoptosis. Cell Calcium. 1999;25(1):19–28. [DOI] [PubMed] [Google Scholar]
- 89. Pinton P, Brini M, Bastianutto C, Tuft RA, Pozzan T, Rizzuto R. New light on mitochondrial calcium. Biofactors. 1998;8(3-4):243–253. [DOI] [PubMed] [Google Scholar]
- 90. Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20(11):2690–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]