Abstract
Background
The male fitness pathway, from pollen production to ovule fertilization, is thought to strongly influence reproductive trait evolution in animal-pollinated plants. This pathway is characterized by multiple avenues of pollen loss which may lead to reductions in male fitness. However, empirical data on the mechanistic processes leading to pollen loss during transport are limited, and we therefore lack a comprehensive understanding of how male fitness is influenced by each step in the pollination process.
Scope
This review assesses the history of studying male function in plants and identifies critical gaps in our understanding of the ecology and evolution of pollen transport. We explore male reproductive function along the steps of the pathway to paternity and discuss evolutionary options to overcome barriers to siring success. In particular, we present a newly emerging idea that bodies of pollinators function as a dynamic arena facilitating intense male–male competition, where pollen of rival males is constantly covered or displaced by competitors. This perspective extends the pollen-competitive arena beyond the confines of the stigma and style, and highlights the opportunity for important new breakthroughs in the study of male reproductive strategies and floral evolution.
Keywords: Floral traits, male reproductive strategies, male–male competition, paternity, pollen competition, pollen loss, pollen presentation theory, pollen transfer, pollination, pollinator, pollinator-mediated selection, sexual selection
INTRODUCTION
‘…plants are gene donors and gene receivers […] these two activities are not necessarily complementary, compatible, or directed toward the same end.’
It has been more than 40 years since biologists began to appreciate the importance of male fitness in the evolution of plant reproductive traits (Horovitz and Harding, 1972; Willson and Rathcke, 1974; Gilbert, 1975; Janzen, 1977; Lloyd and Webb, 1977; Willson, 1979). In the preceding century, the view that seed production was a sufficient measure of reproductive fitness in hermaphroditic plants went largely unquestioned. However, the increasing realization that some individuals in a population may achieve greater reproductive success through pollen export than pollen receipt (Horovitz and Harding, 1972) catalysed a major paradigm shift; by the end of the 1970s, male fitness could no longer be ignored.
The male fitness awakening was followed by a burst of new theory, much of it highlighting the potential for sexual selection to act on male mating success in plants [as it does in animals (Bateman, 1948)], since large numbers of pollen grains often compete for a limited number of ovules (e.g. Willson, 1979, 1990, 1994; Queller, 1983; Stephenson and Bertin, 1983). To some, it was initially unclear if, or how, sexual selection could act in the early stages of the pollination process (i.e. before pollen deposition onto stigmas) when plants interact indirectly through pollination agents (e.g. Charlesworth et al., 1987; Lyons et al., 1989; Grant, 1995). However, the parallels between pollen-tube races for ovules in plants after pollination, and sperm competition for ova in animals after copulation, were immediately clear. Indeed, in the post-pollination phase, selection on pollen traits that enhance pollen competition and mating success have now been well demonstrated. These traits include pollen size (McCallum and Chang, 2016), pollen provisioning (Delph et al., 1997) and pollen-tube growth rate (Bertin, 1988; Spira et al., 1996; Sorin et al., 2016; Harder et al., 2016a), providing clear functional links between pollen traits and male reproductive success.
The early controversy surrounding sexual selection in plants has since dissipated, with most plant reproductive biologists today recognizing the importance of sexual selection in shaping male reproductive traits that affect both pollen transport and post-deposition fertilization success (Murphy, 1998; Skogsmyr and Lankinen, 2002; Delph and Ashman, 2006; Moore and Pannell, 2011). While traits affecting post-deposition fertilization success have been relatively well studied, fewer studies have directly addressed the potential links between various male reproductive traits and their influence on pollination success. Yet, a large proportion of male reproductive success may be determined by events that occur long before pollen germination on stigmas (Fig. 1). In most plants, the pathway to successful pollen export is highly complex: the combination of pollen transfer mediated by animals (that have no interest in pollinating flowers) and the simultaneous display of multiple flowers introduces substantial variability into the male reproductive pathway (Barrett, 2003). As a result, male gametes can be lost to a dazzling array of fates before ovule fertilization (Inouye et al., 1994), with each avenue of pollen loss potentially acting as a unique selective force on plant reproductive traits. This review will focus on the complex journey undertaken by pollen, from anthers to stigmas, in animal-pollinated plants. Travelling along the different stages of this journey, we explore how male–male competition may drive the evolution of competitive pollen-export strategies. To give context to this journey, we start with a brief history on the study of pollen fates.
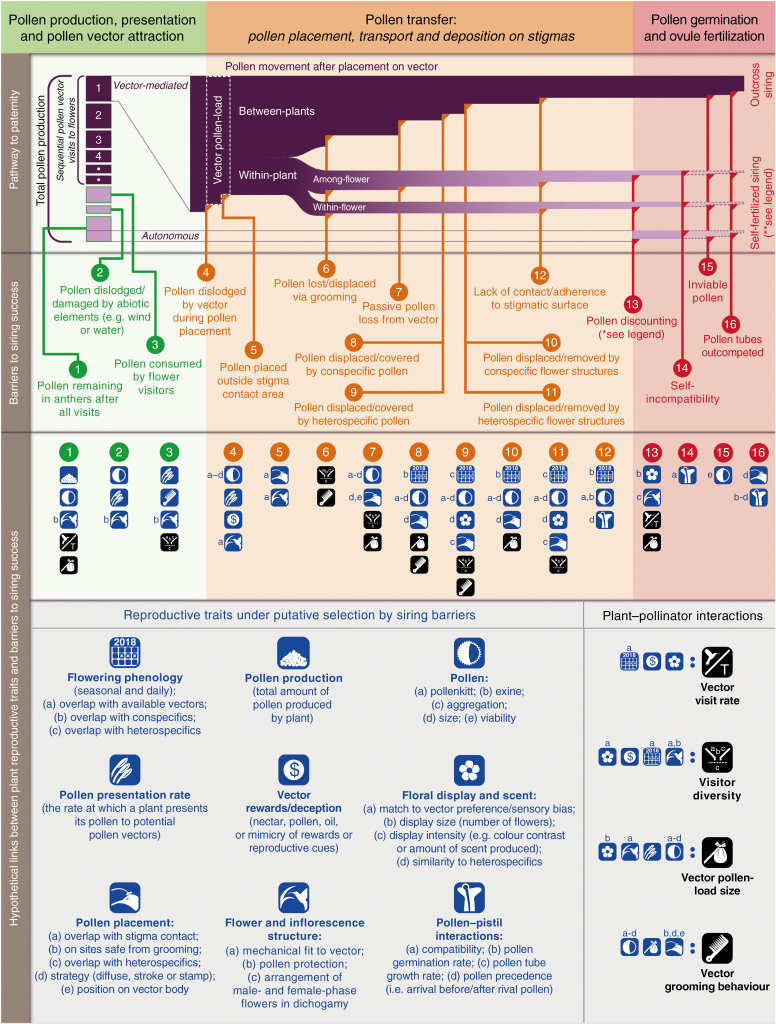
Fig. 1.
A conceptual diagram of the pathway to paternity (row 1). The pathway is divided into three phases: (1) pollen production, presentation, and pollen vector attraction (green); (2) pollen transfer (orange); (3) pollen germination and ovule fertilization (pink). Along these three main phases, a sequence of 16 siring barriers potentially diminish the probability of siring success (row 2). Selection to increase male reproductive success through each siring barrier along the pathway is likely to act on certain suites of reproductive traits (blue icons, row 3) and the products of pollen vector interactions with combinations of these traits (black icons, row 3); the descriptions of these traits and the products of vector–trait interactions can be found in row 4. This review is primarily focused on the first two phases of this pathway (green and orange). *Pollen discounting represents the portion of pollen lost to self-stigmas that could otherwise have been exported to outcross mates (Harder and Wilson, 1998b). Autonomous self-pollination is not discounted if it is delayed and occurs once there is no chance of further visits by pollen vectors. **For self-incompatible plants, all pollen deposited on self-stigmas is lost. However, if self-compatible, the pathway to paternity may continue to self-fertilization (pale purple pathways with dotted-line borders) and may aid in ensuring reproduction when opportunities to export pollen are scarce. The extent to which self-fertilization may contribute to siring success depends on the combination of pollen discounting and inbreeding depression (Harder and Wilson, 1998b). Illustration: C. Minnaar.
THE STUDY OF POLLEN FATES
For species with granular pollen, only a tiny fraction of pollen produced by flowers ever reaches conspecific stigmas. For example, 2.9 % of pollen grains produced in a community of 26 flowering plant species were deposited on conspecific stigmas (Gong and Huang, 2014). Consequently, pollen export represents a significant challenge to male fertility, and a potentially significant opportunity for selection to act on traits that optimize pollen production, transport and delivery (Fig. 1).
Molecular paternity assignment techniques have allowed biologists to start linking variation in pollination-relevant traits [e.g. floral display size (Harder and Barrett, 1995; Karron et al., 2012); flowering phenology (Austen and Weis, 2016); corolla-tube shape (Kulbaba and Worley, 2012, 2013); and petal area (Briscoe Runquist et al., 2017)] to variation in siring success. However, because so few pollen grains ever sire seeds, it is difficult to make direct links between paternity and the multitude of possible non-reproductive pollen fates which may explain a large proportion of variation in male reproductive success (Fig. 1). To understand how male reproductive traits function in determining siring success during the pollination phase (Fig. 1) requires explicit tracking of pollen movement from individual plants.
Empirical studies of pollen movement have historically been limited to the few species for which pollen tracking has been possible [e.g. orchids, for which pollinia can be dyed (e.g. Peakall, 1989; Johnson and Harder, 2018), or species with pollen-colour or size polymorphisms (e.g. Thomson and Plowright, 1980; Nichols, 1985; Holsinger and Thomson, 1994; Stone, 1995; Keller et al., 2014)]. Several important aspects of male reproductive function have been explored in orchids by direct tracking of massulae dispersed from dyed pollinaria (Johnson and Harder, 2018). These include trade-offs between pollination quantity and quality for rewarding and deceitful plants (Johnson et al., 2004; Jersáková and Johnson, 2006; Walsh and Michaels, 2017), the detriment of self-pollination to male outcrossing success (i.e. pollen discounting) (Johnson et al., 2005), and contrasting selection for floral morphology through male and female components of fitness (Ellis and Johnson, 2010).
Pollen movement studies exploring male function in plants with granular pollen dispersal are comparatively rare. Among these few studies, the landmark experiments conducted by Harder and Thomson (1989) and Thomson and Thomson (1989) highlight the importance of tracking pollen movement: by exploiting a pollen-colour polymorphism, Harder and Thomson tracked dispersal of Erythronium grandiflorum pollen and generated detailed quantitative estimates of various components of the pollen export process. A surprising finding of these experiments was that total pollen export (from a single bumble bee visit) to multiple recipient stigmas did not increase linearly with the amount of pollen initially placed on bumble bees. Instead, flowers experienced diminishing returns on pollen export success: the larger a pollen load placed on a bee, the greater the proportional pollen loss during transport. This pattern may be common in animal-pollinated plants for several reasons. For example, pollen loss and displacement may increase with the amount of pollen placed on pollinators if large pollen loads increase the probability and intensity of grooming (as found by Harder, 1990 for bumble bees). A greater proportion of large pollen loads may fall off during transport since smaller proportions of pollen are in direct contact with the pollinator (Harder and Wilson, 1997; Johnson et al., 2005; Harder and Johnson, 2008). The potential ubiquity of diminishing returns associated with large pollen loads may therefore broadly favour restricted pollen presentation, leading to small pollen loads placed on individual pollinators. However, the extent to which plants should restrict their rate of pollen presentation will depend on pollinator visit rates—restricting pollen presentation when pollinator visits are rare would result in lost mating opportunities and wasted pollen production (Harder and Thomson, 1989; Thomson and Thomson, 1992; Harder and Wilson, 1994, 1998a; Thomson, 2003). These two predictions form the core of pollen presentation theory.
Pollen presentation theory has provided much needed insight into the evolution of pollen dispersal strategies and the role of male fitness in the evolution of plant reproductive traits. However, several aspects of the pollination phase remain poorly explored. As new technology improves our ability to identify pollen donors and track movement of individual pollen grains [e.g. pollen grain sequencing (Matsuki et al., 2007, 2008; Chen et al., 2008; Hasegawa et al., 2009, 2015) and pollen labelling (Minnaar and Anderson, 2018)], we envisage that the empirical study of male reproductive function will become ever-more feasible, allowing biologists to fill some of the long-standing gaps between empirical studies and the 40+ years of theoretical work on male fitness in plants. In this review, we endeavour to identify some of these gaps and the progress made in addressing them, while also highlighting emerging lines of enquiry that promise to yield exciting results in the near future. Because post-pollination processes have been comparatively well studied, and thoroughly reviewed elsewhere (Snow, 1994; Delph et al., 1997; Delph and Havens, 1998; Harder et al., 2016a, b; Williams and Mazer, 2016; Williams et al., 2016), we limit the scope of our review to the pollination phase of the pathway to paternity. However, where applicable, we discuss how processes in the pollination phase of the pathway may alter post-pollination success.
The rest of the review will take the reader on a journey along the pathway from pollen production to pollen deposition onto stigmas. Along this pathway, we discuss multiple functional steps in the pollination process likely to influence eventual siring success, including mechanisms that divert or block pollen flow, resulting in an ever-narrowing pathway to paternity. In this review, we refer to the mechanisms that divert or block pollen flow as ‘siring barriers’ because they act as successive barriers that limit the siring potential of a donor’s pollen grains. Through each barrier, fewer and fewer of the donor’s pollen grains remain available for ovule fertilization. Of course, in most cases, a plant’s total pollen export does not occur in a single pollen export event from a single flower, and therefore the pathway to paternity consists of several sub-pathways from successive pollinator visits to multiple flowers on individual plants. We therefore also consider how plants should allocate pollen to these sub-pathways among visiting pollinators—the basic tenet of pollen presentation theory. We expand on prior reviews which highlight the pathway to paternity (Inouye et al., 1994; Harder, 2000; Harder and Routley, 2006; Barrett and Harder, 2017) by discussing potential male reproductive strategies that may mitigate siring barriers, thereby increasing potential siring success. Furthermore, at each step along the pathway, we present hypotheses, expose interesting questions and suggest avenues for future research. In particular, over and above competition for pollinator visits or pollen-tube competition in styles, we emphasize other mechanisms through which plants are likely to compete for male mating success. Specifically, we highlight the largely neglected possibility for pollinator bodies to act as competitive arenas where plants have the opportunity to displace, cover and remove pollen grains of their competitors and increase their siring success. At the end of the journey, we hope that the reader will share our excitement about a research field filled with opportunities.
PATHWAY TO PATERNITY
The pathway to paternity can be divided into three phases: (1) pollen production and presentation; (2) pollen transfer (pollen placement, transport and deposition); and (3) pollen germination and ovule fertilization (Fig. 1). Each phase in the pathway can be further divided into sub-phases representing the sequential steps to siring success. Our review focuses on the first two phases of the pathway only; however, to better illustrate the context of the first two phases, we have included pollen germination and ovule fertilization in our depiction of the pathway to paternity (Fig. 1). From this point onwards, ‘male’ or ‘males’ refer to the male reproductive function of an individual hermaphroditic plant or the separate male functions of several hermaphroditic plants. For example, ‘male reproductive success’ refers to the male component of a hermaphroditic plant’s reproductive success, while ‘competition between males’ refers to competition between the male reproductive functions of separate hermaphroditic individuals. References to ‘female’ and ‘females’ should be treated similarly. We also refer to pollinators as pollen vectors. Although the term ‘pollinator’ is more commonly used in pollination biology, we prefer the term ‘pollen vector’ as it places emphasis on plants as the agents manipulating floral visitors to transport pollen, instead of floral visitors themselves having agency in the pollination process.
Pollen production and presentation
Pollen production.
Pollen production per flower and per plant varies widely within and among species of animal-pollinated plants (Stanton and Preston, 1986; Devlin, 1989; Young and Stanton, 1990a; Stanton et al., 1991; Ashman, 1998; Gong and Huang, 2014). Since pollen production, in both quantity and quality, is often resource limited (Goldman and Willson, 1986;Rameau and Gouyon, 1991; Ashman, 1994; Delph et al., 1997; Obeso, 2002), a significant proportion of this variation may be explained by environmental factors such as soil nutrient availability (Young and Stanton, 1990b; Lau and Stephenson, 1993, 1994), extent of herbivory (Quesada et al., 1995; Mutikainen and Delph, 1996) and environmental temperature (Johannsson et al., 1994). Nevertheless, selection may act on the remaining heritable variation in pollen production (Young et al., 1994; Queller, 1997) to increase male mating success, since total pollen production sets the upper limit of a plant’s reproductive potential (Stephenson and Bertin, 1983).
In one of the earliest molecular paternity-assignment studies in plants, Schoen and Stewart (1986) found that increased cone production resulted in increased siring success for wind-pollinated white spruce trees. However, since then, very few studies have empirically tested the relationship between pollen production and realized siring success, especially in animal-pollinated plants; those that have, provide contrasting results. For example, Stanton et al. (1991) found that wild radish plants which produce more pollen, also sired more seeds. However, the link between pollen production and siring success was indirect: plants with high pollen production received more visits from small native bees relative to honeybees, thereby limiting the detrimental effects of honeybee visitation on siring success. Surprisingly, Ashman (1998) found no relationship between pollen production per plant (displaying a single flower) and realized paternity in wild strawberry plants. Instead, siring success was correlated with the total amount of pollen removed from anthers in a plant. These two studies highlight the importance of the fundamental functional link between pollen production and realized siring success—pollen removal and transport by pollen vectors—which may limit the potential for pollen production to influence siring success directly in animal-pollinated plants. For example, a male producing more pollen than its rivals may simply end up with more pollen left in its anthers ❶ (encircled numbers refer to siring barriers in Fig. 1) than rivals if excess pollen production is not accompanied by an increased vector visit rate, or placement of larger vector pollen loads than rivals. Still, increased vector attraction and placement of large vector pollen loads may respectively aggravate diminishing returns on pollen production through increased self-pollination (Klinkhamer and de Jong, 1993) and increased proportional pollen loss during transport (Harder and Thomson, 1989). Evidently, the interaction between plants and biotic pollen vectors demands male reproductive strategies that go beyond simply increasing investment in gamete production.
Pollen presentation.
Animal-pollinated plants face a unique challenge in delivering pollen to mates. They cannot directly deliver gametes in measured doses through copulation, nor can they release their gametes into a surrounding medium for diffuse transport. Instead, available pollen needs to be presented and placed on individual pollen vectors in a way that maximizes siring success. Since plants cannot anticipate the arrival of vectors, they face strategic ‘decisions’ on how to present and place vector pollen loads of the correct size, which may vary with time and vector visit frequency (Harder and Wilson, 1994). Therefore, in most species, the total amount of pollen produced by a plant is not simply presented all at once: a limited number of flowers may be displayed simultaneously over a long flowering period, or anthers may release pollen gradually (Fig. 3) (Castellanos et al., 2006). The degree to which plants restrict pollen presentation depends on the frequency of visits over time (Harder and Wilson, 1994) and the severity of diminishing returns, which may vary widely between pollen vector types (Thomson, 2003). Plants appear to vary vector pollen-load sizes dynamically in response to vector visit frequency (Harder and Barclay, 1994; Harder and Wilson, 1994), or dynamically alter floral display size (and therefore the amount of pollen presented) with pollination rate (Harder and Johnson, 2005). A recent study also found geographic divergence in pollen presentation rate for Claytonia virginica, likely reflecting local adaptation to pollen vectors that differ in visitation and pollen depletion rate (Parker et al., 2018). The generally accepted explanation for the evolution of restricted pollen presentation (while accounting for vector visit frequency) is to mitigate diminishing returns associated with increasing vector pollen-load size (i.e. pollen presentation theory: Harder and Thomson, 1989; Thomson and Thomson, 1992; Harder and Wilson, 1994). However, plants do not always benefit from placing small vector pollen loads (see ‘Pollen placement and deposition competition’ below), and pollen presentation traits may also be under selection through processes that occur prior to pollen transport (e.g. abiotic pollen loss).
Fig. 3.
Plants have evolved various mechanisms to control the rate of pollen presentation to plants. Plants may stagger the opening of flowers or anthers within flowers to control the amount of pollen exposed to individual pollinators. (A) For example, the anthers of some species (e.g. Lillium longiflorum) expose pollen quickly so that large vector pollen loads are placed onto vectors with each visit. Other species expose their pollen over the course of many days so that pollen is placed onto vectors in small doses through multiple visits. Here, we show the slow release of Gethyllis verticillata pollen as the anthers roll up and dehisce over several days: (B) day 1, (C) day 3. Photos: B. Anderson. (D) Although pollen from Asclepias verticillata is aggregated in pollinia and presented to pollen vectors all at once, the rate of pollen removal may be controlled by the low probability of the pollinarium’s corpusculum attaching to a pollinator. This can be influenced by floral features such as floral structure or nectar abundance which impact visit duration. Each pollinarium comprises joined pollinaria from adjacent anthers (there are five anthers in Asclepias), and the attachment of a single pollinarium to the hairs or bristles of visiting pollinators removes one-fifth of the flower’s pollen in a single visit. Note the paired pollinaria (indicated by an arrow in D) on the left front leg of the bee Bombus griseocollis, magnified in (E). Photos: J. Karron.
By presenting pollen, plants face the risk of losing it through abiotic mechanisms ❷ (Fig. 1) such as wind dislodgement [e.g. in a single night, approx. 50 % of pollen grains were lost from the anthers of Silene plants under pollen vector exclusion (Reynolds et al., 2009)] (Fig. 2A) and water damage (Mao and Huang, 2009). Consequently, floral structures thought to function in vector attraction and mechanical aspects of pollen presentation and placement may, in part, be under selection to protect pollen from wind and rain (Mao and Huang, 2009). Reductions in siring potential as a result of abiotic pollen loss or damage, and the traits putatively evolved to prevent such loss [e.g. sticky pollenkitt and large pollen grains (Pacini and Hesse, 2005) or floral orientation (Wang et al., 2010)], have rarely been explored.
Fig. 2.
Pollen that is dislodged from the reproductive parts of flowers either by the abiotic environment or by pollinators has a much lower probability of ever reaching the stigma of another flower. (A) Pollen shows up yellow against the contrasting petal background of Pauridia capensis after a gust of wind dislodged it from the anther. Photo: M. de Jager. (B) Pollen from the anthers of Salvia greggii falls to the ground after being dislodged by a visiting rufous hummingbird. Photo: S. Tekiela – Nature Smart Images.
While pollen is frequently offered as a reward to vectors to promote pollen export, some animals consume large quantities of pollen without transferring appreciable amounts between flowers (Hargreaves et al., 2009). These so-called pollen thieves or robbers lower male fitness by reducing the amount of pollen available for export ❸ (Fig. 1) (e.g. do Carmo and Franceschinelli, 2004; Koski et al., 2018). Pollen presentation mechanisms not only cause variation in the amount of pollen presented to vectors per visit but they also cause variation in the quantity of pollen exposed to potential theft (Hargreaves et al., 2009). Thus, traits controlling pollen release could also have evolved to protect pollen from thieves. For example, buzz-pollinated anthers could have evolved to increase pollen export efficiency by controlling the amount of pollen removed by pollen-collecting vectors per visit (Harder and Barclay, 1994). However, buzz-pollinated anthers may also have evolved to prevent pollen theft by insects incapable of buzzing at the correct frequency (Hargreaves et al., 2009).
Pollen vector attraction.
Attractive floral traits are often thought to be largely under selection through male function because male reproductive success is more likely to be enhanced by frequent visitation, while female reproductive success is more likely to be limited by resources for provisioning fertilized ovules (Willson and Rathcke, 1974; Burd and Callahan, 2000). Initial studies found support for male-biased function in large floral displays (Willson and Rathcke, 1974; Queller, 1983). However, these two studies used pollen removal as a proxy for male fitness, which may not relate directly or linearly to eventual siring success. These studies also focused on milkweeds which disperse pollen in the form of pollinaria that take almost 2 min to dry and move into the orientation best suited for insertion into stigmatic slits (Queller, 1983). Since pollen vectors typically spend <2 min foraging on individual plants (Queller, 1983), the risk of geitonogamous pollen transfer may be low compared with species with granular pollen. Subsequent studies on species with granular pollen add to a growing body of evidence that increased floral display size may increase pollen loss to self-stigmas (Harder and Barrett, 1995) and therefore favour seed production more than outcross siring success (Karron and Mitchell, 2012).
The argument that male fitness in plants is more dependent on vector visit frequency than female fitness is based on Bateman’s (1948) principle: selection for traits that increase mate acquisition should almost always be stronger in males since their gametes vastly outnumber those of females. However, this direct application of Bateman’s principle fails to address two important aspects of reproduction unique to animal-pollinated plants. First, although plants rely on animal vectors to facilitate reproduction, they do not mate with their vectors. Consequently, visitation frequency cannot be equated with mating frequency. Secondly, most animal-pollinated plants have several independent, but simultaneously functioning male and female reproductive organs. This creates opportunity for self-pollination and self-fertilization within and between a plant’s flowers, which may counter selection for increased attractiveness through large floral displays (Barrett, 2002; Mitchell et al., 2004). Therefore, when considering selection for increased attractiveness, we need to examine how traits influence pollen-vector foraging patterns at the plant and flower level. For instance, a rewarding plant with a relatively large number of simultaneously receptive flowers will probably attract more pollen vectors than a plant with fewer flowers (Conner and Rush, 1996; Mitchell et al., 2004). However, pollen vectors are likely to visit several flowers on the same plant in succession (Mitchell et al., 2004). Consequently, plants with larger floral displays increase their risk of geitonogamous pollen transfer (Harder and Barrett, 1996; Karron et al., 2009). Production of smaller floral displays over a longer flowering period should therefore increase reproductive success (Karron and Mitchell, 2012).
Much of the debate surrounding male-biased selection for attractive floral displays has been informed by studies on floral display size and the associated costs of geitonogamy. Yet, several other floral traits may influence pollen-vector visit rates without an increased risk of geitonogamy or pollen discounting. These traits include: flower size (e.g. Conner and Rush 1996), scent (e.g. Kessler et al. 2008; Larue et al. 2015), colour [e.g. overall colour (Stanton et al., 1986), colour pattern (de Jager et al., 2016; Kemp et al., 2019), and colour brightness and contrast (Sletvold et al., 2016)], shape and symmetry (e.g. Møller 1995; Gómez et al., 2006), and height above ground (e.g. Peakall and Handel, 1993). Since these traits do not directly influence geitonogamy, they are more likely to show male-biased selection following Bateman’s predictions, and therefore deserve more detailed study in the context of male fitness.
Plants may also increase floral reward quality and quantity to increase visitation rates and siring success (Thomson, 1988). However, the potential male fitness benefits of offering greater rewards to vectors may be limited: increased floral nectar quantity may increase the number of flowers visited per plant, as well as the amount of time spent on a single flower (Zimmerman, 1983; Thomson, 1986; Klinkhamer et al., 1991), leading to increased self-pollination and pollen discounting (Klinkhamer and de Jong, 1993; Hodges, 1995; Jersáková and Johnson, 2006). Another way of increasing vector recruitment and, potentially, siring success is to attract, reward and place pollen on a greater variety of potential flower visitors (generalization). However, plants with relatively generalized pollination strategies may risk increased pollen loss during pollen transport ❻❼❽❾
 . For example, plants with more diffuse pollen placement may place pollen on a larger sub-set of available flower visitors but may in turn suffer increased pollen wastage if more of their pollen is placed outside of grooming safe sites ❻ or if pollen is placed on pollinators that visit heterospecific flowers ❾ more frequently. The various siring barriers in the pathway to paternity are thus closely linked to the evolution of specialization and generalization.
. For example, plants with more diffuse pollen placement may place pollen on a larger sub-set of available flower visitors but may in turn suffer increased pollen wastage if more of their pollen is placed outside of grooming safe sites ❻ or if pollen is placed on pollinators that visit heterospecific flowers ❾ more frequently. The various siring barriers in the pathway to paternity are thus closely linked to the evolution of specialization and generalization.
Pollen transfer
The process of pollen transfer is often depicted as sequential (pollen placement, transport and deposition). However, the reality is far more complex. Once pollen is placed on a vector, the number of potential fates for individual pollen grains is immense. Pollen may move within plants, between plants and between species, from anthers to vectors and back to other anthers again, onto stigmas only to be removed again and deposited onto other stigmas. Pollen grains might move across vector bodies through grooming and displacement, or coverage by other pollen grains, and most pollen grains never reach plants. In fact, nearly all pollen produced by a plant may be lost before reaching compatible stigmas, and many grains that do eventually reach stigmas may no longer be viable (Thomson et al., 1994; Dafni and Firmage, 2000; Parker et al., 2015). The movement of pollen is therefore highly stochastic, with successful pollen transfer being strongly dependent on chance (Richards et al., 2009; Harder et al., 2016b). Yet, in this complex chaos of pollen movement, males may still increase their siring success through various strategies that influence the fate of their pollen and the pollen of their rivals. In this section we examine these strategies. The sub-sections presented below address various important aspects of the pollen transfer process which, in addition to siring success, may also influence the distance of pollen moved, mate diversity, and pollen carryover.
Mechanical fit of pollen vectors.
After producing and presenting pollen, and attracting a potential pollen vector, a plant still needs to physically manipulate the vector to place pollen on their bodies. This manipulation requires a reward (or the promise of it) (Pyke, 2016) which acts as a lure, drawing pollen vectors into the flower’s morphological structure, where they are forced to make contact with anthers and pollen (Fig. 4). Thus, while the amount of pollen available for placement on a vector depends primarily on pollen presentation traits (e.g. pollen aggregation and anther dehiscence rate), every other aspect of pollen placement (realized vector pollen-load size and pollen placement position, direction, and accuracy) depends on the extent to which a flower’s morphology and offered rewards manipulate and restrict the movement of a potential vector relative to the plant’s sexual organs (Muchhala, 2007; Armbruster et al., 2009a, b; de Jager and Peakall, 2019). The importance of mechanical fit in plant–pollinator interactions has been well demonstrated by several examples of plant species that show consistent geographic covariation in floral-tube length and local vector proboscis length (Pauw et al., 2009; Anderson et al., 2014; Newman et al., 2014, 2015) (Fig. 4A, B). However, most flowers do not show mechanical fit to one specific vector, but rather a sub-set of available pollen vectors in their environment. Flower morphology may therefore reflect selection to exclude inefficient pollen vectors in favour of a more efficient sub-set of vectors (Thomson, 2003), or selection to balance the fitness trade-offs between relatively efficient and inefficient pollen vectors (Aigner, 2001, 2004).
Fig. 4.
The architecture and mechanics of flowers and inflorescences often manipulate foraging visitors in different ways to maximize contact between visitors and anthers and/or stigmas. (A) Representative of many interactions between long-tubed angiosperm species and their floral visitors; the proboscis of the long proboscid fly Prosoeca longipennis is closely matched to the floral tube length of many flowers from which it it forages (e.g. Tritoniopsis revoluta, tube length approx. 65 mm). To obtain nectar at the base of the tube, the fly must insert its entire proboscis and crawl inside the gullet of the flower (B) where the stigma and/or anthers make contact with the thorax and abdomen of the fly. Photos: C. von Witt. (C) Similarly, the unique architecture of Babiana ringens depicts another of the varied ways in which plant morphology is able to manipulate the behaviour of floral visitors to enhance contact with reproductive structures. Sunbirds (Nectarinia famosa) visit B. ringens flowers while perching on a highly modified, naked inflorescence axis. This forces the birds to lean over the exerted stigmas and anthers in order to probe the tubular flowers on the ground. In doing so, the anthers place pollen on the chests of visiting birds (Anderson et al., 2005). Photo: B. Anderson.
The amount of pollen placed on vectors (and lost due to dislodgement ❹) may further be affected by the duration of a flower visit (Harder and Thomson, 1989), since this is likely to influence the probability of contact between anther and pollen vector, as well as the area of the vector body available for pollen placement. Visit duration tends to increase with the amount of reward offered (Zimmerman, 1983; Thomson, 1986; Klinkhamer and de Jong, 1993), and is further influenced by the ease of access to rewards (Harder, 1983; De Kock et al., 2018).
Pollen landscapes on pollen vector bodies.
Pollen landscapes that vary in 2- or 3-D structure and pollen donor composition may be generated on the bodies of pollen vectors due to sequential pollen placement by different males, sequential pollen capture by recipient females and grooming by pollen vectors (Lertzman and Gass, 1983; Morris et al., 1995; Harder and Wilson, 1998a). The concept of multidonor pollen landscapes is a crucial prerequisite for understanding the potential for pollen-vector bodies to serve as platforms for pollen transfer, interfaces for competitive male–male interactions and interspecific competition. In particular, the interaction between vector pollen-load size and the distribution and position of pollen placement on vectors potentially influences a male’s position and dominance within a pollen landscape, thus affecting almost every subsequent aspect of the pathway to paternity including siring success.
Theoretical studies of pollen landscapes on vector bodies are rare, and empirical studies are practically non-existent. The first studies to address pollen landscape structure (Lertzman and Gass, 1983; Morris et al., 1995) predicted that a layered pollen landscape would extend pollen carryover because deeply buried layers may resurface and deposit pollen on recipients, long after pollen placement. Harder and Wilson (1998a) added more biological realism to these initial models by comparing pollen dispersal in vertically structured landscapes (layered) to horizontally heterogeneous pollen landscapes that result from pollen placement across pollinator bodies within sites exposed to, or safe from, pollen grooming. While mean pollen dispersal characteristics were similar for both scenarios, they found that variation in female characters had a greater influence on pollen redistribution and carryover in vertically structured landscapes than in horizontally structured landscapes—pollen capture by stigmas determined the rate at which buried pollen layers were subsequently exposed. However, male characteristics influenced the pollen landscape in both scenarios. These models clearly demonstrate that successive vector pollen loads placed by different plants are the fundamental building blocks that form pollen landscapes, and that the structure of these landscapes is likely to have a significant impact on an individual’s pollen dispersal in space and time.
Thus, to understand how pollen landscapes might form and how they might influence siring success, we need to understand pollen placement mechanisms in plants. While detailed work has been done on the adaptive accuracy of pollen placement on vectors (Armbruster et al., 2009a), the link between pollen placement strategies, pollen landscapes and how males might influence these landscapes to increase siring success has received little study. As it stands, almost nothing is known about the structure and donor composition of actual pollen landscapes.
Thus, we can only present tentative hypotheses about how different pollen placement strategies and their associated pollen landscape structures may influence male reproductive success. We do so by limiting our discussion to three distinct pollen placement strategies and the respective pollen landscapes they may produce. Similar to the more comprehensive classification of Armbruster et al. (2009a), our three pollen placement strategies—diffuse placement, stroke placement and stamp placement (Fig. 5)—differ with respect to two variables: pollen placement area and pollen vector movement with respect to plant sexual organs. The three strategies roughly represent the mid- and end-points along continuums of these two characteristics. Although not inclusive of all pollen placement systems (see Armbruster et al., 2009a for a detailed treatment), this simplified classification provides a useful functional basis from which to generate possible pollen landscape scenarios and explore the potential fitness consequences of those landscapes. Diffuse placement includes any mechanisms that place pollen over large, undefined areas of vectors. This strategy may typically be associated with actinomorphic flowers that diffusely place pollen using multiple, spatially-separated anthers (Fig. 6A, B) [cf. floral class 1 in Armbruster et al. (2009a)]. In contrast, stroke placement will require the pollen vector to drag a part of its body across tightly packed anthers in a consistent direction, leaving a streak of pollen (Fig. 6C, D) [cf. floral classes 2–4 in Armbruster et al. (2009a)]. Stroke placement is more likely to be associated with zygomorphic flowers where plants have greater control over the relative body position and approach direction of pollen vectors (Macior, 1974; Muchhala, 2007; Westerkamp and Claßen-Bockhoff, 2007; Armbruster et al., 2009a). Stamp placement includes any pollen placement strategy where anthers are not dragged across vector bodies, but instead stamp pollen onto vector bodies in a single contact event (Fig. 6E, F) [cf. floral class 5–7 in Armbruster et al. (2009a)].
Fig. 5.
Graphic depiction of pollen landscapes (top row) that may form on a pollen vector’s body after visiting a sequence of five different plants. Each plant’s contribution to the pollen landscape is depicted in a different colour, ranging from blue (first plant visited) to red (the final plant visited—the focal male). Underneath these graphic depictions, we provide a comparative table of potential male fitness implications for each of the three, hypothetical pollen placement strategies. The rows of the table correspond to various potential plant–pollinator interactions that may influence siring success within the pollen transfer (orange) and pollen germination and ovule fertilization (red) phases of the paternity depicted in Fig. 1.
Fig. 6.
Three common strategies for placing pollen on floral visitors, diffuse (A, B), stroke (C, D) and stamp (E, F). (A) The open flowers and circular anther arrangement of species such as Drosera cistiflora place pollen diffusely all over the bodies of monkey beetle pollinators like this Lepisia rupicola. Photo: B. Anderson. (B) Alternatively, a central anther arrangement and wide floral tube (as depicted in Roella ciliata) can also result in diffuse pollen placement when visitors circle the anthers as they forage for rewards. The blue pollen can clearly be seen, diffusely covering the hairs on the bodies of two foraging monkey beetles. Photo: I. Minnaar. (C) The anthers of Salvia chamaedryoides (Lamiales) deposit pollen on the head and thorax of visiting honeybees in a stroke-like motion. The stroke starts on the head and ends on the thorax, forming a distinct stripe of pollen. The stroking or pushing motion of the anthers appears to displace pollen towards the back of the stroke as predicted in Fig. 5 for stroke pollen placement. Photo: Christine Dimech. (D) Narrow, tubular flowers can also result in stroke placement. In this example, as the proboscis of Moegistorhynchus longirostris reaches into the depth of a Lapeirousia anceps floral tube, the anthers first make contact with the fly’s head and then stroke towards the thorax as the fly tilts its abdomen in order to get the last remaining nectar at the very base of the deep tube. Photo: C. Minnaar. (E) The anthers of Campsomeris plumipes push against the head of a visiting Hyptis alata forming a very discrete, circular pollen signature characteristic of stamp placement. Photo: J. Lampkin. (F) Stamp placement in Stylidium occurs actively when the anthers and stigma forcibly slap floral visitors with surprising accuracy. Here Stylidium tenue stamps Urocolletes rhodurus with pollen. Photo: F. Hort and J. Hort.
The three pollen placement strategies are likely to yield different pollen landscape structures (Fig. 5). Diffuse placement strategies are most likely to produce an unlayered pollen-landscape structure since pollen grains are distributed over a large area of vector bodies and unlikely to build up in layers. Stroke placement may result in a layered pollen-landscape structure; however, the layered structure may vary along the length of the stroke placement area. When anthers stroke against vector bodies to place pollen, they may also displace pollen from previous donors towards the back of the stroke (Fig. 5). Stigmas may similarly capture more pollen at the start of the stroke and push some of the previously placed pollen towards the back of the stroke. We therefore hypothesize that pollen landscapes resulting from stroke placement may be more layered towards the back of the stroke and consist of more recent pollen, and less layering, towards the front of the stroke. Stamp placement strategies are most likely to result in symmetrically distributed and layered pollen landscapes since pollen loads are deposited in succession on top of each other (Fig. 5).
The number of flowers visited per plant is also likely to affect the donor composition of pollen landscapes. For example, several stroke layers from a single plant may combine to form a very thick layer, or in the case of diffuse placement strategies, multiple visits to the same donor may generate vertical structure. The following sections examine how these placement strategies are likely to influence siring success at different points along the paternity to pathway. By the end of this review, it will be clear that understanding many aspects of male fitness may rely on accurate depictions of real pollen landscapes, providing an exciting new direction of study for plant reproductive biologists.
Intrafloral pollen transfer and pollen placement strategies.
Pollen placement strategies influence the degree to which male reproductive success may be limited through sexual conflict arising from simultaneous male and female reproductive functions in hermaphroditic plants (Barrett, 2002). The primary source of sexual conflict is vector-mediated self-pollination (Barrett, 2002) which can reduce male and female fitness through inbreeding depression and pollen discounting (reduction in pollen available for export) (Harder and Wilson, 1998b). While we consider the consequences of geitonogamous pollen transfer and pollen discounting  for the evolution of male reproductive strategies (Fig. 1), we do not address the full complexities of the costs and potential benefits of selfing and the joint effects of inbreeding depression and pollen discounting—these have been reviewed extensively elsewhere (Holsinger and Thomson, 1994; Harder and Wilson, 1998b; Porcher and Lande, 2005; Devaux et al., 2014). Here, we focus on differences between pollen placement strategies in terms of their likelihood to produce sexual conflict, and male reproductive traits selected to reduce the potential costs of sexual conflict. We divide sexual conflict into intrafloral processes (discussed here) and interfloral processes (discussed below in ‘Geitonogamous pollen transfer’).
for the evolution of male reproductive strategies (Fig. 1), we do not address the full complexities of the costs and potential benefits of selfing and the joint effects of inbreeding depression and pollen discounting—these have been reviewed extensively elsewhere (Holsinger and Thomson, 1994; Harder and Wilson, 1998b; Porcher and Lande, 2005; Devaux et al., 2014). Here, we focus on differences between pollen placement strategies in terms of their likelihood to produce sexual conflict, and male reproductive traits selected to reduce the potential costs of sexual conflict. We divide sexual conflict into intrafloral processes (discussed here) and interfloral processes (discussed below in ‘Geitonogamous pollen transfer’).
Within-flower sexual conflict is primarily determined by the proximity between male and female reproductive structures in flowers, which increases the probability of self-pollination and may reduce the likelihood of pollen export (Karron et al., 1997; Fetscher, 2001; Barrett, 2002). Adaptive strategies thought to resolve this conflict primarily involve spatial separation of male and female reproductive organs (herkogamy) (Webb and Lloyd, 1986), or separation in the timing of pollen presentation and stigma receptivity (dichogamy) (Lloyd and Webb, 1986). While sufficient herkogamy may completely eliminate autonomous selfing, the efficacy of herkogamy in preventing vector-mediated pollen transfer within flowers may vary with different pollen placement strategies.
Herkogamy is likely to be less effective in reducing vector-mediated pollen transfer within flowers with diffuse pollen placement, because vectors move less predictably between anthers and stigmas when compared with stroke and stamp placement (Fig. 5A). For example, the splayed anthers and stigmas of the flower in Fig. 6A are clearly herkogamous, which likely prevents autonomous pollen transfer within a flower. However, when a pollen vector visits the flower, it may mediate self-pollination if it contacts the stigma after crawling around the flower while foraging for pollen rewards. Temporal (dichogamy) rather than spatial separation may therefore be a more common mechanism of reducing pollen vector-mediated sexual conflict in flowers with diffuse pollen-placement strategies (Fig. 5A).
Herkogamy might also introduce inefficiency in pollen transfer if anthers and stigmas do not place and receive pollen on the same parts of the pollen vector’s body (Lloyd and Webb, 1992). However, with stroke placement, spatial separation between anthers and stigmas may reduce within-flower sexual conflict without significantly reducing pollen transfer efficiency: stigmas that are exerted beyond anthers may make first contact with pollen vectors and drag along vector bodies to capture pollen from previous donors. Behind the stigma, anthers contact the vector secondarily and place pollen by dragging across the vector’s body. In this way, stigmas and anthers still largely overlap in pollen-vector contact area, allowing spatial separation between anthers and stigma without a substantial cost to pollen transfer efficiency. Consequently, herkogamy may frequently be associated with flowers characterized by strong stroke pollen placement.
Stamp placement represents the least diffuse of all the placement strategies and, therefore, pollen transfer success will depend on accurate matching of pollen placement and capture sites on vector bodies. As a result, the reduced pollen transfer efficiency associated with herkogamy will be most acute for stamp placement relative to other pollen placement strategies (Fig. 5A). Instead, Armbruster et al. (2009a) predicted that flowers with stamp pollen placement are most likely to separate male and female functions in time, not space.
Geitonogamous pollen transfer.
When pollen vectors visit multiple flowers sequentially on a plant (Darwin, 1876; de Jong et al., 1993; Snow et al., 1996) they frequently cause within-plant pollen movement (Lloyd and Schoen, 1992; Harder and Barrett, 1996; Eckert, 2000). This process, known as geitonogamous pollen transfer, reduces the amount of pollen available for export (i.e. pollen discounting  ) (Harder and Barrett, 1995; Karron et al., 2004) and the extent of pollen carryover (Mitchell et al., 2013). For self-compatible plants, geitonogamy also increases the male selfing rate (Harder and Barrett, 1995; Karron and Mitchell, 2012) which can lower male fitness through inbreeding depression (Holsinger and Thomson, 1994; Harder and Wilson, 1998b; Devaux et al., 2014). The risk of geitonogamy is likely to be highest in stroke and stamp pollination because these mechanisms layer pollen onto vectors, and most pollen transfer onto stigmas will be from the last few flowers visited (often from the same plant) (Karron et al., 2009) (Fig. 5). In contrast, with diffuse pollen placement, stigmas capture pollen from a more diffuse, mixed donor pollen landscape, thus reducing the risk of geitonogamy, as long as the proportion of self-pollen in the pollen landscape is not very high (Fig. 5B). When inbreeding depression is severe, selection on plants with multiflowered displays should favour traits which reduce the extent of within-plant pollen transfer, such as longer flowering windows with small floral displays (Karron and Mitchell, 2012), reciprocal herkogamy (Jesson and Barrett, 2002), dichogamy (Lloyd and Webb, 1986) or even rewardlessness (Johnson and Nilsson, 1999).
) (Harder and Barrett, 1995; Karron et al., 2004) and the extent of pollen carryover (Mitchell et al., 2013). For self-compatible plants, geitonogamy also increases the male selfing rate (Harder and Barrett, 1995; Karron and Mitchell, 2012) which can lower male fitness through inbreeding depression (Holsinger and Thomson, 1994; Harder and Wilson, 1998b; Devaux et al., 2014). The risk of geitonogamy is likely to be highest in stroke and stamp pollination because these mechanisms layer pollen onto vectors, and most pollen transfer onto stigmas will be from the last few flowers visited (often from the same plant) (Karron et al., 2009) (Fig. 5). In contrast, with diffuse pollen placement, stigmas capture pollen from a more diffuse, mixed donor pollen landscape, thus reducing the risk of geitonogamy, as long as the proportion of self-pollen in the pollen landscape is not very high (Fig. 5B). When inbreeding depression is severe, selection on plants with multiflowered displays should favour traits which reduce the extent of within-plant pollen transfer, such as longer flowering windows with small floral displays (Karron and Mitchell, 2012), reciprocal herkogamy (Jesson and Barrett, 2002), dichogamy (Lloyd and Webb, 1986) or even rewardlessness (Johnson and Nilsson, 1999).
Pollen placement and deposition competition.
‘Pollen competition is a post-pollination phenomenon just as sperm competition is a post-copulation phenomenon. The process of fertilization in plants is most closely related to that which occurs in animals that have internal fertilization.’
Until recently, all research on pollen competition was narrowly focused upon competition between pollen grains germinating on stigmas and growing pollen tubes within styles of flowers (Stephenson and Bertin, 1983; Snow, 1994; Willson, 1994; Delph and Havens, 1998; Skogsmyr and Lankinen, 2002; Moore and Pannell, 2011). This limited scope for competitive pollen interactions comes from the notion that pollen competition is similar to sperm competition in internally fertilizing animals, i.e., it only occurs after pollination or copulation (see quote above, Delph and Havens, 1998). As a result, only two phases of male–male competition in animal-pollinated plants are typically recognized. The first is competition between plants for pollen vector visits, with strong parallels to competition for access to mates in animals (Stephenson and Bertin, 1983). Pollen competition, the next phase of male–male competition, is typically thought to occur after pollen deposition onto stigmas, where races between rival pollen grains to germinate and grow pollen tubes inside the stylar tissue of recipient flowers may ensue (Harder et al., 2016b), just as sperm race to ova in reproductive tracts of animals.
Here we identify a newly emerging realization that pollen competition is not just a ‘post-pollination phenomenon’, and that the body of a pollen vector represents the first opportunity for males to interact directly with pollen of other male competitors (Cocucci et al., 2014), potentially allowing them to alter pollen landscapes to their advantage. In this respect, pollination is less similar to the internal fertilization process of animals (see quote above, Delph and Havens, 1998) than it is to the sperm casting of marine invertebrates which also use a vector (water) for gamete transport. In sperm casting, sperm competition is thought to occur from the time of sperm production and release until the time of fertilization (Parker, 1984; Bode and Marshall, 2007; Beekman et al., 2016). We suggest that if sperm can compete in a shared vector (water) for access to eggs, pollen grains could compete on a shared vector (pollinators). In fact, it could be argued that competition between pollen grains should be more intense in animal-pollinated plants, as the total amount of vector space for pollen transport is far more limited than the vast amounts of water available to sperm-casting marine animals. Moreover, pollen placement on vector bodies requires physical contact between plant and vector, and therefore physical contact between the plant and pollen previously placed by rivals, providing ample opportunity for physical interactions between plants, their pollen, and their rival’s pollen prior to pollen germination.
The potential for pollen competition prior to germination has historically been neglected—we know of only one explicit, although brief, statement considering competitive interactions between pollen grains on pollen vectors by Lertzman and Gass (1983) (p. 488): ‘For instance, flooding a pollen pool [i.e. pollen landscape] with one’s own pollen may increase success as a male…’. However, two recent studies have since provided the first evidence of physical competition between pollinaria of different plants for space on pollen vector bodies (Cocucci et al., 2014; Duffy and Johnson, 2014). Importantly, Cocucci et al. (2014) revealed that such ‘physical struggles’ between pollinaria may have led to the evolution of pollinaria horns that function in preventing unwanted attachment to pollinaria from rival males (because it interferes with deposition on stigmas). This finding provides the first evidence of sexually selected male weaponry in plants—once considered an exclusively animal phenomenon—contradicting the widely held notion of pollen competition as a post-pollination phenomenon (e.g. Lloyd and Webb, 1977; Stanton, 1994; Grant, 1995; Murphy, 1998; Delph and Ashman, 2006; Moore and Pannell, 2011).
There seems to be no sound theoretical reason to continue the historic restriction of pollen competition to interactions that involve germination and pollen-tube growth only—the scope of pollen competition should include the pollen transfer process, where pollen-placement and pollen-deposition competition may occur. While evidence for pollen placement competition on vector bodies is currently limited to two studies on species that disperse pollen in the form of pollinaria, we suggest that this form of pollen competition should also occur in plants dispersing granular pollen. In this review, we would like to expand on the idea of pollen competition on vector bodies by exploring hypothetical mechanisms of pollen-placement and pollen-deposition competition using the three different granular pollen-placement strategies as a foundation. However, since the composition and structure of multidonor pollen landscapes on vectors have never been studied, we make the following predictions with caution and encourage their future empirical exploration.
The potential for males to alter pollen landscapes is likely to vary among the three pollen placement strategies (Fig. 5). In diffuse pollen placement, a male’s ability to alter the structure of a pollen landscape is limited, due to the diffuse distribution of competitor pollen grains across the vector’s body. Plants with diffuse pollen placement may change pollen landscapes by contributing more pollen than their competitors. However, this may increase the risk of geitonogamous pollination because it increases the proportion of potential self-pollen on a pollen vector (see ‘Geitonogamous pollen transfer’ above).
In contrast to diffuse pollen-placement strategies, stroke and stamp placement provide males with more opportunity to alter pollen landscape composition and structure to their advantage. With stroke placement, anthers can physically displace competitor pollen (or have their own pollen displaced ❽) towards the back of the stroke (Figs 5 and 6C) as a result of the dragging motion of anthers and pollen placement in a single direction. By displacing rival pollen, males may increase their relative contribution to the pollen landscape without increasing pollen load size. Intense pollen placement competition may drive selection for traits that amplify the relatively passive displacement effect inherent in stroke placement systems. While speculative, it is not difficult to conceive of simple, secondary anther structures or floral appendages that could potentially scrape, sweep or scoop rival pollen from vector bodies prior to pollen placement. This would be analogous to ancillary structures on the penises of male animals that remove rival sperm from female reproductive tracts (Waage, 1979; Hosken and Stockley, 2004).
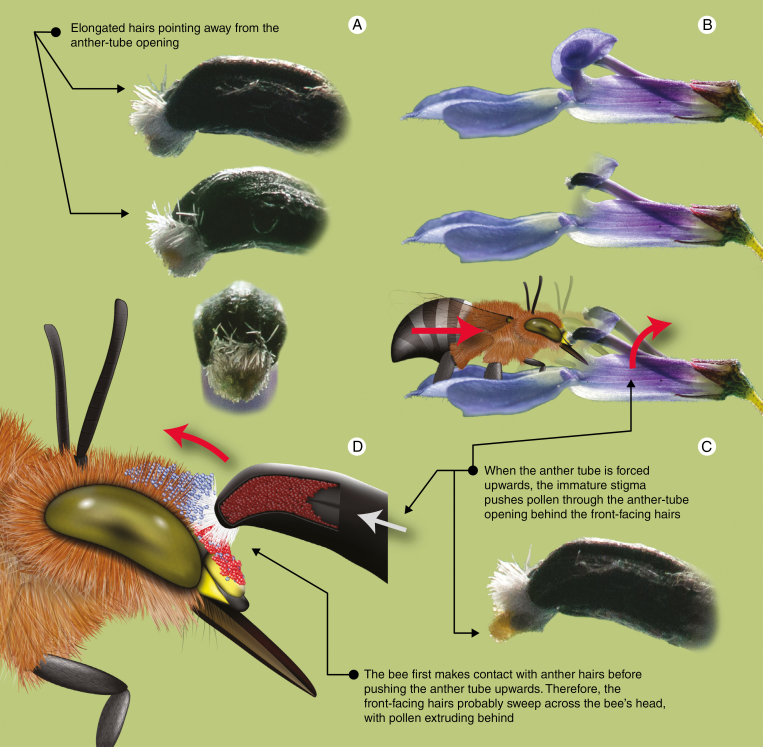
A possible example of a flowering-plant structure that may potentially scrape or remove rival pollen from vector bodies are the hairs surrounding anther tubes in Lobelia tomentosa (Fig 7A). In Lobelia, pollen is extruded through the end of an anther tube where pollen is placed, usually in an accurate stroke fashion, along the head and thorax of visiting pollen vectors (Macior, 1967; Johnston, 1991; Yeo, 1992; Howell et al., 1993). Many Lobelia have hairs surrounding the anther-tube opening which appear to control pollen extrusion (Ladd, 1994). In L. tomentosa, pollen is stroked onto the head of visiting bees as the anther tube is levered upwards and pushed from the front, causing the undeveloped stigma to push pollen through the anther-tube opening (Fig. 7B, C). While doing so, the hairs surrounding the anther-tube opening, especially the elongated front-facing hairs (Fig. 7A), may also sweep away rival pollen on vectors before the donor’s pollen is laid down (Fig. 7D). We performed a preliminary test of this hypothesis by placing quantum-dot (q-dot)-labelled L. tomentosa pollen (Minnaar and Anderson, 2018) on an Amegilla bee’s head and pushing it into a virgin flower to simulate a visit. We found q-dot-labelled pollen grains from the bee’s head on the front-facing hairs of the anther tube and a stroke of unlabelled pollen on the bee’s head where the q-dot-labelled pollen appeared to be partially removed. While this may represent an intriguing example of rival pollen removal, we stress that we do not have enough data to support this hypothesis, and alternative functions of the elongated front-facing hairs need to be considered (e.g. concealment of pollen from pollen thieves or protection of pollen from rain). We only present this as one of many putative examples of structures that may function in rival pollen removal that deserve further exploration.
Fig. 7.
In this review we hypothesize that plants may have evolved secondary anther structures or floral appendages that function in displacing or removing rival pollen from pollen vector bodies. Here we demonstrate a putative example of competitive pollen scraping. (A) The hairs surrounding the anther-tube opening in Lobelia tomentosa are elongated towards the front. These elongated hairs also appear to point away from the anther-tube opening, in the same direction that pollen is placed on bees. (B) To reach the nectar in L. tomentosa flowers, bees are forced to push against the anther tube and, in doing so, force the anther tube upwards. This causes the immature stigma to push pollen, like a piston, through the anther-tube opening. (C) Since pollen is extruded behind the forward-facing hairs (D), we hypothesize that bees entering L. tomentosa flowers will make contact with the anther hairs first, potentially allowing the forward-facing hairs to scrape away rival pollen, while the plant’s own pollen is extruded onto the bee behind these sweeping hairs. Preliminary experiments suggest that this hypothetical scenario may be likely (see text); however, we do not have sufficient empirical data to confirm this mechanism, and caution readers to view this example as speculative. Illustration and photos: C. Minnaar.
The evolution of competitive pollen removal in plants  may be limited in species with large floral displays because the risk of removing the focal donor’s pollen (i.e. self-pollen removal) likely increases with the number of flowers visited on the same plant. We therefore expect that, if present, pollen scrapers may be most developed in species with small floral displays, or in dichogamous plants where the risk of self-pollen removal is reduced.
may be limited in species with large floral displays because the risk of removing the focal donor’s pollen (i.e. self-pollen removal) likely increases with the number of flowers visited on the same plant. We therefore expect that, if present, pollen scrapers may be most developed in species with small floral displays, or in dichogamous plants where the risk of self-pollen removal is reduced.
Stamp pollen-placement strategies may also displace rival pollen concentrically outward if the force of the stamp action is great enough. Forceful stamp displacement of competitor pollen may have contributed to the evolution of triggered-hammer pollination mechanisms (Scott Armbruster, pers. comm.). For example, Stylidium species swing their anthers (or stigmas in the female phase) forcefully onto vectors during visits (Fig. 6F) (Armbruster et al., 1994).
In addition to displacement and removal, pollen may also be covered/buried by large loads of pollen ❽, denying rivals access to stigmas until the pollen covering is sufficiently depleted. Large vector pollen loads may also saturate a stigmatic surface, limiting access to subsequent rival pollen  (Ashman et al., 1993). This form of pollen deposition competition is analogous to mating plugs in animals (Alcock, 1994). Large vector pollen loads may also facilitate rapid pollen export allowing males to gain siring priority in species where ovules become available relatively synchronously [e.g. species with single-day flowers or short vector-activity periods (Harder and Johnson, 2008)]. However, if pollen vectors visit multiple flowers on an individual plant, placing large vector pollen loads may be costly, since self-stigma saturation could lead to a reduction in outcross pollen export
(Ashman et al., 1993). This form of pollen deposition competition is analogous to mating plugs in animals (Alcock, 1994). Large vector pollen loads may also facilitate rapid pollen export allowing males to gain siring priority in species where ovules become available relatively synchronously [e.g. species with single-day flowers or short vector-activity periods (Harder and Johnson, 2008)]. However, if pollen vectors visit multiple flowers on an individual plant, placing large vector pollen loads may be costly, since self-stigma saturation could lead to a reduction in outcross pollen export  and receipt. Therefore, this strategy is most likely in plants with small floral displays, dichogamous plants with daily flowers or dichogamous plants that manipulate flower visitor behaviour through inflorescence architecture so that flowers in male phase are visited last.
and receipt. Therefore, this strategy is most likely in plants with small floral displays, dichogamous plants with daily flowers or dichogamous plants that manipulate flower visitor behaviour through inflorescence architecture so that flowers in male phase are visited last.
Placing large vector pollen loads may also increase pollen-germination and tube-growth competition among a plant’s own pollen grains on outcross stigmas. This may amplify diminishing returns on pollen production, limiting selection for placing large numbers of pollen grains in vector pollen loads (Charnov, 1982). However, plants do not compete only for ovules in a population—they first compete for access to pollen vectors, then access to stigmas, and only then do they potentially compete for access to ovules. If access to vectors or stigmas is limited in a population, selection may still favour males that place large vector pollen loads in terms of volume and proportion of total pollen production, as they are most likely to capitalize, and potentially monopolize, available mating opportunities. For example, orchids, which are typically poorly visited by pollen vectors, place vector pollen loads that are large both in volume and proportion of total production, but with low pollen grain numbers relative to ovules (Harder and Johnson, 2008).
Different pollen placement strategies and their associated pollen landscape structures may also influence the donor composition of stigmatic pollen loads and, therefore, the likelihood of pollen-germination and tube-growth competition. For example, diffuse placement is more likely to generate multimale stigmatic pollen loads and therefore strong pollen-tube competition, than stroke placement, where stigmas potentially capture pollen from top layers in the pollen landscape that consist of pollen from one or a few donors (Fig. 5F).
Pollen grooming and passive pollen loss.
A major source of pollen loss is the displacement or removal of pollen from the original placement site due to pollen vector grooming ❻ (Thomson, 1986; Harder, 1990; Holmquist et al., 2012). However, pollen vectors may not be able to groom all areas of their bodies effectively and with equal ease (Macior, 1967, 1974; Kimsey, 1984; Thorp, 2000). Many bees, for example, seem to struggle to reach and groom the mid-line along the dorsal and ventral surfaces of their thorax and abdomen (Koch et al., 2017; Tong and Huang, 2018; Fig 8B). Pollen placed within these hard-to-reach areas is relatively safe from grooming (pollen ‘safe sites’), and selection may therefore favour pollen placement within these areas (Macior, 1974; Westerkamp and Claßen-Bockhoff, 2007). The widespread occurrence of bilabiate flowers among angiosperms, and especially Lamiales (Fig. 6C), may reflect a convergent pollen protection and placement strategy: bilabiate flowers are able to protect pollen from pollen thieves by hiding anthers under the top lip of the flowers (away from the nectar source), while simultaneously stroking pollen onto the dorsal midline of pollen vectors, thereby limiting pollen lost to grooming (Macior, 1967, 1974; Westerkamp and Claßen-Bockhoff, 2007).
Fig. 8.
The accumulation of pollen on pollinators can be strongly affected by variation in the grooming patterns of different pollinators. (A) Rhigioglossa nitens grooms infrequently and its entire body is densely covered with Dimorphotheca sinuata pollen. Photo: B. Anderson. (B) Other pollinators such as Bombus vagans groom regularly but are only able to access pollen from certain parts of their bodies. Pollen ‘safe sites’ (see stripe of Mimulus ringens pollen on the head and thorax) often lie along the midlines of bees’ bodies because they are unable to reach those areas with their legs (Macior, 1974; Westerkamp and Claßen-Bockhoff, 2007; Koch et al., 2017). Photo: J. Karron.
Pollen loss as a result of grooming ❻ may also be ameliorated by reducing vector pollen-load sizes so that stimulation of grooming behaviour is reduced (Harder, 1990). Pollen morphology, nutrient content, and pollenkitt composition could reduce the ease or incentive to groom. For example, spines and pollenkitt on pollen grains inhibit the ability of corbiculate bees to package pollen and reduce the incentive to collect it (Lunau et al., 2015). Specialization or pollinator shifts towards non-grooming (less wasteful) vectors may ameliorate grooming-related pollen loss (Stebbins, 1970; Thomson, 2003). For example, directional trait evolution such as tube length elongation or colour shifts to red are frequently associated with shifts from grooming vectors such as bees to less frequently grooming vectors such as flies (Anderson et al., 2014) (Fig. 8A) or birds (Castellanos et al., 2004; Wilson et al., 2006).
The three pollen placement strategies potentially differ in their susceptibility to pollen loss through grooming (Fig. 5D). Diffuse pollen placement is most susceptible to grooming, as there is a high probability of pollen placement on areas that vectors are able to groom. However, because the area of stigma pollen capture is also large, the detrimental effect of pollen being displaced from one area of the vector body to another by grooming may be small. Diffusely placed pollen may mainly be lost when it is groomed off vector bodies, consumed or packed into corbiculae where pollen grains may be rendered inviable (Parker et al., 2015) and unlikely to be captured by stigmas (Thomson, 1986).
In contrast, pollen placed by stroking or stamping would likely be lost as soon as it is groomed from the relatively small stigma-contact area (Fig. 5D). The potential costs of grooming-related pollen loss in stroke and, in particular, stamp pollen placement should therefore select for placement on pollen grooming safe sites (Koch et al., 2017; Tong and Huang, 2018) (Fig. 8B), or traits that increase recruitment and pollen placement on non-grooming pollen vectors.
Even if pollen vectors do not groom regularly, pollen may still be lost passively from vectors during transport ❼. As with grooming, the extent of passive pollen loss may vary among pollen vectors. For example, bat fur may hold more pollen for a longer period during transport than feathers on birds (Muchhala and Thomson, 2010), and selection to decrease passive pollen loss during transport may therefore drive specialization on, or shifts to, different pollen vectors. Selection may also favour stickier pollen grains, smaller pollen load sizes or less exposed pollen placement sites.
Interspecific interactions: reproductive interference and isolation.
Most flowering plants share pollen vectors with other co-flowering species, potentially lowering male and female fitness through heterospecific pollen transfer (Flanagan et al., 2009; Mitchell et al., 2009). Receipt of heterospecific pollen on stigmas may reduce female fitness by preventing conspecific pollen attachment or by interfering with pollen germination and pollen-tube growth (Waser, 1978; Ashman and Arceo-Gómez, 2013; Briggs et al., 2016). However, interactions between co-flowering species may have even greater consequences for male function; for example, pollen wastage (pollen loss)  can occur if pollen is deposited on stigmas or other floral parts of co-flowering species (Bell et al., 2005; Morales and Traveset, 2008). Furthermore, every visit to a heterospecific flower could result in a male’s vector pollen load being covered or displaced by heterospecific pollen ❾. Muchhala and Thomson (2012) elegantly measured both pollen loss to heterospecific stigmas and the additional decrease in pollen transfer associated with pollen displacement and covering, following a visit to a single flower of a co-flowering species in male phase. They found that pollen loss to heterospecific stigmas reduced pollen export to the next conspecific flower by 43.1 %
can occur if pollen is deposited on stigmas or other floral parts of co-flowering species (Bell et al., 2005; Morales and Traveset, 2008). Furthermore, every visit to a heterospecific flower could result in a male’s vector pollen load being covered or displaced by heterospecific pollen ❾. Muchhala and Thomson (2012) elegantly measured both pollen loss to heterospecific stigmas and the additional decrease in pollen transfer associated with pollen displacement and covering, following a visit to a single flower of a co-flowering species in male phase. They found that pollen loss to heterospecific stigmas reduced pollen export to the next conspecific flower by 43.1 %  , while pollen displacement/covering by heterospecific pollen resulted in a 66.1 % reduction in pollen export to the next conspecific flower
, while pollen displacement/covering by heterospecific pollen resulted in a 66.1 % reduction in pollen export to the next conspecific flower  . This study highlights the substantial costs to pollen export potential when pollen vectors visit co-flowering species. Moreover, every visit to a heterospecific flower increases the time spent away from potential conspecific recipients, and therefore the amount of pollen lost passively or through grooming (Flanagan et al., 2009). This study also suggests that if different species can compete through pollen displacement or covering on pollen vectors, then pollen displacement/covering may lead to even more intense intraspecific male–male competition because pollen placement sites of intraspecific rival males are expected to have greater overlap.
. This study highlights the substantial costs to pollen export potential when pollen vectors visit co-flowering species. Moreover, every visit to a heterospecific flower increases the time spent away from potential conspecific recipients, and therefore the amount of pollen lost passively or through grooming (Flanagan et al., 2009). This study also suggests that if different species can compete through pollen displacement or covering on pollen vectors, then pollen displacement/covering may lead to even more intense intraspecific male–male competition because pollen placement sites of intraspecific rival males are expected to have greater overlap.
To avoid interspecific reproductive interference ❾  , selection may act on several traits (Fig. 1). First, selection may reduce the overlap in flowering time between heterospecifics that share a common pollen vector (Rathcke, 1983). Secondly, interspecific reproductive interference may drive specialization on, or shifts to, vectors that visit fewer heterospecifics (Muchhala et al., 2010). Interspecific reproductive interference can also promote character displacement to reduce the overlap in sites of pollen placement and receipt (Armbruster et al., 1994; Muchhala and Potts, 2007; Muchhala and Thomson, 2012) (Fig. 9). However, plants that place pollen diffusely are unlikely to reduce overlap in pollen placement with heterospecifics through shifts in pollen placement position. Instead, we predict that selection to avoid interspecific reproductive interference ❾
, selection may act on several traits (Fig. 1). First, selection may reduce the overlap in flowering time between heterospecifics that share a common pollen vector (Rathcke, 1983). Secondly, interspecific reproductive interference may drive specialization on, or shifts to, vectors that visit fewer heterospecifics (Muchhala et al., 2010). Interspecific reproductive interference can also promote character displacement to reduce the overlap in sites of pollen placement and receipt (Armbruster et al., 1994; Muchhala and Potts, 2007; Muchhala and Thomson, 2012) (Fig. 9). However, plants that place pollen diffusely are unlikely to reduce overlap in pollen placement with heterospecifics through shifts in pollen placement position. Instead, we predict that selection to avoid interspecific reproductive interference ❾  in diffuse pollen-placement systems would act on traits that promote constancy (sensuWaser, 1986) in vector foraging sequences (Fig. 5E). Bees are often described as generalist flower visitors in pollination network studies (e.g. Alarcón et al., 2008). However, individual bees often forage exclusively from a single flowering species during a foraging bout (Grant, 1950; Waser, 1986), limiting the probability of interspecific reproductive interference. Consequently, selection may favour dissimilarity in floral display colour, colour pattern or scent amongst co-flowering species to promote individual pollen-vector constancy in bee pollen vectors (Jones, 1978; Grant, 1994) (Fig. 1) [however, see Ellis and Johnson (2012) and Kemp et al. (2019) for discussion regarding non-constant pollen vectors and convergence in display traits for co-flowering species].
in diffuse pollen-placement systems would act on traits that promote constancy (sensuWaser, 1986) in vector foraging sequences (Fig. 5E). Bees are often described as generalist flower visitors in pollination network studies (e.g. Alarcón et al., 2008). However, individual bees often forage exclusively from a single flowering species during a foraging bout (Grant, 1950; Waser, 1986), limiting the probability of interspecific reproductive interference. Consequently, selection may favour dissimilarity in floral display colour, colour pattern or scent amongst co-flowering species to promote individual pollen-vector constancy in bee pollen vectors (Jones, 1978; Grant, 1994) (Fig. 1) [however, see Ellis and Johnson (2012) and Kemp et al. (2019) for discussion regarding non-constant pollen vectors and convergence in display traits for co-flowering species].
Fig. 9.
Pollen competition between species may drive the evolution of unique pollen placement sites on pollinators. Here, the yellow pollen of Burmeistera ceratocarpa is placed mostly between the eyes of a bat (Anoura geoffroyi) while the white pollen of Burmeistera borjensis is placed between the ears. This is thought to be the product of character displacement and, in sympatry, it minimizes interspecific pollen interference (Muchhala and Potts, 2007). Photos: N. Muchhala.
Autonomous pathway to paternity
Although our review has focused on vector-mediated male reproductive strategies, it is important to note that not all reproduction by animal-pollinated plants is facilitated by vectors. Plants may transfer pollen autonomously within flowers, leading to a vectorless portion of the pathway to male reproductive success (Lloyd and Schoen, 1992; Kalisz et al., 2004). The proportion of offspring sired through this pathway varies widely among species (Busch and Delph, 2012), and the evolution of autonomous selfing from outcrossing ancestors is one of the most frequent evolutionary transitions in plants (Wright et al., 2013). Although many factors [e.g. the automatic selection advantage of selfing (Fisher, 1941)] are thought to contribute to an increase in the rate of autonomous selfing, considerable evidence suggests that poor pollinator service often plays a major role (Kalisz et al., 2004; Bodbyl Roels and Kelly, 2011; Yin et al., 2016).
CONCLUSION
‘[C]ompetition among plants to pollinate other plants does not involve struggles, or even contact, between competitors…’
The use of biotic gamete vectors is unique to plants (Bishop and Pemberton, 2006; Beekman et al., 2016) and so comparisons with fertilization processes in animals need to be made with care. In particular, the notion that pollen competition only occurs after deposition onto stigmas, as with post-copulation sperm competition in animals, is a false equivalence. Pollen competition is likely to occur along most of the pathway to paternity, starting at the time of pollen production and placement, continuing all the way through to pollen deposition on stigmas, pollen germination and pollen-tube growth, and ovule fertilization. Every time a plant places pollen on a pollinator, it has the opportunity to displace, cover, and remove pollen grains of its competitors and increase its siring success. When access to stigmas or pollen vectors is limited, plants may benefit from gaining exclusive access to stigmas by placing large vector pollen loads that saturate recipient stigmas upon deposition. This form of pollen deposition competition may be similar to mate guarding in animals. Pollen placement and deposition competition on vectors may be as important as pollen-germination and tube-growth competition, and selection for increased competitiveness could influence pollen placement strategies and pollen presentation, as well as several siring barriers further along the pathway. Unlike internally fertilizing animals, the pathway to paternity in animal-pollinated plants is a complicated obstacle course along which pollen can be lost at multiple stages before the final stylar race to fertilize ovules. Nevertheless, after considering the potential ramifications of pollen placement and deposition competition, it is perhaps possible to make some comparisons with animal reproductive strategies, and Janzen’s jarring parallels between plant and animal reproduction appear to hold more than ever:
‘…plants are not trying to maximize outcrossing but rather to optimize it. In doing so they perform courtship displays, […] promiscuity, and fickleness just as do animals.’
ACKNOWLEDGEMENTS
We would like to sincerely thank Steven Johnson, Lawrence Harder, James Thomson, and an anonymous reviewer for their detailed and constructive comments on the various iterations of this manuscript. We thank Christine Dimech, Fred Hort, Jean Hort, Ingrid Minnaar, John Lampkin, Nathan Muchhala, and Stan Tekiela for the use of their photographic images. This work was supported by the Claude Leon Foundation [M.D.J.]; the Eva Crane Trust [ECTA_20170609 to C.M. and ECTA_20170905 to M.D.J.]; the National Research Foundation (South Africa) [105987 to B.A. and C.M.; 111979 to C.M.]; and the National Science Foundation [1654943 to J.K.].
A GLOSSARY OF TERMS USED THROUGHOUT THE REVIEW
Fitness: the lifetime reproductive output of an individual. In hermaphroditic plants that produce both male and female gametes, this is the sum of the individual’s male fitness (number of viable seeds sired) and female fitness (number of viable seeds produced).
Geitonogamous pollen transfer: the dispersal of pollen between flowers of a hermaphroditic plant.
Pathway to paternity: the voyage of a pollen grain from production and release in an anther to fertilization of an ovule.
Pollen competition: the competitive interactions between pollen of rival individuals to fertilize a limited set of ovules. Different forms of competition can occur at several phases along the pathway to paternity, pollen placement competition on biotic pollen vector bodies, pollen deposition competition on stigmas and pollen-germination and tube-growth competition (progammic pollen competition) within styles.
Pollen discounting: the reduction of pollen available for export following self-pollination.
Pollen landscape: the 2- or 3-D structure and composition of multidonor pollen on the bodies of biotic pollen vectors. Pollen landscapes arise as a result of successive pollen placement by different rival individuals on pollen vector bodies and provide an interface for competitive male–male interactions that can include the displacement, covering, or removal of competing pollen grains.
Pollen presentation: the process through which plants make pollen available for placement on pollen vectors.
Pollen presentation rate: the rate at which pollen is presented at the level of the plant. Various pollen presentation traits can influence pollen presentation rates; for example, the number of flowers simultaneously on display or the rate of anther dehiscence.
Siring barriers: the mechanisms along the pathway to paternity that reduce the likelihood of an individual’s pollen siring seeds.
Siring success: the relative success of an individual’s pollen at fertilizing ovules.
Stigmatic pollen load: the quantity of pollen deposited on a stigma.
Strategies: adaptive solutions to increase siring success by mitigating siring barriers along the pathway to paternity.
Vector pollen load: the quantity of pollen placed on a biotic vector.
LITERATURE CITED
- Aigner PA. 2001. Optimality modeling and fitness trade-offs: when should plants become pollinator specialists?Oikos 95: 177–184. [Google Scholar]
- Aigner PA. 2004. Floral specialization without trade-offs: optimal corolla flare in contrasting pollination environments. Ecology 85: 2560–2569. [Google Scholar]
- Alarcón R, Waser NM, Ollerton J. 2008. Year-to-year variation in the topology of a plant–pollinator interaction network. Oikos 117: 1796–1807. [Google Scholar]
- Alcock J. 1994. Postinsemination associations between males and females in insects: the mate-guarding hypothesis. Annual Review of Entomology 39: 1–21. [Google Scholar]
- Anderson B, Cole WW, Barrett SCH. 2005. Specialized bird perch aids cross-pollination. Nature 435: 41–42. [DOI] [PubMed] [Google Scholar]
- Anderson B, Ros P, Wiese TJ, Ellis AG. 2014. Intraspecific divergence and convergence of floral tube length in specialized pollination interactions. Proceedings of the Royal Society B: Biological Sciences 281: 20141420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster WS, Edwards ME, Debevec EM. 1994. Floral character displacement generates assemblage structure of Western Australian triggerplants (Stylidium). Ecology 75: 315–329. [Google Scholar]
- Armbruster WS, Hansen TF, Pélabon C, Pérez-Barrales R, Maad J. 2009a The adaptive accuracy of flowers: measurement and microevolutionary patterns. Annals of Botany 103: 1529–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster WS, Pélabon C, Hansen TF, Bolstad GH. 2009b Macroevolutionary patterns of pollination accuracy: a comparison of three genera. New Phytologist 183: 600–617. [DOI] [PubMed] [Google Scholar]
- Ashman T-L. 1994. A dynamic perspective on the physhiological costs of reproduction in plants. American Naturalist 144: 300–316. [Google Scholar]
- Ashman T-L. 1998. Is relative pollen production or removal a good predictor of relative male fitness? An experimental exploration with a wild strawberry (Fragaria virginiana, Rosaceae). American Journal of Botany 85: 1166–1171. [PubMed] [Google Scholar]
- Ashman T-L, Arceo-Gómez G. 2013. Toward a predictive understanding of the fitness costs of heterospecific pollen receipt and its importance in co-flowering communities. American Journal of Botany 100: 1061–1070. [DOI] [PubMed] [Google Scholar]
- Ashman T-L, Galloway LF, Stanton ML. 1993. Apparent vs. effective mating in an experimental population of Raphanus sativus. Oecologia 96: 102–107. [DOI] [PubMed] [Google Scholar]
- Austen EJ, Weis AE. 2016. The causes of selection on flowering time through male fitness in a hermaphroditic annual plant. Evolution 70: 111–125. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. 2002. Sexual interference of the floral kind. Heredity 88: 154–159. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. 2003. Mating strategies in flowering plants: the outcrossing–selfing paradigm and beyond. Philosophical Transactions of the Royal Society B: Biological Sciences 358: 991–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH, Harder LD. 2017. The ecology of mating and its evolutionary consequences in seed plants. Annual Review of Ecology, Evolution, and Systematics 48: 135–157. [Google Scholar]
- Bateman AJ. 1948. Intra-sexual selection in Drosophila. Heredity 2: 349–368. [DOI] [PubMed] [Google Scholar]
- Beekman M, Nieuwenhuis B, Ortiz-Barrientos D, Evans JP. 2016. Sexual selection in hermaphrodites, sperm and broadcast spawners, plants and fungi. Philosophical Transactions of the Royal Society B: Biological Sciences 371: 20150541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JM, Karron JD, Mitchell RJ. 2005. Interspecific competition for pollination lowers seed production and outcrossing in Mimulus ringens. Ecology 86: 762–771. [Google Scholar]
- Bertin RI. 1988. Paternity in plants. In: Lovett-Doust J, Lovett-Doust L, eds. Plant reproductive ecology: patterns and strategies. Oxford: Oxford University Press, 30–59. [Google Scholar]
- Bishop JDD, Pemberton AJ. 2006. The third way: spermcast mating in sessile marine invertebrates. Integrative and Comparative Biology 46: 398–406. [DOI] [PubMed] [Google Scholar]
- Bode M, Marshall DJ. 2007. The quick and the dead? Sperm competition and sexual conflict in sea. Evolution 61: 2693–2700. [DOI] [PubMed] [Google Scholar]
- Bodbyl Roels SA, Kelly JK. 2011. Rapid evolution caused by pollinator loss in Mimulus guttatus. Evolution 65: 2541–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs HM, Anderson LM, Atalla LM, Delva AM, Dobbs EK, Brosi BJ. 2016. Heterospecific pollen deposition in Delphinium barbeyi: linking stigmatic pollen loads to reproductive output in the field. Annals of Botany 117: 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe Runquist RD, Geber MA, Pickett-Leonard M, Moeller DA. 2017. Mating system evolution under strong pollen limitation: evidence of disruptive selection through male and female fitness in Clarkia xantiana. American Naturalist 189: 549–563. [DOI] [PubMed] [Google Scholar]
- Burd M, Callahan HS. 2000. What does the male function hypothesis claim?Journal of Evolutionary Biology 13: 735–742. [Google Scholar]
- Busch JW, Delph LF. 2012. The relative importance of reproductive assurance and automatic selection as hypotheses for the evolution of self-fertilization. Annals of Botany 109: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo RM, Franceschinelli EV. 2004. Introduced honeybees (Apis mellifera) reduce pollination success without affecting the floral resource taken by native pollinators. Biotropica 36: 317–376. [Google Scholar]
- Castellanos MC, Wilson P, Thomson JD. 2004. ‘Anti-bee’ and ‘pro-bird’ changes during the evolution of hummingbird pollination in Penstemon flowers. Journal of Evolutionary Biology 17: 876–885. [DOI] [PubMed] [Google Scholar]
- Castellanos MC, Wilson P, Keller SJ, Wolfe AD, Thomson JD. 2006. Anther evolution: pollen presentation strategies when pollinators differ. American Naturalist 167: 288–96. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Schemske DW, Sork VL. 1987. The evolution of plant reproductive characters; sexual versus natural selection. In: Stearns, SC, ed. The evolution of sex and its consequences. Basel: Springer Basel AG, 317–335. [DOI] [PubMed] [Google Scholar]
- Charnov EL. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press. [Google Scholar]
- Chen PH, Pan YB, Chen RK. 2008. High-throughput procedure for single pollen grain collection and polymerase chain reaction in plants. Journal of Integrative Plant Biology 50: 375–383. [DOI] [PubMed] [Google Scholar]
- Cocucci AA, Marino S, Baranzelli M, Wiemer AP, Sérsic A. 2014. The buck in the milkweed: evidence of male–male interference among pollinaria on pollinators. New Phytologist 203: 280–286. [DOI] [PubMed] [Google Scholar]
- Conner JK, Rush S. 1996. Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum. Oecologia 105: 509–516. [DOI] [PubMed] [Google Scholar]
- Dafni A, Firmage D. 2000. Pollen viability and longevity: practical, ecological and evolutionary implications. In: Dafni A, Hesse M, Pacini E, eds. Pollen and pollination. Vienna: Springer, 113–132. [Google Scholar]
- Darwin C. 1876. The effects of cross and self fertilisation in the vegetable kingdom. London: John Murrray. [Google Scholar]
- de Jager ML, Peakall R. 2019. Experimental examination of pollinator-mediated selection in a sexually deceptive orchid. Annals of Botany doi:10.1093/aob/mcy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager ML, Willis-Jones E, Critchley S, Glover BJ. 2016. The impact of floral spot and ring markings on pollinator foraging dynamics. Evolutionary Ecology 31: 1–12. [Google Scholar]
- De Kock C, Minnaar C, Lunau K, et al. 2018. The functional role of the keel crest in Polygala myrtifolia (Polygalaceae) and its effects on pollinator visitation success. South African Journal of Botany 118: 105–111. [Google Scholar]
- Delph LF, Ashman TL. 2006. Trait selection in flowering plants: how does sexual selection contribute?Integrative and Comparative Biology 46: 465–472. [DOI] [PubMed] [Google Scholar]
- Delph LF, Havens K. 1998. Pollen competition in flowering plants. In: Birkhead TR, Moller AR, eds. Sperm competition and sexual selection. San Diego, CA: Academic Press, 149–173. [Google Scholar]
- Delph LF, Johannsson MH, Stephenson AG. 1997. How environmental factors affect pollen performance: ecological and evolutionary perspectives. Ecology 78: 1632–1639. [Google Scholar]
- Devaux C, Lepers C, Porcher E. 2014. Constraints imposed by pollinator behaviour on the ecology and evolution of plant mating systems. Journal of Evolutionary Biology 27: 1413–1430. [DOI] [PubMed] [Google Scholar]
- Devlin B. 1989. Components of seed and pollen yield of Lobelia cardinalis: variation and correlations. American Journal of Botany 76: 204–214. [Google Scholar]
- Duffy KJ, Johnson SD. 2014. Male interference with pollination efficiency in a hermaphroditic orchid. Journal of Evolutionary Biology 27: 1751–1756. [DOI] [PubMed] [Google Scholar]
- Eckert CG. 2000. Contributions of autogamy and geitonogamy to self‐fertilization in a mass‐flowering, clonal plant. Ecology 81: 532–542. [Google Scholar]
- Ellis AG, Johnson SD. 2010. Gender differences in the effects of floral spur length manipulation on fitness in a hermaphrodite orchid. International Journal of Plant Sciences 171: 1010–1019. [Google Scholar]
- Ellis AG, Johnson SD. 2012. Lack of floral constancy by bee fly pollinators: implications for ethological isolation in an African daisy. Behavioral Ecology 23: 729–734. [Google Scholar]
- Fetscher AE. 2001. Resolution of male–female conflict in an hermaphroditic flower. Proceedings of the Royal Society B: Biological Sciences 268: 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. 1941. Average excess and average effect of a gene substitution. Annals of Eugenics 11: 53–63. [Google Scholar]
- Flanagan RJ, Mitchell RJ, Knutowski D, Karron JD. 2009. Interspecific pollinator movements reduce pollen deposition and seed production in Mimulus ringens (Phrymaceae). American Journal of Botany 96: 809–815. [DOI] [PubMed] [Google Scholar]
- Gilbert LE. 1975. Ecological consequences of a coevolved mutualism between butterflies and plants. In: Gilbert LE, Raven PH, eds. Coevolution of animals and plants. Austin, TX: University of Texas Press, 210–240. [Google Scholar]
- Goldman DA, Willson MF. 1986. Sex allocation in functionally hermaphroditic plants: a review and critique. Botanical Review 52: 157–194. [Google Scholar]
- Gómez JM, Perfectti F, Camacho JPM. 2006. Natural selection on Erysimum mediohispanicum flower shape: insights into the evolution of zygomorphy. American Naturalist 168: 531–545. [DOI] [PubMed] [Google Scholar]
- Gong Y-B, Huang S-Q. 2014. Interspecific variation in pollen–ovule ratio is negatively correlated with pollen transfer efficiency in a natural community. Plant Biology 16: 843–847. [DOI] [PubMed] [Google Scholar]
- Grant V. 1950. The flower constancy of bees. Botanical Review 16: 379–398. [Google Scholar]
- Grant V. 1994. Modes and origins of mechanical and ethological isolation in angiosperms. Proceedings of the National Academy of Sciences, USA 91: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant V. 1995. Sexual selection in plants: pros and cons. Proceedings of the National Academy of Sciences, USA 92: 1247–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD. 1983. Flower handling efficiency of bumble bees: morphological aspects of probing time. Oecologia 57: 274–280. [DOI] [PubMed] [Google Scholar]
- Harder LD. 1990. Behavioral responses by bumble bees to variation in pollen availability. Oecologia 85: 41–47. [DOI] [PubMed] [Google Scholar]
- Harder LD. 2000. Pollen dispersal and the floral diversity of Monocotyledons. In: Wilson KL, Morrison D, eds. Monocots: systematics and evolution. Melbourne, Australia: CSIRO Publishing, 243–257. [Google Scholar]
- Harder LD, Barclay RMR. 1994. The functional significance of poricidal anthers and buzz pollination: controlled pollen removal from Dodecatheon. Functional Ecology 8: 509–517. [Google Scholar]
- Harder LD, Barrett SCH. 1995. Mating cost of large floral displays in hermaphrodite plants. Nature 373: 512–515. [Google Scholar]
- Harder LD, Barrett SCH. 1996. Pollen dispersal and mating patterns in animal-pollinated plants. In: Lloyd DG, Barrett SCH, eds. Floral biology: studies on floral evolution in animal-pollinated plants. New York, NY: Chapman & Hall, 140–190. [Google Scholar]
- Harder LD, Johnson SD. 2005. Adaptive plasticity of floral display size in animal-pollinated plants. Proceedings of the Royal Society B: Biological Sciences 272: 2651–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Johnson SD. 2008. Function and evolution of aggregated pollen in angiosperms. International Journal of Plant Sciences 169: 59–78. [Google Scholar]
- Harder LD, Routley MB. 2006. Pollen and ovule fates and reproductive performance by flowering plants. In: Barrett SCH, Harder LD, eds. Ecology and evolution of flowers. Oxford: Oxford University Press, 61–80. [Google Scholar]
- Harder LD, Thomson JD. 1989. Evolutionary options for maximizing pollen dispersal of animal-pollinated plants. American Naturalist 133: 323–344. [Google Scholar]
- Harder LD, Wilson WG. 1994. Floral evolution and male reproductive success: optimal dispensing schedules for pollen dispersal by animal-pollinated plants. Evolutionary Ecology 8: 542–559. [Google Scholar]
- Harder LD, Wilson WG. 1997. Theoretical perspectives on pollination. Acta Horticulturae 437: 83–101. [Google Scholar]
- Harder LD, Wilson WG. 1998a Theoretial consequences of heterogeneus transport conditions for pollen dispersal by animals. Ecology 79: 2789–2807. [Google Scholar]
- Harder LD, Wilson WG. 1998b A clarification of pollen discounting and its joint effects with inbreeding depression on mating system evolution. Tmerican Naturalist 152: 684–695. [DOI] [PubMed] [Google Scholar]
- Hargreaves AL, Harder LD, Johnson SD. 2009. Consumptive emasculation: the ecological and evolutionary consequences of pollen theft. Biological Reviews 84: 259–276. [DOI] [PubMed] [Google Scholar]
- Harder LD, Aizen MA, Richards SA. 2016a The population ecology of male gametophytes: the link between pollination and seed production. Ecology Letters 19: 497–509. [DOI] [PubMed] [Google Scholar]
- Harder LD, Aizen MA, Richards SA, Joseph MA, Busch JW. 2016b Diverse ecological relations of male gametophyte populations in stylar environments. American Journal of Botany 103: 484–497. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Suyama Y, Seiwa K. 2009. Pollen donor composition during the early phases of reproduction revealed by DNA genotyping of pollen grains and seeds of Castanea crenata. New Phytologist 182: 994–1002. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Suyama Y, Seiwa K. 2015. Variation in pollen-donor composition among pollinators in an entomophilous tree species, Castanea crenata, revealed by single-pollen genotyping. PLoS One 10: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges SA. 1995. The influence of nectar production on hawkmoth behavior, self pollination, and seed production in Mirabilis multiflora (Nyctaginaceae). American Journal of Botany 82: 197–204. [Google Scholar]
- Holmquist KG, Mitchell RJ, Karron JD. 2012. Influence of pollinator grooming on pollen-mediated gene dispersal in Mimulus ringens (Phrymaceae). Plant Species Biology 27: 77–85. [Google Scholar]
- Holsinger KE, Thomson JD. 1994. Pollen discounting in Erythronium grandiflorum: mass-action estimates from pollen transfer dynamics. American Naturalist 144: 799–812. [Google Scholar]
- Horovitz A, Harding J. 1972. The concept of male outcrossing in hermaphrodite higher plants. Heredity 29: 223–236. [Google Scholar]
- Hosken DJ, Stockley P. 2004. Sexual selection and genital evolution. Trends in Ecology & Evolution 19: 87–93. [DOI] [PubMed] [Google Scholar]
- Howell GJ, Slater AT, Knox RB. 1993. Secondary pollen presentation in angiosperms and its biological significance. Australian Journal of Botany 41: 417–438. [Google Scholar]
- Inouye DW, Gill DE, Dudash MR, Fenster CB. 1994. A model and lexicon for pollen fate. American Journal of Botany 81: 1517–1530. [Google Scholar]
- Janzen DH. 1977. A note on optimal mate selection in plants. American Naturalist 111: 365–371. [Google Scholar]
- Jersáková J, Johnson SD. 2006. Lack of floral nectar reduces self-pollination in a fly-pollinated orchid. Oecologia 147: 60–68. [DOI] [PubMed] [Google Scholar]
- Jesson LK, Barrett SCH. 2002. Solving the puzzle of mirror-image flowers. Nature 417: 707. [DOI] [PubMed] [Google Scholar]
- Johannsson MH, Winsor JA, Stephenson AG. 1994. Genetic and environmental effects on in vitro pollen tube growth in Cucurbita. In: Stephenson AJ, Kao T-H, eds. Pollen–pistil interactions and pollen tube growth. Volume 12. Current Topics in Plant Physiology. Rockville, MD: American Society of Plant Physiologists, 307–309. [Google Scholar]
- Johnson SD, Harder LD. 2018. Tracking pollen fates in orchid populations. In: Lee Y, Yeung EC, eds. Orchid propagation: from laboratories to greenhouses – methods and protocols. Humana Press, NY, 227–239. [Google Scholar]
- Johnson SD, Nilsson LA. 1999. Pollen carryover, geitonogamy, and the evolution of deceptive pollination systems in orchids. Ecology 80: 2607–2619. [Google Scholar]
- Johnson SD, Peter CI, Jon A. 2004. The effects of nectar addition on pollen removal and geitonogamy in the non-rewarding orchid Anacamptis morio. Proceedings of the Royal Society B: Biological Sciences 271: 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SD, Neal PR, Harder LD. 2005. Pollen fates and the limits on male reproductive success in an orchid population. Biological Journal of the Linnean Society 86: 175–190. [Google Scholar]
- Johnston MO. 1991. Natural selection on floral traits in two species of Lobelia with different pollinators. Evolution 45: 1468. [DOI] [PubMed] [Google Scholar]
- Jones CE. 1978. Pollinator constancy as a pre-pollination isolating mechanism between sympatric species of Cercidium. Evolution 32: 189–198. [DOI] [PubMed] [Google Scholar]
- de Jong TJ, Waser NM, Klinkhamer PGL. 1993. Geitonogamy: the neglected side of selfing. Trends in Ecology & Evolution 8: 321–325. [DOI] [PubMed] [Google Scholar]
- Kalisz S, Vogler DW, Hanley KM. 2004. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature 430: 884–887. [DOI] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ. 2012. Effects of floral display size on male and female reproductive success in Mimulus ringens. Annals of Botany 109: 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron JD, Jackson RT, Thumser NN, Schlicht SL. 1997. Outcrossing rates of individual Mimulus ringens genets are correlated with anther–stigma separation. Heredity 79: 365–370. [Google Scholar]
- Karron JD, Mitchell RJ, Holmquist KG, Bell JM, Funk B. 2004. The influence of floral display size on selfing rates in Mimulus ringens. Heredity 92: 242–248. [DOI] [PubMed] [Google Scholar]
- Karron JD, Holmquist KG, Flanagan RJ, Mitchell RJ. 2009. Pollinator visitation patterns strongly influence among-flower variation in selfing rate. Annals of Botany 103: 1379–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron JD, Ivey CT, Mitchell RJ, Whitehead MR, Peakall R, Case AL. 2012. New perspectives on the evolution of plant mating systems. Annals of Botany 109: 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B, Thomson JD, Conti E. 2014. Heterostyly promotes disassortative pollination and reduces sexual interference in Darwin’s primroses: evidence from experimental studies. Functional Ecology 28: 1413–1425. [Google Scholar]
- Kemp JE, Bergh NG, Soares M, Ellis AG. 2019. Dominant pollinators drive non-random community assembly and shared flower colour patterns in daisy communities. Annals of Botany doi: 10.1093/aob/mcy126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D, Gase K, Baldwin IT. 2008. Field experiments with transformed plants reveal the sense of floral scents. Science 321: 1200–1202. [DOI] [PubMed] [Google Scholar]
- Kimsey LS. 1984. The behavioural and structural aspects of grooming and related activities in euglossine bees (Hymenoptera: Apidae). Journal of Zoology 204: 541–550. [Google Scholar]
- Klinkhamer PGL, de Jong TJ. 1993. Attractiveness to pollinators: a plant’s dilemma. Oikos 66: 180. [Google Scholar]
- Klinkhamer PGL, De Jong TJ, Wesselingh RA. 1991. Implications of differences between hermaphrodite and female flowers for attractiveness to pollinators and seed production. Netherlands Journal of Zoology 41: 130–143. [Google Scholar]
- Koch L, Lunau K, Wester P. 2017. To be on the safe site – ungroomed spots on the bee’s body and their importance for pollination. PLoS One 12: e0182522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski MH, Ison JL, Padilla A, Pham AQ, Galloway LF. 2018. Linking pollinator efficiency to patterns of pollen limitation: small bees exploit the plant–pollinator mutualism. Proceedings of the Royal Society B: Biological Sciences 285: 20180635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulbaba MW, Worley AC. 2012. Selection on floral design in Polemonium brandegeei (Polemoniaceae): female and male fitness under hawkmoth pollination. Evolution 66: 1344–1359. [DOI] [PubMed] [Google Scholar]
- Kulbaba MW, Worley AC. 2013. Selection on Polemonium brandegeei (Polemoniaceae) flowers under hummingbird pollination: in opposition, parallel, or independent of selection by hawkmoths?Evolution 67: 2194–2206. [DOI] [PubMed] [Google Scholar]
- Ladd PG. 1994. Pollen presenters in the flowering plants – form and function. Botanical Journal of the Linnean Society 115: 165–195. [Google Scholar]
- Larue A-AC, Raguso RA, Junker RR. 2015. Experimental manipulation of floral scent bouquets restructures flower–visitor interactions in the field. Journal of Animal Ecology 85: 396–408. [DOI] [PubMed] [Google Scholar]
- Lau T-C, Stephenson AG. 1993. Effects of soil nitrogen on pollen production, pollen grain size, and pollen performance in Cucurbita pepo. American Journal of Botany 80: 763–768. [Google Scholar]
- Lau T-C, Stephenson AG. 1994. Effects of soil phosphorus on pollen production, pollen size, pollen phosphorus content, and the ability to sire seeds in Cucurbita pepo (Cucurbitaceae). Sexual Plant Reproduction 7: 215–220. [Google Scholar]
- Lertzman KP, Gass CL. 1983. Alternate models of pollen transfer, In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold, 474–489. [Google Scholar]
- Lloyd DG, Schoen DJ. 1992. Self- and cross-fertilization in plants. I. Functional dimensions. International Journal of Plant Sciences 153: 358–369. [Google Scholar]
- Lloyd DG, Webb CJ. 1977. Secondary sex characters in plants. Botanical Review 43: 177–216. [Google Scholar]
- Lloyd DG, Webb CJ. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms I. Dichogamy. New Zealand Journal of Botany 24: 135–162. [Google Scholar]
- Lloyd DG, Webb CJ. 1992. The selection of heterostyly. In: Barrett, SCH, ed. Evolution and function of heterostyly. Berlin: Springer-Verlag, 179–207. [Google Scholar]
- Lunau K, Piorek V, Krohn O, Pacini E. 2015. Just spines – mechanical defense of malvaceous pollen against collection by corbiculate bees. Apidologie 46: 144–149. [Google Scholar]
- Lyons EE, Waser NM, Price MV, Antonovics J, Motten AF. 1989. Sources of variation in plant reproductive success and implications for concepts of sexual selection. American Naturalist 134: 409–433. [Google Scholar]
- Macior LW. 1967. Pollen-foraging behavior of Bombus in relation to pollination of nototribic flowers. American Journal of Botany 54: 359–364. [Google Scholar]
- Macior LW. 1974. Behavioral aspects of coadaptations between flowers and insect pollinators. Annals of the Missouri Botanical Garden 61: 760. [Google Scholar]
- Mao Y-Y, Huang S-Q. 2009. Pollen resistance to water in 80 angiosperm species: flower structures protect rain-susceptible pollen. New Phytologist 183: 892–899. [DOI] [PubMed] [Google Scholar]
- Matsuki Y, Isagi Y, Suyama Y. 2007. The determination of multiple microsatellite genotypes and DNA sequences from a single pollen grain: technical article. Molecular Ecology Notes 7: 194–198. [Google Scholar]
- Matsuki Y, Tateno R, Shibata M, Isagi Y. 2008. Pollination efficiencies of flower-visiting insects as determined by direct genetic analysis of pollen origin. American Journal of Botany 95: 925–930. [DOI] [PubMed] [Google Scholar]
- McCallum B, Chang S-M. 2016. Pollen competition in style: effects of pollen size on siring success in the hermaphroditic common morning glory, Ipomoea purpurea. American Journal of Botany 103: 460–470. [DOI] [PubMed] [Google Scholar]
- Minnaar C, Anderson B. 2018. A novel pollen-tracking method: using quantum dots as pollen labels. bioRxiv doi: 10.1101/286047. [Google Scholar]
- Mitchell RJ, Karron JD, Holmquist KG, Bell JM. 2004. The influence of Mimulus ringens floral display size on pollinator visitation patterns. Functional Ecology 18: 116–124. [Google Scholar]
- Mitchell RJ, Flanagan RJ, Brown BJ, Waser NM, Karron JD. 2009. New frontiers in competition for pollination. Annals of Botany 103: 1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RJ, Wilson WG, Holmquist KG, Karron JD. 2013. Influence of pollen transport dynamics on sire profiles and multiple paternity in flowering plants. PLoS One 8: e76312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP. 1995. Bumblebee preference for symmetrical flowers. Proceedings of the National Academy of Sciences, USA 92: 2288–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JC, Pannell JR. 2011. Sexual selection in plants. Current Biology 21: R176–R182. [DOI] [PubMed] [Google Scholar]
- Morales CL, Traveset A. 2008. Interspecific pollen transfer: magnitude, prevalence and consequences for plant fitness. Critical Reviews in Plant Sciences 27: 221–238. [Google Scholar]
- Morris WF, Mangel M, Adler FR. 1995. Mechanisms of pollen deposition by insect pollinators. Evolutionary Ecology 9: 304–317. [Google Scholar]
- Muchhala N. 2007. Adaptive trade‐off in floral morphology mediates specialization for flowers pollinated by bats and hummingbirds. American Naturalist 169: 494–504. [DOI] [PubMed] [Google Scholar]
- Muchhala N, Potts MD. 2007. Character displacement among bat-pollinated flowers of the genus Burmeistera: analysis of mechanism, process and pattern. Proceedings of the Royal Society B: Biological Sciences 274: 2731–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchhala N, Thomson JD. 2010. Fur versus feathers: pollen delivery by bats and hummingbirds and consequences for pollen production. American Naturalist 175: 717–726. [DOI] [PubMed] [Google Scholar]
- Muchhala N, Thomson JD. 2012. Interspecific competition in pollination systems: costs to male fitness via pollen misplacement. Functional Ecology 26: 476–482. [Google Scholar]
- Muchhala N, Brown Z, Armbruster WS, Potts MD. 2010. Competition drives specialization in pollination systems through costs to male fitness. American Naturalist 176: 732–743. [DOI] [PubMed] [Google Scholar]
- Murphy CG. 1998. Interaction-independent sexual selection and the mechanisms of sexual selection. Evolution 52: 8–18. [DOI] [PubMed] [Google Scholar]
- Mutikainen P, Delph LF. 1996. Effects of herbivory on male reproductive success in plants. Oikos 75: 353. [Google Scholar]
- Newman E, Manning J, Anderson B. 2014. Matching floral and pollinator traits through guild convergence and pollinator ecotype formation. Annals of Botany 113: 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, Manning J, Anderson B. 2015. Local adaptation: mechanical fit between floral ecotypes of Nerine humilis (Amaryllidaceae) and pollinator communities. Evolution 69: 2262–2275. [DOI] [PubMed] [Google Scholar]
- Nichols MS. 1985. Pollen flow, population composition, and the adaptive significance of distyly in Linum tenuifolium L. (Linaceae). Biological Journal of the Linnean Society 25: 235–242. [Google Scholar]
- Obeso JR. 2002. The costs of reproduction in plants. New Phytologist 155: 321–348. [DOI] [PubMed] [Google Scholar]
- Pacini E, Hesse M. 2005. Pollenkitt – its composition, forms and functions. Flora: Morphology, Distribution, Functional Ecology of Plants 200: 399–415. [Google Scholar]
- Parker GA. 1984. Sperm competition and the evolution of animal mating strategies. In: Smith, RL, ed. Sperm competition and the evolution of animal mating systems. Orlando, FL: Academic Press, 1–60. [Google Scholar]
- Parker AJ, Tran JL, Ison JL, Bai JDK, Weis AE, Thomson JD. 2015. Pollen packing affects the function of pollen on corbiculate bees but not non-corbiculate bees. Arthropod-Plant Interactions 9: 197–203. [Google Scholar]
- Parker AJ, Williams NM, Thomson JD. 2018. Geographic patterns and pollination ecotypes in Claytonia virginica. Evolution 72: 202–210. [DOI] [PubMed] [Google Scholar]
- Pauw A, Stofberg J, Waterman RJ. 2009. Flies and flowers in Darwin’s race. Evolution 63: 268–279. [DOI] [PubMed] [Google Scholar]
- Peakall R. 1989. A new technique for monitoring pollen flow in orchids. Oecologia 79: 361–365. [DOI] [PubMed] [Google Scholar]
- Peakall R, Handel SN. 1993. Pollinators discriminate among floral heights of a sexually deceptive orchid: implications for selection. Evolution 47: 1681–1687. [DOI] [PubMed] [Google Scholar]
- Porcher E, Lande R. 2005. The evolution of self-fertilization and inbreeding depression under pollen discounting and pollen limitation. Journal of Evolutionary Biology 18: 497–508. [DOI] [PubMed] [Google Scholar]
- Pyke GH. 2016. Floral nectar: pollinator attraction or manipulation?Trends in Ecology and Evolution 31: 339–341. [DOI] [PubMed] [Google Scholar]
- Queller DC. 1983. Sexual selection in a hermaphroditic plant. Nature 305: 706–707. [Google Scholar]
- Queller D. 1997. Pollen removal, paternity, and the male function of flowers. American Naturalist 149: 585–594. [Google Scholar]
- Quesada M, Bollman K, Stephenson AG. 1995. Leaf damage decreases pollen production and hinders pollen performance in Cucurbita texana. Ecology 76: 437–443. [Google Scholar]
- Rameau C, Gouyon P-H. 1991. Resource allocation to growth, reproduction and survival in Gladiolus: the cost of male function. Journal of Evolutionary Biology 4: 291–307. [Google Scholar]
- Rathcke B. 1983. Competition and facilitation among plants for pollination. In: Real, L, ed. Pollination biology. New York: Academic Press, 305–329. [Google Scholar]
- Reynolds RJ, Westbrook MJ, Rohde AS, Cridland JM, Fenster CB, Dudash MR. 2009. Pollinator specialization and pollination syndromes of three related North American Silene. Ecology 90: 2077–2087. [DOI] [PubMed] [Google Scholar]
- Richards SA, Williams NM, Harder LD. 2009. Variation in pollination: causes and consequences for plant reproduction. American Naturalist 174: 382–398. [DOI] [PubMed] [Google Scholar]
- Schoen DJ, Stewart SC. 1986. Variation in male reproductive investment and male reproductive success in white spruce. Evolution 40: 1109–1120. [DOI] [PubMed] [Google Scholar]
- Skogsmyr I, Lankinen Å. 2002. Sexual selection: an evolutionary force in plants?Biological Reviews of the Cambridge Philosophical Society 77: 537–562. [DOI] [PubMed] [Google Scholar]
- Sletvold N, Trunschke J, Smit M, Verbeek J, Ågren J. 2016. Strong pollinator-mediated selection for increased flower brightness and contrast in a deceptive orchid. Evolution 70: 716–724. [DOI] [PubMed] [Google Scholar]
- Snow AA. 1994. Postpollination selection and male fitness in plants. American Naturalist 144: S69–S83. [Google Scholar]
- Snow AA, Spira TP, Simpson R, Klips RA. 1996. The ecology of geitonogamous pollination. In: Lloyd DG, Barrett SCH, eds. Floral biology. Boston, MA: Springer, 191–216. [Google Scholar]
- Sorin YB, Mitchell RJ, Trapnell DW, Karron JD. 2016. Effects of pollination and postpollination processes on selfing rate in Mimulus ringens. American Journal of Botany 103: 1524–1528. [DOI] [PubMed] [Google Scholar]
- Spira TP, Snow AA, Puterbaugh MN. 1996. The timing and effectiveness of sequential pollinations in Hibiscus moscheutos. Oecologia 105: 230–235. [DOI] [PubMed] [Google Scholar]
- Stanton ML. 1994. Male–male competition during pollination in plant populations. American Naturalist 144: S40–S68. [Google Scholar]
- Stanton ML, Preston RE. 1986. Pollen allocation in wild radish: variation in pollen grain size and number. In: Mulcahy DL, Mulcahy GB, Ottaviano E, eds. Biotechnology and ecology of pollen. New York: Springer New York, 461–466. [Google Scholar]
- Stanton ML, Snow AA, Handel SN. 1986. Floral evolution: attractiveness to pollinators increases male fitness. Science 232: 1625–1627. [DOI] [PubMed] [Google Scholar]
- Stanton ML, Young HJ, Ellstrand NC, Clegg JM. 1991. Consequences of floral variation for male and female reproduction in experimental populations of wild radish, Raphanus sativus L. Evolution 45: 268–280. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. 1970. Adaptive radiation of reproductive characteristics in angiosperms, I: pollination mechanisms. Annual Review of Ecology and Systematics 1: 307–326. [Google Scholar]
- Stephenson AG, Bertin RI. 1983. Male competition, female choice, and sexual selection in plants. In: Real, L, ed. Pollination biology. Orlando, FL: Academic Press, Inc. [Google Scholar]
- Stone JL. 1995. Pollen donation patterns in a tropical distylous shrub (Psychotria suerrensis; Rubiaceae). American Journal of Botany 82: 1390–1398. [Google Scholar]
- Thomson JD. 1986. Pollen transport and deposition by bumble bees in Erythronium: influences of floral nectar and bee grooming. Journal of Ecology 74: 329–341. [Google Scholar]
- Thomson JD. 1988. Effects of variation in inflorescence size and floral rewards on the visitation rates of traplining pollinators of Aralia hispida. Evolutionary Ecology 2: 65–76. [Google Scholar]
- Thomson JD. 2003. When is it mutualism?American Naturalist 162: S1–S9. [DOI] [PubMed] [Google Scholar]
- Thomson JD, Plowright RC. 1980. Pollen carryover, nectar rewards, and pollinator behaviour with special reference to Diervilla lonicera. Oecologia 46: 68–74. [DOI] [PubMed] [Google Scholar]
- Thomson JD, Thomson BA. 1989. Dispersal of Erythronium grandiflorum pollen by bumblebees: implications for gene flow and reproductive success. Evolution 43: 657–661. [DOI] [PubMed] [Google Scholar]
- Thomson JD, Thomson BA. 1992. Pollen presentation in animal-pollinated plants. In: Wyatt, R, ed. Ecology and evolution of plant reproduction. London: Chapman & Hall, 1–24. [Google Scholar]
- Thomson JD, Rigney LP, Karoly KM, Thomson BA. 1994. Pollen viability, vigor, and competitive ability in Erythronium grandiflorum (Liliaceae). American Journal of Botany 81: 1257–1266. [Google Scholar]
- Thorp RW. 2000. The collection of pollen by bees. In: Dafni A, Hesse M, Pacini E, eds. Pollen and pollination. Vienna: Springer, 211–223. [Google Scholar]
- Tong Z-U, Huang S-Q. 2018. Safe sites of pollen placement: a conflict of interest between plants and bees?Oecologia 186: 163–171. [DOI] [PubMed] [Google Scholar]
- Waage JK. 1979. Dual function of the damselfly penis: sperm removal and transfer. Science 203: 916–918. [DOI] [PubMed] [Google Scholar]
- Walsh RP, Michaels HJ. 2017. When it pays to cheat: examining how generalized food deception increases male and female fitness in a terrestrial orchid. PLoS One 12: e0171286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Meng LL, Yang YP, Duan YW. 2010. Change in floral orientation in Anisodus luridus (Solanaceae) protects pollen grains and facilitates development of fertilized ovules. American Journal of Botany 97: 1618–1624. [DOI] [PubMed] [Google Scholar]
- Waser NM. 1978. Competition for hummingbird pollination and sequential flowering in two Colorado wildflowers. Ecology 59: 934–944. [Google Scholar]
- Waser NM. 1986. Flower constancy: definition, cause, and measurement. American Naturalist 127: 593–603. [Google Scholar]
- Webb CJ, Lloyd DG. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. II. Herkogamy. New Zealand Journal of Botany 24: 163–178. [Google Scholar]
- Westerkamp C, Claßen-Bockhoff R. 2007. Bilabiate flowers: the ultimate response to bees?Annals of Botany 100: 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JH, Mazer SJ. 2016. Pollen – tiny and ephemeral but not forgotten: new ideas on their ecology and evolution. American Journal of Botany 103: 365–374. [DOI] [PubMed] [Google Scholar]
- Williams JH, Edwards JA, Ramsey AJ. 2016. Economy, efficiency, and the evolution of pollen tube growth rates. American Journal of Botany 103: 471–483. [DOI] [PubMed] [Google Scholar]
- Willson MF. 1979. Sexual selection in plants. American Naturalist 113: 777–790. [Google Scholar]
- Willson MF. 1990. Sexual selection in plants and animals. Trends in Ecology & Evolution 5: 210–214. [DOI] [PubMed] [Google Scholar]
- Willson MF. 1994. Sexual selection in plants: perspectives and overview. American Naturalist 144: S13–S39. [Google Scholar]
- Willson MF, Rathcke BJ. 1974. Adaptive design of the floral display in Asclepias syriaca L. American Midland Naturalist 92: 47–57. [Google Scholar]
- Wilson P, Castellanos MC, Wolfe AD, Thomson JD. 2006. Shifts between bee and bird pollination in Penstemons. In: Waser NM, Ollerton J, eds. Plant–pollinator interactions: from specialization to generalization. Chicago: The University of Chicago Press, 47–68. [Google Scholar]
- Wright SI, Kalisz S, Slotte T. 2013. Evolutionary consequences of self-fertilization in plants. Proceedings of the Royal Society B: Biological Sciences 280: 20130133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo PF. 1992. Secondary pollen presentation: form, function and evolution. Vienna: Springer. [Google Scholar]
- Yin G, Barrett SCH, Luo Y-B, Bai W-N. 2016. Seasonal variation in the mating system of a selfing annual with large floral displays. Annals of Botany 117: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HJ, Stanton ML. 1990a Influences of floral variation on pollen removal and seed production in wild radish. Ecology 71: 536–547. [Google Scholar]
- Young HJ, Stanton ML. 1990b Influence of environmental quality on pollen competitive ability in wild radish. Science 248: 1631–1633. [DOI] [PubMed] [Google Scholar]
- Young HJ, Stanton ML, Ellstrand NC, Clegg JM. 1994. Temporal and spatial variation in heritability and genetic correlations among floral traits in Raphanus sativus, wild radish. Heredity 73: 298–308. [Google Scholar]
- Zimmerman M. 1983. Plant reproduction and optimal foraging: experimental nectar manipulations in Delphinium nelsonii. Oikos 41: 57–63. [Google Scholar]