Abstract
Uricosuria and crystallization are increasingly recognized risk factors for diabetic tubulopathy. This pilot clinical trial aimed to determine the acute effect of urinary alkalinization using oral sodium bicarbonate [NaHCO3] on UA crystals in adults with type 1 diabetes (T1D). Adults with T1D ages 18–65 years (n=45, 60% female, HbA1c 7.5±1.2%, 20.2±9.3 years duration) without chronic kidney disease (eGFR ≥60ml/min/1.73m2 and albumin-to-creatinine ratio <30mg/g) received two doses of 1950 mg oral NaHCO3 over 24 hours. Fasting urine and serum were collected pre- and post-intervention. UA crystals were identified under polarized microscopy. Urine measurements included: osmolality, pH, UA, creatinine, and kidney injury molecule-1 [KIM-1].
NaHCO3 therapy increased mean±SD urine pH from 6.1±0.7 to 6.5±0.7 (p<0.0001). Pre-therapy, 31.0% of participants had UA crystals vs. 6.7% post-therapy (p=0.005). Change in urine pH inversely correlated with change in urine KIM-1 (r:−0.51, p=0.0003). In addition, change in urine UA over 24 hours correlated with change in urine KIM-1 (r:0.37, p=0.01). In conclusion, oral NaHCO3 normalized urine pH and decreased UA crystals, and may hold promise as an inexpensive and safe tubulo-protective intervention in people with T1D.
Keywords: Uric acid, urine uric acid crystals, sodium bicarbonate, type 1 diabetes
Introduction:
Diabetic kidney disease (DKD) remains the leading cause of dialysis in the developed world (1). While diabetic glomerulopathy has received significant attention from researchers, determinants of diabetic tubulopathy are less well examined. Compared to glomerular injury, tubular injury is more strongly associated with renal function (2). Whereas the mechanisms underlying diabetic tubulopathy are still incompletely understood, there is growing evidence that a glucosuria mediated increase in urine uric acid (UA) is a major determinant (3, 4).
Glucosuria is associated with activation of the polyol pathway and consequent increased fructose concentration (3). Intracellular fructose is further metabolized, generating UA as a byproduct (3). People with type 1 diabetes (T1D) have more acidic urine than their non-diabetic peers (4), which may predispose to UA crystallization and UA-mediated tubulopathy by inflammation and apoptosis of tubular cells (5–7). In animal studies, inhibition of UA production protects the kidney from tubular injury, suggesting a causal role for UA in the development of diabetic tubulopathy (8, 9).
Despite the compelling evidence implicating UA in the pathogenesis of vascular disease (10) and diabetic tubulopathy, it is unknown whether urinary alkalinization can attenuate UA crystals and tubular injury in the setting of T1D. Accordingly, the goal of this pilot study was to determine the effect of urinary alkalinization with oral sodium bicarbonate (NaHCO3) on UA crystals and markers of tubular injury in adults with T1D. We hypothesized that NaHCO3 supplementation would raise urine pH and reduce UA crystals in adults with T1D.
Materials and Methods
Study design
Participants (n=45, 60% females, mean age 33.6±8.5 years and duration 20.2±9.3 years) in the non-randomized non-controlled Effect of Urinary Alkalinization on Urine Uric Acid Precipitation and Crystallization in Adults with Type 1 Diabetes (Alk-UA Study) pilot trial (NCT02502071) received 2 doses of 1950 mg oral NaHCO3 over 24 hours, and were examined with fasting urine and blood collected over two consecutive days (day 1: pre-therapy, and day 2: post-therapy). Inclusion criteria included age 18–50 years, T1D and ability to fast and provide informed consent. Exclusion criteria included history of estimated GFR (eGFR) <60 ml/min/1.73m2 or albumin-to-creatinine ratio (ACR) ≥30mg/g, history of hypocalcemia, taking allopurinol or other UA altering medications, phosphorus binders, SGLT2 inhibitors, blood pressure medications, or medications which may interact with sodium bicarbonate (phentermine, pseudoephedrine, antifungal medication, cephalosporin antibiotics [e.g. Keflex], tetracycline antibiotics [e.g. doxycycline], steroids or lithium). The study was approved by the Colorado Multiple Institutional Review Board (protocol #: 15–0541) and all participants provided informed consent. All clinical experimentation adheres to the Declaration of Helsinki.
Pre-study diet, fasting instructions and intervention
Participants were asked to maintain a moderate protein (1.5g/kg of weight), and high sodium diet (3,450 mg of sodium per day) for one week prior to the first study visit, and between study visits 1 and 2. They were also instructed to fast for 8 hours prior to each study visit. Fasting and insulin dosing instructions were provided for the participants to reduce risk of hypoglycemia. After completion of day 1 assessment, all participants received 2 doses of 1950 mg of oral NaHCO3 (three tablets of 650 mg NaHCO3). The first dose was administered in the Clinical & Translational Research Center (CTRC) outpatient clinic, and the participants were instructed to take the second dose at home 12 hours later. All participants returned empty pill containers on day 2.
Clinical and Laboratory Measurements
Physical examination measurements including height, weight, body mass index (BMI), systolic (SBP) and diastolic blood pressure (DBP) were performed at both study visits. All subjects were given standardized questionnaires to obtain demographics, medical history and medication use. After an 8 hour fast, blood and urine were collected, centrifuged, and separated. Urine samples were centrifuged at 3900 rpm for 10 minutes at 4o C and the urine pH was measured from supernatant by Accumet basic AB 15 plus pH meter (Fisher Scientific, New Hampshire, USA). Urine osmolality was measured using freezing point method with Micro-Osmometer Model 3300 (Advanced Instruments, Massachusetts, USA). Urine and serum UA were evaluated using a QuantiChrom UA kit assay (DIUA-250) with quantitative colorimetric UA determination at 590 nm (BioAssay System, California, USA). Urine and serum creatinine were analyzed by high-performance liquid chromatography–tandem mass spectrometry (Prominence Liquid Chromatograph LC 20 AD Shimadzu 3200 Q-TRAP, Applied Biosystem, California, USA). Serum glucose was measured by Hexokinase, UV methodology (Brea, California, USA). Serum cystatin C was measured using the commercially available Dade-Behring assay following package insert instructions on a BNII instrument. Urine NGAL was measured with Human Lipocalin 2/NGAL ELISA Kit and KIM-1 measured with Human KIM-1 ELISA Kit (R&D System, Minnesota, USA). Urine NGAL and KIM-1 was normalized for urine creatinine. Estimated GFR (eGFR) were calculated by CKD-EPI creatinine and CKD-EPI cystatin C. UA crystals were identified by polarized microscopy (Polarized light imaging Zeiss Axiovert 135; 0.3NA objective), and pictures were captured from each urine sample. UA crystals were defined dichotomously as being present or absent.
Statistical analysis
Per our power analyses, a sample size of 45 would provide greater than 80% power to detect a change in urine pH of 0.50 following 24 hours of NaHCO3 assuming a SD of change of 0.42 and a correlation of pre- and post- urine pH greater than 0.5 (4). Variables were checked for the distributional assumption of normality using normal plots. Variables that were positively skewed were natural log-transformed for the analyses. Paired t-test were used for normally distributed continuous parameters, and McNemar’s test for categorical variables. Pearson and Spearman correlation employed to evaluate the relationships between continuous variables. A two-sided p<0.05 was considered statistically significant. Data are presented as mean ± SD for normally distributed variables, and median (p25, p75) for positively skewed variables. Analyses were performed in SAS (version 9.4 for Windows; SAS Institute, Cary, NC).
Results:
Clinical characteristics of the study cohort are shown in Table 1. Thirty-one percent of participants had evidence of UA crystals pre-therapy. Supplementary Table 2 shows the characteristics stratified by the presence of UA crystals at baseline.
Table 1.
Baseline Characteristics
| Adults with Type 1 Diabetes (n=45) | |
|---|---|
| Age (years) | 33.6±8.5 |
| Type 1 Diabetes duration (years) | 20.2±9.3 |
| Sex (% female) | 60% |
| Weight (kg) | 77.5±15.6 |
| BMI (kg/m2) | 25.9±3.5 |
| Serum glucose (mg/dl) | 151±60 |
| Point of care glucose (mg/dl) | 146±54 |
| HbA1c (%) | 7.5±1.2 |
| HbA1c (mmol/mol) | 58±13 |
| Total Daily Insulin (units) | 40.8±16.3 |
| Total Daily Insulin per weight (units/kg) | 0.5±0.2 |
| MDI (%) | 29% |
| CSII or HCL (%) | 71% |
| SBP (mm Hg) | 120±9 |
| DBP (mm Hg) | 74±8 |
| HR (min−1) | 70±12 |
| Serum uric acid (mg/dl) | 4.1±0.9 |
| Albumin-to-creatinine ratio (mg/g)a | 4.1 (3.0–9.0) |
| CKD-EPI Creatinine (ml/min/1.73m2) | 98±16 |
| CKD-EPI Cystatin C (ml/min/1.73m2) | 113±15 |
| Urine pH | 6.1±0.7 |
| Complianceb | 100% |
Median, p25–75
Based on returning empty pill containers
BMI = body mass index; HbA1c = hemoglobin A1c; MDI = multiple daily injections; CSII = continuous subcutaneous insulin infusion; HCL = hybrid-closed loop; SBP = systolic blood pressure; DBP = diastolic blood pressure; HR = heart rate; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration
Oral NaHCO3 therapy increased urine pH (6.1±0.7 vs. 6.5±0.7, p<0.0001, Figure 1) and decreased UA crystals (31.0% vs. 6.7%, p=0.0045, Figure 1). There was also a modest increase in SBP in response to oral NaHCO3, but we observed no significant effects on DBP, HR, eGFR, serum glucose and UA (Supplementary Table 3). As expected, there were no significant differences in serum and urine UA, urine osmolality or eGFR pre- and post-NaHCO3 (Supplementary Table 3).
Figure 1. Effect of NaHCO3 on Urine pH and UA Crystals in Adults with Type 1 Diabetes.
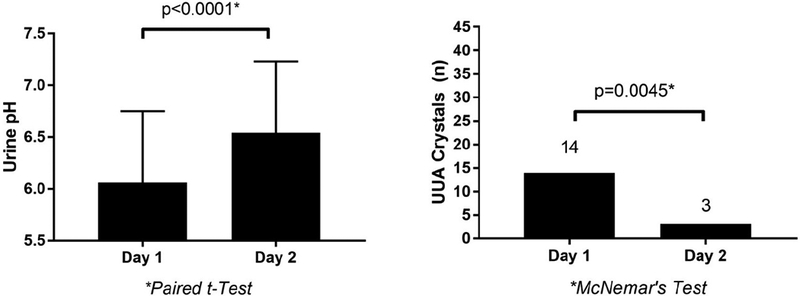
UUA = urine uric acid
The change in urine UA over 24 hours correlated with the change in urine KIM-1 (r: 0.37, p=0.01). Participants who experienced an increase in urine UA over 24 hours therefore demonstrated an increase in urine KIM-1 excretion. Furthermore, there was an inverse correlation between change in urine pH over 24 hours and change in urine KIM-1 (r: −0.51, p=0.0003). Participants who experienced a decrease in their urine pH over 24 hours exhibited an increase in urine KIM-1 excretion. These relationships were not evident with urine NGAL (Supplementary Table 4). Conversely, participants who experienced resolution of UA crystals in response to NaHCO3 experienced a greater decrease in urine NGAL over 24 hours compared to those who had no UA crystals present before or after intervention (Supplementary Table 5).
Discussion:
In this pilot study, almost a third of adults with T1D without CKD had evidence of UA crystals. Oral NaHCO3 therapy normalized urine pH and decreased the prevalence of UA crystals. Furthermore, an increase in urine pH and decrease in UA in response to NaHCO3 were associated with a reduction in KIM-1 excretion, a marker of tubular injury. While this pilot study cannot imply causality between UA crystals and tubular injury, these observations support the hypothesis that urinary alkalinization dissolves UA crystals. NaHCO3 therefore holds promise as an inexpensive and safe tubule-protective intervention in T1D.
The positive effects of NaHCO3 supplementation were observed in the absence of any acute effect on serum UA, urine UA or eGFR, suggestive of an independent effect on UA solubility and crystallization. We decided on a total dose of 3900 mg of oral NaHCO3 a priori for the following reasons: usual adult daily doses range between 1,200–8,000mg, doses for urinary alkalinization with a goal urine pH of 8.0 tend to be up to 12,000 mg daily (11). We did not aim to raise the urine pH above 8.0, but rather normalize the relatively acidic urine found in participants with T1D, by increasing the urine pH by 0.5–1.0.
NaHCO3 supplementation has been shown to preserve renal function in patients with CKD (12). Experimental data suggest that the benefit of NaHCO3 may relate to prevention of tubular injury by decreasing cellular β2-microglobulin generation and renal ammoniogenesis (13, 14). While these data may support our findings, the findings may not be generalizable to our cohort of adults with T1D without CKD. To our knowledge there are no large interventional trials of alkali therapy in adults with early kidney disease.
Diabetic tubulopathy may precede glomerulopathy. For example, tubular proteinuria has been shown to precede elevated albumin excretion in children with T1D (2). Exactly how UA contributes to the development of DKD remains incompletely understood. What is known is that glucosuria results in elevated UA load in T1D by the uricosuric effect (15), and that elevated UA may precipitate and crystalize in the setting of urinary acidification. We have previously demonstrated that youth with T1D have more acidic urine than their normoglycemic peers (4). The UA crystals activate tubular cells via both crystalline and non-crystalline effects, resulting in inflammation, oxidative stress, epithelial-mesenchymal cell transformation and apoptosis with resultant tubulopathy (5–7).
Limitations to the present study include the small sample size and the non-randomized non-controlled study design. We are also unable to determine whether urinary alkalinization resulted in decreased UA crystals through decreased crystal formation, crystal dissolution or both. Furthermore, we are unable to determine whether the relationships between change in urine UA, KIM-1 and NGAL are independent of urine pH, as urine UA and urine pH are collinear. For these reasons, the data should be viewed as hypothesis generating, and needs to be validated in longer and larger clinical trials. Strengths of the study include strict eligibility criteria and a pre-study diet to control for medication and dietary effects on uricosuria and urine pH. Furthermore, we also directly quantified urine osmolality pre- and post-intervention to control for the differences in hydration status.
In summary, UA crystals were common in adults with T1D without CKD, which may predispose them to diabetic tubulopathy. NaHCO3 supplementation over 24 hours normalized urine pH and decreased the prevalence of UA crystals. Finally, an increase in urine pH over 24 hours was associated with lower urine KIM-1, a tubular injury marker. Further research is needed to define the effect of long term bicarbonate therapy in people with T1D, and also whether such supplementation can delay or prevent the development of renal function impairment.
Supplementary Material
Acknowledgements
The Alk-UA Study was funded by a University of Colorado Clinical & Translational Research Center (CTRC) grant (UL1TR001082), in addition to support from Drs. Snell-Bergeon, Garg, Rewers, Maahs and Johnson. P.B. is funded by NIH training grant (T32-DK063687, K23-DK116720–01), Thrasher Foundation Early Career Award, JDRF-ISPAD Research Fellowship and Center for Women’s Health Research at University of Colorado. P.B. is the corresponding author and guarantor of the manuscript, and designed, researched, collected data, performed statistical analysis of the data and prepared the manuscript. D.M. helped with study design and reviewed the manuscript for scholarly content. C.A.R. researched, collected data, ran assays for urine and serum biomarkers, measured urine pH and urine osmolality with T.H. and P.B., and performed polarized microscopy with M.H., and reviewed the manuscript for scholarly content. J.K.S.B. helped with study design and reviewed the manuscript for scholarly content. V.S. helped with study design, patient recruitment and reviewed the manuscript for scholarly content. T.H. researched, collected data, ran assays for urine and serum biomarkers, measured urine pH and urine osmolality with C.A.R and P.B., and reviewed the manuscript for scholarly content. S.E. helped with study design, dispensed sodium bicarbonate and reviewed the manuscript for scholarly content. M.H. researched, performed polarized microscopy with C.A.R, and reviewed the manuscript for scholarly content. L.T.C. researched, helped with RedCap database data entry, data cleaning, and reviewed the manuscript for scholarly content. M.J.R. helped with study design, patient recruitment, and reviewed the manuscript for scholarly content. D.Z.C. helped data interpretation and reviewed the manuscript for scholarly content. S.G. helped with study design, patient recruitment, and reviewed the manuscript for scholarly content. L.P. helped with statistical analyses and study design and reviewed the manuscript for scholarly content. K.J.N. helped with study design and reviewed the manuscript for scholarly content. R.J.J. researched, helped with study design and data interpretation and reviewed the manuscript for scholarly content.
D.Z.C. has received consulting fees or speaking honorarium or both from Janssen, Boehringer Ingelheim-Eli, Lilly, AstraZeneca, Merck, Abbvie and Sanofi, and has received operating funds from Janssen, Boehringer Ingelheim-Eli, Lilly, AstraZeneca and Merck. VNS’ employer received research funding from Sanofi US, Dexcom Inc and Type 1 Diabetes Exchange. VNS received consulting fees from Sanofi and Dexcom. DMM has research support from the NIH (including 1P30DK116074), JDRF, NSF, Helmsley Charitable Trust and his institution has research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, and Roche. DMM has consulted for Abbott and the Helmsley Charitable Trust and is on an advisory board for Insulet. RJJ has patents and patent applications related to uric acid and fructose and metabolic and renal diseases. He also has equity with two startup companies (XORT therapeutics and Colorado Research Partners, LLC) that are interested in developing novel inhibitors of fructose and uric acid metabolism. RJJ also has received honorarium from Danone Research Foundation, Horizon Pharmaceuticals and Astra Zeneca. Finally, RJJ has funding from the National Institute of Health.
Funding Source: Clinical & Translational Research Center (CTRC) grant (UL1TR001082) and support from Drs. Snell-Bergeon, Satish, Rewers, Maahs and Johnson
References:
- 1.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016;67(3 Suppl 1):Svii, S1–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginevri F, Piccotti E, Alinovi R, DeToni T, Biagini C, Chiggeri GM, et al. Reversible tubular proteinuria precedes microalbuminuria and correlates with the metabolic status in diabetic children. Pediatr Nephrol. 1993;7(1):23–6. [DOI] [PubMed] [Google Scholar]
- 3.Bjornstad P, Lanaspa MA, Ishimoto T, Kosugi T, Kume S, Jalal D, et al. Fructose and uric acid in diabetic nephropathy. Diabetologia. 2015;58(9):1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjornstad P, Roncal C, Milagres T, Pyle L, Lanaspa MA, Bishop FK, et al. Hyperfiltration and uricosuria in adolescents with type 1 diabetes. Pediatr Nephrol. 2016;31(5):787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verzola D, Ratto E, Villaggio B, Parodi EL, Pontremoli R, Garibotto G, et al. Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4. PLoS One. 2014;9(12):e115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schepers MS, van Ballegooijen ES, Bangma CH, Verkoelen CF. Crystals cause acute necrotic cell death in renal proximal tubule cells, but not in collecting tubule cells. Kidney Int. 2005;68(4):1543–53. [DOI] [PubMed] [Google Scholar]
- 7.Ryu ES, Kim MJ, Shin HS, Jang YH, Choi HS, Jo I, et al. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol. 2013;304(5):F471–80. [DOI] [PubMed] [Google Scholar]
- 8.Kim SM, Choi YW, Seok HY, Jeong KH, Lee SH, Lee TW, et al. Reducing serum uric acid attenuates TGF-beta1-induced profibrogenic progression in type 2 diabetic nephropathy. Nephron Exp Nephrol. 2012;121(3–4):e109–21. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Pan Y, Zhang QY, Wang FM, Kong LD. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS ONE. 2012;7(6):e38285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cicero AF, Rosticci M, Bove M, Fogacci F, Giovannini M, Urso R, et al. Serum uric acid change and modification of blood pressure and fasting plasma glucose in an overall healthy population sample: data from the Brisighella heart study. Ann Med. 2017;49(4):275–82. [DOI] [PubMed] [Google Scholar]
- 11.Cohen B, Laish I, Brosh-Nissimov T, Hoffman A, Katz LH, Braunstein R, et al. Efficacy of urine alkalinization by oral administration of sodium bicarbonate: a prospective open-label trial. Am J Emerg Med. 2013;31(12):1703–6. [DOI] [PubMed] [Google Scholar]
- 12.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010;78(3):303–9. [DOI] [PubMed] [Google Scholar]
- 13.Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985;76(2):667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonikian M, Gogusev J, Zingraff J, Loric S, Quednau B, Bessou G, et al. Potential effect of metabolic acidosis on beta 2-microglobulin generation: in vivo and in vitro studies. J Am Soc Nephrol. 1996;7(2):350–6. [DOI] [PubMed] [Google Scholar]
- 15.Lytvyn Y, Skrtic M, Yang GK, Yip PM, Perkins BA, Cherney DZ. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2014:ajprenal 00555 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


