Abstract
Background
Timing and stability of the sleep-wake cycle are potential modifiable risk factors for cardiometabolic disease. The aim of this study was to evaluate the relationship between objective measures of sleep-wake timing and stability with cardiometabolic disease risk.
Methods
In this multicenter, cross-sectional, population-based study, actigraphy data were obtained from the 2,156 adults, aged 18 to 64 years, recruited from the Sueño ancillary study of the Hispanic Community Health Study/Study of Latinos (2010-2013). These data were correlated with measures of cardiometabolic disease risk, including systolic and diastolic BPs, homeostatic assessment of insulin resistance, glycosylated hemoglobin, BMI, and hypertension and diabetes status.
Results
Each 10% decrease in interdaily stability was associated with a 3.0% absolute increase in the prevalence of hypertension (95% CI, 0.6-5.3; P < .05), an increase in systolic BP by 0.78 mm Hg (95% CI, 0.12-1.45; P < .05) and an increase in diastolic BP by 0.80 mm Hg (95% CI, 0.28-1.32; P < .05). In addition, delaying the midpoint of sleep by 1 h was associated with an increase in systolic BP by 0.73 mm Hg (95% CI, 0.30-1.16; P < .01) and diastolic BP by 0.53 mm Hg (95% CI, 0.17-0.90; P < .01). These associations were not significant after adjusting for shift work status. No association was found between interdaily stability or sleep timing and diabetes, BMI, or insulin resistance.
Conclusions
These results suggest that beyond sleep duration, the timing and regularity of sleep-wake schedules are related to hypertension prevalence and BP.
Key Words: cardiovascular diseases, circadian rhythm, hypertension, sleep
Abbreviations: AHI, apnea-hypopnea index; CMD, cardiometabolic disease; DBP, diastolic BP; SBP, systolic BP
Cardiometabolic diseases (CMDs), including obesity, diabetes, and hypertension, are leading causes of morbidity and mortality worldwide, and they disproportionately affect Hispanic/Latino individuals.1, 2 Many risk factors for CMDs can be addressed with varying degrees of success through a combination of medication and lifestyle changes.3, 4 There is growing interest in identifying novel risk factors for CMDs that can serve as additional targets for prevention and disease modification.
Short sleep duration has previously been shown to be associated with an increased incidence of hypertension in healthy individuals5 and in those with insomnia.6 In addition, short sleep duration has been associated with increased BMI,7 impaired glucose tolerance,8 and increased odds of developing diabetes.9, 10 More recently, decreased sleep efficiency and increased sleep fragmentation were also shown to be associated with an increased prevalence of hypertension.11
Along with sleep duration and quality, there is growing evidence that the stability and timing of the sleep-wake cycle are important predictors of cardiometabolic risk. Greater day-to-day instability of the daily activity rhythm, as assessed by the interdaily stability measure, has been associated with a higher risk of obesity, diabetes, hypertension, and hyperlipidemia in older adults.12 In addition, the timing of sleep-wake patterns may also contribute to CMD risk. Many individuals experience the phenomenon of “social jet lag,” in which sleep-wake timing differs significantly between work days and nonwork days.13 Increased social jet lag has been associated with higher BMI in individuals who are overweight or obese.14 In addition, individuals with diabetes and a later midpoint of sleep have higher glycosylated hemoglobin values, indicating poorer glucose control.15 Finally, shift work is one of the most obvious sources of disruption to sleep-wake timing and has also been shown to increase the risk of CMD. Among Hispanic/Latino individuals, 27.7% work alternative (non-day) shifts,16 and thus this population is likely to have a higher instability of sleep-wake timing, which may contribute to the observed CMD risk.
In a large community-based sample of Hispanic/Latino adults from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), the present study used objective measures of interdaily stability and sleep-wake timing to examine the cross-sectional associations of stability and timing of sleep with CMD risk factors. We hypothesized that greater disruption of the timing of the sleep-wake schedule, reflected in either later sleep timing, increased social jet lag, or lower interdaily stability of actigraphically measured sleep timing, would be associated with higher CMD risk as measured according to BP, BMI, and glucose control. Furthermore, it was assumed that this group would be a population with a high prevalence of shift work.
Patients and Methods
Study Cohort
The HCHS/SOL is a community-based cohort study of 16,415 self-identified Hispanic/Latino adults, recruited between 2008 and 2011 from randomly selected households at four US field centers (Bronx, New York; Chicago, Illinois; Miami, Florida; and San Diego, California). The multistage sampling design and cohort selection procedures have been previously described.17, 18
As part of the baseline examination, all participants not previously diagnosed with diabetes underwent an oral glucose tolerance test. Fasting blood samples were collected on arrival for assay of glucose, insulin, and glycosylated hemoglobin, with glucose measured a second time 120 min following oral glucose ingestion. BP was measured in the seated position by using an automated sphygmomanometer following an initial 5-min rest period. Results were reported as the average of three BP readings, taken 1 min apart. All BP recordings occurred in the morning, during the second hour of the 7-h baseline study examination. In addition, sleep apnea severity was assessed by using a home monitoring system as previously described.19 Levels of education and acculturation (defined based on place of birth and duration of time living in the United States), as well as current work schedule, were assessed through the use of questionnaires.
The Sueño sleep ancillary study recruited 2,252 individuals who were within 30 months of their baseline HCHS/SOL examination (median, 823 days) from December 2010 to December 2013. Participants were aged 18 to 64 years, did not have narcolepsy (according to history), an apnea-hypopnea index (AHI) ≥ 50 events per hour, and were not using nocturnal positive airway pressure therapy. Individuals were not excluded if they had insomnia, restless leg syndrome, shift work, or other sleep disorders not specified earlier. Both the HCHS/SOL study and the Sueño ancillary study were approved by the institutional review boards at all participating institutions (sites and reading/coordinating center) (e-Appendix 1), and all participants provided written informed consent.
Sueño Study Protocol
As part of the Sueño ancillary study, all participants completed a questionnaire that included self-reported medical history, current medications, Hispanic/Latino background, household income, and employment status. Height and weight were measured at the time of this visit. Staff-administered questionnaires were completed in either English or Spanish depending on the language preference of the participant.
Actigraphy
All participants in the Sueño ancillary study were asked to wear an Actiwatch Spectrum (Philips Respironics) device on their nondominant wrist for 7 days. Participants were asked to not remove the watch for the duration of the study and were instructed to press the event marker button upon getting into or out of bed. They also completed a sleep diary each morning indicating time into bed, time out of bed, any naps taken the previous day, and whether each day was a free day or a work day.
The actigraph units were programmed to collect activity data in 30-s epochs. Each actigraph was manually scored for the main rest interval and any nap intervals using standardized criteria detailed previously.20 Once rest intervals were defined, sleep was scored by using the Actiware 5.59 algorithm (5 immobile minutes for sleep onset, 0 immobile minutes for sleep offset, wake threshold count of 40).21 Any day of recording with > 4 h of missing data during the 24-h window, or > 2 min of missing data during a main rest interval, was considered invalid. For analyses of sleep midpoint, only individuals with ≥ 5 valid days were included (n = 2,156); for analyses of interdaily stability, only individuals with 7 consecutive days of valid data were included (n = 1,694) to allow for sampling of both work and nonwork days. If participants collected > 7 days of data, only the first 7 consecutive days of valid actigraphy data were used.
Definitions
Actigraphic variables measured were as follows: mean sleep onset time, mean sleep offset time, mean sleep midpoint time, and interdaily stability. Sleep midpoint was calculated as the midpoint between sleep onset and sleep offset, determined for both weekdays (Sunday through Thursday) and weekends (Friday and Saturday). Social jet lag was defined as the difference between the mid-sleep point on weekdays and weekends.14 Sleep duration was calculated as the sum of all epochs of sleep between sleep onset and sleep offset, averaged across all days. These measures were all based on the primary sleep period. The interdaily stability index quantifies the regularity of sleep patterns across days and provides a measure of how stable sleep-wake rhythms are from day to day.
A similar metric has been previously described for assessing stability of activity rhythms across the day, where each day is divided into 24 one-hour bins, and the variance in activity explained by these bins is compared with the total variance.22, 23 The focus of the present study was on the variance in the dichotomous variable of the sleep/wake state; we divided the variance into two components: one explained by the hour of the day (between-hour variance) and the other the residual variance within each hour bin (within-hour variance). The interdaily stability was calculated as the between-hour variance to total (between-hour plus within-hour) variance. Interdaily stability can range from 0% to 100%, with 0% indicating very unstable sleep-wake patterns from day to day, and 100% indicating very stable sleep-wake patterns from day to day. This measure incorporates both the primary sleep period and any napping that might occur during the day.
BMI was calculated by using height and weight measured at the time of the Sueño study visit. Obesity was defined as BMI ≥ 30 kg/m2. Hypertension was defined from information provided at the time of the Sueño study visit, based on self-report of a diagnosis of hypertension or currently taking antihypertensive medications. Information regarding glycemic control and BP were obtained from the baseline HCHS/SOL study examination for each participant. Diabetes was defined as fasting glucose levels ≥ 126 mg/dL at > 8 h of fasting or > 200 mg/dL at < 8 h of fasting, glucose levels ≥ 200 mg/dL following the oral glucose tolerance test, glycosylated hemoglobin values ≥ 6.5%, or the use of an antidiabetes medication.
Statistical Methods
Survey linear regression was performed to examine the associations between sleep timing variables and continuous response variables, including systolic BP (SBP), diastolic BP (DBP), and BMI using sampling weights to account for the study design and probability of participating in the study. Values for skewed response variables (homeostatic assessment of insulin resistance and glycosylated hemoglobin) were log transformed prior to regression analysis. Survey linear regression was also used for dichotomous outcomes (eg, hypertension, diabetes) modeling the prevalence of each response.24 We first considered a regression model adjusting for age, sex, Hispanic/Latino background, and study site (Model 1). Model 2 adjusted for covariates in Model 1, plus income, acculturation, education level, sleep duration, and AHI. Finally, Model 3 adjusted for covariates in Model 2, plus shift work status. All statistical tests were 2-sided at a significance level of .05. Analyses were performed by using SAS version 9.4 (SAS Institute, Inc).
Results
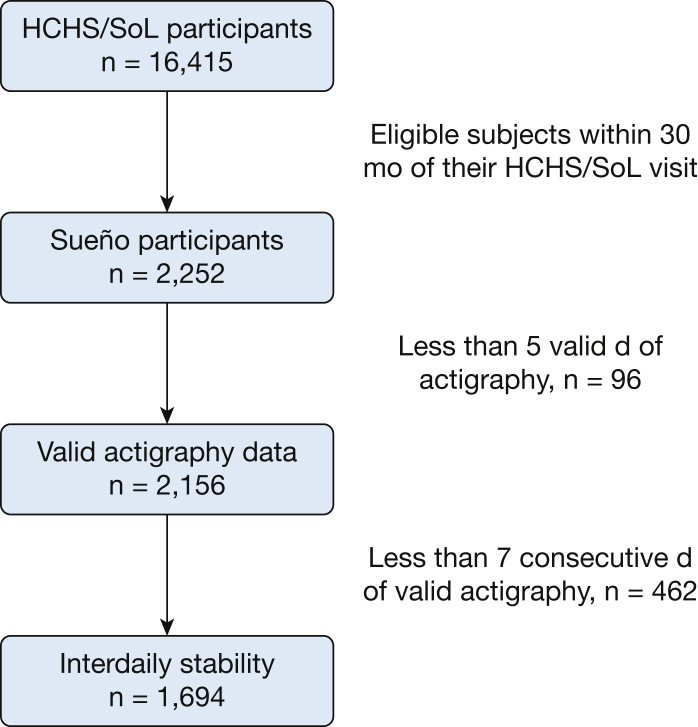
A total of 2,156 participants had actigraphy data available for this analysis (Fig 1). Their mean age was 47 years, 65% were women, and 767 (36%) had a diagnosis of hypertension. Table 1 provides age- and sex-standardized (based on the 2010 US Census) comparisons of those with and without hypertension. Individuals with hypertension were more likely to be unemployed, have lower household income, and to have not been born in the United States. Notably, 21% of the participants were shift workers, whereas 42% were unemployed. Table 2 presents age- and sex-standardized sleep measures according to hypertension status. Average sleep onset was 0:01 ± 0:04 on weekdays and 0:43 ± 0:04 on weekends. Average sleep midpoint was 3:48 ± 0:04 on weekdays, and 4:38 ± 0:04 on weekends. Interdaily stability ranged from 5% to 97%, with a mean of 75%. Individuals with hypertension had a significantly lower interdaily stability (73% vs 76%; P < .05).
Figure 1.
Consort table outlining participant recruitment and inclusion for this study. HCHS/SOL = Hispanic Community Health Study/Study of Latinos.
Table 1.
Sociodemographic and Acculturation Characteristics: Sueño Ancillary Study (2010-2013)
| Characteristic | No Hypertension (n = 1,389) | Hypertension (n = 767) | P Value |
|---|---|---|---|
| Education, % | |||
| < High school | 30.7 | 35.1 | .0362 |
| At least high school | 69.3 | 64.9 | |
| Shift work, % | |||
| Not currently employed | 36.6 | 51.6 | < .0001 |
| Employed, day shift | 41.7 | 29.6 | |
| Employed, shift worker | 21.7 | 18.8 | |
| Annual household income, % | |||
| < $20,000 | 44.0 | 48.8 | < .0001 |
| ≥ $20,000 | 47.7 | 44.9 | |
| Missing | 8.3 | 6.4 | |
| Hispanic/Latino background, % | |||
| Central American | 15.1 | 10.7 | < .0001 |
| Cuban | 17.7 | 18.8 | |
| Dominican | 11.0 | 15.3 | |
| Mexican | 27.3 | 25.7 | |
| Puerto Rican | 18.7 | 25.2 | |
| South American | 10.2 | 4.4 | |
| Site, % | |||
| Bronx, NY | 22.9 | 29.9 | .0023 |
| Chicago, IL | 27.3 | 26.5 | |
| Miami, FL | 33.0 | 27.4 | |
| San Diego, CA | 16.8 | 16.3 | |
| Acculturation, % | |||
| US born | 18.9 | 12.6 | < .0001 |
| Foreign born, > 10 y in the United States | 51.8 | 67.9 | |
| Foreign born, <10 y in the United States | 29.3 | 19.4 | |
| Diabetes, % | |||
| Yes | 9.3 | 31.4 | < .0001 |
| Apnea-hypopnea index, events per hour | 15.5 | 18.4 | .016 |
Values were weighted for survey design and nonresponse and adjusted to the age and sex distribution of the 2010 US Census.
Table 2.
Description of Sleep Timing Variables Based on Actigraphy Data, Stratified According to Hypertensive Status: Sueño Ancillary Study (2010-2013)
| Measure | Day of the Week | No Hypertension | Hypertension |
|---|---|---|---|
| Sleep onset, | Weekday | 0:01 ± 0:04 | 0:02 ± 0:08 |
| HH:MM | Weekend | 0:46 ± 0:04 | 0:37 ± 0:09 |
| Sleep offset, | Weekday | 7:33 ± 0:04 | 7:40 ± 0:06 |
| HH:MM | Weekend | 8:34 ± 0:04 | 8:29 ± 0:10 |
| Sleep duration, | Weekday | 6:38 ± 0:02 | 6:36 ± 0:04 |
| HH:MM | Weekend | 6:56 ± 0:03 | 6:45 ± 0:06 |
| Sleep midpoint, | Weekday | 3:46 ± 0:04 | 3:51 ± 0:07 |
| HH:MM | Weekend | 4:40 ± 0:04 | 4:33 ± 0:08 |
| Social jet lag, HH:MM | … | 1:09 ± 0:03 | 1:07 ± 0:05 |
| Interdaily stability | All days | 76% ± 1.2%a | 73% ± 0.5%a |
Data are presented as mean ± SE. Values are weighted for survey design and nonresponse and adjusted to the age and sex distribution of the 2010 US Census. HH:MM = hour hour: minute minute.
P < .05.
Table 3 presents regression coefficients of the association between sleep variables and hypertension prevalence and SBP and DBP. Each 10% decrease in interdaily stability was associated with a 3.0% increase in the prevalence of hypertension (95% CI, 0.6-5.3; P < .05), a 0.78 mm Hg increase in SBP (95% CI, 0.12-1.45; P < .05), and a 0.80 mm Hg increase in DBP (95% CI, 0.28-1.32; P < .01). Associations remained significant after adjusting for age, sex, background, and site. When income, acculturation, education, sleep duration, and AHI were added to the models, associations with DBP remained significant (but not hypertension and SBP). Associations did not remain significant after adding shift work to the model.
Table 3.
Linear Regression Coefficients From Models Predicting Hypertension and BP From Sleep Midpoint, Social Jet Lag, and Interdaily Stability
| Model | Hypertension Prevalence (%) |
Systolic BP (mm Hg) |
Diastolic BP (mm Hg) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Interdaily Stability (Per 10% Change) | Midpoint (Per Hour) | Social Jet Lag (Per Hour) | Interdaily Stability (Per 10% Change) | Midpoint (Per Hour) | Social Jet Lag (Per Hour) | Interdaily Stability (Per 10% Change) | Midpoint (Per Hour) | Social Jet Lag (Per Hour) | |
| Model 1 | –3.0 (–5.3 to –0.6)a | 0.29 (–1.68 to 2.26) | –0.52 (–3.49 to 2.45) | –0.78 (–1.45 to –0.12)a | 0.73 (0.30 to 1.17)b | –0.05 (–0.71 to 0.62) | –0.80 (–1.32 to –0.28)b | 0.53 (0.17 to 0.90)b | 0.14 (–0.39 to 0.68) |
| Model 2 | –2.4 (–4.9 to 0.1) | –0.17 (–2.19 to 1.86) | –0.66 (–3.66 to 2.34) | –0.50 (–1.16 to 0.15) | 0.55 (0.12 to 0.99)a | –0.05 (–0.72 to 0.63) | –0.54 (–1.06 to –0.02)a | 0.41 (0.06 to 0.76)a | 0.16 (–0.38 to 0.69) |
| Model 3 | –2.2 (–4.9 to 0.4) | –1.0 (–3.2 to 1.1) | 0.05 (–3.05 to 3.15) | –0.40 (–1.06 to 0.25) | 0.27 (–0.21 to 0.74) | 0.11 (–0.58 to 0.80) | –0.48 (–0.99 to 0.04) | 0.25 (–0.13 to 0.63) | 0.24 (–0.29 to 0.77) |
Data are presented as β (95% CI). Model 1: Adjusted for age, sex, Hispanic/Latino background, and study site. Model 2: Adjusted for Model 1 covariates plus income, acculturation, education, sleep duration, and apnea-hypopnea index. Model 3: Adjusted for model 2 covariates plus shift work status.
P < .05.
P < .01.
Each 1-h delay in sleep midpoint was associated with a 0.73 mm Hg increase in SBP (95% CI, 0.30-1.16; P < .01) and a 0.53 mm Hg increase in DBP (95% CI, 0.06-0.76; P < .01). These associations remained significant after adjusting for age, sex, background, site, income, acculturation, sleep duration, and AHI but not after adjusting for shift work status. There was no association between social jet lag and any of the BP measures.
Table 4 presents regression coefficients of the association between the actigraphically measured sleep variables with BMI, glucose control, and the prevalence of diabetes. No association was found between actigraphically measured sleep timing variables and diabetes, BMI, log of homeostatic assessment of insulin resistance, or log of glycosylated hemoglobin.
Table 4.
Results From Survey Linear Regression Models Predicting Cardiometabolic Disease Risk Factors From Sleep Midpoint, Social Jet Lag, and Interdaily Stability
| Model | Diabetes Prevalence (%) |
BMI (kg/m2) |
Log of HOMA-IR |
Log of HbA1c |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interdaily Stability (Per 10% Change) | Midpoint (Per Hour) | Social Jet Lag (Per Hour) | Interdaily Stability (Per 10% Change) | Midpoint (Per Hour) | Social Jet Lag (Per Hour) | Interdaily Stability (Per 10% Change) | Midpoint (Per Hour) | Social Jet Lag (Per Hour) | Interdaily Stability (Per 10% Change) | Midpoint (Per Hour) | Social Jet Lag (Per Hour) | |
| Model 1 | –1.0 (–2.4 to 0.4) | 0.60 (0.73 to 1.94) | –0.49 (–1.83 to 0.85) | –0.18 (–0.52 to 0.17) | 0.01 (–0.29 to 0.30) | 0.26 (–0.16 to 0.68) | –0.00 (–0.04 to 0.03) | 0.01 (–0.02 to 0.04) | –0.01 (–0.06 to 0.04) | –0.01 (–0.01 to 0.00) | –0.00 (–0.01 to 0.00) | –0.00 (–0.01 to 0.01) |
| Model 2 | –1.4 (–3.0 to 0.1) | 0.42 (–0.96 to 1.80) | –0.40 (–1.74 to 0.93) | 0.11 (–0.25 to 0.48) | –0.08 (–0.37 to 0.22) | 0.22 (–0.21 to 0.65) | 0.00 (–0.04 to 0.05) | 0.00 (–0.02 to 0.03) | –0.00 (–0.05 to 0.05) | –0.00 (–0.01 to 0.00) | –0.00 (–0.01 to 0.00) | –0.00 (–0.01 to 0.00) |
| Model 3 | –1.4 (–2.9 to 0.1) | 0.13 (–1.29 to 1.55) | –0.03 (–1.36 to 1.31) | 0.12 (–0.26 to 0.50) | –0.15 (–0.47 to 0.17) | 0.28 (–0.15 to 0.72) | 0.01 (–0.04 to 0.05) | –0.01 (–0.03 to 0.02) | 0.00 (–0.05 to 0.05) | –0.01 (–0.01 to 0.00) | –0.00 (–0.01 to 0.00) | –0.00 (–0.01 to 0.01) |
Data are presented as β (95% CI). Model 1: Adjusted for age, sex, Hispanic/Latino background, and study site. Model 2: Adjusted for Model 1 covariates and income, acculturation, education, sleep duration, and apnea-hypopnea index. Model 3: Adjusted for Model 2 covariates and shift work status. HbA1c = glycosylated hemoglobin; HOMA-IR = homeostatic assessment of insulin resistance.
Table 5 presents regression coefficients of the association between sleep variables and hypertension prevalence and SBP and DBP, stratified according to shift work status. Associations between hypertension and interdaily stability remained significant in the non-shift-work group. In addition, associations between SBP and sleep midpoint, and DBP and sleep midpoint, remained significant in the non-shift-work group.
Table 5.
Linear Regression Coefficients From Models Predicting Hypertension and BP From Sleep Midpoint, Social Jet Lag, and Interdaily Stability, Stratified According to Shift Work Status
| Shift Work Status | Hypertension Prevalence (%) |
Systolic BP (mm Hg) |
Diastolic BP (mm Hg) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Interdaily Stability (Per 10% Change) | Midpoint (Per Hour) | Social Jet Lag (Per Hour) | Interdaily Stability (Per 10% Change) | Midpoint (Per Hour) | Social Jet Lag (Per Hour) | Interdaily Stability (Per 10% Change) | Midpoint (Per Hour) | Social Jet Lag (Per Hour) | |
| Non-shift worker | –4.5 (–7.6 to –1.4)a | 0.3 (–1.46 to 2.06) | 0.9 (–2.82 to 4.62) | –0.49 (–1.18 to 0.20) | 0.65 (0.01 to 1.28)b | –0.25 (–0.94 to 0.44) | –0.51 (–1.13 to 0.11) | 0.52 (0.02 to 1.02)b | –0.01 (–0.61 to 0.60) |
| Shift worker | –0.1 (–3.1 to 3.0)b | 1.2 (–1.35 to 3.75) | 0.4 (–2.93 to 3.73) | –0.69 (–2.03 to 0.66) | 0.34 (–0.38 to 1.060) | 0.43 (–1.29 to 2.15) | –0.69 (–1.64 to 0.26) | 0.24 (–0.30 to 0.78) | 0.36 (–0.80 to 1.52) |
Data are presented as β (95% CI). Adjusted for age, sex, Hispanic/Latino background, and study site.
P < .01.
P < .05.
Discussion
In this observational study using objective data to evaluate sleep timing and stability in US Hispanic/Latino subjects, decreased interdaily stability was associated with an increased risk of hypertension and greater objectively measured SBP and DBP. In addition, later sleep-wake timing, as measured by the midpoint of sleep, was associated with higher objectively measured SBP and DBP. Although the clinical impact of sleep-wake timing and stability on BP may not be apparent, at a population level, these differences can be meaningful. For example, a large meta-analysis evaluating the use of antihypertensive agents in the prevention of cardiovascular disease showed that decreases in SBP by 1 mm Hg and DBP by 0.5 mm Hg (similar to the magnitude of difference seen in our study) were associated with a 4% decrease in stroke and a 2% decrease in coronary heart disease events.25 These results reflect the importance of optimizing both the timing and regularity of the sleep-wake cycle in promoting overall cardiovascular health.
BP is under prominent circadian control, typically measuring higher during the day and dipping in the middle of the night. Loss of this normal dipping pattern is believed to be a better predictor of CMD risk and mortality than an isolated elevated BP reading during the day.26 A potential mechanism for interdaily stability to contribute to hypertension risk is through disruption of this normal dipping pattern. With increased interdaily stability, day-to-day timing of exposure to light and other circadian environmental entraining signals, which are important for proper alignment between the internal circadian clock and environment, becomes more regular. As a result, it is more likely that the circadian clock will be stably aligned to the environment, in turn leading to better sleep quality. In support of this hypothesis, data have shown loss of nocturnal dipping of BP in shift workers, a group more likely to have decreased interdaily stability and circadian misalignment.27 Experimental models of shift work have shown that mean arterial BP increases by 3% in individuals with sleep-wake cycles that are misaligned with the external environment, independent of changes in norepinephrine or epinephrine.28 There are far fewer data regarding interdaily stability and CMD risk in healthy non-shift workers. Our results are similar to those found in the Rush Memory and Aging Project, which reported an association between decreased interdaily stability and increased risk for hypertension12; however, we have reported greater generalizability of those findings through our population-based study.
As expected, the associations between sleep timing and hypertension did not remain after adjusting for shift work status but remained present in the non-shift-work group, which included individuals who worked day shifts or were unemployed. Of note, 42% of the overall study population was unemployed, and 52% of the hypertensive population was unemployed, suggesting that the unemployed group was the primary driver of this association. Although many factors may contribute to higher levels of hypertension in the unemployed population, these data suggest that the greater variability of sleep-wake timing that accompanies the lack of a fixed daily schedule imposed by work may be an important additional factor.
Unlike previous studies, our data found no associations between sleep timing or interdaily stability and diabetes, BMI, or insulin resistance.14, 15 Of note, several of the cardiometabolic risk factors in our study were obtained up to 30 months prior to actigraphy. However, this factor does not explain the lack of association with BMI, which was obtained at the same time as the actigraphy assessment. Because interdaily stability and circadian misalignment (either experimentally induced28 or measured by using melatonin levels as a proxy29) have previously been shown to precede the development of adverse health effects, it is possible that sampling actigraphy several months following the assessment of insulin resistance may have underestimated the magnitude of the association.
The primary limitation of the present study is that many of the cardiometabolic assessments, including BP measurements, were obtained several months prior to the time of actigraphy assessment. Although we are presumably assessing the long-term health effects of these behaviors in this study, it is possible that sleep-wake timing and stability may have changed between the initial assessment and the time of the actigraphy measurements. In addition, with a single time point, it is not possible to determine whether the observed changes in sleep-wake timing and stability are the cause or a downstream effect of hypertension. However, the advantage of this longitudinal cohort is that we have the opportunity to perform additional assessments for prospective analyses in the future.
In addition, it should be noted that objective BP measurements were based on averaged readings obtained at a single time point, rather than a reflection of the 24-h profile of BP. It is known that the loss of a circadian pattern of nocturnal dipping of BP may be associated with greater risk for CMD than an isolated high reading during the day.30 With that in mind, future studies evaluating the association between actigraphically measured rest-activity patterns and 24 h profiles of BP would be useful. We are also unable to comment specifically on the use of beta-blockers and hypnotic agents in this population, which could potentially affect sleep and circadian rhythms in these individuals.31 Finally, it should be noted that although the unique aspect of this cohort is that it provides information regarding cardiometabolic risk factors in a Hispanic population with a large proportion of individuals who are either shift workers, unemployed, or not following a conventional work schedule, these factors also potentially limit the generalizability of the findings to the general population.
In future directions, further characterization is needed of the mechanisms underlying these observed associations between disrupted sleep-wake timing and hypertension, including evaluation of 24-h BP profiles, melatonin levels, and the use of medications that may disrupt melatonin production. In addition, interventions are needed to determine whether therapies aimed at improving interdaily stability and adjusting sleep midpoint can improve BP.
Conclusions
We observed that the timing and regularity of sleep-wake schedules were associated with hypertension. These findings suggest that interventions aimed at improving the timing of sleep may be important targets for decreasing cardiometabolic disease risk.
Acknowledgments
Author contributions: S. M. A. confirms that the study objectives and procedures are honestly disclosed; moreover, she has reviewed study execution data and confirms that procedures were followed to an extent that convinces all authors that the results are valid and generalizable to a population similar to that enrolled in this study. S. M. A. also contributed to drafting/revising the manuscript for content, study content or design and analysis, and interpretation of data. J. W. contributed to statistical analysis and drafting/revising the manuscript. K. J. R., M. L. D., L. C. G., J. S. L., S. M. N., A. R. R., N. A. S., D. S.-A., S. R. P., and P. C. Z. contributed to drafting/revising the manuscript for content and study concept or design and analysis or interpretation of data.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the staff and participants of HCHS/SOL for their important contributions. A complete list of staff and investigators has been provided by Lavange et al17 and is also available on the study website (http://www.cscc.unc.edu/hchs/).
Additional information: The e-Appendix can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Patel and Zee are co-senior authors.
FUNDING/SUPPORT: This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) [Grants HL098297 and HL127307]. The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) was conducted as a collaborative study supported by contracts from the NHLBI to the University of North Carolina [N01-HC65233], the University of Miami [N01-HC65234], Albert Einstein College of Medicine [N01-HC65235], Northwestern University [N01-HC65236], and San Diego State University [N01-HC65237]. The following institutes/centers/offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: the National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements.
Supplementary Data
References
- 1.Ogden C.L., Carroll M.D., Curtin L.R., McDowell M.A., Tabak C.J., Flegal K.M. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Ong K.L., Cheung B.M., Wong L.Y., Wat N.M., Tan K.C., Lam K.S. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999-2004. Ann Epidemiol. 2008;18(3):222–229. doi: 10.1016/j.annepidem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 3.St-Onge M.P., Grandner M.A., Brown D. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–e386. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox C.S., Golden S.H., Anderson C. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2015;38(9):1777–1803. doi: 10.2337/dci15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Mei H., Jiang Y.R. Relationship between duration of sleep and hypertension in adults: a meta-analysis. J Clin Sleep Med. 2015;11(9):1047–1056. doi: 10.5664/jcsm.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bathgate C.J., Edinger J.D., Wyatt J.K., Krystal A.D. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39(5):1037–1045. doi: 10.5665/sleep.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel S.R., Malhotra A., White D.P., Gottlieb D.J., Hu F.B. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164(10):947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 9.Yaggi H.K., Araujo A.B., McKinlay J.B. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29(3):657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 10.Cespedes E.M., Dudley K.A., Sotres-Alvarez D. Joint associations of insomnia and sleep duration with prevalent diabetes: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) J Diabetes. 2016;8(3):387–397. doi: 10.1111/1753-0407.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos A.R., Weng J., Wallace D.M. Sleep patterns and hypertension using actigraphy in the Hispanic Community Health Study/Study of Latinos. Chest. 2018;153(1):87–93. doi: 10.1016/j.chest.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohail S., Yu L., Bennett D.A., Buchman A.S., Lim A.S. Irregular 24-hour activity rhythms and the metabolic syndrome in older adults. Chronobiol Int. 2015;32(6):802–813. doi: 10.3109/07420528.2015.1041597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roenneberg T., Merrow M. The circadian clock and human health. Curr Biol. 2016;26(10):R432–R443. doi: 10.1016/j.cub.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Roenneberg T., Allebrandt K.V., Merrow M., Vetter C. Social jetlag and obesity. Curr Biol. 2012;22(10):939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 15.Reutrakul S., Hood M.M., Crowley S.J. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36(9):2523–2529. doi: 10.2337/dc12-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alterman T., Luckhaupt S.E., Dahlhamer J.M., Ward B.W., Calvert G.M. Prevalence rates of work organization characteristics among workers in the U.S.: data from the 2010 National Health Interview Survey. Am J Ind Med. 2013;56(6):647–659. doi: 10.1002/ajim.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavange L.M., Kalsbeek W.D., Sorlie P.D. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):642–649. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorlie P.D., Aviles-Santa L.M., Wassertheil-Smoller S. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):629–641. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redline S., Sotres-Alvarez D., Loredo J. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189(3):335–344. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel S.R., Weng J., Rueschman M. Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep. 2015;38(9):1497–1503. doi: 10.5665/sleep.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marino M., Li Y., Rueschman M.N. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Someren E.J., Swaab D.F., Colenda C.C., Cohen W., McCall W.V., Rosenquist P.B. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505–518. doi: 10.3109/07420529908998724. [DOI] [PubMed] [Google Scholar]
- 23.Luik A.I., Zuurbier L.A., Hofman A., Van Someren E.J., Tiemeier H. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int. 2013;30(10):1223–1230. doi: 10.3109/07420528.2013.813528. [DOI] [PubMed] [Google Scholar]
- 24.Hellevik O. Linear versus logistic regression when the dependent variable is a dichotomy. Qual Quant. 2009;43:59–74. [Google Scholar]
- 25.Law M.R., Morris J.K., Wald N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagard R.H. Dipping pattern of nocturnal blood pressure in patients with hypertension. Expert Rev Cardiovasc Ther. 2009;7(6):599–605. doi: 10.1586/erc.09.35. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura T., Onishi K., Dohi K. Circadian rhythm of blood pressure is transformed from a dipper to a non-dipper pattern in shift workers with hypertension. J Hum Hypertens. 2002;16(3):193–197. doi: 10.1038/sj.jhh.1001328. [DOI] [PubMed] [Google Scholar]
- 28.Scheer F.A., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forman J.P., Curhan G.C., Schernhammer E.S. Urinary melatonin and risk of incident hypertension among young women. J Hypertens. 2010;28(3):446–451. doi: 10.1097/HJH.0b013e3283340c16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staessen J.A., Thijs L., Fagard R. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282(6):539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 31.Cowen P.J., Bevan J.S., Gosden B., Elliott S.A. Treatment with beta-adrenoceptor blockers reduces plasma melatonin concentration. Br J Clin Pharmacol. 1985;19(2):258–260. doi: 10.1111/j.1365-2125.1985.tb02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.