Abstract
Rationale: Sepsis is a leading cause of death and disability whose heterogeneity is often cited as a key impediment to translational progress.
Objectives: To test the hypothesis that there are consequential and significant differences in sepsis outcomes that result from differences in a patient’s clinical course leading up to sepsis hospitalization.
Methods: We conducted an observational cohort study of U.S. Health and Retirement Study (HRS) participants in Medicare (1998–2012) and U.S. Department of Veterans Affairs beneficiaries (2009). Using latent profile analysis, we identified patient subtypes based on trajectory of presepsis healthcare facility use. Subtypes were identified in the derivation cohort (1,512 sepsis hospitalizations among earlier HRS participants), then validated them in two additional cohorts (1,992 sepsis hospitalizations among later HRS participants; 32,525 sepsis hospitalizations among U.S. Department of Veterans Affairs beneficiaries). We measured the association between presepsis path and 90-day mortality using chi-square tests and multivariable logistic regression.
Results: We identified three subtypes: low use of inpatient healthcare facilities, comprising 84% of the derivation cohort; rising use, 12%; and high use, 4%. The shape and distribution of presepsis trajectories were similar in all three cohorts. In the derivation cohort, 90-day mortality differed by presepsis trajectory as follows: 38% (low use), 63% (rising use), and 48% (high use) (P < 0.001). This association persisted in the validation cohorts (P < 0.001 for each). The rising use class remained an independent predictor of mortality after adjustment for potential confounders, including detailed physiologic data.
Conclusions: In national cohorts of patients with sepsis, we have shown that several distinct paths into sepsis exist. These paths, identified by trajectories of presepsis healthcare use, are predictive of 90-day mortality.
Keywords: cluster analysis, patient outcomes assessment, hospitalization, infection
The understanding of the pathobiology of sepsis has advanced (1); yet, sepsis remains among the most lethal, costly, and morbid reasons for acute hospitalization in the United States (2–5). Increasingly sophisticated understanding of the molecular mechanisms and epidemiology of sepsis has not translated into successful clinical trials, and sepsis mortality remains unacceptably high (1). Increasingly, experts have argued that this lack of translational progress may be due to the heterogeneity of sepsis (1, 6), although there is no consensus regarding which factors are most important to that heterogeneity.
We hypothesized that relevant heterogeneity in sepsis might be made more visible by considering an acute episode of sepsis within the context of the patient’s presepsis clinical trajectory. This approach contrasts with many current searches for heterogeneity, which have emphasized differences in inflammatory phenotypes measured during the acute episode. Our hypothesis is motivated in part by two contrasting clinical stories of sepsis—the archetypes of 1) the otherwise healthy patient who is struck down by an acute infection and 2) the frail and chronically ill patient who develops sepsis while residing in a nursing home. No population-based data are available to distinguish the relative frequencies of such diverse patients.
We sought to test the hypothesis that there are consequential and significant differences in acute sepsis outcomes that result from differences in a patient’s clinical course leading up to sepsis hospitalization. Furthermore, we hypothesized that a small number of empirically derived and reproducible subtypes of sepsis could be identified on the basis of different paths leading up to sepsis. In order to test these hypotheses, we first identified archetypical paths into sepsis, as defined by distinct patterns of healthcare facility use leading up to sepsis hospitalization. We then validated these presepsis paths in two additional cohorts and tested the association between patients’ presepsis paths and 90-day outcomes. We previously reported some of these results in a minisymposium at the American Thoracic Society International Conference (7).
Methods
Study Cohorts
We studied three cohorts of patients hospitalized with sepsis. The derivation cohort comprised participants in the U.S. Health and Retirement Study (HRS) (8) in fee-for-service Medicare and hospitalized with sepsis during 1998–2005. A similar cohort was used in prior studies, as described in Appendix E1 in the online supplement. HRS is also described further in Appendix E1. The first validation cohort comprised HRS participants in fee-for-service Medicare hospitalized in 2006–2012. The second cohort, an external validation cohort, comprised U.S. Department of Veterans Affairs (VA) beneficiaries hospitalized with sepsis at any VA hospital in 2009. Sepsis hospitalizations were identified in administrative data using modified Angus criteria, as described in Appendix E1 (9, 10).
Healthcare Use Before Sepsis
The goal of our analysis was to identify subgroups of patients with sepsis based on their path into sepsis, as measured by trajectory of inpatient healthcare use in the year before hospitalization. To do this, we first determined each patient’s daily inpatient healthcare facility use for the year before sepsis hospitalization using claims for acute hospitalizations, long-term acute care hospitalizations, and nursing facility stays, as described in Appendix E1.
Covariates
We abstracted patient characteristics (e.g., age, sex, date of death) and hospitalization characteristics (e.g., length of stay, intensive care unit use, and mechanical ventilation) from HRS surveys, Medicare data, and/or national VA datasets. We ascertained comorbid conditions from a 1-year look-back in inpatient and outpatient claims data (11, 12). For HRS cohorts, we abstracted presepsis disability (count of limitations of activities and instrumental activities of daily living [13]) and household wealth (described in Appendix E1) from the HRS survey immediately before sepsis hospitalization. For the VA cohort, we calculated illness severity upon hospital presentation (predicted 90-d mortality) using a composite score similar to Acute Physiology and Chronic Health Evaluation IV (14), as in prior analyses (15, 16) and as described further in Appendix E1.
Identifying Subgroups of Patients with Different Paths into Sepsis
Subgroups of patients with sepsis, defined by their trajectory of presepsis healthcare facility use, were identified using latent profile analysis (LPA) (17). LPA is a type of probabilistic cluster analysis that identifies subgroups (also known as latent profiles or classes) of patients on the basis of their characteristics. The optimal solution identifies a parsimonious number of subgroups in which differences between patients in the same group are minimized and differences between groups are maximized (17). Conceptually, this analysis was used to find the optimal number and shape of presepsis healthcare use trajectories that best fit the data, without consideration of any outcome measure. LPA allows for nonlinear trajectories, and unlike conventional growth curve models using multilevel random-effects models, it does not assume a functional form for the trajectories (17).
LPA was implemented using measures of average healthcare use over 12 time periods, as described in Appendix E1. In the derivation cohort, we fitted models with 1 to 15 trajectories. We determined the optimal number of trajectories by considering both model fit, as measured by Bayesian information criterion, and clinical interpretability of the model (i.e., whether each trajectory appeared to be represent a distinct path into sepsis). After selecting the optimal number of trajectories in the derivation cohort, we then applied the same model to each of the validation cohorts to estimate the predicted trajectories in each of the validation samples. The models determined the optimal shape of the trajectories and the optimal assignment of patients to classes separately for each cohort.
We assessed for differences in patient characteristics and outcome (90-d mortality) between subgroups using one-way analysis of variance, the Kruskal-Wallis rank test, and chi-square tests. We also abstracted charts for two patients from each class in the VA derivation cohort, and we present these as vignettes.
Association of Path into Sepsis with Clinical Outcomes
To measure the association of presepsis path with 90-day mortality, we first compared mortality by class assignment. Next, we measured the association using multivariable logistic regression, adjusting for important patient characteristics. In the multivariable models, trajectory class assignment was entered as a categorical variable, and patients were assigned to a single class on the basis of greatest probability. For the HRS cohorts, we adjusted for age, race, sex, presepsis functional limitations, and chronic disease burden (weighted Elixhauser score [11, 12]). For the VA cohort, we adjusted for age, race, sex, and acute illness severity. We calculated the adjusted mortality by trajectory class from the multivariable model using the effects command in the effects package in R. (This is equivalent to Stata’s margins command, with the “atmeans” option.) To visually display differences in outcomes by path into sepsis, we graphed patients’ daily locations over 90 days from sepsis admission, similar to prior analyses (18, 19), as described in Appendix E1.
Sensitivity Analyses
We performed several sensitivity analyses. First, we evaluated alternative time bins (e.g., weekly) for measuring healthcare facility use. Second, we limited the HRS cohorts to one randomly selected sepsis hospitalization per patient. Third, in the multivariable regression model, we excluded patients without complete information and alternatively imputed missing data using multiple imputation.
Data management and analysis were performed using SAS version 9.2 (SAS Institute), Stata MP version 14 (StataCorp), and R version 3.4.3 (R Foundation for Statistical Computing) software. The University of Michigan and Ann Arbor VA institutional review boards approved this study. HRS participants provided informed consent at enrollment and again for Medicare linkage.
Results
There were 1,512 sepsis hospitalizations in the derivation cohort, 1,992 in the first validation cohort, and 32,525 in the second validation cohort (Table 1). The cohorts were older (median ages, 79, 79, and 69 yr, respectively) with significant comorbidity burden (median Elixhauser score, 7–16). Median hospital length of stay was 7 days; 35–49% of patients used intensive care; and 16–27% were mechanically ventilated. The 90-day mortality was 27–41% by cohort. Patients had spent a median of 4–7 days in an inpatient healthcare facility in the prior year.
Table 1.
Derivation and validation cohort demographics
| Characteristic | Derivation Cohort (1998–2005) (n = 1,512) | Validation Cohort 1 (2006–2012) (n = 1,992) | Validation Cohort 2 (2009) (n = 32,525) |
|---|---|---|---|
| Age at admission, yr | |||
| Median | 79 | 79 | 69 |
| Interquartile range | 71–85 | 72–86 | 61–80 |
| Male sex, n (%) | 700 (46) | 860 (43) | 31,536 (97) |
| Race, n (%) | |||
| White | 1,134 (75) | 1,531 (77) | 23,414 (72) |
| Black | 319 (21) | 369 (19) | 5,924 (18) |
| Other | 59 (4) | 89 (5) | 449 (1) |
| Unknown | 0 (0) | 3 (0) | 2,738 (8) |
| Elixhauser comorbidity index | |||
| Median | 15 | 16 | 7 |
| Interquartile range | 9–21 | 9–23 | 3–13 |
| Functional limitations | |||
| Median | 1 | 1 | — |
| Interquartile range | 0–4 | 0–4 | — |
| Inpatient facility use during prior year, d | |||
| Median | 5 | 4 | 7 |
| Interquartile range | 0–26 | 0–26 | 0–22 |
| Length of hospitalization, d | |||
| Median | 8 | 6 | 7 |
| Interquartile range | 4–14 | 4–11 | 4–15 |
| Used intensive care, n (%) | 736 (49) | 692 (35) | 13,795 (42) |
| Used mechanical ventilation, n (%) | 413 (27) | 327 (16) | 5,917 (18) |
| In-hospital mortality, n (%) | 348 (23) | 322 (16) | 4,496 (14) |
| 90-d mortality, n (%) | 621 (41) | 667 (34) | 8,787 (27) |
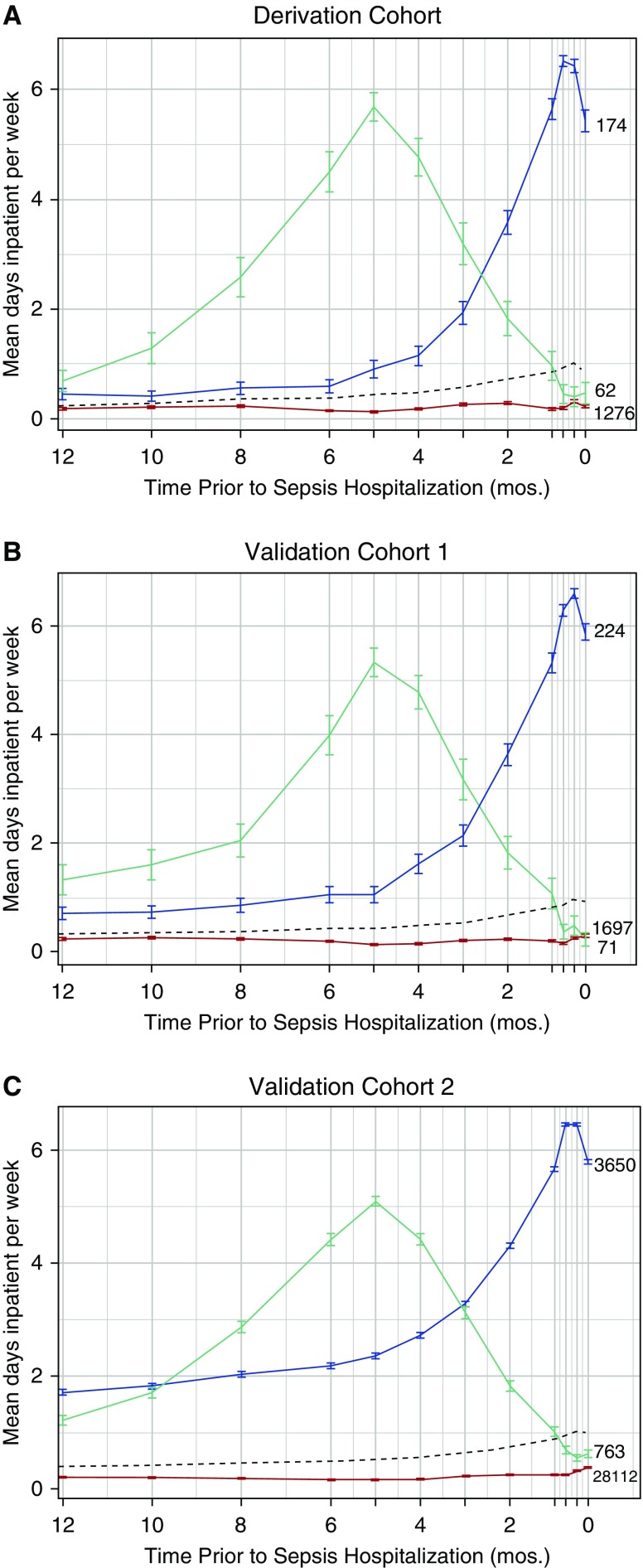
In the derivation cohort, a three-class model best characterized trajectories of presepsis healthcare use (Figure 1). Class 1 (“low use”) had little inpatient healthcare use, with half (n = 610) spending 0 days in an inpatient healthcare facility. Class 2 (“rising use”) had increased healthcare facility use in the months immediately preceding sepsis hospitalization, with the median patient spending 55 days spent in a healthcare facility. Class 3 (“high use”) had marked use of inpatient healthcare facilities over the year, with the median patient spending 118.5 days in a healthcare facility. Importantly, none of these three trajectories resembled the overall mean trajectory (Figures 1 and E2).
Figure 1.
Trajectory classes and frequencies for the derivation and validation cohorts. The black dashed line shows the mean inpatient days per week for the entire cohort. Mean use over time for the low-use, rising-use, and high-use classes are shown in red, blue, and green, respectively, for (A) the derivation cohort, (B) validation cohort 1, and (C) validation cohort 2. Vertical lines depict the 95% confidence intervals.
Of 1,512 patients in the derivation cohort, 1,276 (84%) were assigned to the low-use class, 174 (12%) to the rising-use class, and 62 (4%) to the high-use class. Patients were classified easily, with 99% mean probability of class assignment. Only 15 (0.1%) patients were assigned with less than 95% certainty (Table 2).
Table 2.
Two illustrative vignettes for each presepsis trajectory class
| Low use |
| An 80-yr-old man with congestive heart failure, COPD, stage III CKD, and no hospitalizations in the preceding year presented to the emergency department with fevers, altered mental status, acute-on-chronic renal failure, and respiratory distress. He was intubated in the emergency department, started on mechanical ventilation, and admitted to the hospital with diagnoses of sepsis and respiratory failure. |
| A 60-yr-old man with poorly controlled type 2 diabetes presented with acute nausea, vomiting, abdominal pain, and confusion. He was diagnosed with acute cholecystitis and admitted to the hospital with a diagnosis of severe sepsis. In the prior year, he had been hospitalized for 3 d after a syncopal episode about 6 mo before sepsis hospitalization. |
| Rising use |
| A 75-yr-old man with coronary artery disease and ischemic cardiomyopathy was hospitalized for 1 wk with a heart failure exacerbation after several months of progressive lower extremity edema and weight gain. Three weeks later, he was rehospitalized with another heart failure exacerbation, this time with new atrial fibrillation with rapid ventricular response. After 2 wk, he was discharged to a nursing facility for rehabilitation owing to progressive deconditioning over the course of his hospitalizations. Two weeks later, he was transferred from the nursing facility back to the emergency department with acute confusion, hypotension, fever, and abdominal pain, at which point he was admitted to the hospital for presumed sepsis. |
| A 75-yr-old man with type 2 diabetes and diabetic neuropathy was hospitalized for a nonhealing diabetic foot ulcer and ultimately underwent a below-the-knee amputation. After 2 wk, he was discharged to a nursing facility for rehabilitation. One week later, he was transferred back to the hospital with fevers and somnolence, at which point he was diagnosed with severe sepsis secondary to healthcare-associated pneumonia. |
| High use |
| An 85-yr-old man with congestive heart failure and osteoarthritis underwent a total knee replacement surgery for knee osteoarthritis, followed by 2 months in a nursing facility for rehabilitation. Two months after discharge from rehabilitation, he presented to the emergency department with dysuria, leukocytosis, and acute renal failure, and he was admitted to the hospital with a diagnosis of urosepsis. |
| A 50-yr-old-man with end-stage cirrhosis had frequent hospitalizations for hepatic encephalopathy. After one hospitalization for decompensated cirrhosis and hepatic encephalopathy, he was discharged to a nursing facility for physical therapy and medical optimization. After spending 3 mo in the nursing facility, he was discharged to home. One month later, he had a short hospitalization for another bout of hepatic encephalopathy. Two and one-half months later, he presented to the emergency department with nausea, vomiting, fevers, and abdominal pain, at which point he was diagnosed with acute cholecystitis and severe sepsis. |
Definition of abbreviations: CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease.
Vignettes were abstracted by chart review. Patient age was rounded to the nearest 5-year category to preserve patient anonymity.
A two-class model that dichotomized patients into those with some versus little/no inpatient healthcare facility use (Figure E3) was also considered. The two-class model fit substantially better than a one-class model but was outperformed by the three-class model (Figure E4). A four-class model split the rising-use class into a very small additional class with an even later and steeper rise in healthcare use (Figure E5). However, the clinical significance of this additional class was questionable, and it yielded minimal additional improvement in model fit (Figure E4). Thus, the three-class model was selected. In sensitivity analyses, when healthcare facility use was examined by week, findings were unchanged (Figures E6 and E7).
When the three-class model was then applied to validation cohorts, the findings were robust. Specifically, the shape of presepsis trajectories and the distribution of trajectory classes were similar across the three cohorts (Figure 1, Table E1), which is not guaranteed if the model estimated in the derivation sample does not fit the data in the validation samples. We present illustrative patient vignettes by class assignment in Table 2.
None of the models identified a class with persistent use. In a post hoc analysis, we looked for patients with inpatient healthcare facility use during each of the 12 time periods leading up to sepsis hospitalization, but we found that these patients were exceedingly rare (n = 44 in derivation cohort [2.9%]), presumably because most patients either die or recover after sustained time in a healthcare facility.
Patient characteristics differed by subtype (Table 3). Although the overall study population consisted of older patients with chronic comorbidity, the low-use class was relatively healthier before developing sepsis. These patients had fewer medical comorbidities and fewer functional limitations before sepsis. The high-use class appeared chronically ill, with high comorbidity burden (median Elixhauser score, 24) and presepsis disability (median functional limitations, 5). The rising-use class patients were the oldest, but they had less comorbidity and less functional disability than the high-use class patients. These patterns were similar across all three cohorts (Tables E2 and E3). The proportion of use that was due to skilled nursing facilities or long-term acute care hospitals also differed by trajectory class; as total use increased, the proportion due to skilled nursing facilities or long-term acute care hospitals also increased (Table E4). There were no differences in either intensive care unit or mechanical ventilation use between classes.
Table 3.
Cohort characteristics by class assignment in the derivation cohort
| Characteristics | Low Use (n = 1,276) | Rising Use (n = 174) | High Use (n = 62) | P Value |
|---|---|---|---|---|
| Age, yr | 0.02 | |||
| Median | 78 | 82 | 80 | |
| Interquartile range | 71–88 | 72–88 | 82–84 | |
| Male sex, n (%) | 600 (47) | 76 (44) | 24 (39) | 0.34 |
| Race, n (%) | 0.02 | |||
| White | 964 (76) | 129 (74) | 41 (66) | |
| Black | 256 (20) | 44 (25) | 19 (31) | |
| Other | 56 (4) | 1 (1) | 2 (3) | |
| Household wealth | 0.013 | |||
| Quartile 4 (most assets) | 188 (14.7) | 18 (10.3) | 3 (4.8) | |
| Quartile 3 | 224 (17.6) | 23 (13.2) | 7 (11.3) | |
| Quartile 2 | 275 (21.6) | 39 (22.4) | 12 (19.4) | |
| Quartile 1 | 389 (30.5) | 61 (35.1) | 26 (42.0) | |
| Net negative or zero assets | 163 (12.8) | 33 (19.0) | 14 (22.6) | |
| Elixhauser comorbidity index | <0.001 | |||
| Median | 14 | 19 | 24 | |
| Interquartile range | 8–20 | 14–25 | 19–29 | |
| Functional limitations | <0.001 | |||
| Median | 1 | 2 | 5 | |
| Interquartile range | 0–4 | 0–6 | 1–8 | |
| Total inpatient facility use during prior year, d |
NA | |||
| Median | 3 | 55 | 118.5 | |
| Interquartile range | 0–12 | 34–89 | 81.5–149 | |
| Hospital use during prior year, d | NA | |||
| Median | 2 | 23 | 37.5 | |
| Interquartile range | 0–10 | 13–40 | 19–58 | |
| SNF use during prior year, d | NA | |||
| Median | 0 | 22.5 | 92 | |
| Interquartile range | 0–0 | 6–49 | 40–99 | |
| LTAC use during prior year, d | NA | |||
| Median | 0 | 0 | 0 | |
| Interquartile range | 0–0 | 0–0 | 0–34 | |
| Length of hospitalization, d | 0.05 | |||
| Median | 8 | 8 | 11 | |
| Interquartile range | 4–14 | 4–15 | 6–19 | |
| Used intensive care, n (%) | 623 (49) | 86 (49) | 27 (44) | 0.70 |
| Used mechanical ventilation, n (%) | 349 (27) | 47 (27) | 17 (27) | 0.995 |
| In-hospital mortality, n (%) | 277 (21.7) | 56 (32.2) | 15 (24.2) | 0.01 |
| 90-d mortality, n (%) | 481 (38) | 110 (63) | 30 (48) | <0.001 |
Definition of abbreviations: LTAC = long-term acute care hospital; NA = not applicable; SNF = skilled nursing facility.
In unadjusted analysis, class assignment was strongly associated with 90-day mortality. In the derivation cohort, 90-day mortality was 63% for the rising-use class versus 48% for the high-use class and 38% for low-use class (P < 0.001) (Table 3). Mortality was lower in the derivation cohorts, consistent with other studies showing declining sepsis mortality over time (15, 20). However, the differences in mortality by class assignment persisted and followed the same pattern, with the rising-use class having worst survival and the low use class having the best survival (Tables E2 and E3).
In adjusted analyses that accounted for important confounders such as age, comorbidity burden, functional limitations (in HRS cohorts), and an acute illness severity measure (in the VA cohort), the rising-use class remained independently predictive of 90-day mortality (Table E5). Adjusted odds of 90-day mortality were 1.3- to 2.2-fold higher in the rising-use class than in the low-use class. This translated to an adjusted mortality of 58% for the rising-use class versus 39% for the low-use class in the derivation cohort, 44% versus 31% in the first validation cohort and 27% versus 23% in the second validation cohort (Table E6).
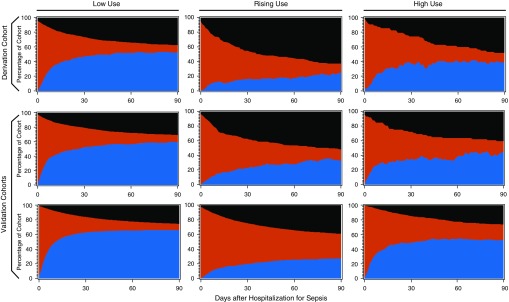
Visual examination of 90-day outcomes by class assignment (Figure 2) revealed distinct patterns of mortality and healthcare facility use, which were consistent across cohorts. Patients in the rising-use class experienced greater mortality in the first 30 days, with a high burden of healthcare facility use.
Figure 2.
Ninety-day outcomes by path into sepsis. Each panel depicts the proportion of patients who are at home (blue), in an inpatient healthcare facility (red), and dead (black) during the 90 days after hospital admission for sepsis. The cohorts are presented by row: The derivation cohort is the top row; the first validation cohort is the middle row; and the second validation cohort is the bottom row. Patients assigned to the low-use class are presented in the left column; patients assigned to rising use are presented in the middle column; and patients assigned to high use are assigned to the right column.
Discussion
Using multiple national cohorts of more than 35,000 hospitalized patients, we have shown that several distinct paths into sepsis exist. Moreover, these paths are broadly conserved across different study populations, including earlier and later HRS participants cared for under fee-for-service Medicare as well as a national cohort of VA beneficiaries cared for in that public integrated system. Specifically, both the distribution and the shape of presepsis paths were robust across the three cohorts. Importantly, none of the paths into sepsis resembled the mean trajectory, indicating that the mean trajectory is a poor proxy for any individual patient’s experience.
Across all three cohorts, paths into sepsis were strongly associated with 90-day mortality. A presepsis path characterized by increasing healthcare facility use in the weeks to months preceding sepsis hospitalization was independently associated with an increased risk for 90-day mortality. The association persisted even after adjusting for potential confounders, such as a multivariable illness severity score that is itself strongly predictive of mortality. Our findings indicate that a patient’s presepsis path provides additional information about 90-day mortality above and beyond current mortality prediction models. Although the high-use class spent significantly more days in a healthcare facility, the rising-use class had the greatest 90-day mortality, underscoring the importance of trajectory (and not merely magnitude) of recent healthcare use.
Our findings are consistent with the long-standing recognition among clinicians and researchers that sepsis is a heterogeneous disease, as well as that the context in which sepsis occurs has important implications for disease severity, progression, and outcome (1). Prior attempts to incorporate patients’ longitudinal clinical history, however, have identified hundreds of distinct trajectories of comorbidity before sepsis (21), limiting the potential for translation into clinical practice.
Recent attempts to identify meaningful subtypes of patients with sepsis have focused on gene expression soon after arrival to the hospital (22–24). The goal has been to identify pathobiological derangements on which to base delivery of therapies targeted at these aberrant processes (6). This approach, however, has met with limited success thus far. Patients may have many pathobiological derangements during sepsis, not all of which are important. Moreover, patients’ physiology changes rapidly. The ability to distinguish the primary mechanisms driving sepsis progression from secondary derangements, which may merely be markers of disease severity, or transient disruptions, is limited (25). This challenge, among others, has limited the role of precision medicine in sepsis diagnosis and management (26).
We hypothesize that a patient’s path into sepsis may not merely be predictive of 90-day mortality, as we have demonstrated, but may also reflect underlying differences in the pathobiology of sepsis. Host defense against sepsis involves two strategies: immunity (decreasing pathogen load) and “disease tolerance” (limiting the negative impact of infection on tissue structure and function through mechanisms other than reducing pathogen load) (27). The disease tolerance strategy relies on stress responses, which trigger metabolic adaptations that limit tissue damage (28). It is possible that patients with rapidly declining health (e.g., rising-use patients who spend an increasing amount of time in healthcare facilities in the weeks leading up to sepsis) may develop impaired immunity and/or impaired disease tolerance, which result in worse outcomes independent of age, comorbidity burden, and illness upon initial presentation. In addition, recent high use may result in microbiome disruption, transiently increasing patients’ vulnerability to sepsis (29, 30). Future studies are needed to test whether presepsis path is indeed associated with differences in pathobiology.
The presepsis paths identified in this study may have several applications. First, trajectory classes could be used immediately for risk adjustment, because hospitals caring for a higher proportion of high-use or rising-use patients would be expected to have worse outcomes. Second, the trajectory classes could be used for prognostic enrichment to identify patients at higher risk of 90-day mortality for inclusion in (or exclusion from) clinical trials. Third, the trajectory classes could be assessed further in translational studies to understand whether host response in sepsis differs based on presepsis trajectory, which broadly characterizes the context in which sepsis occurred.
Our findings should be interpreted in the setting of several limitations. First, sepsis hospitalizations were identified by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, which may misclassify patients in both directions (31). There is no ideal method for identifying sepsis (31). We used the approach described by Angus and colleagues (9) rather than explicit ICD-9-CM codes for sepsis because our study included patients hospitalized over a span of 15 years, before the advent of explicit sepsis codes. Second, there are many methods by which to identify trajectory subclasses (e.g., growth mixture modeling, group-based trajectory modeling). Alternative modeling approaches, as well as alternative specifications of the healthcare facility use data (e.g., binning by month or by quarter), may have yielded slightly different results. Furthermore, because presepsis paths were defined on the basis of inpatient healthcare facility use alone, over 80% of each cohort was assigned to the low-use class. Including additional information, such as outpatient healthcare use or pharmacy data, may in the future facilitate the identification of additional subclasses. Finally, patient comorbidities were determined from claims data, which may incompletely capture comorbidities, particularly in the VA healthcare system.
Conclusions
Using multiple national cohorts of more than 35,000 hospitalized patients, we have shown that several distinct paths into sepsis exist. These paths, which can be identified from healthcare facility use before sepsis hospitalization, are predictive of 90-day mortality. Future studies are needed to determine whether these distinct paths into sepsis are also associated with differences in pathobiology of sepsis.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Ryan McCammon, M.S., at the University of Michigan and Kyle Kepreos, M.A., formerly at the VA Center for Clinical Management Research, for their expert programming. They were not compensated beyond salary support.
Footnotes
Supported by grants K08 GM115859 (H.C.P.) and L30 GM116118 (H.C.P.) from the National Institutes of Health, IIR 11-109 (T.J.I.) from the U.S. Department of Veterans Affairs Health Services Research and Development Service, and an unrestricted critical care research grant from the American Thoracic Society Foundation (H.C.P.). The Health and Retirement Study is sponsored by the National Institute on Aging (grant U01 AG009740) and performed at the Institute for Social Research, University of Michigan. The funders were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. government.
Author Contributions: Conception and design: all authors; data acquisition: H.C.P., K.M.L., and T.J.I.; data analyses: H.C.P., A.G.C., and R.G.; interpretation of data: all authors; drafting of manuscript: H.C.P.; revision the manuscript for important intellectual content: all authors; and approval of the manuscript for submission: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 2.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 3.Torio C, Moore B. Rockville, MD: Agency for Healthcare Research and Quality; National inpatient hospital costs: the most expensive hospital conditions by payer, 2013. HCUP Statistical Brief 204. May 2016 [accessed 2017 Feb 14]. Available from: https://hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.pdf. [PubMed] [Google Scholar]
- 4.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. doi: 10.1136/bmj.i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med. 2016;194:147–155. doi: 10.1164/rccm.201512-2544CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prescott HC, Carmichael A, Langa KM, Gonzalez R, Iwashyna TJ. Paths into sepsis: trajectories of pre-sepsis healthcare use [abstract] Am J Respir Crit Care Med. 2018;197:A2716. [Google Scholar]
- 8.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the Health and Retirement Study (HRS) Int J Epidemiol. 2014;43:576–585. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Iwashyna TJ, Odden A, Rohde J, Bonham C, Kuhn L, Malani P, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52:e39–e43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 12.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 13.Fonda S, Herzog AR. HRS/AHEAD documentation report: documentation of physical functioning measured in the Health and Retirement Study and the Asset and Health Dynamics among the Oldest Old Study [updated 2004 Dec 21; accessed 2018 Apr 17] Available from: http://hrsonline.
- 14.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 15.Prescott HC, Kepreos KM, Wiitala WL, Iwashyna TJ. Temporal changes in the influence of hospitals and regional healthcare networks on severe sepsis mortality. Crit Care Med. 2015;43:1368–1374. doi: 10.1097/CCM.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prescott HC. Variation in postsepsis readmission patterns: a cohort study of Veterans Affairs beneficiaries. Ann Am Thorac Soc. 2017;14:230–237. doi: 10.1513/AnnalsATS.201605-398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraley C, Raftery AE. Model-based clustering, discriminant analysis, and density estimation. J Am Stat Assoc. 2002;97:611–631. [Google Scholar]
- 18.Prescott HC, Langa KM, Liu V, Escobar GJ, Iwashyna TJ. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190:62–69. doi: 10.1164/rccm.201403-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeMerle KM, Vincent BM, Iwashyna TJ, Prescott HC. Increased healthcare facility use in veterans surviving sepsis hospitalization. J Crit Care. 2017;42:59–64. doi: 10.1016/j.jcrc.2017.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014;42:625–631. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck MK, Jensen AB, Nielsen AB, Perner A, Moseley PL, Brunak S. Diagnosis trajectories of prior multi-morbidity predict sepsis mortality. Sci Rep. 2016;6:36624. doi: 10.1038/srep36624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maslove DM, Tang BM, McLean AS. Identification of sepsis subtypes in critically ill adults using gene expression profiling. Crit Care. 2012;16:R183. doi: 10.1186/cc11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong HR, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Willson DF, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davenport EE, Burnham KL, Radhakrishnan J, Humburg P, Hutton P, Mills TC, et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir Med. 2016;4:259–271. doi: 10.1016/S2213-2600(16)00046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 26.Maslove DM, Lamontagne F, Marshall JC, Heyland DK. A path to precision in the ICU. Crit Care. 2017;21:79. doi: 10.1186/s13054-017-1653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soares MP, Teixeira L, Moita LF. Disease tolerance and immunity in host protection against infection. Nat Rev Immunol. 2017;17:83–96. doi: 10.1038/nri.2016.136. [DOI] [PubMed] [Google Scholar]
- 29.Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ. Hospitalization type and subsequent severe sepsis. Am J Respir Crit Care Med. 2015;192:581–588. doi: 10.1164/rccm.201503-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baggs J, Jernigan JA, Halpin AL, Epstein L, Hatfield KM, McDonald LC. Risk of subsequent sepsis within 90 days after a hospital stay by type of antibiotic exposure. Clin Infect Dis. 2018;66:1004–1012. doi: 10.1093/cid/cix947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jolley RJ, Sawka KJ, Yergens DW, Quan H, Jetté N, Doig CJ. Validity of administrative data in recording sepsis: a systematic review. Crit Care. 2015;19:139. doi: 10.1186/s13054-015-0847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.