To the Editor:
Cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction is the major pathophysiologic defect leading to bronchiectasis in cystic fibrosis (CF) (1). Recently published data indicated that cigarette smoking causes acquired CFTR dysfunction, manifested by increased sweat chloride (2), altered sweat rate (3), and reduced CFTR activity in the upper (4) and lower respiratory tracts (5) measured by nasal and lower airway potential difference, respectively, in subjects with chronic obstructive pulmonary disease (COPD) and smokers without fixed airflow obstruction. The defect continues despite smoking cessation, which may be related to smoking intensity (2). COPD-associated bronchiectasis is a common COPD endotype, affecting between 25% and 69% of patients. It is identified by computed tomography (CT) and is associated with greater sputum production, poorer lung function, more frequent exacerbations, greater bacterial colonization, and worse outcomes compared with COPD patients without bronchiectasis (6, 7). We hypothesized that acquired CFTR dysfunction in smokers would be associated with CT-identified bronchiectasis in patients with COPD, defining a particular subphenotype and an indicator of the clinical impact of CFTR dysfunction in individuals without CF.
Methods
This single-center study of current and former smokers without a known history of CF was conducted between January 1 and December 31, 2015, in participants previously enrolled in COPDGene (Genetic Epidemiology of COPD), a study intended to define subphenotypes of the disease (8). We collected demographic data, body mass index, smoking status (current vs. former), smoking history in pack-years, and self-reported chronic bronchitis. Spirometry was performed according to American Thoracic Society/European Respiratory Society standards (9), and severity of airflow limitation was grouped according to Global Initiative for Chronic Obstructive Lung Disease recommendations (10). Inspiratory and expiratory volumetric computed tomographic scans were acquired using multidetector CT scanners and image reconstruction algorithms as previously reported (8). The presence or absence of bronchiectasis was scored visually by a radiologist blinded to clinical characteristics as previously reported and validated by a second blinded scorer (11). Bronchiectasis was defined by the presence of bronchial dilation (bronchial diameter greater than the diameter of the accompanying pulmonary vessel). Intraobserver agreement for visual bronchiectasis was good (Cohen’s κ = 0.746). Sweat chloride was measured by quantitative pilocarpine iontophoresis using the Macroduct collection system (Wescor) (2). CFTR genetics were analyzed by complete CFTR sequencing if the sweat chloride level was abnormal (≥40 mmol/L) (12). Never smokers and subjects who had withdrawn from COPDGene were ineligible. Fisher’s exact test and a nonparametric test (Wilcoxon rank-sum test) were used to assess the bivariate relationships between groups. The results were reported as median (interquartile range) for continuous variables. Statistical analyses were performed using SAS 9.4 software (SAS Institute Inc.). This study was approved by the University of Alabama at Birmingham Institutional Review Board (F111209002), and all subjects provided written informed consent.
Results
Ninety-eight subjects were screened; 11 were excluded owing to the lifetime absence of smoking (n = 6) or because of missing radiologic data (n = 5). The baseline characteristics of the 87 subjects included in the study are presented in Table 1. About one-half (49%) of the cohort were current smokers with a median (interquartile range) 40 (30–57)–pack-year history. Post-bronchodilator forced expiratory volume in 1 second (FEV1) percent predicted was 67.5 (53–88), and FEV1/forced vital capacity ratio was 0.69 (0.5–0.78). Twelve (24%) of the patients had chronic bronchitis based on self-report; none used roflumilast, which can activate CFTR (13, 14).
Table 1.
Baseline characteristics
| Variables | Total (N = 87) | Presence of Bronchiectasis (n = 12) | Absence of Bronchiectasis (n = 75) | P Value* |
|---|---|---|---|---|
| Age, yr | 64 (57–71) | 68.5 (61–74) | 68.5 (57–70) | 0.20 |
| Sex | 1.00 | |||
| Male | 50 (57%) | 7 (58%) | 43 (57%) | |
| Female | 37 (43%) | 5 (42%) | 32 (43%) | |
| Race | 1.00 | |||
| Non-Hispanic white | 54 (62%) | 8 (67%) | 46 (61%) | |
| African American | 33 (38%) | 4 (33%) | 29 (39%) | |
| Body mass index, kg/m2 | 28.1 (24–32.6) | 27.4 (21.2–31.4) | 28.7 (24–33.2) | 0.28 |
| Smoking status | 0.76 | |||
| Current smoker | 43 (49%) | 5 (42%) | 38 (51%) | |
| Former smoker | 44 (52%) | 7 (58%) | 37 (49%) | |
| Smoking intensity, pack-years | 39.8 (29.5–57) | 43.8 (35–76.5) | 39.7 (28.5–52.6) | 0.29 |
| COPD severity | 0.45 | |||
| GOLD 1 | 2 (2.3%) | 1 (8.33%) | 1 (1.33%) | |
| GOLD 2 | 23 (26.4%) | 2 (16.67%) | 21 (28%) | |
| GOLD 3 | 13 (14.9%) | 1 (8.33%) | 12 (16%) | |
| GOLD 4 | 6 (6.9%) | 0 | 6 (8%) | |
| No COPD | 43 (49.5%) | 8 (66.67%) | 35 (46.67%) | |
| Symptoms of chronic bronchitis | 21 (24%) | 2 (17%) | 19 (26%) | 0.72 |
| Spirometry | ||||
| FEV1, % predicted | 67.5 (53–88) | 86 (56.5–97.5) | 66.5 (52–87) | 0.14 |
| FEV1/FVC | 0.69 (0.5–0.78) | 0.72 (0.58–0.77) | 0.65 (0.48–0.78) | 0.61 |
| Sweat chloride level, mmol/L | 30 (13–43) | 44 (24.5–53) | 24 (12–40) | 0.03 |
| Sweat chloride ≥40 mmol/L | 27 (31%) | 8 (67%) | 19 (25%) | 0.007 |
| Chest CT findings | ||||
| Bronchial wall thickening | 23 (26%) | 5 (42%) | 18 (24%) | 0.29 |
| Percent emphysema | 6.56 (1.9–17.1) | 4.29 (1.4–10.1) | 6.96 (2.1–18.3) | 0.30 |
| Pi10 | 3.68 (3.6–3.8) | 3.69 (3.6–3.8) | 3.68 (3.6–3.8) | 0.47 |
| Mosaicism | 9 (10.3%) | 1 (8.3%) | 8 (10.7%) | 1.00 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; CT = computed tomography; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; GOLD = Global Initiative for Chronic Obstructive Lung Disease; Pi10 = square root of wall area of an airway of 10-mm internal perimeter.
Data are expressed as median (interquartile range) or frequency (percent).
Estimated using Wilcoxon rank-sum test and Fisher’s exact test for continuous and categorical variables, respectively.
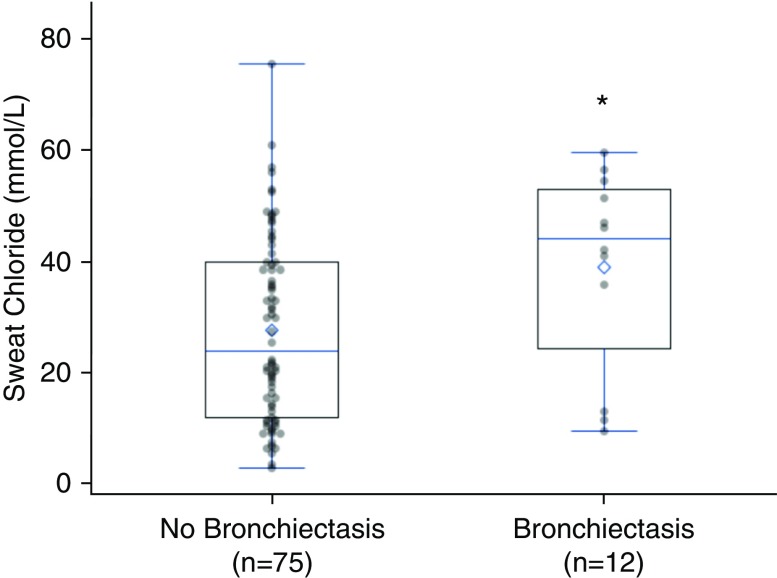
Twelve subjects (14%) had bronchiectasis visualized by CT. Among those with bronchiectasis, 33% had COPD defined by spirometry. Sweat chloride was measured in all subjects. The median (interquartile range) was 30 (13–43) mmol/L. Sweat chloride was greater in subjects with bronchiectasis than in individuals without bronchiectasis (44 [24.5–53] vs. 24 [12–40] mmol/L; P = 0.03) (Figure 1). Elevated sweat chloride was associated with a 4.4-fold increased prevalence of visual bronchiectasis (prevalence ratio, 4.4; 95% confidence interval, 1.5, 13.5; P = 0.008). Among those with visual bronchiectasis, 8 (67%) of 12 patients had elevated sweat chloride (≥40 mmol/L) (15), whereas 19 (25%) of 75 of those without bronchiectasis exceeded this threshold (P = 0.004). Elevated sweat chloride was also more common in subjects with mosaic attenuation visualized by CT (43 [40–49] vs. 22.5 [12–40] mmol/L; P = 0.03).
Figure 1.
Comparison of sweat chloride levels in subjects with or without visual bronchiectasis on computed tomographic scans. Sweat chloride was significantly higher in those with visual bronchiectasis on computed tomographic scans. The horizontal line corresponds to the median, and the diamond represents the mean sweat chloride value. *P = 0.03 by Wilcoxon rank-sum test.
Subjects with elevated sweat chloride underwent full-gene CFTR sequencing (n = 25). No individuals carried two CF-causing variants on separate chromosomes. Four carried a variant that required further consideration, although none had visual bronchiectasis. One subject carried the common CF-causing variant p.Phe508del (c.1521_1523delCTT), which was not unexpected, given the carrier frequency. A second subject had two variants (p.Arg74Trp [c.220C>T] and p.Asp1270Asn [c.3808G>A]), which are likely to be in cis and which have been associated with CFTR-related disorders (16). The remaining two variants identified (p.Glu528 [c.1584G>A] and p.Ser1235Arg (c.3705T>G]) are rare and unlikely to be deleterious (17). CFTR variants of unknown significance (n = 5) were not more prevalent in patients with visual bronchiectasis. We also assessed for the presence of variants in the epithelial sodium channel genes (SCNN1A, SCNN1B, SCNN1D, and SCNN1G) and CA12, which are known or suspected to associate with bronchiectasis. Overall, seven patients had one or more variants in the epithelial sodium channel; these were not more prevalent in patients with visual bronchiectasis. The lack of detectable genetic contribution is consistent with experimental modeling (18), although heterozygosity may serve as a susceptibility risk factor and should be evaluated in well-powered studies.
Discussion
CFTR dysfunction identified by elevated sweat chloride is associated with CT bronchiectasis among current and former smokers. To our knowledge, this is the first study to evaluate this relationship, and it suggests a possible underlying pathophysiologic mechanism that contributes to the development of bronchiectasis in COPD (and in smokers without recognized COPD). The degree of sweat chloride abnormality reflects a 20–30% decrement in CFTR function based on genotype–phenotype correlations, levels comparable to those of individuals with CF who have CFTR-related disorders that manifest with bronchiectasis later in life (19). Of note, this association was independent of chronic bronchitis symptoms by self-report.
In addition to bronchiectasis, mosaic attenuation visualized by high-resolution CT is a sign of airway disease by inducing gas trapping and heterogeneity of the parenchyma. The association of mosaic attenuation with elevated sweat chloride further supports the concept that CFTR abnormality may contribute to airway disease. Of note, air trapping was rapidly reversible in G551D CFTR patients with CF with administration of the CFTR potentiator ivacaftor (20).
Our study has important implications for the understanding of the relationship between acquired CFTR dysfunction and CT bronchiectasis. Smoke-induced acquired CFTR dysfunction should be considered when evaluating the etiology of bronchiectasis, especially when patients have elevated sweat chloride but normal CFTR genetic analysis. Recent studies suggested that CFTR potentiators can reverse acquired CFTR abnormalities in vitro (2, 4, 21, 22) and that ivacaftor may restore CFTR function and improve symptoms in patients with COPD with chronic bronchitis (22). Thus, these results have potential therapeutic implications, given that CFTR potentiators used for treatment of CF may also restore CFTR activity in patients with COPD (22).
Limitations of the present study to consider include the observational design; the relatively small number of individuals with bronchiectasis; and that alternative etiologies of bronchiectasis, such as infection, immunodeficiency, and chronic aspiration (23), were not available for evaluation. Although our study did not reveal an association of bronchiectasis with lung function or chronic bronchitis (2, 4, 21, 22), the present analysis was underpowered for this analysis. The potential for CFTR dysfunction to contribute to worsened airway disease in patients with smoking-related lung disease and to identify a specific subphenotype warrants further investigation.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Gerald McGwin, M.S., Ph.D., for providing a number of helpful suggestions; Briana Vecchio-Pagan, Ph.D., for her work on the CFTR variant annotation pipeline; and the patients who participated in this study.
Footnotes
Supported by National Institutes of Health grants K08 HL123940 (J.M.W.), R01 HL105487 (S.M.R.), P30 DK072482 (S.M.R.), R01 DK44003 (G.R.C.), UL1TR001417, and UL1TR001079 and by Cystic Fibrosis Foundation grants CUTTIN13A1 and CUTTIN13A2. The Genetic Epidemiology of COPD (COPDGene) study is funded by the National Heart, Lung, and Blood Institute (grants R01 HL089897 and R01 HL089856) and by the COPD Foundation through contributions made to an industry advisory board comprised of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
Author Contributions: Study conception and design: K.T., J.M.W., S.P.B., P.H.N., M.T.D., and S.M.R.; sample acquisition and analysis: K.T., J.M.W., S.V.R., P.H.N., K.S.R., M.A.A., G.R.C., and L.R.; data interpretation: K.T., J.M.W., K.S.R., G.R.C., S.P.B., P.H.N., M.T.D., and S.M.R.; composition of the manuscript: K.T., J.M.W., K.S.R., and S.M.R.; revision and approval of the manuscript: all authors; accountability agreement: S.M.R.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188:1321–1330. doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courville CA, Tidwell S, Liu B, Accurso FJ, Dransfield MT, Rowe SM. Acquired defects in CFTR-dependent β-adrenergic sweat secretion in chronic obstructive pulmonary disease. Respir Res. 2014;15:25. doi: 10.1186/1465-9921-15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One. 2012;7:e39809. doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novosad SA, Barker AF. Chronic obstructive pulmonary disease and bronchiectasis. Curr Opin Pulm Med. 2013;19:133–139. doi: 10.1097/MCP.0b013e32835d8312. [DOI] [PubMed] [Google Scholar]
- 7.Ni Y, Shi G, Yu Y, Hao J, Chen T, Song H. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1465–1475. doi: 10.2147/COPD.S83910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic Epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 10.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur Respir J. 2017;49:1700214. doi: 10.1183/13993003.00214-2017. [DOI] [PubMed] [Google Scholar]
- 11.Barr RG, Berkowitz EA, Bigazzi F, Bode F, Bon J, Bowler RP, et al. COPDGene CT Workshop Group. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD. 2012;9:151–159. doi: 10.3109/15412555.2012.654923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vecchio-Pagán B, Blackman SM, Lee M, Atalar M, Pellicore MJ, Pace RG, et al. Deep resequencing of CFTR in 762 F508del homozygotes reveals clusters of non-coding variants associated with cystic fibrosis disease traits. Hum Genome Var. 2016;3:16038. doi: 10.1038/hgv.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raju SV, Rasmussen L, Sloane PA, Tang LP, Libby EF, Rowe SM. Roflumilast reverses CFTR-mediated ion transport dysfunction in cigarette smoke-exposed mice. Respir Res. 2017;18:173. doi: 10.1186/s12931-017-0656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert JA, Raju SV, Tang LP, McNicholas CM, Li Y, Courville CA, et al. Cystic fibrosis transmembrane conductance regulator activation by roflumilast contributes to therapeutic benefit in chronic bronchitis. Am J Respir Cell Mol Biol. 2014;50:549–558. doi: 10.1165/rcmb.2013-0228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe SM, Hoover W, Solomon GM, Sorscher EJ. Cystic fibrosis. In: Broaddus VC, editor. Murray & Nadel’s textbook of respiratory medicine. 6th ed. Vol. 1. Philadelphia: Elsevier/Saunders; 2016. pp. 822–852. [Google Scholar]
- 16.Terlizzi V, Castaldo G, Salvatore D, Lucarelli M, Raia V, Angioni A, et al. Genotype–phenotype correlation and functional studies in patients with cystic fibrosis bearing CFTR complex alleles. J Med Genet. 2017;54:224–235. doi: 10.1136/jmedgenet-2016-103985. [DOI] [PubMed] [Google Scholar]
- 17.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raju SV, Solomon GM, Dransfield MT, Rowe SM. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in chronic bronchitis and other diseases of mucus clearance. Clin Chest Med. 2016;37:147–158. doi: 10.1016/j.ccm.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilschanski M, Dupuis A, Ellis L, Jarvi K, Zielenski J, Tullis E, et al. Mutations in the cystic fibrosis transmembrane regulator gene and in vivo transepithelial potentials. Am J Respir Crit Care Med. 2006;174:787–794. doi: 10.1164/rccm.200509-1377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adam RJ, Hisert KB, Dodd JD, Grogan B, Launspach JL, Barnes JK, et al. Acute administration of ivacaftor to people with cystic fibrosis and a G551D-CFTR mutation reveals smooth muscle abnormalities. JCI Insight. 2016;1:e86183. doi: 10.1172/jci.insight.86183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raju SV, Lin VY, Liu L, McNicholas CM, Karki S, Sloane PA, et al. The cystic fibrosis transmembrane conductance regulator potentiator ivacaftor augments mucociliary clearance abrogating cystic fibrosis transmembrane conductance regulator inhibition by cigarette smoke. Am J Respir Cell Mol Biol. 2017;56:99–108. doi: 10.1165/rcmb.2016-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon GM, Hathorne H, Liu B, Raju SV, Reeves G, Acosta EP, et al. Pilot evaluation of ivacaftor for chronic bronchitis. Lancet Respir Med. 2016;4:e32–e33. doi: 10.1016/S2213-2600(16)30047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao YH, Guan WJ, Liu SX, Wang L, Cui JJ, Chen RC, et al. Aetiology of bronchiectasis in adults: a systematic literature review. Respirology. 2016;21:1376–1383. doi: 10.1111/resp.12832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.