Abstract
Circulating levels of Brain Derived Neurotrophic Factor (BDNF) are lower in coronary heart disease (CHD) than in healthy subjects and are associated with coronary events and mortality. However, the mechanism(s) underling this association is not fully understood. We hypothesize that BDNF may influence fibrin fiber structure and clot stability, favoring clot lysis and thrombus resolution. We showed that recombinant BDNF (rh-BDNF) influenced with clot formation in a concentration-dependent manner in both purified fibrinogen and plasma from healthy subjects. In particular, rh-BDNF reduced the density of fibrin fibers, the maximum clot firmness (MCF) and the maximum clot turbidity, and affected the lysis of clot. In addition, both thrombin and reptilase clotting time were prolonged by rh-BDNF, despite the amount of thrombin formed was greater. Intriguingly, CHD patients had lower levels of BDNF, greater fibrin fibers density, higher MCF than control subjects, and a negative correlation between BDNF and MCF was found. Of note, rh-BDNF markedly modified fibrin clot profile restoring physiological clot morphology in CHD plasma. In conclusion, we provide evidence that low levels of BDNF correlate with the formation of bigger thrombi (in vitro) and that this effect is mediated, at least partially, by the alteration of fibrin fibers formation.
Introduction
Brain-derived neurotrophic factor (BDNF), a member of neurotrophin family consisting of 118 amino acids, with a molecular weight of ∼14 kDa and a high charge (pI [isoelectric point] = ∼9–10)1, promotes growth, survival, and maintenance of neurons2. It is expressed not only in neurons, but also in other types of cells including endothelial cells3, cardiomyocytes4, vascular smooth muscle cells5, leukocytes6, platelets7 and megakaryocytes8. BDNF plays a key role not only in the brain but also in the cardiovascular system, and its alterations have been related to pathological changes in the cardiovascular system, suggesting its potential role in the pathogenesis of coronary heart disease (CHD). Indeed, BDNF promotes development of cardiac vasculature, modulates cardiac endothelium survival and proliferation, enhances capillary formation, sustains angiogenesis and maintains integrity of vascular system9. Interestingly, modifications of BDNF gene, including rs10767664 and rs 6265 polymorphisms, are associated with coronary artery disease and myocardial infarction10–12. Similarly, lower circulating BDNF levels are detected in cardiovascular disease13,14 and related disorders15, are associated with increased risk of atrial fibrillation and with future coronary events16, and are an independent predictor of 4-year coronary and all-cause mortality17. Finally, circulating BDNF is negatively associated with triglyceride, LDL-cholesterol and fibrinogen, and positively associated with HDL-cholesterol in patients with angina pectoris18.
However, the relationship between circulating levels of BDNF and CHD has not been fully understood as yet.
Accumulating evidence shows that both hypercoagulable state and hypo-fibrinolytic conditions are related to the increased risk of cardiovascular events and severity of CHD19–23, suggesting that the unbalance between clotting and lysis proteins plays an important role into CHD pathogenesis. In particular, higher plasma levels of fibrinogen as well as fibrin clot properties predict adverse clinical outcome in CHD patients and are associated with the extent of coronary atherosclerosis24–27. Similarly, an inadequate function of fibrinolytic system, with lower plasma levels of tissue plasminogen activator (tPA) and greater concentration of plasminogen activator inhibitor type 1 (PAI-1), has been observed in coronary artery disease patients28.
Interestingly, tPA not only catalyzes the conversion of plasminogen to plasmin, the key modulator of fibrin clot degradation, but also regulates the proteolytic cleavage of pro-BDNF to mature BDNF29,30, suggesting the fibrinolytic system as a potential bridge between BDNF and CHD. Indeed, in pathological conditions, circulating BDNF is positively associated with tPA/plasmin activity31 and negatively with fibrinogen17.
In addition, since fibrin(ogen) binds BDNF by its heparin-binding domain and since fibrin matrix retains BDNF into the clot32, we hypothesized that BDNF may modify fibrin fiber structure and affect clot stability favoring clot lysis and thrombus resolution.
Methods
Study population
Twenty one healthy men (age between 29 and 78 years) with normal sinus rhythm, no electrocardiographic alterations and history of atrial fibrillation were screened from those attending the clinic for global control of cardiovascular risk at Centro Cardiologico Monzino, IRCCS. Forty-one coronary heart disease (CHD, age between 39 and 79 years) patients, candidate for coronary artery bypass grafting (CABG), were also recruited at Centro Cardiologico Monzino. Preoperative inclusion criteria were: need for elective, isolated surgical procedure, age between 18–80 years, ejection fraction >30%, normal sinus rhythm and no history of atrial fibrillation. Individuals suffering from renal or liver disease or taking antioxidants within 30 days prior to surgery were excluded. Clinical and demographical features of controls and CHD patients are listed in Supplementary Table 1.
Blood was collected from CHD patients the day of in-hospital admittance while controls underwent sample collection at a scheduled visit. Both CHD and control subjects were not under anticoagulant drugs.
The study complies with the Declaration of Helsinki and was approved by the Institutional Review Board and Ethical Committee of Centro Cardiologico Monzino IRCCS. All participants provided written informed consent.
Biochemical analysis
Peripheral blood sample was collected from patients and controls while fasting into a vacutainer tubes containing EDTA (ethylenediaminetetraacetic acid) disodium salt (9.3 mM; Vacutainer System, Becton Dickinson, Franklin Lakes, NJ, USA.) for detection of BDNF and fibrinogen, or into a vacutainer tubes containing Sodium Citrate (0.105 M; Vacutainer System, Becton Dickinson, Franklin Lakes, NJ, USA) for clot and thromboelastographic analysis, and then centrifuged within 30 min at 3000 g for 10 min at 4 °C. Plasma thus obtained was collected, aliquoted and immediately stored at −80 °C until analysis.
BDNF and fibrinogen were measured in plasma by kits commercially available: BDNF by Emax Immunoassay system (Promega, Madison, WI, USA), and Human FG (Fibrinogen) by ELISA assay (Whuan Fine Biotech Co., China), respectively33.
Clot analysis
Fibrin clot characterization was performed (a) in a purified system, (b) in five different plasma pools obtained mixing four by four plasma from 20 healthy subjects, and (c) in 9 and 12 plasma pools from control subjects and CHD patients, respectively, obtained mixing two or three plasma, accordingly to plasma BDNF levels (±20 pg/ml) to minimize biological variability. (d) The effect of rh-BDNF on fibrin clot structure in CHD patients was performed directly in plasma sample of 12 CHD patients with BDNF < 100 pg/ml as specified in figure legends.
Fibrin clots analysis
Fibrin clot structure: Fibrinogen-Alexa Fluor 488 conjugated (fibrinogen-AF, Invitrogen, St Louis, MO) was reconstituted in sodium bicarbonate 0.1 M pH 8.3 at a concentration of 1.5 mg/ml by layering the protein on top of the saline solution and solubilizing it with occasional gentle mixing. Then fibrinogen was aliquoted and frozen until the use. Fibrin clots were prepared in chambered coverslips using 100 µl of clotting solution with the following final concentrations: (a) In purified systems, the experiments were performed without adding of Factor XIII34 to focus exclusively on the reaction of BDNF on fibrin mesh. In particular, clots were prepared in HEPES buffer (HEPES 20 mM pH 7.4, NaCl 110 mM) with 1 mg/ml fibrinogen, 2.5 mM CaCl2 and 0.1 U/ml human thrombin (SIGMA-Aldrich, St Louis, MO) in the presence of 1 µl of PBS containing different concentrations (0–300 pg/ml) of recombinant BDNF (rh-BDNF, prepared according to manufacturer instructions in distilled water; Invitrogen, Waltham, MA, USA) or an equal volume of PBS containing BSA (1 mg/ml: control). (b) Citrated Plasma samples were diluted 1:10 in imidazole buffer35, then 1 µl of PBS containing scalar concentration of rh-BDNF (as indicated into the figure legend) plus or equal volume of PBS containing BSA (1 mg/ml: control) and then 10 µl of 1 mg/ml fibrinogen-AF were added. After 5 min of incubation at 37 °C, clot formation was induced by 2.5 mM CaCl2 and 0.1 U/ml human thrombin36.
The samples were allowed to polymerize for 2 hours in incubator at 37 °C, and then the images were acquired using apotome microscope (Carl Zeiss, Milano, Italy) or laser scanning confocal microscope (LSM710, Carl Zeiss, Milano, Italy) at 20X magnification. Quantization of fibers density was accomplished in blind by Image J software program according to method described previously with some modifications. Briefly, “the density of the fibers in each slide of Z-stack acquisition were measured after entering the “Image” menu, clicking on the “Adjust” box and isolating the area covered by fibers using “Threshold” tool. The threshold was automatically adjusted until the entire green area was highlighted in red. Then, measurement of the threshold area was performed as follows: we entered the set measurement dialog under the “Analyze” menu, and after checking the “Area”, “Integrity Intensity” and “Limit to Treshold”, we have clicked the “Measurement” button under the “Analyze” menu, and data have been found in the “Results” Window”37.
Fibrin polymerization assay: 100 µl of citrated plasma samples, diluted 1:2 in imidazole buffer, were pre incubated for 15 min at 37 °C with 1 µl of PBS containing scalar concentrations of rh-BDNF (0, 60 and 120 pg/ml) or with equal volume of PBS containing BSA (1 mg/ml: control). Plasma clotting was initiated by Thrombin (1 U/ml) and CaCl2 (2.5 mM). Fibrin polymerization was assessed by TECAN spectrophotometer at 350 nm with the interval of 23 sec38.
For Clot lysis studies, recombinant tPA 1,8 µM (Creative Enzymes, NY, USA) was added to each sample and fibrinolysis was monitored by TECAN spectrophotometer at 350 nm with the interval of 23 sec. The lysis time: time taken for turbidity to drop by 50% from maximum as a measure of lysis potential, and maximum turbidity: turbidity refers to the scattering of light as a measure of fibrin clot density27.
Clotting time
For thrombin clotting time 100 µl of citrated plasma sample were incubated (for 15 min at 37 °C) with the same volume of diluent containing scalar concentrations of BDNF (0, 60 and 120 pg/ml) or BSA (1 mg/ml: control), then, 100 µl of human thrombin (1 U/ml; SIGMA-ALDRICH, St Louis, MO, USA) were added39.
For reptilase clotting time measurement, 180 µl of citrated plasma sample were incubated for 15 min at 37°Cwith the same volume of diluent containing scalar concentrations of BDNF (0, 60 and 120 pg/ml) or BSA (1 mg/ml: control), then, 60 µl of reptilase (2 BU/100 ml; Creatie Enzymes, NY, USA) and 60 µl CaCl2 (20 mM) were added40.
Time to reach the formation of a stable fibrin clot was recorded. All samples were measured in triplicates.
Thrombin generation
Whole blood (WB) was drawn from healthy subjects with a 19-gauge needle without venous stasis into citrate (1/10 volume of 0.129M sodium citrate)-containing tubes (Vacutainer, Becton Dickinson) in the presence or absence of corn trypsin inhibitor (CTI, 50 µg/ml) to inhibit the intrinsic coagulation pathway.
Thrombin generation was measured using the Calibrated Automated Thrombogram (CAT) assay (Thrombinoscope BV, Maastricht, the Netherlands) on platelet-free plasma, prepared by two sequential centrifugations at 2500 × g for 15 minutes. Triplicate plasma samples (80 µl/well) were incubated for 15 minutes with 120 pg/ml BDNF or BSA 1 mg/ml (control) in PBS in the presence of 1pM Tissue Factor and 4 µM phospholipids (PPP Low reagent, Stago) in round-bottom 96-well microtiter plates (Immulon 2HB). Thrombin generation was started by the addition of a CaCl2/fluorogenic substrate mixture (20 µl/well FluCa reagent).
In order to correct for inner-filter effects and substrate consumption, each thrombin generation measurement was calibrated against the fluorescence curve obtained in the same plasma to which a fixed amount of thrombin-α2-macroglobulin complex was added (Thrombin Calibrator, Thrombinoscope BV). Fluorescence was read in a Fluoroskan Ascent reader (Thermo Labsystems OY, Helsinki, Finland) equipped with a 390/460 nm filter set and thrombin generation curves were calculated with Thrombinoscope software (Thrombinoscope BV). ETP (Endogenous Thrombin Potential, nM x min), Peak Height (nM Thrombin) and Velocity Index (nM/min) were used as main parameters describing thrombin generation.
Rotational thromboelastography
The clotting potential of plasma was evaluated by Rotational Thromboelasto- graphy (ROTEM®)41. Briefly, plasma pools were incubated for 15 min at 37 °C with different amount rh-BDNF (0, 60 and 120 pg/ml) or equal volume of PBS containing BSA (1 mg/ml: control) and then analyzed. Three hundred µL of plasma for each condition was recalcified with star-tem® solution (20 µL CaCl2/HEPES buffer 200 mM) and activated with Thrombin (SIGMA-ALDRICH, St Louis, MO) 1 U/ml. Recording has been started immediately and has been allowed to proceed for 60 min according to manufacturer’s instructions. Clot characterization in terms of Maximum Clot Firmness (MCF, measure of the consistence of the clot) was assessed. Before testing, an aliquot of each condition was stored at −80 °C to determine BDNF concentration.
Statistical analysis
Statistical analyses were performed with SAS v. 9.4 software (SAS Institute). Data were compared between groups by Wilcoxon rank-sum test. Main effects of treatment and time were analyzed by repeated measures ANOVA, followed by a paired t-test with Bonferroni correction. Partial Spearman correlation was employed to assess associations between variables adjusting for group of subjects. Values of p < 0.05 were considered statistically significant. Data are expressed as mean/ ± SEM or median and interquartile range.
Results
BDNF affects fibrin clot network
To evaluate the potential impact of exogenous BDNF on fibrin clot structure, Alexa Fluor 488-labeled fibrinogen in presence of recombinant BDNF (rh-BDNF) was used.
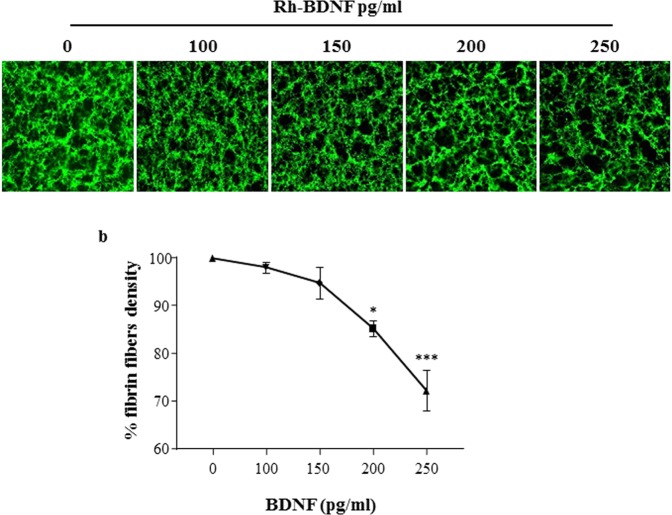
The rh-BDNF affected the fibrin network in a concentration-dependent manner. Changes in clot structure, in terms of non-uniform, densely packed fibers and presence of large holes, are observed when 200 pg/ml rh-BDNF are added to purified fibrin(ogen)/thrombin-clot, whereas the density of fibers decreased significantly with 250 pg/ml rh-BDNF. In particular, at the highest doses used rh-BDNF reduced of about 15% and 30% fibrin fiber density compared to control (Fig. 1).
Figure 1.
rh-BDNF reduces fibrin fibers density in vitro. Purified fibrin/thrombin clots were formed with recombinant BDNF (rh-BDNF: 100–250 pg/ml) or with BSA (1 mg/ml; control: point 0). (a) Representative image of clots using Alexa Fluor 488–labeled fibrinogen (20X magnification), and (b) percentage of fibrin fibers versus control. Fibrin fibers were analyzed using Image J software. All samples were performed in triplicate. Data are expressed as mean ± SEM; n = 9 *p < 0.05 ***p < 0.005.
Plasma BDNF inversely correlates with fibrin fiber density and in vitro clot dimension in healthy subjects’ plasma
To investigate the ability of BDNF to modify clot morphology in a physiological system, rh-BDNF was added to pools of plasma from healthy subjects. Structural analyses and polymerization of clot were assessed by visualization of fluorescent fibrin(ogen) fibers and turbidity assay, respectively, and viscoelastic properties were analysed by thromboelastography.
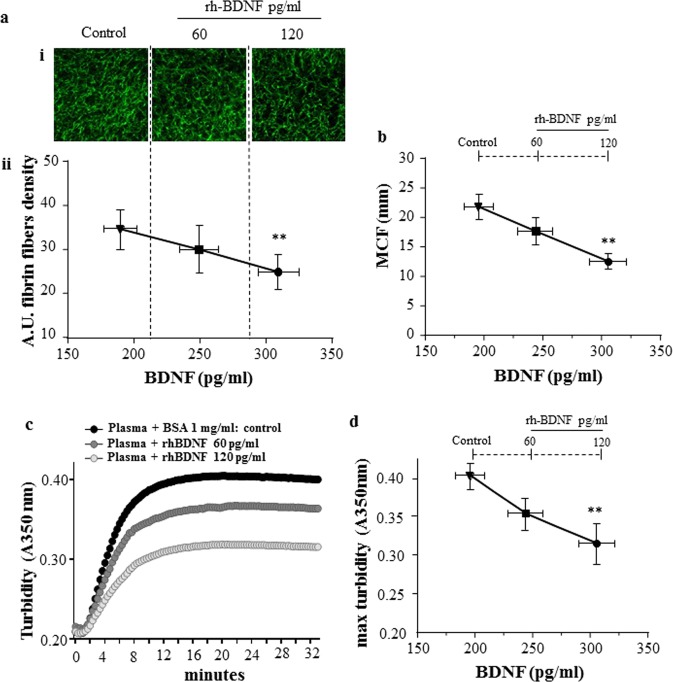
Remarkably, a reduction of about 30% of fibrin fiber density was measured when the total amount of BDNF present in plasma (endogenous plasma levels plus rhBDNF added) reached 303.9 ± 4.93 pg/ml (Fig. 2a, and Supplementary Fig. 1a).
Figure 2.
rh-BDNF influences fibrin density and polymerization, and in vitro clot dimension in healthy subjects’ plasma. Recombinant BDNF (rh-BDNF; 60, 120 pg/ml) or BSA (1 mg/ml: control) was added to plasma pools from healthy subjects before induction of coagulation with thrombin, then fibrin density and polymerization, and viscoelastic property of clot were analyzed. (ai) Visualization images (20X magnification) with Alexa Fluor 488–labeled method and (aii) quantization of fibrin fibers using Image J software. (b) Maximum Clot firmness (MCF) assessed by thromboelastographic analyses. All samples were performed in triplicate. (c) Representative kinetic and (d) maximum turbidity detected at A350 nm at 37 °C and monitored every 23 sec by spectrophotometric method. Data are expressed as mean ± SEM; horizontal bars indicate variation of BDNF levels measured in plasma pools analyzed; n = 5 different pools. **p < 0.01.
Similarly, rh-BDNF reduced the dimension of clot in all samples as shown by the progressive reduction in MCF (Fig. 2b, and Supplementary Fig. 1b). As expected, a positive correlation between density of fibrin fibers and MCF was found (r = 0.986, p < 0.0001, Supplementary Fig. 1c).
In addition, rh-BDNF modified the polymerization rate, defined as the slope of the turbidimetric curve, (control: 0.6198 ± 0.067, rh-BDNF 60 pg/ml: 0.547 ± 0.073 and rh-BDNF 120 pg/ml: 0.458 ± 0.053; rh-BDNF 120 pg/ml versus control p < 0.05), and the maximum optical density (Fig. 2c, and Supplementary Fig. 2a), reflecting the lateral aggregation of protofibrils and the fibre-cross-sectional area, respectively42.
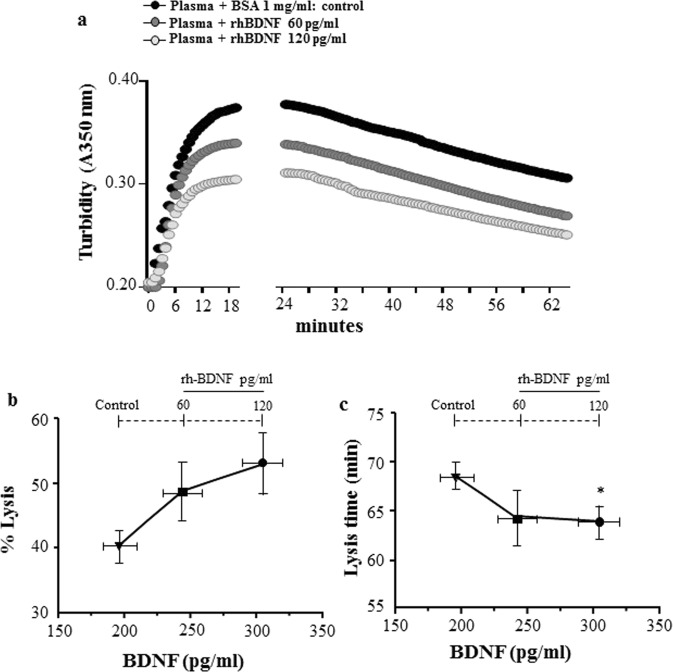
Rh-BDNF affected the fibrinolysis, slightly in terms of % of lysis reached at 60 minutes (control: 40.35 ± 2.48, rh-BDNF 60 pg/ml: 48.59 ± 4.71 and rh-BDNF 120 pg/ml: 53.41 ± 4.05; rh-BDNF 120 pg/ml versus control p = 0.073) and significantly the lysis time (control: 68.6 ± 1.46, rh-BDNF 60 pg/ml: 64.2 ± 3.077 and rh-BDNF 120 pg/ml: 63.5 ± 1.351; rh-BDNF 120 pg/ml versus control p = 0.043) (Fig. 3 and Supplementary Fig. 2b,c).
Figure 3.
Effect of rh-BDNF on lysis of fibrin clot in healthy subjects’ plasma. Recombinant BDNF (rh-BDNF; 60, 120 pg/ml) or BSA (1 mg/ml: control) were added to five plasma pools from healthy subjects before induction of coagulation with thrombin and tPA, consequently polymerization of clot were analyzed. (a) Representative turbidity curves monitored by spectrophotometric method every 23 sec (A350 nm at 37 °C), (b) % of Lysis at 60 minutes and (c) Lysis time. All samples were performed in triplicate. Data are expressed as mean ± SEM; n = 5 different pools.
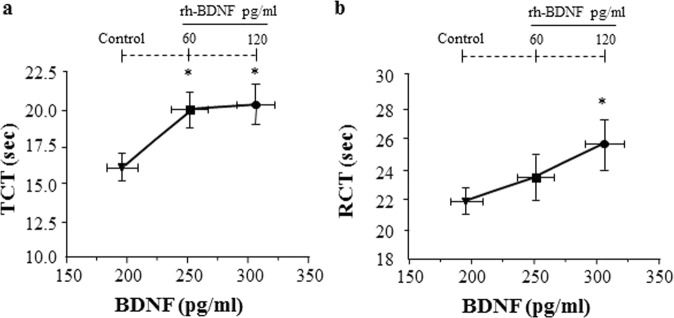
Interestingly, both the concentrations of rh-BDNF prolonged thrombin clotting time (Fig. 4a and Supplementary Fig. 3a), whether only the highest concentration of rh-BDNF was able to lengthen the clotting time when experiments were performed with reptilase (Fig. 4b and Supplementary Fig. 3b).
Figure 4.
rh-BDNF influences thrombin (TCT) and e) reptilase (RCT) clotting time in healthy subjects’ plasma. Recombinant BDNF (rh-BDNF; 60, 120 pg/ml) or BSA (1 mg/ml: control) were added to plasma pools from healthy subjects, then (a) thrombin (TCT) and (b) reptilase (RCT) clotting time were measured. All samples were performed in triplicate. Data are expressed as mean ± SEM; horizontal bars indicate variation of BDNF levels measured in plasma pools analyzed; n = 5 different pools. *p < 0.05 and **p < 0.01.
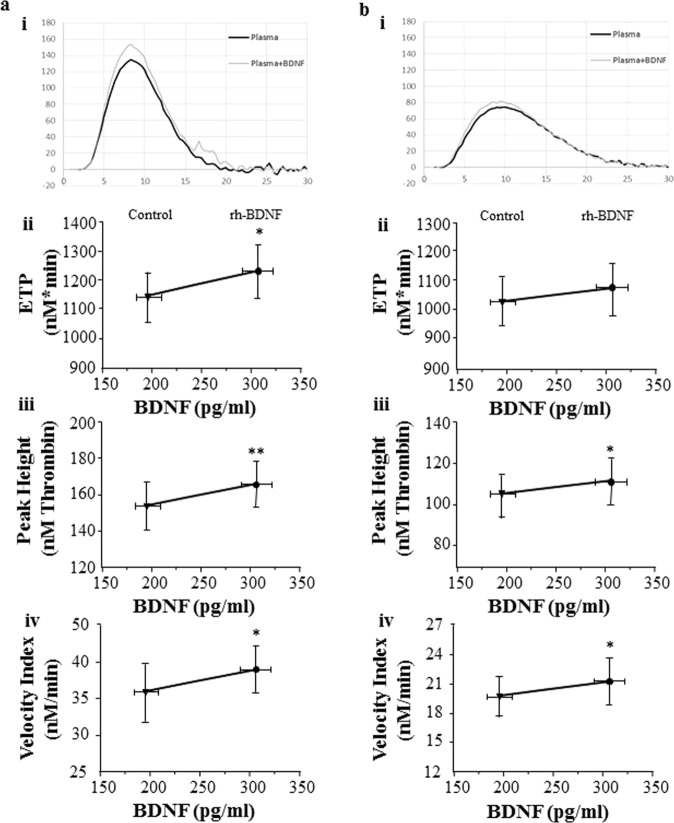
Finally, the effect of BDNF on thrombin generation in human plasma from healthy donors (n = 5) was assessed by CAT assay. Data indicate that, when both the intrinsic and the extrinsic pathway are contributing to the generation of thrombin, BDNF slightly but significantly increased thrombin formation (+~8% compared to control) in all the samples tested. The ETP as well as the Peak Height and the Velocity Index were, indeed, increased in the presence of 120 pg/ml rh-BDNF compared to control samples (ETP: control: 1140 ± 195 nM x min and rh-BDNF: 1232 ± 210 nMxmin; Peak Height: control: 154 ± 29 nM and rh-BDNF: 166 ± 28 nM thromb; Vel Ind: control: 36 ± 9 nM/min and rhBDNF: 39 ± 8 nM) (Fig. 5a and Supplementary Fig. 4a).
Figure 5.
Effect of BDNF on thrombin generation. Recombinant BDNF (rh-BDNF; 120 pg/ml) or BSA (1 mg/ml: control) was added to platelet-free plasma and thrombin formation was measured by CAT assay. Thrombin generated (a) by the concomitant activation of both intrinsic and extrinsic coagulation pathways and (b) only by the extrinsic pathway. (i) Representative curves of the kinetic of thrombin formation. (ii) Endogenous thrombin potential (ETP, area under the curve), (iii) Peak Height (maximum concentration of generated thrombin) and (iv) Velocity Index (velocity of thrombin formation) were used as main parameters describing thrombin generation. Data are expressed as mean ± SEM; horizontal bars indicate variation of BDNF levels measured in plasma pools analyzed n = 5 different pools. *p < 0.05 and **p < 0.01.
By contrast, when the intrinsic coagulation cascade was inhibited, the TF-dependent thrombin formation was not affected by BDNF (Fig. 5b and Supplementary Fig. 4b).
Reduced levels of BDNF in coronary heart disease patients favors the formation of bigger and more dense fiber clot
In order to assess the relationship between BDNF and fibrin clot morphology in clinical setting, firstly we measured levels of BDNF and fibrinogen in plasma of 41 coronary heart disease (CHD) patients and of 21 healthy subjects (Control).
Plasma BDNF levels were significantly lower in patients with CHD than controls (CHD: 126.83 ± 15.39 pg/ml versus controls: 208.90 ± 25.13 pg/ml; p < 0.0125; n = 41 and n = 21 patients and control, respectively), whereas fibrinogen was similar in both groups (CHD: 2.77 ± 0.63 g/l versus controls: 2.63 ± 0.51 g/l)
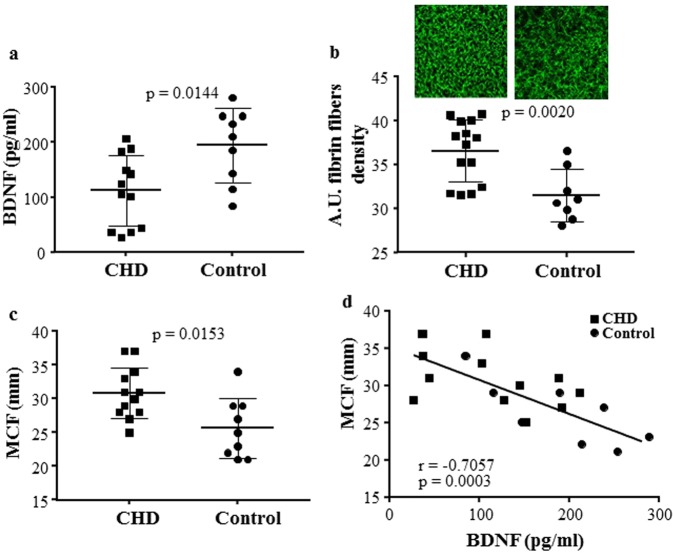
Clot morphology analysis was carried out in pools of plasma from Control and CHD patients (see methods section). As expected, BDNF levels were lower in pools of plasma from CHD patients with respect to controls (p = 0.0144, Fig. 6a).
Figure 6.
Low circulating BDNF levels are associated with higher density of fibrin fibers and greater MCF in CHD patients. Plasma pools of two or three CHD patients or healthy subjects (Control) were obtained according to similar BDNF levels (±20 pg/ml): (a) BDNF levels, (b) fibrin fibers density, and (c) Maximum Clot firmness (MCF) have been analysed by ELISA kit, Alexa Fluor 488–labeled method (20X magnification) and thromboelastographic analyses, respectively. Correlation between BDNF concentrations and (d) MCF. All samples were performed in triplicate, and representative images are shown. Data are expressed as mean ± SEM. n = 12 and 9 pools of CHD and healthy subjects, respectively.
Interestingly, both density of fibrin fibers (p = 0.0020; Fig. 6b) and MCF (p = 0.0153; Fig. 6c) were greater in CHD than in plasma pools of controls. In addition, in the whole group (CHD and Control) a negative correlation between BDNF and MCF (r = − 0.7057, p = 0.0003) was found (Fig. 6d), and this difference remained significant after adjustment for group of subjects (r = − 0.64252, p = 0.003).
Of note, the addition of rh-BDNF to plasma samples of CHD patients with BDNF < 100 pg/ml markedly reduced fibrin clot profile (Fig. 4). In particular, a reduction of about 30% in density of fibrin fibers was detected when BDNF reached the concentration of about 300 pg/ml (p = 0.0017) (Fig. 7 and Supplementary Fig. 5).
Figure 7.
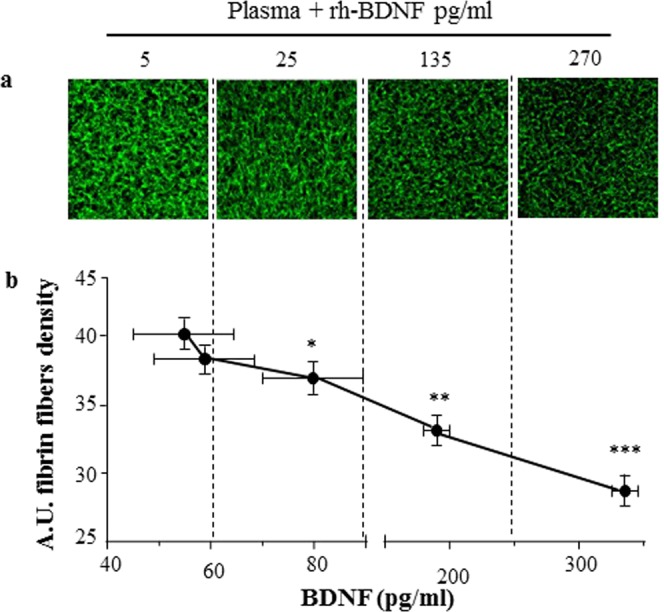
rh-BDNF reduces fibrin clot profile of CHD patients in vitro. Recombinant BDNF (rh-BDNF; 5, 25, 135 and 270 pg/ml) or BSA (1 mg/ml: control) was added to plasma from CHD patients before induction of coagulation with thrombin, and fibrin fibers were (a) visualized with Alexa Fluor 488–labeled (20X magnification) and (b) quantified using Image J software. All samples were performed in triplicate and representative images are shown. Data are expressed as mean ± SEM; horizontal bars indicate variation of BDNF levels measured in plasma pools analyzed. n = 12 plasma sample from CHD patients with BDNF < 100 pg/ml. *p < 0.05, **p < 0.01 and ***p < 0.005.
Discussion
In this study, we provide evidence that BDNF affects viscoelastic fibrin clot property and fibrin fibers density and that low levels of BDNF are associated in vitro with formation of bigger clot. In particular, we found that CHD patients have lower BDNF levels, higher fibrin clot strength and greater fibrin fibers complexity compared to healthy control subjects, all data consistent with previous reports41,43. In particular, others have showed that the range of MCF detected in control subjects and CHD patients (∼20–30 mm44,45 and ∼25–38 mm45, respectively), which confirms our data. In addition, the negative correlation between plasma BDNF levels and fibrin clot properties is suggestive of a new mechanism by which BDNF influences cardiovascular prognosis.
Several mechanisms aiming at explaining how lower BDNF levels may predict adverse cardiovascular events, have been proposed13,14,46. A special attention has been paid to the antioxidant, pro-survivor and pro-angiogenic effects of BDNF. Indeed, recent evidence suggests that BDNF plays a cardio-protective role by promoting the activation of enzymes involved in the detoxification of ROS47 and by decreasing cardiomyocytes death in hypoxic conditions4. In addition, it is well known that BDNF, binding to heparin-binding domain of fibrin(ogen), is retained within fibrin matrix32. The release of BDNF from fibrin matrix may partially explain both direct and indirect angiogenic effect of this neurothrophin. Indeed, BDNF promotes the local assembly of new vessels by induction/activation of TrkB receptor on endothelial cells48, and it favors the release of pro-angiogenic factors from bone marrow-derived progenitors recruited to the site of injury49. However, the potential involvement of BDNF in clot structure and stability has not been evaluated yet, although it is well known that BDNF binds to fragment 15–66 of fibrinogen β (Fgβ) chain32. This fragment is exposed after thrombin cleavage and it is involved in fibrin monomer self-association and clot formation50.
Interestingly, modification of Fgβ chain (e.g. tyrosine nitration and BβArg448Lys single-nucleotide polymorphism) are associated with increased fiber density and with greater resistance to fibrinolysis51–53. In addition, fibrin(ogen)-derived peptide β15–42 as well as fibrinogen gene deletion inhibit leukocyte infiltration, infarct size and subsequent scar formation in a mouse model of myocardial damage and reperfusion injury54. Overall, these findings provide compelling evidence that supports an important role of the Fgβ chain in the pathogenesis of myocardial infarction.
Thus, we can speculate that in CHD patients, by virtue of the lower BDNF levels, there is a great amount of free Fg β15–66 fragment that might induce stitches of cross-linking fiber with consequent generation of more dense fiber clots. In support to this hypothesis, we showed that when exogenous BDNF is added to CHD plasma, reaching physiological levels of BDNF, the density of fibrin fibers decreased as well as the amplitude of clot.
The relationship between BDNF and MCF observed in healthy subjects, as well as the ability of BDNF to affect structure, polymerization and viscoelastic properties of clot and to favor the formation of unstable clot more prone to lysis, suggests the key relevance of this neurotrophin in physiological hemostasis processes. Remarkably, the prolonged thrombin and reptilase clotting time induced by the highest concentration of rh-BDNF suggests that BDNF influences fibrin clot formation also by a mechanism independent of its binding with fibrinogen beta-chain. In particular, we can hypothesize that the high charge of BDNF might affect fibrin mesh55,56. Future studies will be designed in order to explain this effect.
Interestingly, the amount of thrombin generated in the presence of rh-BDNF was slightly, although significantly, increased. Thrombin plays a key role not only in the conversion of soluble fibrinogen into insoluble strands of fibrin, but catalyzes also anticoagulation related reaction, the most relevant being protein C activation. Activated protein C then inactivates FVIIIa and FVa57,58.
Considering the overall effect of BDNF on fibrin structure reported in the present study, it is tempting to speculate that physiological concentration of BDNF influences the thrombin-mediated anticoagulation process. This hypothesis needs to be tested in ad hoc studies and therefore it will be matter of future investigations.
The clinical relevance of fibrin clot properties as independent predictor of adverse clinical outcome following acute coronary syndrome has been recently highlighted by PLATO study. Indeed, Sumaya and colleagues have shown that fibrin clot properties independently predict the risk of spontaneous myocardial infarct and cardiovascular death after initial in-hospital management. In particular, enhanced resistance to lysis is associated with the increasing of established cardiovascular biomarkers (e.g. Troponin T and N-terminal pro b-type natriuretic peptide). All these findings point out to the potential need of an additional therapy in those patients with unfavorable fibrin clot structure and time of lysis27.
Our data would support the potential use of BDNF as biomarker of disease. It has to be considered, however, that different anti-coagulants, temperature and delay in sample centrifugation, and stability of sample storage can modify its levels33,59,60, thus limiting its use until standard procedures will be established.
Finally, the identification of mechanisms involved in fibrinogen modification and fibrin intrafibrillar structure are particularly attractive because the regulation of the cross-linking of the fibrin fiber as well as the preservation of endogenous thrombolytic mechanism represent crucial strategies to prevent the formation of occlusive thrombi in pathological conditions.
Supplementary information
Acknowledgements
We are indebted to individuals who have participated in, or have helped with this article. In particular, we thank Miss Helen Mc Pherson for her advices and support in technical setting of in vitro experiments. The present work was supported by the Italian Ministry of Health (Ricerca Corrente: BIO37-2016: 2613074; BIO37-2017: 2631213). Cofunding was provided by the contribution of the Italian “5 × 1000” tax (2014 and 2015). Sandrini L is supported by the 32nd cycle PhD program in Scienze Farmacologiche Sperimentali e Cliniche, Università degli Studi di Milano.
Author Contributions
Amadio P. performed experiments, analyzed the data and wrote the manuscript; Sandrini L. created the figures. The materials and datasets are available from Porro B. and Cavalca V. Fiorelli S. performed confocal microscope experiments. Brambilla M. and Camera M. performed the experiments and analyzed data of thrombin generation. Bonomi A and Veglia F performed statistical analyses. Porro B., Cavalca V. and Tremoli E. revised the manuscript; Barbieri S.S. designed study, analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37117-1.
References
- 1.Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. The EMBO journal. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poo MM. Neurotrophins as synaptic modulators. Nature reviews. Neuroscience. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 3.Nakahashi T, et al. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS letters. 2000;470:113–117. doi: 10.1016/S0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- 4.Okada S, et al. Brain-derived neurotrophic factor protects against cardiac dysfunction after myocardial infarction via a central nervous system-mediated pathway. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1902–1909. doi: 10.1161/ATVBAHA.112.248930. [DOI] [PubMed] [Google Scholar]
- 5.Donovan MJ, et al. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. The American journal of pathology. 1995;147:309–324. [PMC free article] [PubMed] [Google Scholar]
- 6.Kerschensteiner M, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? The Journal of experimental medicine. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimura H, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thrombosis and haemostasis. 2002;87:728–734. doi: 10.1055/s-0037-1613072. [DOI] [PubMed] [Google Scholar]
- 8.Chacon-Fernandez P, et al. Brain-derived Neurotrophic Factor in Megakaryocytes. The Journal of biological chemistry. 2016;291:9872–9881. doi: 10.1074/jbc.M116.720029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pius-Sadowska E, Machalinski B. BDNF - A key player in cardiovascular system. Journal of molecular and cellular cardiology. 2017;110:54–60. doi: 10.1016/j.yjmcc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 10.de Luis DA, Fernandez Ovalle H, Izaola O, Primo D, Aller R. RS 10767664 gene variant in Brain Derived Neurotrophic Factor (BDNF) affect metabolic changes and insulin resistance after a standard hypocaloric diet. Journal of diabetes and its complications. 2018;32:216–220. doi: 10.1016/j.jdiacomp.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Bozzini S, et al. Coronary artery disease and depression: possible role of brain-derived neurotrophic factor and serotonin transporter gene polymorphisms. International journal of molecular medicine. 2009;24:813–818. doi: 10.3892/ijmm_00000297. [DOI] [PubMed] [Google Scholar]
- 12.Amadio P, et al. BDNFVal66met polymorphism: a potential bridge between depression and thrombosis. European heart journal. 2017;38:1426–1435. doi: 10.1093/eurheartj/ehv655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin H, et al. Association between brain-derived neurotrophic factor and von Willebrand factor levels in patients with stable coronary artery disease. BMC cardiovascular disorders. 2018;18:23. doi: 10.1186/s12872-018-0762-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaess BM, et al. Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. Journal of the American Heart Association. 2015;4:e001544. doi: 10.1161/JAHA.114.001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaldakov GN, et al. NGF, BDNF, leptin, and mast cells in human coronary atherosclerosis and metabolic syndrome. Archives of physiology and biochemistry. 2001;109:357–360. doi: 10.1076/apab.109.4.357.4249. [DOI] [PubMed] [Google Scholar]
- 16.Rahman F, et al. Serum brain-derived neurotrophic factor and risk of atrial fibrillation. American heart journal. 2017;183:69–73. doi: 10.1016/j.ahj.2016.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Liu Y, Zhang Y, Chen ZY. Association of plasma brain-derived neurotrophic factor and cardiovascular risk factors and prognosis in angina pectoris. Biochemical and biophysical research communications. 2011;415:99–103. doi: 10.1016/j.bbrc.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Tasci I, Kabul HK, Aydogdu A. Brain derived neurotrophic factor (BDNF) in cardiometabolic physiology and diseases. Anadolu kardiyoloji dergisi: AKD = the Anatolian journal of cardiology. 2012;12:684–688. doi: 10.5152/akd.2012.221. [DOI] [PubMed] [Google Scholar]
- 19.Meade TW, Ruddock V, Stirling Y, Chakrabarti R, Miller GJ. Fibrinolytic activity, clotting factors, and long-term incidence of ischaemic heart disease in the Northwick Park Heart Study. Lancet. 1993;342:1076–1079. doi: 10.1016/0140-6736(93)92062-X. [DOI] [PubMed] [Google Scholar]
- 20.Lisman T, de Groot PG, Meijers JC, Rosendaal FR. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005;105:1102–1105. doi: 10.1182/blood-2004-08-3253. [DOI] [PubMed] [Google Scholar]
- 21.Meltzer ME, Doggen CJ, de Groot PG, Rosendaal FR, Lisman T. Reduced plasma fibrinolytic capacity as a potential risk factor for a first myocardial infarction in young men. British journal of haematology. 2009;145:121–127. doi: 10.1111/j.1365-2141.2008.07569.x. [DOI] [PubMed] [Google Scholar]
- 22.Guimaraes AH, et al. Hypofibrinolysis is a risk factor for arterial thrombosis at young age. British journal of haematology. 2009;145:115–120. doi: 10.1111/j.1365-2141.2008.07568.x. [DOI] [PubMed] [Google Scholar]
- 23.Lima LM, Carvalho MD, Sousa Mde O. Plasminogen and fibrinogen plasma levels in coronary artery disease. Revista brasileira de hematologia e hemoterapia. 2012;34:298–301. doi: 10.5581/1516-8484.20120075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortellaro M, et al. The PLAT Study: hemostatic function in relation to atherothrombotic ischemic events in vascular disease patients. Principal results. PLAT Study Group. Progetto Lombardo Atero-Trombosi (PLAT) Study Group. Arteriosclerosis and thrombosis: a journal of vascular biology. 1992;12:1063–1070. doi: 10.1161/01.ATV.12.9.1063. [DOI] [PubMed] [Google Scholar]
- 25.Thompson SG, et al. Antithrombin III and fibrinogen as predictors of cardiac events in patients with angina pectoris. Arteriosclerosis, thrombosis, and vascular biology. 1996;16:357–362. doi: 10.1161/01.ATV.16.3.357. [DOI] [PubMed] [Google Scholar]
- 26.Benderly M, et al. Fibrinogen is a predictor of mortality in coronary heart disease patients. The Bezafibrate Infarction Prevention (BIP) Study Group. Arteriosclerosis, thrombosis, and vascular biology. 1996;16:351–356. doi: 10.1161/01.ATV.16.3.351. [DOI] [PubMed] [Google Scholar]
- 27.Sumaya W, et al. Fibrin clot properties independently predict adverse clinical outcome following acute coronary syndrome: a PLATO substudy. European heart journal. 2018;39:1078–1085. doi: 10.1093/eurheartj/ehy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drinane MC, Sherman JA, Hall AE, Simons M, Mulligan-Kehoe MJ. Plasminogen and plasmin activity in patients with coronary artery disease. Journal of thrombosis and haemostasis: JTH. 2006;4:1288–1295. doi: 10.1111/j.1538-7836.2006.01979.x. [DOI] [PubMed] [Google Scholar]
- 29.Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing research reviews. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Pang PT, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 31.Jiang H, et al. The serum protein levels of the tPA-BDNF pathway are implicated in depression and antidepressant treatment. Translational psychiatry. 2017;7:e1079. doi: 10.1038/tp.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbel JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within synthetic matrix. Proceeding of the National Academy of Sciences of the United States of America. 2013;110:4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amadio, P., Sandrini, L., Ieraci, A., Tremoli, E. & Barbieri, S. S. Effect of Clotting Duration and Temperature on BDNF Measurement in Human Serum. International journal of molecular sciences18, 10.3390/ijms18091987 (2017). [DOI] [PMC free article] [PubMed]
- 34.Konings J, et al. Factor XIIa regulates the structure of the fibrin clot independently of thrombin generation through direct interaction with fibrin. Blood. 2011;118:3942–3951. doi: 10.1182/blood-2011-03-339572. [DOI] [PubMed] [Google Scholar]
- 35.Hooper JM, et al. Thyroid dysfunction and fibrin network structure: a mechanism for increased thrombotic risk in hyperthyroid individuals. The Journal of clinical endocrinology and metabolism. 2012;97:1463–1473. doi: 10.1210/jc.2011-2894. [DOI] [PubMed] [Google Scholar]
- 36.Olorundare OE, Peyruchaud O, Albrecht RM, Mosher DF. Assembly of a fibronectin matrix by adherent platelets stimulated by lysophosphatidic acid and other agonists. Blood. 2001;98:117–124. doi: 10.1182/blood.V98.1.117. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Yu Q, Xu CB. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. International journal of clinical and experimental medicine. 2017;10:14904–14910. [Google Scholar]
- 38.Huang D, Chen H, Hu X, Wang X, Wang H. Identification of a novel splicing mutation in the fibrinogen gamma chain gene leading to dysfibrinogenaemia in a Chinese pedigree. Pathology. 2015;47:145–150. doi: 10.1097/PAT.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 39.Sinauridze EI, Gorbatenko AS, Seregina EA, Lipets EN, Ataullakhanov FI. Moderate plasma dilution using artificial plasma expanders shifts the haemostatic balance to hypercoagulation. Scientific reports. 2017;7:843. doi: 10.1038/s41598-017-00927-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karapetian H. Reptilase time (RT) Methods Mol Biol. 2013;992:273–277. doi: 10.1007/978-1-62703-339-8_20. [DOI] [PubMed] [Google Scholar]
- 41.Kreutz RP, et al. Fibrin clot strength measured by thrombelastography and outcomes after percutaneous coronary intervention. Thrombosis and haemostasis. 2017;117:426–428. doi: 10.1160/TH16-10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zubairova LD, et al. Circulating Microparticles Alter Formation, Structure, and Properties of Fibrin Clots. Scientific reports. 2015;5:17611. doi: 10.1038/srep17611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takashio S, et al. Significance of low plasma levels of brain-derived neurotrophic factor in patients with heart failure. The American journal of cardiology. 2015;116:243–249. doi: 10.1016/j.amjcard.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Schoergenhofer C, et al. The use of frozen plasma samples in thromboelastometry. Clinical and experimental medicine. 2017;17:489–497. doi: 10.1007/s10238-017-0454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schols SE, et al. Effects of plasma dilution on tissue-factor-induced thrombin generation and thromboelastography: partly compensating role of platelets. Transfusion. 2008;48:2384–2394. doi: 10.1111/j.1537-2995.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 46.Kadowaki S, et al. Additive clinical value of serum brain-derived neurotrophic factor for prediction of chronic heart failure outcome. Heart and vessels. 2016;31:535–544. doi: 10.1007/s00380-015-0628-6. [DOI] [PubMed] [Google Scholar]
- 47.Hildreth V, Anderson RH, Henderson DJ. Autonomic innervation of the developing heart: origins and function. Clin Anat. 2009;22:36–46. doi: 10.1002/ca.20695. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Sun L, Huan Y, Zhao H, Deng J. Application of bFGF and BDNF to improve angiogenesis and cardiac function. The Journal of surgical research. 2006;136:85–91. doi: 10.1016/j.jss.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 49.Kermani P, et al. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. The Journal of clinical investigation. 2005;115:653–663. doi: 10.1172/JCI22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira M, et al. The incorporation of fibrinogen into extracellular matrix is dependent on active assembly of a fibronectin matrix. Journal of cell science. 2002;115:609–617. doi: 10.1242/jcs.115.3.609. [DOI] [PubMed] [Google Scholar]
- 51.Ajjan R, et al. Common variation in the C-terminal region of the fibrinogen beta-chain: effects on fibrin structure, fibrinolysis and clot rigidity. Blood. 2008;111:643–650. doi: 10.1182/blood-2007-05-091231. [DOI] [PubMed] [Google Scholar]
- 52.Wypasek E, et al. Fibrinogen beta-chain -C148T polymorphism is associated with increased fibrinogen, C-reactive protein, and interleukin-6 in patients undergoing coronary artery bypass grafting. Inflammation. 2012;35:429–435. doi: 10.1007/s10753-011-9332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenhalgh KA, et al. BbetaArg448Lys polymorphism is associated with altered fibrin clot structure and fibrinolysis in type 2 diabetes. Thrombosis and haemostasis. 2017;117:295–302. doi: 10.1160/TH16-07-0554. [DOI] [PubMed] [Google Scholar]
- 54.Wiedemann D, et al. The fibrin-derived peptide Bbeta(15-42) significantly attenuates ischemia-reperfusion injury in a cardiac transplant model. Transplantation. 2010;89:824–829. doi: 10.1097/TP.0b013e3181ccd822. [DOI] [PubMed] [Google Scholar]
- 55.Edsall JT, Lever WF. Effects of ions and neutral molecules on fibrin clotting. The Journal of biological chemistry. 1951;191:735–756. [PubMed] [Google Scholar]
- 56.Pratt KP, Cote HC, Chung DW, Stenkamp RE, Davie EW. The primary fibrin polymerization pocket: three-dimensional structure of a 30-kDa C-terminal gamma chain fragment complexed with the peptide Gly-Pro-Arg-Pro. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7176–7181. doi: 10.1073/pnas.94.14.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Cera E. Thrombin as procoagulant and anticoagulant. Journal of thrombosis and haemostasis: JTH. 2007;5(Suppl 1):196–202. doi: 10.1111/j.1538-7836.2007.02485.x. [DOI] [PubMed] [Google Scholar]
- 58.Esmon CT. Molecular events that control the protein C anticoagulant pathway. Thrombosis and haemostasis. 1993;70:29–35. [PubMed] [Google Scholar]
- 59.Tsuchimine S, Sugawara N, Ishioka M, Yasui-Furukori N. Preanalysis storage conditions influence the measurement of brain-derived neurotrophic factor levels in peripheral blood. Neuropsychobiology. 2014;69:83–88. doi: 10.1159/000358061. [DOI] [PubMed] [Google Scholar]
- 60.Sandrini, L. et al. Association between Obesity and Circulating Brain-Derived Neurotrophic Factor (BDNF) Levels: Systematic Review of Literature and Meta-Analysis. International journal of molecular sciences19, 10.3390/ijms19082281 (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.