Abstract
Borderline personality disorder (BPD) is characterized by impairments in the cognitive control of negative information. These impairments in cognitive control are presumably due to blunted activity of the dorsolateral prefrontal cortex (dlPFC) along with enhanced activations of the limbic system. However, the impact of an excitatory stimulation of the dlPFC still needs to be elucidated. In the present study, we therefore assigned 50 patients with BPD and 50 healthy controls to receive either anodal or sham stimulation of the right dlPFC in a double-blind, randomized, between-subjects design. Participants performed a delayed working memory task with a distracter period during which a grey background screen, or neutral, or negative stimuli were presented. This experimental paradigm was first evaluated in a pilot study with 18 patients with BPD and 19 healthy controls. In both studies, patients with BPD showed an impairment of cognitive control when negative distracters were presented in the delay period of a working memory task. However, excitatory stimulation of the right dlPFC did not ameliorate cognitive control of negative stimuli in BPD, which raises questions about the specific role of the right dlPFC for the understanding of BPD psychopathology. Methodological limitations are discussed.
Introduction
Borderline personality disorder (BPD) is a serious mental disorder characterized by affective disturbances, impulsivity, self-injury, and chronic suicidal tendencies1,2. Of particular interest for the understanding of BPD psychopathology are impairments in cognitive control that allow individuals to process and maintain goal-relevant information, while adapting flexibly to changing environmental demands. Cognitive control is particularly important when individuals are presented with salient but irrelevant information that distracts resources from their current tasks, such as the presence of emotionally evocative information. Individual differences in the ability to control such irrelevant information are associated with different aspects of psychosocial functioning and mental health3–6.
A multitude of experimental studies investigated cognitive control in BPD. Experimental studies have hitherto mostly illustrated that patients with BPD do not show general deficits in cognitive control e.g.7–9. It was rather suggested that pronounced impairments in the cognitive control of negative affective material are characteristic for BPD. For instance, patients with BPD show an impaired inhibition of negatively valenced material in comparison to healthy controls10,11. Additional findings suggest that patients with BPD are more susceptible to interference from negative, schema-related stimuli12,13. The presentation of such negative distracting information was found to enhance response latencies or decrease accuracy scores in patients with BPD compared to healthy controls9,14,15. However, findings of an enhanced interference of negative stimuli with cognitive processes in BPD are not unequivocal. Several experimental studies found no valence-specific effects of emotional distracters on cognitive control10,16,17.
Functional imaging studies elucidated the neural basis of impaired cognitive control of negative material in BPD. These studies illustrated congruently prefrontal dysfunctions in orbitofrontal and dorsolateral regions of patients with BPD compared to healthy controls16–22. A recent meta-analysis concluded that BPD patients’ impairments in the cognitive control of negative stimuli are presumably the result of blunted activity of the dorsolateral prefrontal cortex (dlPFC) along with enhanced activation of the limbic system23.
However, despite the centrality of dlPFC abnormalities for neurobiological models of BPD24, no study to date has investigated the behavioral effects of an excitatory stimulation of this brain region in BPD. Transcranial direct current stimulation (tDCS) represents a simple and presumably effective way to alter cortical brain activity25–27. Beneficial effects of excitatory dlPFC stimulation on executive functioning have been reported for healthy and clinical samples28. Notably, experimental studies have also provided promising results that excitatory stimulation of the dlPFC ameliorates cognitive control of aversive stimuli not only in healthy controls29, but also in patients with major depression30.
In the present study, we investigated whether excitatory stimulation of the right dlPFC (compared to a sham condition) results in an amelioration of cognitive control of negative stimuli in BPD. To this end, participants performed a delayed working memory task with a distracter period during which either a grey background screen, or neutral, or negative stimuli were presented. This task was first evaluated in a pilot study (Study 1). We hypothesized that negative distracters result in prolonged response latencies in patients with BPD compared to healthy controls. The main study (Study 2) assessed the effects of anodal stimulation of the right dlPFC in patients with BPD and healthy controls. We expected an amelioration of cognitive control of negative stimuli in BPD during excitatory stimulation of the right dlPFC compared to a sham condition.
Study 1
Research questions
This pilot study investigated whether the presentation of negative distracters in a delayed working memory task interferes with behavioral performance in patients with BPD9,14. More specifically, we expected prolonged response latencies in patients with BPD compared to healthy controls. Furthermore, we explored whether valence-dependent interference with behavioral performance in patients with BPD is modulated by the length of the distracter presentation (i.e. interference duration).
Methods
Participants
We enrolled a convenience sample of 20 patients with borderline personality disorder and 20 healthy controls in this study. One patient did not finish the experimental paradigm. Another patient and one healthy control had a general hit rate below 65% indicating insufficient engagement in the experimental task; both participants were excluded from the analysis. Thus, the final sample comprised 18 patients with BPD and 19 healthy controls.
Healthy controls were recruited via public advertising. BPD patients were recruited at the Department of Psychiatry, Charité - Universitätsmedizin Berlin. At the time of study participation, patients were part of a specialized psychotherapeutic inpatient treatment program for BPD. These patients were on a waiting list prior to treatment and none of them was admitted for acute psychiatric care. All participants underwent diagnostic interviews with German versions of the Mini-International Neuropsychiatric Interview for DSM-IV Axis-I Mental Disorders31 and the Structured Clinical Interview for DSM-IV Axis-II Personality Disorders32. Clinical psychologists holding a master’s degree in psychology conducted the clinical interviews. Interviewers were trained in the use of these instruments and supervised by the senior author. We did assess interrater reliabilities of this procedure for SCID-II personality disorder diagnoses in our research group33. We found acceptable interrater reliabilities of κ = 0.82 for a diagnosis of BPD, and acceptable internal consistencies with Cronbach’s α = 0.88 for the sum of BPD criteria. Participants recruited via media advertisements were initially screened by telephone, before undergoing the clinical interview in the lab directly before the experiment. Furthermore, participants were screened regarding basic cognitive abilities (LPS-4)34. Healthy controls were only included if they did not take any psychotropic medication and had neither a current nor a lifetime diagnosis of any mental or neurological disorders (e.g., traumatic diseases of the central nervous system). Exclusion criteria for BPD patients were comorbid diagnosis of past or present psychotic disorder, bipolar disorder, cognitive disorders (e.g., delirium, dementia), or neurological disorders as well as substance-associated disorders within three months prior to study participation.
Patients with BPD and healthy controls did not differ with regard to basic socio-demographic variables, such as age (BPD: M = 29.67, SD = 8.85; HC: M = 33.05, SD = 7.15; W = 118.5, p = 0.11), gender (BPD: 15 female, 3 male; HC: 18 female, 1 male; p = 0.34, Fisher’s exact test), and intellectual abilities (raw score LPS-4, BPD: M = 26.11, SD = 5.04; HC: M = 26.32, SD = 3.30; t(29.08) = 0.15, p = 0.89). A total of 13 patients received psychotropic medication at the time of the study. The most frequent (n > 2) comorbid mental disorders in our sample were major depression (n = 6), posttraumatic stress disorder (n = 4), panic disorder (n = 4) as well as avoidant personality disorder (n = 4), and antisocial personality disorder (n = 3).
All participants gave written informed consent prior to participation. The ethics committee of the Charité-Universitätsmedizin Berlin approved the study protocol. The experiment was performed in accordance with the Declaration of Helsinki. The study took place at the Department of Psychiatry, Charité-Universitätsmedizin Berlin between February 2013 and July 2013.
Experimental Paradigm
Participants performed a delayed working memory task14,35. Each trial started with a fixation cross (1000 ms), followed by the presentation of six target letters (1500 ms), which participants were asked to memorize. After a variable distracter period (i.e., interference duration of 1000, 2000, or 4000 ms), participants were presented a recognition display (until a response was made) and had to decide whether the presented letter was part of the initial set of letters. In half of the trials, the recognition display contained a previously presented target. Participants were asked to respond as quickly and accurately as possible.
The distracter period of the experimental paradigm was manipulated with regard to the factors valence (grey background screen, or neutral, or negative stimuli) and interference duration (1000, 2000, or 4000 ms). Neutral and negative affective stimuli were selected from the International Affective Picture System36. Valence ratings (rated from 1 - very negative to 9 - very positive) were M = 5.01, SD = 0.35 for neutral stimuli, and M = 2.33, SD = 0.42 for negative stimuli. Arousal ratings (rated from 1 - not arousing at all to 9 - highly arousing) were M = 3.75, SD = 0.86 for neutral stimuli, and M = 5.86, SD = 0.82 for negative stimuli. Neutral and negative stimuli were matched with regard to luminance and visual complexity as determined from jpeg size in bytes (all p’s > 0.40)37.
The experiment contained 180 trials, divided into nine blocks with 20 trials each. Each block contained a unique experimental condition (e.g., negative stimuli presented for 1000 ms). Visual stimuli in these blocks were matched regarding valence, arousal, luminance, and visual complexity (all p’s > 0.55). Experimental blocks were presented in pseudo-random order.
The experiment was conducted on a standard notebook connected with a 15-inch screen (screen resolution of 1024 × 768). We also recorded participants’ eye movements and skin conductance, but data will not be reported here. Presentation of visual stimuli and collection of behavioral data was realized using PsychoPy38. All participants underwent a training session to familiarize them with the experimental task.
Statistical Analyses
Reaction times (RT) from erroneous responses and below <300 ms were filtered. Next, response latencies below or above three times the interquartile range from each individual’s median value in each experimental condition were excluded from the analysis (BPD: 15.2%, HC: 13.5%). Based on the remaining trials, median response times were calculated for each condition.
It was also analyzed whether the percentage of accurate responses and response latencies for accurate responses were correlated. There was no significant association in the control group (r = 0.05, p = 0.75), but we found a negative correlation of r = −0.38, p = 0.03 in the BPD group. Less accurate responding was associated with longer response latencies in BPD participants.
Our primary analyses focused on response times as a dependent variable. First, RTs were subjected to a mixed-design analysis with the within-subject factors valence and interference duration, and the between-subject factors group. Significant interactions were followed by simple effect analyses. Second, response latencies from neutral conditions were subtracted from response latencies for negative conditions. The respective difference score was entered into a Welch t-test with the factor group and subsequent one-sample t-tests per group. Finally, condition-wise hit rates were analyzed.
All analyses were conducted with the System for Statistical Computation and Graphics R39, applying the packages afex40, and emmeans41. Statistical tests were conducted at a 5% significance level. Note, we applied Greenhouse-Geisser corrections regardless of violations of sphericity.
Data availability
The full data set, syntax, and statistical results are available at https://osf.io/g43bh/iew_only=f647fa67773041669f0a670c234dd150.
Results
Response Times
As hypothesized, our analysis highlighted a significant interaction of valence and diagnostic group (F(1.90, 66.57) = 3.84, p = 0.03, generalized η2 = 0.01). Follow-up tests of estimated marginal means showed enhanced response latencies in patients with BPD compared to the control group only when negative stimuli were presented (t(48.93) = 2.42, p = 0.02, r = 0.33; BPD: M = 1383 ms, SE = 52; HC: M = 1206 ms, SE = 51). No group differences were observed for neutral stimuli or the control condition (all p’s > 0.21).
Except for a significant main effect of valence (F(1.90, 66.57) = 13.05, p < 0.001, generalized η2 = 0.04), there were no further main effects or interactions in this analysis (all p’s > 0.14). Descriptive results are presented in Supplementary Table 1.
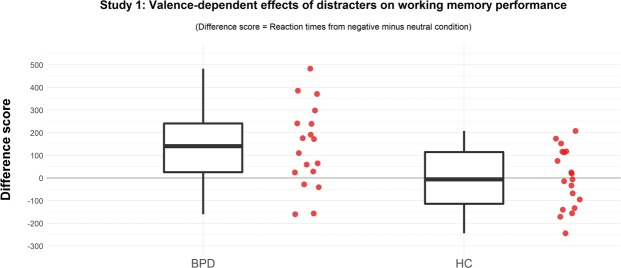
Our second (complementary) analysis illustrated that patients with BPD show a stronger interference of negative stimuli (in comparison to healthy controls) even when controlling for response latencies to neutral stimuli (t(30.83) = 2.68, p = 0.008, r = 0.43). This valence-dependent increase in response latencies differed significantly from zero in patients with BPD (M: 137 ms [SD: 181], t(17) = 3.20, p < 0.001), but not in healthy controls (M: −3 ms [SD: 131], t(18) = −0.10, p = 0.92). See Fig. 1 for a visualization.
Figure 1.
Separate boxplots and individual results of response latencies for trials with negative distracters controlled for response latencies to neutral stimuli for patients with BPD and healthy controls.
Hit Rates
Analyses of hit rates showed that accuracy scores were significantly lower in the BPD group compared to healthy controls (F(1,35) = 4.60, p = 0.04, generalized η2 = 0.06; BPD: M = 78.56%, SE = 16.93; HC: M = 83.70%, SE = 16.93). Furthermore, there was a main effect of interference duration (F(1.52,53.25) = 8.03, p = 0.002, generalized η2 = 0.03). Accuracy scores were significantly higher for 1000 ms (M = 83.41%, SE = 13.44) compared to 2000ms (M = 80.76, SE = 13.44, p = 0.04), and 4000 ms (M = 79.21, SE = 13.44, p < 0.001), whereas accuracy for 2000 ms and 4000 ms did not differ significantly (p = 0.45). Descriptive results are presented in Supplementary Table 2.
Summary of Study 1
In line with our hypotheses, the main analyses of response latencies showed that patients with BPD were more easily distracted by negative stimuli than healthy controls. We did not find empirical evidence that this valence-dependent interference with working memory processes in BPD was further modulated by the length of the interference duration. With respect to accuracy rates, we observed that BPD patients were less accurate than healthy controls and that hit rates were lower for longer presentation times.
In sum, our results reinforce previous findings that patients with BPD are more susceptible to interference by negative stimuli.
Study 2
Research questions
The results of our pilot study demonstrate that the experimental paradigm is able to assess valence-dependent impairments of cognitive control in BPD. In Study 2, we thus investigated whether excitatory stimulation of the right dlPFC (compared to a sham condition) results in an attenuation of this valence-dependent interference effect in patients with BPD, i.e., we examined the three-way interaction of valence by group and stimulation.
Methods
Participants
Fifty in- and outpatients with BPD and 50 healthy controls matched for age, gender, and intelligence were enrolled in this study. Power analyses yielded a total sample size of 80 individuals for the detection of a significant interaction with an assumed effect size of 0.06 (partial η2 = 0.06, f(U) = 0.25) and a power of 80%. To account for potential data loss, we aimed for a sample size of 25 individuals per group (in total 100 participants). Two patients had a mean hit rate below 65% and were excluded from all statistical analyses. Thus, the final sample comprised 48 patients with BPD and 50 healthy controls. Demographic and clinical characteristics are presented in Table 1.
Table 1.
Demographic and clinical characteristics of BPD patients and control participants, separated by sham and verum stimulation of the right dorsolateral prefrontal cortex.
| Borderline personality disorder | Healthy controls | Statistics | |||
|---|---|---|---|---|---|
| Sham stimulation (n = 25) | Verum stimulation (n = 23) | Sham stimulation (n = 26) | Verum stimulation (n = 24) | ||
| Demographical characteristics | |||||
| Age | 32.56 (8.57) | 31.61 (8.50) | 30.50 (7.45) | 32.29 (8.41) | all p’s > 0.40a |
| LPS-4 | 28.60 (4.92) | 26.26 (5.88) | 26.88 (5.15) | 27.63 (5.76) | all p’s > 0.15a |
| Gender | 2 male, 23 female | 2 male, 21 female | 3 male, 23 female | 2 male, 22 female | all p’s > 0.65b |
| Medication intake | 16 yes, 9 no | 12 yes, 11 no | p > 0.55c | ||
| Clinical characteristics | |||||
| BSL-95 | 2.12 (0.57) | 2.02 (0.77) | 0.29 (0.17) | 0.41 (0.35) | p < 0.001a (diagnostic group) |
| BDI | 25.38 (9.51) | 26.52 (11.79) | 2.88 (3.68) | 3.63 (4.84) | p < 0.001a (diagnostic group) |
| GSI | 2.03 (0.72) | 1.94 (0.75) | 0.17 (0.19) | 0.25 (0.32) | p < 0.001a (diagnostic group) |
| ALS | 74.52 (25.96) | 66.02 (27.87) | 139.04 (24.36) | 134.53 (24.22) | p < 0.001a (diagnostic group) |
| DERS | 132.36 (20.49) | 124.22 (22.48) | 61.50 (11.28) | 65.69 (16.13) | p < 0.001a (diagnostic group) |
ALS – Affective Lability Scale, BDI – Beck Depression Inventory, BSL-95 – Borderline Symptom List, DERS – Difficulties in emotion regulation scale, GSI – Global Severity Index, LPS-4 - Leistungspruefsystem, subtest 4.
aBased on an univariate general linear model with the factors: group (BPD and HC), and stimulation (sham and verum); bbased on a loglinear analysis with the factors group (BPD and HC), and stimulation (sham and verum) – please note the main effect of gender is significant (p < 0.001); cbased on Pearsons Chi-Square-Test with the factor stimulation (sham and verum)
Healthy controls and patients with BPD were recruited via public advertising. BPD patients were also recruited at the Department of Psychiatry, Charité – Universitätsmedizin, Berlin.
All participants underwent diagnostic screening with German versions of the Structured Clinical Interview for DSM-IV Axis-I Mental Disorders and Axis-II Personality Disorders32,42. Clinical psychologists holding at least a bachelor’s degree in psychology conducted the clinical interviews. Interviewers were trained in the use of these instruments and supervised by the senior author. We did assess interrater reliabilities of this procedure for SCID-II personality disorder diagnoses in our research group33. We found acceptable interrater reliabilities of κ = 0.82 for a diagnosis of BPD, and acceptable internal consistencies with Cronbach’s α = 0.88 for the sum of BPD criteria. Participants recruited via media advertisements were initially screened by telephone, before undergoing the clinical interview in the lab directly before the experiment.
We used the exclusion criteria applied in Study 1, but additionally excluded participants with possible tDCS contraindications, such as a cardiac pacemaker, metal in or around the head, pregnancy, or tattoos or scarred skin on the scalp or left deltoid muscle. Furthermore, BPD patients with a current diagnosis of a major depressive episode were also excluded from study participation. The most frequent current comorbid mental disorders (n > 2) were posttraumatic stress disorder (n = 19), eating disorders including anorexia and bulimia nervosa (n = 14), social anxiety disorder (n = 4), substance abuse (n = 3), and paranoid personality disorder (n = 3).
All participants gave written informed consent prior to participation. The ethics committee of the Charité-Universitätsmedizin Berlin approved the study protocol. The experiment was performed in accordance with relevant guidelines and regulations. The study took place at the Department of Psychiatry, Charité-Universitätsmedizin Berlin between January 2016 and June 2017.
Transcranial Direct Current Stimulation
Participants were pseudo-randomly assigned to receive either sham or verum stimulation of the right dorsolateral prefrontal cortex within a double-blind, between-subjects design. Direct electrical current was applied by a saline-soaked pair of surface sponge electrodes with a surface of 35 mm² connected to a battery-driven constant current stimulator (DC-Stimulator, NeuroConn GmbH, Ilmenau, Germany). For anodal stimulation of the right dlPFC, the electrode was positioned over F4 according to the 10–20 international system for EEG electrode placement43. The cathode was placed on the left deltoid muscle.
During active stimulation a constant current of 1.0 mA was applied for the duration of the experimental paradigm (or a maximum of 20 minutes). To mimic the sensation of tDCS in the sham condition, the current was ramped up and down for 30 seconds respectively at the beginning and end of the experimental session. In the sham condition the stimulator was turned off during the experiment. The stimulation device contained a study mode for double-blind trials. The principal investigator generated numeric codes for active and sham stimulation sessions prior to the experimental sessions. Sequences were generated with in-house functions based on randperm (Matlab). The experimenter entered these preassigned codes and was unaware of the experimental condition.
Participants were asked for the presence of possible side effects of the stimulation. Statistical analyses showed that perception of tingling or burning sensations, pain under the electrodes, light flashes during the stimulation, or headaches and nausea after the stimulation did not differ between sham and verum stimulation of the right dlPFC (all p’s > 0.1). A subsample (n = 44) was asked to guess which stimulation condition they were assigned to. Participants mainly assumed to have received verum stimulation of the right dlPFC (sham condition: 70.83%, verum condition: 75.00%), but groups did not differ significantly.
Experimental Paradigm
We used the same experimental procedures as described in Study 1.
Statistical Analyses
We used the same statistical procedures as described in Study 1. In short, median response times were calculated after outlier correction (BPD sham: 14.7%, BPD verum: 13.9%, HC sham: 13.4%, HC verum: 13.4%). There were no significant correlations between the percentage of accurate responses and response latencies for accurate responses in the experimental groups (r’s: −0.04 to 0.01, p’s: 0.78–1).
RTs were subjected to a mixed-design analysis with the within-subject factors valence and interference duration, and the between-subject factors group and stimulation. Furthermore, difference scores (negative - neutral condition) were entered into a univariate analysis with the factors stimulation and diagnostic group. Finally, accuracy rates were analyzed with a mixed-design analysis comprising the within-subject factors valence and interference duration, and the between-subject factors group and stimulation.
Data Availability
Hypotheses, sample size, exclusion criteria (i.e., based on general accuracy scores), and statistical analyses were pre-registered. The pre-registration, full data set, syntax, and statistical results are available at https://osf.io/g43bh/?view_only=f647fa67773041669f0a670c234dd150. At the request of this journal, Study 2 was registered as a clinical trial after the initial submission of the manuscript (16/08/2018; clinicaltrials.gov: NCT03636139). This also applies to the research protocol and the CONSORT checklist provided in the supplementary materials.
Results
Main analyses
Response Times: As predicted, the analysis showed a significant interaction of valence and diagnostic group (F(1.93, 180.97) = 5.38, p = 0.006, generalized η2 = 0.006). Follow-up tests of estimated marginal means showed that BPD patients were significantly slower than healthy controls when presented with negatively valenced stimuli (t(116.75) = 2.54, p = 0.012, r = 0.23). No group differences were found for neutral stimuli or the baseline condition (all p’s > 0.26).
In contrast to our hypothesis, this valence by diagnostic group effect was not further modulated by an excitatory stimulation of the right dlPFC (F(1.93, 180.97) = 1.18, p = 0.31, generalized η2 = 0.001). Rather we found a significant, but unpredicted, three-way interaction of interference duration, diagnostic group, and stimulation (F(1.97, 185.35) = 4.24, p = 0.02, generalized η2 = 0.003). Follow-up tests showed neither significant differences between diagnostic groups at specific interference durations (all p’s > 0.09), nor between stimulation conditions (all p’s > 0.14). Furthermore, main effects of valence (F(1.93, 180.97) = 31.49, p < 0.001, generalized η2 = 0.03), and interference duration (F(1.97, 185.35) = 14.83, p < 0.001, generalized η2 = 0.009) were significant. Descriptive results are presented in Supplementary Table 3.
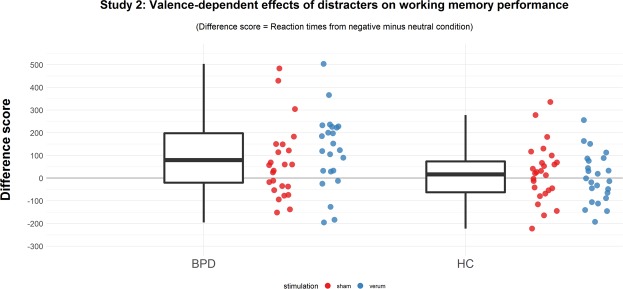
The secondary analysis of individual difference scores showed again a stronger interference of negative stimuli in patients with BPD (in comparison to healthy controls) even when controlling for response latencies to neutral stimuli (F(1,94) = 8.60, p = 0.004, generalized η2 = 0.08, see Fig. 2). This additional analysis yielded neither support for a general effect of stimulation nor an interaction of stimulation by diagnostic group (all p’s > 0.47). The valence-dependent increase in response latencies differed significantly from zero in patients with BPD (M: 105 ms [SD: 190], t(47) = 3.85, p < 0.001), but not in healthy controls (12.5 ms [SD: 118], t(49) = 0.75, p = 0.46).
Figure 2.
Separate boxplots and individual results of response latencies for trials with negative distracters controlled for response latencies to neutral stimuli for patients with BPD and healthy controls. Individual results are presented separately for the stimulation condition. Please note, one participant had a value of 831 ms and is not presented in the figure. Findings remain significant when excluding this individual from data analysis.
Hit Rates: Compared to healthy controls, percentage hit rates of the BPD sample was significantly lower in the delayed working memory task (BPD: 82.28 (10.03), HC: 85.57 (10.07); F(1,94) = 5.06, p = 0.03, generalized η2 = 0.03). Results also showed a main effect of valence (F(1.96, 183.88) = 9.75, p < 0.001, generalized η2 = 0.02). Accuracy scores were significantly higher for the control condition (85.63 [10.20]) compared to neutral (83.66 [10.27], p = 0.02), and negative (82.59 [10.27], p < 0.001) stimuli. Accuracy did not differ between neutral and negative stimuli (p > 0.37).
There were no further significant main effects or interactions of the experimental factors (all p’s > 0.07). Descriptive results are presented in Supplementary Table 4.
Additional exploratory analyses
BPD groups only: We repeated the statistical analyses presented above, but focused exclusively on patients with BPD. Thus, we did not include the samples of healthy controls in the analyses presented below.
Analyses with response latencies as dependent variable yielded a significant main effect of valence (F(1.73, 79.42) = 22.09, p < 0.001, generalized η2 = 0.04) and interference duration (F(1.82, 83.61) = 6.64, p = 0.003, generalized η2 = 0.008) as well as a significant interaction of tDCS stimulation and interference duration (F(3.53, 162.35) = 5.42, p = 0.008, generalized η2 = 0.007). Follow-up tests showed no significant differences between stimulation conditions at specific interference durations (all p’s > 0.15). The secondary analysis of individual difference scores (negative – neutral) did not show a significant difference between sham and verum conditions (t(44.79) = −0.48, p = 0.64).
Analyses with accuracy rates as dependent variable yielded only a significant main effect of valence (F(1.92, 88.43) = 3.47, p < 04, generalized η2 = 0.01).
Summary
The results show again that negative distracters impair cognitive control in BPD. However, in contrast to our hypotheses, excitatory stimulation of the right dlPFC did not significantly attenuate valence-dependent impairments of cognitive control in patients with BPD.
Discussion
In this project, we investigated the effects of negative valence as well as of an excitatory stimulation of the right dlPFC on cognitive control in BPD. As predicted, patients with BPD showed an impairment of cognitive control when negative distracters were presented in the delay period of a working memory task. However, in contrast to our hypotheses, excitatory stimulation of the right dlPFC did not ameliorate cognitive control of negative stimuli in BPD.
Cognitive control in BPD
Response latencies of the BPD group differed significantly from healthy controls only when negative distracters were presented, whereas no group differences were observed during the control condition (i.e. grey background screen) or the presentation of neutral distracters. It is noteworthy that we observed this pattern in two different study samples. Our results reinforce claims that patients with BPD do not exhibit general deficits in cognitive control, but are best characterized by circumscribed impairments in the cognitive control of negatively valenced material.
Notably, enhanced response latencies during the presentation of negative compared to neutral distracters were exclusively found in patients with BPD (contrast negative - neutral, Study 1: M = 137 ms; Study 2: M = 105 ms). Healthy controls did not show valence-dependent behavioral effects (contrast negative - neutral, Study 1: M = −3 ms; Study 2: M = 12.5 ms). This is in line with a recent meta-analysis of the effects of affective information on working memory performance44. In that study, only negligible effects of affective task-irrelevant distracters on working memory performance in healthy individuals were found. In contrast, affective stimuli had substantially larger effects in individuals with mental health problems44. Negative affective distracters seem to bind cognitive resources in psychopathology, and consequentially impact task-relevant processes.
It remains unclear whether such valence-dependent impairments of cognitive control are uniform or diverse across different forms of psychopathology. This is due to the fact that most experimental studies do not compare subjects with different mental disorders. Rather, and admittedly like our work presented here, most studies compared healthy controls and patients with a specific form of psychopathology (e.g., ADHD, BPD, depression). We decided against inclusion of a clinical control group, since our studies focused exclusively on the replication of impaired cognitive control of negative material in BPD, and the modulation of this effect by means of transcranial direct current stimulation. Future studies are needed to provide empirical answers to questions of disorder-specificity or which specific forms of psychopathology, like repetitive negative thinking, or impairments in daily emotion regulation, are associated with valence-dependent impairments of cognitive control45. Such studies might also help to disentangle the role of more general factors, such as mental distress. With regard to disorder-specificity, previous studies compared different facets of behavioral impulsivity in BPD and patients with ADHD46,47. Recent reviews of these studies concluded that impulsivity in ADHD reflects deficits in general behavioral inhibition, whereas impulsivity in BPD is mainly driven by affective and interpersonal aspects for reviews see22,48.
Effects of transcranial direct current stimulation
Excitatory stimulation of the right dlPFC did neither ameliorate cognitive control of negative stimuli in patients with BPD, nor in the control group. The lack of a stimulation effect in the control group was expected, since these participants usually do not show a valence-dependent modulation of cognitive control in experimental paradigms. However, previous findings led us to assume a modulatory effect of excitatory stimulation of the right dlPFC on the cognitive control of negative stimuli in BPD. This assumption was based on a number of functional neuroimaging studies, which highlighted blunted activity of the dorsolateral prefrontal cortex during negative emotion processing in BPD23. A recent update of that earlier meta-analysis yielded again an attenuated functioning of the right dlPFC in patients with BPD49. However, this specific prefrontal abnormality might not be consistently replicable in experimental studies as suggested by the additional results of a robustness analysis (i.e. Jackknife analysis). Future studies should are needed to assess which experimental paradigms or patient characteristics contribute to an attenuated activation of the dlPFC in patients with BPD.
There are some limitations of our study that should be considered in the interpretation of this null-finding. First, we used a between-subjects design. In other words, participants were randomly allocated to receive either sham or verum stimulation of the right dlPFC. The decision for a between-subjects design came at the cost of lower statistical power (compared to a within-subjects design), but had the benefit that there are no order or carryover effects between experimental sessions. Such confounds were previously reported in within-subjects studies of tDCS30. Our results indicate the absence of large or medium effect sizes with regard to a three-way interaction of valence by group and stimulation. An additional sensitivity analysis (assuming 80% power) yielded an effect size (Cohens D) of ≥0.73 (one-tailed) or ≥0.83 (two-tailed) for a significant group comparison between BPD patients with and without stimulation of the right dlPFC in the present study. Thus, substantially bigger sample sizes or within-subject designs would be needed to establish the absence or presence of smaller effects. Second, the cathodal electrode was positioned on the left deltoid muscle. Extracephalic positioning of the cathodal electrode allowed unambiguous interpretation of anodal tDCS, since results were not confounded by cathodal effects on another brain region. However, extracephalic positioning of the reference electrode affects the stimulation intensity effective at the dlPFC50. Future studies might consider to adapt stimulation intensity accordingly. Third, it remains an empirical question to explore whether left-lateralized or bilateral excitatory stimulation of the dlPFC might ameliorate cognitive control in BPD.
After the start of this study, questions were raised about the general effectiveness of tDCS for the manipulation of executive functioning51,52. For instance, Medina and Cason (2017) conclude that their analysis shows minimal evidence (at best) that tDCS influences working memory processes. The debate about the effectiveness of tDCS will be further fueled by a recent finding that about 75% of scalp-applied currents are attenuated by soft tissue and skull53. The authors of this work state that higher intensity currents than those of conventional protocols would be necessary to affect neuronal circuits. Further work is necessary to provide reliable manipulations of brain activity and to establish a causal role of specific brain abnormalities for the understanding of BPD-related psychopathology.
Conclusion
In sum, our results illustrate reliable impairments in the cognitive control of negatively valenced material in BPD. However, excitatory stimulation of the right dlPFC by means of tDCS did not ameliorate deficits in cognitive control of negative stimuli in patients with BPD. Further research is needed to understand the specific role of the right dlPFC in BPD.
Supplementary information
Supplementary Information: CONSORT checklist
Supplementary Information: Research Protocol
Acknowledgements
LS is supported by a grant from the German Research Foundation (DFG-SCHU 2961/2-1). We thank Isabelle Dühn and Anne Wentworth-Perry for assistance with data acquisition, and Marina Benoit as well as Anna Weinbrecht for valuable feedback on a previous version of this manuscript.
Author Contributions
L.S. and S.R. designed the study. S.T. programmed the experimental paradigm. M.G. contributed to implementation of the study and data collection. L.S. analyzed the data and wrote the first draft of the manuscript. M.G., B.R. and S.R. contributed to the interpretation of the data. All authors contributed to writing, reviewing and editing of the manuscript and approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37315-x.
References
- 1.Ansell EB, Sanislow CA, McGlashan TH, Grilo CM. Psychosocial impairment and treatment utilization by patients with borderline personality disorder, other personality disorders, mood and anxiety disorders, and a healthy comparison group. Compr. Psychiatry. 2007;48:329–336. doi: 10.1016/j.comppsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. Borderline personality disorder. Lancet Lond. Engl. 2004;364:453–461. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- 3.De Panfilis C, Meehan KB, Cain NM, Clarkin JF. The relationship between effortful control, current psychopathology and interpersonal difficulties in adulthood. Compr. Psychiatry. 2013;54:454–461. doi: 10.1016/j.comppsych.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Nigg JT, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- 5.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Zetsche U, Bürkner P-C, Schulze L. Shedding light on the association between repetitive negative thinking and deficits in cognitive control – A meta-analysis. Clin. Psychol. Rev. 2018;63:56–65. doi: 10.1016/j.cpr.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Barker V, et al. Impulsivity in borderline personality disorder. Psychol. Med. 2015;45:1955–1964. doi: 10.1017/S0033291714003079. [DOI] [PubMed] [Google Scholar]
- 8.Jacob GA, et al. Impulsivity in borderline personality disorder: impairment in self-report measures, but not behavioral inhibition. Psychopathology. 2010;43:180–188. doi: 10.1159/000304174. [DOI] [PubMed] [Google Scholar]
- 9.Prehn K, et al. Effects of emotional stimuli on working memory processes in male criminal offenders with borderline and antisocial personality disorder. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry. 2013;14:71–78. doi: 10.3109/15622975.2011.584906. [DOI] [PubMed] [Google Scholar]
- 10.Domes G, et al. The influence of emotions on inhibitory functioning in borderline personality disorder. Psychol. Med. 2006;36:1163–1172. doi: 10.1017/S0033291706007756. [DOI] [PubMed] [Google Scholar]
- 11.Korfine L, Hooley JM. Directed forgetting of emotional stimuli in borderline personality disorder. J. Abnorm. Psychol. 2000;109:214–221. doi: 10.1037/0021-843X.109.2.214. [DOI] [PubMed] [Google Scholar]
- 12.Arntz A, Appels C, Sieswerda S. Hypervigilance in borderline disorder: a test with the emotional Stroop paradigm. J. Personal. Disord. 2000;14:366–373. doi: 10.1521/pedi.2000.14.4.366. [DOI] [PubMed] [Google Scholar]
- 13.Sieswerda S, Arntz A, Mertens I, Vertommen S. Hypervigilance in patients with borderline personality disorder: specificity, automaticity, and predictors. Behav. Res. Ther. 2007;45:1011–1024. doi: 10.1016/j.brat.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Krause-Utz A, et al. Influence of emotional distraction on working memory performance in borderline personality disorder. Psychol. Med. 2012;42:2181–2192. doi: 10.1017/S0033291712000153. [DOI] [PubMed] [Google Scholar]
- 15.Krause-Utz A, et al. Susceptibility to Distraction by Social Cues in Borderline Personality Disorder. Psychopathology. 2014;47:148–157. doi: 10.1159/000351740. [DOI] [PubMed] [Google Scholar]
- 16.Holtmann J, et al. Trait anxiety modulates fronto-limbic processing of emotional interference in borderline personality disorder. Front. Hum. Neurosci. 2013;7:54. doi: 10.3389/fnhum.2013.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wingenfeld K, et al. Neural correlates of the individual emotional Stroop in borderline personality disorder. Psychoneuroendocrinology. 2009;34:571–586. doi: 10.1016/j.psyneuen.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Lang S, et al. Cognitive reappraisal in trauma-exposed women with borderline personality disorder. NeuroImage. 2012;59:1727–1734. doi: 10.1016/j.neuroimage.2011.08.061. [DOI] [PubMed] [Google Scholar]
- 19.Schulze L, et al. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biol. Psychiatry. 2011;69:564–573. doi: 10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Silbersweig D, et al. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am. J. Psychiatry. 2007;164:1832–1841. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- 21.Soloff PH, Abraham K, Ramaseshan K, Burgess A, Diwadkar VA. Hyper-modulation of brain networks by the amygdala among women with Borderline Personality Disorder: Network signatures of affective interference during cognitive processing. J. Psychiatr. Res. 2017;88:56–63. doi: 10.1016/j.jpsychires.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebastian A, et al. Frontal dysfunctions of impulse control - a systematic review in borderline personality disorder and attention-deficit/hyperactivity disorder. Front. Hum. Neurosci. 2014;8:698. doi: 10.3389/fnhum.2014.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulze L, Schmahl C, Niedtfeld I. Neural Correlates of Disturbed Emotion Processing in Borderline Personality Disorder: A Multimodal Meta-Analysis. Biol. Psychiatry. 2016;79:97–106. doi: 10.1016/j.biopsych.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Krause-Utz A, Winter D, Niedtfeld I, Schmahl C. The latest neuroimaging findings in borderline personality disorder. Curr. Psychiatry Rep. 2014;16:438. doi: 10.1007/s11920-014-0438-z. [DOI] [PubMed] [Google Scholar]
- 25.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/WNL.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 27.Nitsche MA, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimulat. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Dedoncker J, Brunoni AR, Baeken C, Vanderhasselt M-A. A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters. Brain Stimulat. 2016;9:501–517. doi: 10.1016/j.brs.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Feeser M, Prehn K, Kazzer P, Mungee A, Bajbouj M. Transcranial direct current stimulation enhances cognitive control during emotion regulation. Brain Stimulat. 2014;7:105–112. doi: 10.1016/j.brs.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Wolkenstein L, Plewnia C. Amelioration of cognitive control in depression by transcranial direct current stimulation. Biol. Psychiatry. 2013;73:646–651. doi: 10.1016/j.biopsych.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):34–57. [PubMed] [Google Scholar]
- 32.Fydrich, T., Renneberg, B., Schmitz, B. & Wittchen, H.-U. Strukturiertes Klinisches Interview für DSM-IV Achse II: Persönlichkeitsstörungen. (Hogrefe, 1997).
- 33.Ritter K, et al. Shame in patients with narcissistic personality disorder. Psychiatry Res. 2014;215:429–437. doi: 10.1016/j.psychres.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Horn, W. L. -P. -S. Leistungsprüfsystem. (Hogrefe, 1983).
- 35.Oei NYL, Tollenaar MS, Spinhoven P, Elzinga BM. Hydrocortisone reduces emotional distracter interference in working memory. Psychoneuroendocrinology. 2009;34:1284–1293. doi: 10.1016/j.psyneuen.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Lang, P. J., Bradley, M. M. & Cuthbert, B. N. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. (2008).
- 37.Donderi DC. Visual complexity: a review. Psychol. Bull. 2006;132:73–97. doi: 10.1037/0033-2909.132.1.73. [DOI] [PubMed] [Google Scholar]
- 38.Peirce JW. PsychoPy–Psychophysics software in Python. J. Neurosci. Methods. 2007;162:8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.R. Core Team. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, 2018).
- 40.Singmann H, Bolker B, Westfall J, Aust F. afex: Analysis of Factorial Experiments. R package version. 2018;0:20–2. [Google Scholar]
- 41.Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. (2018).
- 42.Wittchen, H.-U., Wunderlich, U., Gruschwitz, S. & Zaudig, M. SKID I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearb. d. amerikanischen Originalversion des SKID I. (Hogrefe, 1997).
- 43.Jasper HH. The ten-twenty electrode system of the international federation. Electroencephalogr. Clin. Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- 44.Schweizer, S. et al. The behavioral and neural effects of affective information on working memory performance: A pair of meta-analytic reviews. PsyArxiv (2018).
- 45.Schulze, L., Bürkner, P.-C., Bohländer, J. & Zetsche, U. Cognitive control and daily affect regulation in major depression and borderline personality disorder: a study protocol for an experimental ambulatory assessment study in Berlin, Germany. BMJ Open8, e022694 (2018). [DOI] [PMC free article] [PubMed]
- 46.Krause-Utz A, et al. Delay discounting and response disinhibition under acute experimental stress in women with borderline personality disorder and adult attention deficit hyperactivity disorder. Psychol. Med. 2016;46:3137–3149. doi: 10.1017/S0033291716001677. [DOI] [PubMed] [Google Scholar]
- 47.Lampe K, et al. Neuropsychological and behavioural disinhibition in adult ADHD compared to borderline personality disorder. Psychol. Med. 2007;37:1717–1729. doi: 10.1017/S0033291707000517. [DOI] [PubMed] [Google Scholar]
- 48.Matthies SD, Philipsen A. Common ground in Attention Deficit Hyperactivity Disorder (ADHD) and Borderline Personality Disorder (BPD)-review of recent findings. Borderline Personal. Disord. Emot. Dysregulation. 2014;1:3. doi: 10.1186/2051-6673-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schulze, L., Schulze, A., Renneberg, B., Schmahl, C. & Niedtfeld, I. Neural Correlates of Affective Disturbances: A Comparative Meta-analysis Of Negative Affect Processing in Borderline Personality Disorder, Major Depression, and Posttraumatic Stress Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging (in press). [DOI] [PubMed]
- 50.Moliadze V, Antal A, Paulus W. Electrode-distance dependent after-effects of transcranial direct and random noise stimulation with extracephalic reference electrodes. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2010;121:2165–2171. doi: 10.1016/j.clinph.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 51.Medina J, Cason S. No evidential value in samples of transcranial direct current stimulation (tDCS) studies of cognition and working memory in healthy populations. Cortex J. Devoted Study Nerv. Syst. Behav. 2017;94:131–141. doi: 10.1016/j.cortex.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 52.Westwood SJ, Romani C. Transcranial direct current stimulation (tDCS) modulation of picture naming and word reading: A meta-analysis of single session tDCS applied to healthy participants. Neuropsychologia. 2017;104:234–249. doi: 10.1016/j.neuropsychologia.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 53.Vöröslakos M, et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat. Commun. 2018;9:483. doi: 10.1038/s41467-018-02928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information: CONSORT checklist
Supplementary Information: Research Protocol
Data Availability Statement
The full data set, syntax, and statistical results are available at https://osf.io/g43bh/iew_only=f647fa67773041669f0a670c234dd150.
Hypotheses, sample size, exclusion criteria (i.e., based on general accuracy scores), and statistical analyses were pre-registered. The pre-registration, full data set, syntax, and statistical results are available at https://osf.io/g43bh/?view_only=f647fa67773041669f0a670c234dd150. At the request of this journal, Study 2 was registered as a clinical trial after the initial submission of the manuscript (16/08/2018; clinicaltrials.gov: NCT03636139). This also applies to the research protocol and the CONSORT checklist provided in the supplementary materials.