Cronobacter sakazakii is a pathogen of importance to neonatal health and is known to persist in dry food matrices, such as powdered infant formula (PIF) and its associated production environment. When infections are reported in neonates, mortality rates can be high. The success of this bacterium in surviving these low-moisture environments suggests that Cronobacter species can respond to a variety of environmental signals. Therefore, understanding those signals that aid the persistence of this pathogen in these ecological niches is an important step toward the development of strategies to reduce the risk of contamination of PIF. This research led to the identification of candidate genes that play a role in the persistence of this pathogen in desiccated conditions and, thereby, serve as a model target to design future strategies to mitigate PIF-associated survival of C. sakazakii.
KEYWORDS: Cronobacter sakazakii, RNA-seq, transcriptome, xerotolerance, desiccation
ABSTRACT
Cronobacter sakazakii is a xerotolerant neonatal pathogen epidemiologically linked to powdered infant food formula, often resulting in high mortality rates. Here, we used transcriptome sequencing (RNA-seq) to provide transcriptional insights into the survival of C. sakazakii in desiccated conditions. Our RNA-seq data show that about 22% of the total C. sakazakii genes were significantly upregulated and 9% were downregulated during desiccation survival. When reverse transcription-quantitative PCR (qRT-PCR) was used to validate the RNA-seq data, we found that the primary desiccation response was gradually downregulated during the tested 4 hours of desiccation, while the secondary response remained constitutively upregulated. The 4-hour desiccation tolerance of C. sakazakii was dependent on the immediate microenvironment surrounding the bacterial cell. The removal of Trypticase soy broth (TSB) salts and the introduction of sterile infant formula residues in the microenvironment enhanced the desiccation survival of C. sakazakii SP291. The trehalose biosynthetic pathway encoded by otsA and otsB, a prominent secondary bacterial desiccation response, was highly upregulated in desiccated C. sakazakii. C. sakazakii SP291 ΔotsAB was significantly inhibited compared with the isogenic wild type in an 8-hour desiccation survival assay, confirming the physiological importance of trehalose in desiccation survival. Overall, we provide a comprehensive RNA-seq-based transcriptional overview along with confirmation of the phenotypic importance of trehalose metabolism in Cronobacter sakazakii during desiccation.
IMPORTANCE Cronobacter sakazakii is a pathogen of importance to neonatal health and is known to persist in dry food matrices, such as powdered infant formula (PIF) and its associated production environment. When infections are reported in neonates, mortality rates can be high. The success of this bacterium in surviving these low-moisture environments suggests that Cronobacter species can respond to a variety of environmental signals. Therefore, understanding those signals that aid the persistence of this pathogen in these ecological niches is an important step toward the development of strategies to reduce the risk of contamination of PIF. This research led to the identification of candidate genes that play a role in the persistence of this pathogen in desiccated conditions and, thereby, serve as a model target to design future strategies to mitigate PIF-associated survival of C. sakazakii.
INTRODUCTION
Water is crucial for many biomolecular processes, such as enzyme-substrate interactions, protein folding and stability, cell structure maintenance, and others. Water activity (aw) is defined as the ratio of the water vapour pressure of any matrix to the pressure of pure water measured under the same conditions. In food, lowering the aw limits the amount of net water available to support microbial growth, thereby making desiccation one of the traditional methods of food preservation. Even though the minimum aw for the growth of most bacteria ranges from 0.88 to 0.91 (1), certain food-borne pathogens can survive for extended periods at a aw of <0.85 (2).
Bacterial tolerance to desiccation is well characterized (3, 4). The loss of water during desiccation triggers many biochemical, physiological, and metabolic responses in bacteria, including a rapid loss in cytoplasmic volume, surface area reduction, and capsular layer shrinkage (5). These responses increase the metabolite concentration and macromolecule crowding within the cytoplasm (6). The van der Waals interaction between phospholipids is consequently increased, compromising the membrane integrity (7). This is followed by membrane fusion, disruption, irreversible protein cross-linking, and extracellular macromolecule leakage (8). Major pathways involved in biosynthesis, transport, and repair are then disrupted, promoting the accumulation of reactive oxygen species (ROS) and the generation of oxidative stress (4). Oxidative stress leads to DNA hydroxylation, protein denaturation, lipid peroxidation, and apoptosis, ultimately compromising cell viability (9).
Cronobacter sakazakii is a Gram-negative non-spore-forming facultatively anaerobic pathogenic bacterium belonging to the family Enterobacteriaceae. Originally classified as a yellow-pigmented Enterobacter cloacae, the bacterium was later reclassified as Enterobacter sakazakii in 1980 (10). More recently, molecular taxonomic approaches necessitated yet another reclassification of the pathogen into a new genus, Cronobacter (11, 12). The genus Cronobacter currently contains seven species, namely C. malonaticus, C. turicensis, C. muytjensii, C. dublinensis, C. condimenti, C. universalis, and C. sakazakii. Cronobacter sakazakii is widely regarded as an opportunistic pathogen causing life-threatening invasive infections, particularly in neonates (13–15) and the elderly (16). Clinical manifestations include necrotizing enterocolitis, septicemia, and meningitis, with fatality rates as high as 80% being reported. The first death was reported in 1961 (17).
C. sakazakii has been demonstrated to be ubiquitous (13, 15, 18) and has been isolated from a variety of foods, such as dairy-based food products, dried meats, rice, water, and others (19–21). Among these foods, C. sakazakii-contaminated powdered infant formula (PIF) has been epidemiologically linked to infections and illnesses (22, 23) and consequently is a major challenge for the infant formula industry (24). PIF is produced by spray drying pasteurized milk, providing an aw of 0.35 to 0.25, while preserving the nutritional and organoleptic qualities of this food (25). The capacity of C. sakazakii to survive under desiccation conditions allows the bacterium to tolerate low aw conditions in foods, such as powdered infant formula (26). This finding is reflected in outbreaks linking Cronobacter spp. to contaminated milk powder or infant formula; there were 3 cases in Iceland (1986), 10 cases in the United States (4 cases in 1988, 2 cases in 2008, and 4 cases in 2011), 12 cases in Belgium (1998), and 3 cases in France (2004) (27). Since normal processing steps, such as pasteurization, can eliminate the pathogen, the presence of C. sakazakii in PIF is likely due to cross contamination from immediate postproduction environments (28, 29). A high care area is defined as an area of high hygienic standard where practices relating to personnel, ingredients, equipment, packaging, and environment are carefully controlled so as to minimize produce contamination by pathogenic microorganisms. It is noteworthy that final product packaging areas are predominantly maintained in a dry state and, therefore, are classified as “high care areas.” Drying inhibits the survival of many microorganisms while also enhancing the persistence of many xerotolerant pathogens (25).
In this paper, we evaluated the survival kinetics of C. sakazakii SP291, an isolate that was repeatedly collected from a dry high care area of a PIF production environment (30). We further used RNA-seq to unveil the transcriptional responses exhibited by this pathogen during adaptation to desiccated conditions and provide clues about how this bacterium modulates metabolic responses to survive these adverse conditions. Using qRT-PCR and gene deletion assays, we provide evidence confirming the physiological importance of trehalose accumulation in desiccated C. sakazakii SP291.
RESULTS AND DISCUSSION
Rationale behind strain selection and experimental methodology.
We used C. sakazakii SP291 (O:2; sequence type 4 [ST-4]) as the model organism. Our earlier study had repeatedly isolated C. sakazakii SP291 from a PIF production facility under surveillance (30). The isolate was subjected to whole-genome sequencing that identified the presence of a 4.3-Mb chromosome with three resident plasmids (GenBank accession numbers CP004091 to CP004094). A total of 4,129 genes were identified on the chromosome, including 82 tRNA and 22 rRNA genes (31).
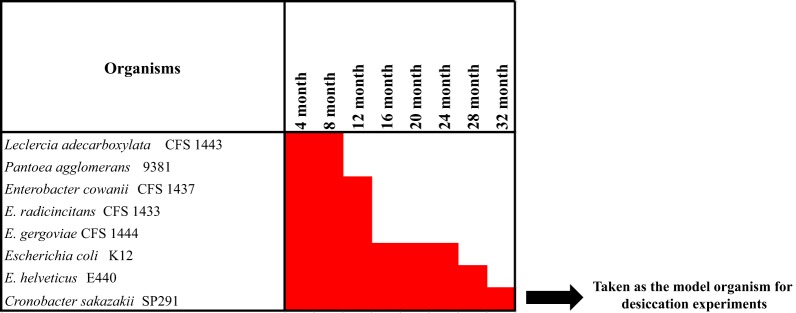
Since C. sakazakii SP291 was repeatedly isolated from a PIF production environment, we hypothesized that C. sakazakii SP291 could be adapted for long-term survival in a desiccated environment. To test this hypothesis, we undertook a 32-month desiccation tolerance study of C. sakazakii SP291 and seven other Enterobacteriaceae bacteria isolated in the same surveillance study, including Enterobacter cowanii CFS 1437, Enterobacter helveticus E440, Enterobacter radicincitans CFS 1433, Enterobacter gergoviae CFS 1444, Escherichia coli K12, Leclercia adecarboxylata CFS 1443, and Pantoea agglomerans 9381 (Fig. 1). All 8 isolates were cultured from infant formula and survived desiccation for a minimum period of 8 months, consistent with survival in a high care environment of a PIF production facility. Leclercia adecarboxylata and Pantoea agglomerans survived desiccated conditions for a period of 8 months, while Enterobacter gergoviae, Enterobacter cowanii, and Enterobacter radicincitans tolerated desiccation for 12 months, after which the strains became nonrecoverable. Notable xerotolerant strains recovered after longer periods of desiccation included Escherichia coli (24 months), Franconibacter helveticus (28 months), and C. sakazakii SP291 (32 months). The propensity of C. sakazakii to survive desiccation stress for multiple days has been noted earlier (32). The longer survival of C. sakazakii SP291 than the other 7 isolates could be due to the capability of the former to survive in xerotolerant conditions.
FIG 1.
Progression of the 32-month desiccation tolerance assay conducted on the members of Enterobacteriaceae isolated from a PIF production facility under surveillance. C. sakazakii SP291 survived desiccation for a period of 32 months, which was greater than other strains; therefore, it was taken as a model organism for downstream desiccation analysis.
We hypothesized that the transcriptional response of C. sakazakii SP291 could play a key role in the adaptability of this pathogen to survive desiccated conditions. To this end, we first enumerated the viability of C. sakazakii SP291 when subjected to laboratory-based desiccation conditions.
Growth kinetics show that C. sakazakii SP291 cells maintain viability during desiccation stress.
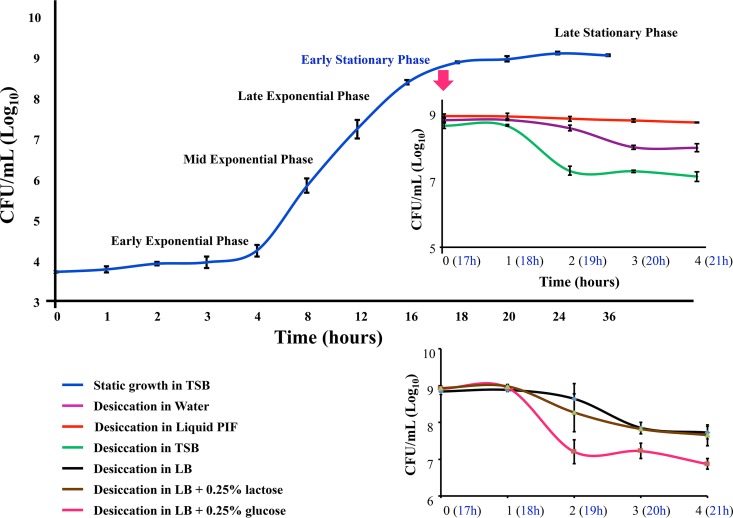
We followed previously described protocols (33, 34) to assess the desiccation viability of C. sakazakii SP291. C. sakazakii SP291 was first grown until early stationary phase (ESP) in nutrient-rich TSB medium at room temperature (25°C) under static conditions to simulate the natural food production environment in the laboratory. ESP was selected as the comparator condition in our desiccation experiments primarily because of the following two reasons: ESP when used as a comparator condition in the study of gene expression profiling in desiccated Salmonella spp. correctly identified the upregulation of secondary desiccation responses (33) and lag phase cells of C. sakazakii are more tolerant to desiccation conditions than exponentially growing cells (32). An aliquot of the ESP-grown bacterial cells were transferred onto sterile stainless-steel coupons and subjected to desiccation for a period of 4 h. At periodic time intervals, the coupons were washed with sterile TSB, and the viable counts were enumerated by serial dilution and plating. A 4-h window was selected for desiccation for the following three basic reasons: (i) mechanisms used for survival in the initial stages of desiccation may contribute to long-term survival, (ii) prolonged periods of desiccation may lead to signals being lost due to RNA degradation, and (iii) like Salmonella spp. (35), C. sakazakii may enter a dormant phase due to the lack of water necessary to support biological reactions. After 1 h of desiccation, approximately 95% of the initial ESP-grown C. sakazakii SP291 cells remained viable, while after 4 h, about 70% were viable (Fig. 2). This result confirmed our hypothesis that C. sakazakii SP291 is tolerant to desiccation stress. The viability pattern of desiccated C. sakazakii SP291 cells was very similar to Salmonella enterica serovar Typhimurium 4/74 grown in similar conditions (33).
FIG 2.
The desiccation-associated growth curve of C. sakazakii SP291. The blue curve represents the static growth of C. sakazakii SP291 in TSB at 25°C. TSB-grown cells were directly subjected to desiccation, and the survival for 4 h is shown in the green curve. TSB-grown cells were resuspended in TSB, sterile water, sterile infant formula, Luria-Bertani broth (LB), LB plus 0.25% glucose, and LB plus 0.25% lactose and then subjected to desiccation on sterile stainless steel coupons. The viability of bacterial cells associated with desiccation in the presence of different media are depicted in different colored curves, and the representation of each color is described in the key.
The influence of microenvironments on the desiccation tolerance of C. sakazakii SP291.
When TSB-grown C. sakazakii SP291 cells are subjected to desiccation, the loss of water from the microenvironment can lead to the increased concentration of TSB salts around the bacterial cell. We hypothesized that the accumulation of the TSB salts in the microenvironment of C. sakazakii SP291 cells might affect the desiccation tolerance of the bacterial cells. To test this, and to specifically study the effect of desiccation of C. sakazakii SP291 cells without hindrance from the TSB salt accumulation, we resuspended the ESP-grown C. sakazakii SP291 in sterile water and subjected the cells to desiccation for 4 h on stainless steel coupons. The lack of TSB salts improved the cell viability of desiccated C. sakazakii SP291 during desiccation from 70% to 90% (Fig. 2). The above observation that the microenvironment influences desiccation viability prompted us to hypothesize that the presence of milk residues in the microenvironment might influence desiccation survival. To test this, ESP-grown C. sakazakii SP291 was resuspended in reconstituted sterile PIF and subjected to desiccation. At 4 h, approximately 97% of C. sakazakii SP291 cells maintained viability, which is significantly (P < 0.005) higher than that of cells desiccated in the presence of TSB or water (Fig. 2). Our observation showed that the presence of milk or milk residues in the processing environment improve the desiccation survival of C. sakazakii. We obtained commercially available infant formula for this experiment. The label of this commercial infant formula mentioned the presence of 7% lactose and 7% additional unspecified sugars. Our RNA-seq data on 4-hour desiccated C. sakazakii SP291 (explained below) show the high upregulation of genes responsible for lactose utilization lacIZYAI, and genes responsible for glucose uptake/utilization were either down-regulated or not differentially regulated. We hypothesized that glucose may not be the preferred sugar source for C. sakazakii SP291 during desiccation. This finding compels us to believe that the utilization of lactose (and other sugars) could be responsible for the improved desiccation survival in the presence of milk or milk residues in the microenvironment of C. sakazakii SP291. To investigate our hypothesis, we subjected C. sakazakii SP291 cells to desiccation in the presence of LB salts, LB plus 0.25% glucose, or LB plus 0.25% lactose. We found that the presence of glucose in the media inhibited desiccation survival while the presence of lactose promoted survival (Fig. 2). However, more-specific experiments are needed to mechanistically characterize the glucose-based inhibition of desiccation survival in C. sakazakii SP291.
RNA-seq unveils the differential expression of C. sakazakii SP291 genes during desiccation.
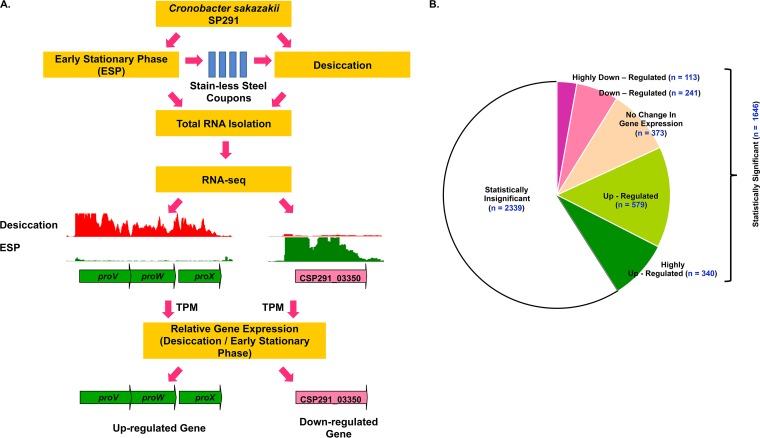
RNA-seq was used to explore the transcriptomic architecture of C. sakazakii SP291 during desiccation, following the strategy depicted in Fig. 3A. Total bacterial RNA was purified from three biological replicates of ESP-grown and 4-h desiccation stressed C. sakazakii SP291 and subjected to deep-level sequencing on the Illumina HiSeq 2500 platform. A total of 158 million reads were generated from the six cDNA libraries, with an average of 26 million reads/library (see Table S1 in the supplementary material, WS1). Approximately 5 to 10 million reads per library is sufficient for robust transcriptomic data analysis (36). The expression profiles of ESP grown- and desiccation stressed-C. sakazakii SP291 genes were calculated using the transcripts per million (TPM) approach (Materials and Methods) (37). A threshold TPM value of 10 was used as a cutoff to define gene expression, and any gene with an expression level of 10 or less was considered either minimally or not expressed (38, 39). The expression values of C. sakazakii SP291 genes identified during desiccation were compared with those expressed during ESP growth, and the fold changes in gene expression desiccation/ESP were used to determine the desiccation transcriptome. Genes with a fold change of >2 were categorized as upregulated, while those genes with a fold change of <0.5 were categorized as downregulated. Genes with >4-fold upregulation/downregulation were classified as highly upregulated/downregulated, respectively (Table S1, WS2).
FIG 3.
(A) A figure summarizing the experimental strategy deployed for the RNA-seq experiment. ESP-grown C. sakazakii SP291 cells were subjected to desiccation for 4 h on sterile stainless steel coupons, and total RNA was purified from both ESP-grown and desiccated cells. The graphs represent the RNA-seq reads mapped uniquely against the C. sakazakii SP291 genome in the respective condition, as visualized in Integrated Genome Browser. The genes on the C. sakazakii SP291 genome were categorized as upregulated (green arrows) or downregulated (pink arrows) based on the ratio of gene expression values, desiccation versus ESP. (B) Number of differentially regulated genes in desiccated C. sakazakii SP291 (Table S1, WS2). The differential expression was calculated from the ratio of TPM-based gene expression data, desiccation versus ESP. The thresholds for the selection of upregulated and downregulated genes are mentioned in Materials and Methods. TPM, transcripts per million approach.
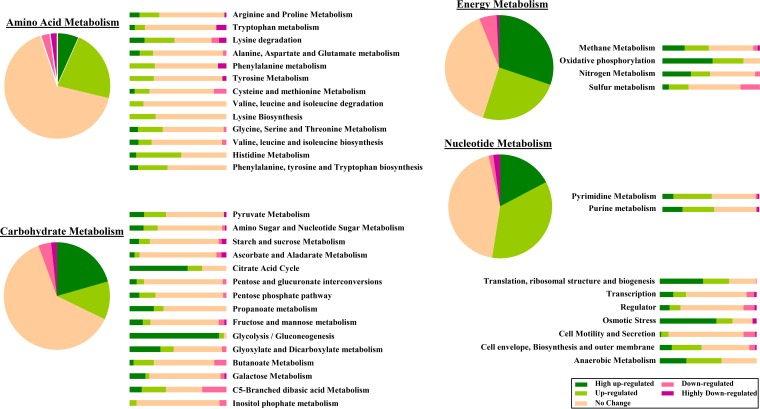
From a total of 4,015 protein-coding sequences (CDS) identified in the C. sakazakii SP291 genome (31), we obtained statistically significant data for 1,646 (41%) genes (Fig. 3B). Of the statistically significant genes, 1,273 genes were differentially regulated during desiccation stress, with 919 (23% of total) upregulated genes and 345 (8.6% of total) downregulated genes. A total of 340 genes were highly upregulated, while 113 genes were highly downregulated (Fig. 3B; see also Table S1, WS2). In an earlier report, computational approaches were used to identify the genes responsible for osmoprotection in the C. sakazakii genome (40). Of these genes, 21 were found to be either upregulated or highly upregulated and 3 were downregulated (Fig. 4). In addition, C. sakazakii SP291 genes were categorized into 11 functional groups, namely amino acid metabolism, carbohydrate metabolism, energy metabolism, nucleotide metabolism, translation ribosomal structure and biosynthesis, transcription, regulator, osmotic stress, cell motility and secretion, cell envelope biosynthesis and outer membrane, and anaerobic metabolism from the functional annotations presented in PATRIC (https://www.patricbrc.org/). These genes were characterized based on their expression levels (Table S1, WS2), and corresponding values were expressed as percentages (Fig. 5).
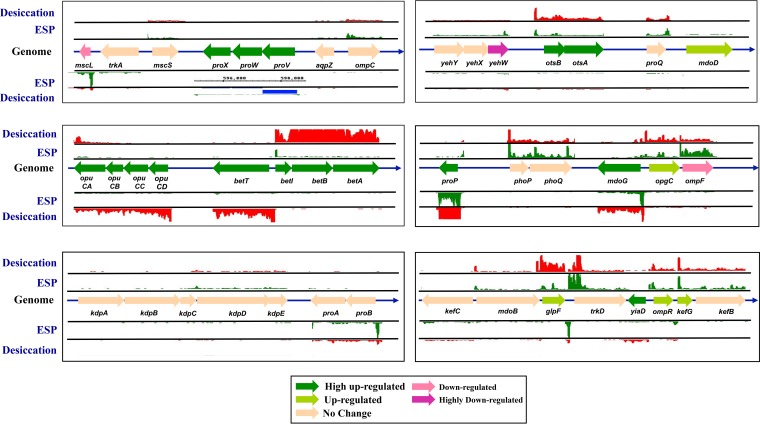
FIG 4.
Differential expression of selected genes associated with osmoprotection in C. sakazakii SP291. The list of genes was obtained from a previous publication (41). The locations of genes are shown according to the order as they appear in the C. sakazakii SP291 genome. Each gene is in scale with each other. The tracks up and below each gene represent the RNA transcripts that map against the location as visualized in IGB (60).
FIG 5.
Differential expression of functionally categorized genes in desiccated C. sakazakii SP291. The list of genes belonging to each category was obtained online (www.patricbrc.org). The genes were arranged according to their differential expression desiccation/ESP and expressed as percentage. The list of genes belonging to each category can be obtained from Table S1, WS3. The gene expression values can be obtained from Table S1, WS2.
Primary and secondary desiccation response in C. sakazakii.
Since gene expression data on desiccated C. sakazakii are scarce, the discussions related to our RNA-seq data must rely, for now, on related xerotolerant organisms whose desiccation-related phenotypic responses are well characterized. Lowering water activity (aw) will affect many vital metabolic functions within bacterial cells, and bacteria initiate several responses to combat these effects. The primary response is characterized by the rapid accumulation of potassium glutamate to provide temporary protection against desiccation stress by increasing the internal osmotic pressure of the bacterial cell, a step that is designed to counteract the high external osmolarity (41). C. sakazakii possesses two constitutive low-affinity K+ transport systems, specifically TrkH and TrkD, and one inducible high-affinity system, Kdp (40). While TrkH is an integral membrane protein, trkA and trkE encode regulatory proteins (42). In E. coli, the Kdp system is an inducible P-type ATPase system which has a high specificity for K+ and is encoded by the kdpFABC operon (43). Although the above genes were identified in the C. sakazakii genome (31, 41), their expression patterns have not been comprehensively studied to date. According to our RNA-seq data, after 4 hours of desiccation, genes encoding all of the above-described K+ uptake systems were not differentially regulated during desiccation (Table S1, WS2; Fig. 4).
In osmotically stressed bacteria, the rapid accumulation of potassium glutamate can lead to disruption in cellular metabolism, making the primary response unsuitable for long-term protection against desiccation stress. The primary response-based cellular disruption initiates a secondary response wherein potassium glutamate is rapidly replaced by more compatible neutral osmoprotectants that can accumulate to elevated levels without affecting cellular metabolism (44). The principle osmoprotectants accumulated in osmotically stressed E. coli are proline, glycine betaine, carnitine, and trehalose. In E. coli, it is known that the uptake of these osmoprotectants is facilitated by the ProP, ProU, and the OpuC systems. Our RNA-seq data showed that in C. sakazakii SP291, the gene proP is highly upregulated (about 5-fold desiccation versus ESP; Table S1, WS2). In bacteria, membrane-bound ProP can transport glycine betaine and ecotine with similar affinities (45). The gene proP was found to be upregulated when Salmonella Typhimurium was subjected to desiccation stress, and its role in facilitating desiccation survival was demonstrated previously (33). The second major osmoprotectant system is the ProU system, comprising of two membrane-bound proteins (ProV and ProW) and a periplasmic bound protein, ProX, encoded by the proVWX operon (46). The proVWX operon is highly upregulated (60-fold to 71-fold desiccation versus ESP) in desiccated C. sakazakii SP291 (Table S1, WS2). The proline biosynthetic genes, including proB (encoding a γ-glutamyl kinase), proA (γ-glutamyl phosphate reductase), and proC (Δ1-pyrroline-5-carboxylate reductase) were identified in E. coli (47) but were not reported to be differentially regulated during desiccation stress in C. sakazakii SP291. This result shows that proline was not endogenously synthesized in C. sakazakii SP291 but was likely to be imported exogenously into the cell by different uptake systems. A third osmoprotectant uptake system, the OpuC system, was identified in C. sakazakii based on gene homology with Listeria monocytogenes (48). This system is composed of the ATP-binding OpuCA protein, extracellular substrate-binding protein OpuCC, and two membrane-associated proteins, OpuCB and OpuCD. The OpuC system encoded by the opuCABCD operon in C. sakazakii SP291 was highly upregulated (14-fold to 28-fold) during desiccation and can transport a wide variety of osmoprotectants, including proline, glycine betaine, choline, ecotine, and carnitine (48). Since E. coli is incapable of carrying out betaine biosynthesis, the precursor choline is transported into the bacterial cell and converted into betaine in a two-step enzymatic reaction. BetT and ProU systems are used to first transport choline into the cell. BetA functions as a choline dehydrogenase which oxidizes the choline into glycine betaine aldehyde and BetB, a glycine betaine aldehyde dehydrogenase, and converts glycine betaine aldehyde to glycine betaine (49). Homologues of the E. coli betT and betIAB were identified in C. sakazakii SP291 (31) and are highly upregulated (10-fold to 55-fold, respectively) during desiccation.
The upregulation of many osmoprotectant uptake systems in desiccated C. sakazakii SP291 shows the increased bacterial activity in transporting osmoprotectants in the cytoplasm to counter the deleterious effects of water loss associated with desiccation. However, this hypothesis works under the assumption that the transcriptional response is followed by efficient translation and consequent phenotypic biochemical processes. It should be taken into account that RNA-seq will only give a measure of RNA transcripts and, therefore cannot provide an absolute picture of the physiological processes underlying desiccation. Our RNA-seq data plus the previously demonstrated importance of secondary responses in E. coli and Salmonella spp. suggest that the same could be true for C. sakazakii SP291.
qRT-PCR-based validation of primary and secondary desiccation responses in C. sakazakii SP291.
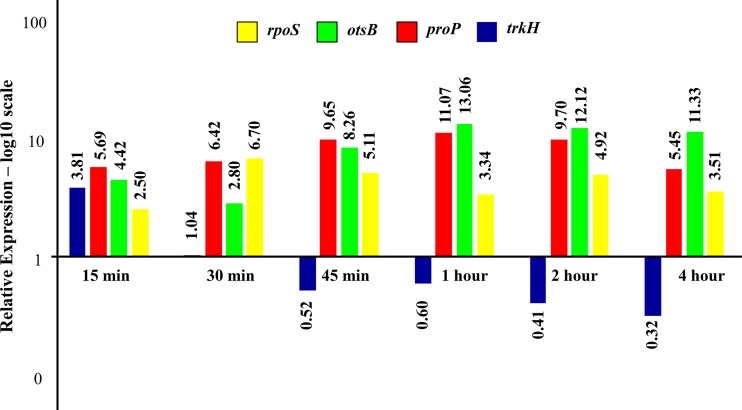
In our RNA-seq data, we observed that the primary desiccation responses were not differentially regulated in C. sakazakii SP291 4-hours postdesiccation. Since the RNA-seq experiment was conducted 4-h postdesiccation, we hypothesized that the primary response signals may be lost during the early phase of desiccation and that at 4 h, secondary responses had already triggered. We tested this hypothesis using qRT-PCR on total RNA isolated from cells subjected to 0 h (ESP) and 15, 30, 45, 60, 120, and 480 min of desiccation (Fig. 6). Representative genes from the primary (trkH) and secondary responses (proP and otsB) were evaluated.
FIG 6.
qRT-PCR validation of RNA-seq-based differential expression data. The trkH gene represents the primary desiccation response, while the proP and otsB represent the secondary desiccation response.
In osmotically stressed E. coli, the primary and secondary responses followed a time-dependent expression pattern (44). The primary response was initiated immediately after exposure to osmotic stress and was replaced by a secondary response 30-min postexposure. qRT-PCR data on C. sakazakii SP291 showed that the trkH gene was upregulated at 15-min postdesiccation but was not differentially regulated after 30 min and was subsequently downregulated at the remaining time points, leading to desiccation (Fig. 6). The RNA-seq data obtained after 4 hours of desiccation did not show any change in the expression pattern for the trkH gene (Table S1, WS2). Overall, the initial upregulation of trkH at the 15-min time point and downregulation at the subsequent time points observed in the qRT-PCR data show that the primary desiccation responses were active only during the early phases of desiccation. In contrast, the upregulation of proP and otsB was observed during all time points of desiccation. The gene otsB encodes a protein that converts glucose-6-phosphate to a neutral osmoprotectant called trehalose. Trehalose accumulation is an important secondary desiccation response observed in many xerotolerant bacteria, including C. sakazakii (32). Our qRT-PCR results on proP and otsB gene expression contradict an earlier report in E. coli stating that cells start synthesizing trehalose only after 30 min following an osmotic shock (44). However, it should be noted that our data measure transcriptional response alone, while in E. coli, high-pressure liquid chromatography (HPLC)-based phenotypic measurements of trehalose accumulation were made. Overall, our qRT-PCR results on C. sakazakii SP291 demonstrated a time-dependent expression paradigm for genes responsible for primary response but not for secondary response, a marked departure from E. coli. As stated earlier, throughout this discussion, we hypothesized that the transcriptional response will be followed by effective translation, leading to a phenotypic response. We tested our hypothesis by evaluating the role of otsAB, two genes encoding trehalose accumulation, an important secondary response in osmotically stressed C. sakazakii (41).
Physiological role of otsAB in desiccated C. sakazakii SP291.
Trehalose, a disaccharide formed by α,α-1,1-glucosidic bonds between two α-glucose units, is a compatible solute accumulated by osmotically stressed E. coli as a part of the secondary response (50, 51). Being inert, the molecule can be accumulated at high intracellular concentrations without affecting biochemical processes. During desiccation, trehalose replaces water at the surface of macromolecules, holding proteins and membranes in native conformation until water is restored (52). Initially, trehalose-6-phosphate is synthesized from glucose-6-phosphate and UDP-glucose, a reaction catalyzed by a trehalose-6-phosphate synthase encoded by otsA. Trehalose-6-phosphate is then converted to trehalose by a trehalose-6-phosphate phosphatase encoded by otsB (51). Our RNA-seq data showed that, at 4 hours, both otsA and otsB were highly upregulated, and the expression of otsB was confirmed by qRT-PCR (Fig. 6). This demonstrated that the mechanism associated with the conversion of glucose-6-phosphate into trehalose is active in C. sakazakii SP291 during desiccation. Similar responses were reported in E. coli during osmotic stress (53) and in the stationary phase of growth (54).
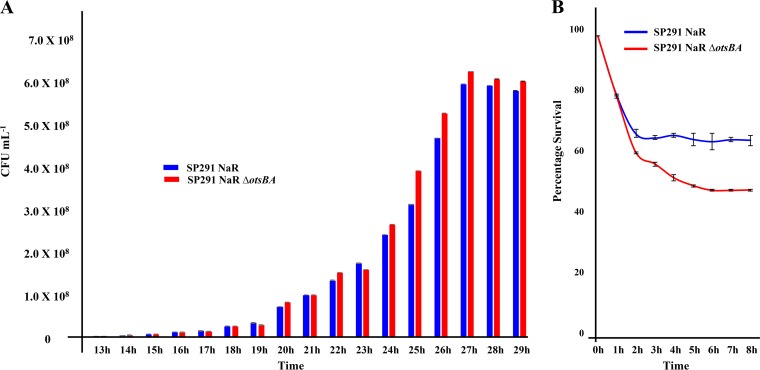
The importance of trehalose as an osmoprotectant in desiccated C. sakazakii was demonstrated very early in the literature. Results from trehalose extraction coupled with HPLC-based measurements showed that dried stationary-phase C. sakazakii SP291 accumulated trehalose 5-fold more than cells in nutrient medium (32). The importance of trehalose was emphasized more recently when trehalose biosynthesis genes otsA and otsB were identified in C. sakazakii BAA-894 (40). However, these publications did not examine the physiological importance of trehalose accumulation when C. sakazakii was subjected to desiccation. Since our RNA-seq data identified that otsBA was highly upregulated in desiccated C. sakazakii SP291, it was of interest to check whether the high expression of these genes translated into an altered phenotype. First, a C. sakazakii SP291 ΔotsBA mutant was created. However, since the deletion protocol necessitated antibiotic selection (Materials and Methods), the wild-type C. sakazakii SP291 was modified to introduce nalidixic acid resistance (C. sakazakii SP291-NalR). otsB and otsA were then deleted in C. sakazakii SP291-NalR to create C. sakazakii SP291-NalR ΔotsBA. Before subjecting both of these strains to a desiccation assay, the growth of C. sakazakii SP291-NalR was first assayed in TSB to check whether the introduction of nalidixic acid resistance introduced any changes in the biology of the organism. We found that C. sakazakii SP291-NalR had an increased lag phase of 14 hours (Fig. 7A) compared with 4 hours in wild-type C. sakazakii SP291 (Fig. 2). Furthermore, the ESP was obtained 27-hours postinoculation for C. sakazakii SP291-NalR, while for C. sakazakii SP291, it was 17 hours. To understand why the C. sakazakii SP291-NalR strain behaved differently than the isogenic wild-type C. sakazakii SP291, we sequenced the genome of C. sakazakii SP291-NalR using the Illumina MiSeq platform, and compared it with a previously published sequence of the C. sakazakii SP291 (30). We identified a 1-base pair single nucleotide polymorphism (SNP) in the DNA repair protein gene recN, a 27-bp insertion in the cell division protein gene zipA, and DNA topoisomerase subunit A (Table S1, WS4), which could account for the very slow growth rate of C. sakazakii SP291-NalR.
FIG 7.
Physiological importance of trehalose accumulation in desiccated C. sakazakii. (A) Growth of C. sakazakii NalR and C. sakazakii NalR ΔotsBA in TSB. (B) Desiccation survival of C. sakazakii NalR and C. sakazakii NalR ΔotsBA on desiccated stainless steel coupons. All experiments were conducted in independent triplicates, and error bars were generated from standard deviations. Statistical significance was calculated from Student’s t test.
Since the biology of C. sakazakii SP291-NalR was very different than that of the wild-type C. sakazakii SP291, the desiccation of C. sakazakii SP291-NalR ΔotsBA was tested against C. sakazakii SP291-NalR as the comparator to specifically investigate the effect of ots genes on desiccation. We subjected both C. sakazakii SP291-NalR and C. sakazakii SP291-NalR ΔotsBA strains to desiccation on sterile stainless steel coupons following standardized protocols. The desiccation of C. sakazakii SP291-NalR followed the same pattern as that of the isogenic wild-type C. sakazakii SP291, with a sharp drop in viability, and then the viability was maintained throughout the tested period (Fig. 2 and 7B). These data showed that the introduction of nalidixic acid resistance did not affect desiccation survival. First, we tested the growth of C. sakazakii SP291-NalR and C. sakazakii SP291-NalR ΔotsBA in TSB medium to check whether the deletion of otsAB had any phenotype during growth in an isotonic medium. Both strains had similar patterns of growth in TSB (Fig. 7A), demonstrating that the deletion of ots genes did not affect growth in isotonic medium. This finding reflects an earlier observation that osmotically unstressed C. sakazakii cells did not accumulate trehalose intracellularly (32). However, when C. sakazakii SP291-NarR ΔotsBA was tested for desiccation survival, there was a reduction in viability to 50% compared with 65% for the wild-type C. sakazakii SP291-NarR. This significant (P = 0.0019) 15% drop in bacterial viability shows the functional indispensability of otsAB in the desiccation survival of C. sakazakii. Since otsAB is closely involved in trehalose accumulation and, therefore, important for the secondary desiccation response, we show indirectly that trehalose accumulation is important for the desiccation survival of C. sakazakii SP291. Taken together with our RNA-seq data, our desiccation-associated viability assays provide a transcriptomic and phenotypic overview of trehalose biosynthesis in C. sakazakii when subjected to desiccation stress.
Elucidation of a transcriptional response model of trehalose metabolism in desiccated C. sakazakii.
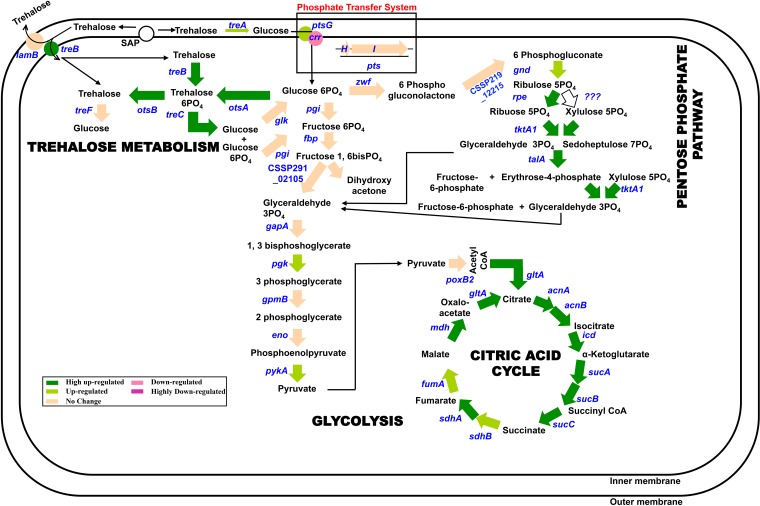
In E. coli, the lack of feedback inhibition in trehalose synthesis shows that the bacterium depends on the overproduction, excretion, and periplasmic degradation of trehalose to control the levels of trehalose within the cell. RNA-seq data showed that desiccated C. sakazakii SP291 also follows similar patterns as described previously for E. coli to regulate trehalose accumulation within the cytoplasm. Strech-associated proteins, such as MscL and MscS, excrete excess trehalose to the periplasm (55), although this observation remains to be experimentally confirmed. The trehalose in the periplasm is catabolized by periplasmic trehalase encoded by treA to produce glucose which is then transported back into the cytoplasm (56). In desiccated C. sakazakii SP291, treA is moderately upregulated, showing the periplasmic degradation of trehalose and uptake of glucose (Fig. 8). Alternatively, periplasmic trehalose can either be secreted to the external medium by the LamB protein or transported back into the cytoplasm as trehalose-6-phosphate by the TreB PTSTre system. No change in the expression of lamB and the high upregulation of treB (8-fold) suggest that trehalose is preferentially transported back into the cytoplasm of desiccated C. sakazakii SP291. Trehalose-6-phosphate can be catabolized to glucose and glucose-6-phosphate by the cytoplasmic amylotrehalase encoded by treC which is highly upregulated (13-fold). The glucose-6-phosphate can either be cycled to the glycolysis/pentose phosphate pathway (PPP) or converted to trehalose by the otsAB pathway. Since the otsAB pathway is highly upregulated compared with the lack of expression of zwf encoding glucose-6-phosphate-1-dehydrogenase, we speculate that the metabolic flux of glucose-6-phosphate is being channeled to trehalose synthesis rather than to PPP. However, this observation needs to be experimentally verified.
FIG 8.
The transcriptional model on the desiccation of C. sakazakii SP291. All the arrows depict the genes encoding the enzyme needed in the conversion step. The color of each arrow depicts the expression pattern of the gene in desiccated C. sakazakii SP291 based on the key given below.
C. sakazakii is epidemiologically linked to contaminated powder infant formula (PIF). The persistence of this pathogen in the PIF production environment has perplexed researchers for decades. We used growth kinetics, RNA-seq, qRT-PCR, and gene deletion assays to investigate the desiccation survival of Cronobacter sakazakii SP291. Our growth kinetics data show that the presence of milk residues in the microenvironment of the pathogen improves persistence by at least 20%. These data show that the presence of milk/milk residues in the PIF facility provide a rich source of alternate sugars which are actively utilized by the pathogen, aiding the long-term persistence of this pathogen. It is also possible that the protein stabilization by increased salt concentrations aids improved desiccation survival in C. sakazakii SP291 (57). The next challenge is to mitigate this pathogen in a PIF facility. Even though the accumulation of the disaccharide trehalose was shown before in desiccated C. sakazakii, the physiological role of the accumulation has not been demonstrated. By inhibiting the trehalose biosynthesis genes, we provide conclusive evidence that preventing trehalose production attenuated the ability of the pathogen to survive desiccated conditions. It should be noted that the desiccation survival of C. sakazakii was not completely inhibited even when trehalose biosynthesis was inhibited. This emphasizes the potential role of other secondary osmolytes, such as proline and choline, in aiding desiccation survival. In the future, researchers should focus on developing unique methodologies for the holistic inhibition of secondary desiccation responses, including trehalose accumulation, to bring about the complete inhibition of this pathogen in a PIF production facility.
MATERIALS AND METHODS
Bacterial strain and growth conditions used in this study.
Cronobacter sakazakii SP291 (O:2; ST-4) is a persistent xerotolerant strain isolated from a facility producing powdered infant formula (PIF), and the complete genome sequence was determined previously (30, 31). C. sakazakii SP291, originally preserved as glycerol stocks at −80°C, was resuscitated by streaking onto to tryptic soy agar (TSA) and incubating overnight at 37°C. The bacteriological medium used was tryptic soy broth (TSB)/TSA (Sigma-Aldrich), and growth conditions were used following previous studies (33, 34). Unless mentioned otherwise, bacterial growth was carried out statically at 25°C, representing a food production setting. For desiccation experiments, grade 304 stainless steel coupons (SSC) cut into dimensions of 5 cm by 0.7 cm by 0.1 cm were used. These coupons were sterilized in a 1% (wt/vol) solution of TriGene disinfectant by soaking overnight. The coupons were then placed in 70% (vol/vol) ethanol for 3 h to remove all residues of the disinfectant and were further rinsed with sterile water. The coupons were then wrapped in aluminum foils and sterilized using dry heat at 200°C for 1 h.
Testing desiccation tolerance.
Members of the Enterobacteriaceae family (n = 8) were grown to approximately 109 CFU/ml in TSB. One milliliter of the bacterial suspension was centrifuged at 11,000 × g at 37°C, and the cell pellet was resuspended in 1 ml PIF milk. Ten-microliter aliquots were dispensed into individual wells of a 96-well microtiter plate and dried in a laminar-flow cabinet for 4 h. Plates were then stored in a sealed box containing Drierite (Sigma). The viable bacterial count of these inoculum was determined by the Miles & Misra method on TSA (58). Every 3 months, the 96-well plates were rehydrated in 50 μl of buffered peptone water (BPW) and incubated at 37°C for 3 h. An aliquot of 200 μl base broth (10 g/liter peptone, 5 g/liter NaCl, 1 g/liter yeast extract, and 0.04 mg/liter bromocresol purple) with 20% (wt/vol) glucose was added to each well and incubated at 37°C for 16 h. A change in color from purple to yellow was considered an indicator of growth.
Growth curve of C. sakazakii SP291 and enumeration of viable cell counts during desiccation on stainless steel.
A single colony of C. sakazakii SP291 was picked from a TSA plate, inoculated in 10 ml TSB, and incubated statically at 25°C for 48 h. The culture was then serially diluted 1:100 in TSB, and 200 μl of this diluted culture was transferred into 500 ml fresh TSB in a 1-liter Stericup flask and was incubated statically at 25°C for a 48-h period. This culture served as the standard inoculum for all subsequent experiments, and the growth curve was generated by normal dilution and plating. The experiment was conducted in biological triplicate. The 17-h time point was designated the early stationary phase. An aliquot of 10 ml of the control phase was centrifuged at 8,000 × g for 5 min, and the pellet was resuspended in 1 ml fresh TSB medium. To test the desiccation viability in sterile water and in infant milk, the pellet was resuspended in either 1 ml of sterile water or infant milk, respectively. Commercially available Aptamil 1 First Milk was used as the source of infant milk. Heat-sterilized stainless steel coupons were placed aseptically in a sterile petri dish, with one coupon representing each hour of analysis. One hundred microliters of this resuspended culture was spread in a zig-zag fashion onto the sterilized stainless steel coupons and allowed to dry aseptically for 0 to 4 h at 25°C. At designated time points, individual coupons were placed in 5 ml sterile PBS and vortexed vigorously for 1 min to suspend the desiccated cells on the coupons into the PBS solution. Viable cell counts postdesiccation were calculated from this resuspended PBS solution by serial dilution and plating. The whole assay was conducted in triplicate, and standard deviations were incorporated in the graph. The statistical significance was calculated using Student’s t test.
RNA purification from desiccated C. sakazakii.
RNA was purified from ESP and desiccation-associated C. sakazakii SP291 using TRIzol (Thermo Fisher Scientific Inc., MA, USA). The purified RNA was then subjected to DNase I digestion (New England Biolabs, Herts, UK). The experiment was carried out in biological triplicates. Purified RNA was stored at −80°C until required. The library preparation and RNA-seq were carried out commercially (vertis Biotechnologie AG, Freising, Germany). Briefly, six RNA samples were treated with antarctic phosphatase and rephosphorylated with polynucleotide kinase (PNK). Thereafter, the RNA fragments were poly(A)-tailed using poly(A) polymerase, and an RNA adapter was ligated to the 5′-phosphate of the RNA. First-strand cDNA synthesis was performed using an oligo(dT)-adapter primer and Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega, WI, USA). The resulting cDNA was PCR-amplified to about 20 to 30 ng/μl using a high-fidelity DNA polymerase. The cDNA was then purified using the Agencourt AMPure XP kit (Beckman Coulter Genomics, MA, USA) and analyzed by capillary electrophoresis. The primers used for PCR amplification were designed according to the manufacturer's instructions (Illumina, CA, USA). Six cDNA samples were pooled in equimolar concentrations. A cDNA size range of 200 to 400 bp was eluted from preparative agarose gels. An aliquot of the size-fractionated cDNA pool was then analyzed by capillary electrophoresis. The cDNA pool was sequenced on an Illumina HiSeq 2500 system to generate reads with a 50 bp length.
Mapping of the libraries and differential gene expression.
The sequence reads obtained from the different libraries were mapped against the C. sakazakii SP291 reference genome (31) using the Segemehl software with an accuracy set at 100% (59). The coverage was increased by sequentially removing the mismatched nucleotide from the 3′ end and mapping the read again on the C. sakazakii SP291 genome. This process was repeated until the read mapped to a single location on the chromosome (uniquely mapped reads) or a minimum read length of 20 nucleotides was reached (39). These uniquely mapped reads were formatted graphically and visualized on the Integrated Genome Browser (IGB) (60). The raw expression values of each gene were normalized using the transcript per million (TPM) method (37, 61, 62). The lowest indicator of expression was fixed at the TPM value of 10, and any gene having a TPM of <10 was either not or very minimally expressed (39). The differential expression and statistical significance of the expression of each gene during desiccation were calculated using the Limma R package (63) on log2-transformed TPM values.
qRT-PCR analysis.
RNA-seq data were validated using reverse transcription-quantitative PCR (qRT-PCR). RNA purified from each time point was transcribed to cDNA using the Applied Biosystems high-capacity RNA-to-cDNA kit (Thermo Fisher, Dublin, Ireland). Primers targeting the genes were designed using the PrimerQuest tool (Integrated DNA Technologies, Leuven, Belgium) and were synthesized incorporating 6-FAM/ZEN/IBFQ (6-carboxyfluorescein/internal ZEN dark quencher/Iowa Black FQ) double-quenched probes (Integrated DNA Technologies, Belgium) (Table 1). The synthesized cDNA was then subjected to qRT-PCR using the PrimeTime gene expression master mix (Integrated DNA Technologies) in a Mastercycler ep realplex gradient (Eppendorf, Arlington, UK). Samples obtained as three biological replicates from each time point were run as three technical replicates, and data were analyzed using realplex software. The relative fold changes in expression levels (threshold cylcle [ΔCT]) were normalized based on the gene expression levels of the housekeeping gene dnaK. Comparative quantification was carried out using the ΔΔCT approach at the different time points versus the comparator (ESP) samples.
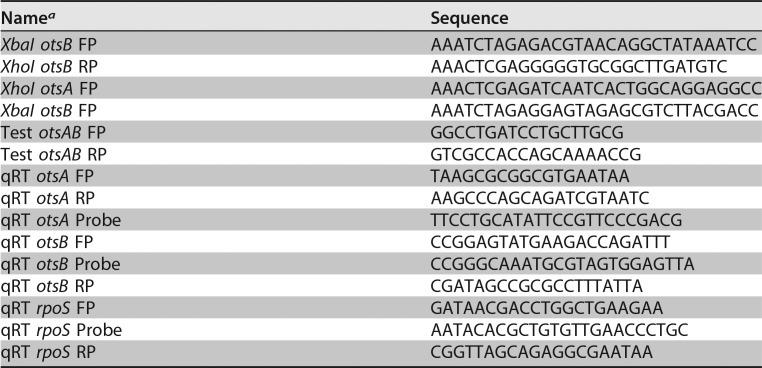
TABLE 1.
Primers used to generate the C. sakazakii SP291 ΔotsBA mutant and in qRT-PCR assays
aFP, forward primer; RP, reverse primer.
Construction of a deletion strain.
The otsBA mutant of C. sakazakii SP291 (C. sakazakii SP291-NalR ΔotsBA) was constructed following the protocol described earlier (64). Briefly, the upstream (XbaI otsB FP and XhoI otsB RP) and downstream (XhoI otsA FP and XbaI otsA RP) flanking regions of otsBA genes were amplified using target-specific primers (Table 1). The resulting fragments were digested using XbaI and XhoI. The digested fragments were ligated into the XbaI-digested suicide vector pDS132. The construct pDS132::ΔotsBA was transformed into SM10λpir via electroporation. The resulting strain, SM10λpir pDS132::ΔotsBA, served as a donor strain for the conjugative transfer of the plasmid into C. sakazakii SP291-NalR (the nalidixic acid-resistant construct of C. sakazakii). The transconjugants were selected on LB plates supplemented with both nalidixic acid (256 mg/liter) and chloramphenicol (30 mg/liter) and confirmed by PCR using test primers (Table 1). Outcrossing was performed by plating serial dilutions of PCR-confirmed transconjugants onto LB plates supplemented with 5% sucrose and no NaCl. Successful allelic exchange was verified in selected chloramphenicol-sensitive and sucrose-resistant strains for the presence of a mutant allele by PCR using test primers.
Accession number(s).
The RNA-seq data generated from this study are deposited in NCBI's Gene Expression Omnibus (GEO) and are available under the accession number GSE118188.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the Department of Agriculture, Food and Marine (DAFM) (SMART-pif; 13/F/423), and Enterprise Ireland (IP 2015 0380) for funding Y.C. and S.S., respectively. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We declare that there is no conflict of interest.
The experiments were designed by S.S., S.C., Y.C., Q.Y., and S.F. The wet lab experiments were carried out by S.S., Y.C., A.L., and Q.Y. S.K.S. conducted the RNA-seq bioinformatic analysis. Y.C. and S.S. carried out the improvisations in the annotations and RNA-seq data analysis. The whole-genome library preparation and sequencing was carried out by K.V.H. Sequence/SNP analysis was carried out by S.N. S.S. and S.F. wrote the manuscript (MS). Y.C., B.D.T., G.R.G., R.S., and A.L. helped in the critical reading and revision of the MS. All authors read and approved the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01993-18.
REFERENCES
- 1.Lund BM, Baird-Parker TC, Gould GW. 2000. The microbiological safety and quality of food. Aspen Publishers, Gaithersburg, MD. [Google Scholar]
- 2.Carrasco E, Morales-Rueda A, García-Gimeno RM. 2012. Cross-contamination and recontamination by Salmonella in foods: a review. Food Res Int 45:545–556. doi: 10.1016/j.foodres.2011.11.004. [DOI] [Google Scholar]
- 3.Lebre PH, De Maayer P, Cowan DA. 2017. Xerotolerant bacteria: surviving through a dry spell. Nat Rev Microbiol 15:285–296. doi: 10.1038/nrmicro.2017.16. [DOI] [PubMed] [Google Scholar]
- 4.Potts M. 1994. Desiccation tolerance of prokaryotes. Microbiol Rev 58:755–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Mortel M, Halverson LJ. 2004. Cell envelope components contributing to biofilm growth and survival of Pseudomonas putida in low-water-content habitats. Mol Microbiol 52:735–750. doi: 10.1111/j.1365-2958.2004.04008.x. [DOI] [PubMed] [Google Scholar]
- 6.Harding T, Brown MW, Simpson AG, Roger AJ. 2016. Osmoadaptative strategy and its molecular signature in obligately halophilic heterotrophic protists. Genome Biol Evol 8:2241–2258. doi: 10.1093/gbe/evw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billi D, Potts M. 2002. Life and death of dried prokaryotes. Res Microbiol 153:7–12. doi: 10.1016/S0923-2508(01)01279-7. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe J, Bryant G. 1999. Freezing, drying, and/or vitrification of membrane-solute-water systems. Cryobiology 39:103–129. doi: 10.1006/cryo.1999.2195. [DOI] [PubMed] [Google Scholar]
- 9.Schieber M, Chandel NS. 2014. ROS function in redox signaling and oxidative stress. Curr Biol 24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farmer JJ, Asbury MA, Hickman FW, Brenner DJ. 1980. Enterobacter sakazakii: a new species of Enterobacteriaceae isolated from clinical specimens. Int J Syst Bacteriol 30:569–584. doi: 10.1099/00207713-30-3-569. [DOI] [Google Scholar]
- 11.Iversen C, Lehner A, Mullane N, Bidlas E, Cleenwerck I, Marugg J, Fanning S, Stephan R, Joosten H. 2007. The taxonomy of Enterobacter sakazakii: proposal of a new genus Cronobacter gen. nov. and descriptions of Cronobacter sakazakii comb. nov. Cronobacter sakazakii subsp. sakazakii, comb. nov., Cronobacter sakazakii subsp. malonaticus subsp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov. and Cronobacter genomospecies 1. BMC Evol Biol 7:64. doi: 10.1186/1471-2148-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iversen C, Mullane N, McCardell B, Tall BD, Lehner A, Fanning S, Stephan R, Joosten H. 2008. Cronobacter gen. nov., a new genus to accommodate the biogroups of Enterobacter sakazakii, and proposal of Cronobacter sakazakii gen. nov., comb. nov., Cronobacter malonaticus sp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov., Cronobacter genomospecies 1, and of three subspecies, Cronobacter dublinensis subsp. dublinensis subsp. nov., Cronobacter dublinensis subsp. lausannensis subsp. nov. and Cronobacter dublinensis subsp. lactaridi subsp. nov. Int J Syst Evol Microbiol 58:1442–1447. doi: 10.1099/ijs.0.65577-0. [DOI] [PubMed] [Google Scholar]
- 13.Bar-Oz B, Preminger A, Peleg O, Block C, Arad I. 2001. Enterobacter sakazakii infection in the newborn. Acta Paediatr 90:356–358. doi: 10.1080/080352501300067857. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Kim YT, Yoon H, Lee JH, Ryu S. 2017. The complete genome sequence of Cronobacter sakazakii ATCC 29544T, a food-borne pathogen, isolated from a child’s throat. Gut Pathog 9:2. doi: 10.1186/s13099-016-0150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullane NR, Iversen C, Healy B, Walsh C, Whyte P, Wall PG, Quinn T, Fanning S. 2007. Enterobacter sakazakii an emerging bacterial pathogen with implications for infant health. Minerva Pediatr 59:137–148. [PubMed] [Google Scholar]
- 16.See KC, Than HA, Tang T. 2007. Enterobacter sakazakii bacteraemia with multiple splenic abscesses in a 75-year-old woman: a case report. Age Ageing 36:595–596. doi: 10.1093/ageing/afm092. [DOI] [PubMed] [Google Scholar]
- 17.Urmenyi AM, Franklin AW. 1961. Neonatal death from pigmented coliform infection. Lancet 1:313–315. [DOI] [PubMed] [Google Scholar]
- 18.Kilonzo-Nthenge A, Chen FC, Godwin SL. 2008. Occurrence of Listeria and Enterobacteriaceae in domestic refrigerators. J Food Prot 71:608–612. doi: 10.4315/0362-028X-71.3.608. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner A, Grand M, Liniger M, Iversen C. 2009. Detection and frequency of Cronobacter spp. (Enterobacter sakazakii) in different categories of ready-to-eat foods other than infant formula. Int J Food Microbiol 136:189–192. doi: 10.1016/j.ijfoodmicro.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Chap J, Jackson P, Siqueira R, Gaspar N, Quintas C, Park J, Osaili T, Shaker R, Jaradat Z, Hartantyo SH, Abdullah Sani N, Estuningsih S, Forsythe SJ. 2009. International survey of Cronobacter sakazakii and other Cronobacter spp. in follow up formulas and infant foods. Int J Food Microbiol 136:185–188. doi: 10.1016/j.ijfoodmicro.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Healy B, Cooney S, O'Brien S, Iversen C, Whyte P, Nally J, Callanan JJ, Fanning S. 2010. Cronobacter (Enterobacter sakazakii): an opportunistic foodborne pathogen. Foodborne Pathog Dis 7:339–350. doi: 10.1089/fpd.2009.0379. [DOI] [PubMed] [Google Scholar]
- 22.Bowen AB, Braden CR. 2006. Invasive Enterobacter sakazakii disease in infants. Emerg Infect Dis 12:1185–1189. doi: 10.3201/eid1208.051509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.FAO/WHO. 2008. Enterobacter sakazakii (Cronobacter) in powdered infant follow-up formulae. Meeting Report. Microbiological Risk Assessment Series no. 15. FAO/WHO, Rome, Italy. [Google Scholar]
- 24.Yan QQ, Condell O, Power K, Butler F, Tall BD, Fanning S. 2012. Cronobacter species (formerly known as Enterobacter sakazakii) in powdered infant formula: a review of our current understanding of the biology of this bacterium. J Appl Microbiol 113:1–15. doi: 10.1111/j.1365-2672.2012.05281.x. [DOI] [PubMed] [Google Scholar]
- 25.Beuchat LR, Komitopoulou E, Beckers H, Betts RP, Bourdichon F, Fanning S, Joosten HM, Ter Kuile BH. 2013. Low-water activity foods: increased concern as vehicles of foodborne pathogens. J Food Prot 76:150–172. doi: 10.4315/0362-028X.JFP-12-211. [DOI] [PubMed] [Google Scholar]
- 26.Gurtler JB, Beuchat LR. 2007. Survival of Enterobacter sakazakii in powdered infant formula as affected by composition, water activity, and temperature. J Food Prot 70:1579–1586. doi: 10.4315/0362-028X-70.7.1579. [DOI] [PubMed] [Google Scholar]
- 27.Burgess CM, Gianotti A, Gruzdev N, Holah J, Knochel S, Lehner A, Margas E, Esser SS, Sela Saldinger S, Tresse O. 2016. The response of foodborne pathogens to osmotic and desiccation stresses in the food chain. Int J Food Microbiol 221:37–53. doi: 10.1016/j.ijfoodmicro.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Mullane NR, Whyte P, Wall PG, Quinn T, Fanning S. 2007. Application of pulsed-field gel electrophoresis to characterise and trace the prevalence of Enterobacter sakazakii in an infant formula processing facility. Int J Food Microbiol 116:73–81. doi: 10.1016/j.ijfoodmicro.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 29.Mullane N, Healy B, Meade J, Whyte P, Wall PG, Fanning S. 2008. Dissemination of Cronobacter spp. (Enterobacter sakazakii) in a powdered milk protein manufacturing facility. Appl Environ Microbiol 74:5913–5917. doi: 10.1128/AEM.00745-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Power KA, Yan Q, Fox EM, Cooney S, Fanning S. 2013. Genome sequence of Cronobacter sakazakii SP291, a persistent thermotolerant isolate derived from a factory producing powdered infant formula. Genome Announc 1:e0008213. doi: 10.1128/genomeA.00082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan Q, Power KA, Cooney S, Fox E, Gopinath GR, Grim CJ, Tall BD, McCusker MP, Fanning S. 2013. Complete genome sequence and phenotype microarray analysis of Cronobacter sakazakii SP291: a persistent isolate cultured from a powdered infant formula production facility. Front Microbiol 4:256. doi: 10.3389/fmicb.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breeuwer P, Lardeau A, Peterz M, Joosten HM. 2003. Desiccation and heat tolerance of Enterobacter sakazakii. J Appl Microbiol 95:967–973. doi: 10.1046/j.1365-2672.2003.02067.x. [DOI] [PubMed] [Google Scholar]
- 33.Finn S, Handler K, Condell O, Colgan A, Cooney S, McClure P, Amezquita A, Hinton JC, Fanning S. 2013. ProP is required for the survival of desiccated Salmonella enterica serovar Typhimurium cells on a stainless steel surface. Appl Environ Microbiol 79:4376–4384. doi: 10.1128/AEM.00515-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolfe MD, Rice CJ, Lucchini S, Pin C, Thompson A, Cameron AD, Alston M, Stringer MF, Betts RP, Baranyi J, Peck MW, Hinton JC. 2012. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J Bacteriol 194:686–701. doi: 10.1128/JB.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng X, Weerapana E, Ulanovskaya O, Sun F, Liang H, Ji Q, Ye Y, Fu Y, Zhou L, Li J, Zhang H, Wang C, Alvarez S, Hicks LM, Lan L, Wu M, Cravatt BF, He C. 2013. Proteome-wide quantification and characterization of oxidation-sensitive cysteines in pathogenic bacteria. Cell Host Microbe 13:358–370. doi: 10.1016/j.chom.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas BJ, Chin M, Nusbaum C, Birren BW, Livny J. 2012. How deep is deep enough for RNA-seq profiling of bacterial transcriptomes? BMC Genomics 13:734. doi: 10.1186/1471-2164-13-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner GP, Kin K, Lynch VJ. 2012. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci 131:281–285. doi: 10.1007/s12064-012-0162-3. [DOI] [PubMed] [Google Scholar]
- 38.Srikumar S, Kroger C, Hebrard M, Colgan A, Owen SV, Sivasankaran SK, Cameron AD, Hokamp K, Hinton JC. 2015. RNA-seq brings new insights to the intra-macrophage transcriptome of Salmonella Typhimurium. PLoS Pathog 11:e1005262. doi: 10.1371/journal.ppat.1005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroger C, Colgan A, Srikumar S, Handler K, Sivasankaran SK, Hammarlof DL, Canals R, Grissom JE, Conway T, Hokamp K, Hinton JC. 2013. An infection-relevant transcriptomic compendium for Salmonella enterica serovar Typhimurium. Cell Host Microbe 14:683–695. doi: 10.1016/j.chom.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Cayley S, Lewis BA, Guttman HJ, Record MT Jr.. 1991. Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. Implications for protein-DNA interactions in vivo. J Mol Biol 222:281–300. doi: 10.1016/0022-2836(91)90212-O. [DOI] [PubMed] [Google Scholar]
- 41.Feeney A, Sleator RD. 2011. An in silico analysis of osmotolerance in the emerging gastrointestinal pathogen Cronobacter sakazakii. Bioeng Bugs 2:260–270. doi: 10.4161/bbug.2.5.17238. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura T, Yamamuro N, Stumpe S, Unemoto T, Bakker EP. 1998. Cloning of the trkAH gene cluster and characterization of the Trk K+-uptake system of Vibrio alginolyticus. Microbiology 144:2281–2289. doi: 10.1099/00221287-144-8-2281. [DOI] [PubMed] [Google Scholar]
- 43.Roe AJ, McLaggan D, O'Byrne CP, Booth IR. 2000. Rapid inactivation of the Escherichia coli Kdp K+ uptake system by high potassium concentrations. Mol Microbiol 35:1235–1243. doi: 10.1046/j.1365-2958.2000.01793.x. [DOI] [PubMed] [Google Scholar]
- 44.Dinnbier U, Limpinsel E, Schmid R, Bakker EP. 1988. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch Microbiol 150:348–357. doi: 10.1007/BF00408306. [DOI] [PubMed] [Google Scholar]
- 45.Wood JM. 1988. Proline porters effect the utilization of proline as nutrient or osmoprotectant for bacteria. J Membr Biol 106:183–202. doi: 10.1007/BF01872157. [DOI] [PubMed] [Google Scholar]
- 46.Doige CA, Ames GF. 1993. ATP-dependent transport systems in bacteria and humans: relevance to cystic fibrosis and multidrug resistance. Annu Rev Microbiol 47:291–319. doi: 10.1146/annurev.mi.47.100193.001451. [DOI] [PubMed] [Google Scholar]
- 47.Csonka LN, Leisinger T. 2007. Biosynthesis of proline. EcoSal Plus 2. doi: 10.1128/ecosalplus.3.6.1.4. [DOI] [PubMed] [Google Scholar]
- 48.Sleator RD, Wouters J, Gahan CG, Abee T, Hill C. 2001. Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl Environ Microbiol 67:2692–2698. doi: 10.1128/AEM.67.6.2692-2698.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landfald B, Strom AR. 1986. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol 165:849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruhal R, Kataria R, Choudhury B. 2013. Trends in bacterial trehalose metabolism and significant nodes of metabolic pathway in the direction of trehalose accumulation. Microb Biotechnol 6:493–502. doi: 10.1111/1751-7915.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riedel K, Lehner A. 2007. Identification of proteins involved in osmotic stress response in Enterobacter sakazakii by proteomics. Proteomics 7:1217–1231. doi: 10.1002/pmic.200600536. [DOI] [PubMed] [Google Scholar]
- 52.Cytryn EJ, Sangurdekar DP, Streeter JG, Franck WL, Chang WS, Stacey G, Emerich DW, Joshi T, Xu D, Sadowsky MJ. 2007. Transcriptional and physiological responses of Bradyrhizobium japonicum to desiccation-induced stress. J Bacteriol 189:6751–6762. doi: 10.1128/JB.00533-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purvis JE, Yomano LP, Ingram LO. 2005. Enhanced trehalose production improves growth of Escherichia coli under osmotic stress. Appl Environ Microbiol 71:3761–3769. doi: 10.1128/AEM.71.7.3761-3769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sola-Penna M, Meyer-Fernandes JR. 1998. Stabilization against thermal inactivation promoted by sugars on enzyme structure and function: why is trehalose more effective than other sugars? Arch Biochem Biophys 360:10–14. doi: 10.1006/abbi.1998.0906. [DOI] [PubMed] [Google Scholar]
- 55.Giaever HM, Styrvold OB, Kaasen I, Strom AR. 1988. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J Bacteriol 170:2841–2849. doi: 10.1128/jb.170.6.2841-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hengge-Aronis R, Klein W, Lange R, Rimmele M, Boos W. 1991. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J Bacteriol 173:7918–7924. doi: 10.1128/jb.173.24.7918-7924.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohno T, Roth J. 1979. Electrolyte effects on the activity of mutant enzymes in vivo and in vitro. Biochemistry 18:1386–1392. doi: 10.1021/bi00574a041. [DOI] [PubMed] [Google Scholar]
- 58.Miles AA, Misra SS, Irwin JO. 1938. The estimation of the bactericidal power of the blood. J Hyg (Lond) 38:732–749. doi: 10.1017/S002217240001158X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strom AR, Kaasen I. 1993. Trehalose metabolism in Escherichia coli: stress protection and stress regulation of gene expression. Mol Microbiol 8:205–210. doi: 10.1111/j.1365-2958.1993.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 60.Nicol JW, Helt GA, Blanchard SG Jr, Raja A, Loraine AE. 2009. The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics 25:2730–2731. doi: 10.1093/bioinformatics/btp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffmann S, Otto C, Kurtz S, Sharma CM, Khaitovich P, Vogel J, Stadler PF, Hackermuller J. 2009. Fast mapping of short sequences with mismatches, insertions and deletions using index structures. PLoS Comput Biol 5:e1000502. doi: 10.1371/journal.pcbi.1000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagner GP, Kin K, Lynch VJ. 2013. A model based criterion for gene expression calls using RNA-seq data. Theory Biosci 132:159–164. doi: 10.1007/s12064-013-0178-3. [DOI] [PubMed] [Google Scholar]
- 63.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Philippe N, Alcaraz JP, Coursange E, Geiselmann J, Schneider D. 2004. Improvement of pCVD442, a suicide plasmid for gene allele exchange in bacteria. Plasmid 51:246–255. doi: 10.1016/j.plasmid.2004.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.