Abstract
Plant pathogenic smut fungi in the broader sense can be divided into the Ustilaginomycetes, which cause classical smut symptoms with masses of blackish spores being produced in a variety of angiosperms, and the Exobasidiomycetes, which are often less conspicuous, as many do not shed large amounts of blackish spores. The leaf-spot causing members of the genus Entyloma (Entylomatales, Exobasidiomycetes) belong to the latter group. Currently, 172 species that all infect eudicots are included in the genus. Vánky (2012) recognised five Entyloma species on species of Ranunculus s.lat. Two have been reported only from Ficaria verna s.lat., while three, E. microsporum, E. ranunculi-repentis, E. verruculosum, have been reported to have a broad host range, encompassing 30, 26, and 5 species of Ranunculus, respectively. This broad host range is in contrast to the generally high host specificity assumed for species of Entyloma, indicating that they may represent complexes of specialised species. The aim of this study was to investigate Entyloma on Ranunculus s.lat. using multigene phylogenies and morphological comparisons. Phylogenetic analyses on the basis of up to four loci (ITS, atp2, ssc1, and map) showed a clustering of Entyloma specimens according to host species. For some of these Entyloma lineages, names not currently in use were available and reinstated. In addition, Entyloma microsporum s.str. is neotypified. Six novel species are described in this study, namely, Entyloma jolantae on Ranunculus oreophilus, E. klenkei on R. marginatus, E. kochmanii on R. lanuginosus, E. piepenbringiae on R. polyanthemos subsp. nemorosus (type host) and R. repens, E. savchenkoi on R. paludosus, and E. thielii on R. montanus. For all species diagnostic bases and morphological characteristics are provided. The results in this study once more highlight the importance of detailed re-investigation of broad host-range pathogens of otherwise specialised plant pathogen groups.
Keywords: Entyloma microsporum complex, Entyloma ranunculi-repentis complex, host specificity, multigene analyses, new primers, six new taxa, smut fungi
INTRODUCTION
The smut fungi in a broad sense (Ustilaginomycotina) contain more than 1 600 plant parasitic species in two major classes, the Ustilaginomycetes, the smut fungi in a strict sense and the Exobasidiomycetes, many of which do not cause typical smut symptoms with huge amounts of blackish spores being shed from sori in their host plants. Two more classes have been proposed recently (Wang et al. 2014), but as they might be embedded within the Exobasidiomycetes (Wang et al. 2015) or the sister group to the Ustilaginomycetes (Mishra et al. 2018), we do not treat them as separate classes here. Entyloma (Entylomatales, Exobasidiomycetes) is a species-rich genus with species that cause mostly inconspicuous, white to brown leaf spots. Entyloma currently comprises 172 species, restricted to dicotyledonous host plants belonging to 26 families (Vánky 2012, Denchev et al. 2013, Savchenko et al. 2014a, Rooney-Latham et al. 2017, Savchenko & Carris 2017). Because of their simple spore morphology, species delimitation in Entyloma is difficult (Savile 1947). A combination of spore morphology and host plant species is currently the most useful way to delineate species of Entyloma (Vánky 1994, 2012). Molecular phylogenetics has resolved species boundaries for many smut fungi (Vánky & Lutz 2007, Pia¸tek et al. 2011, 2013, 2015a, b, 2016, Savchenko et al. 2013, 2014a, b, Vasighzadeh et al. 2014, Li et al. 2017, Kruse et al. 2018), including Entyloma (Begerow et al. 2002, Vánky & Lutz 2010, Savchenko et al. 2014a, Lutz & Pia¸tek 2016). However, sequences of many Entyloma species are poorly represented in publicly available databases and many currently recognised species lack sequence data.
With about 600 species, Ranunculus is the largest genus of the family Ranunculaceae (Tamura 1995). Ranunculus species have a cosmopolitan distribution and mostly occur in temperate to arctic zones, where they grow in forests, meadows, peat bogs, on wet soils, as well as in lakes and rivers. Most species are herbaceous, some are annual, but the vast majority of species are perennial (Rastipishe et al. 2011). In the world monograph of smut fungi, Vánky (2012) recognised five different Entyloma species on Ranunculus s.lat., namely, E. ficariae, E. majewskii, E. microsporum, E. ranunculi-repentis, and E. verruculosum. Two species, Entyloma ficariae and E. majewskii, infect hosts in the genus Ficaria that is closely related to Ranunculus (Hörandl et al. 2005, Emadzade et al. 2010). Only three Entyloma species, E. microsporum, E. ranunculi-repentis, and E. verruculosum, were reported to infect species of the genus Ranunculus s.str. (Vánky 2012). Considering the narrow host specificity for the species occurring on Ficaria, it is remarkable that these three Entyloma species are reported from about 46 mostly yellow flowered Ranunculus species, worldwide (Savchenko et al. 2012, Vánky 2012). Entyloma microsporum and E. ranunculi-repentis have the widest reported host range with 30 and 26 different Ranunculus host species, respectively (Vánky 2012). However, it is still to be demonstrated, whether these Entyloma species are indeed generalist species, like some biotrophic pathogens (Choi et al. 2009, Runge et al. 2011, Scholler et al. 2011, Morin et al. 2012), or represent complexes of specialised species that justify earlier attempts to split them into several species with narrow host spectra, specifically Caeoma bullosum on R. chaerophyllos and E. pygmaeum on R. pygmaeus (Saccardo 1915, Ciferri 1928), E. ranunculacearum on R. acris, E. ranunculi-scelerati on R. sceleratus, E. ranunculorum on R. auricomus, and E. wroblewskii on R. polyanthemos (Kochman 1934, 1936, Liro 1938). Only a small number of Entyloma spp. on Ranunculus species have been included in phylogenetic analyses (e.g., Begerow et al. 2000, 2002, 2006, Savchenko et al. 2014a, Savchenko & Carris 2017).
The aim of this study was to resolve the species boundaries of Entyloma species on Ranunculus, based on the combination of morphological, biological, and molecular markers, including four loci (ITS, atp2, ssc1, and map). For this, a broad set of host-fungus combinations was studied, including Entyloma specimen from eleven different Ranunculus species, mostly from Germany but also from the Mediterranean (Greece, Italy, Slovenia, Spain), and Central Europe (Austria, Poland, Slovakia).
MATERIALS AND METHODS
Specimen sampling, documentation, and nomenclature
This study is based on morphological and/or phylogenetic analyses of 96 Entyloma specimens from eleven different Ranunculus species and one Ficaria species that were either collected in different regions of Europe or obtained from private herbaria (Table 1). They were deposited in the herbarium Senckenbergianum Görlitz (GLM) and in the herbarium of the W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków (KRAM F). The nomenclature of the host plant species is according to Euro+Med PlantBase (Euro+Med 2006–onwards), the nomenclature of the fungi is according to Index Fungorum (http://www.indexfungorum.org/) and Vánky (2012). The Entyloma microsporum complex and the E. ranunculi-repentis complex are defined as species complexes having sori forming swollen pustules filled with spores with cracked surfaces and sori forming flat leaf spots with tissue-embedded smooth spores, respectively.
Table 1.
Smut specimens used for phylogenetic analysis.
| Species | Host | Location | Location details | Date | Collector | DNA-no | Fungarium no. | GenBank no. |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ITS | atp2 | ssc1 | map | ||||||||
| Entyloma bullosum | Ranunculus paludosus | Greece, Rhodes | eastcoast, SE of Archangelos: c. 1.5 km S Stegna, Phrygana, northeast slope, N36°11'49" E28°08'06", elev. c. 70 m a.s.l. | 09.03.2016 | J. Kruse | 3471 | GLM-F107632 | MF924658 | MH022782 | MF939230 | MF939296 |
| R. paludosus | Spain, Andalusia | Cazorla, Parque Natural Sierras de Cazorla, 2.2 km E of Burunchel, A-319, slip rocks at wayside, N37°56'50" W02°56'28", elev. c. 1200 m a.s.l | 23.04.2015 | J. Kruse | 3211 | GLM-F107633 | MF924651 | MH022775 | MF939223 | MF939289 | |
| R. paludosus | Greece, Rhodes | c. 2.8 km NW of Lindos, Phrygana, way up to the mountain, hiking path, N36°05'48" W28°03'13", elev. c. 145 m a.s.l. | 10.03.2016 | J. Kruse | 3467 | GLM-F107634 | MF924654 | MH022778 | MF939226 | MF939292 | |
| R. paludosus | Greece, Rhodes | eastcoast, c. 3.5 km NE of Archangelos: Tsambika, way up Kloster, northern slope, Phrygana, N36°14'16" E28°09'16", elev. c. 160 m a.s.l. | 11.03.2016 | J. Kruse | 3468 | GLM-F107635 | MF924655 | MH022779 | MF939227 | MF939293 | |
| R. paludosus | Greece, Rhodes | c. 1 km S of Salakos, way up Mt Profitis Ilias, Phrygana, N36°17'03" E27°56'38", elev. c. 275 m a.s.l. | 13.03.2016 | J. Kruse | 3469 | GLM-F107636 | MF924656 | MH022780 | MF939228 | MF939294 | |
| R. paludosus | Greece, Rhodes | c. 1 km NW of Siana, way up to Akramitis, open Phrygana, plateau, N36°09'23" E27°45'59", elev. c. 650 m a.s.l. | 15.03.2016 | J. Kruse | 3470 | GLM-F107637 | MF924657 | MH022781 | MF939229 | MF939295 | |
| E. eburneum | R. bulbosus | Germany, Baden-Württemberg | Hegau, county Konstanz, NE of Neuhausen, near Schoren, dry grasland, MTB/Q: 8118/41, elev. c. 500 m a.s.l. | 28.05.2013 | J. Kruse | 107 | GLM-F107639 | MF924630 | MH022754 | MF939209 | MF939275 |
| R. bulbosus | Germany, Baden-Württemberg | county Konstanz, peninsula Reichenau in the Undersea, E Oberzell, littoral, MTB/Q: 8320/2, elev. c. 400 m a.s.l. | 31.05.2013 | J. Kruse | 108 | GLM-F107640 | MF924631 | MH022755 | MF939210 | MF939276 | |
| R. bulbosus | Germany, Bavaria | Oberfranken, S of Bayreuth, Swedebridge in direction to Studentwood, wayside, MTB/Q: 6035/34, elev. c. 360 m a.s.l. | 12.06.2013 | J. Kruse | 109 | GLM-F107641 | MF924632 | MH022756 | – | – | |
| R. bulbosus | Germany, Baden-Württemberg | Swabian Alps, county Sigmaringen, Beuron, Leibertingen-Wildenstein, castle Wildenstein, N48°03'21" E9°00'0", MTB/Q: 7919/13, elev. c. 760 m a.s.l. | 07.06.2014 | J. Kruse | 3049 | GLM-F107642 | MF924649 | MH022773 | MF939221 | MF939287 | |
| R. bulbosus | Germany, Hesse | Taunus, Bad Nauheim, Bad Nauheimer street, wayside, N50°22'50" E08°44'45", MTB/Q: 5618/12, elev. c. 175 m a.s.l. | 09.11.2015 | J. Kruse | 3496 | GLM-F107643 | MF924665 | MH022789 | MF939237 | MF939303 | |
| R. bulbosus | Germany, Hesse | Main-Taunus-county, Hattersheim at Main, grasland at Welschenstream, Kuckuckspfad, wayside, N50°03'54" E08°30'03", MTB/Q: 5917/13, elev. c. 90 m a.s.l. | 30.04.2016 | J. Kruse | 3621 | GLM-F107644 | MF924666 | MH022790 | MF939238 | MF939304 | |
| R. bulbosus | Italy, Liguria | Lower Varavalley, c. 1.5 km SW of Tavarone, circular path, Monte Alpe from Agriturismo Giandriale, east slope, meadow, N44°18'28" E09°31'58", elev. c. 725 m a.s.l. | 10.05.2016 | J. Kruse | 3622 | GLM-F107645 | MF924667 | MH022791 | MF939239 | MF939305 | |
| R. bulbosus | Germany, Saxony-Anhalt | Kyffhäuser-northern area, county Sangerhausen: SW of Kelbra, Großes Rabental, wayside, N51°25'33" E11°01'14", MTB/Q: 4532/33 | 13.05.2008 | H. Jage | 2317 | GLM-F095089 | MF924639 | MH022763 | – | – | |
| R. repens | Germany, Baden-Württemberg | Swabian Alps, county Sigmaringen, Leibertingen-Wildenstein, S of Beuron, ascent castle Wildenstein, mixed forest, wayside, N48°02'49" E08°58'17", MTB/Q: 7919/42, elev. c. 682 m a.s.l. | 06.06.2014 | J. Kruse | 3045 | GLM-F107638 | MF924646 | MH022770 | MF939218 | MF939284 | |
| R. repens | Germany, Lower Saxony | county Northeim, at the bottom of the Katlencastle, wayside near river, MTB/Q: 4326/21, elev. c. 110 m a.s.l. | 23.04.2010 | J. Kruse | 110 | GLM-F107648 | MF924633 | MH022757 | MF939211 | MF939277 | |
| R. repens | Germany, Schleswig-Holstein | county Rendsburg-Eckernförde, Barkelsby, Schusterredder, wayside, MTB/Q: 1425/33, elev. c. 22 m a.s.l. | 25.04.2011 | J. Kruse | 113 | GLM-F107649 | MF924634 | MH022758 | – | – | |
| R. repens | Germany, Hesse | Franfurt at Main, Sachsenhausen, Landwehrstreet,South-Cementery, N50°05'20" E08°41'43", MTB/Q: 5918/11, elev. c. 150 m a.s.l. | 22.03.2014 | J. Kruse | 3048 | GLM-F107654 | MF924648 | MH022772 | MF939220 | MF939286 | |
| R. repens | Germany, Saxony-Anhalt | county Wittenberg, Zahna-Elster, E of Bad Zahna, Oßnitzbach, wet grasland, N51°55'24" E12°46'10", MTB/Q: 4042/41, elev. c. 100 m a.s.l. | 17.08.2014 | J. Kruse | 3046 | GLM-F107653 | MF924647 | MH022771 | MF939219 | MF939285 | |
| R. repens | Germany, Bavaria | Upper Bavaria, Chiemgauer Alps, county Rosenheim, Priener cabin, climb down towards Berg, Via Alpina, firsforest, wayside, N47°41'41" E12°18'24", MTB/Q: 8339/22, elev. c. 1290 m a.s.l. | 22.07.2014 | J. Kruse | 3044 | GLM-F107652 | MF924645 | MH022769 | – | – | |
| R. repens | Germany, Hesse | Rüsselsheim, county Groß-Gerau, Varkaustreet, forest cementary, wayside, N49°59'22" E08°26'10", MTB/Q: 6016/21, elev. c. 100 m a.s.l. | 08.03.2015 | J. Kruse | 3641 | GLM-F107651 | MF924680 | MH022804 | MF939245 | MF939311 | |
| R. repens | Germany, Hesse | Wiesbaden, Stationstraße, Reisingeranlage, wayside, N50°04'21" E08°14'38", MTB/Q: 5915/12, elev. c. 110 m a.s.l. | 21.03.2015 | J. Kruse | 3640 | GLM-F107650 | MF924679 | MH022803 | MF939244 | MF939310 | |
| R. repens | Poland | Małopolska Province: Kraków-Pleszów, at Suchy Jar street | 20.11.2010 | M. Pia¸tek | 3652 | KRAM F-59037 | MF924689 | MH022813 | – | – | |
| R. repens | Poland | Małopolska Province: near Bukowica Reserve, close to Wygiełzów | 10.09.2014 | J. & M. Pia¸tek | 3653 | KRAM F-59038 | MF924690 | MH022814 | – | – | |
| R. polyanthemos subsp. nemorosus | Germany, Bavaria | Upper Bavaria, county Garmisch-Partenkirchen, c. 4.9 km NE of Mittenwald, Karwendel mountains, hiking path 266 from Rehbergalm to Hochland cabin, mixed mountain-forest, N47°27'37" E11°18'36", MTB/Q: 8533/24, elev. c. 1575 m a.s.l. | 11.07.2016 | J. Kruse | 3659 | GLM-F107647 | MF924696 | MH022820 | MF939255 | MF939321 | |
| R. polyanthemos subsp. nemorosus | Austria, Salzburg | county Salzburg, Lungau, Prebersee, MTB/Q: 8849/1 | 14.08.2013 | C. & F. Klenke | 3631 | GLM-F107646 | MF924674 | MH022798 | – | – | |
| E. ficariae | Ficaria verna | Germany, Schleswig-Holstein | Barkelsby, Schusterredder, wayside, MTB/Q: 1425/33, elev. c. 20 m a.s.l. | 27.04.2008 | J. Kruse | 70 | GLM-F107655 | MF924702 | MH022826 | – | – |
| Ficaria verna | Germany, Lower Saxony | Hannover, Misburg-North, Ludwig-Jahn-Street, country lane nearby the marlpit, wayside, MTB/Q: 3625/11, elev. c. 60 m a.s.l. | 18.04.2011 | J. Kruse | 73 | GLM-F107656 | MF924703 | MH022827 | – | – | |
| Ficaria verna | Germany, Schleswig-Holstein | county Rendsburg-Eckernförde, Ascheffel, Old Station, near exit to the Asch Mt, mixed forest, wayside, MTB/Q: 1524/31, elev. c. 6 m a.s.l. | 24.04.2011 | J. Kruse | 74 | GLM-F107658 | MF924704 | MH022828 | – | – | |
| Ficaria verna subsp. chrysocephala | Italy, Liguria | Varavalley, c. 2.5 km S of Varese Ligure, E of Stora, street from Sant Pietro Vare to Teviggo, shady wayside, N44°22'01" E09°37'39", elev. c. 530 m a.s.l. | 08.05.2016 | J. Kruse | 3638 | GLM-F107657 | MF924677 | MH022801 | MF939242 | MF939308 | |
| E. jolantae | R. oreophilus | Poland | Małopolska Province: Tatra Mts, Mała Dolinka valley – northern slopes of Giewont Mt, elev. c. 1230 m a.s.l. | 25.08.2008 | J. & M. Pia¸tek | 3650 | KRAM F-59030 | MF924688 | MH022812 | MF939250 | MF939316 |
| R. oreophilus | Poland | Małopolska Province: Tatra Mts, Mała Dolinka valley – northern slopes of Giewont Mt, elev. c. 1260 m a.s.l. | 25.08.2008 | J. & M. Pia¸tek | ML1535 | KRAM F-59031 | MF924714 | MH022838 | – | – | |
| E. klenkei | R. marginatus | Greece, Rhodes | c. 0.7 km W of Archipoli, Eparchiaki Odos Pastidas-Mesanagrou, field beneath street, N36°15'58" E28°03'11", elev. c. 185 m a.s.l. | 13.03.2016 | J. Kruse & V. Kummer | 3476 | GLM-F107659 | MF924663 | MH022787 | MF939235 | MF939301 |
| E. kochmanii | R. lanuginosus | Italy, Liguria | Varavalley, c. 2 km NE of Caranza, Strada Provinciale from Caranza to passo della Cappelletta, canyon alluvial forest, N44°23'33" E09°38'44", elev. c. 840 m a.s.l. | 09.05.2016 | J. Kruse | 3639 | GLM-F107660 | MF924678 | MH022802 | MF939243 | MF939309 |
| E. majewskii | Ficaria verna | Iran | Tehran Prov., 60 km E Tehran, Mts Elburz, ‘Emamzadeh-Haskei’, N35°50' E52°02', elev. c. 2610 m a.s.l. | 17.05.1990 | D. Ershad, T. Vánky & K. Vánky | Efc34 | BRIP: HUV14888 | MF924713 | MH022837 | MF939265 | MF939331 |
| E. microsporum | R. repens | Germany, Lower Saxony | county Hildesheim, Brüggen, Kirschweg, Sieben Bergen, Mt Hohe Tafel, wayside, MTB/Q: 3924/42, elev. c. 395 m a.s.l. | 08.05.2011 | J. Kruse | 95 | GLM-F107667 | MF924708 | MH022832 | MF939262 | MF939328 |
| R. repens | Germany, Bavaria | Oberfranken, Bayreuth, cemetery Saas, Bärenleite, wayside, MTB/Q: 6035/3, elev. c. 360 m a.s.l. | 24.05.2012 | J. Kruse | 96 | GLM-F107668 | MF924709 | MH022833 | MF939263 | MF939329 | |
| R. repens | Germany, Bavaria | Oberpfalz, national park Bavarian Wood, county Regen, W of Zwieseler Waldhaus, Watzlikhain, mixed mountain-forest on granite, MTB/Q: 6945/1, elev. c. 650 m a.s.l. | 21.08.2012 | J. Kruse | 97 | GLM-F107669 | MF924710 | MH022834 | MF939264 | MF939330 | |
| R. repens | Germany, Bavaria | Oberpfalz, national park Bavarian Wood, county Regen, Zwieseler Waldhaus, Mittelsteig cabin, mixed mountain-forest on granite, MTB/Q: 6945/2, elev. c. 700 m a.s.l. | 24.08.2012 | J. Kruse | 98 | GLM-F107670 | MF924711 | MH022835 | – | – | |
| R. repens | Germany, Bavaria | Oberfranken, Bayreuth, Eremitage, W of river Red Main, mixed forest, MTB/Q: 6035/42, elev. c. 375 m a.s.l. | 02.05.2013 | J. Kruse | 99 | GLM-F107671 | MF924712 | MH022836 | – | – | |
| R. acris | Germany, Bavaria | Oberfranken, between Horbach at the Steinach and Leutendorf, flood hollow, MTB/Q: 5733/3, elev. c. 290 m a.s.l. | 10.05.2013 | J. Kruse | 92 | GLM-F107662 | MF924705 | MH022829 | MF939261 | MF939327 | |
| R. repens | Germany, Baden-Württemberg | county Konstanz, Hegau, W of Singen, way up Mt Hohentwiel, wayside, MTB/Q: 8218/2, elev. c. 600 m a.s.l. | 29.05.2013 | J. Kruse | 101 | GLM-F107672 | MF924624 | MH022748 | MF939205 | MF939271 | |
| R. repens | Germany, Hesse | county Groß-Gerau, Ginsheim-Gustavsburg, Radweg zum Mainspitzdreieck, wayside circular path, N49°59'37" E08°17'46", MTB/Q: 6015/22 | 17.11.2013 | J. Kruse | 1631 | GLM-F107661, KRAM F-59043 | MF924636 | MH022760 | MF939213 | MF939279 | |
| R. repens | Austria, Tyrol | district Kufstein, county Walchsee, Kaiserwinkel, hiking track, Wandberg cabin towards Niederkaseralm, firsforest, slope, wayside, N47°41'16" E12°19'07", MTB/Q: 8339/22, elev. c. 1380 m a.s.l. | 21.07.2014 | J. Kruse | 3040 | GLM-F107675 | MF924643 | MH022767 | MF939216 | MF939282 | |
| R. repens | Germany, Bavaria | Upper Bavaria, Chiemgauer Alps, county Rosenheim, way up Priener cabin, N47°42'00" E12°17'54", MTB/Q: 8239/44, elev. c. 1280 m a.s.l. | 22.07.2014 | J. Kruse | 3038 | GLM-F107674 | MF924642 | MH022766 | – | – | |
| R. acris | Germany, Bavaria | county Rottal-Inn, Simbach, road St 2112, grasland at roundabout, N48°16'23" E13°00'53", elev. c. 370 m a.s.l. | 14.08.2014 | J. Kruse | 3037 | GLM-F107663 | MF924641 | MH022765 | MF939215 | MF939281 | |
| R. repens | Austria, Upper Austria | Braunau am Inn, Hagenau in Inncounty, Hagenauer street, grasland, sidearm of Inn, wayside, N48°16'23" E13°06'01", MTB/Q: 7744/2, elev. c. 340 m a.s.l. | 18.08.2014 | J. Kruse | 3036 | GLM-F107673 | MF924640 | MH022764 | – | – | |
| R. repens | Germany, Baden-Württemberg | Eschberg, county Waldshut, Buckmattstraße, meadow and forest edge around Liederbach, N08°10'49" E08°10'49", MTB/Q: 8315/31, elev. c. 400 m a.s.l. | 03.07.2015 | J. Kruse | 3643 | GLM-F107665 | MF924682 | MH022806 | MF939246 | MF939312 | |
| R. repens | Austria, Carinthia | Völkermarkt, SW of Bad Eisenkappel-Vellach, Koschuta, Trögener land road,Trögener Klamm, towards Trögern, wet slope at wayside, N46°27'53" E14°30'18", elev. c. 720 m a.s.l. | 06.07.2015 | J. Kruse | 3644 | GLM-F107666 | MF924683 | MH022807 | MF939247 | MF939313 | |
| R. repens | Germany, Hesse | Gießen, Lahntal, county Gießen, c. 5.5 km SW of Gießen, Allendorf at river Lahn, above parking at TSV Allendorf, street „In der Lache“, Slopeforest at stream Kleebach, wayside, N50°33'18" E08°36'55", MTB/Q: 5417/23, elev. c. 165 m a.s.l. | 21.05.2016 | J. Kruse | 3642 | GLM-F107664 | MF924681 | MH022805 | – | – | |
| R. repens | Poland | Małopolska Province: Tatra Mts, between Hala Kalatówki glade and Hala Kondratowa glade, elev. c. 1250 m a.s.l. | 17.07.2005 | J. & M. Pia¸tek | 3646 | KRAM F-59039 | MF924685 | MH022809 | – | – | |
| R. repens | Slovakia | on tourist track from Lucky to Choc Mt | 28.06.2008 | J. & M. Pia¸tek | 3647 | KRAM F-59040 | MF924686 | MH022810 | – | – | |
| R. repens | Poland | Małopolska Province, Tatra Mts, Hala Ga¸sienicowa glade (near Murowaniec mountain hut), elev. c. 1510 m a.s.l. | 24.09.2005 | J. & M. Pia¸tek | 3649 | KRAM F-59041 | MF924687 | MH022811 | MF939249 | MF939315 | |
| E. piepenbringiae | R. polyanthemos agg. | Germany, Bavaria | Upper Bavaria, county Weilheim, N Pähl, E of Hartschimmelhof, ‘Goaslweide’, region F3, MTB/Q: 8033/31, elev. c. 730 m a.s.l. | 14.05.2013 | J. Kruse | 93 | GLM-F107688 | MF924706 | MH022830 | – | – |
| R. polyanthemos agg. | Germany, Bavaria | Upper Bavaria, county Weilheim, N Pähl, E Hartschimmelhof, ‘Goaslweide’, region F4, MTB/Q: 8033/31, elev. c. 730 m a.s.l. | 13.05.2013 | J. Kruse | 94 | GLM-F107689 | MF924707 | MH022831 | – | – | |
| R. repens | Germany, Baden-Württemberg | county Konstanz, communal Moos, S of Weiler, nearby Grey Reed, wayside, MTB/Q: 8219/4, elev. c. 445 m a.s.l. | 30.05.2013 | J. Kruse | 102 | GLM-F107694 | MF924625 | MH022749 | MF939206 | MF939272 | |
| R. sp. | Spain, Andalusia | Cazorla, Parque Natural Sierras de Cazorla, c. 2.2 km S of Cazorla, hicking track, ascent Gilillo, slip rock, N37°53'30" W02°59'49", elev. c. 1185 m a.s.l. | 24.04.2015 | J. Kruse | 3210 | GLM-F107695 | MF924650 | MH022774 | MF939222 | MF939288 | |
| R. polyanthemos subsp. nemorosus | Germany, Bavaria | Oberallgäu, Einödsbach, Allgäu Alps, hiking path from Black Cabin to Rappensee cabin, meadow W of Rappensee cabin, N47°17'24" E10°14'40", MTB/Q: 8727/12, elev. c. 1900 m a.s.l. | 26.07.2015 | J. Kruse | 3493 | GLM-F107687 | MF924664 | MH022788 | MF939236 | MF939302 | |
| R. polyanthemos subsp. nemorosus | Germany, Bavaria | Upper Bavaria, county Garmisch-Partenkirchen, c. 2.8 km SE of Mittenwald, Karwendel mountains, hiking path 290 towards Brunnstein cabin, serpentines, light mixed mountain-forest, N47°24'44" E11°16'23", MTB/Q: 8533/43, c. 1260 m a.s.l. | 06.07.2016 | J. Kruse | 3664 | GLM-F107693 | MF924701 | MH022825 | MF939260 | MF939326 | |
| R. polyanthemos subsp. nemorosus | Germany, Bavaria | Upper Bavaria, county Garmisch-Partenkirchen, c. 2.8 km SE of Mittenwald, Karwendel mountains, hiking path 290 towards Brunnstein cabin, serpentines, light mixed mountain-forest, N47°24'48" E11°16'33", MTB/Q: 8533/43, elev. c. 1380 m a.s.l. | 06.07.2016 | J. Kruse | 3663 | GLM-F107692 | MF924700 | MH022824 | MF939259 | MF939325 | |
| R. polyanthemos subsp. nemorosus | Germany, Bavaria | Upper Bavaria, county Garmisch-Partenkirchen, c. 3.2 km SE of Mittenwald, Karwendel mountains, hiking path 291 from Brunnstein cabin towards Mt Brunnsteinspitze, scree, N47°24'33", E11°16'59", MTB/Q: 8533/43, elev. c. 1760 m a.s.l. | 07.07.2016 | J. Kruse | 3662 | GLM-F107691 | MF924699 | MH022823 | MF939258 | MF939324 | |
| R. polyanthemos subsp. nemorosus | Germany, Bavaria | Upper Bavaria, county Garmisch-Partenkirchen, c. 2.8 km SE of Mittenwald, Karwendel mountains, meadow around Brunnstein cabin, N47°24'49" E11°16'41", MTB/Q: 8533/43, elev. c. 1475 m a.s.l. | 08.07.2016 | J. Kruse | 3661 | GLM-F107690 | MF924698 | MH022822 | MF939257 | MF939323 | |
| R. polyanthemos agg. | Austria, Tyrol | Lechtal, N of Elbigenalp, wayside in mixed forest | 26.08.2002 | U. Fischer & M. Lutz | ML523 | TUB-012566 | MF924716 | MH022840 | MF939267 | MF939333 | |
| R. sp. | Switzerland | Kanton Bern, north bottom slope of Sustenpass, c. 4 km to Hotel Steingletscher, meadow | 12.06.2003 | W. Maier & M. Lutz | ML614 | TUB-012567 | MF924717 | MH022841 | MF939268 | MF939334 | |
| R. polyanthemos subsp. nemorosus | Slovenia | Triglav National Park, Lopucnica, way to Siebenseen cabin, tall herbaceous vegetation | 02.08.2005 | M. Kemler | ML838 | TUB-012568 | MF924718 | MH022842 | MF939269 | MF939335 | |
| R. repens | Germany, Baden-Württemberg | Tübingen, Bebenhausen, Goldersbachtal, littoral of lake, N48°33'30" E09°02'48", elev. c. 370 m a.s.l. | 14.06.2002 | M. Lutz | ML471 | TUB-012570 | MF924715 MF924626 | MH022839 MH022750 | MF939266 MF939207 | MF939332 MF939273 | |
| E. ranunculacearum | R. acris | Germany, Bavaria | Oberfranken, county Bamberg, SE of Sandhof, Mönchsweiher, mixed forest on Keuper-Sandstone, MTB/Q: 6030/2, elev. c. 290 m a.s.l. | 05.05.2012 | J. Kruse | 103 | GLM-F107676 | MF924627 | MH022751 | – | – |
| R. acris | Germany, Bavaria | Oberpfalz, national park Bavarian Wood, county Regen, Zwieseler Waldhaus, Mittelsteig cabin, mixed mountainous forest on granite, MTB/Q: 6945/2, elev. c. 700 m a.s.l. | 24.08.2012 | J. Kruse | 104 | GLM-F107677 | MF924628 | MH022752 | MF939208 | MF939274 | |
| R. acris | Germany, Baden-Württemberg | county Konstanz, Lake Constance, Radolfzell, SE of Möggingen, Mindelsea, circular path around sea, littoral and wayside, MTB/Q: 8220/1, elev. c. 420 m a.s.l. | 30.05.2013 | J. Kruse | 105 | GLM-F107678 | MF924637 | MH022761 | MF939214 | MF939280 | |
| R. acris | Germany, Saxony-Anhalt | county Wittenberg, Kemberg, district Rotta-Gniest, Heidestreet, wayside, N51°45'4" E12°35'33", MTB/Q: 4241/23, elev. c. 105 m a.s.l. | 13.11.2013 | J. Kruse | 1632 | GLM-F107680 | MF924635 | MH022759 | MF939212 | MF939278 | |
| R. acris | Germany, Hesse | Rheingau-Taunus-county, Eltville at river Rhein, Rheinsteig direction forest- restaurant Rausch, N50°02'46", E08°05'44", MTB/Q: 5914/41, elev. c. 160 m a.s.l. | 08.03.2014 | J. Kruse | 1373i | GLM-F107679 | MF924644 | MH022768 | MF939217 | MF939283 | |
| R. acris | Germany, Bavaria | Kirchdorf at Inn, Lower Bavaria, county Rottal-Inn, Hitzenau, Eckener street, wayside, N48°15'56" E12°58'53", MTB/Q: 7743/24, elev. c. 400 m a.s.l. | 17.08.2014 | J. Kruse | 3043 | GLM-F107681 | MF924668 | MH022792 | – | – | |
| R. acris | Germany, Saarland | Mettlach-Orscholz, county Merzig-Wadern, Cloef-Street, surroundings of Cloef-Atrium and Varadeser Park, N49°30'20" E06°32'06", MTB/Q: 6405/33, elev. c. 395 m a.s.l. | 29.09.2014 | J. Kruse | 3623 | GLM-F107684 | MF924653 | MH022777 | MF939225 | MF939291 | |
| R. acris | Germany, Hesse | Hoher Meißner, Meißner eastern slope, Fulda-Werra-uplands, Werra-Meißner county, Frau Holle lake, circular path, N51°13'09" E09°52'07", MTB/Q: 4725/33, elev. c. 640 m a.s.l. | 09.06.2015 | J. Kruse | 3315 | GLM-F107683 | MF924652 | MH022776 | MF939224 | MF939290 | |
| R. acris | Germany, Hesse | Hoher Meißner, Meißner eastern slope, Fulda-Werra-uplands, Werra-Meißner county, Frau Holle lake, circular path, alpine meadow, N51°13'06" E09°52'13", MTB/Q: 4725/33, elev. c. 620 m a.s.l. | 09.06.2015 | J. Kruse | 3314 | GLM-F107682 | MF924676 | MH022800 | MF939241 | MF939307 | |
| E. ranunculiscelerati | R. sceleratus | Germany, Bavaria | Oberpfalz, county Grafenwöhr, E of Hütten, littoral of lake, N49°40'52" E11°58'42", MTB/Q: 6337/22, elev. c. 410 m a.s.l. | 01.05.2016 | G. Hübner | 3637 | GLM-F107685 | MF924669 | MH022793 | – | – |
| R. sceleratus | Germany, Saxony-Anhalt | SSE of Elsnigk, S of Würflauer Schachtlake, near road B 185, MTB/Q: 4238/12 | 03.11.2004 | H. Jage | 3624 | GLM-F074573 | MF924670 | MH022794 | – | – | |
| R. sceleratus | Germany, Saxony-Anhalt | SE of Allstedt, Ziegelrodaer forest (N-part), aiport Allstedt (NW edge), MTB/Q: 4634/21 | 23.10.2005 | H. Jage | 3625 | GLM-F076138 | MF924671 | MH022795 | – | – | |
| R. sceleratus | Germany, Saxony-Anhalt | SW Sülldorf, Sülzetal, wet ditch right next to the brook Sülze (nearby salty area), MTB/Q: 3935/34 | 04.11.2005 | H. Jage | 3626 | GLM-F076159 | MF924629 | MH022753 | – | – | |
| R. sceleratus | Germany, Saxony-Anhalt | Lodersleben, near castle, in the Querne, MTB/Q: 4635/12 | 06.05.2005 | H. John & H. Jage | 3627 | GLM-F076186 | MF924673 | MH022797 | – | – | |
| R. sceleratus | Germany, Saxony-Anhalt | Friedersdorf near Lohsa (South), WSW of Neuhof, near Ballackmill, Maxlake (part of Ballacklakes), surceased, MTB/Q: 4652/14 | 26.05.2006 | H. Jage | 3628 | GLM-F086008 | MF924691 | MH022815 | – | – | |
| R. sceleratus | Poland | Mazowieckie Province: Warszawa-Wesoła | 17.07.2015 | P. Me¸dykowski | 3654 | KRAM F-59032 | MF924672 | MH022796 | – | – | |
| E. ranunculorum | R. auricomus | Germany, Bavaria | Oberfranken, county Kulmbach, Lindau, Mt chain Rough Mt, wayside, MTB/Q: 5934/2, elev. c. 410 m a.s.l. | 12.05.2012 | J. Kruse | 106 | GLM-F107686 | MF924638 | MH022762 | – | – |
| R. auricomus | Germany, Saxony-Anhalt | E of Dölkau, Burgholz (E-part) Jagen 29, alluvial forest, MTB/Q: 4638/24, elev. c. 25 m a.s.l. | 19.04.1998 | H. Jage | 1768 | GLM-F048093 | MF924659 | MH022783 | MF939231 | MF939297 | |
| E. savchenkoi | R. paludosus | Greece, Rhodes | c. 1 km S of Salakos, way up to Mt Profitis Ilias, Quercus coccifera forest, N36°16'59" E27°56'42", elev. c. 320 m a.s.l. | 13.03.2016 | J. Kruse | 3472 | GLM-F107696 | MF924660 | MH022784 | MF939232 | MF939298 |
| R. paludosus | Greece, Rhodes | c. 1 km NW of Siana, way up Akramitis, open Phrygana, plateau, N36°09'23" E27°45'59", elev. c. 650 m a.s.l. | 15.03.2016 | J. Kruse | 3473 | GLM-F107697 | MF924661 | MH022785 | MF939233 | MF939299 | |
| R. paludosus | Greece, Rhodes | c. 1.2 km SE of Theologos, olive grove, N36°22'00" E28°02'45", elev. c. 40 m a.s.l. | 16.03.2016 | J. Kruse | 3474 | GLM-F107698 | MF924662 | MH022786 | MF939234 | MF939300 | |
| R. paludosus | Greece, Rhodes | eastcoast, c. 2.5 km N of Kalathos, street towards Masari, wayside, olive grove, N36°08'47" E28°03'33", elev. c. 15 m a.s.l. | 20.03.2016 | J. Kruse | 3475 | GLM-F107699 |
MF924675 MF924697 |
MH022799 MH022821 |
MF939240 MF939256 |
MF939306 MF939322 |
|
| E. thielii | R. montanus | Germany, Bavaria | Oberallgäu, Einödsbach, Rappensee cabin, near Rappensea, wayside, N47°17'11" E10°15'19", MTB/Q: 8727/21, elev. c. 2080 m a.s.l. | 29.07.2015 | J. Kruse | 3632 | GLM-F107705 | MF924695 | MH022819 | MF939254 | MF939320 |
| R. montanus | Germany, Bavaria | Upper Bavaria, county Garmisch-Partenkirchen, c. 4.9 km NE Mittenwald, Karwendel mountains, hiking path 266 from Rehbergalm to Hochland cabin, mixed mountainous forest, N47°27'37" E11°18'36", MTB/Q: 8533/24, elev. c. 1575 m a.s.l. | 11.07.2016 | J. Kruse | 3660 | GLM-F107704 | MF924694 | MH022818 | MF939253 | MF939319 | |
| R. montanus | Germany, Bavaria | Upper Bavaria, county Garmisch-Partenkirchen, c. 4.9 km NE of Mittenwald, Karwendel mountains, hiking path 266 from Rehbergalm to Hochland cabin, mixed mountain-forest, N47°27'37" E11°18'36", MTB/Q: 8533/24, elev. c. 1575 m a.s.l. | 11.07.2016 | J. Kruse | 3658 | GLM-F107703 | MF924693 | MH022817 | MF939252 | MF939318 | |
| R. montanus | Germany, Bavaria | Upper Bavaria, county Garmisch-Partenkirchen, c. 2.8 km SE of Mittenwald, Karwendel mountains, meadows around Brunnstein cabin, N47°24'49" E11°16'41", MTB/Q: 8533/43, elev. c. 1475 m a.s.l. | 08.07.2016 | J. Kruse | 3657 | GLM-F107702 | MF924692 | MH022816 | MF939251 | MF939317 | |
| R. montanus | Germany, Bavaria | Upper Bavaria, county Garmisch-Partenkirchen, c. 2.8 km SE of Mittenwald, Karwendel mountains, meadows around Brunnstein cabin, N47°24'49" E11°16'41", MTB/Q: 8533/43, elev. c. 1475 m a.s.l. | 08.07.2016 | J. Kruse | 3656 | GLM-F107701 | MF924719 | MH022843 | MF939270 | MF939336 | |
| R. montanus | Germany, Bavaria | Upper Bavaria, county Garmisch-Partenkirchen, c. 2.8 km SE of Mittenwald, Karwendel mountains, hiking path 290 from Brunnstein cabin towards Mittenwald, serpentines, thin mixed mountainous forest, N47°24'48" E11°16'33", MTB/Q: 8533/43, elev. c. 1380 m a.s.l. | 08.07.2016 | J. Kruse | 3655 | GLM-F107700 | MF924684 | MH022808 | MF939248 | MF939314 | |
| R. montanus | Germany, Bavaria | Oberjoch, Iseler, elev. c. 1500 m a.s.l. | 29.09.1997 | M. Piepenbring | rr502 | TUB-012586 | MF924658 | MH022782 | MF939230 | MF939296 | |
| Entyloma verruculosum | R. lanuginosus | Italy, Apulia | Monte Sant Angelo, Provinz Foggia, c. 12 km N of Monte Sant Angelo, road SP52b, Foresta Umbra, beech forest, N41°47'52" E15°58'44", elev. c. 720 m a.s.l. | 19.04.2016 | J. Kruse | 3645 | GLM-F107706 | MF924651 | MH022775 | MF939223 | MF939289 |
Type specimens are printed in bold face.
Morphological examination
The morphology of sori and spores was studied using dry herbarium specimens. For each of the host species of the two presumed complexes, up to five specimens were analysed in detail, using those specimens for which four loci (ITS, atp2, ssc1, and map) could be obtained, with four exceptions: for Entyloma sp. on Ranunculus auricomus specimens included in the two loci (ITS and atp2) dataset were used; for Entyloma sp. on R. oreophilus, one of two specimens had only two loci available; for Entyloma sp. on R. sceleratus, four of five specimens had only two loci available; and for E. eburneum one of six specimens had only two loci available. The specimens morphologically analysed are listed in the respective species descriptions.
Preparations for light microscopy (LM) were done as follows. Thin freehand sections of sori with spores and conidiophores and conidia (if present) were mounted in 80 % lactic acid, heated to the boiling point, and then immediately examined using a Nikon Eclipse 80i light microscope (Nikon) at ×1000. Thirty spores were measured using the Nikon NIS-Elements BR 3.0 imaging software (Nikon). Measurements were rounded to the nearest 0.5 μm. LM micrographs were taken with a Nikon DS-Fi1 camera (Nikon). The species descriptions include combined values from all analysed specimens of the respective species.
DNA extraction, primer design, PCR, and sequencing
Genomic DNA was isolated from 96 Entyloma herbarium specimens (Table 1). For methods regarding isolation, homogenisation of fungal material, and DNA extraction see Lutz et al. (2004) as well as Kruse et al. (2017a). PCR amplification of the complete ITS nrDNA (internal transcribed spacers) was performed with the conditions outlined in White et al. (1990), using M-ITS1 (Stoll et al. 2003) as forward and ITS4 (White et al. 1990) or smITS-R2 (Kruse et al. 2017a) as reverse primers. Plant ITS was amplified using primer pair ITS1P/ITS4 (Ridgway et al. 2003) with an annealing temperature of 53 °C. The amplification of the atp2 (ATP synthase subunit 2) locus was done according to Kruse et al. (2017b), using the F8/R4 primer combination with an annealing temperature of 54 °C. For the ssc1 (member of the heat shock protein family) and map (methionine aminopeptidase) locus used in Kruse et al. (2017b) two new primer sets specific for the Exobasidiomycetes were designed in this study on the basis of unpublished genome sequences of Exobasidium vaccinii and Pseudomicrostroma juglandis. The set of primers designed along the lines described in Kruse et al. (2017b) was tested on a variety of Exobasidiomycetes genera (Entyloma, Exobasidium, and Tilletia) and Ustilaginomycetes (Urocystis) with an annealing temperature of 53 °C. For the primer combinations providing best results gradient PCRs were conducted (50 °C to 60 °C and 60 °C to 72 °C) using Entyloma sp. samples and the optimal temperature was selected based on amplification strength and the absence of unspecific amplification. For the amplification of the ssc1 locus of Entyloma spp. this revealed the optimal primer pair to be ssc1_F3ex (5’GWGGWGAAGACTTYGACTTGT3’) and ssc1_R5ex (5’ACACCACCYTGRATSGAAGC3’) with an annealing temperature of 58 °C. For the amplification of the map locus of Entyloma spp. map_F3ex (5’AGYTGCTRATRTCGTTCCACCA3’) and map_R3ex (5’CCAYGCCAAYTTGGCCAAGAC3’) with an annealing temperature of 60 °C gave the best results.
PCR conditions were according to Kruse et al. (2017b), but with 46 PCR cycles. The resulting amplicons were sequenced at the sequencing laboratory of the Senckenberg Biodiversity and Climate Research Centre (BiK-F, Senckenberg, Germany) using the primers used in PCR, except for the map_F3ex/map_R3ex amplicons which were sequenced with a shortened reverse primer: map_R3exShort (5’CCAAYTTGGCCAAGAC3’). Sequences were deposited in GenBank (accession numbers are given in Table 1).
Molecular phylogenetic reconstruction
In total 91 ITS, 91 atp2, 64 ssc1, and 64 map sequences from Entyloma species affecting members of the genus Ranunculus were used for phylogenetic reconstructions in two different datasets. In addition to Entyloma on Ranunculus some Entyloma species on Ficaria verna were included because initial analyses suggested that Entyloma species on Ficaria verna might belong to the E. ranunculi-repentis complex. The first dataset comprised all four loci for 66 Entyloma specimens. The second comprised only ITS and atp2 sequences for 96 Entyloma specimens. Alignments were done for each locus independently using MAFFT (Katoh & Standley 2013) v. 7, employing the G-INS-i algorithm, and subsequently leading and trailing gaps were removed. After this and after checking for supported phylogenetic conflicts between the loci using Minimum Evolution analysis as outlined below, the aligned sequences of the individual loci were concatenated to obtain the datasets for phylogenetic analyses. For dataset 1 the resulting total alignment contained 1 871 characters (ITS: 523, atp2: 480, ssc1: 394, map: 474) for dataset 2 the resulting total alignment contained 1 003 characters (ITS: 523, atp2: 480). The methods for phylogenetic analyses were according to Kruse et al. (2018) for reconstructions using Minimum Evolution, Maximum Likelihood, and Bayesian Inference. To determine diagnostic bases for the different Entyloma species, alignments were checked manually for differences between the different host-fungus combinations. Host plant determination was verified comparing their ITS sequences to those deposited in GenBank (https://www.ncbi.nlm.nih.gov/genbank/) using BLASTN (Altschul et al. 1997).
RESULTS
Molecular phylogenetic reconstruction
There were no strongly supported conflicts between the topologies of the trees obtained from single loci. Minimum Evolution, Maximum Likelihood, and Bayesian Analyses yielded consistent topologies for both datasets. The results of the phylogenetic reconstructions based on four and two loci are given in Fig. 1 and Fig. 2, respectively.
Fig. 1.
Phylogenetic relationships of Entyloma species on Ranunculus spp., rooted with the specimens of the Entyloma microsporum complex, based on Minimum Evolution analyses of four loci (ITS, atp2, ssc1, and map). Numbers on branches denote bootstrap support in Minimum Evolution and Maximum Likelihood, as well as a posteriori probabilities from Bayesian Analyses, in the respective order. Values below 60 % are not shown. The scale bar indicates the number of substitutions per site.
Fig. 2.

Phylogenetic relationships of Entyloma species on Ranunculus spp., rooted with the specimens of the Entyloma microsporum complex, based on Minimum Evolution analyses of two loci (ITS and atp2). Numbers on branches denote bootstrap support in Minimum Evolution and Maximum Likelihood, as well as a posteriori probabilities from Bayesian Analyses. Values below 60 % are not shown. The scale bar indicates the number of substitutions per site.
All analyses revealed three strongly supported major lineages. The first lineage corresponded to the E. microsporum complex and included specimens from Ranunculus acris, R. paludosus, R. polyanthemos subsp. nemorosus, and R. repens, with gross morphology that matched E. microsporum. The second lineage corresponded to the E. ranunculi-repentis complex and included specimens from Ficaria verna, Ranunculus acris, R. auricomus, R. bulbosus, R. lanuginosus, R. marginatus, R. montanus, R. oreophilus, R. paludosus, R. polyanthemos subsp. nemorosus, R. repens, and R. sceleratus, with gross morphology that matched E. ranunculi-repentis. The third lineage was represented by E. verruculosum on R. lanuginosus. Within both the Entyloma microsporum complex and the E. ranunculi-repentis complex, specimens from the same host plant species grouped together, with few exceptions. Within the E. microsporum complex the majority of specimens from Ranunculus repens formed a clade together with two accessions on R. acris. Two specimens from R. repens (ML471, 102) clustered with specimens from R. polyanthemos subsp. nemorosus. Within the E. ranunculi-repentis complex, specimens from Ranunculus repens clustered together with specimens from R. bulbosus and R. polyanthemos subsp. nemorosus.
Comparing the results from both datasets, support values for the topology inferred from two loci (ITS and atp2) were mostly lower than from four loci (ITS, atp2, ssc1, and map), and the topology was generally more resolved in the latter. Within the E. microsporum complex, a group of specimens on both Ranunculus polyanthemos subsp. nemorosus and R. repens, were a sister lineage to specimens on R. acris and R. repens. The specimens on R. paludosus formed the sister group to all specimens mentioned so far. Within the E. ranunculi-repentis complex support values for the relationships of the well-supported host-specific clades were generally low.
Diagnostic bases enable the molecular identification of species given on the basis of a defined alignment (Bennett et al. 2017, Kruse et al. 2018). Diagnostic bases for the different Entyloma species are given as an overview in Fig. 3 and detailed in Table 2.
Fig. 3.
Consensus sequences for atp2, ITS, map, and ssc1, with diagnostic positions for Entyloma species on Ranunculus highlighted in bold type.
Table 2.
The diagnostic bases within the Entyloma microsporum complex and the Entyloma ranunculi-repentis complex, apart from the type host of the respective complex.
| Gene Loci |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
atp2 |
map |
ssc1 |
ITS |
||||||
| Position | Base | Position | Base | Position | Base | Position | Base | ||
| E. microsporum complex | |||||||||
| on Ranunculus paludosus | 232 | T / G | 28 | G / A | 36, 171 | G / A | 196 | T / C | |
| 364, 388, 440, 467 | G / A | 115, 289 | A / G | 68 | T / C | ||||
| 375 | C / T | 256 | T / G | 237 | T / G | ||||
| 382 | A / G | 322, 343 | T / C | 255 | C / T | ||||
| 282 | C / G | ||||||||
| E. ranunculi-repentis complex | |||||||||
| on Ranunculus acris | x | x | x | x | 27, 72, 96 | A / G | x | x | |
| 57 | T / C | ||||||||
| on Ranunculus auricomus | 142 | C / G | x | x | x | x | 168 | G / A | |
| 358 | T / A o. G | ||||||||
| 437 | G / C | ||||||||
| 440 | G / A | ||||||||
| 473 | A / G | ||||||||
| on Ficaria verna (E. ficariae) | x | x | 22, 169 | A / G | 142 | C / T | 43 | T / – | |
| 364 | T / G | 44 | G / – | ||||||
| 209 | G / A | ||||||||
| on Ficaria verna (E. majewskii) | 328, 336, 428 | A / G | 4 | T / G o. C | 1, 261 | G / A | 226, 413 | G / T | |
| 19, 172 | A / G | 2 | A / T | ||||||
| 111 | T / G o. A | ||||||||
| 258, 389 | A / G | ||||||||
| on Ranunculus lanuginosus | 389 | T / C | x | x | 144 | A / G | 462 | C / A | |
| on Ranunculus marginatus | 458 | C / G | 226, 235 | G / A | 345 | A / G | x | x | |
| 253 | A / G o. R | ||||||||
| 274 | C / T | ||||||||
| on Ranunculus montanus | 22, 28, 220, 241 | G / A | 1, 211 | A / C o. G | 75, 267 | A / G o. T | 130, 143, 171, 202, 207 | A / G | |
| 139 | A / T | 28, 58 | G / T o. A | 81, 108, 264, 267 | A / G | 47, 123,162, 429, 461 | C / T | ||
| 274 | C / A | 37, 92, 175, 208, 220 | A / G | 189 | T / C | 55, 124, 169, 172, 444 | G / A | ||
| 313, 327, 337, 352, 378 | C / T | 96 | T / C | 309 | G / C | 181 | C / G | ||
| 325, 391 | – /A o. G | 166 | G / T | 457, 480 | G / T | ||||
| 354 | T / G o. C | 352 | G / A | 158 | T / G | ||||
| 339 | C / A o. G | 427 | G / T o. C | 161 | A / T | ||||
| 355 | T / G | 164 | – / G | ||||||
| 362 | G / T | 211 | G / T o. C | ||||||
| 372 | C / T o. A | 392 | – / A | ||||||
| 380, 461 | T / A | ||||||||
| 384 | A / G o. C | ||||||||
| 392 | – / T | ||||||||
| 422, 433 | A / G | ||||||||
| on Ranunculus oreophilus | 295, 374 | A / G | 31 | A / G | 192 | A / G | 447 | A / T | |
| 330 | C / G | 194 | C / T | ||||||
| on Ranunculus paludosus | 333 | T / G o. – | x | x | 207 | A / G o. C | 395 | G / T | |
| 464 | C / T | ||||||||
| on Ranunculus sceleratus | 1, 169 | A / G | 25, 82 | C / G | 51 | A / G | 448, 466 | A / G | |
| 34, 357 | T / C | 40 | T / G | 174 | C / T | 453 | – / G | ||
| 349 | G / C | 94 | A / G o. C | 180, 336 | G / A | ||||
| 354 | C / G o. T | 157 | T / C | 210, 303 | C / A | ||||
| 358 | G / A o. T | 265 | G / T o. C | ||||||
| 371 | T / G | 406 | A / G | ||||||
| 384 | C / A o. G | ||||||||
| 393 | T / C o. – | ||||||||
*/ = instead of; x = no diagnostic bases; o. = or.
Morphology
The three major phylogenetic lineages could be distinguished by teliospore surface ornamentation. Spores from species in the E. microsporum complex had a cracked surface; those from the E. ranunculi-repentis complex were smooth; and those from E. verruculosum were verrucose. Species in the E. microsporum complex always formed sori in hard, swollen galls. Most species-specific lineages of the E. ranunculi-repentis complex produced an asexual morph, which was not observed in the E. microsporum and the E. verruculosum complexes. Morphological differences within the two species complexes were generally low. The morphological characterisation of the species is included in species descriptions, depicted in Fig. 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, and summarised in Table 3.
Fig. 4.
Entyloma bullosum on Ranunculus paludosus. a. Macroscopic symptoms of infection; b–d. spores, as seen in light microscopy, median and superficial views (from neotype). — Scale bars = 10 μm.
Fig. 5.
Entyloma microsporum on Ranunculus repens. a–b. Macroscopic symptoms of infection; c–e. spores, as seen in light microscopy, median and superficial views (from neotype). — Scale bars: b = 5 mm; c–e = 10 μm.
Fig. 6.
Entyloma piepenbringiae on Ranunculus polyanthemos subsp. nemorosus. a. Macroscopic symptoms of infection; b–d. spores, as seen in light microscopy, median and superficial views (from holotype). — Scale bars = 10 μm.
Fig. 7.
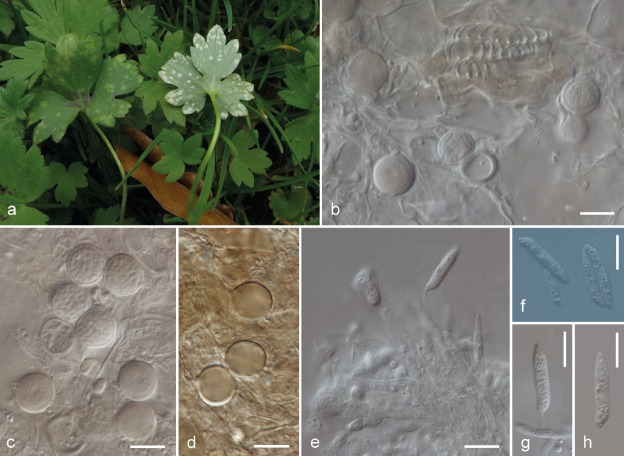
Entyloma eburneum on Ranunculus repens. a–b. Macroscopic symptoms of infection; c–e. spores, as seen in light microscopy; f. conidiophores emerging through the stoma, as seen in light microscopy; g–i. cylindrical conidia (with one conidiophore on ‘h’), as seen in light microscopy; j–l. acicular conidia, as seen in light microscopy (from neotype). — Scale bars = 10 μm.
Fig. 8.
Entyloma jolantae on Ranunculus oreophilus. a. Macroscopic symptoms of infection (two leaves to the left from holotype, one leaf to the right from paratype); b–d. spores as seen in light microscopy (from holotype). — Scale bars = 10 μm.
Fig. 9.
Entyloma klenkei on Ranunculus marginatus. a. Macroscopic symptoms of infection; b–d. spores, as seen in light microscopy (from holotype). — Scale bars = 10 μm.
Fig. 10.
Entyloma kochmanii on Ranunculus lanuginosus. a. Macroscopic symptoms of infection; b–d. spores, as seen in light microscopy; e. conidiophores emerging through the stoma, as seen in light microscopy; f. conidium, as seen in light microscopy (from holotype). — Scale bars = 10 μm.
Fig. 11.
Entyloma ranunculacearum on Ranunculus acris. a. Macroscopic symptoms of infection; b–d. spores, as seen in light microscopy; e. conidiophores emerging through the stoma (with one conidium attached to the conidiophore), as seen in light microscopy; f–h. conidia, as seen in light microscopy. Note conidium attached to the conidiophore seen on ‘g’ (from reference specimen). — Scale bars = 10 μm.
Fig. 12.
Entyloma ranunculi-scelerati on Ranunculus sceleratus. a. Macroscopic symptoms of infection; b–c. spores, as seen in light microscopy; d. conidiophores emerging through the stoma, as seen in light microscopy; e–f. cylindrical conidia, as seen in light microscopy; g–i. acicular conidia, as seen in light microscopy (from reference specimen). — Scale bars = 10 μm.
Fig. 13.
Entyloma ranunculorum on Ranunculus auricomus. a. Macroscopic symptoms of infection; b–d. spores, as seen in light microscopy; e–g. conidia, as seen in light microscopy (from reference specimen). — Scale bars = 10 μm.
Fig. 14.
Entyloma savchenkoi on Ranunculus paludosus. a. Macroscopic symptoms of infection; b–c. spores, as seen in light microscopy; d. conidiophores emerging through the stoma, as seen in light microscopy; e–g. conidia, as seen in light microscopy. Note conidium attached to the conidiophore seen on ‘g’ (from reference specimen). — Scale bars = 10 μm.
Fig. 15.
Entyloma thielii on Ranunculus montanus. a. Macroscopic symptoms of infection; b–d. spores, as seen in light microscopy (from holotype). — Scale bars = 10 μm.
Fig. 16.
Entyloma verruculosum on Ranunculus lanuginosus. a. Macroscopic symptoms of infection; b–e. spores as seen in light microscopy, median and superficial views (from GLM-F107706). — Scale bars = 10 μm.
Table 3.
Main diagnostic ecological (host species) and morphological characters for Entyloma species on Ranunculus. E. = Entyloma, R. = Ranunculus.
| Species | Host plant | Arrangement of spores in the sori (between the leaf mesophyll cells) | Spore sizes (μm) | Mean spore sizes and standard deviation (μm) | Spore wall thickness (μm) | Asexual morph | Conidia |
|---|---|---|---|---|---|---|---|
| Entyloma microsporum complex (sori forming swollen pustules and spores with cracked surface) | |||||||
| E. bullosum | R. paludosus | very densely crowded | (11.5–)15.0–21.5(–26.5) ×(10.5–)12.0–16.5(–19.5) | 18.1 ± 2.9 × 14.9 ± 1.8 | 2.5–7.0(–8.0) | absent | absent |
| E. microsporum | R. acris, R. repens (type host) | very densely crowded | 10.0–18.5(–24.0) ×(9.5–)10.0–13.5(–17.5) | 14.6 ± 2.8 × 12.2 ± 1.7 | (1.5–)2.0–4.5 | absent | absent |
| E. piepenbringiae | R. polyanthemos subsp. nemorosus (type host), R. repens | very densely crowded | (10.5–)12.0–17.5(–21.0) × (9.0–)10.0–15.5(–16.0) | 14.5 ± 2.4 × 12.5 ± 1.4 | (1.5–)2.5–4.0(–6.0) | absent | absent |
| Entyloma ranunculi-repentis complex (sori forming flat leaf spots and smooth spores) | |||||||
| E. eburneum | R. bulbosus, R. polyanthemos, R. repens | loosely scattered or moderately densely crowded | (9.5–)11.0–13.5(–16.0) × (9.0–)9.5–13.5(–14.5) | 12.3 ± 1.4 × 11.3 ± 1.3 | 1.0–1.5(–2.0) | present | dimorphic, cylindrical, 15–22 × 2.5–4.0 μm, and acicular, 30.0–45.0(–60.0) × (2.0–)2.5–3.5 μm |
| E. jolantae | R. oreophilus | densely crowded | 10.5–15.5(–16.5) × 10.0–13.5(–14.5) | 13.2 ± 1.4 × 11.6 ± 1.1 | 1.5–2.0 | absent | absent |
| E. klenkei | R. marginatus | loosely scattered | 10.5–13.0 × 10.0–12.5 | 11.7 ± 0.9 × 11.1 ± 0.7 | 1.0–1.8 | absent | absent |
| E. kochmanii | R. lanuginosus | loosely scattered | (9.0–)11.0–13.0 × (9.0–)10.0–12.5 | 11.7 ± 0.9 × 10.9 ± 0.8 | 0.5–1.5 | present | cylindrical, 20–24 × 3.0–3.5(–4.0) μm |
| E. ranunculacearum | R. acris | loosely scattered | 10.0–13.5(–14.5) × (9.0–)10.0–12.5(–13.5) | 11.8 ± 1.1 × 10.9 ± 0.8 | 0.8–1.5 | present | cylindrical, (10–)15–19(–25) × 2.5–3.5(–4.0) μm |
| E. ranunculi-scelerati | R. sceleratus | loosely scattered | (9.5–)10.0–12.5(–13.5) ×(9.0–)10.0–12.5(–13.0) | 11.7 ± 0.9 × 11.0 ± 0.9 | 1.0–1.5 | present | dimorphic, acicular, rarely cylindrical, 20–60 × (2.0–)2.5–3.5(–4.0) μm |
| E. ranunculorum | R. auricomus | loosely scattered or moderately densely crowded | 10.0–12.5(–14.5) × (9.0–)10.0–12.5(–13.0) | 11.8 ± 0.9 × 10.9 ± 0.9 | 1.0–1.5(–1.8) | present | cylindrical, 16–28 × 2.5–3.5(–4.0) μm |
| E. savchenkoi | R. paludosus | loosely scattered or moderately densely crowded | (10.0–)12.0–16.5(–18.0) × (9.0–)11.0–14.5(–15.0) | 13.9 ± 1.4 × 12.3 ± 1.2 | 1.5–2.5(–3.0) | present | acicular-cylindrical, 25–40 × 2.5–3.0(–3.5) μm |
| E. thielii | R. montanus | densely crowded, often in compact groups | (9.5–)11.0–14.5(–16.5) × 9.0–12.5(–13.0) | 12.5 ± 1.5 × 10.8 ± 1.0 | 0.8–1.5 | absent | absent |
| Entyloma verruculosum (indistinct sori and distinctly tuberculate spores) | |||||||
| E. verruculosum | Ranunculus spp. | densely crowded in the intercellular spaces | (11.0–)12.0–14.5(–16.0) × (10.5–)11.0–14.5(–15.0) | 13.4 ± 1.4 × 13.1 ± 1.4 | 1.5–2.5 | absent | absent |
TAXONOMY
In this section an overview on accepted Entyloma species on Ficaria and Ranunculus is given, and six new species are introduced. We have refrained from designating formal epitypes in the current study. The progress in sequencing technologies has already enabled the sequencing of the whole genome of specimens from the mid-19th century Irish Potato Famine (Yoshida et al. 2013, 2014). Thus, it seems to be only a matter of time until cheap and reliable whole genome sequencing from historic specimens will become routine. However, if the historic specimens turn out to be demonstrably devoid of DNA that can be used for sequencing, the reference specimens given in this section could be designated as epitypes.
Entyloma microsporum complex
Entyloma bullosum (Sacc.) J. Kruse, M. Lutz, Pia¸tek & Thines, comb. nov. — MycoBank MB823957; Fig. 4
Basionym. Caeoma bullosum Sacc., Nuovo Giorn. Bot. Ital., n.s. 22: 32. 1915.
Type. Malta, Uied il Kleigha, on Ranunculus ‘chaerophyllos’ (= R. paludosus), Mar. 1914, A. Caruana-Gatto (type could not be located, probably lost); – Greece, Rhodes, eastern coast, SE of Archangelos, c. 1.5 km S Stegna, Phrygana, northeast slope, N36°11'49″ E28°08'06″, elevation c. 70 m a.s.l., on Ranunculus paludosus, 9 Mar. 2016, J. Kruse (GLM-F107632 neotype designated here; MycoBank MBT380639; ex-type sequences available in GenBank: MF924658 (ITS), MH022782 (atp2), MF939296 (ssc1), MF939230 (map)).
Sori in the leaves, rarely leaf petioles, forming distinct, rounded, hard, swollen pustules on leaves, 1–2 mm diam, markedly delineated from the healthy host tissue, at first yellow-greenish, later brownish, usually closed but sometimes old pustules cracked. Spores embedded in the leaf tissue, single, very densely crowded in the intercellular space between the mesophyll cells, which, in older pustules are destroyed; spores subhyaline (in young sori), pale yellow to yellow (in mature sori), very variable in shape and size, globose, subglobose, broadly ellipsoidal, rarely elongated, usually more or less polyangular, (11.5–)15.0–21.5(–26.5) × (10.5–)12.0–16.5(–19.5) μm (av. ± SD, 18.1 ± 2.9 × 14.9 ± 1.8 μm, n = 150/5), with smooth surface; teliospore wall 2-layered, 2.5–7.0(–8.0) μm thick (including inner layer c. 0.8–1.0 μm thick), layers well visible in LM, often with angles, inner layer evenly thickened, outer layer unevenly thickened, spore surface rough or superficially cracked, rarely smooth. Asexual morph not found.
Diagnostic bases — Within the E. microsporum complex there are 19 diagnostic bases across all four loci (Fig. 3, Table 2).
Host plant — Parasitic on Ranunculus paludosus.
Additional specimens examined. Greece, Rhodes, c. 2.8 km NW of Lindos, Phrygana, way up Mountain, hiking path, N36°05'48″ E28°03'13″, elevation c. 145 m a.s.l., on Ranunculus paludosus, 10 Mar. 2016, J. Kruse (GLM-F107634); eastern coast, c. 3.5 km NE of Archangelos, Tsambika, way up to the monastery, northern slope, phrygana, N36°14'16″ E28°09'16″, elevation c. 160 m a.s.l., on Ranunculus paludosus, 11 Mar. 2016, J. Kruse (GLM-F107635); c. 1 km S of Salakos, way up to Mt Profitis Ilias, phrygana, N36°17'03″ E27°56'38″, elevation c. 275 m a.s.l., on Ranunculus paludosus, 13 Mar. 2016, J. Kruse (GLM-F107636); c. 1 km NW of Siana, way up to Akramitis, open phrygana, plateau, N36°09'23″ E27°45'59″, elevation c. 650 m a.s.l., on Ranunculus paludosus, 15 Mar. 2016, J. Kruse (GLM-F107637).
Notes — The smut specimens with swollen pustules on the leaves of Ranunculus paludosus are usually assigned to Entyloma microsporum (Vánky 2012), but the molecular analyses in the present study reveal that they form a distinct lineage, for which the name Caeoma bullosum is available. This species was described by Saccardo (1915) from leaves of Ranunculus chaerophyllos collected in Malta. Sydow (1924) considered that Caeoma bullosum was identical with E. microsporum. In the protologue, Saccardo (1915) did not provide the author of the name Ranunculus chaerophyllos, and in the current usage this name can be applied to three species, namely R. chaerophyllos, R. gracilis, and R. paludosus, of which only the latter occurs in Malta (Euro+Med 2006–onwards). Thus, the host plant for Caeoma bullosum is assumed to have been Ranunculus paludosus. No authentic material of this species is currently preserved in the herbarium of P.A. Saccardo deposited in PAD (R. Marcucci, pers. comm.) or in the herbarium of H. Sydow in B (R. Lücking, pers. comm.). The morphological characters provided in the protologue (swollen sori, 1–2 mm diam, yellow, angular, globose, as well as ellipsoidal spores 20–23 × 18–20 μm, spore wall of 3–5 μm thickness with a more or less warty surface; – excerpt from the Latin description) agree well with the morphology of the specimens analysed in the current study. Therefore, a neotype was designated from among the sequenced specimens to fix the application of this name. Entyloma bullosum differs from the other currently recognized species in the E. microsporum complex by a larger mean spore size and thicker spore walls.
Entyloma microsporum (Unger) J. Schröt., in Rabenhorst, Fungi Europ. no. 1872. 1874 — Fig. 5
Basionym. Protomyces microsporus Unger, Die Exantheme der Pflanzen, etc.: 343. 1833.
Synonym. Entyloma ungerianum de Bary, Bot. Zeitung (Berlin) 32: 101. 1874, nom. nov. superfl. pro P. microsporus.
Type. Austria, Tirol, Kitzbühel, on Ranunculus repens, F. Unger (type could not be located, probably lost). – Germany, Hesse, county Groß-Gerau, Ginsheim-Gustavsburg, bikeway to Mainspitzdreieck, wayside, N49°59'37″ E08°17'46″, on Ranunculus repens, 17 Nov. 2013, J. Kruse (GLM-F107661 neotype designated here; KRAM F-59043 isoneotype; MycoBank MBT380061; ex-type sequences available in GenBank: MF924636 (ITS), MH022760 (atp2), MF939279 (ssc1), MF939213 (map)).
Sori in the leaves, rarely leaf petioles, on the leaves forming distinct, rounded or elongated, hard, swollen pustules, 1–6 mm diam, markedly delineated from the healthy host tissue, at first yellow-cream, later brownish, pustules at first closed but at the maturity cracked. Spores embedded in the leaf tissue, single, very densely crowded in the intercellular space between the mesophyll cells, which in mature pustules are totally destroyed; spores subhyaline or rarely pale yellow, variable in shape and size, globose, subglobose, broadly ellipsoidal, rarely elongated, often more or less irregular, 10.0–18.5(–24.0) × (9.5–)10.0–13.5(–17.5) μm (av. ± SD, 14.6 ± 2.8 × 12.2 ± 1.7 μm, n = 150/5), with smooth or granular context; wall 2-layered, (1.5–)2.0–4.5 μm, occasionally 7.0 μm thick (including inner layer c. 0.5–1.0 μm thick), sometimes with angles, layers well visible in LM, inner layer evenly thickened, outer layer evenly or unevenly thickened, spore surface rough or superficially cracked, rarely smooth. Asexual morph not found.
Host plants — Parasitic on Ranunculus acris and R. repens.
Additional specimens examined. Germany, Baden-Württemberg, county Konstanz, Hegau, W of Singen, way up to Mt Hohentwiel, wayside, elevation c. 600 m a.s.l., on Ranunculus repens, 29 May 2013, J. Kruse (GLM-F107672); Bavaria, county Rottal-Inn, Simbach, road St 2112, grassland at roundabout, N48°16'23″ E13°00'53″, elevation c. 370 m a.s.l., on Ranunculus acris, 14 Aug. 2014, J. Kruse (GLM-F107663); Lower Saxony, county Hildesheim, Brüggen, Kirschweg, Sieben Bergen, Mt Hohe Tafel, wayside, elevation c. 395 m a.s.l., on Ranunculus repens, 8 May 2011, J. Kruse (GLM-F107667). – Poland, Małopolska Province, Tatra Mts, Hala Ga¸sienicowa glade (near Murowaniec cabin), elevation c. 1510 m a.s.l., on Ranunculus repens, 24 Sept. 2005, J. Pia¸tek & M. Pia¸tek (KRAM F-59041).
Notes — This species has been first described as Protomyces microsporus. In the protologue, Unger (1833) contrasted it with Protomyces macrosporus (Ascomycota, Taphrinales) as a species forming pustules on stems and leaf veins of Ranunculus repens and having very small, rounded and pale sporidia (= spores). De Bary (1874) obtained spore germination of this species and concluded that it is not a member of Protomyces but a smut fungus, for which he described the distinct genus, Entyloma. He introduced the new name Entyloma ungerianum for this species. However, this was superfluous and Schröter (in Rabenhorst 1874) combined the species in Entyloma as E. microsporum. The original material probably does not exist anymore. Piepenbring (2003) could not locate it in BPI, GJO, M, and W. The current species concept of E. microsporum is based on a long tradition of application of this name to any specimen of Ranunculus displaying the characters reported by Unger (1833). However, spore sizes were not reported in the protologue (Unger 1833). Also De Bary (1874) did not provide spore sizes for material examined by him. Schröter (1887) finally measured the spores of this species, reporting the following values: spores 15–24 μm long and 12–17 μm wide, wall up to 7 μm thick. Similar counts were reported more recently, e.g., Vánky (1994, 2012: spores 11–23 × 10–16 μm, wall 1–9 μm thick), Scholz & Scholz (1988: spores 10–25 μm diam, wall 1–9 μm thick), but Kochman (1936) reported that spores were 10–20 μm diam (with mean 14 μm) and the wall thickness was reported as 1.5–5 μm. The latter observations are in agreement with our observations, and it seems possible that the larger spore sizes reported by other authors result from the presentation of extreme values without indicating which values predominated in the overall spore counts.
In the phylogenetic analyses the specimens forming swollen pustules on Ranunculus repens clustered in two lineages: one containing the majority of accessions on R. repens and two accessions on R. acris, and the other containing the minority of accessions on R. repens with predominance of accessions on R. polyanthemos subsp. nemorosus. The specimens in both lineages were morphologically similar, and it is not clear to which of the two lineages the name E. microsporum could be applied. Therefore, to stabilize this fungus name we designate a neotype from specimens from the lineage where most accessions on R. repens were placed. The specimens on R. acris were inseparable morphologically and only very weakly separated genetically, and are therefore currently remain in E. microsporum. The specimens forming the second lineage are accommodated in the novel species, E. piepenbringiae.
Entyloma piepenbringiae J. Kruse, M. Lutz, Pia¸tek & Thines, sp. nov. — MycoBank MB824511; Fig. 6
Etymology. Named in honour of Prof. dr Meike Piepenbring (Frankfurt a. Main), for her contributions to the knowledge of temperate and tropical smut fungi.
Type. Germany, Bavaria, Oberallgäu, Einödsbach, Allgäu Alps, hiking path from Black cabin to Rappensee cabin, meadow W Rappensee cabin, N47°17'24″ E10°14'40″, elevation c. 1900 m a.s.l., on Ranunculus polyanthemos subsp. nemorosus, 26 July 2015, J. Kruse (GLM-F107687 holotype; ex-type sequences available in GenBank: MF924664 (ITS), MH022788 (atp2), MF939302 (ssc1), MF939236 (map)).
Sori in the leaves, rarely leaf petioles, on the leaves forming distinct, rounded or elongated, hard, swollen pustules, 1–5 mm diam, markedly delineated from the healthy host tissue, at first creamy yellow, later brownish, usually closed but sometimes old pustules cracked. Spores embedded in the leaf tissue, single, very densely crowded in the intercellular space between the mesophyll cells, which in older pustules are totally destroyed; spores subhyaline or rarely pale yellow, variable in shape and size, globose, subglobose, broadly ellipsoidal, rarely elongated, often more or less irregular, (10.5–)12.0–17.5(–21.0) × (9.0–)10.0–15.5(–16.0) μm (av. ± SD, 14.5 ± 2.4 × 12.5 ± 1.4 μm, n = 150/5), with smooth context; wall 2-layered, (1.5–)2.5–4.0(–6.0) μm thick (including inner layer c. 0.7–1.0 μm thick), sometimes with angles, layers well visible in LM, inner layer evenly thickened, outer layer evenly or unevenly thickened, spore surface rough or superficially cracked, rarely smooth. Asexual morph not found.
Host plants — Parasitic on Ranunculus polyanthemos subsp. nemorosus and R. repens.
Additional specimens examined. Germany, Baden-Württemberg, county Konstanz, communal Moos, S of Weiler, near Grey Reed, wayside, elevation c. 445 m a.s.l., on Ranunculus repens, 30 May 2013, J. Kruse (GLM-F107694); Bavaria, Upper Bavaria, county Garmisch-Partenkirchen, c. 2.8 km SE of Mittenwald, Karwendel mountains, meadow around Brunnstein cabin, N47°24'49″ E11°16'41″, elevation c. 1475 m a.s.l., on Ranunculus polyanthemos subsp. nemorosus, 8 July 2016, J. Kruse (GLM-F107690); hiking path 290 towards Brunnstein cabin, serpentines, open mixed mountainous forest, N47°24'44″ E11°16'23″, elevation c. 1260 m a.s.l., on Ranunculus polyanthemos subsp. nemorosus, 6 July 2016, J. Kruse (GLM-F107693); c. 3.2 km SE of Mittenwald, Karwendel mountains, hiking path 291 from Brunnstein cabin towards Mt Brunnsteinspitze, scree, N47°24'33″ E11°16'59″, elevation c. 1760 m a.s.l., on Ranunculus polyanthemos subsp. nemorosus, 7 July 2016, J. Kruse (GLM-F107691).
Notes — The specimens on Ranunculus polyanthemos subsp. nemorosus and R. repens were morphologically similar and clustered together, and are therefore considered as belonging to the same species.
Entyloma ranunculi-repentis complex
Entyloma eburneum (J. Schröt.) J. Kruse, M. Lutz, Pia¸tek & Thines, comb. nov. — MycoBank MB824512; Fig. 7
Basionym. Fusidium eburneum J. Schröt., Beitr. Biol. Pflanzen 2 (3): 373. 1877.
Type. On Ranunculus repens, (further details not included in the protologue, but probably the material was collected in Silesia, now in Poland, by J. Schröter, before 1877 (type could not be located, probably lost). – Poland, Małopolska Province, Kraków-Pleszów, at Suchy Jar street, on Ranunculus repens, 20 Nov. 2010, M. Pia¸tek (KRAM F-59037 neotype designated here; MycoBank MBT380062; ex-type sequences available in GenBank: MF924689 (ITS), MH022813 (atp2)).
Synonyms. Ramularia repentis Oudem., Beih. Bot. Centralbl.: 15. 1902.
Type. The Netherlands, Valkenberg, on Ranunculus repens, 1900, C.A.J.A. Oudemans (L, see Braun 1998).
Entyloma ranunculi-repentis Sternon, L’hétérogenité du genre Ramularia, These, Nancy: 34, 45. 1925.
Type. Belgium, Gembloux, Virton and Rochefort, on Ranunculus repens, 1917, F. Sternon (no type designated, see Vánky 2012).
Entyloma wroblewskii Kochman, Acta Soc. Bot. Poloniae 11 (Suppl.): 291. 1934.
Type. Poland, Anin near Warszawa, on Ranunculus polyanthemos, 15 Sept. 1933, J. Kochman (KRAM F-2658 holotype; KRAM F-2656 and KRAM F-2657 isotypes).
Sori in the leaves, forming very distinct, flat, rounded, polyangular or irregular spots, 0.5–4 mm long, 0.5–2 mm wide, usually partly delineated by the leaf veins of the host, at first whitish or cream-coloured due to the presence of the conidiophores and conidia of the asexual morph, later pale brown on both sides of the leaf. Spores embedded in the leaf tissue, single, loosely scattered or moderately densely crowded in the intercellular space between the mesophyll cells; spores pale yellow to yellow, globose, subglobose or rarely broadly ellipsoidal, regular in shape, (9.5–)11.0–13.5(–16.0) × (9.0–)9.5–13.5(–14.5) μm (av. ± SD, 12.3 ± 1.4 × 11.3 ± 1.3 μm, n = 200/6), with smooth context; wall 2-layered, 1.0–1.5(–2.0) μm thick (including inner layer c. 0.5–0.8 μm thick), without angles, layers well visible in LM, both layers evenly thickened, spore surface smooth. Asexual morph entylomella-like, very well developed. Caespituli both hypophyllous and epiphyllous, conidiophores in dense, agglutinated fascicles, emerging through stomata, hyaline, conidiogenous loci inconspicuous. Conidia solitary, hyaline, dimorphic, cylindrical, straight or somewhat curved, 15–22 × 2.5–4.0 μm, and acicular, straight or somewhat curved, 30.0–45.0(–60.0) × (2.0–)2.5–3.5 μm, non-septate, hilum inconspicuous, not darkened.
Host plants — Parasitic on Ranunculus bulbosus, R. polyanthemos, and R. repens.
Additional specimens examined. Germany, Baden-Württemberg, Swabian Alps, county Sigmaringen, Leibertingen-Wildenstein, S of Beuron, ascent to castle Wildenstein, mixed forest, wayside, N48°02'49″ E08°58'17″, elevation c. 682 m a.s.l., on Ranunculus repens, 6 June 2014, J. Kruse (GLM-F107638); Bavaria, Upper Bavaria, county Garmisch-Partenkirchen, c. 4.9 km NE of Mittenwald, Karwendel mountains, hiking path 266 from Rehbergalm to Hochland cabin, mixed mountainous forest, N47°27'37″ E11°18'36″, elevation c. 1575 m a.s.l., on Ranunculus polyanthemos subsp. nemorosus, 11 July 2016, J. Kruse (GLM-F107647); Hesse, Main-Taunus-county, Hattersheim on Main, grassland at Welschenstream, Kuckuckspfad, wayside, N50°03'54″ E08°30'03″, elevation c. 90 m a.s.l., on Ranunculus bulbosus, 30 Apr. 2016, J. Kruse (GLM-F107644); Lower Saxony, county Northeim, at the bottom of the Katlencastle, wayside near river, elevation c. 110 m a.s.l., on Ranunculus repens, 23 Apr. 2010, J. Kruse (GLM-F107648). – Italy, Liguria, Lower Varavalley, c. 1.5 km SW Tavarone, circular path, Monte Alpe from Agriturismo Giandriale, east slope, meadow, N44°18'28″ E09°31'58″, elevation c. 725 m a.s.l., on Ranunculus bulbosus, 10 May 2016, J. Kruse (GLM-F107645).
Notes — The Entyloma species on Ranunculus repens causing flat spots is usually referred to as Entyloma ranunculi-repentis, which is the earliest available name for the teleomorph (Vánky 2012). Ramularia gibba (= Entylomella gibba) was considered to be the earliest name for the asexual morph (Braun 1998, Vánky 2012), which is an earlier name than Entyloma ranunculi-repentis, and following the ‘one fungus, one name rule’ (Hawksworth et al. 2011), Rossman & Castlebury (in Rossman et al. 2016) proposed the new combination Entyloma gibbum. However, they were apparently not aware that the original description and type material of Ramularia gibba were based on mixed elements of two fungi: the entylomella-like asexual morph of E. eburneum, and the sexual morph of E. microsporum. Due to the inseparable chimeric description and material, Kruse & Thines (2017) proposed the rejection of Ramularia gibba. The oldest available name for a flat-spotting Entyloma species on Ranunculus repens is Fusidium eburneum. This species has been described by Schröter (1877) for a conidial fungus on Ranunculus repens resembling the conidial state of Entyloma ranunculi (= Entyloma ficariae), producing whitish or yellowish spots, 1.5–2 mm diam and having hyaline, filamentous conidia 40–50 μm long and 2.5–3.0 μm wide. This morphological characterisation agrees well with the morphology of the asexual state in the holomorphic specimens analysed in the current study. Schröter (1877) did not observe corresponding Entyloma-like spores in the leaves. He thus might have analysed a young infection in which leaf spots and conidia are prominently developed, but teliospores are lacking. Fusidium eburneum is an earlier name than Entyloma ranunculi-repentis, and in line with the current International Code of Nomenclature for algae, fungi, and plants (McNeill et al. 2012) should be applied for the holomorph. In the protologue, Schröter (1877) did not provide a specific localization of the collected material, but in the monograph dealing with Silesian fungi (Schröter 1887), he enumerated several collections from Silesia. Authentic material of Fusidium eburneum is not preserved in the herbarium of J. Schröter deposited in WRSL (M. Halama, pers. comm.). Likewise, we could not locate any original material in other herbaria where some specimens of J. Schröter might have been deposited (e.g., in HBG; T. Feuerer, pers. comm.). Therefore, we are designating a neotype from among the specimens that were sequenced in this study. The neotype represents a holomorphic specimen with an asexual morph having characters that perfectly fit with the description in the protologue.
The present molecular and morphological analyses suggest that Entyloma specimens on Ranunculus bulbosus, R. polyanthemos subsp. nemorosus, and R. repens p.p. represent a single species. Entyloma on Ranunculus polyanthemos was previously described as a distinct species, Entyloma wroblewskii (Kochman 1934), which is considered as synonym with Entyloma eburneum, here. In the protologue of E. wroblewskii, Kochman (1934) reported one collection on Ranunculus polyanthemos collected in September 1933 in Anin near Warszawa (now within the borders of Warszawa) in Poland. In the herbarium KRAM F there are three specimens of E. wroblewskii having labels matching all information from the protologue, with the exception that the date of collection is given precisely as 15 September 1933 – these specimens apparently represent one original gathering. The label on one of these specimens is written in Latin and the species name is given as ‘Entyloma Wróblewskii n. sp. Kochman’ – this specimen should be considered as holotype. The labels on two remaining specimens are written in Polish and lack ‘n. sp.’ next to the species name – these specimens should be considered as isotypes. Vánky (2012) mentioned that type of E. wroblewskii is deposited in the herbarium WA. However, the corresponding herbarium specimen in WA was apparently collected in Anin a year later, on 15 September 1934 (M. Graniszewska, pers. comm.) and distributed in Kochman's exsiccates, Ustilaginales Poloniae no. 28 – therefore, this specimen does not represent the original gathering.
Entyloma eburneum is morphologically distinct from most other Entyloma species infecting Ranunculus spp. in having prominently developed leaf spots, relatively large spores and dimorphic conidia (cylindrical and acicular). Entyloma ranunculi-scelerati is the most similar species, but differs in having somewhat smaller spores and longer, predominantly acicular conidia.
Entyloma jolantae J. Kruse, M. Lutz, Pia¸tek & Thines, sp. nov. — MycoBank MB824513; Fig. 8
Etymology. Named after Jolanta Pia¸tek (Kraków, Poland), Polish phycologist, who together with the second author of this work collected this smut and many other smut fungi during joint field trips in Europe and Africa.
Type. Poland, Małopolska Province, Tatra Mts, Mała Dolinka valley – northern slopes of Giewont Mt, elevation c. 1230 m a.s.l., on Ranunculus oreophilus, 25 Aug. 2008, J. Pia¸tek & M. Pia¸tek (KRAM F-59030 holotype; ex-type sequences available in GenBank: MF924688 (ITS), MH022812 (atp2), MF939316 (ssc1), MF939250 (map)).
Sori in the leaves, forming distinct flat spots, 0.5–3 mm long, 0.5–2 mm wide, rounded or more or less polyangular – usually well delineated by the leaf veins of the host, at first creamcoloured, later brownish on both sides of the leaf, finally necrotic. Spores embedded in the leaf tissue, single, densely crowded in the intercellular space between the mesophyll cells; spores subhyaline to pale yellow, globose, subglobose or broadly ellipsoidal and often somewhat irregular due to mutual pressure, 10.5–15.5(–16.5) × 10.0–13.5(–14.5) μm (av. ± SD, 13.2 ± 1.4 × 11.6 ± 1.1 μm, n = 60/2), with smooth context; wall 2-layered, 1.5–2.0 μm thick (including inner layer c. 0.5–0.8 μm thick), layers well visible in LM, inner layer evenly thickened, outer layer unevenly thickened, spore surface smooth. Asexual morph not found.
Diagnostic bases — Within the E. ranunculi-repentis complex there are seven diagnostic bases distributed among all loci (Fig. 3, Table 2).
Host plant — Parasitic on Ranunculus oreophilus.
Additional specimen examined. Poland, Małopolska Province, Tatra Mts, Mała Dolinka valley – northern slopes of Giewont Mt, elevation c. 1260 m a.s.l., on Ranunculus oreophilus, 25 Aug. 2008, J. Pia¸tek & M. Pia¸tek (KRAM F-59031).
Notes — This species differs from most other species in the E. ranunculi-repentis complex by having larger spores with larger mean spore sizes, somewhat thicker spore walls, and lacking the asexual morph. Entyloma savchenkoi is the most similar species that differs in having an asexual morph.
Entyloma klenkei J. Kruse, M. Lutz, Pia¸tek & Thines, sp. nov. — MycoBank MB824514; Fig. 9
Etymology. Named after Friedemann Klenke (Naundorf, Germany), for his eminent contributions as field mycologist to the knowledge on plant pathogenic fungi, e.g. as the lead author of the reference work Pflanzenparasitische Kleinpilze (Klenke & Scholler 2015).
Type. Greece, Rhodes, c. 0.7 km W of Archipoli, Eparchiaki Odos Pastidas-Mesanagrou, field beneath street, N36°15'58″ E28°03'11″, elevation c. 185 m a.s.l., on Ranunculus marginatus, 13 Mar. 2016, J. Kruse & V. Kummer (GLM-F107659 holotype; ex-type sequences available in GenBank: MF924663 (ITS), MH022787 (atp2), MF939301 (ssc1), MF939235 (map)).
Sori in the leaves, forming indistinct, flat, polyangularly rounded spots, 1.5–2 mm diam, dirty yellow in colour. Spores embedded in the leaf tissue, single, loosely scattered in the intercellular space between the mesophyll cells; spores subhyaline to pale yellow, globose or subglobose, regular in shape, 10.5–13.0 × 10.0–12.5 μm (av. ± SD, 11.7 ± 0.9 × 11.1 ± 0.7 μm, n = 30/1), with smooth context; wall 2-layered, 1.0–1.8 μm thick (including inner layer c. 0.5 μm thick), without angles, layers hardly visible in LM, both layers evenly thickened, spore surface smooth. Asexual morph not found.
Diagnostic bases — Within the E. ranunculi-repentis complex there are six diagnostic bases distributed among all loci except ITS (Fig. 3, Table 2).
Host plant — Parasitic on Ranunculus marginatus.
Notes — This species differs from most other species in the E. ranunculi-repentis complex by the combination of small spores (with thin walls), which are loosely scattered between leaf mesophyll cells, and lacking an asexual morph. The most similar species is Entyloma thielii, which differs in having densely crowded spores, often in compact groups, in the intercellular space between the mesophyll cells.
Entyloma kochmanii J. Kruse, M. Lutz, Pia¸tek & Thines, sp. nov. — MycoBank MB824515; Fig. 10
Etymology. Dedicated to the memory of Józef Kochman (1903–1995), Polish smut taxonomist, who first challenged the view that Entyloma specimens on Ranunculus represent just three species.
Type. Italy, Liguria, Varavalley, c. 2 km NE of Caranza, Strada Provinciale from Caranza to Passo della Cappelletta, alluvial canyon forest, N44°23'33″ E09°38'44″, elevation c. 840 m a.s.l., on Ranunculus lanuginosus, 9 May 2016, J. Kruse (GLM-F107660 holotype; ex-type sequences available in GenBank: MF924678 (ITS), MH022802 (atp2), MF939309 (ssc1), MF939243 (map)).
Sori in the leaves, forming small, moderately distinct, flat, rounded or somewhat polyangular spots, 0.5–1 mm diam, usually delineated by the leaf veins of the host, yellow or cream-coloured on the upper side of the leaf, whitish on the lower side of the leaf due to the presence of the conidiophores and conidia of the asexual morph. Spores embedded in the leaf tissue, single, loosely scattered in the intercellular space between the mesophyll cells; spores pale yellow, globose or subglobose, regular in shape, (9.0–)11.0–13.0 × (9.0–)10.0–12.5 μm (av. ± SD, 11.7 ± 0.9 × 10.9 ± 0.8 μm, n = 30/1), with smooth context; wall 2-layered, 0.5–1.5 μm thick (including inner layer c. 0.2–0.5 μm thick), without angles but sometimes with hyaline appendage, layers hardly visible in LM, both layers evenly thickened, spore surface smooth. Asexual morph entylomella-like, weakly developed. Caespituli hypophyllous, conidiophores in dense fascicles, emerging through stomata, hyaline, conidiogenous loci inconspicuous. Conidia solitary, hyaline, cylindrical, 20–24 × 3.0–3.5(–4.0) μm, non-septate, hilum inconspicuous, not darkened.
Diagnostic bases — Within the E. ranunculi-repentis complex there are three diagnostic bases distributed among all loci, except map (Fig. 3, Table 2).
Host plant — Parasitic on Ranunculus lanuginosus.
Notes — This species differs from most other species in the E. ranunculi-repentis complex by the combination of small spores (with thin walls) and cylindrical conidia. Entyloma ranunculacearum differs in having shorter conidia, while E. ranunculorum differs in having longer conidia.
Entyloma ranunculacearum Kochman, Pl. Polon. 4: 105. 1936 — Fig. 11
Type. Ukraine, district Mościska, Krukienice, on Ranunculus acris, 17 Aug. 1935, J. Kochman (KRAM F-2606 lectotype indicated by Lindeberg 1959: 41, but precisely designated here; MycoBank MBT380645).
Reference specimen. Germany, Saxony-Anhalt, county Wittenberg, Kemberg, district Rotta-Gniest, Heidestreet, wayside, N51°45'04″ E12°35'33″, elevation c. 105 m a.s.l., on Ranunculus acris, 13 Nov. 2013, J. Kruse (GLM-F107680 reference specimen designated here; ex-reference specimen sequences available in GenBank: MF924637 (ITS), MH022761 (atp2), MF939280 (ssc1), MF939214 (map)).
Sori in the leaves, forming distinct, flat, rounded or somewhat irregular spots, 0.5–4 mm diam, usually partly delineated by the leaf veins of the host, yellowish on the upper side of the leaf, whitish on the lower side of the leaf due to the presence of the conidiophores and conidia of the asexual morph. Spores embedded in the leaf tissue, single, loosely scattered in the intercellular space between the mesophyll cells; spores subhyaline to pale yellow, globose, subglobose or rarely broadly ellipsoidal, regular in shape, 10.0–13.5(–14.5) × (9.0–)10.0–12.5(–13.5) μm (av. ± SD, 11.8 ± 1.1 × 10.9 ± 0.8 μm, n = 150/5), with smooth context; wall 2-layered, 0.8–1.5 μm thick (including inner layer c. 0.3–0.5(–0.8) μm thick), without angles, layers hardly visible in LM, both layers evenly thickened, spore surface smooth. Asexual morph entylomella-like, prominently developed. Caespituli hypophyllous, conidiophores in dense, agglutinated fascicles, emerging through stomata, hyaline, conidiogenous loci inconspicuous. Conidia solitary, hyaline, cylindrical, straight, sometimes slightly curved, (10–)15–19(–25) × 2.5–3.5(–4.0) μm, non-septate, hilum inconspicuous, not darkened.
Diagnostic bases — Within the E. ranunculi-repentis complex there are four diagnostic bases within the ssc1 locus (Fig. 3, Table 2).
Host plant — Parasitic on Ranunculus acris.
Additional specimens examined. Germany, Baden-Württemberg, county Konstanz, Lake Constance, Radolfzell, SE of Möggingen, Mindelsee, circular path around lake, littoral and wayside, elevation c. 420 m a.s.l., on Ranunculus acris, 30 May 2013, J. Kruse (GLM-F107678); Bavaria, Oberfranken, county Bamberg, SE of Sandhof, Mönchsweiher, mixed forest on Keuper-Sandstone, elevation c. 290 m a.s.l., on Ranunculus acris, 5 May 2012, J. Kruse (GLM-F107676); Hesse, Rheingau-Taunus-county, Eltville on Rhine, Rheinsteig, direction to forest-restaurant Rausch, N50°02'46″ E08°05'44″, elevation c. 160 m a.s.l., on Ranunculus acris, 8 Mar. 2014, J. Kruse (GLM-F107679); Saarland, Mettlach-Orscholz, county Merzig-Wadern, Cloef-Street, surroundings of Cloef-Atrium and Varadeser Park, N49°30'20″ E06°32'06″, elevation c. 395 m a.s.l., on Ranunculus acris, 29 Sept. 2014, J. Kruse (GLM-F107684).
Notes — When describing this species, Kochman (1936) reported three collections: two on R. acris and one on R. lanuginosus. In the Polish text he wrote that the typical form of this species infects R. acris and an additional host is R. lanuginosus. In the Latin diagnosis, Kochman (1936) reported only R. acris as type host without an indication of the specific collection. Lindeberg (1959) designated the lectotype from one of the two collections on R. acris (collected in 1935 in Krukienice, district Mościska, then in Poland but now in Ukraine), but she did not mention where the specimen was deposited. This specimen is currently preserved in the herbarium KRAM F. Kochman (1936) reported the date of collection as 1935, but on the lectotype specimen the exact date is given as 17 August 1935. Entyloma sp. on R. lanuginosus belongs to a distinct species, described here as E. kochmanii, which is phylogenetically closely related but distinct from E. ranunculacearum in having longer conidia.
Entyloma ranunculi-scelerati Kochman, Pl. Polon. 4: 104. 1936 — Fig. 12
Type. Poland, Skierniewice-Glinianki, on Ranunculus sceleratus, 2 July 1927, W. Konopacka (BRIP: HUV 974 lectotype, isolectotypes in Kochman, Ust. Pol. no. 29; lectotype designated by Lindeberg 1959: 41, corrected and narrowed by Vánky 1985: 66).
Reference specimen. Poland, Mazowieckie Province, Warszawa-Wesoła, on Ranunculus sceleratus, 17 July 2015, P. Me¸dykowski (KRAM F-59032 reference specimen designated here; ex-reference specimen sequences available in GenBank: MF924691 (ITS), MH022815 (atp2)).
Sori in the leaves, forming distinct, flat, rounded spots, 1–4 mm diam, yellow or light brown on the upper side of the leaf, whitish or cream coloured on the lower side of the leaf due to the presence of the conidiophores and conidia of the asexual morph, surrounded by brownish rim, finally necrotic – starting from the centre of the sori. Spores embedded in the leaf tissue, single, loosely scattered in the intercellular space between the mesophyll cells; spores subhyaline, pale yellow or yellow, globose or subglobose, regular in shape, (9.5–)10.0–12.5(–13.5) × (9.0–)10.0–12.5(–13.0) μm (av. ± SD, 11.7 ± 0.9 × 11.0 ± 0.9 μm, n = 150/5), with smooth context; wall 2-layered, 1.0–1.5 μm thick (including inner layer c. 0.3–0.8 μm thick), without angles, layers hardly visible in LM, both layers evenly thickened, spore surface smooth. Asexual morph entylomella-like, prominently developed. Caespituli both hypophyllous and epiphyllous, conidiophores in dense fascicles, emerging through stomata, hyaline, conidiogenous loci inconspicuous. Conidia solitary, hyaline, acicular, rarely cylindrical, usually straight, 20–60 × (2.0–)2.5–3.5(–4.0) μm, non-septate, hilum inconspicuous, not darkened.
Diagnostic bases — Within the E. ranunculi-repentis complex there are 26 diagnostic bases distributed among all loci (Fig. 3, Table 2).
Host plant — Parasitic on Ranunculus sceleratus.
Additional specimens examined. Germany, Saxony-Anhalt, SE of Allstedt, Ziegelrodaer forest (N-part), airport Allstedt (NW-side), on Ranunculus sceleratus, 23 Oct. 2005, H. Jage (GLM-F076138); Lodersleben, near castle, in Querne, on Ranunculus sceleratus, 6 May 2005, H. John & H. Jage (GLM-F076186); Friedersdorf near Lohsa S, WSW Neuhof, near Ballackmühle, Maxsee (part of Ballacksee), on Ranunculus sceleratus, 26 May 2006, H. Jage (GLM-F086008); Bavaria, Oberpfalz, county Grafenwöhr, E of Hütten, littoral of lake, N49°40'52″ E11°58'42″, elevation c. 410 m a.s.l., on Ranunculus sceleratus, 1 May 2016, G. Hübner (GLM-F107685).
Notes — In the protologue, Kochman (1936) reported two collections on Ranunculus sceleratus: one collected in Skierniewice-Glinianki in 1927 by W. Konopacka, and another, collected in Skierniewice-Zwierzyniec in 1925 by W. Siemaszko, both in Poland. Lindeberg (1959) designated the collection in Skierniewice-Glinianki as the lectotype, but erroneously wrote that the material was collected in 1925 by W. Siemaszko – apparently mixing data from both original collections. Also, Lindeberg (1959) did not mention where the specimen is deposited. Vánky (1985) corrected her mistake and narrowed the lectotype to the specimen in HUV. Kochman (1936) reported the date of collection as 1927 but on the lectotype specimen the exact date is given as 2 July 1927. KRAM F-2628 is a specimen labelled as Entyloma ranunculi-scelerati collected on Ranunculus sceleratus in Skierniewice-Glinianki by W. Konopacka on 28 May 1927. This specimen may represent authentic material but in the light of Vánky's (1985) lectotypification its status remains unclear.
Entyloma ranunculi-scelerati is most similar to E. eburneum, which differs in having somewhat larger spores and shorter conidia.
Entyloma ranunculorum Liro, Mycoth. Fennic. Die Etiketten. No. 301–600: 25. 1939 — Fig. 13
Synonym. Entyloma ranunculorum Liro, Ann. Acad. Sci. Fenn., Ser. A, 42 (1): 111. 1938, invalid name, no Latin description or diagnosis.
Type. Sweden, Härjedalen, Fjellnäs, on Ranunculus auricomus, July 1897, G. Lagerheim (BRIP: HUV 894 lectotype, isolectotypes in Sydow, Ust. no. 233, as Entyloma ranunculi; lectotype designated by Vánky 1985: 66).
Reference specimen. Germany, Bavaria, Oberfranken, county Kulmbach, Lindau, Mountain chain Rough Mt, wayside, elevation c. 410 m a.s.l., on Ranunculus auricomus agg., 12 May 2012, J. Kruse (GLM-F107686 reference specimen designated here, ex-reference specimen sequences available in GenBank: MF924629 (ITS), MH022753 (atp2)).
Sori in the leaves, forming distinct, flat, rounded or somewhat polyangular spots, 1–4 mm diam, whitish or cream coloured on both sides of the leaf. Spores embedded in the leaf tissue, single, loosely scattered or moderately densely crowded in the intercellular space between the mesophyll cells; spores subhyaline, pale yellow to yellow, globose, subglobose or broadly ellipsoidal, usually regular but sometimes somewhat irregular due to mutual pressure, 10.0–12.5(–14.5) × (9.0–)10.0–12.5(–13.0) μm (av. ± SD, 11.8 ± 0.9 × 10.9 ± 0.9 μm, n = 60/2), with smooth context; wall 2-layered, 1.0–1.5(–1.8) μm thick (including inner layer c. 0.5–0.8 μm thick), without angles, layers hardly visible in LM, both layers evenly thickened, spore surface smooth. Asexual morph entylomella-like, well-developed. Caespituli hypophyllous, conidiophores in densely agglutinated fascicles, emerging through stomata, hyaline, conidiogenous loci inconspicuous. Conidia solitary, hyaline, cylindrical, usually curved, rarely almost straight, 16–28 × 2.5–3.5(–4.0) μm, non-septate, hilum inconspicuous, not darkened.
Diagnostic bases — Within the E. ranunculi-repentis complex there are six diagnostic bases within ITS and the atp2 locus (Fig. 3, Table 2).
Host plant — Parasitic on Ranunculus auricomus.
Additional specimen examined. Germany, Saxony-Anhalt, E of Dölkau, Burgholz (E-part) Jagen 29, alluvial forest, elevation c. 25 m a.s.l., on Ranunculus auricomus, 19 Apr. 1998, H. Jage (GLM-F048093).
Notes — The most similar species are Entyloma kochmanii and E. ranunculacearum, which differ in having shorter conidia.
Entyloma savchenkoi J. Kruse, M. Lutz, Pia¸tek & Thines, sp. nov. — MycoBank MB824516; Fig. 14
Etymology. Named after Kyrylo G. Savchenko (Pullman, United States), Ukrainian mycologist, for his contributions to Entyloma phylogeny and taxonomy.
Type. Greece, Rhodes, eastern coast, c. 2.5 km N of Kalathos, street towards Masari, wayside, olive grove, N36°08'47″ E28°03'33″, elevation c. 15 m a.s.l., on Ranunculus paludosus, 20 Mar. 2016, J. Kruse (GLM-F107699 holotype; ex-type sequences available in GenBank: MF924662 (ITS), MH022786 (atp2), MF939300 (ssc1), MF939234 (map)).
Sori in the leaves, forming rather indistinct, flat, rounded or somewhat polyangular spots, 1–3 mm long, 1–2 mm wide, yellow or light brown on the upper side of the leaf, whitish or cream coloured on the lower side of the leaf. Spores embedded in the leaf tissue, single, loosely scattered or moderately densely crowded in the intercellular space between the mesophyll cells; spores subhyaline, pale yellow to yellow, globose, subglobose or broadly ellipsoidal, usually regular but sometimes somewhat irregular due to mutual pressure, (10.0–)12.0–16.5(–18.0) × (9.0–)11.0–14.5(–15.0) μm (av. ± SD, 13.9 ± 1.4 × 12.3 ± 1.2 μm, n = 120/4), with smooth context; wall 2-layered, 1.5–2.5(–3.0) μm thick (including inner layer c. 0.5–1.0 μm thick), without angles, layers well visible in LM, both layers evenly thickened, spore surface smooth. Asexual morph entylomella-like, weakly developed. Caespituli hypophyllous, conidiophores in densely agglutinated fascicles, emerging through stomata, hyaline, conidiogenous loci inconspicuous. Conidia solitary, hyaline, acicular-cylindrical, straight, 25–40 × 2.5–3.0(–3.5) μm, non-septate, hilum inconspicuous, not darkened.
Diagnostic bases — Within the E. ranunculi-repentis complex there are four diagnostic bases distributed among all loci, except map (Fig. 3, Table 2).
Host plant — Parasitic on Ranunculus paludosus.
Additional specimens examined. Greece, Rhodes, c. 1 km S of Salakos, way up to Mt Profitis Ilias, Quercus coccifera forest, N36°16'59″ E27°56'42″, elevation c. 320 m a.s.l., on Ranunculus paludosus, 13 Mar. 2016, J. Kruse (GLM-F107696); c. 1 km NW of Siana, way up to Akramitis, open Phrygana, plateau, N36°09'23″ E27°45ʹ59ʺ, elevation c. 650 m a.s.l., on Ranunculus paludosus, 15 Mar. 2016, J. Kruse (GLM-F107697); c. 1.2 km SE of Theologos, olive grove, N36°22'00″ E28°02'45″, elevation c. 40 m a.s.l., on Ranunculus paludosus, 16 Mar. 2016, J. Kruse (GLM-F107698).
Notes — This species is most similar to Entyloma jolantae, which differs in lacking an asexual morph.
Entyloma thielii J. Kruse, M. Lutz, Pia¸tek & Thines, sp. nov. — MycoBank MB824517; Fig. 15
Etymology. Named after Hjalmar Thiel from Jameln (Germany), for his contributions to the knowledge of phytopathogenic fungi and for enabling well-sampled phylogenetic investigations in various plant pathogen groups by his collections.
Type. Germany, Bavaria, Upper Bavaria, county Garmisch-Partenkirchen, c. 2.8 km SE of Mittenwald, Karwendel mountains, meadows around Brunnstein cabin, N47°24'49″ E11°16'41″, elevation c. 1475 m a.s.l., on Ranunculus montanus, 8 July 2016, J. Kruse (GLM-F107702 holotype; ex-type sequences available in GenBank: MF924694 (ITS), MH022818 (atp2), MF939319 (ssc1), MF939253 (map)).
Sori in the leaves, forming indistinct, flat, polyangular spots, 1–3 mm long, 0.5–2 mm wide, partly delineated by the leaf veins of the host, yellow or light brown on the upper side of the leaf, greyish or cream coloured on the lower side of the leaf. Spores embedded in the leaf tissue, single, densely crowded, often in compact groups, in the intercellular space between the mesophyll cells; spores hyaline, subhyaline to pale yellow, globose, subglobose or broadly ellipsoidal, often somewhat irregular due to mutual pressure, (9.5–)11.0–14.5(–16.5) × 9.0–12.5(–13.0) μm (av. ± SD, 12.5 ± 1.5 × 10.8 ± 1.0 μm, n = 150/5), with smooth context; wall 2-layered, 0.8–1.5 μm thick (including inner layer c. 0.3–0.5 μm thick), without angles, layers very hardly visible in LM, both layers evenly thickened, spore surface smooth. Asexual morph not found.
Diagnostic bases — Within the E. ranunculi-repentis complex there are 68 diagnostic bases distributed among all loci (Fig. 3, Table 2).
Host plant — Parasitic on Ranunculus montanus.
Additional specimen examined. Germany, Bavaria, Upper Bavaria, county Garmisch-Partenkirchen, c. 2.8 km SE of Mittenwald, Karwendel mountains, hiking path 290 from Brunnstein cabin towards Mittenwald, serpentines, sparse mixed mountainous forest, N47°24'48″ E11°16'33″, elevation c. 1380 m a.s.l., on Ranunculus montanus, 8 July 2016, J. Kruse (GLM-F107700); meadows around Brunnstein cabin, N47°24'49″ E11°16'41″, elevation c. 1475 m a.s.l., on Ranunculus montanus, 8 July 2016, J. Kruse (GLM-F107701); c. 4.9 km NE of Mittenwald, Karwendel mountains, hiking path 266 from Rehbergalm to Hochland cabin, mixed mountainous forest, N47°27'37″ E11°18'36″, elevation c. 1575 m a.s.l., on Ranunculus montanus, 11 July 2016, J. Kruse (GLM-F107704); Oberallgäu, Einödsbach, Rappensee cabin, near Rappensee, wayside, N47°17'11″ E10°15'19″, elevation c. 2080 m a.s.l., on Ranunculus montanus, 29 July 2015, J. Kruse (GLM-F107705).
Notes — This species differs from the other species in the Entyloma ranunculi-repentis complex in having densely crowded spores, often in compact groups, in the intercellular space between the mesophyll cells.
Entyloma verruculosum
Entyloma verruculosum Pass., Nuovo Giorn. Bot. Ital. 9: 239. 1877; in Rabenhorst, Fungi Europ. no. 2253. 1877; in Fischer von Waldheim, Bull. Soc. Imp. Naturalistes Moscou 52: 310. 1877 — Fig. 16
Type. Italy, Parma, on Ranunculus velutinus, May 1873, G. Passerini (BRIP: HUV 1307 lectotype, isolectotypes in Rabenhorst, Fungi Europ. no. 2253; lectotype designated by Vánky 1985: 80).
Sori in the leaves, forming indistinct, flat, polyangular spots, 1–5 mm long, 1–3 mm wide, partly delineated by the leaf veins of the host, yellow or light brown on the upper side of the leaf, cream coloured on the lower side of the leaf. Spores embedded in the leaf tissue, single, densely crowded in the intercellular space between the mesophyll cells; spores subhyaline to pale yellow, globose or subglobose, regular in shape, (11.0–)12.0–14.5(–16.0) × (10.5–)11.0–14.5(–15.0) μm (av. ± SD, 13.4 ± 1.4 × 13.1 ± 1.4 μm, n = 30/1), with granular context; wall apparently 1-layered, 1.5–2.5 μm thick, without angles, spore surface distinctly tuberculate. Asexual morph not found.
Host plant — Parasitic on Ranunculus spp.
Specimen examined. Italy, Apulia, Monte Sant’Angelo, Provinz Foggia, c. 12 km N of Monte Sant’Angelo, road SP52b, Foresta Umbra, beech forest, N41°47'52″ E15°58'44″, elevation c. 720 m a.s.l., on Ranunculus lanuginosus, 19 Apr. 2016, J. Kruse (GLM-F107706).
Notes — The specimens of Entyloma verruculosum on the type host (Ranunculus velutinus) were not available for molecular analyses, and the morphological description is based on the sequenced specimen on R. lanuginosus. The smut species was additionally reported on Ranunculus acris, R. repens, and R. sceleratus (Vánky 2012), which indicates that E. verruculosum may represent a species complex, too, to be resolved in future studies.
DISCUSSION
The analyses of the morphology and molecular phylogenetics presented in this study indicate that most of the Entyloma species on Ranunculus spp. are specific at the host species level. This provides evidence for two more assumed broad-range biotrophic pathogens to be species complexes, rather than single species, similar to the situation observed in other pathogens (e.g., Lutz et al. 2005, Beenken et al. 2012, Choi et al. 2015, Scholler et al. 2016, Kruse et al. 2018, Ziegler et al. 2018). The three major lineages found within Entyloma (the E. microsporum complex, the E. ranunculi-repentis complex, and E. verruculosum) are readily distinguished by teliospore surface ornamentation. Species in the E. microsporum complex have cracked spore surfaces, those in the E. ranunculi-repentis complex are smooth, and spores of E. verruculosum are verrucose. In addition, species in the E. microsporum complex cause swollen galls readily distinguishing them from the other two lineages. Entyloma verruculosum, for which we examined only a single specimen, may represent yet another complex to be resolved in future studies, as it has been reported on five different Ranunculus species (Vánky 2012).
For the Entyloma ranunculi-repentis complex the four-gene dataset (with ITS, atp2, ssc1, and map sequences) recovered 11 mostly highly supported host-specific lineages (nine on Ranunculus spp. and two on Ficaria verna). These lineages are also correlated with (sometimes subtle) morphological characters. The most informative morphological and biological characters were the arrangement of spores within the leaf spot; size of spores; mean size of spores; spore wall thickness; presence of an asexual morph; and the shape and size of conidia (see Table 3).
For some of the lineages in the E. ranunculi-repentis complex validly published names are available, previously often listed as synonyms of E. ranunculi-repentis s.lat. (Vánky 2012). The results of this study support E. ranunculacearum (on R. acris), E. ranunculi-scelerati (on R. sceleratus), and E. ranunculorum (on R. auricomus) as distinct species (Kochman 1936, Liro 1938). For six other lineages, each associated with a single host plant species, new species were introduced.
In addition to these host-specific Entyloma species, one additional clade with specimens from related species (Paun et al. 2005), R. bulbosus, R. polyanthemos subsp. nemorosus, and R. repens, has been assigned to a new combination in Entyloma for Fusidium eburneum. Further study is needed to determine if this clade represents a recently-differentiated species complex. If it contained distinct species, the name Entyloma wroblewskii (Kochman 1934) could be adopted for the Entyloma pathogen on Ranunculus polyanthemos. As even more loci or microsatellites would be needed to resolve this question, we have taken a conservative approach, considering the whole clade to represent E. eburneum.
The species Ramularia gibba, which was thought to be connected with the asexual morph-forming species of Entyloma on Ranunculus repens (Braun 1998), is a chimera that contains the diagnostic features of both the E. microsporum and the E. ranunculi-repentis species complexes (De Bary 1874). An inspection of the type specimen revealed a dual infection was present on the leaves, explaining the chimeric nature of the description. Consequently, the name cannot be applied to a species in either group and has been proposed for rejection (Kruse & Thines 2017).
There was less resolution of species in the E. microsporum complex than in the E. ranunculi-repentis complex with the four loci used in the present study. However, as specimens from Ranunculus paludosus were clearly distinct, the name Caeoma bullosum should be reinstated in its combination in Entyloma. The additional two clades found in the E. microsporum complex were each represented by specimens from different host species. Both lineages include morphologically similar specimens and both include specimens from Ranunculus repens, the type host of Entyloma microsporum. To fix the application of the name E. microsporum, a neotype was selected from among the specimens in the clade containing most accessions on Ranunculus repens, and a new species is introduced for the specimens of the other clade. Both clades with specimens from Ranunculus repens showed some internal differentiation according to the host species and thus might be revealed to be species complexes in future studies.
The relationships of the Entyloma species covered in this study do not correspond to the relationships of the respective hosts (Paun et al. 2005). It is, thus, conceivable that, similar to the situation in obligate biotrophic downy mildews (Choi & Thines 2015), species of Entyloma do not diversify by long-term co-evolution, but rather by host jumps, subsequent radiation, and finally specific adaptation, leading to diversification into distinct species. As there are numerous additional hosts for Entyloma in the genus Ranunculus (Vánky 2012) that could not be included in the current study, it seems likely that additional species await discovery and more detailed patterns regarding the evolution of Entyloma await revelation.
Acknowledgments
This study was supported by the LOEWE excellence initiative in the framework of the cluster for Integrative Fungal Research (IPF). The work of the second author was supported by the statutory funds of the W. Szafer Institute of Botany, Polish Academy of Sciences in Kraków. We thank T. Feuerer (HGB), M. Graniszewska (WA), M. Halama (WRSL), R. Lücking (B), and R. Marcucci (PAD) for searching for potential type specimens in their herbaria and U. Damm (GLM) for providing several herbarium numbers for private smut collections. We are grateful to the curators of GLM, BRIP, and TUB for allowing the investigation of specimens in their keeping, and the private collectors H. Jage, H. John, U. Richter, and H. Richter for submitting parts of their collections to GLM, and P. Me¸dykowski for submitting one specimen to KRAM F. We also thank the private collectors G. Hübner, F. Klenke, C. Klenke, and V. Kummer for allowing us to include some of their collections in this study. We also thank V. Kummer for allowing us to use his picture of macroscopic infection of Ranunculus sceleratus.
REFERENCES
- Altschul SF, Madden TL, Schäffer AA, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25:3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenken L, Zoller S, Berndt R. 2012. Rust fungi on Annonaceae II: the genus Dasyspora Berk. & M.A. Curtis. Mycologia 104:659–681. [DOI] [PubMed] [Google Scholar]
- Begerow D, Bauer R, Boekhout T. 2000. Phylogenetic placements of ustilaginomycetous anamorphs as deduced from nuclear LSU rDNA sequences. Mycological Research 104:53–60. [Google Scholar]
- Begerow D, Bauer R, Oberwinkler F. 2002. The Exobasidiales: an evolutionary hypothesis. Mycological Progress 1:187–199. [Google Scholar]
- Begerow D, Stoll M, Bauer R. 2006. A phylogenetic hypothesis of Ustilaginomycotina based on multiple gene analyses and morphological data. Mycologia 98:906–916. [DOI] [PubMed] [Google Scholar]
- Bennett RM, De Cock AW, Lévesque CA, et al. 2017. Calycofera gen. nov., an estuarine sister taxon to Phytopythium, Peronosporaceae. Mycological Progress 16:947–954. [Google Scholar]
- Braun U. 1998. A monograph of Cercosporella, Ramularia and allied genera (Phytopathogenic Hyphomycetes). Vol. 2 IHW-Verlag Eching, Germany. [Google Scholar]
- Choi Y-J, Klosterman SJ, Kummer V, et al. 2015. Multi-locus tree and species tree approaches toward resolving a complex clade of downy mildews (Straminipila, Oomycota), including pathogens of beet and spinach. Molecular Phylogenetics and Evolution 86:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y-J, Shin HD, Thines M. 2009. The host range of Albugo candida extends from Brassicaceae through Cleomaceae to Capparaceae. Mycological Progress 8:329–335. [Google Scholar]
- Choi Y-J, Thines M. 2015. Host jumps and radiation, not co-divergence drives diversification of obligate pathogens. A case study in downy mildews and Asteraceae. PloS One 10: e0133655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri R. 1928. Quarta contribuzione allo studio degli Ustilaginales. Annales Mycologici 26:1–68. [Google Scholar]
- De Bary A. 1874. Protomyces microsporus und seine Verwandten. Botanische Zeitung (Berlin) 32:97–108. [Google Scholar]
- Denchev TT, Denchev CM, Shivas RG. 2013. Two new Entyloma species (Entylomatales, Ustilaginomycotina) from the USA. Mycobiota 3:35–39. [Google Scholar]
- Emadzade K, Lehnebach C, Lockhart P, et al. 2010. A molecular phylogeny, morphology and classification of genera of Ranunculeae (Ranunculaceae). Taxon 59:809–828. [Google Scholar]
- Euro+Med. 2006 onwards. Euro+Med PlantBase – the information resource for Euro-Mediterranean plant diversity. Published on the Internet: http://ww2.bgbm.org/EuroPlusMed/ (accessed 15 May 2017). [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, et al. 2011. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E, Paun O, Johansson JT, et al. 2005. Phylogenetic relationships and evolutionary traits in Ranunculus s.l. (Ranunculaceae) inferred from ITS sequence analysis. Molecular Phylogenetics and Evolution 36:305–327. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenke F, Scholler M. 2015. Pflanzenparasitische Kleinpilze. Bestimmungsbuch für Brand-, Rost-, Mehltau-, Flagellatenpilze und Wucherlingsverwandte in Deutschland, Österreich, der Schweiz und Südtirol. Springer Verlag Berlin Heidelberg, Germany. [Google Scholar]
- Kochman J. 1934. Contribution to the knowledge of the Polish Ustilaginales. Acta Societatis Botanicorum Poloniae 11 (Suppl): 285–303. [In Polish.] [Google Scholar]
- Kochman J. 1936. Grzyby głowniowe Polski (Ustilaginales Poloniae). Planta Polonica 4: 1–161. [In Polish.] [Google Scholar]
- Kruse J, Choi Y-J, Thines M. 2017a. New smut-specific primers for the ITS barcoding of smut fungi (Ustilaginomycotina). Mycological Progress 16:213–221. [Google Scholar]
- Kruse J, Dietrich W, Zimmermann H, et al. 2018. Ustilago species causing leaf-stripe smut revisited. IMA Fungus 9:49–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse J, Mishra B, Choi Y-J, et al. 2017b. New smut-specific primers for multilocus genotyping and phylogenetics of Ustilaginales. Mycological Progress 16:917–925. [Google Scholar]
- Kruse J, Thines M. 2017. Proposal to reject the name Ramularia gibba (Entylomatales, Ustilaginomycotina). Taxon 66:515–516. [Google Scholar]
- Li Y-M, Shivas RG, Cai L. 2017. Cryptic diversity in Tranzscheliella spp. (Ustilaginales) is driven by host switches. Scientific Reports 7: 43549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg B. 1959. Ustilaginales of Sweden (exclusive of the Cintractias on Caricoideae). Symbolae Botanicae Upsalienses 16:1–175. [Google Scholar]
- Liro JI. 1938. Die Ustilagineen Finnlands. II. Annales Academiæ Scientiarum Fennicæ Series A, 42:1–720. [Google Scholar]
- Lutz M, Bauer R, Begerow D, et al. 2004. Tuberculina, rust relatives attack rusts. Mycologia 96:614–626. [PubMed] [Google Scholar]
- Lutz M, Göker M, Pia¸tek M, et al. 2005. Anther smuts of Caryophyllaceae: molecular characters indicate host-dependent species delimitation. Mycological Progress 4:225–238. [Google Scholar]
- Lutz M, Pia¸tek M. 2016. Phylogenetic placement, DNA barcoding, morphology and evidence for the spreading of Entyloma cosmi, a species attacking Cosmos bipinnatus in temperate climate gardens. European Journal of Plant Pathology 145:857–869. [Google Scholar]
- McNeill J, Barrie FR, Buck WR, et al. 2012. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). Regnum Vegetabile 154. A.R.G. Ruggell, Gantner Verlag, Liechtenstein. [Google Scholar]
- Mishra B, Choi Y-J, Thines M. 2018. Phylogenomics of Bartheletia paradoxa reveals its basal position in Agaricomycotina and that the early evolutionary history of basidiomycetes was rapid and probably not strictly bifurcating. Mycological Progress 17:333–341. [Google Scholar]
- Morin L, Aveyard R, Lidbetter JR, et al. 2012. Investigating the host range of Puccinia psidii sensu lato across tribes of the family Myrtaceae present in Australia. PLoS One 7: e35434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paun O, Lehnebach C, Johansson JT, et al. 2005. Phylogenetic relationships and biogeography of Ranunculus and allied genera (Ranunculaceae) in the Mediterranean region and in the European alpine system. Taxon 54:911–932. [Google Scholar]
- Pia¸tek M, Lutz M, Chater AO. 2013. Cryptic diversity in the Antherospora vaillantii complex on Muscari species. IMA Fungus 4:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pia¸tek M, Lutz M, Nobis M, et al. 2015a. Phylogeny and morphology of Anthracoidea pamiroalaica sp. nov. infecting the endemic sedge Carex koshewnikowii in the Pamir Alai Mts (Tajikistan). Mycological Progress 14: 120. [Google Scholar]
- Pia¸tek M, Lutz M, Smith PA, et al. 2011. A new species of Antherospora supports the systematic placement of its host plant. IMA Fungus 2:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pia¸tek M, Lutz M, Yorou NS. 2015b. A molecular phylogenetic framework for Anthracocystis (Ustilaginales), including five new combinations (inter alia for the asexual Pseudozyma flocculosa), and description of Anthracocystis grodzinskae sp. nov. Mycological Progress 14: 88. [Google Scholar]
- Pia¸tek M, Riess K, Karasiński D, et al. 2016. Integrative analysis of the West African Ceraceosorus africanus sp. nov. provides insights into the diversity, biogeography, and evolution of the enigmatic Ceraceosorales (Fungi: Ustilaginomycotina). Organisms Diversity and Evolution 16:743–760. [Google Scholar]
- Piepenbring M. 2003. Smut fungi (Ustilaginomycetes p.p. and Microbotryales, Basidiomycota). Flora Neotropica 86: iv + 1–291. [PubMed] [Google Scholar]
- Rabenhorst L. 1874. Fungi europaei exs. Cent. XIX. Nr. 1801–1900. Hedwigia 13: 174–175, 184–189. [Google Scholar]
- Rastipishe S, Pakravan M, Tavassoli A. 2011. Phylogenetic relationships in Ranunculus species (Ranunculaceae) based on nrDNA ITS and cpDNA trnL-F sequences. Progress in Biological Sciences 1:41–87. [Google Scholar]
- Ridgway KP, Duck JM, Young JPW. 2003. Identification of roots from grass swards using PCR-RFLP and FFLP of the plastid trn L (UAA) intron. BMC Ecology 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney-Latham S, Lutz M, Blomquist CL, et al. 2017. Entyloma helianthi: identification and characterization of the causal agent of sunflower white leaf smut. Mycologia 109:520–528. [DOI] [PubMed] [Google Scholar]
- Rossman AY, Allen WC, Castlebury LA. 2016. New combinations of plant-associated fungi resulting from the change to one name for fungi. IMA Fungus 7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge F, Choi YJ, Thines M. 2011. Phylogenetic investigations in the genus Pseudoperonospora reveal overlooked species and cryptic diversity in the P. cubensis species cluster. European Journal of Plant Pathology 129:135–146. [Google Scholar]
- Saccardo PA. 1915. Fungi ex insula Melita (Malta) lecti a Doct. A. Caruana-Gatto et Doct. G. Borg annis MCMXII et MCMXIV. Series III. Nuovo Giornale Botanico Italiano 22:24–76. [Google Scholar]
- Savchenko KG, Carris LM. 2017. Two new Entyloma (Entylomataceae, Basidiomycota) species on North American Sanicula. Phytotaxa 327:191–195. [Google Scholar]
- Savchenko KG, Carris LM, Castlebury LA, et al. 2014a. Revision of Entyloma (Entylomatales, Exobasidiomycetes) on Eryngium. Mycologia 106:797–810. [DOI] [PubMed] [Google Scholar]
- Savchenko KG, Carris LM, Castlebury LA, et al. 2014b. Stripe smuts of grasses: one lineage or high levels of polyphyly? Persoonia 33: 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savchenko KG, Heluta VP, Wasser SP, et al. 2012. A contribution to the study of smut fungi of Israel. Mycotaxon 118:303–309. [Google Scholar]
- Savchenko KG, Lutz M, Pia¸tek M, et al. 2013. Anthracoidea caricis-meadii is a new North American smut fungus on Carex sect. Paniceae. Mycologia 105:181–193. [DOI] [PubMed] [Google Scholar]
- Savile DBO. 1947. A study of the species of Entyloma on North American composites. Canadian Journal of Research 25:105–120. [Google Scholar]
- Scholler M, Lutz M, Wood AR, et al. 2011. Taxonomy and phylogeny of Puccinia lagenophorae: a study using rDNA sequence data, morphological and host range features. Mycological Progress 10:175–187. [Google Scholar]
- Scholler M, Schmidt A, Siahaan SAS, et al. 2016. A taxonomic and phylogenetic study of the Golovinomyces biocellatus complex (Erysiphales, Ascomycota) using asexual state morphology and rDNA sequence data. Mycological Progress 15: 56. [Google Scholar]
- Scholz H, Scholz I. 1988. Die Brandpilze Deutschlands (Ustilaginales). Englera 8:1–691. [Google Scholar]
- Schröter J. 1877. Bemerkungen und Beobachtungen über einige Ustilagineen. Beiträge zur Biologie der Pflanzen 2 (3): 349–385. [Google Scholar]
- Schröter J. 1887. Die Pilze Schlesiens: Ustilaginei. In: Cohn F. (ed), Kryptogamen-Flora von Schlesien 3 (1): 261–290. J.U. Kern's Verlag, Breslau. [Google Scholar]
- Stoll M, Piepenbring M, Begerow D, et al. 2003. Molecular phylogeny of Ustilago and Sporisorium species (Basidiomycota, Ustilaginales) based on internal transcribed spacer (ITS) sequences. Canadian Journal of Botany 81:976–984. [Google Scholar]
- Sydow H. 1924. Notizen über Ustilagineen. Annales Mycologici 22:277–291. [Google Scholar]
- Tamura M. 1995. Angiospermae. Ordnung Ranunculales. Fam. Ranunculaceae. II. Systematic Part. In: Hiepko P. (ed), Natürliche Pflanzenfamilien. 2nd edn: 223–519. Duncker & Humblot Berlin, Germany. [Google Scholar]
- Unger F. 1833. Die Exantheme Pflanzen, etc. K. Gerold, Wien, Austria. [Google Scholar]
- Vánky K. 1985. Carpathian Ustilaginales. Symbolae Botanicae Upsalienses 24 (2): 1–309. [Google Scholar]
- Vánky K. 1994. European smut fungi. Gustav Fischer Verlag, Stuttgart, Jena, New York. [Google Scholar]
- Vánky K. 2012. Smut fungi of the world. APS Press, St. Paul, US. [Google Scholar]
- Vánky K, Lutz M. 2007. Revision of some Thecaphora species (Ustilaginomycotina) on Caryophyllaceae. Mycological Research 111:1207–1219. [DOI] [PubMed] [Google Scholar]
- Vánky K, Lutz M. 2010. Entyloma majewskii sp. nov. (Entylomataceae) on Ranunculus ficaria from Iran. Polish Botanical Journal 55:271–279. [Google Scholar]
- Vasighzadeh A, Zafari D, Selçuk F, et al. 2014. Discovery of Thecaphora schwarzmaniana on Rheum ribes in Iran and Turkey: implications for the diversity and phylogeny of leaf smuts on rhubarbs. Mycological Progress 13:881–892. [Google Scholar]
- Wang QM, Begerow D, Groenewald M, et al. 2015. Multigene phylogeny and taxonomic revision of yeasts and related fungi in the Ustilaginomycotina. Studies in Mycology 81:55–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Theelen B, Groenewald M, et al. 2014. Moniliellomycetes and Malasseziomycetes, two new classes in Ustilaginomycotina. Persoonia 33:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA sequences for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: A guide to methods and applications: 315–322. Academic Press, Inc., US. [Google Scholar]
- Yoshida K, Burbano HA, Krause J, et al. 2014. Mining herbaria for plant pathogen genomes: back to the future. PLoS Pathogens 10: e1004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Schuenemann VJ, Cano LM, et al. 2013. The rise and fall of the Phytophthora infestans lineage that triggered the Irish potato famine. eLife 2: e00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler R, Lutz M, Pia¸tek J, et al. 2018. Dismantling a complex of anther smuts (Microbotryum) on carnivorous plants in the genus Pinguicula. Mycologia in press. 10.1080/00275514.2018.1451697. [DOI] [PubMed] [Google Scholar]