Abstract
Objective:
Adenomyosis is a clinical disorder defined by the presence of endometrial glands and stroma within the myometrium, the pathogenesis of which is poorly understood. We postulate that dysregulation of genes and pathways in eutopic endometrium may predispose to ectopic implantation. No study, to our knowledge, has examined the global transcriptome of isolated eutopic endometrium from women with clinically significant adenomyosis.
Design:
Laboratory-based study with full institutional review board approval and consents.
Material and Methods:
Endometrial sampling was performed on hysterectomy specimens (proliferative phase) from symptomatic women with pathologically confirmed diffuse adenomyosis (n = 3). Controls (n = 5) were normo-ovulatory patients without adenomyosis. All patients were free from leiomyoma, endometriosis, and hormonal exposures. Isolated purified total RNA was subjected to microarray analysis using the Gene 1.0 ST Affymetrix platform. Data were analyzed with GeneSpring and Ingenuity Pathway analysis. Validation of several genes was undertaken by quantitative real-time reverse transcriptase polymerase chain reaction.
Results:
Comparison of transcriptomes of proliferative endometrium from women with and without adenomyosis revealed 140 upregulated and 884 downregulated genes in samples from women with adenomyosis compared to controls. Highly differentially expressed genes include those involved in regulation of apoptosis, steroid hormone responsiveness, and proteins involved in extracellular matrix remodeling as well as microRNAs of unknown significance. Affected canonical pathways included eukaryotic initiation factor 2 signaling, oxidative phosphorylation, mitochondrial dysfunction, estrogen receptor signaling, and mammalian target of rapamycin signaling.
Conclusion:
The eutopic endometrium in patients with adenomyosis has fundamental abnormalities that may predispose to invasion and survival beyond the myometrial interface.
Keywords: adenomyosis, eutopic endometrium, microarray, apoptosis, signaling pathways
Introduction
Adenomyosis is a common and clinically significant condition causing abnormal uterine bleeding, dysmenorrhea, and pelvic pain.1,2 The disease is defined by the histologic presence of endometrial glands and stroma within the uterine musculature, with associated hypertrophy and hyperplasia of adjacent myometrium.3 The pathogenesis of the disorder is not well understood, and the condition is often refractory to medical therapy, sometimes necessitating hysterectomy for complete alleviation of symptoms. The implication of adenomyosis to fertility is contested but may impair implantation.1,4
Previous data from several investigators have established that eutopic endometrium is abnormal in patients with endometriosis, another complex and multifactorial condition characterized by ectopic implants of endometrial glands and stroma.5,6 As there is often histologic continuity between the basal and the ectopic endometrium in adenomyosis, it is reasonable to postulate the existence of intrinsic abnormalities in the eutopic endometrium of patients with adenomyosis. Indeed, previous immunohistochemical and selected gene expression analyses indicate endometrial abnormalities in the pathophysiology of adenomyosis, including proliferation, apoptosis, angiogenesis, steroid responsiveness, and oxidative damage.7–12
To date, most investigations of eutopic endometrium from women with adenomyosis have focused on expression of single gene or a limited number of genes.13–15 One study examined the global transcriptome of extracted uterine tissue in women with adenomyosis, although the patients with adenomyosis had coexisting uterine fibroids which can also affect endometrial gene expression,16 and it was not clear whether the extracted RNA was derived from isolated ectopic endometrium or combination of adenomyosis tissue with adjacent myometrium.14 Despite these methodological limitations, endometrial gene expression and pathway analyses clustered together by principal component analysis (PCA) in comparison with normal controls without uterine pathology.14 Interestingly, of the top 9 pathways dysregulated, the most significant was impairment of apoptosis.14
We hypothesized that eutopic endometrium in women with adenomyosis is abnormal and exhibits dysregulation of pathways that globally predispose toward the development, migration, and survival of ectopic endometrial implants beyond the myometrial interface. In the current study, we undertook the first global transcriptomic analysis of eutopic endometrium in women undergoing surgical treatment with histologically demonstrated adenomyosis and no other uterine or pelvic pathologies, compared to controls without adenomyosis or any uterine or pelvic pathologies. Through global gene expression profiling, we sought to identify pathways and candidate genes implicated in the pathogenesis of this complex, clinically significant, but poorly understood disorder.
Materials and Methods
Sample Collection and Processing
Endometrial tissue biopsies were obtained from 8 reproductive-age women. Three patients had pathologically confirmed diffuse adenomyosis on hysterectomy performed during the proliferative phase. Participating patients were 37 to 41 years old (mean 39.6 ± 1.3). Controls were 5 normo-ovulatory premenopausal patients 31-40 years old (mean 36.6 ± 1.6) undergoing endometrial biopsy for nonmalignant surgical indication in proliferative phase of the menstrual cycle (Table 1). There was no significant difference in the age of patients in the adenomyosis group compared to the control group (P = .2). Neither cohort had the evidence of endometriosis or fibroids at the time of surgery. All participants were documented not to be pregnant and did not receive hormonal therapies or gonadotropin-releasing hormone agonist (GnRHa) suppression for at least 3 months before tissue sampling.
Table 1.
Demographics and Characteristics of Patients.
| Patient Identifier | Age | Ethnicity | Gravity and Parity | Surgical Procedure | Surgical Indication | Cycle Phase Histology | Used in Experiments |
|---|---|---|---|---|---|---|---|
| Adenomyosis | |||||||
| UC-065 | 41 | Black | G3P3 | Laparoscopic supracervical hysterectomy | Abnormal uterine bleeding, adenomyosis | Proliferative | Microarray, QRT-PCR validation |
| 421 | 37 | Black | G8P5 | Total abdominal hysterectomy | Pelvic pain, adenomyosis | Proliferative | Microarray, QRT-PCR validation |
| UC-034 | 41 | Black | G4P3013 | Total vaginal hysterectomy | Dysmenorrhea, chronic pelvic pain, mild cystocele, adenomyosis | Proliferative | Microarray, QRT-PCR validation |
| Controls | |||||||
| UC-137 | 31 | White | G0 | LEEP, laparoscopic bilateral tubal ligation | Cervical HSIL, undesired fertility | Proliferative | Microarray, QRT-PCR validation |
| UC-207 | 40 | Hispanic | G5P2032 | Mini lap bilateral tubal ligation | Undesired fertility | Proliferative | Microarray, QRT-PCR validation |
| UC-251 | 39 | Asian | G5P2 | Laparoscopic bilateral tubal ligation | Undesired fertility | Proliferative | Microarray, QRT-PCR validation |
| UC-230 | 36 | Asian | G4P3 | Laparoscopic bilateral tubal ligation and retropubic midurethral sling w/mesh | Undesired fertility, stress urinary incontinence | Proliferative | Microarray, QRT-PCR validation |
| UC-182 | 37 | Asian | G2P2 | Laparoscopic bilateral tubal ligation | Undesired fertility | Proliferative | Microarray, QRT-PCR validation |
Abbreviations: HSIL, high-grade squamous intraepithelial lesions; LEEP, loop electrosurgical excision procedure; QRT-PCR, real-time reverse transcriptase polymerase chain reaction.
Endometrial samples were collected using a Pipelle catheter (Cooper Surgical, Trumbull, Connecticut) or curetting the endometrial functionalis layer from hysterectomy specimens. Tissues were immediately flash frozen in liquid nitrogen. Portions of the tissues were saved in 10% formalin and were examined by up to 4 independent pathologists for dating of cycle phase, according to histological gold standard.17
The University of California, San Francisco, Committee on Human Research approved the study. Written informed consent was obtained from patients. Samples were also obtained through the University of California, San Francisco, National Institutes of Health Human Endometrial Tissue and DNA bank with appropriate institutional review, approvals, and informed consent from all patients.
Total RNA Isolation, Microarray Hybridization
Total RNA was isolated from samples using RNeasy Plus mini kit (QIAGEN, Valencia, California), quantified by spectroscopy, and purity was analyzed by the 260:280 absorbance ratio. RNA quality and integrity were assessed using Bioanalyzer 2100 (Agilent Technologies, Santa Clara, California) with all samples having high-quality RNA (RNA integrity number [RIN] = 9.7-10). Hybridization was performed with Human Gene 1.0 ST arrays (Affymetrix Inc, Santa Clara, California). Total RNA of 100 ng from each sample were reverse transcribed to complementary DNA (cDNA) followed by overnight in vitro transcription to generate complementary RNA. The latter was reverse transcribed, and the 5.5 μg of sense cDNA were fragmented and labeled. The quality of cDNA and fragmented cDNA was assessed in the Bioanalyzer 2100 (Agilent Technologies). Microarrays were hybridized, washed, and scanned at the Gladstone Genomics Core Facility, according to the protocol described in whole transcript sense target labeling assay manual from Affymetrix (version 4; FS450_0007). Raw data files have been uploaded to the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database under accession number GSE78851.
Microarray Gene Expression Data Analysis and Statistical Analysis
To minimize technical (nonbiological) variability among arrays, densitometry values between arrays were normalized using the robust multichip average function and further transformed to the logarithmic scale (log 2). Probes with a known GenBank accession ID correspondence were selected for functional analysis. Statistically significant differences between groups were determined using statistical analysis of microarrays using Genespring (version 12.1), applying 1-way analysis of variance (ANOVA) with Tukey post hoc test and Benjamini-Hochberg multiple testing correction for false discovery rate. Fold change (FC) ≥2.0-FCand P < .05 were accepted. Functional annotations were carried out using the ingenuity pathway analysis (IPA; Ingenuity Systems, Redwood City, California), in which gene symbols and FCs of the up- and downregulated genes were imported.
Principal Component and Heirarchical Clustering Analysis
Principal component analysis of the expression profiles distributes samples into the 3-dimensional space based on variance in gene expression. Samples clustering together indicate similar gene expression profiles. Hierarchical clustering is an unsupervised way of grouping samples based only on their gene expression similarities.18 We conducted hierarchical cluster analysis of differentially expressed genes from all samples in the combined gene list using the smooth correlation for the distance measure algorithm (GeneSpring 12.1) to identify samples with similar patterns of gene expression. The output data are also displayed graphically as a dendrogram of adenomyosis versus control samples. The complete .cel data files were uploaded to the NCBI GEO database and are also available on request.
Microarray Validation by Real-Time Polymerase Chain Reaction
Several genes were selected for validation by quantitative real-time reverse transcriptase polymerase chain reaction (QRT-PCR) using the same tissue sample set. Briefly, 1 μg of RNA was converted to cDNA using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, California). The real-time RT-PCR reaction was carried out for 40 cycles using the primers listed in Table 2. Each sample was run in duplicate, and the relative expression of the target genes was normalized with ribosomal protein (RP) L19 as the internal reference. Differences in the expression levels between samples were analyzed using 1-way ANOVA. P ≤ .05 was considered statistically significant.
Table 2.
Primer Sequences Used in Real-Time RT-PCR Validation.
| Gene | Sense Primer 5′-3′ | Antisense Primer 5′-3′ |
|---|---|---|
| SNORD116-5 | ACATTCCTTGGAAAGCTGAACA | CCTCAGTTTGACGAGGATGAC |
| MMP7 | TGTATGGGGAACTGCTGACA | ATGAGCCAGCGTGTTTCC |
| LOX | TTTCTTACCCAGCCGACCAA | TCAAGCAGGTCATAGTGGCTAA |
| VCAN | GGTGCCTCTGCCTTCCAA | TTGTGCCAGCCATAGTCACA |
| VIM | CCTGTGAAGTGGATGCCCTTA | CAACGGCAAAGTTCTCTTCCA |
| DIO2 | AGCTTCCTCCTCGATGCCTA | GAGACATGCACCACACTGGAA |
| RPL19 | CCTGTGACGGTCCATTCCC | GCGCAAAATCCTCATTCTCC |
Abbreviations: DIO2, thyroxine deiodinase 2; LOX, lysyl oxidase; MMP7, matrix metalloproteinase 7; RPL19, ribosomal protein L19; RT-PCR, reverse transcriptase polymerase chain reaction; SNORD, small nucleolar RNA C/D box; VCAN, versican; VIM, vimentin.
Results
Principal Component Analysis and Hierarchical Clustering Analysis
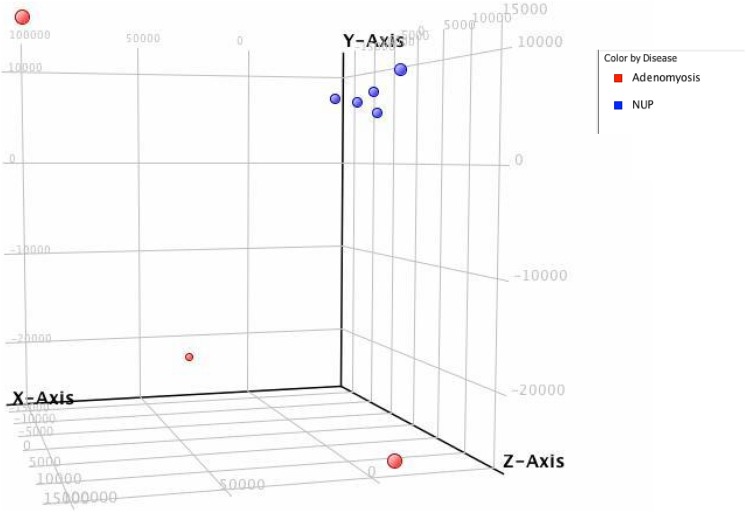
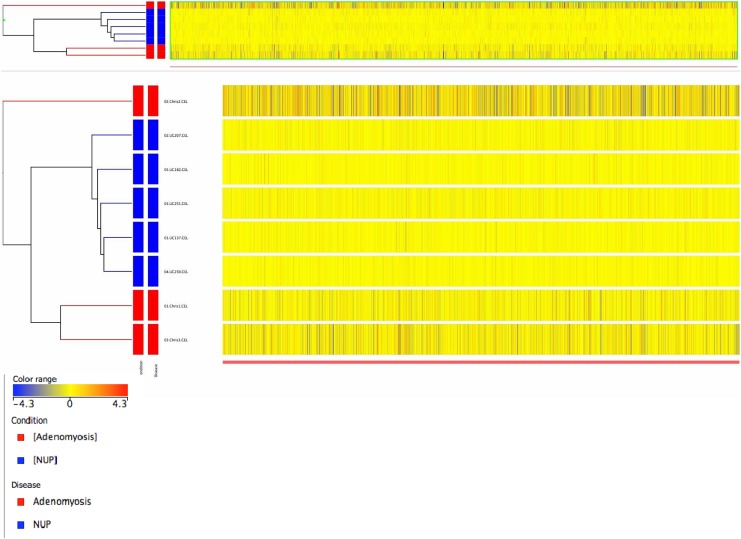
To comprehensively assess potential differences in gene expression in eutopic endometrium from women with and without adenomyosis, we performed comparative microarray analysis between these 2 groups. By PCA, samples of normal eutopic endometrium clustered together, whereas samples from patients with adenomyosis segregated distinctly separate from controls (Figure 1). The microarray gene expression profiles of the 8 samples of endometrium from women with and without adenomyosis were also subjected to unsupervised hierarchical clustering analysis based on differentially expressed genes. We observed segregation of samples into 2 major branches, and, as by PCA, samples self-segregated into the groups based on disease state, demonstrating that at the global transcriptome level, eutopic endometrium from women with diffuse adenomyosis is molecularly distinct from control endometrium. Figure 2 shows a heatmap of relative expression levels of genes in the endometrial samples of women with and without adenomyosis.
Figure 1.
Principal component analysis of eutopic endometrium in patients with adenomyosis (red, n = 3) in comparison to normal controls (blue, n = 5).
Figure 2.
Heatmap representation of relative expression levels of genes in the endometrium of subjects with adenomyosis in comparison to controls, using the profiles of significantly regulated genes. Yellow = no difference in gene expression, blue = downregulated genes, red = upregulated genes. Each horizontal row represents a single sample, and each vertical line represents a single gene.
Gene expression and IPA Pathway Analysis
Comparison of transcriptomes of the proliferative endometrium from women with and without adenomyosis revealed 140 upregulated and 884 downregulated genes (P < .05) >2-fold in adenomyosis compared to disease-free samples (Table 3), indicating the overall state of gene deregulation of the tissue.
Table 3.
List of Top 50 Genes Differentially Regulated in Proliferative Phase Endometrial Biopsy Samples From Women With Adenomyosis Versus Healthy Controls.
| Gene Symbol | Fold Change | Gene Name | |||
|---|---|---|---|---|---|
| LOC100293539 | 28.28 | ||||
| SNORD116-5|SNORD116-7|SNORD116-3|SNORD116-9 | 15.72 | small nucleolar RNA, C/D box 116-5 | small nucleolar RNA, C/D box 116-7 | small nucleolar RNA, C/D box 116-3 | small nucleolar RNA, C/D box 116-9 | |||
| SNORD116-5|SNORD116-7|SNORD116-3|SNORD116-9 | 15.70 | small nucleolar RNA, C/D box 116-5 | small nucleolar RNA, C/D box 116-7 | small nucleolar RNA, C/D box 116-3 | small nucleolar RNA, C/D box 116-9 | |||
| ND2 | 12.59 | ||||
| SNORD116-3|SNORD116-9|SNORD116-5|SNORD116-7|SNORD116-8 | 10.52 | small nucleolar RNA, C/D box 116-3 | small nucleolar RNA, C/D box 116-9 | small nucleolar RNA, C/D box 116-5 | small nucleolar RNA, C/D box 116-7 | small nucleolar RNA, C/D box 116-8 | |||
| SNORD116-3|SNORD116-9|SNORD116-5|SNORD116-7|SNORD116-8 | 10.52 | small nucleolar RNA, C/D box 116-3 | small nucleolar RNA, C/D box 116-9 | small nucleolar RNA, C/D box 116-5 | small nucleolar RNA, C/D box 116-7 | small nucleolar RNA, C/D box 116-8 | |||
| SNORD116-8|SNORD116-3|SNORD116-9 | 8.72 | small nucleolar RNA, C/D box 116-8 | small nucleolar RNA, C/D box 116-3 | small nucleolar RNA, C/D box 116-9 | |||
| SNORD116-4|SNRPN | 8.47 | small nucleolar RNA, C/D box 116-4 | small nuclear ribonucleoprotein polypeptide N | |||
| SNORD33|RPL13A | 7.85 | small nucleolar RNA, C/D box 33 | ribosomal protein L13a | |||
| SNORA73A|SNHG3 | 7.64 | small nucleolar RNA, H/ACA box 73A | small nucleolar RNA host gene 3 (nonprotein coding) | |||
| SNORD41 | 6.94 | small nucleolar RNA, C/D box 41 | |||
| SNORD116-1 | 6.75 | small nucleolar RNA, C/D box 116-1 | |||
| YIPF4 | 4.53 | Yip1 domain family, member 4 | |||
| DUX4L4|DUX4L7|DUX4L2|DUX4L3|DUX4L5|DUX4L6 | 4.41 | double homeobox 4 like 4 | double homeobox 4 like 7 | double homeobox 4 like 2 | double homeobox 4 like 3 | double homeobox 4 like 5 | double homeobox 4 like 6 | |||
| DUX4L4|DUX4L7|DUX4L2|DUX4L3|DUX4L5|DUX4L6 | 4.38 | double homeobox 4 like 4 | double homeobox 4 like 7 | double homeobox 4 like 2 | double homeobox 4 like 3 | double homeobox 4 like 5 | double homeobox 4 like 6 | |||
| DUX4L4|DUX4L7|DUX4L2|DUX4L3|DUX4L5|DUX4L6 | 4.38 | double homeobox 4 like 4 | double homeobox 4 like 7 | double homeobox 4 like 2 | double homeobox 4 like 3 | double homeobox 4 like 5 | double homeobox 4 like 6 | |||
| DUX4L4|DUX4L7 | 4.37 | double homeobox 4 like 4 | double homeobox 4 like 7 | |||
| DUX4L4|DUX4L7|DUX4L2|DUX4L3|DUX4L5|DUX4L6 | 4.37 | double homeobox 4 like 4 | double homeobox 4 like 7 | double homeobox 4 like 2 | double homeobox 4 like 3 | double homeobox 4 like 5 | double homeobox 4 like 6 | |||
| DUX4L4|DUX4L7|DUX4L2|DUX4L3|DUX4L5|DUX4L6 | 4.37 | double homeobox 4 like 4 | double homeobox 4 like 7 | double homeobox 4 like 2 | double homeobox 4 like 3 | double homeobox 4 like 5 | double homeobox 4 like 6 | |||
| DUX4L4|DUX4L7|DUX4L3|DUX4L5|DUX4L6 | 4.35 | double homeobox 4 like 4 | double homeobox 4 like 7 | double homeobox 4 like 3 | double homeobox 4 like 5 | double homeobox 4 like 6 | |||
| RFC1 | 4.33 | replication factor C (activator 1) 1, 145 kDa | |||
| GP1BB | 4.23 | glycoprotein Ib (platelet), β polypeptide | |||
| DUX4|DUX4L2|DUX4L3|DUX4L5|DUX4L6|DUX4L4|DUX4L7 | 4.19 | double homeobox 4 | double homeobox 4 like 2 | double homeobox 4 like 3 | double homeobox 4 like 5 | double homeobox 4 like 6 | double homeobox 4 like 4 | double homeobox 4 like 7 | |||
| SNORD95|GNB2L1 | 4.18 | small nucleolar RNA, C/D box 95 | guanine nucleotide binding protein (G protein), β polypeptide 2-like 1 | |||
| DUX4|DUX4L2|DUX4L3|DUX4L5|DUX4L6|DUX4L4|DUX4L7 | 4.18 | double homeobox 4 | double homeobox 4 like 2 | double homeobox 4 like 3 | double homeobox 4 like 5 | double homeobox 4 like 6 | double homeobox 4 like 4 | double homeobox 4 like 7 | |||
| DUX4L4|DUX4L2|DUX4L3|DUX4L5|DUX4L6|DUX4L7|DUX2 | 3.96 | double homeobox 4 like 4 | double homeobox 4 like 2 | double homeobox 4 like 3 | double homeobox 4 like 5 | double homeobox 4 like 6 | double homeobox 4 like 7 | double homeobox 2 | |||
| DUX4|DUX4L2|DUX4L3|DUX4L5|DUX4L6|DUX4L4|DUX4L7|DUX2 | 3.89 | double homeobox 4 | double homeobox 4 like 2 | double homeobox 4 like 3 | double homeobox 4 like 5 | double homeobox 4 like 6 | double homeobox 4 like 4 | double homeobox 4 like 7 | double homeobox 2 | |||
| SIAE | 3.83 | sialic acid acetylesterase | |||
| DUX4L4|DUX4L7 | 3.74 | double homeobox 4 like 4 | double homeobox 4 like 7 | |||
| KRTAP5-1 | 3.53 | keratin associated protein 5-1 | |||
| DUX4|DUX4L2|DUX4L3|DUX4L5|DUX4L6 | 3.52 | double homeobox 4 | double homeobox 4 like 2 | double homeobox 4 like 3 | double homeobox 4 like 5 | double homeobox 4 like 6 | |||
| CXorf18 | 3.37 | chromosome X open reading frame 18 | |||
| PLCL1 | 3.35 | phospholipase C-like 1 | |||
| LOC100132147 | 3.26 | ||||
| HLA-DOB|TAP2 | 3.25 | major histocompatibility complex, class II, DO β | transporter 2, ATP-binding cassette, sub-family B (MDR/TAP) | |||
| MIR197 | 3.22 | microRNA 197 | |||
| ZBTB34 | 3.13 | zinc finger and BTB domain containing 34 | |||
| MIR339 | 3.07 | microRNA 339 | |||
| HLA-DOB|TAP2 | 2.98 | major histocompatibility complex, class II, DO β | transporter 2, ATP-binding cassette, sub-family B (MDR/TAP) | |||
| MIR326 | 2.93 | microRNA 326 | |||
| SNORD55 | 2.92 | small nucleolar RNA, C/D box 55 | |||
| POU5F1B | 2.86 | POU class 5 homeobox 1B | |||
| FAM58B | 2.85 | family with sequence similarity 58, member B | |||
| LOC440518 | 2.84 | golgin A2 pseudogene | |||
| PGM5P2 | 2.80 | phosphoglucomutase 5 pseudogene 2 | |||
| VHL | 2.77 | von Hippel-Lindau tumor suppressor | |||
| CXorf18 | 2.76 | chromosome X open reading frame 18 | |||
| MIR139 | 2.73 | microRNA 139 | |||
| C2orf27B | 2.61 | chromosome 2 open reading frame 27B | |||
| ZNRF2 | 2.59 | zinc and ring finger 2 | |||
| HOXA11 | −7.11 | homeobox A11 | |||
| EIF3K | −7.21 | eukaryotic translation initiation factor 3, subunit K | |||
| UQCRQ|GDF9 | −7.29 | ubiquinol-cytochrome c reductase, complex III subunit VII, 9.5 kDa | growth differentiation factor 9 | |||
| RPL35 | −7.37 | ribosomal protein L35 | |||
| TGFBI | −7.55 | transforming growth factor, β-induced, 68 kDa | |||
| TYMS | −7.66 | thymidylate synthetase | |||
| RPN2|EEF1A2 | −7.68 | ribophorin II | eukaryotic translation elongation factor 1 α 2 | |||
| HSPA5 | −7.83 | heat shock 70 kDa protein 5 (glucose-regulated protein, 78 kDa) | |||
| ATP5I|MYL5 | −7.91 | ATP synthase, H+ transporting, mitochondrial Fo complex, subunit E | myosin, light chain 5, regulatory | |||
| C11orf10 | −7.94 | chromosome 11 open reading frame 10 | |||
| TOMM7|C4orf46 | −8.04 | translocase of outer mitochondrial membrane 7 homolog (yeast) | chromosome 4 open reading frame 46 | |||
| GSTP1 | −8.04 | glutathione S-transferase pi 1 | |||
| UQCR11 | −8.05 | ubiquinol-cytochrome c reductase, complex III subunit XI | |||
| RPS19 | −8.06 | ribosomal protein S19 | |||
| NDUFAB1 | −8.13 | NADH dehydrogenase (ubiquinone) 1, α/β subcomplex, 1, 8 kDa | |||
| SNRPF | −8.19 | small nuclear ribonucleoprotein polypeptide F | |||
| CALR | −8.34 | calreticulin | |||
| ADAM12 | −8.39 | ADAM metallopeptidase domain 12 | |||
| CD74 | −8.42 | ||||
| GLIPR1|KRR1 | −8.46 | GLI pathogenesis-related 1 | KRR1, small subunit (SSU) processome component, homolog (yeast) | |||
| MT2A | −8.47 | metallothionein 2A | |||
| RPL38|UBE2J2 | −8.51 | ribosomal protein L38 | ubiquitin-conjugating enzyme E2, J2 (UBC6 homolog, yeast) | |||
| ECM1 | −8.62 | extracellular matrix protein 1 | |||
| ATP5E | −8.68 | ATP synthase, H+ transporting, mitochondrial F1 complex, epsilon subunit | |||
| PFDN5 | −8.89 | prefoldin subunit 5 | |||
| COX7C | −9.07 | cytochrome c oxidase subunit VIIc | |||
| RPS12|SNORA33 | −9.15 | ribosomal protein S12 | small nucleolar RNA, H/ACA box 33 | |||
| TNC | −9.22 | tenascin C | |||
| SCD | −9.33 | stearoyl-CoA desaturase (delta-9-desaturase) | |||
| VCAN | −9.33 | versican | |||
| C3orf78 | −9.56 | chromosome 3 open reading frame 78 | |||
| RPS15A | −9.89 | ribosomal protein S15a | |||
| RPS25 | −10.03 | ribosomal protein S25 | |||
| NUCKS1 | −10.04 | nuclear casein kinase and cyclin-dependent kinase substrate 1 | |||
| VIM | −10.11 | vimentin | |||
| POM121|POM121C | −10.13 | POM121 membrane glycoprotein | POM121 membrane glycoprotein C | |||
| RPS28 | −10.17 | ribosomal protein S28 | |||
| RBP7 | −10.54 | retinol binding protein 7, cellular | |||
| RBP1 | −10.59 | retinol binding protein 1, cellular | |||
| RPLP0 | −10.69 | ribosomal protein, large, P0 | |||
| MMP7 | −10.72 | matrix metallopeptidase 7 (matrilysin, uterine) | |||
| RPS28 | −11.27 | ribosomal protein S28 | |||
| NDUFA1|RNF113A | −11.45 | NADH dehydrogenase (ubiquinone) 1 α subcomplex, 1, 7.5 kDa | ring finger protein 113A | |||
| GJA1 | −12.38 | gap junction protein, α 1, 43 kDa | |||
| RNASEK | −13.16 | ribonuclease, RNase K | |||
| COX6C | −13.28 | cytochrome c oxidase subunit VIc | |||
| RPL27 | −13.73 | ribosomal protein L27 | |||
| SEC61G | −14.43 | Sec 61 γ subunit | |||
| ZNF778 | −14.97 | zinc finger protein 778 | |||
| RPL41 | −16.03 | ribosomal protein L41 | |||
Abbreviation: ATP, Adenosine triphosphate. See Appendix 1.
The most highly upregulated genes were small nucleolar RNA C/D box (SNORD) genes, which were increased by 2- to 15-fold (Table 3). The SNORDs are small RNA molecules that regulate modifications of other RNAs, such as ribosomal RNAs, transfer RNAs, and small nuclear RNA and can guide methylation.19 Several microRNA (miRNA) transcripts were also upregulated, including miR-9 -1, -139, -149, -197, -326, and -339, whereas none of miRNAs were downregulated.
Gene symbols with FCs in dysregulated genes were imported into IPA. The IPA identified several regulated canonical pathways and grouped dysregulated genes into networks. Only networks with the highest score were selected for the analysis. It is worth mentioning that several molecules can participate in different pathways and networks simultaneously.
The major canonical pathways (Table 4) included eukaryotic initiation factor 2 signaling (ratio 33 of 181 [0.182; number of regulated genes/total number of genes in the pathway; P > .05 is significant]); oxidative phosphorylation (ratio 21 of 105 [0.2]); mitochondrial dysfunction (ratio 24 of 165 [0.145]); estrogen receptor (ER) signaling (ration 20 of 133 [0.15]); and mammalian target of rapamycin (mTOR) signaling (ratio 23 of 189 [0.122]).
Table 4.
Canonical Pathways Regulated in Proliferative Phase Endometrium From Women With Adenomyosis Versus Healthy Controls.
| Ingenuity Canonical Pathways | −Log (P Value) | P Value | Ratio | Molecules |
|---|---|---|---|---|
| EIF2 signaling | 1.06E01 | .00000 | 1.82E-01 | RAF1, RPL22, RPS18, RPS8, RPL14, EIF4A2, RPS21, RPS7, SHC1, RPL35, MAPK3, RPL36, RPL12, RPS24, RPL34, RPL27, RPS28, RPS19, RPL23A, RPLP0, RPS12, RPS29, FAU, RPS26, RPL26L1, RPS15A, RPS25, RPL39L, RPL38, RPLP1, RPL13A, RPL41, EIF3K |
| Oxidative phosphorylation | 7.55E00 | .00000 | 2.00E-01 | COX6B1, SDHB, ATP5O, UQCRH, COX6A1, UQCR11, ATP5G2, NDUFA1, ATP5E, COX7C, ATP5G1, NDUFAB1, NDUFA3, MT-ND2, UQCRQ, COX4I1, NDUFB2, ATP5I, NDUFA8, COX6C, NDUFS4 |
| Mitochondrial dysfunction | 5.86E00 | .00000 | 1.45E-01 | SDHB, COX6B1, ATP5O, UQCRH, COX6A1, UQCR11, XDH, NCSTN, ATP5G2, NDUFA1, ATP5E, COX7C, ATP5G1, NDUFAB1, CYB5R3, NDUFA3, MT-ND2, COX4I1, NDUFB2, UQCRQ, ATP5I, NDUFA8, COX6C, NDUFS4 |
| Estrogen receptor signaling | 5.36E00 | .00000 | 1.5E-01 | RAF1, CREBBP, RBFOX2, MED12, SMARCA4, MED27, G6PC3, MED14, TAF9B, PGR, EP300, SHC1, POLR2A, MED15, MAPK3, NCOR2, POLR2I, MED24, MED4, TAF15 |
| mTOR signaling | 4.37E00 | .00004 | 1.22E-01 | RPS28, RPS18, RPS19, FKBP1A, RPS8, EIF4A2, RPS21, PDGFC, RPS12, RPS7, RPS29, HMOX1, FAU, RPS26, MAPK3, RPS15A, PPP2R2C, RPS25, RHOF, PRKD1, EIF3K, EIF4B, RPS24 |
| Hepatic fibrosis/hepatic stellate cell activation | 4.22E00 | .00006 | 1.36E-01 | MYH10, IGFBP4, MYL6, TNFRSF1A, IGFBP5, MMP2, NFKB1, PDGFC, MET, CCL2, TIMP1, TGFB1, IGFBP3, IGF1R, MYH9, EDNRA, ECE1, A2M, TIMP2 |
| Inhibition of matrix metalloproteases | 4.09E00 | .00008 | 2.37E-01 | TIMP3, MMP7, ADAM12, TIMP1, MMP16, MMP14, MMP2, A2M, TIMP2 |
| Regulation of eIF4 and p70S6K signaling | 3.96E00 | .00011 | 1.2E-01 | RAF1, RPS28, RPS18, RPS19, RPS8, EIF4A2, RPS21, RPS12, RPS7, RPS29, SHC1, FAU, RPS26, MAPK3, RPS15A, PPP2R2C, RPS25, RPS24, EIF3K |
| Apoptosis signaling | 2.93E00 | .00117 | 1.3E-01 | ENDOG, ACIN1, TP53, RAF1, CAPN6, TNFRSF1A, MAPK3, CAPN1, PLCG1, NFKB1, MAP4K4, PARP1 |
| Huntington disease signaling | 2.84E00 | .00145 | 9.61E-02 | TP53, CAPN6, SDHB, REST, CREBBP, GNB2L1, HSPA5, VTI1B, SIN3A, EP300, TAF9B, GNG10, TGM2, SHC1, POLR2A, MAPK3, CAPN1, IGF1R, NCOR2, POLR2I, BET1L, PRKD1 |
| Aryl hydrocarbon receptor signaling | 2.46E00 | .00347 | 1.06E-01 | TP53, GSTM3, NFKB1, SMARCA4, EP300, TGM2, GSTM2, ALDH1A1, CYP1A2, MGST2, TGFB1, MAPK3, NCOR2, GSTP1, MCM7 |
| PPARα/RXRα activation | 2.42E00 | .00380 | 9.77E-02 | RAF1, ACOX1, CREBBP, CKAP5, PLCG1, NFKB1, MED12, TGS1, EP300, SHC1, TGFB1, MAPK3, PRKACA, NCOR2, PLCL1, MED24, MAP4K4 |
| Androgen signaling | 2.41E00 | .00389 | 9.92E-02 | CALR, GNB2L1, CREBBP, NFKB1, GNG10, EP300, GNAI2, SHC1, POLR2A, MAPK3, PRKACA, POLR2I, PRKD1 |
| Leukocyte extravasation signaling | 2.37E00 | .00427 | 9.69E-02 | TIMP3, MMP7, MYL6, MMP16, MMP14, JAM2, CXCL12, PLCG1, MMP2, GNAI2, TIMP1, CYBB, ARHGAP35, VCL, ACTN4, ARHGAP1, CTTN, PRKD1, TIMP2 |
| Bladder cancer signaling | 2.35E00 | .00447 | 1.24E-01 | TP53, RAF1, CDH1, MMP7, THBS1, MMP16, MMP14, MAPK3, MMP2, PDGFC, SIN3A |
Abbreviations: ATP, Adenosine triphosphate; EIF, eukaryotic initiation factor; HSPA, heat shock 70 kDa protein; mTOR, mammalian target of rapamycin; RP, ribosomal protein; COX, cytochrome c oxidase; UQCR, ubiquinol-cytochrome c reductase; NDUFAB1, NADH dehydrogenase (ubiquinone) 1, α/β subcomplex; MMP, matrix metalloproteinase; CALR, calreticulin; MYL, myosin light chain; GNB, guanine nucleotide binding protein. See Appendix 1.
The major networks (Table 5) included connective tissue disorders, developmental disorder, and neurological disease; embryonic development, organismal development, and tissue development; cellular movement, cancer, cell-to-cell signaling, and interaction; and cell death and survival, cancer, cellular movement, tissue morphology, organismal functions. The scores and number of molecules involved are presented in Table 5.
Table 5.
Molecular Networks Regulated in Proliferative Phase Endometrium in Women With Adenomyosis Versus Healthy Controls.
| Molecules in Network | Score | Focus Molecules | Top Diseases and Functions |
|---|---|---|---|
| 26s proteasome, APOBEC3G, caspase, CREBBP, EP300, GCLC, GSTP1, HIPK1, HIPK2, HNRNPC, HUWE1, IFITM3, IGFBP7, KMT2A, LTBP1, mir-145, MSH6, NUMB, PCGF2, PLAUR, PP2A, PRPF8, REST, RPS7, SAP130, SIN3A, Smad2/3, SMARCA4, SOX4, SYVN1, TEAD2, TGS1, TP53, UBL5, WWTR1 | 35 | 31 | Connective tissue disorders, developmental disorder, and neurological disease |
| 14-3-3, Actin, ALDH1A1, ANP32A, ATXN1, CBY1, CD3, CST4, ECM1, EDN3, ELAVL1, GPI, IFITM1, IHH, KCNIP4, LEF1, Mapk, MSX2, NUP214, NXF1, PCOLCE, PCSK6, PRKACA, PTCH1, RBFOX2, RCN1, RLIM, RPN2, SHC1, SNRPG, STAT2, TCF, TIMM17A, TSC22D1, VIM | 33 | 30 | Embryonic development, organismal development, and tissue development |
| ADAM12, c-Src, C9orf3, CCL2, collagen(s), CTTN, CYP19, DIO2, ECE1, EDNRA, ETV5, FBLN1, GNB2L1, HEXA, HMOX1, IGF1R, IGFBP3, IL1, ILF3, ITPA, MAP4K4, MET, MMP2, P38 MAPK, PGR, Pkc(s), PKP4, PRKD1, RBP1, SFRP1, TGFB1, TIMP2, TJP1, TNC, VCAN | 31 | 29 | Cellular movement, cancer, and cell-to-cell signaling and interaction |
| Alpha catenin, AMPK, ATP6V0C, BAG4, CD74, CDH1, CDH11, CLU, CXCL12, DES, DPAGT1, Fcer1, FOXO3, growth hormone, Hsp70, IER3, IFNβ, LDB2, MT-ND2, NFkB (complex), NOB1, PFKFB3, PI3K (family), PLCG1, PTPRS, RTF1, SLC39A6, SRCAP, TGM2, TIMP3, TNFRSF1A, TRAF7, UNC93B1, VCL, VHL | 27 | 27 | Cell death and survival, cancer, and cellular movement |
| Akt, BSG, CALR, CAV1, CTSA, EFNB2, ERK1/2, F2R, focal adhesion kinase, GJA1, GLB1, GMFG, GRN, HSPA5, IGFBP5, Jnk, LDL, LRRN1, MAPK3, MMP7, MMP14, PAK1, PI3K (complex), Ppp2c, PSMB10, RAF1, Ras, SERPINA1, SLCO2A1, SPHK1, SRC (family), SULF2, TAPBP, TIMP1, Vegf | 24 | 25 | Cellular movement, tissue morphology, and organismal functions |
| ADCY, ARHGAP1, ARHGAP35, ATP9A, AUTS2, CAP2, Cg, cyclin A, DROSHA, ERK, FJX1, FSH, GNAI2, GPR56, histone h3, Hsp90, IGFBP4, interferon α, LAMP1, Lh, PEAK1, PEBP1, PLAT, PLCL1, PODXL, PRDM1, PSMD3, RNA polymerase II, RPL12, RPL23A, SETD2, SH3KBP1, SKP2, TCR, THBS1 | 22 | 24 | Cardiovascular system development and function, cell-to-cell signaling and interaction, and cellular compromise |
| BCL9, C1orf112, CASP2, DECR1, DPF2, EIF4B, ERMP1, MGLL, NUCKS1, NUPR1, PCYOX1, PTPRJ, RELB, TNS3, ZBTB34, ZFP36L1, ZSWIM6 | 13 | 13 | Cardiovascular system development and function, embryonic development, and organismal injury and abnormalities |
| AGTR1, DKC1, DYNC1H1, HTR2B, KPNA1, MAP3K7, MOB2, MOB1B, MYH10, PBX1, RPL14, RPL35, RPL36, RPS8, SPTBN1, STAT1, STK38L, TNRC6A, UBR5, WEE1, YWHAG | 13 | 14 | Cellular growth and proliferation, hematological system development and function, and hematopoiesis |
| ABI2, BRCA1, COL1A2, COX7C, CST3, E2F1, E2F4, FBN1, HIST1H3A (includes others), HIST2H2BE (includes others), HSF1, KIF4A, LOX, MLH1, MMP25, MTHFD1, NDC80, POLA2, PPARG, PPP2R2B, PRKDC, RB1, RBL1, RFX5, RFXANK, RFXAP, SMAD3, SMAD4, SMC2, SMC1A, SRRM2, UBAP2L, VPS39, YIPF3, YLPM1 | 12 | 17 | Cancer, antigen presentation, and developmental disorder |
| CAV1, CD68, CD163, CSNK2A2, DCN, DDIT3, FCGR1A, HMOX1, HP, HSPA1A/HSPA1B, HSPD1, IL6, IL10, IL24, KAT5, KDM5B, LGALS3, LGALS3BP, LGR5, MEX3C, MIA3, mir-155, miR-155-5p (miRNAs w/seed UAAUGCU), NEUROG1, PDCD1, PPIC, PPP2R2C, PSIP1, PXDN, RBP7, SBDS, THBS1, TIMP1, Tlr, VPS53 | 12 | 17 | Cell death and survival, organismal injury and abnormalities, and inflammatory response |
Abbreviations: ATP, Adenosine triphosphate; CALR, calreticulin; COX, cytochrome c oxidase; DIO, thyroxine deiodinase; ECM, extracellular matrix; EIF, eukaryotic initiation factor; GJA, gap junction protein α; GNB, guanine nucleotide binding protein; GP, glycoprotein; GSTP, glutathione S-transferase pi; Hsp, heat shock protein; IL, interleukin; IFN, interferon; LOX, lysyl oxidase; MMP, matrix metalloproteinase; RBP, retinol binding protein; RPN, ribophorin; RP, ribosomal protein; SNRPG, small nuclear ribonucleoprotein polypeptide G; TNC, tenascin C; VCAN, versican; VIM, vimentin; VHL, von Hippel-Lindau tumor suppressor; YIPF, Yip1 domain family; ZBTB34, zinc finger and BTB domain containing 34. See Appendix 1.
Validation of Microarray Results by Quantitative Real-Time PCR
For validation by QRT-PCR, we tested the most highly up- and downregulated genes in the microarray data analysis. These genes had an FC of 2.0 or higher by comparison of endometrial gene expression from women with versus without adenomyosis.
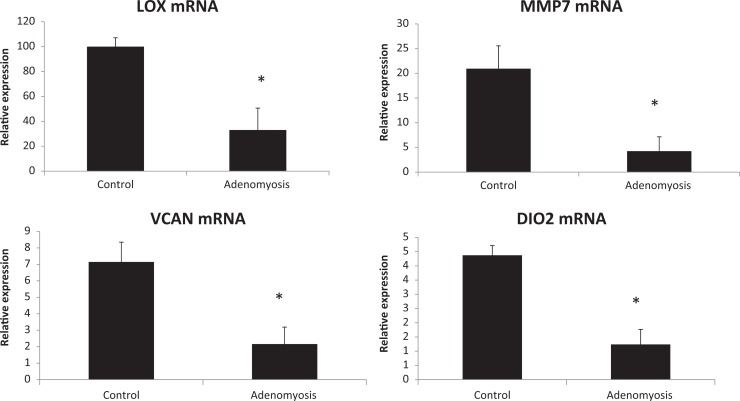
The QRT-RT-PCR validated versican (VCAN), lysyl oxidase (LOX), matrix metalloproteinase (MMP) 7 (MMP7), and thyroxine deiodinase 2 genes (Figure 3). The SNORD116-5 gene expression did not reach statistical significance upon validation.
Figure 3.
Quantitative real-time reverse transcriptasepolymerase chain reaction (QPCR) validation of microarray data in proliferative phase adenomyosis (n = 3) and control samples (n = 5) expressed as relative expression to the levels of endogenous control RPL19. *Statistically significant differences (p < 0.05) determined by one-way ANOVA. Error bars represent standard error of mean (SEM). LOX, lysyl oxidase; MMP7, matrix metalloproteinase 7; VCAN, versican; DIO2, thyroxine deiodinase 2.
Discussion
Endometrial infiltration into the myometrium eliciting an inflammatory and proliferative response is a hallmark of adenomyosis. The pathobiology of the endometrium in the setting of an inflammatory milieu in the adjacent myometrium in this disorder is poorly understood. This is the first study to evaluate global gene expression in women with diffuse adenomyosis and no other uterine or pelvic pathology, compared to healthy, fertile, and normal controls. The results of the current study suggest a global disturbance of the endometrium involving extracellular matrix (ECM), proliferation, apoptosis, and steroid hormone signaling.
Steroid Responsiveness
Estrogen receptor signaling is one of the top canonical pathways dysregulated in our study (Table 4). Superficial foci of adenomyosis have been shown to be more estrogen sensitive than implants deep in the myometrium.20 Of significance, expression of progesterone receptor (PR)-B is reduced in both eutopic and ectopic endometrium in women with adenomyosis.10 Mehasseb et al showed decreased ER-α and PR expression, but increased ER-β expression, in the adenomyotic compared to that of the normal endometrium, suggesting an explanation for the resistance of the condition to progestational agents.21 The fact that all of our samples were biopsied in the proliferative phase may provide an explanation to why PR did not come up among dysregulated genes in our study. Another dysregulated gene also involved in ER signaling is gene regulated in breast cancer 1, a chromatin-bound ER coactivator essential for ER-mediated transcription by stabilizing interactions between ER and additional cofactors.22,23 Interestingly, while herein it was almost 3-fold downregulated, it was upregulated in ectopic endometriotic lesions.24
Leyendecker et al postulated that ectopic diseases of the endometrium result in part from the physiological mechanism of “tissue injury and repair” involving local estrogen production in an estrogen-sensitive environment.25 Activity of aromatase and estrone sulfatase has been identified in both eutopic and ectopic endometrium of women with adenomyosis.26 Chen et al postulated that estradiol may stimulate epithelial–mesenchymal transition of cells with invasive properties,27 and serum estradiol levels negatively correlated with E-cadherin expression in both eutopic and ectopic endometrium. In that study, raloxifene inhibited estrogen-dependent persistence and growth of xenotransplanted eutopic or ectopic endometrium from patients with adenomyosis in ovariectomized, severe combined immunodeficient mice. Recent data indicate that estrogen promotes angiogenesis in endometrium by activating the slug–vascular endothelial growth factor axis in endometrial stromal cells.28 The activation of ER signaling pathway in the current study supports the previous research while warranting more functional studies on this matter.
MicroRNA
Several miRNAs were upregulated in the current study, namely, miR-9 -1, -139, -149, -197, -326, and -339. To the best of our knowledge, there are no published data on endometrial miRNA expression and involvement in adenomyosis. Adenomyosis was observed histologically in uteri in a murine knockout model of Dicer, a ribonuclease required for miRNA biosynthesis, indicating that miRNA have an important role in adenomyosis.29 MicroRNAs have been extensively studied in other uterine pathologies, for example, endometriosis and fibroids.30–32 An earlier study demonstrated dysregulation of miR-9 and miR-34 miRNA families in eutopic, early secretory endometrial tissue from women with endometriosis.33 The dysregulation of endometrial miR-9 in proliferative phase of women with adenomyosis herein suggests its involvement in gene expression regulation in the pathobiology of this disorder. Dysregulation of miR-9 has been implicated in several human malignancies and is thought to be involved in the migration and proliferation of different cell types.34 Further studies are warranted to explore the role of miRNA in the pathogenesis and pathophysiology of adenomyosis.
Apoptosis and Proliferation
Herein, we found that apoptosis pathway was significantly dysregulated in endometrium of women with adenomyosis, compared to controls (Table 4). The most downregulated gene in our study, RPL14, is a RP that regulates casein kinase II (CK2), a protein serine/threonine kinase involved in cell survival, growth, and proliferation. Downregulation of CK2 confirms an earlier report in eutopic endometrium in patients with adenomyosis.35 Impaired apoptosis and proliferation of the eutopic endometrium are believed to play an important role in the pathogenesis of adenomyosis and endometriosis.15 Stromal B-cell lymphoma 2 (BCL-2) levels were lower in endometrium of patients with adenomyosis,36 and endometrial stromal cells from women with adenomyosis proliferate more rapidly than controls, cultured alone or in the presence of estradiol, medroxyprogesterone acetate, interleukin 6, or interferon γ.8 Apoptosis was one of the top significantly regulated networks in the global gene expression analysis of adenomyosis versus uterine fibroid or control samples, as mentioned earlier.14
We additionally observed dysregulation of mTOR signaling in proliferative eutopic endometrium from women with adenomyosis. The mTOR is a serine/threonine protein kinase that regulates cell proliferation and survival, which is upregulated in endometriosis.37,38 Dysregulation of mTOR is noted in human malignancies inhibitors of mTOR are currently in development and clinical investigation as novel anticancer agents.39–42 Induction of apoptoptic pathways may be a therapeutic target for endometrial dysfunction in women with symptomatic adenomyosis. Currently existing therapy with GnRHas has been demonstrated to act in part through induction of apoptosis in eutopic and ectopic endometrium in women with adenomyosis.43
Dysregulation of Genes Involved in ECM Function
Prior studies suggest that the endometrium of women with adenomyosis may have an enhanced predisposition for invasiveness.2,44 One of the most downregulated genes in our array study, validated by QRT-PCR, was MMP7, a member of the MMP family of enzymes that participate in ECM remodeling in endometrium, accompanying proliferation, and at the time of tissue desquamation.45 A band of smooth muscle and ECM creates a barrier between the endometrium and the myometrium,46 and cell invasion is mediated by interaction of adhesion receptors with ECM proteins.47,48 While MMP7 was downregulated herein, others have reported upregulation of genes for other MMPs, MMP2, MMP3, and MMP9, in eutopic endometrium from patients with adenomyosis, compared to the controls.49,50 The MMP2 messenger RNA (mRNA) was more highly expressed in the proliferative compared to the secretory phase, suggesting a higher propensity for invasion in an estrogen-dominant milieu.
Lysyl oxidase was one of the most highly downregulated genes herein. It encodes an extracellular copper enzyme that initiates crosslinking of collagens and elastin. In addition to crosslinking ECM proteins, LOX may have a role in tumor suppression as well as being involved in the embryo-endometrial cross talk.51 A less rigid ECM in the eutopic endometrium may enable enhanced endometrial cell migration to the endomyometrial junction. Another gene relevant to endometrial tissue integrity that was downregulated was gap junction protein α 1, important in connexin 43 functionality. This finding supports an earlier report of decreased connexin 43 function in eutopic endometrium of women with adenomyosis compared to controls.52,53 Interestingly, in our study, mRNA for VCAN, a major ECM component, was significantly downregulated in eutopic endometrium of patients with adenomyosis. This is in contrast to its overexpression in endometrial stromal cells and endometrial tissue from women with moderate and severe endometriosis,54 suggesting presence of molecular differences between these 2 diseases of ectopic endometrial location.
Study Limitations
The primary limitation of our study relates to the small sample size. As other investigators have noted (see subsequently), the high prevalence of other uterine pathologies with adenomyosis makes it difficult to obtain significant numbers of tissue specimens from patients free of confounding pathologies or hormonal exposures. In one study, leiomyoma was found in 50% of patients with adenomyosis.55 The association of endometriosis has been variably reported from 27 to as high as 90% of patients with adenomyosis.56–58 For this reason, our sample size, even drawing from a large multisite tissue bank, was limited.59 Also, samples analyzed herein were from the proliferative phase, and key pathways and gene dysregulation in the secretory phase may add additional insights into endometrial abnormalities in the pathogenesis of adenomyosis. It is also plausible that gene expression in adenomyosis is different in early stages than later stages of the disease as has been shown for endometriosis.54 In addition, all of our adenomyosis samples were obtained from black women, whereas control group was comprised of women of other races. Whether this has a significant impact on the data is unclear.
Conclusion
This study presents the first genome-wide gene expression profile of eutopic endometrium of patients with clinical adenomyosis without confounders of other uterine or pelvic pathologies in the adenomyosis or the control groups. The results support prior focal studies that reveal fundamental abnormalities in eutopic endometrium in patients with adenomyosis. The implications and biological significance of the differentially expressed genes and altered pathways provide a platform for further investigation to elucidate the mechanisms and improve the molecular understanding of this complex disorder.
Appendix 1
LOC100293539 hypothetical protein LOC100293539
LOC100132147 uncharacterized LOC100132147
ND2 NADH dehydrogenase subunit 2
POU (Pit-1, Oct-1/2, Unc86)
POM Pore membrane protein
NADH nicotinamide adenine dinucleotide (reduced)
ADAM a disintegrin and metalloproteinase
CD cluster of differentiation
GLI Glioma associated
ACA ACA
MDR Multi Drug Resistance
TAP Transporter associated with antigen processing
KRR not acronym
UBC Ubiquitin conjugating
HLA-DOB HLA class II molecule is a heterodimer consisting of an alpha (DOA) and a beta chain (DOB)
Vlc V light chain
PPAR Peroxisome Proliferator-Activated Receptor
RXR Retinoid X Receptor
RAF Rapidly Accelerated Fibrosarcoma
SHC Src Homology 2 Domain Containing
MAPK Mitogen Activated Protein Kinase
FAU Finkel-Biskis-Reilly Murine Sarcoma Virus (FBR- MuSV) Ubiquitously Expressed
SDHB Succinate Dehydrogenase Complex, Subunit B
MT Metallothionien
CREBBP (CAMP Responsive Element Binding Protein) Binding Protein
TAF TATA Box Binding Protein (TBP)-Associated Factor
CCL Chemokine (C-C Motif) Ligand
NFKB Nuclear Factor Of Kappa Light Polypeptide Gene Enhancer In B-Cells
PRKD Protein Kinase D
PPP Protein Phosphatase
HMOX Heme Oxygenase
PDGFC Platelet Derived Growth Factor C
FKBP FK506 Binding Protein
NCOR Nuclear Receptor Corepressor
PGR Progesterone Receptor
SMARCA SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin, Subfamily A
RBFOX RNA Binding Protein, Fox-1 Homolog
ARHGAP RhoA GTPase activating protein
GSTM Glutathione S-Transferase Mu
EP E1A binding protein
SIN SWI Independent
VTI Vesicle Transport Through Interaction With T-SNAREs
PLCG Phospholipase C Gamma
MET Met protooncogene
MED Mediator complex subunit
POLR Polymerase (RNA) III (DNA Directed) Polypeptide
RHOF Ras Homolog Family Member
MYH Myosin, Heavy Chain
IGFBP Insulin-Like Growth Factor Binding Protein
TNFRSF Tumor Necrosis Factor Receptor Superfamily
TIMP Tissue inhibitor of metalloproteinase
TGFB Transforming Growth Factor, Beta
EDNRA Endothelin Receptor Type A
CAPN Calpain
ECE Endothelin Converting Enzyme
ENDOG Endonuclease G
ACIN Apoptotic Chromatin Condensation Inducer
TP Tumor protein
PARP Poly (ADP-Ribose) Polymerase
GNG Guanine Nucleotide Binding Protein (G Protein), Gamma
CXCL Chemokine (C-X-C Motif) Ligand
JAM Junctional Adhesion Molecule
GNA Guanine Nucleotide Binding Protein (G Protein), Alpha
CYP Cytochrome P450
ALDH Aldehyde Dehydrogenase
TGM Transglutaminase
BET Bromo and Extra Terminal
MGST Microsomal Glutathione S-Transferase
PLCL Phospholipase C-Like
ACOX Acyl-CoA Oxidase
CTTN Cortactin
PRKACA Protein Kinase, CAMP-Dependent, Catalytic, Alpha
CYBB Cytochrome B-245, Beta
VCL Viculin
ACTN Actinin, Alpha
CDH Cadherin
NCSTN Nicastrin
REST RE1-Silencing Transcription Factor
THBS Thrombospondin
APOBEC Apolipoprotein B MRNA Editing Enzyme, Catalytic
ANP acidic (leucine-rich) nuclear phosphoprotein
ATXN Ataxin
AMPK adenosine monophosphate-activated protein kinase
Akt Akt
ADCY Adenylate Cyclase
AUTS Autism, susceptibility to
AGTR Angiotensin II Receptor
ABI Abl-Interactor
BAG BCL2-Associated Athanogene
BSG Basigin
BRCA Breast Cancer
CBY Chibby Homolog
DES Desmin
DPAGT Dolichyl-Phosphate (UDP-N-Acetylglucosamine) N-Acetylglucosamine phosphotransferase 1 (GlcNAc-1-P Transferase)
DROSHA Drosha, Ribonuclease Type III
DECR 2,4-Dienoyl CoA Reductase
DPF Dipeptidyl-Peptidase
DKC Dyskeratosis Congenita
DYNC Dynein, Cytoplasmic
DCN Decorin
DDIT DNA-Damage-Inducible Transcript
CST Cystatin
c-Src (cytoplasmic) Sarcoma
CLU Clusterin
CAV Caveolin
CAP CAP Adenylate Cyclase-Associated Protein
SRCAP Snf2-Related CREBBP Activator Protein
Cg cathepsin G
CASP Caspase
PCOLCE Procollagen C-Endopeptidase Enh
COL Collagen
CSNK Casein Kinase
EDN Endothelin
ELAVL ELAV Like RNA Binding Protein
ETV Ets Variant
EFNB Ephrin-B
ERK Extracellular signal related kinase
ERMP endoplasmic reticulum metallopeptidase
GCLC Glutamate-Cysteine Ligase, Catalytic Subunit
GLB Galactosidase, Beta
GMFG Glia Maturation Factor, Gamma
GRN Granulin
GPR G-Protein Coupled Receptor
FBLN Fibulin
Fcer Fc fragment of IgE, high affinity I, receptor
FOXO Forkhead Box O
FJX four jointed box
FSH Follicle Stimulating Hormone
FBN Fibrillin
FCGR Fc Fragment Of IgG, Low Affinity, Receptor
HIPK Homeodomain Interacting Protein Kinase
HNRNPC Heterogeneous Nuclear Ribonucleoprotein C
HUWE HECT, UBA And WWE Domain Containing, E3 Ubi quitin Protein Ligase
HEXA Hexosaminidase
HTR 5-hydroxytryptamine (serotonin) receptor
HIST histocompatibility
HSF Heat Shock Transcription Factor
HP Haptoglobin
HSPD Heat Shock 60kDa Protein 1
IFITM Interferon Induced Transmembrane
IHH Indian Hedgehog
ILF interleukin enha
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/NIH, National Centers for Translational Research in Reproduction, and Infertility P50 HD 055764-09 (LCG).
References
- 1. Tomassetti C, Meuleman C, Timmerman D, D’Hooghe T. Adenomyosis and subfertility: evidence of association and causation. Semin Reprod Med. 2013;31(2):101–108. [DOI] [PubMed] [Google Scholar]
- 2. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril. 2012;98(3):572–579. [DOI] [PubMed] [Google Scholar]
- 3. Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyosis of the uterus—revisited. Am J Obstet Gynecol. 1972;112(5):583–593. [DOI] [PubMed] [Google Scholar]
- 4. Fischer CP, Kayisili U, Taylor HS. HOXA10 expression is decreased in endometrium of women with adenomyosis. Fertil Steril. 2011;95(3):1133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinol. 2007;148(8):3814–3826. [DOI] [PubMed] [Google Scholar]
- 6. Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinol. 2003;144(7):2870–2881. [DOI] [PubMed] [Google Scholar]
- 7. Matsumoto Y, Iwasaka T, Yamasaki F, Sugimori H. Apoptosis and Ki-67 expression in adenomyotic lesions and in the corresponding eutopic endometrium. Obstet Gynecol. 1999;94(1):71. [DOI] [PubMed] [Google Scholar]
- 8. Yang JH, Wu MY, Chen CD, Chen MJ, Yang YS, Ho HN. Altered apoptosis and proliferation in endometrial stromal cells of women with adenomyosis. Hum Reprod. 2007;22(4):945–952. [DOI] [PubMed] [Google Scholar]
- 9. Goteri G, Lucarini G, Montik N, et al. Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor-1α (HIF-1α), and microvessel density in endometrial tissue in women with adenomyosis. Int J Gynecol Path. 2009;28(2):157–163. [DOI] [PubMed] [Google Scholar]
- 10. Nie J, Lu Y, Liu X, Guo S-W. Immunoreactivity of progesterone receptor isoform B, nuclear factor κB, and IκBα in adenomyosis. Fertil Steril. 2009;92(3):886–889. [DOI] [PubMed] [Google Scholar]
- 11. Ota H, Igarashi S, Kato N, Tanaka T. Aberrant expression of glutathione peroxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil Steril. 2000;74(2):313–318. [DOI] [PubMed] [Google Scholar]
- 12. Ota H. Xanthine oxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil Steril. 2001;75(4):785–790. [DOI] [PubMed] [Google Scholar]
- 13. Xiaoyu L, Weiyuan Z, Ping J, Anxia W, Liane Z. Serum differential proteomic analysis of endometriosis and adenomyosis by iTRAQ technique. Eur J Obstet Gynecol Reprod Biol. 2014;182:62–65. [DOI] [PubMed] [Google Scholar]
- 14. Hever A, Roth RB, Hevezi PA, et al. Molecular characterization of human adenomyosis. Mol Hum Reprod. 2006;12(12):737–748. [DOI] [PubMed] [Google Scholar]
- 15. Benagiano G, Brosens I. The endometrium in adenomyosis. Women’s Health 2012;8(3):301–312. [DOI] [PubMed] [Google Scholar]
- 16. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1(0):3–25. [DOI] [PubMed] [Google Scholar]
- 18. Talbi S, Hamilton AE, Vo KC, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinol. 2006;147(3):1097–1121. [DOI] [PubMed] [Google Scholar]
- 19. Kiss-László Z, Henry Y, Kiss T. Sequence and structural elements of methylation guide snoRNAs essential for site-specific ribose methylation of pre-rRNA. EMBO J. 1998;17(3):797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585–596 [DOI] [PubMed] [Google Scholar]
- 21. Mehasseb MK, Panchal R, Taylor AH, Brown L, Bell SC, Habiba M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil Steril. 2011;95(7):2228–2235.e1. [DOI] [PubMed] [Google Scholar]
- 22. Mohammed H, D’Santos C, Serandour AA, et al. Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Reports. 2013;3(2):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chand AL, Wijayakumara DD, Knower KC, et al. The orphan nuclear receptor LRH-1 and ERα activate GREB1 expression to induce breast cancer cell proliferation. PLoS ONE. 2012;7(2):e31593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pellegrini C, Gori I, Achtari C, et al. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil Steril. 2012;98(5):1200–1208. [DOI] [PubMed] [Google Scholar]
- 25. Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet. 2009;280(4):529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamamoto T, Noguchi T, Tamura T, Kitawaki J, Okada H. Evidence for estrogen synthesis in adenomyotic tissues. Am J Obstet Gynecol. 1993;169(3):734–738. [DOI] [PubMed] [Google Scholar]
- 27. Chen Y-J, Li H-Y, Huang CH, et al. Oestrogen-induced epithelial–mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J Pathol. 2010;222(3):261–270. [DOI] [PubMed] [Google Scholar]
- 28. Huang T-S, Chen Y-J, Chou T-Y, et al. Oestrogen-induced angiogenesis promotes adenomyosis by activating the Slug-VEGF axis in endometrial epithelial cells. J Cell Mol Med. 2014;18(7):1358–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gonzalez G, Behringer RR. Dicer is required for female reproductive tract development and fertility in the mouse. Mol Reprod Dev. 2009;76(7):678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marsh EE, Lin Z, Yin P, Milad M, Chakravarti D, Bulun SE. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil Steril. 2008;89(6):1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pan Q, Luo X, Chegini N. Differential expression of microRNAs in myometrium and leiomyomas and regulation by ovarian steroids. J Cell Mol Med. 2008;12(1):227–240. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Santamaria X, Taylor H. MicroRNA and gynecological reproductive diseases. Fertil Steril. 2014;101(6):1545–1551. [DOI] [PubMed] [Google Scholar]
- 33. Burney RO, Hamilton AE, Aghajanova L, et al. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod. 2009;15(10):625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yuva-Aydemir Y, Simkin A, Gascon E, Gao F-B. MicroRNA-9. RNA Biol. 2014;8(4):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sa R, Song J. Relationship between ultrastructural features with the expression of connexin 43 in the uterine junction zone and pathogenesis of adenomyosis. Zhonghua Fu Chan Ke Za Zhi. 2010;45(10):762–766. [PubMed] [Google Scholar]
- 36. Jones RK, Searle RF, Bulmer JN. Apoptosis and bcl-2 expression in normal human endometrium, endometriosis and adenomyosis. Hum Reprod. 1998;13(12):3496–3502. [DOI] [PubMed] [Google Scholar]
- 37. Makker A, Goel MM, Das V, Agarwal A. PI3K-Akt-mTOR and MAPK signaling pathways in polycystic ovarian syndrome, uterine leiomyomas and endometriosis: an update. Gynecol Endocrinol. 2012;28(3):175–181. [DOI] [PubMed] [Google Scholar]
- 38. Erikson D, Chen JC, Piltonen TT, Conti M, Irwin JC, Giudice LC. Inhibition of epidermal growth factor receptor restores decidualization markers in stromal fibroblasts from women with endometriosis. JEPPD. 2014;6(4):196–211. [Google Scholar]
- 39. Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729–734. [DOI] [PubMed] [Google Scholar]
- 40. Fasolo A, Sessa C. mTOR inhibitors in the treatment of cancer. Expert Opin Investig Drugs. 2008;17(11):1717–1734. [DOI] [PubMed] [Google Scholar]
- 41. deGraffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clin Cancer Res. 2004;10(23):8059–8067. [DOI] [PubMed] [Google Scholar]
- 42. Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27(13):2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khan KN, Kitajima M, Hiraki K, et al. Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum Reprod. 2010;25(11):2878–2890. [DOI] [PubMed] [Google Scholar]
- 44. Mehasseb MK, Taylor AH, Pringle JH, Bell SC, Habiba M. Enhanced invasion of stromal cells from adenomyosis in a three-dimensional coculture model is augmented by the presence of myocytes from affected uteri. Fertil Steril. 2010;94(7):2547–2551. [DOI] [PubMed] [Google Scholar]
- 45. Li T, Li YG, Pu DM. Matrix metalloproteinase-2 and -9 expression correlated with angiogenesis in human adenomyosis. Gynecol Obstet Invest. 2006;62(4):229–235. [DOI] [PubMed] [Google Scholar]
- 46. Yang J-H, Wu M-Y, Chen M-J, Chen S-U, Yang Y-S, Ho H-N. Increased matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 secretion but unaffected invasiveness of endometrial stromal cells in adenomyosis. Fertil Steril. 2009;91(5):2193–2198. [DOI] [PubMed] [Google Scholar]
- 47. Symington BE, Takada Y, Carter WG. Interaction of integrins alpha 3 beta 1 and alpha 2 beta 1: potential role in keratinocyte intercellular adhesion. J Cell Biol. 1993;120(2):523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prifti S, Zourab Y, Koumouridis A, Bohlmann M, Strowitzki T, Rabe T. Role of integrins in invasion of endometrial cancer cell lines. Gynecol Oncol. 2002;84(1):12–20. [DOI] [PubMed] [Google Scholar]
- 49. Qiu F, XM G, GL L, et al. Expression of matrix metalloproteinase and tissue inhibitor of metalloproteinase in adenomyosis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2006;37(1):118–122. [PubMed] [Google Scholar]
- 50. Tokyol C, Aktepe F, Dilek FH, Sahin O, Arioz DT. Expression of cyclooxygenase-2 and matrix metalloproteinase-2 in adenomyosis and endometrial polyps and its correlation with angiogenesis. Int J Gynecol Path. 2009;28(2):148–156. [DOI] [PubMed] [Google Scholar]
- 51. Aghajanova L, Shen S, Rojas AM, Fisher SJ, Irwin JC, Giudice LC. Comparative transcriptome analysis of human trophectoderm and embryonic stem cell-derived trophoblasts reveal key participants in early implantation. Biol Reprod. 2011;86(1):1–21. [DOI] [PubMed] [Google Scholar]
- 52. Yu J, Boicea A, Barrett KL, et al. Reduced connexin 43 in eutopic endometrium and cultured endometrial stromal cells from subjects with endometriosis. Mol Hum Reprod. 2014;20(3):260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Regidor PA, Regidor M, Schindler AE, Winterhager E. Aberrant expression pattern of gap junction connexins in endometriotic tissues. Mol Hum Reprod. 1997;3(5):375–381. [DOI] [PubMed] [Google Scholar]
- 54. Aghajanova L, Giudice LC. Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod Sci. 2010;18(3):229–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McElin TW, Bird CC. Adenomyosis of the uterus. Obstet Gynecol Annu. 1974;3(0):425–441. [PubMed] [Google Scholar]
- 56. Gonzales M, de Matos LA, da Costa Gonçalves MO, et al. Patients with adenomyosis are more likely to have deep endometriosis. Gynecol Surg. 2012;9(3):259–264. [Google Scholar]
- 57. Kunz G, Beil D, Huppert P, Noe M, Kissler S, Leyendecker G. Adenomyosis in endometriosis—prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod. 2005;20(8):2309–2316. [DOI] [PubMed] [Google Scholar]
- 58. Larsen SB, Lundorf E, Forman A, Dueholm M. Adenomyosis and junctional zone changes in patients with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2011;157(2):206–211. [DOI] [PubMed] [Google Scholar]
- 59. Sheldon E, Vo KC, McIntire RA, et al. Biobanking human endometrial tissue and blood specimens: standard operating procedure and importance to reproductive biology research and diagnostic development. Fertil Steril. 2011;95(6):2120–2122. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]