Abstract
Preeclampsia (PE) is a pregnancy-specific disease characterized by the new onset of hypertension and proteinuria. Mothers with PE are known to develop endothelial dysfunction, but its effect on infants has been understudied, as newborns are often asymptomatic. Recent studies indicate that infants born from preeclamptic pregnancies develop endothelial dysfunction including higher blood pressure during childhood and an increased risk of stroke later in life. We hypothesize that PE reduces the number and function of fetal angiogenic progenitor cells and may contribute to this increased risk. We quantified 2 distinct types of angiogenic progenitors, pro-angiogenic circulating progenitor cells (CPCs) and endothelial colony-forming cells (ECFCs), from the umbilical cord blood of preeclamptic pregnancies and normotensive controls. Pro-angiogenic and nonangiogenic CPCs were enumerated via flow cytometry and ECFCs by cell culture. Additionally, we studied the growth, migration, and tube formation of ECFCs from PE and gestational age–matched normotensive control pregnancies. We found that PE resulted in decreased cord blood pro-angiogenic CPCs and ECFCs. Nonangiogenic CPCs were also decreased. Preeclamptic ECFCs demonstrated decreased growth and migration but formed tube-like structures in vitro similar to controls. Our results suggest that the preeclamptic environment alters the number and function of angiogenic progenitor cells and may increase the risk of later vascular disease.
Keywords: preeclampsia, endothelial progenitor cells, endothelial colony-forming cells, pro-angiogenic circulating progenitor cells, cord blood
Introduction
Preeclampsia (PE), a pregnancy-specific disease, annually affects 8 million women–infant pairs worldwide, resulting in significant morbidity and mortality for both mothers and infants.1,2 Women who develop PE typically present with hypertension and proteinuria. These symptoms are indicative of maternal systemic endothelial dysfunction. It was long thought that PE only affected the mother, as the infant does not manifest similar features. Yet, recent studies indicate that infants born to PE pregnancies also develop endothelial dysfunction, resulting in higher blood pressure during childhood and young adulthood as well as an increased long-term risk of stroke.3–6 Mechanisms leading to this increased risk are unknown but could potentially be related to fetal programming of endothelial progenitor cells (EPCs).
Many disorders characterized by endothelial dysfunction have been associated with reduced circulating EPC number and function.7–10 Endothelial progenitor cells have been implicated in cardiovascular disease, stroke, and hypertension.11–14 Prior studies have shown that PE is associated with a decrease in the number of circulating progenitors (referred to as EPCs) in both the maternal and fetal circulation.15–17 However, subsequent studies have shown that the flow cytometry protocols used (CD34+, VEGFR-2+, and ACC133+) are identifying angiogenic macrophages rather than progenitors of the endothelial lineage.18,19 The term “EPC” itself thus remains the subject of considerable debate.20 The more inclusive term, “angiogenic progenitor cell” pertains to all cell populations of either endothelial or hematopoietic lineage that stimulate angiogenesis whether or not these cells differentiate into mature endothelial cell populations themselves.21–23 To date, only late-outgrowth endothelial colony-forming cells (ECFCs) have been shown to be true endothelial cell precursors.20 Therefore, we hypothesized that PE alters the number and function of pro-angiogenic circulating progenitor cells (CPCs) and ECFCs, 2 distinct types of angiogenic progenitor cells.
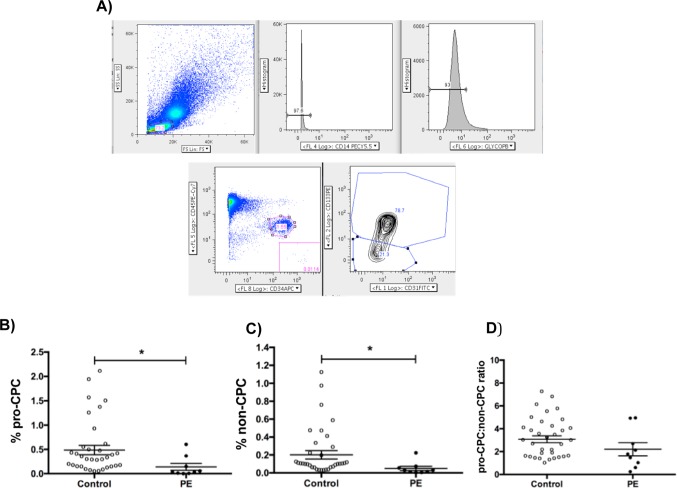
Circulating progenitor cells are a unique subpopulation of mononuclear cells (MNCs) that express hematopoietic, endothelial, and progenitor markers.24,25 They are identified by flow cytometry as a CD31-positive/CD34-positive/CD45-dim/glycophorin-A-negative/CD14-negative population.24 Based on their expression of the immaturity marker, AC133 (Figure 1A), CPCs have been previously shown to be either pro-angiogenic (AC133 positive; pro-CPCs) or nonangiogenic (AC133 negative; non-CPCs) as demonstrated by the ability to promote vascular growth in tumor models.25 However, this same study confirmed that neither the pro-CPC nor the non-CPC populations demonstrated engraftment or the capacity for differentiation into mature endothelial cells. The pro-CPC:non-CPC ratio, a marker of angiogenic potential, is decreased in the cord blood of infants who subsequently develop bronchopulmonary dysplasia and in those delivered from pregnancies affected by gestational diabetes.23,26 Analysis of cord blood pro-CPCs and non-CPCs in PE has yet to be reported.
Figure 1.
A, Cord blood mononuclear cells (MNCs) were analyzed by polychromatic flow cytometry (PFC). Live MNCs were selected and then gated for CD14− and glycophorin A− cells to exclude erythrocytes and macrophages. Next, the CD45-dim and CD34+ population was selected, from which the pro-angiogenic (CD45-dim CD34+ CD31+ AC133+) and nonangiogenic (CD45-dim CD34+ CD31+ AC133-) circulating progenitor cells (CPCs) were identified. B, Pro-CPCs are identified as the percentage of the grandparent MNC population via PFC. The control patients have significantly increased pro-CPCs, 0.49% ± 0.10% (mean ± SEM), P = .01, when compared to the preeclamptic cohort, 0.14% ± 0.07%. C, The percentage of non-CPC is also significantly higher in control versus preeclamptic participants (controls 0.20 ± 0.05 vs preeclamptic 0.05% ± 0.02%; P = .01). D, When comparing the ratio of pro-CPC:non-CPC of controls versus preeclamptics, there is no significant change (controls 3.08 ± 0.30 vs preeclamptic 2.20 ± 0.57, P = .20) *P < .05. SEM indicates standard error of the mean.
Late-outgrowth ECFCs are abundant in umbilical cord blood, have an endothelial morphology, stain positively for endothelial markers, are highly proliferative, and are capable of self-renewal.27 Endothelial colony-forming cells do not express hematopoietic markers19 and are thus distinct from pro-CPCs. Additionally, unlike CPCs that currently can’t be passaged in culture in vitro, ECFCs are utilized to assess cellular function. Preterm infants who subsequently develop chronic lung disease have decreased cord blood ECFCs,23,28 and recent studies have demonstrated that ECFCs are also decreased in PE.29,30 One study showed that the number of ECFC colonies in proportion to the number of peripheral blood MNCs was decreased in PE and that PE-ECFCs demonstrated impaired in vitro angiogenesis, growth, and migration.30 In contrast, another recent study showed that the concentration of ECFCs in proportion to cord blood volume is decreased in PE but showed no difference in the in vitro angiogenic potential of PE-ECFCs.29 Neither of these studies examined the CPC populations, which may provide additional insight into the impact PE has on the health of the offspring.
Given the above findings, we aimed to measure pro-CPC and non-CPC populations using flow cytometry, to enumerate late-outgrowth ECFCs in the cord blood in proportion to both the MNC number and the blood volume and to study the growth, migration, and in vitro angiogenesis of ECFCs in culture from PE compared to controls. We specifically hypothesized that pro-CPCs as well as the number and function of cord blood ECFCs will be decreased in PE.
Materials and Methods
Participant Enrollment and Data Collection
The Colorado Multiple Institutional Review Board approved all study protocols. Eligible mothers were those admitted to the University Hospital Anschutz Inpatient Pavilion, delivered a newborn of 24 to 40 weeks’ gestation, and provided informed consent. Participants were prospectively enrolled from October 2009 until July 3013. Exclusion criteria were known human immunodeficiency virus/hepatitis B virus/hepatitis C virus infection, chorioamnionitis diagnosed by standard clinical criteria,31 and congenital anomalies. Preeclampsia was diagnosed using standard definitions based on the American College of Obstetrics and Gynecology bulletin and the National Institutes of Health Working Group on High Blood Pressure in Pregnancy as the new onset of systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg on at least 2 occasions for at least 6 hours apart and proteinuria of ≥300 mg per 24-hour period or ≥1+ on dipstick.32 For multiple gestations, only dichorionic/diamniotic twins were included. Maternal and infant clinical data were collected until hospital discharge. Data were stored in a secure Research Electronic Data Capture database.33
Cord Blood Collection
Samples were collected in heparinized tubes from the umbilical vein minutes after the placenta was delivered, maintained at room temperature, and analyzed within 24 hours. Mononuclear cells were isolated by Ficoll gradient centrifugation for both polychromatic flow cytometry (PFC) and the ECFC primary culture assay as previously described.23
Polychromatic Flow Cytometry
Cord blood MNCs were analyzed by PFC as previously described.23,25 For analysis, 0.5 to 1.0 × 106 cells were stained with antibodies to CD31, CD34, CD45, AC133, glycophorin A (erythrocyte exclusion), CD14 (monocyte/macrophage exclusion), and a LIVE/DEAD viability marker. Compensation beads, fluorescence minus one controls, and biexponential gating were utilized to facilitate accurate compensation and gating. Pro-angiogenic (CD45dimCD34+CD31+AC133+) and nonangiogenic (CD45dimCD34+CD31+AC133−) CPCs were measured and each reported as the percentage of the grandparent population of LIVE MNCs (Figure 1A). The pro-CPC:non-CPC ratio was then calculated.24 Polychromatic flow cytometry analysis was performed using a CyAn 9-color flow cytometer (Beckman Coulter, Pasadena, CA) and FlowJo software (version 9.3.2). Due to the technical issues during staining or processing, CPC populations could not be properly identified by PFC in all samples. Polychromatic flow cytometry data only met quality control criteria for analysis of 43 of the participants.
Endothelial Colony-Forming Cell Isolation and Functional Analysis
Mononuclear cells were cultured on type 1 rat tail collagen (BD Biosciences, San Jose, CA) in complete endothelial cell growth medium (EGM-2, Lonza, Basel, Switzerland) with 10% fetal bovine serum (FBS). Endothelial colony-forming cell colonies were identified daily and enumerated on day 14 in proportion to both the number of MNCs plated and the volume of cord blood collected.
Low-passage (p3-5) ECFCs from PE (n = 3-5) and gestational age (GA)– and gender-matched normotensive controls (n = 3-5) were analyzed for growth, migration, and angiogenic capacity. We performed functional analysis with representative ECFCs from singleton pregnancies without clinical chorioamnionitis. Cells (25 000/well) were plated in a 6-well plate coated with type 1 rat tail collagen (BD biosciences). Growth was determined at 1, 2, 4, and 6 days after day 0 (represented as the fold increase: day n cell number/day 0 cell number, day 0 is 24 hours postplating). Trypan blue 0.4% (Thermo Fisher, Rockford, IL) was used to identify live cells; cell counts were performed in triplicate by hemocytometer.
Endothelial colony-forming cell migration was analyzed using a previously described wound-healing assay.30 Images were captured at baseline and 20 hours after the initial scratch, and the percentage closure was calculated as follows: (mean initial width − mean final width)/mean final width) × 100%.
Endothelial colony-forming cell in vitro angiogenesis was assayed by plating cells (20 000) on cross-linked rat tail collagen. As Matrigel (BD Biosciences, San Jose, CA) has been shown to support the formation of tube-like structures of nonendothelial cell types, a collagen matrix was used.34 Cells were plated in 0.5% FBS complete EGM-2 media on 24-well plates for 20 hours before fixation with 4% paraformaldehyde. Images were taken using an IX71 inverted microscope (Olympus, Center Valley, PA), and the number of branch points, complete loop structures, and total line length were counted in 4 high-power fields per well. Each experiment was performed 3 times.
Statistical Analyses
Nonparametric data were analyzed using Mann-Whitney U testing and are presented as medians with interquartile ranges. Normally distributed data were analyzed using unpaired t tests and are presented as means with standard deviations (SDs). Spearman correlation coefficients were utilized to compare ECFC colony per MNC and per volume. Fisher exact test was used to analyze categorical data. Analysis was performed using the Prism software package (version 5.0; GraphPad Software, La Jolla, California). The significance level was set at α = .05.
Results
Participant Characteristics
A total of 106 mother–infant pairs were enrolled, of those 13 had PE and 93 were normotensive (Table 1). Maternal age, gravidity, parity, race, preexisting diabetes mellitus, gestational diabetes, tobacco use, premature rupture of the membranes, prolonged rupture of the membranes, preterm labor, documented perinatal infection (other than chorioamnionitis), and antenatal corticosteroids were not different between the 2 groups. Cesarean sections were increased in PE (P = .006), however, there was no significant difference in labor between the 2 groups. Neonatal characteristics including GA at birth, gender, small for GA (birth weight <10th percentile), and both 1-minute and 5-minute Apgar scores were not significantly different.
Table 1.
Participant Characteristics.a,b
| Characteristic | Control (n = 93) | Preeclampsia (n = 13) | P Value |
|---|---|---|---|
| Maternal | |||
| Maternal age, years | 26.7 ± 6.3 | 30.08 ± 5.1 | .07 |
| Gravidity | 2.4 ± 1.7 | 2.9 ± 2.3 | .34 |
| Parity | 2.3 ± 1.5 | 2.4 ± 1.4 | .28 |
| Multiple pregnancy | 23 (24.5) | 7 (41.2) | .23 |
| Race | |||
| Asian/Pacific Islander | 5 (5.4) | 3 (23.1) | .06 |
| Black | 14 (15.1) | 1 (7.7) | .69 |
| Hispanic | 31 (33.3) | 3 (23.2) | .54 |
| White | 42 (45.2) | 6 (46.2) | >.99 |
| Unknown/other | 1 (1.1) | 0 (0) | >.99 |
| Diabetes mellitus (preexisting) | 4 (4.3) | 0 (0) | .99 |
| Gestational diabetes | 12 (12.9) | 1 (7.7) | .99 |
| Tobacco use | 13 (14) | 3 (23.1) | .41 |
| Premature rupture of the membranes | 29 (31.2) | 2 (15.4) | .34 |
| Prolonged rupture of the membranes | 10 (10.8) | 1 (7.7) | .99 |
| Preterm labor | 38 (40.9) | 8 (61.5) | .23 |
| Documented perinatal infection | 17 (18.3) | 1 (7.7) | .69 |
| Antenatal corticosteroids | 43 (46.2) | 8 (61.5) | .38 |
| Cesarean section | 45 (48.4) | 12 (92.3) | .006 |
| Labor | 60 (64.5) | 5 (35.5) | .13 |
| Birth to sample processing time, hours | 10.1 (8.0) | 9.2 (6.8) | .71 |
| Neonatal | |||
| Female | 48 (51.6) | 4 (30.8) | .24 |
| Gestational age, weeks | 34.0 ± 4.6 | 33.5 ± 3.2 | .68 |
| Preterm birth | 61 (65.6) | 12 (92.3) | .06 |
| Small for gestational age (birth weight <10th percentile) | 17 (18.4) | 5 (38.5) | .14 |
| Apgar score | |||
| 1 minute | 6.6 ± 2.1 | 6.8 ± 1.7 | .73 |
| 5 minutes | 8.1 ± 1.5 | 8.1 ± 1.1 | .92 |
aData are presented as n (%) or mean ± standard deviation (SD).
bBoldface value indicates statistical significance.
Polychromatic Flow Cytometry Analysis of Pro-Angiogenic and Nonangiogenic Circulating Progenitor Cells
When comparing PE to controls, pro-CPCs were decreased (0.14% ± 0.07% [n = 9] vs 0.49% ± 0.10% [n = 34], P = .01; Figure 1B). Additionally, non-CPCs were decreased (0.05% ± 0.02% [n = 9] vs 0.20% ± 0.05% [n = 34], P = .01; Figure 1C). The pro-CPC:non-CPC ratio did not show a significant difference (2.20 ± 0.57 [n = 9] vs 3.08 ± 0.30 [n = 34], P = .20; Figure 1D). Pro-CPCs, non-CPCs, and the pro-CPC:non-CPC ratio were not significantly different when analyzed by gender, GA, or single/multiple gestation (Figure S1).
Given the difference in cesarean rates between the PE and control groups, the effect of mode of delivery on CPC levels was evaluated. When comparing controls delivered by cesarean section versus vaginal delivery, there was no significant difference in pro-CPCs, non-CPCs, or the pro-CPC:non-CPC ratio (Figure S2).
Cord Blood–Derived ECFC Colonies Are Reduced in PE
By both methods of enumeration, the ECFC colony number was decreased in the PE group. Endothelial colony-forming cell colonies per MNC were decreased in the PE cohort (5.0 ± 0.4 [n = 93] vs 2.0 ± 0.4 [n = 13], P = .002; Figure 2A), and ECFC colonies per volume of cord blood collected were also decreased (2.1 ± 0.2 [n = 72] vs 1.3 ± 0.2 [n = 13], P = .01; Figure 2B). Although ECFCs were decreased when enumerated by both methods, the correlation between the 2 methods was modest (Figure S3). Neither the ECFC colonies per MNC (Figure S4A) nor ECFC colonies per volume (Figure S4B) were significantly different when analyzed by gender, GA, or single/multiple gestation. When comparing controls delivered by cesarean section versus vaginal delivery, there was no significant difference in ECFCs per MNC or per volume (Figure S5).
Figure 2.
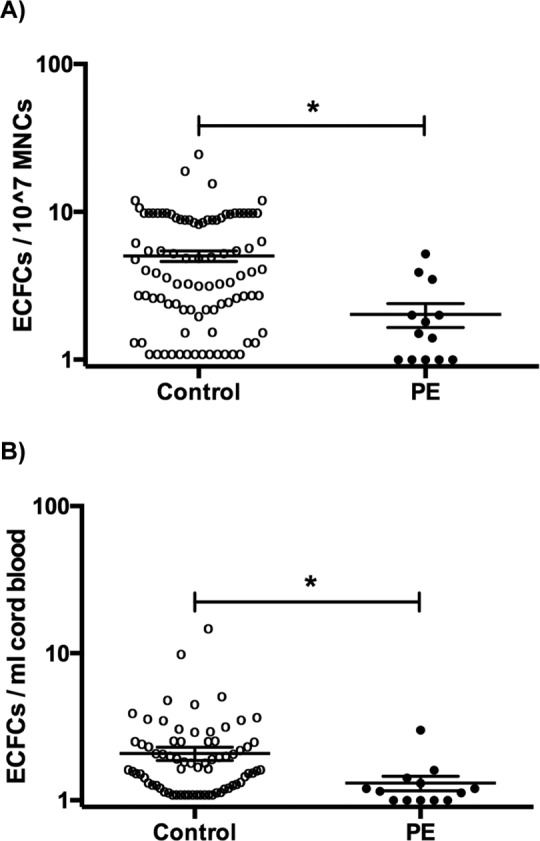
A, Number of colonies after 14 days in culture, represented as the number of colonies per mononuclear cell (MNC) plated. The number of colonies were log transformed so that 0 colonies at day 14 would be clearly represented on the graph. Each point represents log[colony number] + 1. The mean for control colonies at day 14 was 5.0 ± 0.4 (bars represent mean ± SEM), and the preeclamptic colonies, 2.0 ± 0.4, is significantly reduced (P = .002). B, Number of colonies after day 14, represented as colonies per volume of blood collected for processing. The number of colonies were log transformed as described above. The mean for the control colonies is 2.1 ± 0.2, whereas the preeclamptic colonies are significantly reduced, 1.3 ± 0.2, P = .01. *P < .05. SEM indicates standard error of the mean.
Functional In Vitro Analysis of ECFCs
There was a significant decrease in PE-ECFC (n = 5) growth over 6 days as compared to GA– and gender-matched controls (n = 5, fold increase = 22.0 ± 4.4 vs 9.4 ± 2.8, P = .03; Figure 3). Preeclampsia-ECFCs did not show significant changes in growth at earlier time points (day 1, 2, or 4).
Figure 3.
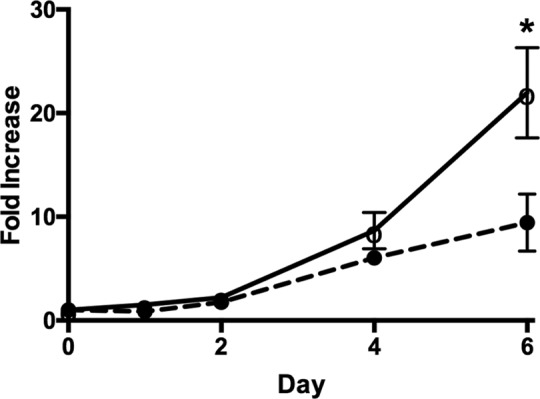
Growth of ECFC cell lines represented as fold increase from day 0 (24 hours after plating). The bars represent fold increase mean ± SEM, the fold increase for control cell lines 22.0 ± 4.4 versus PE cell lines 9.4 ± 2.8. Control cell lines (solid line with open circles) only show a significant increase, P = .03, in growth at day 6 when compared to PE cell lines (dotted line with closed circles). *P < .05. ECFC indicates endothelial colony-forming cells; PE, preeclampsia; SEM, standard error of the mean.
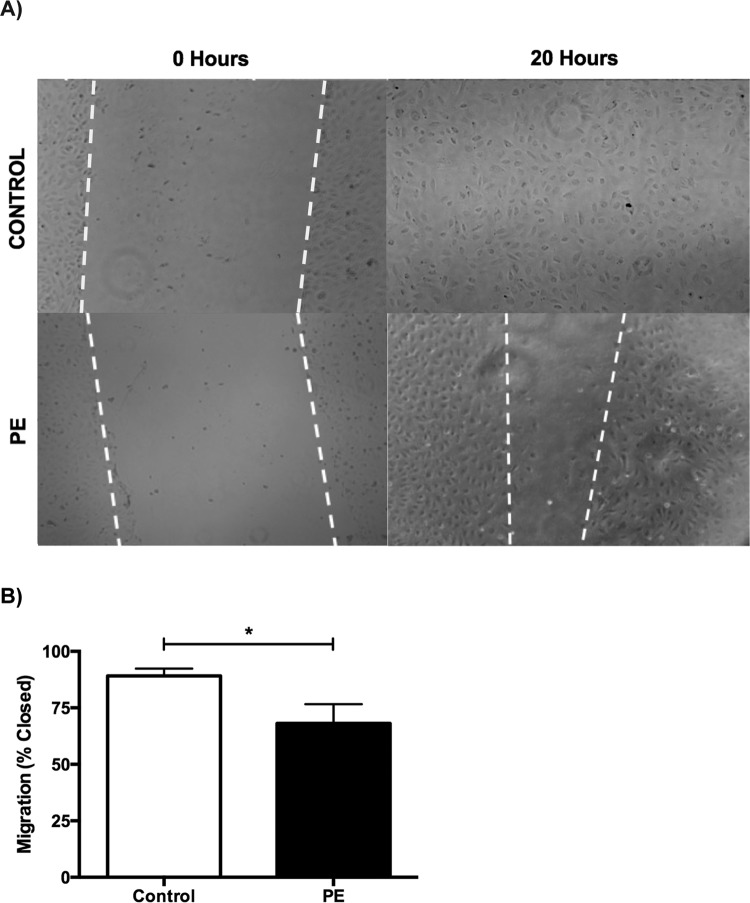
In addition to a decrease in growth, PE-ECFCs also exhibited a significant reduction in migration (89.2% ± 3.2% vs 68.1% ± 8.6%, P = .03, n = 3 per group) 20 hours after the creation of a scratch wound (Figure 4A and B).
Figure 4.
A, A phase-contrast micrograph of the migration assay showing the size of the wound at time 0, immediately after scratch wounding, for representative control (top left) and PE cells (bottom left) and the amount of wound closure 20 hours postscratch for representative control (top right) and PE cells (bottom right). B, Migration represented as percentage of wound closure, and the bars represent mean ± SEM. Control, 89.2% ± 3.2%, had a significantly higher percentage of wound closure at 20 hours when compared to PE cell lines, 68.1% ± 8.6%. *P = .03. PE indicates preeclampsia; SEM, standard error of the mean.
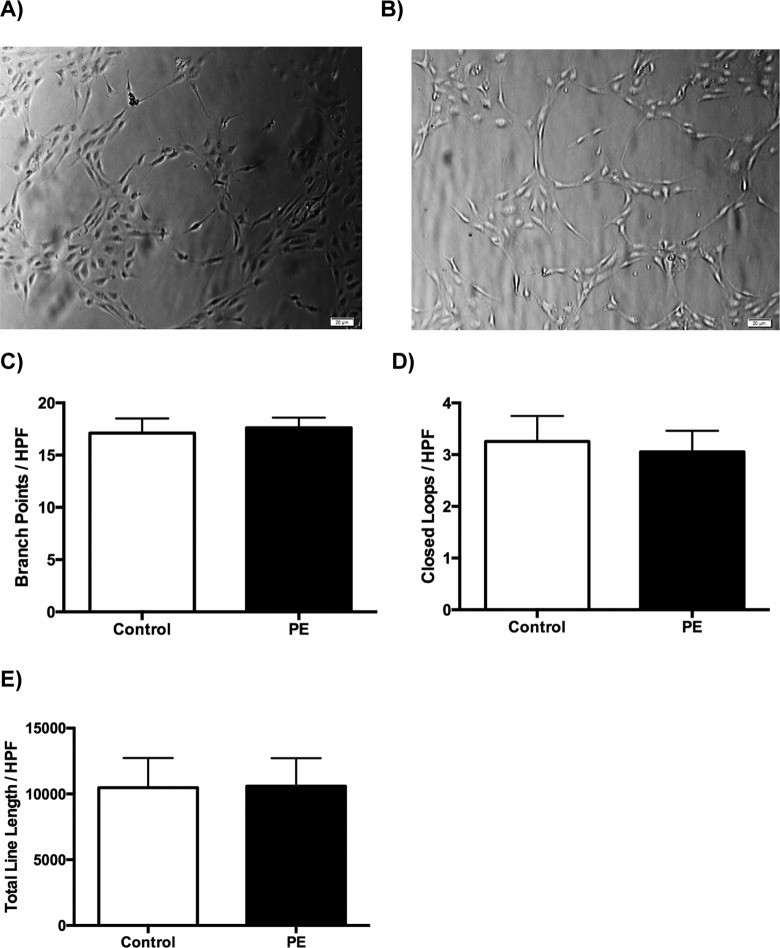
Preeclampsia-ECFCs (n = 3) were able to form tube-like structures in the in vitro angiogenesis assay. However, there was no difference in the number of branch points, closed loops, or total line length when compared to controls (n = 3; Figure 5).
Figure 5.
A, A representative phase-contrast micrograph of tube formation in control cell lines. B, A representative phase-contrast micrograph of tube formation in PE cell lines. C, The number of branch points per high-power field; the bars represent mean ± SEM. Control cell lines did not show any significant difference in the number of branch points, 17.1 ± 1.4 when compared to the PE cell lines, 17.6 ± 1.0 (P =.77). D, Similarly, the number of closed loops (D) and total line length (E) per high-power field also do not show any difference, 3.3 ± 0.5 versus 3.1 ± 0.4 (P = .76) and 10,480 ± 360 versus 10,598 ± 349 (P = .82), respectively. PE indicates preeclampsia; SEM, standard error of the mean.
Discussion
The mechanism through which in utero exposure to PE confers an increased risk for developing adult disease in the offspring is poorly understood. We found that pro-CPCs, non-CPCs, and late-outgrowth ECFCs are all decreased in the cord blood of PE newborns. Furthermore, ECFCs isolated from the cord blood of PE pregnancies demonstrate impaired growth and migration.
Monga et al previously showed a decrease in the number of EPCs in PE cord blood.15 However, these authors utilized a set of antigens that are more likely to identify angiogenic macrophages.18 Using a set of antigens that identify pro-angiogenic and nonangiogenic CPCs, we found that both CPC subsets are decreased in PE, but there was no difference in the pro-CPC:non-CPC ratio, a ratio that is decreased in neonates that develop bronchopulmonary dysplasia and in those born from pregnancies affected by gestational diabetes.23,26 These specific populations of progenitor cells have not been previously characterized in PE cord blood. Preeclampsia generates an in utero stress that appears to decrease both pro-CPCs and non-CPCs. However, whether these reductions impact the later cardiovascular health of the offspring remains a topic worthy of further study.
The incidence of cesarean section was higher in the PE population. We previously reported that cord blood ECFCs are decreased in preterm infants born via cesarean section.23 In order to confirm that the observed reductions in the ECFC colony number, pro-CPCs, and non-CPCs were in fact due to PE and not cesarean section, we determined whether cesarean section resulted in reduced cell numbers within the controls. We showed that there was not a significant reduction in cord blood ECFCs or CPCs by cesarean delivery in this study (Figures S2 and S5). Therefore, the significant reduction in PE-ECFCs and CPCs is likely not related to the increased incidence of cesarean section in the PE group. It is possible that fetal compromise, directly or indirectly related to PE, affects the number and function of cord blood angiogenic progenitor cells.
Two previously published papers reported conflicting results on the effect of PE on ECFC in vitro function.29,30 Both studies tested the growth, migration, and tube formation of PE-ECFCs. One group reported no change in growth, migration, or angiogenesis, whereas the other showed impairment in all 3 assays. In agreement with von Versen-Höynck et al, we showed that the PE-ECFCs have impaired growth and migration,30 and in agreement with Muñoz-Hernandez et al, we found that PE-ECFCs had similar angiogenic capacity as controls.29 Of note, Muñoz-Hernandez et al reported no difference in PE-ECFC growth after 2 days and we did not observe a significant reduction until day 6. Differences in methodology could account for these seemingly conflicting reports.
One of the limitations of this study, and of the studies mentioned above, is that our sample size did not enable us to determine whether the severity of PE influences the effects. We also did not exclude comorbidities, such as intrauterine growth restriction and gestational diabetes, which have been shown to affect ECFC function.35–37 However, the incidence of these conditions was not significantly different between our study groups. Additionally, the ECFCs studied during the in vitro experiments were obtained from GA– and gender-matched preterm pregnancies (31-35 weeks’ GA). Given that PE is often an indication for preterm delivery, we felt it was more relevant to perform functional analyses on preterm ECFCs with and without PE. However, it is possible that PE-ECFC from full-term deliveries will function differently. The effect of PE on ECFC growth and migration was significant in these representative studies and there was no trend in tube formation. The trend toward a decrease in the pro-CPC:non-CPC ratio might have reached statistical significance if we studied more samples, but all PE samples with valid flow cytometry results were included (n = 9).
We conclude that PE decreases pro-CPC, non-CPC, ECFC numbers in the cord blood and decreases ECFC growth and migration in vitro. The impact of PE on these CPC populations may contribute to the offspring’s increased risk of developing adult disease. Further study is needed to better understand this potential mode of fetal programming as well as how PE alters gene and protein expression in ECFCs. Finally, longitudinal studies in children born to mothers with PE will help to elucidate the lasting effect of PE on angiogenic progenitor cell number and function.
Supplementary Material
Acknowledgments
The authors thank the nursing staff of the perinatal Clinical and Translational Research Center for assistance with cord blood collection. They also thank Sharon Ryan and Gregory Seedorf for laboratory support.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research is supported by the National Institutes of Health K12-HL090147-01, K23-HL121090-01 (to C. D. Baker), the University of Colorado, Department of Obstetrics and Gynecology R01-HD60723 (to V. D. Winn) and the Colorado CTSI ( 5UL1 RR025780).
Supplemental Material: Supplementary material is available for this article online.
References
- 1. Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Health of children born to mothers who had preeclampsia: a population-based cohort study. Am J Obstet Gynecol. 2009;201(3):269.e1–269.e10. [DOI] [PubMed] [Google Scholar]
- 2. Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36(1):56–59. [DOI] [PubMed] [Google Scholar]
- 3. Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40(4):1176–1180. [DOI] [PubMed] [Google Scholar]
- 4. Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Pre-eclampsia and offspring’s blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstet Gynaecol. 1991;98(10):1009–1014. [DOI] [PubMed] [Google Scholar]
- 5. Tenhola S, Rahiala E, Halonen P, Vanninen E, Voutilainen R. Maternal preeclampsia predicts elevated blood pressure in 12-year-old children: evaluation by ambulatory blood pressure monitoring. Pediatr Res. 2006;59(2):320–324. [DOI] [PubMed] [Google Scholar]
- 6. Vatten LJ, Romundstad PR, Holmen TL, Hsieh C, Trichopoulos D, Stuver SO. Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet Gynecol. 2003;101(3):529–533. [DOI] [PubMed] [Google Scholar]
- 7. Giannotti G, Doerries C, Mocharla PS, et al. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension. 2010;55(6):1389–1397. [DOI] [PubMed] [Google Scholar]
- 8. Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45(9):1449–1457. [DOI] [PubMed] [Google Scholar]
- 9. Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005;172(7):854–860. [DOI] [PubMed] [Google Scholar]
- 10. Rafat N, Hanusch C, Brinkkoetter PT, et al. Increased circulating endothelial progenitor cells in septic patients: correlation with survival. Crit Care Med. 2007;35(7):1677–1684. [DOI] [PubMed] [Google Scholar]
- 11. Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–E7. [DOI] [PubMed] [Google Scholar]
- 12. Ghani U, Shuaib A, Salam A, et al. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36(1):151–153. [DOI] [PubMed] [Google Scholar]
- 13. Martí-Fàbregas J, Delgado-Mederos R, Crespo J, et al. Circulating endothelial progenitor cells and the risk of vascular events after ischemic stroke. PLoS One. 2015;10(4):e0124895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boos CJ, Goon PK, Lip GY. Endothelial progenitor cells in the vascular pathophysiology of hypertension: arterial stiffness, ageing and more. J Hum Hypertens. 2006;20(7):475–477. [DOI] [PubMed] [Google Scholar]
- 15. Monga R, Buck S, Sharma P, Thomas R, Chouthai NS. Effect of preeclampsia and intrauterine growth restriction on endothelial progenitor cells in human umbilical cord blood. J Matern Fetal Neonatal Med. 2012;25(11):2385–2389. [DOI] [PubMed] [Google Scholar]
- 16. Luppi P, Powers RW, Verma V, Edmunds L, Plymire D, Hubel CA. Maternal circulating CD34+VEGFR-2+ and CD133+VEGFR-2+ progenitor cells increase during normal pregnancy but are reduced in women with preeclampsia. Reprod Sci. 2010;17(7):643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu X, Luo Q, Zheng Y, et al. Notch1 impairs endothelial progenitor cell bioactivity in preeclampsia [published online May 18, 2016]. Reprod Sci. 2016. [DOI] [PubMed] [Google Scholar]
- 18. Case J, Mead LE, Bessler WK, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35(7):1109–1118. [DOI] [PubMed] [Google Scholar]
- 19. Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106(5):1525–1531. [DOI] [PubMed] [Google Scholar]
- 21. Duong HT, Erzurum SC, Asosingh K. Pro-angiogenic hematopoietic progenitor cells and endothelial colony-forming cells in pathological angiogenesis of bronchial and pulmonary circulation. Angiogenesis. 2011;14(4):411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richardson MR, Yoder MC. Endothelial progenitor cells: quo vadis? J Mol Cell Cardiol. 2011;50(2):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baker CD, Balasubramaniam V, Mourani PM, et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J. 2012;40(6):1516–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Estes ML, Mund JA, Ingram DA, Case J. Identification of endothelial cells and progenitor cell subsets in human peripheral blood. Curr Protoc Cytom. 2010;Chapter 9:Unit 9.33.1-11. [DOI] [PubMed] [Google Scholar]
- 25. Estes ML, Mund JA, Mead LE, et al. Application of polychromatic flow cytometry to identify novel subsets of circulating cells with angiogenic potential. Cytometry A. 2010;77(9):831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Acosta JC, Haas DM, Saha CK, Dimeglio LA, Ingram DA, Haneline LS. Gestational diabetes mellitus alters maternal and neonatal circulating endothelial progenitor cell subsets. Am J Obstet Gynecol. 2011;204(3):254.e8–254.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–2760. [DOI] [PubMed] [Google Scholar]
- 28. Borghesi A, Massa M, Campanelli R, et al. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2009;180(6):540–546. [DOI] [PubMed] [Google Scholar]
- 29. Muñoz-Hernandez R, Miranda ML, Stiefel P, et al. Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension. 2014;64(1):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Versen-Höynck F, Brodowski L, Dechend R, Myerski AC, Hubel CA. Vitamin D antagonizes negative effects of preeclampsia on fetal endothelial colony forming cell number and function. PLoS One. 2014;9(6):e98990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tita ATN, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37(2):339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. ACOG Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002 Obstet Gynecol. 2002;99(1):159–167. [DOI] [PubMed] [Google Scholar]
- 33. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bikfalvi A, Cramer EM, Tenza D, Tobelem G. Phenotypic modulations of human umbilical vein endothelial cells and human dermal fibroblasts using two angiogenic assays. Biol Cell. 1991;72(3):275–278. [DOI] [PubMed] [Google Scholar]
- 35. Ingram DA, Lien IZ, Mead LE, et al. In vitro hyperglycemia or a diabetic intrauterine environment reduces neonatal endothelial colony-forming cell numbers and function. Diabetes. 2008;57(3):724–731. [DOI] [PubMed] [Google Scholar]
- 36. Sipos PI, Bourque SL, Hubel CA, et al. Endothelial colony-forming cells derived from pregnancies complicated by intrauterine growth restriction are fewer and have reduced vasculogenic capacity. J Clin Endocrinol Metab. 2013;98(12):4953–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blue EK, DiGiuseppe R, Derr-Yellin E, et al. Gestational diabetes induces alterations in the function of neonatal endothelial colony-forming cells. Pediatr Res. 2014;75(2):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.