Abstract
Excessive daytime sleepiness (EDS) is a prevalent symptom among stroke survivors. This symptom is an independent risk factor for stroke and may reduce stroke survivors’ quality of life, cognitive functioning, and daytime functional performance. The lack of a universally accepted definition of EDS makes it difficult to measure EDS and synthesize research. The purpose of this integrative review is to describe poststroke EDS, ascertain conceptual and operational definitions of EDS, identify factors that contribute to EDS in stroke survivors, and explore outcomes associated with EDS in stroke survivors. We searched the following databases: PubMed and MEDLINE (OvidSP 1946—April; Week 2, 2015), Embase (OvidSP 1974—March; Week 1, 2015), and PsycINFO (OvidSP 1967—April; Week 2, 2015). Our search yielded 340 articles, 27 of which met inclusion criteria. The literature reveals EDS to be a multidimensional construct that is operationalized with both subjective and objective measures. Choosing measures that can quantify both the objective and subjective components is useful for gaining a comprehensive understanding of EDS. The antecedents of EDS are stroke, sleep-disordered breathing, reversed Robin Hood syndrome, and depression. The outcomes associated with EDS in stroke patients are serious and negative. Via synthesis of this research, we propose a possible framework for poststroke EDS, which may be of use in clinical practice and in research to identify valid quantifying methods for EDS as well as to prevent harmful outcomes in stroke survivors.
Keywords: excessive daytime sleepiness, stroke, integrative review
Stroke survivors often experience the symptom of excessive daytime sleepiness (EDS) in the early poststroke phase (Bliwise, Rye, Dihenia, & Gurecki, 2002; Davies, Rodgers, Walshaw, James, & Gibson, 2003). The estimated prevalence rate of EDS in stroke survivors ranges between 18% and 72%, depending on the method used to measure EDS and the type of stroke (C. L. Bassetti, 2005; Masel, Scheibel, Kimbark, & Kuna, 2001; Sterr, Herron, Dijk, & Ellis, 2008). Despite the wide range of estimated prevalence, there is an agreement that poststroke EDS may persist and become a chronic problem in 34% of stroke survivors and continue for at least 6 months after stroke (Herron, Dijk, & Dean, 2014; Schuiling, Rinkel, Walchenbach, & de Weerd, 2005). EDS affects a stroke survivor’s daytime functional performance, lowers overall well-being, and impacts cognitive functioning (Hermann et al., 2008; Sterr et al., 2008; Van Zandvoort, Kappelle, Algra, & De Haan, 1998). It is also an independent risk factor for stroke and contributes to an increased number of driving and industrial accidents (Boden-Albala et al., 2012; Davies et al., 2003; Lundqvist, Alinder, & Ronnberg, 2008; Sagberg, 2006).
Both homeostatic and circadian factors, such as a prolonged period of waking time and staying active during the nocturnal circadian phase, contribute to EDS (Dinges, 1995). EDS can be caused by chronic partial sleep deprivation (i.e., insufficient or fragmented sleep), disorders of the central nervous system, psychological conditions, and neurological disorders (Breslau, Roth, Rosenthal, & Andreski, 1997; Happe, 2003). Sleep-disordered breathing, particularly obstructive sleep apnea (OSA), another independent risk factor for stroke, represents a common cause of fragmented sleep and hence may also be a cause of EDS (Guilleminault & Brooks, 2001). Medications used in disease management also influence EDS, but stroke can cause EDS in the absence of preexisting medical conditions or medication use due to dopaminergic and noradrenergic impulse disruption (C. Bassetti, Mathis, Gugger, Lovblad, & Hess, 1996).
Studying EDS among stroke patients has many challenges. First, although research has supported an association between EDS and stroke, no clear consensus currently exists for a definition of EDS or how it should be measured (Johns, 2010). Many terms, such as fatigue and hypersomnia, have been used synonymously with EDS to indicate the increased homeostatic sleep drive (or accumulated sleep debt) that results from sleep deprivation or sleep restriction (Dinges, 1995; Goel, Basner, Rao, & Dinges, 2013). Second, sleep researchers have proposed different definitions for sleepiness. The most accepted definition is “one’s tendency to fall asleep,” also referred to as “sleep propensity” (Shen, Barbera, & Shapiro, 2006, p. 64). Another definition of sleepiness is “sleep drive,” that is, a “physiological drive usually resulting from sleep deprivation” (Johns, 2010, p. 171). Researchers have also defined sleepiness as the subjective perception of drowsiness and self-estimated behavioral observations of sleepiness (Kim & Young, 2005). Third, investigators have operationalized EDS using three different categories of measurement: the subjective level, the behavioral–performance level, and the electrophysiological level (Cluydts, De Valck, Verstraeten, & Theys, 2002; Curcio, Casagrande, & Bertini, 2001). Authors proposed these evaluation levels based upon an observed distinction between subjective and objective sleepiness, as individuals’ subjective perceptions may not always correspond to objective evidence. The quantifying methods that evaluate EDS at the three levels are summarized in Table 1.
Table 1.
Overview of the Operationalization for Excessive Daytime Sleepiness.
| Measure | Type of Measure | What It Measures | Use in Research | Number of Reviewed Studies Using This Measure |
|---|---|---|---|---|
| Subjective level | ||||
| SSS | Self-report | Degree of alertness at a particular time | Measure acute sleepiness | 2 |
| KSS | Self-report | State of sleepiness over the past few minutes | Measure acute sleepiness | 1 |
| VAS | Self-estimated | Indicates sleepiness on a 100-mm line | Estimate sleepiness state | 0 |
| ESS | Self-report | Likelihood to doze off in different situations in daily life | Estimate average sleepiness Distinguish patients with sleep disorders (score > 12) from normal (score < 10) | 18 |
| Behavioral–performance level | ||||
| Behavioral indicators | Behavioral measure | Behavioral observations (e.g., yawning, eye closing, and oculomotor activity) | Give indications of sleepiness | 3 |
| PVT | Performance measure | Sustained attention and the effect on different aspects of functioning | Evaluate sleepiness caused by sleep deprivation | 0 |
| Driving simulator | Performance measure | Objective sleepiness in specific situations (driving) | Simulate driving situation | 0 |
| Electrophysiological level | ||||
| MSLT | Objective measure | How fast one can fall asleep in the testing situation | Old “gold standard” to measure sleep latency | 3 |
| MWT | Objective measure | One’s ability to maintain wakefulness in testing situation | Quantify sleep latency | 1 |
| Polysomnography | Objective measure | Drowsiness episodes indicated by certain EEG patterns | Evaluate sleep stage | 10 |
| Pupillometry | Objective measure | Change in diameter of pupils | Indicator for sleepiness state | 0 |
| Cerebral evoked potential | Objective measure | Altered cerebral evoked potentials in the drowsy state | Visual evidence of drowsy state | 1 |
Note. EEG = electroencephalogram; ESS = Epworth Sleepiness Scale; KSS = Karolinska Sleepiness Scale; MSLT = multiple sleep latency test; MWT = maintenance of wakefulness test; PVT = psychomotor vigilance test; SSS = Stanford Sleepiness Scale; VAS = Visual Analogue Scale.
Given the high prevalence of EDS among stroke survivors and the evidence that adverse outcomes are associated with EDS, an increased understanding of this symptom in stroke survivors is critical, in spite of the challenges (Masel et al., 2001; Yaggi et al., 2005). Therefore, the purpose of this review is to (a) describe EDS in the context of poststroke survivorship, (b) ascertain conceptual and operational definitions of EDS in stroke, (c) evaluate potential factors contributing to EDS in stroke survivors, (d) explore outcomes associated with EDS in stroke survivors, and (e) discuss implications for future practice and research with respect to EDS and stroke.
Method
We used an integrative review methodology, which allows for the inclusion of experimental and nonexperimental research related to EDS (Whittemore & Knafl, 2005), and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009). We retrieved data from studies with diverse methods, focusing on characteristics of EDS in the context of poststroke, including its conceptual or operational definitions, antecedents, contributing factors, and outcomes.
Search Methods
To identify theoretical and empirical reports available on EDS in the context of poststroke, we conducted a comprehensive search of multiple computerized library databases on April 20, 2015, including PubMed, Embase (OvidSP 1974—April; Week 2, 2015), MEDLINE (OvidSP 1946—March; Week 1, 2015), PsycINFO (OvidSP 1967—March; Week 1, 2015), and CINAHL. We used controlled vocabulary terms and synonymous free-text words to capture the concept of sleepiness or somnolence in persons with stroke, transient ischemic attack, or cerebrovascular accident. In the initial search, we considered articles in all languages. Because the search was intended to expose a large number of reports as well as the earliest literature on the concept of sleepiness in the context of stroke, we applied no time limit to the search. We identified further studies by hand searching relevant journals and examining the reference lists of all articles included in the review. Figure 1 illustrates the study selection process, and the full search strategy may be obtained by contacting the authors.
Figure 1.
Flowchart of literature selection process.
We included articles in the review, if they had been peer reviewed and written in English, measured EDS in the context of the poststroke period, described or defined the phenomenon of EDS in stroke survivors, and/or addressed EDS in the poststroke period. We excluded duplicates, review articles, unpublished manuscripts, electronic articles in nonjournal formats, studies that did not report EDS in their findings, and studies that discussed EDS in the nonstroke population only.
Analysis
We conducted the analysis of the literature in four phases. In Phase 1, we obtained an overall view of the content in the articles and divided the articles into subgroups based on the study design. We evaluated the articles for the conceptual definition of EDS and organized them according to the types of methods used to quantify sleepiness in stroke survivors. In Phase 2, we extracted data from the studies, developing matrices for the recording and displaying of conceptual and operational definitions of EDS, antecedents, influencing factors, and outcomes associated with EDS. If no conceptual definition of EDS was present in an article, we identified implied definitions based on the selected measurement methods. In Phase 3, we compared data across studies using the matrices, identifying patterns and relationships within the variables to help form a visual concept map of EDS. In the fourth and final phase of data analysis, we synthesized the conceptual and operational definitions of EDS in stroke survivors and developed a conceptual model describing this symptom among these patients.
Results
Study Sample
The initial search yielded a total of 3,139 citations. After removing duplicates and articles that did not meet the inclusion criteria, we reviewed a total of 340 articles. We conducted a manual inspection of the full text of the articles to determine whether they met exclusion criteria, including (1) duplicates; (2) editorial or review articles, unpublished manuscripts, or electronic media; (3) lack of measurement or reporting of EDS in the study findings; or (4) report of EDS in the prestroke rather than the poststroke phase. After excluding articles based on these criteria, we included 27 articles in this review. The selected articles were published between 1996 and 2015 and comprised experimental studies (n = 2, 7%), observational studies (n = 18, 67%), and case reports (n = 7, 26%). The observational studies included case-control, cross-sectional, and cohort studies. The sample size for the reviewed studies ranged from n = 1 (case report; Fonseca et al., 2011) to n = 269 (observational study; Mills, Koufali, Sharma, Tennant, & Young, 2013). The samples of stroke survivors varied in terms of age (average age ranged from 46 to 61 ± 11 years), gender (female 10–48%), types or severity of stroke, living environment (home, rehabilitation, or hospital), stage after the stroke (acute or chronic), and comorbidity.
Conceptual and Operational Definitions of EDS in Stroke
Conceptual definition
None of the articles gave explicit conceptual definitions of sleepiness. Rather, they used operational definitions and measurement to quantify EDS in stroke survivors. The following words were used interchangeably between articles to describe EDS, such as excessive daytime sleepiness, hypersomnia (Arzt et al., 2010; C. Bassetti et al., 1996), narcolepsy (Scammell, Nishino, Mignot, & Saper, 2001), fatigue (Falconer, Walsh, & Harbison, 2010), and subjective sleepiness (Mills et al., 2013; Sterr et al., 2008). We noted five methods of identifying the presence of EDS in stroke survivors identified in the articles: (a) retrospective reports of how often a person dozes off in different situations of daily life (Alexandrov et al., 2009; Arzt et al., 2010; C. Bassetti et al., 1996; Brooks et al., 2010; Falconer et al., 2010; Hermann & Bassetti, 2009; Medeiros et al., 2011; Mills et al., 2013; Pasic, Smajlovic, Dostovic, Kojic, & Selmanovic, 2011; Ryan, Bayley, Green, Murray, & Bradley, 2011; Sterr et al., 2008; Vock et al., 2002; Wessendorf, Teschler, Wang, Konietzko, & Thilmann, 2000); (b) observation of sleepy behaviors such as reduced movement or longer hours of sleeping time compared to the person’s routine, which are confirmed by the electroencephalogram (EEG) sleep pattern (C. Bassetti et al., 1996; Fonseca et al., 2011; Goyal, Kumar, & Sahota, 2012; Hermann & Bassetti, 2009; Hermann et al., 2008; Scammell et al., 2001); (c) subjective feeling of a drowsy state or sleep need (Goyal et al., 2012; Hermann et al., 2008; Koch et al., 2007; Ryan et al., 2011; Vock et al., 2002); (d) falling asleep in less than 10 min in a darkened, quiet test situation (C. Bassetti et al., 1996; Bliwise et al., 2002; Hermann & Bassetti, 2009) <5 min indicates a pathological level of daytime sleepiness according to the guideline, thus 10 min is half as fast as the criterion score (Cluydts et al., 2002); (e) inability to maintain wakefulness for a specified period of time (20, 30, or 40 min) in a quiet, darkened room, when instructed to “remain awake” (Hermann & Bassetti, 2009; Shen et al., 2006).
Operational definition
Operationalization of EDS in poststroke patients also varied across studies, with different operational definitions based on different implied conceptual definitions or ways of thinking about sleepiness.
Our interpretation of common themes in the operational definitions across articles showed that authors used quantifying methods from the three primary evaluation levels (subjective, behavioral–performance, and electrophysiological) to measure poststroke EDS (Table 1). In 13 articles, investigators used established instruments that measure EDS subjectively, while in 4 authors used purely objective measures and in 10 they used a combination of both subjective and objective measurements. Table 2 lists the instruments or methods researchers used in the reviewed studies along with the type of study, the localization or type of stroke, and the time since the stroke event.
Table 2.
Summary of Key Features of Reviewed Studies (Ordered by Type of Study).
| Author, Year, Country | EDS Quantifying Methods | Type of Study | Localization, Type, or Severity of Stroke | Time Since the Injury |
|---|---|---|---|---|
| Alexandrov et al., 2009, USA | ESS | Observational | Neurological deterioration and reversed Robin Hood syndrome in acute ischemic stroke | Within 48 hr from symptom onset |
| Arzt et al., 2010, Canada | ESS | Observational | Embolic, thromboembolic, or hemorrhagic stroke | Sudden-onset neurological deficit lasting > 24 hr |
| C. Bassetti et al., 1996, Switzerland | ESS, sleep log, observation of sleepy behaviors, polysomnography, actigraphy, MSLTs, and MWTs | Prospective observational | Paramedian thalamic stroke | Poststroke unspecified |
| Brooks et al., 2010, Canada | ESS | Cross sectional | Ischemic or hemorrhagic stroke | Poststroke, unspecified |
| Camilo et al., 2014, Brazil | ESS | Observational | First-ever ischemic stroke | Within 24 hr of onset of symptoms |
| Chan, Coutts, & Hanly, 2010, Canada | ESS | Observational | Acute TIA or minor stroke | 3 months poststroke |
| Duncan et al., 2015, UK | ESS | Prospective longitudinal | Hemorrhagic or ischemic stroke | Had acute stroke within the previous month |
| Falconer, Walsh, & Harbison, 2010, Ireland | ESS | Observational | Stroke or TIA | 1–6 months poststroke or TIA |
| Hermann et al., 2008, Switzerland | Self-estimated sleep time and tendency to sleep during the day, polysomnography, and actigraphy | Observational | Bilateral thalamic ischemic stroke | 2 weeks–1 year poststroke |
| Herron, Dijk, & Dean, 2014, UK | ESS, Karolinska Sleepiness Scale, and quantitative electroencephalography | Observational | First ever unilateral cortical or subcortical stroke | >12 months poststroke |
| Koch et al., 2007, USA | Berlin questionnaire | Observational | Acute ischemic stroke | Within 5 days of symptom onset |
| Medeiros et al., 2011, Brazil | ESS and Pittsburgh Sleep Quality Index | Longitudinal | Ischemic stroke | 15 days–12 months poststroke |
| Pasic, Smajlovic, Dostovic, Kojic, & Selmanovic, 2011, Bosnia and Herzegovina | ESS and Stanford Sleepiness Scale | Prospective observational | Ischemic or intracerebral hemorrhage | Unspecified time poststroke |
| Schuiling, Rinkel, Walchenbach, & de Weerd, 2005, Netherlands | ESS, sleep diagnostic questionnaire, and polysomnography | Prospective observational | Subarachnoid hemorrhage | 1 year after subarachnoid hemorrhage |
| Sterr, Herron, Dijk, & Ellis, 2008, UK | ESS and Pittsburgh Sleep Quality Index | Observational | Hemispheric lesions | 12–180 months poststroke |
| Suh, Choi-Kwon, & Kim, 2014, South Korea | Self-estimated sleep time and tendency to sleep during the day | Observational | Acute ischemic stroke | Mean of 6.7 ± 1.89 days after the onset of stroke |
| Vock et al., 2002, Switzerland | Self-estimated sleep time, ESS, and polysomnography | Longitudinal | Hemispheric ischemic stroke | 8 days–24 months poststroke onset |
| Wessendorf, Teschler, Wang, Konietzko, & Thilmann, 2000, Germany | ESS and polysomnography | Cross sectional | Ischemic or intracerebral hemorrhage | Mean of 46 ± 19.5 days poststroke |
| Bliwise, Rye, Dihenia, & Gurecki, 2002, USA | MSLTs | Case report | Subcortical stroke | Poststroke, unspecified |
| Fonseca et al., 2011, Portugal | Polysomnography and observation of sleepy behaviors | Case report | Bilateral paramedian thalamic stroke | 4 days–5 months poststroke |
| Goyal, Kumar, & Sahota, 2012, USA | Polysomnography and observation of sleepy behaviors | Case report | Bilateral thalamic ischemic stroke | 20 hr–2 months after onset of symptoms |
| Khairkar & Diwan, 2012, India | ESS and MSLTs | Case report | Thalamo-striatal stroke | 3 days–2 weeks poststroke |
| Rivera, Meyer, Hata, Ishikawa, & Imai, 1986, USA | Self-estimated sleep time and tendency to sleep during the day, and polysomnography | Case report | Cerebral hypoxic ischemia | 3 weeks poststroke |
| Scammell, Nishino, Mignot, & Saper, 2001, USA | Self-estimated sleep time, polysomnography, and MSLTs | Case report | Diencephalic stroke | 5 years poststroke |
| Tosato, Aquila, Della Marca, Incalzi, & Gambassi, 2009, Italy | Polysomnography | Case report | Paramedian pontine ischemic lesions | Between 4 hr of symptom onset and 2 weeks poststroke |
| Mills, Koufali, Sharma, Tennant, & Young, 2013, UK | ESS and self-estimated sleep time | Experimental | Ischemic or intracerebral hemorrhage | Within 4 years after stroke |
| Ryan, Bayley, Green, Murray, & Bradley, 2011, Canada | ESS, Stanford Sleepiness Scale, and polysomnography | Randomized controlled trial | Ischemic or hemorrhagic stroke | Between 3 weeks of stroke onset and 7 weeks poststroke |
Note. EDS = excessive daytime sleepiness; ESS = Epworth Sleepiness Scale; MSLTs = multiple sleep-latency tests; MWTs = maintenance of wakefulness tests; TIA = transient ischemic attack.
The Epworth Sleepiness Scale (ESS), a self-administered questionnaire that standardizes assessment of dozing behavior in eight different situations of daily life (Johns, 1998, 2010), was the most frequently used subjective method (n = 18, 67%) for quantifying poststroke EDS in the reviewed literature, with seven (26%) studies relying solely on the ESS. Among the 18 studies that used the ESS to measure EDS, the average ESS score ranged from 7.7 to 12, with 6–49.5% of stroke patients reporting EDS (ESS score >10; data summarized from Medeiros et al., 2011; Pasic et al., 2011; Vock et al., 2002; Wessendorf et al., 2000).
Other subjective measures of EDS researchers used in the reviewed studies included the Stanford Sleepiness Scale (n = 2, 7%) and the Karolinska Sleepiness Scale (n = 1, 4%). Koch et al. (2007) used items from the second category of the Berlin Questionnaire, which ask about early morning and daytime fatigue and falling asleep while driving, for the identification of EDS in stroke patients.
Polysomnography was the most commonly used objective test for the detection of EDS in stroke patients in these studies (n = 10, 37%). Besides measuring sleep, polysomnography can be used to identify different wakeful states, and researchers have found its parameters to be reliable indicators for sleepiness in real-life situations (Cluydts et al., 2002). In nine of these studies, researchers also used another subjective (i.e., self-estimated sleep time or ESS) or objective measure (i.e., observation of sleepy behaviors) along with polysomnography to measure EDS. The most common findings of nocturnal polysomnography among the stroke populations assessed in these studies were low total sleep time at night, frequent awakening, increased Stage 1 sleep, reduced Stage 2 sleep, relative suppression of rapid eye movement stages, and reduced sleep spindles (findings from Hermann et al., 2008; Rivera, Meyer, Hata, Ishikawa, & Imai, 1986).
The Multiple Sleep Latency Test (MSLT) has been considered the gold standard for measuring EDS (Johns, 2010). It is based on the idea that sleepiness represents the physiological need for sleep and, thus, an increased susceptibility to falling asleep reflects greater sleepiness (Cluydts et al., 2002). However, in spite of the fact that the MSLT is the most widely accepted objective measure of sleep tendency (Wise, 2006), fewer of the studies we reviewed employed this measure (n = 3, 11%) than polysomnography to quantify EDS among stroke patients. Among these studies, the MSLT showed a sleep latency to Stage 1 sleep ranging from 0.5 to 4 min on average (C. Bassetti et al., 1996; Khairkar & Diwan, 2012; Scammell et al., 2001). In one of these studies, investigators did not find significant correlations between the MSLT and a subjective measure of sleep (ESS), with the MSLT indicating mildly abnormal (<10 min) sleep latency in patients with paramedian thalamic stroke, but the ESS indicating severe EDS (ESS > 10 but < 15; C. Bassetti et al., 1996). Thus, MSLT may not be an ideal measure for quantifying sleepiness among survivors of all types of strokes.
In addition to polysomnography and MSLT, investigators used behavioral indicators of sleepiness (n = 3 11%) and the maintenance of wakefulness test ( n = 1 4%) to objectively quantify EDS in stroke survivors. In another of the studies, researchers used quantitative EEG (qEEG) to indicate how sleepy stroke patients were while awake (Herron et al., 2014). This technique involves analysis of the frequency composition of the raw EEG gathered through the MSLT test at a particular time point. The results of the study showed sustained slowing in EEG after stroke. The authors suggest that increased power density in delta and theta frequency bands were not associated with increased perceived sleepiness in stroke survivors.
The Antecedents of EDS in Stroke Survivors
The antecedents of EDS comprise conditions that cause poststroke sleepiness and conditions that influence the severity of sleepiness to produce worse outcomes in stroke survivors. Stroke survivors have specific conditions, such as subtypes of cerebral infarctions, sleep disordered breathing (SDB), and depression that contribute to the development of EDS (C. Bassetti et al., 1996; Brooks et al., 2010; Hermann et al., 2008). Although these antecedents are distinguishable from one another, authors assume them to be interrelated and interacting with each other.
Subtype of stroke
In 13 of the reviewed studies, findings demonstrated an association between stroke and EDS. Patients frequently reported EDS (ESS > 10) after stroke (Falconer et al., 2010; Medeiros et al., 2011; Mills et al., 2013; Pasic et al., 2011; Sterr et al., 2008; Vock et al., 2002). Survivors who suffered from paramedian thalamic stroke (C. Bassetti et al., 1996; Fonseca et al., 2011), bilateral thalamic infarct (Goyal et al., 2012), subcortical stroke (Bliwise et al., 2002), diencephalic stroke (Scammell et al., 2001), or had paramedian pontine ischemic lesions (Tosato, Aquila, Della Marca, Incalzi, & Gambassi, 2009) were more likely than those having stroke in other locations to have severe EDS (ESS > 10). Additionally, in an observational study, Alexandrov et al. (2009) found that reversed Robin Hood syndrome, a hemodynamic steal condition that is associated with neurological deterioration, was also associated with significant daytime sleepiness. These authors observed decreased cerebral blood flow to the ischemic areas of the brain along with increased blood flow to the vessels in the nonaffected regions in stroke survivors during episodes of hypercapnia.
SDB
SDB, a condition that is often thought to contribute to EDS, occurred in 62–91% of stroke patients in the reviewed studies (Brooks et al., 2010; Chan, Coutts, & Hanly, 2010). However, stroke patients with SDB in the reviewed studies and other studies have not tended to have the typical clinical features of SDB, such as obesity and, interestingly, subjectively measured EDS (Brooks et al., 2010; Chan et al., 2010; Lundqvist et al., 2008). Although EDS is recognized as a cardinal symptom of SDB in the general population, the predictive value of subjectively measured EDS for SDB in stroke survivors is unclear. In two of the reviewed studies, authors identified a lack of association between the severity of subjective daytime sleepiness measured by ESS and the severity of SDB in stroke survivors (Arzt et al., 2010; Wessendorf et al., 2000). At a given severity of SDB, these stroke survivors tended to report less severe daytime sleepiness than did the nonstroke sleep-apnea population.
Although these studies did not indicate that SDB leads directly to poststroke daytime sleepiness, EDS and SDB were closely related among survivors of various types of strokes (Arzt et al., 2010; Brooks et al., 2010; Hermann & Bassetti, 2009; Koch et al., 2007; Wessendorf et al., 2000). It is well accepted that stroke can contribute to reduced cerebral perfusion and impairment of the respiratory control center in the brain. The interruption of blood flow to the brain can lead to periodic breathing or nocturnal hypoxia. However, SDB by itself can result in EDS because of the associated fragmented sleep, nocturnal arousal, and sympathetic hyperarousal (Arzt et al., 2010; Balfors & Franklin, 1994; Slater & Steier, 2012; Wessendorf et al., 2000).
Depression
Depression is another condition linked to EDS in stroke survivors (Hermann & Bassetti, 2009). Stroke survivors have an elevated risk for depressive symptoms even 2 or more years after the event (Whyte, Mulsant, Vanderbilt, Dodge, & Ganguli, 2004). Depressive symptoms are commonly associated with EDS, and the two symptoms often coexist among patients with SDB (Santamaria, Iranzo, Ma Montserrat, & de Pablo, 2007). In fact, depressive symptoms are closely interrelated with sleep disturbance more generally, including short or fragmented sleep that can be precursors or outcomes associated with EDS (Fava, 2004).
Although there was no established relationship between subjective sleepiness, as measured by ESS, and depression in adults who had had a stroke, depression did increase poststroke fatigue, and sleepiness may be a manifestation of fatigue (Staub & Bogousslavsky, 2001). It is suggested that treating depression with stimulating antidepressants may help improve EDS among stroke survivors (Hermann & Bassetti, 2009). However, among the studies we reviewed, only two discussed the relationship between depression and EDS. Khairkar and Diwan (2012) reported that depression comorbid with poststreptococcal infection was associated with poststroke EDS, while the other study found that poststroke EDS was more closely associated with fatigue and subcortical lesion location than with depression as measured by the Beck Depression Inventory (Suh, Choi-Kwon, & Kim, 2014).
Physical disability
In one of the studies we reviewed, researchers found physical disability leading to reduction in physical activity levels to be associated with EDS in stroke survivors (Arzt et al., 2010). However, authors also noted that stroke survivors with disability were less likely to report EDS than working adults in the general population. They posit that one potential explanation for the lack of subjective sleepiness in stroke patients may be that, because most of their physical needs are met by caregivers, low-level physical activity does not exhaust them and they may, thus, be less likely to feel sleepy. Further, drugs that stroke survivors commonly use, such as platelet aggregation inhibitors, antiarrhythmic agents, and diuretics, may reduce the perception of EDS through central stimulating effects, which may also explain the finding that subjective daytime sleepiness was not common among stroke survivors in this study.
Dynamic relationships among antecedents for EDS in stroke
The relationships among antecedents of EDS in stroke survivors are complex. For example, SDB can contribute to a higher risk for stroke, while conversely, stroke can lead to SDB (e.g., periodic breathing) in a certain proportion of stroke survivors. In one study we reviewed, Alexandrov et al. (2009) point out that, though we do not yet have the knowledge to cure or reduce the severity of certain types of stroke, we do have well-known strategies for managing depressive symptoms and SDB, two modifiable risk factors for EDS in stroke. Reducing the severity of SDB and depression in these patients could, then, indirectly lessen the severity of EDS (Hermann & Bassetti, 2009).
Outcomes Associated With EDS in Stroke Survivors
The outcomes associated with EDS in stroke survivors were serious and negative in the studies in this review. These outcomes can be divided into three overlapping categories: neurocognitive, functional, and health related. The neurocognitive outcomes consisted of neurological decline (Alexandrov et al., 2009), reduced alertness (C. Bassetti et al., 1996; Bliwise et al., 2002; Goyal et al., 2012; Hermann et al., 2008), learning deficits (Hermann et al., 2008; Sterr et al., 2008), and memory problems (Hermann et al., 2008). Stroke survivors with EDS also experienced declines in physical function (Hermann et al., 2008; Sterr et al., 2008), reduced quality of life (Hermann & Bassetti, 2009), and impaired driving ability (Mills et al., 2013). The health-related outcomes associated with poststroke EDS included early deterioration at the acute-stroke phase (Alexandrov et al., 2009), poor clinical outcomes (Hermann et al., 2008; Vock et al., 2002), increased mortality rate in elderly stroke survivors (Medeiros et al., 2011), mood instability that presented as anxiety or depression (Falconer et al., 2010; Sterr et al., 2008), and elevated risk for recurring stroke (Pasic et al., 2011). It is difficult to determine, however, whether the negative health outcomes were the results of EDS, alone, or of the synergistic effects of EDS and stroke.
Another outcome associated with EDS highlighted in these studies was the need for increased medical treatment. Stroke survivors with EDS often required medical treatment to help improve sleep behavior (Hermann & Bassetti, 2009). Medications such as bromocriptine, modafinil, methylphenidate, and levodopa have been used to improve wakefulness among stroke survivors (C. Bassetti et al., 1996; Hermann & Bassetti, 2009), and positive airway pressure treatment improved EDS in stroke survivors with OSA (Ryan et al., 2011). Treatment of depression in stroke survivors either improved or worsened daytime sleepiness depending on the type of antidepressant (sedating or stimulating; Hermann & Bassetti, 2009).
Discussion
The purpose of this integrative review was to explore the state of the science regarding EDS in stroke survivors. We described EDS in the poststroke context and explored factors as well as outcomes associated with EDS. We found that EDS is highly prevalent in stroke survivors, especially among those with paramedian thalamic, subcortical, diencephalic and pontine lesions. EDS can also be caused by SDB or depression and is associated with negative health outcomes in stroke survivors. Given the established association between EDS and stroke and these known negative outcomes, there is a need to increase awareness of EDS among clinicians and for careful evaluation and management of EDS in stroke survivors.
The conceptual definition of EDS was poorly elucidated in the literature in the context of poststroke patients. The lack of distinction we noted in this review between EDS and other concepts such as fatigue among stroke survivors was similar to the findings of Pigeon, Sateia, and Ferguson (2003) in the medical literature on EDS in the general population.
Further, there is a lack of a standardized method for quantifying EDS in stroke survivors, which makes it difficult to compare findings across studies. Researchers often favor subjective over objective measures for studying EDS in stroke, likely because of the cost associated with objective measurement and the lack of ecological validity due to the fact that these measures are usually applied in the laboratory setting for 1 day only (Paquet, Kawinska, & Carrier, 2007; Stepnowsky, Moore, & Dimsdale, 2003).
In the studies we reviewed, ESS was the most frequently used subjective measure of EDS among stroke patients. Authors, however, have expressed concerns about the suitability of using ESS in stroke survivors because it was not designed for patients with neurological conditions (Arzt et al., 2010; Mills et al., 2013). Only one study tested the construct validity of ESS through statistical analysis models (Mills et al., 2013), with the authors concluding that ESS has a good construct validity for use in stroke patients and is a robust scale for detecting pathological sleepiness in stroke survivors when the item regarding driving is excluded from the driving item. However, in their sample, responders were primarily male (63.2%) with a mean age of 66.2 years, which was significantly younger than the mean age of nonresponders (70.9 years); thus, results might not be generalizable to a broader population. Evidence from clinical studies indicates that ESS is helpful for differentiating OSA from primary snoring and can measure improvement in patients treated for narcolepsy and OSA in the general population (Shen et al., 2006). However, given the available evidence, it is difficult to conclude that ESS is equally useful in assessing sleepy behaviors across genders and age levels (Kim & Young, 2005).
The most common objective measure of EDS in stroke patients in the reviewed studies was polysomnography rather than MSLT, which has been considered the gold standard for measuring EDS.
Conceptualization of EDS in Stroke Survivors
Both the operational and conceptual definitions of poststroke EDS in the reviewed studies consisted of two components, the objective and the subjective, both of which are necessary to get a complete picture of the phenomenon of EDS in stroke survivors. It is thus advisable that investigators use both a subjective and an objective measure of EDS in adults who have had a stroke or choose a measure that can quantify both the objective and subjective components of EDS.
The characteristics of subjective EDS in stroke survivors encompassed self-reported dozing behaviors in daily life and the feeling of a drowsy state. The characteristics of objective EDS included sleep latency (how fast one falls asleep), sleepy behavior indicators, and EEG evidence. Operational definitions can thus be formed by employing instruments to measure latency, sleep behaviors, and EEG patterns.
Proposed Theoretical Framework for EDS in Stroke Survivors
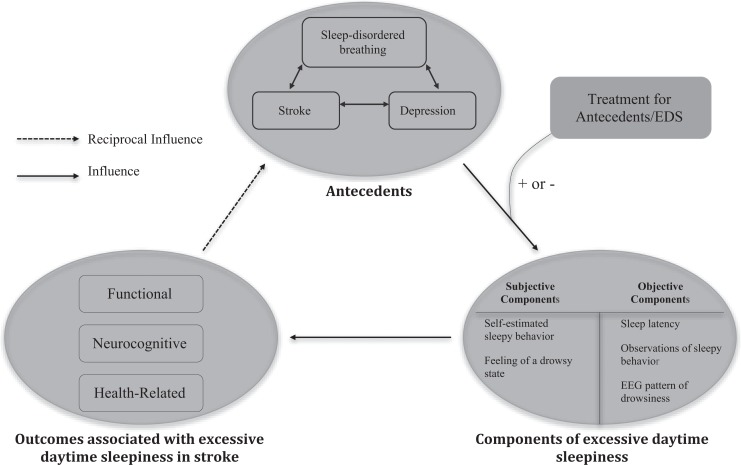
An improved understanding of EDS in stroke survivors would help researchers and clinicians identify reliable, valid, and easy to administer EDS screening measures and could change the focus of poststroke rehabilitation. Based on the results of our literature review, we have created a potential theoretical framework for EDS in stroke survivors, which is depicted in Figure 2. This framework depicts the complex relationships among factors influencing EDS and has the potential to guide both the design of rehabilitation programs to improve outcomes and future research.
Figure 2.
Theoretical framework of excessive daytime sleepiness (EDS) in stroke survivors. ±: Treatments for antecedents or EDS can positively or negatively influence EDS.
The basic tenets of the framework are as follows:
In the poststroke context, EDS is caused and influenced by antecedents including stroke, SDB, reversed Robin Hood syndrome, and depression. These antecedents are closely related to each other. The relationship between EDS and the modifiable antecedent of depression is particularly complex, as depression can also be an outcome associated with EDS due to mood instability;
EDS in stroke survivors is multidimensional, which indicates the need to select measures that can quantify both the objective and subjective components of EDS in order to gain a comprehensive understanding of the phenomenon. It may be wise not to rely solely on ESS, a subjective measure, to assess daytime sleepiness in stroke survivors due to its questionable validity;
the subjective characteristics of EDS in stroke survivors include self-reported dozing behaviors and the subjective feeling of a drowsy state, while the objective characteristics of EDS in stroke include sleep latency, observations of sleepy behavior, and EEG patterns of sleep;
the outcomes associated with EDS in stroke survivors are serious and primarily negative. Treatment can either improve or worsen the symptom. These outcomes, in turn, likely influence the antecedents of poststroke EDS.
Implications for Future Research
Although defining sleepiness and evaluating sleep in brain-injured patients (such as those with stroke or trauma) with disorders of consciousness can be particularly challenging, the systematic and objective evaluation of sleep offers a promising way to refine diagnosis and prognosis in these patients, since consciousness and sleep are intimately connected (Cologan et al., 2010, 2013).
Further research is needed to test the objective and subjective components of the concept of poststroke EDS, thus helping to develop a reliable and valid method for quantifying EDS. For instance, the Psychomotor Vigilance Task (PVT), a behavior-performance measure of the objective component of EDS, defines EDS as one’s tendency to fall into sleep. The feasibility of measuring EDS using PVT, however, has not yet been tested in stroke survivors. Research comparing PVT to other measures in quantifying EDS in this population is thus indicated.
There is also a need for research on the mechanisms by which EDS may cause harmful outcomes in stroke survivors. Does EDS lead directly to negative outcomes in stroke populations? Do the antecedents of EDS—such as depression and SDB—cause poor health outcomes in stroke populations, or is EDS independently associated with these outcomes? In order to allow synthesis of evidence from different studies, the use of consistent and validated measures of EDS is necessary.
Finally, our review of the literature indicates that EDS is potentially modifiable in stroke survivors due to the potential link between EDS and SDB and depression. Thus, research could extend to examining the potential role of behavioral interventions such as cognitive behavioral therapy in managing EDS and its antecedents among stroke survivors.
Limitations
This review of EDS has a number of limitations. There is the possibility that we missed relevant studies despite a careful review with sophisticated search strategies and hand searching reference lists. It is also limited by the small number of relevant studies we could find that described and measured EDS among poststroke patients. Finally, our exclusion of studies not published in English prevented the inclusion of potentially valuable research.
Conclusion
Stroke survivors frequently experience EDS, and this symptom may not always be caused by SDB. Health-care providers and sleep specialists can seek to influence stroke survivors’ functional, neurological, and health-related outcomes through interventions that help to mitigate EDS, depressive symptoms, and SDB. The suspicion of SDB in stroke survivors may indicate the wisdom of choosing both a subjective and objective evaluation of sleep to more fully describe the patient’s symptoms. In particular, future study is needed to evaluate the possibility of adding the PVT as an adjunct to screen for EDS in stroke survivors. Greater attention to EDS in stroke survivors has the potential to improve important physiological and psychological outcomes of stroke.
Footnotes
Author Contribution: Q. Ding contributed to conception, design, and acquisition; drafted and critically revised manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. R. Whittemore contributed to conception, design, and acquisition; critically revised manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. N. Redeker contributed to conception, design, and acquisition; critically revised manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Alexandrov A. V., Nguyen H. T., Rubiera M., Alexandrov A. W., Zhao L., Heliopoulos I.…Tsivgoulis G. (2009). Prevalence and risk factors associated with reversed Robin Hood syndrome in acute ischemic stroke. Stroke, 40, 2738–2742. doi:10.1161/strokeaha.109.547950 [DOI] [PubMed] [Google Scholar]
- Arzt M., Young T., Peppard P. E., Finn L., Ryan C. M., Bayley M., Bradley T. D. (2010). Dissociation of obstructive sleep apnea from hypersomnolence and obesity in patients with stroke. Stroke, 41, e129–e134. doi:10.1161/strokeaha.109.566463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfors E. M., Franklin K. A. (1994). Impairment of cerebral perfusion during obstructive sleep apneas. American Journal of Respiratory and Critical Care Medicine, 150, 1587–1591. doi:10.1164/ajrccm.150.6.7952619 [DOI] [PubMed] [Google Scholar]
- Bassetti C., Mathis J., Gugger M., Lovblad K. O., Hess C. W. (1996). Hypersomnia following paramedian thalamic stroke: A report of 12 patients. Annals of Neurology, 39, 471–480. doi:10.1002/ana.410390409 [DOI] [PubMed] [Google Scholar]
- Bassetti C. L. (2005). Sleep and stroke. Seminars in Neurology, 25, 19–32. doi:10.1055/s-2005-867073 [DOI] [PubMed] [Google Scholar]
- Bliwise D. L., Rye D. B., Dihenia B., Gurecki P. (2002). Greater daytime sleepiness in subcortical stroke relative to Parkinson’s disease and Alzheimer’s disease. Journal of Geriatric Psychiatry and Neurology, 15, 61–67. [DOI] [PubMed] [Google Scholar]
- Boden-Albala B., Roberts E. T., Bazil C., Moon Y., Elkind M. S., Rundek T.…Sacco R. L. (2012). Daytime sleepiness and risk of stroke and vascular disease: Findings from the Northern Manhattan Study (NOMAS). Circulation: Cardiovascular Quality and Outcomes, 5, 500–507. doi:10.1161/circoutcomes.111.963801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N., Roth T., Rosenthal L., Andreski P. (1997). Daytime sleepiness: An epidemiological study of young adults. American Journal of Public Health, 87, 1649–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D., Davis L., Vujovic-Zotovic N., Boulias C., Ismail F., Richardson D., Goldstein R. S. (2010). Sleep-disordered breathing in patients enrolled in an inpatient stroke rehabilitation program. Archives of Physical Medicine and Rehabilitation, 91, 659–662. doi:10.1016/j.apmr.2009.12.019 [DOI] [PubMed] [Google Scholar]
- Camilo M. R., Sander H. H., Eckeli A. L., Fernandes R. M., Dos Santos-Pontelli T. E., Leite J. P., Pontes-Neto O. M. (2014). SOS score: An optimized score to screen acute stroke patients for obstructive sleep apnea. Sleep Medicine, 15, 1021–1024. doi:10.1016/j.sleep.2014.03.026 [DOI] [PubMed] [Google Scholar]
- Chan W., Coutts S. B., Hanly P. (2010). Sleep apnea in patients with transient ischemic attack and minor stroke: Opportunity for risk reduction of recurrent stroke? Stroke, 41, 2973–2975. doi:10.1161/strokeaha.110.596759 [DOI] [PubMed] [Google Scholar]
- Cluydts R., De Valck E., Verstraeten E., Theys P. (2002). Daytime sleepiness and its evaluation. Sleep Medicine Reviews, 6, 83–96. doi:10.1053/smrv.2002.0191 [DOI] [PubMed] [Google Scholar]
- Cologan V., Drouot X., Parapatics S., Delorme A., Gruber G., Moonen G., Laureys S. (2013). Sleep in the unresponsive wakefulness syndrome and minimally conscious state. Journal of Neurotrauma, 30, 339–346. doi:10.1089/neu.2012.2654 [DOI] [PubMed] [Google Scholar]
- Cologan V., Schabus M., Ledoux D., Moonen G., Maquet P., Laureys S. (2010). Sleep in disorders of consciousness. Sleep Medicine Reviews, 14, 97–105. doi:10.1016/j.smrv.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio G., Casagrande M., Bertini M. (2001). Sleepiness: Evaluating and quantifying methods. International Journal of Psychophysiology, 41, 251–263. [DOI] [PubMed] [Google Scholar]
- Davies D. P., Rodgers H., Walshaw D., James O. F., Gibson G. J. (2003). Snoring, daytime sleepiness and stroke: A case-control study of first-ever stroke. Journal of Sleep Research, 12, 313–318. [DOI] [PubMed] [Google Scholar]
- Dinges D. F. (1995). An overview of sleepiness and accidents. Journal of Sleep Research, 4, 4–14. [DOI] [PubMed] [Google Scholar]
- Duncan F., Lewis S. J., Greig C. A., Dennis M. S., Sharpe M., MacLullich A. M., Mead G. E. (2015). Exploratory longitudinal cohort study of associations of fatigue after stroke. Stroke, 46, 1052–1058. doi:10.1161/strokeaha.114.008079 [DOI] [PubMed] [Google Scholar]
- Falconer M., Walsh S., Harbison J. A. (2010). Estimated prevalence of fatigue following stroke and transient ischemic attack is dependent on terminology used and patient gender. Journal of Stroke and Cerebrovascular Diseases, 19, 431–434. doi:10.1016/j.jstrokecerebrovasdis.2009.07.017 [DOI] [PubMed] [Google Scholar]
- Fava M. (2004). Daytime sleepiness and insomnia as correlates of depression. Journal of Clinical Psychiatry, 65, 27–32. [PubMed] [Google Scholar]
- Fonseca A. C., Geraldes R., Pires J., Falcao F., Bentes C., Melo T. P. (2011). Improvement of sleep architecture in the follow up of a patient with bilateral paramedian thalamic stroke. Clinical Neurology and Neurosurgery, 113, 911–913. doi:10.1016/j.clineuro.2011.05.003 [DOI] [PubMed] [Google Scholar]
- Goel N., Basner M., Rao H., Dinges D. F. (2013). Circadian rhythms, sleep deprivation, and human performance. Progress in Molecular Biology and Translational Science, 119, 155–190. doi:10.1016/b978-0-12-396971-2.00007-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M. K., Kumar G., Sahota P. K. (2012). Isolated hypersomnia due to bilateral thalamic infarcts. Journal of Stroke and Cerebrovascular Diseases, 21, 146–147. doi:10.1016/j.jstrokecerebrovasdis.2010.05.013 [DOI] [PubMed] [Google Scholar]
- Guilleminault C., Brooks S. N. (2001). Excessive daytime sleepiness: A challenge for the practising neurologist. Brain, 124, 482–1491. [DOI] [PubMed] [Google Scholar]
- Happe S. (2003). Excessive daytime sleepiness and sleep disturbances in patients with neurological diseases: Epidemiology and management. Drugs, 63, 2725–2737. [DOI] [PubMed] [Google Scholar]
- Hermann D. M., Bassetti C. L. (2009). Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology, 73, 1313–1322. doi:10.1212/WNL.0b013e3181bd137c [DOI] [PubMed] [Google Scholar]
- Hermann D. M., Siccoli M., Brugger P., Wachter K., Mathis J., Achermann P., Bassetti C. L. (2008). Evolution of neurological, neuropsychological and sleep-wake disturbances after paramedian thalamic stroke. Stroke, 39, 62–68. doi:10.1161/strokeaha.107.494955 [DOI] [PubMed] [Google Scholar]
- Herron K., Dijk D. J., Dean P. (2014). Quantitative electroencephalography and behavioural correlates of daytime sleepiness in chronic stroke. Biomed Research International, 2014, 794086 doi:10.1155/2014/794086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M. W. (1998). Rethinking the assessment of sleepiness. Sleep Medicine Reviews, 2, 3–15. [DOI] [PubMed] [Google Scholar]
- Johns M. W. (2010). A new perspective on sleepiness. Sleep and Biological Rhythms, 8, 170–179. doi:10.1111/j.1479-8425.2010.00450.x [Google Scholar]
- Khairkar P., Diwan S. (2012). Late-onset obsessive-compulsive disorder with comorbid narcolepsy after perfect blend of thalamo-striatal stroke and post-streptococcal infection. Journal of Neuropsychiatry & Clinical Neurosciences, 24, E29–E31. doi:10.1176/appi.neuropsych.11100252 [DOI] [PubMed] [Google Scholar]
- Kim H., Young T. (2005). Subjective daytime sleepiness: Dimensions and correlates in the general population. Sleep, 28, 625–634. [DOI] [PubMed] [Google Scholar]
- Koch S., Zuniga S., Rabinstein A. A., Romano J. G., Nolan B., Chirinos J., Forteza A. (2007). Signs and symptoms of sleep apnea and acute stroke severity: Is sleep apnea neuroprotective? Journal of Stroke Cerebrovascular Diseases, 16, 114–118. doi:10.1016/j.jstrokecerebrovasdis.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Lundqvist A., Alinder J., Ronnberg J. (2008). Factors influencing driving 10 years after brain injury. Brain Injury, 22, 295–304. doi:10.1080/02699050801966133 [DOI] [PubMed] [Google Scholar]
- Masel B. E., Scheibel R. S., Kimbark T., Kuna S. T. (2001). Excessive daytime sleepiness in adults with brain injuries. Archives of Physical Medicine and Rehabilitation, 82, 1526–1532. doi:10.1053/apmr.2001.26093 [DOI] [PubMed] [Google Scholar]
- Medeiros C. A. M., De Bruin P. F. C., Paiva T. R., Coutinho W. M., Ponte R. P., De Bruin V. M. S. (2011). Clinical outcome after acute ischaemic stroke: The influence of restless legs syndrome. European Journal of Neurology, 18, 144–149. doi:10.1111/j.1468-1331.2010.03099.x [DOI] [PubMed] [Google Scholar]
- Mills R. J., Koufali M., Sharma A., Tennant A., Young C. A. (2013). Is the Epworth Sleepiness Scale suitable for use in stroke? Topics in Stroke Rehabilitation, 20, 493–499. doi:10.1310/tsr2006-493 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151, 264–269. [DOI] [PubMed] [Google Scholar]
- Paquet J., Kawinska A., Carrier J. (2007). Wake detection capacity of actigraphy during sleep. Sleep, 30, 1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasic Z., Smajlovic D., Dostovic Z., Kojic B., Selmanovic S. (2011). Incidence and types of sleep disorders in patients with stroke. Medical Archives, 65, 225–227. [DOI] [PubMed] [Google Scholar]
- Pigeon W. R., Sateia M. J., Ferguson R. J. (2003). Distinguishing between excessive daytime sleepiness and fatigue: Toward improved detection and treatment. Journal of Psychosomatic Research, 54, 61–69. doi: 10.1016/S0022-3999(02)00542-1 [DOI] [PubMed] [Google Scholar]
- Rivera V. M., Meyer J. S., Hata T., Ishikawa Y., Imai A. (1986). Narcolepsy following cerebral hypoxic ischemia. Annals of Neurology, 19, 505–508. doi:10.1002/ana.410190516 [DOI] [PubMed] [Google Scholar]
- Ryan C. M., Bayley M., Green R., Murray B. J., Bradley T. D. (2011). Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke, 42, 1062–1067. doi:10.1161/strokeaha.110.597468 [DOI] [PubMed] [Google Scholar]
- Sagberg F. (2006). Driver health and crash involvement: A case-control study. Accident Analysis & Prevention, 38, 28–34. doi:10.1016/j.aap.2005.06.018 [DOI] [PubMed] [Google Scholar]
- Santamaria J., Iranzo A., Ma Montserrat J., de Pablo J. (2007). Persistent sleepiness in CPAP treated obstructive sleep apnea patients: Evaluation and treatment. Sleep Medicine Reviews, 11, 195–207. doi:10.1016/j.smrv.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Scammell T. E., Nishino S., Mignot E., Saper C. B. (2001). Narcolepsy and low CSF orexin (hypocretin) concentration after a diencephalic stroke. Neurology, 56, 1751–1753. [DOI] [PubMed] [Google Scholar]
- Schuiling W. J., Rinkel G. J., Walchenbach R., de Weerd A. W. (2005). Disorders of sleep and wake in patients after subarachnoid hemorrhage. Stroke, 36, 578–582. doi:10.1161/01.str.0000154862.33213.73 [DOI] [PubMed] [Google Scholar]
- Shen J., Barbera J., Shapiro C. M. (2006). Distinguishing sleepiness and fatigue: Focus on definition and measurement. Sleep Medicine Reviews, 10, 63–76. doi:10.1016/j.smrv.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Slater G., Steier J. (2012). Excessive daytime sleepiness in sleep disorders. Journal of Thoracic Disease, 4, 608–616. doi:10.3978/j.issn.2072-1439.2012.10.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub F., Bogousslavsky J. (2001). Fatigue after stroke: A major but neglected issue. Cerebrovascular Disease, 12, 75–81. [DOI] [PubMed] [Google Scholar]
- Stepnowsky C. J., Jr., Moore P. J., Dimsdale J. E. (2003). Effect of ethnicity on sleep: Complexities for epidemiologic research. Sleep, 26, 329–332. [DOI] [PubMed] [Google Scholar]
- Sterr A., Herron K., Dijk D. J., Ellis J. (2008). Time to wake-up: Sleep problems and daytime sleepiness in long-term stroke survivors. Brain Injury, 22, 575–579. doi:10.1080/02699050802189727 [DOI] [PubMed] [Google Scholar]
- Suh M., Choi-Kwon S., Kim J. S. (2014). Sleep disturbances after cerebral infarction: Role of depression and fatigue. Journal of Stroke and Cerebrovascular Diseases, 23, 1949–1955. doi:10.1016/j.jstrokecerebrovasdis.2014.01.029 [DOI] [PubMed] [Google Scholar]
- Tosato M., Aquila S., Della Marca G., Incalzi R. A., Gambassi G. (2009). Sleep disruption following paramedian pontine stroke. BMJ Case Reports, 2009 doi:10.1136/bcr.07.2008.0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zandvoort M. J., Kappelle L. J., Algra A., De Haan E. H. (1998). Decreased capacity for mental effort after single supratentorial lacunar infarct may affect performance in everyday life. Journal of Neurology, Neurosurgery & Psychiatry, 65, 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vock J., Achermann P., Bischof M., Milanova M., Müller C., Nirkko A.…Bassetti C. L. (2002). Evolution of sleep and sleep EEG after hemispheric stroke. Journal of Sleep Research, 11, 331–338. [DOI] [PubMed] [Google Scholar]
- Wessendorf T. E., Teschler H., Wang Y. M., Konietzko N., Thilmann A. F. (2000). Sleep-disordered breathing among patients with first-ever stroke. Journal of Neurology, 247, 41–47. [DOI] [PubMed] [Google Scholar]
- Whittemore R., Knafl K. (2005). The integrative review: Updated methodology. Journal of Advanced Nursing, 52, 546–553. doi:10.1111/j.1365-2648.2005.03621.x [DOI] [PubMed] [Google Scholar]
- Whyte E. M., Mulsant B. H., Vanderbilt J., Dodge H. H., Ganguli M. (2004). Depression after stroke: A prospective epidemiological study. Journal of the American Geriatrics Society, 52, 774–778. doi:10.1111/j.1532-5415.2004.52217.x [DOI] [PubMed] [Google Scholar]
- Wise M. S. (2006). Objective measures of sleepiness and wakefulness: Application to the real world? Journal of Clinical Neurophysiology, 23, 39–49. doi:10.1097/01.wnp.0000190416.62482.42 [DOI] [PubMed] [Google Scholar]
- Yaggi H. K., Concato J., Kernan W. N., Lichtman J. H., Brass L. M., Mohsenin V. (2005). Obstructive sleep apnea as a risk factor for stroke and death. New England Journal of Medicine, 353, 2034–2041. doi:10.1056/NEJMoa043104 [DOI] [PubMed] [Google Scholar]