Abstract
Sensory and regulatory domains allow bacteria to adequately respond to environmental changes. The regulatory ACT (Aspartokinase, Chorismate mutase and TyrA) domains are mainly found in metabolic-related proteins as well as in long (p)ppGpp synthetase/hydrolase enzymes. Here, we investigate the functional role of the ACT domain of SpoT, the only (p)ppGpp synthetase/hydrolase of Caulobacter crescentus. We show that SpoT requires the ACT domain to efficiently hydrolyze (p)ppGpp. In addition, our in vivo and in vitro data show that the phosphorylated version of EIIANtr (EIIANtr∼P) interacts directly with the ACT and inhibits the hydrolase activity of SpoT. Finally, we highlight the conservation of the ACT-dependent interaction between EIIANtr∼P and SpoT/Rel along with the phosphotransferase system (PTSNtr)-dependent regulation of (p)ppGpp accumulation upon nitrogen starvation in Sinorhizobium meliloti, a plant-associated α-proteobacterium. Thus, this work suggests that α-proteobacteria might have inherited from a common ancestor, a PTSNtr dedicated to modulate (p)ppGpp levels in response to nitrogen availability.
INTRODUCTION
Bacteria use a wide range of sensory and regulatory domains to integrate environmental signals. In response to nutrient limitation, most bacteria synthesize a second messenger, the guanosine penta- or tetra-phosphate commonly referred to as (p)ppGpp. This molecule helps in reallocating cellular resources notably by reprogramming transcription and interfering with cell cycle progression (1,2). In Escherichia coli, (p)ppGpp levels are regulated by RelA and SpoT, two long RelA/SpoT homologue (RSH) enzymes. SpoT carries a synthetase domain (SD) that is poorly activated by starvation signals (carbon, fatty acids, phosphate, …) and a functional hydrolase domain (HD). By contrast, RelA harbours an SD activated by amino acid scarcity and a degenerated and inactive HD domain (reviewed in (2) and (3)). Despite these differences, both RSH enzymes present the same domain architecture with catalytic domains (SD and HD) located towards the N-terminus and regulatory domains TGS (ThrRS, GTPase and SpoT) and ACT (Aspartokinase, Chorismate mutase and TyrA) at the C-terminal end (Figure 1). The C-terminal domains (CTD) are thought to play a critical role in RSH by sensing the starvation signal and transducing it to the catalytic domains. RelA, for example, is known to be associated with the ribosome, where it detects deacylated transfer RNA (tRNA) in the ribosomal acceptor site (A-site) as a signal for amino acid starvation, which activates the synthetase activity (4). Recent studies using cryo-electron microscopy revealed that E. coli RelA adopts an extended ‘open’ conformation on stalled ribosomes with the A-site/tRNA in contact with the TGS domain in the C-terminal part (5–7). Likewise, activation of SpoT synthetase activity in E. coli cells starved for fatty acids has been shown to require an acyl carrier protein that binds to the TGS of SpoT (8). Although the role of the CTD is undoubtedly critical for regulating catalytic functions of RSH, the mechanisms by which this regulation is mediated remain unknown.
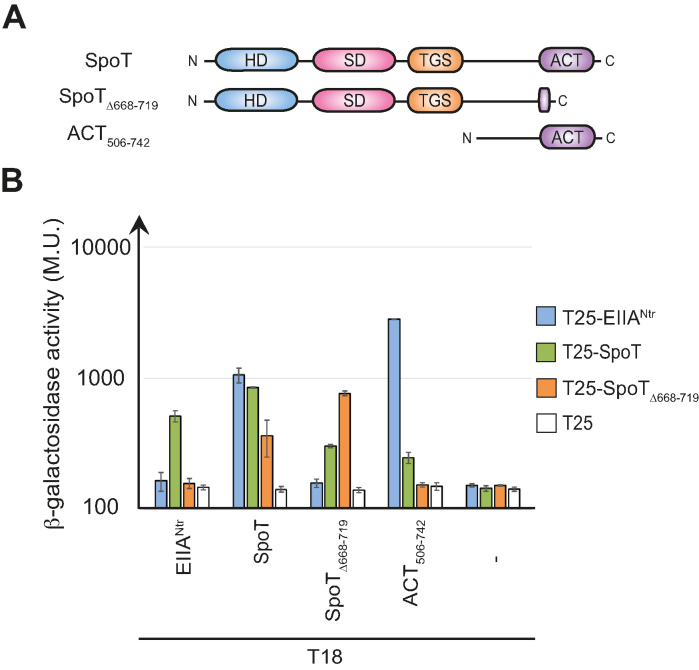
Figure 1.
The ACT domain is required for the interaction between SpoT and EIIANtr∼P in a bacterial two-hybrid assay. (A) Domain organisation of C. crescentus SpoT with the catalytic domains, HD and SD, located at the N-terminal extremity and the regulatory domains, TGS and ACT, located at the C-terminal end. The SpoT variants used in the BTH assay (SpoTΔ668–719 and ACT506–742) are also represented. (B) SpoT and ACT506–742, but not SpoTΔ668–719, directly interact with EIIANtr∼P. β-galactosidase assays were performed on MG1655 cyaA::frt (RH785) strains coexpressing T18- fused to ptsN, spoT, spoTΔ668–719 or ACT506–742 with T25- fused to ptsN, spoT or spoTΔ668–719. Error bars = SD, n = 3.
In contrast to E. coli, most α-proteobacteria encode bifunctional synthetase/hydrolase RSH usually referred to as Rel or SpoT (9). This is the case of Caulobacter crescentus, which harbours a single RSH (SpoT) sensitive to nitrogen or carbon starvation (10,11).Caulobacter crescentus divides asymmetrically to generate two dissimilar progeny, a motile swarmer cell and a sessile stalked cell. The stalked cell initiates a new replication cycle (S phase) immediately at birth whereas the swarmer cell first enters into a non-replicative G1 phase before starting replication and concomitantly differentiating into a stalked cell (12). Once accumulated, (p)ppGpp modulates cell cycle progression by specifically extending the G1/swarmer phase (13,14). Recently, we reported a key role played by the nitrogen-related phosphotransferase system (PTSNtr) in regulating (p)ppGpp accumulation in response to nitrogen starvation (15). We showed that EINtr, the first protein of PTSNtr, uses intracellular glutamine concentration as a proxy for nitrogen availability, since glutamine binds to the GAF domain of EINtr to inhibit its autophosphorylation. Therefore, glutamine deprivation strongly stimulates autophosphorylation of EINtr, which in turn triggers phosphorylation of the downstream components HPr and EIIANtr. Once phosphorylated, both HPr∼P and EIIANtr∼P modulate activities of SpoT to quickly increase (p)ppGpp levels. Whereas HPr∼P stimulates synthetase activity of SpoT by an unknown mechanism, EIIANtr∼P interacts directly with SpoT to likely interfere with its hydrolase activity (15). The plant-associated α-proteobacterium Sinorhizobium meliloti also accumulates (p)ppGpp upon nitrogen or carbon starvation from a single enzyme called Rel (16,17), but the mechanism beyond this regulation remains unknown.
In this work, we investigate the role of the ACT domain in regulating the activity of SpoT-like enzymes. In particular, we show that the ACT is indispensable for the hydrolase activity of C. crescentus SpoT and that EIIANtr∼P inhibits (p)ppGpp hydrolysis likely by directly interacting with the ACT of SpoT. In addition, we show that the EIIANtr∼P-mediated regulation of RSH is conserved in S. meliloti, suggesting PTSNtr plays a critical role in sensing the metabolic state and regulating cell cycle progression in α-proteobacteria.
MATERIALS AND METHODS
Bacterial strains and growth conditions
Oligonucleotides, strains and plasmids used in this study are listed in Supplementary Tables S1–S3, together with construction details provided in the Supplementary Methods. E. coli Top10 was used for cloning purpose, and grown aerobically in Luria-Bertani (LB) broth (Invitrogen) (18). Electrocompetent cells were used for transformation of E. coli. All C. crescentus strains used in this study are derived from the synchronizable (NA1000) wild-type strain, and were grown in Peptone Yeast Extract (PYE) or synthetic M2 (20 mM PO43−, 9.3 mM NH4+; +N) or P2 (20 mM PO43−; -N) supplemented with 0.5 mM MgSO4, 0.5 mM CaCl2, 0.01 mM FeSO4 and 0.2% glucose (respectively M2G or P2G) media at 28–30°C. Growth was monitored by following the optical density at 660 nm (OD660) during 24 h, in an automated plate reader (Epoch 2, Biotek) with continuous shaking at 30°C. Motility was monitored on PYE swarm (0.3% agar) plates. Area of the swarm colonies were quantified with ImageJ software as described previously (15). For kinetic experiments with S. meliloti (Supplementary Figure S5d), bacteria where cultivated overnight at 30°C in LBMC (LB broth with 2.5 mM MgSO4 and 2.5 mM CaCl2) supplemented with kanamycin, then back-diluted in LBMC during 3 h before induction with 0.1 mM isopropyl-β-D-thiogalactoside (IPTG). Samples were taken each hour during 5 h. For E. coli, antibiotics were used at the following concentrations (μg/ml; in liquid/solid medium): ampicillin (50/100), kanamycin (30/50), oxytetracycline (12.5/12.5) where appropriate. For C. crescentus, media were supplemented with kanamycin (5/20), tetracycline (1/2.5) where appropriate. The doubling time of Caulobacter strains was calculated in exponential phase (OD660: 0.2–0.5) using D = [ln(2).(T(B) – T(A))) / (ln(OD660(B))-ln(OD660(A))] and normalized according to the wild-type strain.E. coli S17-1 and MT607 helper strains were used for transferring plasmids to C. crescentus by respectively bi- and tri-parental mating. In-frame deletions were created by using pNPTS138-derivative plasmids and by following the procedure described previously (15).
Bacterial two-hybrid assays
Bacterial two-hybrid (BTH) assays were performed as described previously in (15). Briefly, 2 μl of MG1655 cyaA::frt (RH785) and MG1655 cyaA::frt Δnpr (RH2122) strains expressing T18 and T25 fusions were spotted on MacConkey Agar Base plates supplemented with ampicillin, kanamycin, maltose (1%), and incubed for 1 day at 30°C. All proteins were fused to T25 (pKT25) or T18 (pUT18C) at their N-terminal extremity. The β-galactosidase assays were performed as described in (15). Briefly, 50 μl E. coli BTH strains cultivated overnight at 30°C in LB medium supplemented with kanamycin, ampicillin and IPTG (1 mM) were resuspended in 800 μl of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl and 1 mM MgSO4) and lysed with chloroform. After the addition of 200 μl (o-nitrophenyl-β-D-galactopyranoside (ONPG); 4 mg/ml), reactions were incubated at 30°C until colour turned yellowish. Reactions were then stopped by adding 500 μl of 1 M Na2CO3, and absorbance at 420 nm was measured. Miller Units are defined as (OD420 × 1000)/ (OD590 × t × v), where ‘OD590’ is the absorbance of the cultures at 590 nm before the β-galactosidase assays, ‘t’ is the time of the reaction (min) and ‘v’ is the volume of cultures used in the assays (ml). All the experiments were performed with at least three biological replicates.
Flow cytometry analysis
DNA content was measured using Fluorescence-Activated Cell Sorting (FACS) as described previously in (15). Briefly, cells were fixed in ice-cold 70% Ethanol. Fixed samples were then washed twice in FACS staining buffer (10 mM Tris pH 7.2, 1 mM ethylenediaminetetraacetic acid, 50 mM NaCitrate, 0.01% Triton X-100) containing 0.1 mg/ml RNaseA and incubated at room temperature (RT) for 30 min. Cells were then harvested by centrifugation for 2 min at 8000 × g, resuspended in 1 ml FACS staining buffer containing 0.5 μM Sytox Green Nucleic acid stain (Life Technologies), and incubated at RT in the dark for 5 min. Samples were analyzed in flow cytometer (FACS Calibur, BD Biosciences) at laser excitation of 488 nm. Percentage of gated G1 cells of each strain was then normalized using gated G1 cells of the wild-type strain as reference.
Detection of intracellular (p)ppGpp levels
(p)ppGpp levels were visualized as described previously in (15) for C. crescentus (Cc) and in (16,17) for S. meliloti. Briefly, strains were grown overnight in PYE and then diluted for a second overnight culture in M5GG (Cc) or grown overnight in LBMC medium. Then, cells were diluted a second time in M5GG (Cc) or in LBMC medium and grown for 3 h to reach an OD660 of 0.5 (Cc) or 0.7. Cells were then split into two parts and washed twice with P5G-labelling buffer (Cc (15)) or with MOPS-MGS without glutamate (morpholine propane sulfonic acid; pH 7.4), 55 mM mannitol, 1 mM MgSO4, 0.25 mM CaCl2 and 0.004 mM biotin) (19). One milliliter of cells were then resuspended in 225 μl of P5G-labelling (-N) or M5G-labelling (+N) for C. crescentus and in 225 μl of MOPS-MGS with (+N) or without (-N) glutamate and 0.05% NH4+ for S. meliloti. In addition, media were supplemented with 25 μl of KH232PO4 at 100 μCi ml−1 and incubated for 1 h or 2 h (Cc) with shaking at 30°C. Then, samples were extracted with an equal volume of 2 M formic acid, placed on ice for 20 min and then stored overnight at −20°C. All cell extracts were pelleted at 14 000 rpm (18 000 x g) for 3 min and 6 × 2 μl (Cc) or 3 × 2 μl of supernatant were spotted onto a polyethyleneimine (PEI) plate (Macherey-Nagel). PEI plates were then developed in 1.5 M KH2PO4 (pH 3.4) at RT. Finally, thin layer chromatography (TLC) plates were imaged on a MS Storage Phosphor Screen (GE Healthcare) and analysed with Cyclone Phosphor Imager (PerkinElmer). For hydrolase experiments (Figure 3C), cells were incubated 1 h in P5G supplemented with xylose (0.1%). Then, cells were washed twice with P5G-labelling, resuspended in P5G-labelling supplemented with KH232PO4, xylose (0.1%) and glutamine (9.3 mM) and incubated for 2 h.
Figure 3.
The ACT domain is required in vivo to support the hydrolase activity of SpoT. (A and B) Absence of the ACT domain of SpoT promotes G1 accumulation and decreases growth rate upon artificial exogenous production of (p)ppGpp. (A) Flow cytometry analysis to determine DNA content in asynchronous population of WT (RH50), spoTY323A(RH1844), spoTD81GY323A(RH2193), ΔptsP spoTY323A (RH2196), spoT Y323AΔACT(RH1586) and ΔptsP spoTY323AΔACT(RH2492) strains with (black bars) or without (grey bars) PxylX::relA-FLAG grown for 6 h in PYE medium supplemented with 0.1% of xylose. The flow cytometry data were normalized to the WT without PxylX::relA-FLAG (100%). (B) The growth of strains used in (A) was measured for 24 h in PYE medium supplemented with 0.1% of xylose. Error bars = SD, n = 3. (C) The regulatory ACT domain of SpoT is required in vivo to degrade (p)ppGpp in nitrogen-replete condition (+N). The intracellular levels of (p)ppGpp were evaluated by TLC after nucleotides extraction from spoTY323A(RH1844), spoTD81GY323A(RH2193), ΔptsP spoTY323A (RH2196), spoTY323AΔACT(RH1586) and ΔptsP spoTY323AΔACT(RH2492) strains harbouring PxylX::relA-FLAG and grown for 2 h in glutamine (Q) containing media (+N) supplemented with 0.1% xylose.
β-galactosidase assay
The β-galactosidase assays performed to measure PspoT-lacZ activity were essentiality done as for the BTH assay with the following modifications. One milliter of Caulobacter strains harbouring the PspoT-lacZ fusion was resuspended in 800 μl of Z buffer and the absorbance of the cultures at 660 nm (OD660) instead of 590 nm was measured before the β-galactosidase assays. All the experiments were performed with three biological replicates and were normalized according to the wild-type strain harbouring the PspoT-lacZ fusion cultivated at 30°C.
Immunoblot analysis
Immunoblot analyses were performed as described in (20) with the following primary antibodies: anti-MreB (1:5000) (20), anti-SpoT (1:5000) and secondary antibodies: anti-rabbit linked to peroxidase (GE Healthcare) at 1:5000, and visualized thanks to Western Lightning Plus-ECL chemiluminescence reagent (Biorad) and Amersham Imager 600 (GE Healthcare).
Proteins purification
Genes encoding C. crescentus SpoT, SpoTD81G, SpoT1–373, SpoTΔACT, SpoT ACT domain as well as EINtrΔGAF, HPr, EIIANtr, EIIANtrH66E and EIIANtrH66A were transformed into E. coli BL21 (DE3) for protein production.
Each protein contains an N-terminal 6His-tag for purification by Ni-NTA chromatography. Cells were grown to an OD600 of ∼0.7 in LB medium (1 l) at 37°C. IPTG was added to a final concentration of 0.5 mM and incubated overnight at 28°C. Cells were harvested by centrifugation for 20 min at 4000 × g, 4°C, resuspended in 8 mM KCl, 1 mM tris(2-carboxyethyl)phosphine (TCEP), 2 mM MgCl2, 50 mM Tris at pH 8, Protease Inhibitor Cocktail (Roche) and lysed with a cell disruptor at 60 psi in 500 mM KCl, 2 mM TCEP, 50 mM Tris at pH 8, 500 mM NaCl, 1% glycerol, Protease Inhibitor Cocktail. The lysis extract was centrifuged for 20 min at 40 000 × g, 4°C. Supernatants were loaded onto a HisTrap HP 1 ml column (GE Healthcare) in 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 500 mM KCl, 500 mM NaCl, 2 mM MgCl2, 1 mM TCEP, 2% glycerol, 0.002% mellitic acid, Protease Inhibitor Cocktail (Roche), 5 mM imidazole, pH 7.5 and eluted with imidazole. The fractions recovered from the Ni-NTA were further purified by size exclusion chromatography (Superdex 200, for SpoT, SpoTD81G, SpoT1–373, SpoTΔACT and EINtrΔGAF, and Superdex 75 for SpoT ACT domain, HPr, EIIANtr, EIIANtrH66E and EIIANtrH66A). Purified samples of SpoT were also used to immunize rabbits in order to produce anti-SpoT polyclonal antibodies.
In vitro phosphorylation of EIIANtr
To produce phosphorylated EIIANtr (EIIANtr∼P), EINtrΔGAF, HPr and EIIANtr were mixed at final concentrations of 2.5, 2.5 and 50 μM, respectively, in phosphorylation buffer [25 mM Tris pH 7.4, 10 mM KCl, 10 mM MgCl2, 1 mM dithiothreitol (DTT)] and incubated at 37°C for 10 min. Samples concentrations were determined by absorbance measurement at 280 nm. The phosphorylation mix was then incubated in 2 mM of phosphoenopyruvate (PEP) at 37°C for 30 min. EIIANtr∼P was further purified by size exclusion chromatography on a Superdex 75 and dialyzed for 72 h at 4°C, in 50 mM HEPES, 500 mM KCl, 500 mM NaCl, 2 mM MgCl2, 1 mM TCEP, 2% glycerol and stored in 20% glycerol at −20°C.
In vitro hydrolase assay
To assess SpoT hydrolase activity, 32PppGpp was synthesized by enzymatic reaction catalyzed by RelA from Chlorobaculum tepidum. To synthesize 32PppGpp, 1 μM of RelACtep was incubated in 1 mM TCEP, 1 mM MgCl2, 50 mM NaCl, 10 mM Tris at pH 7.4. Then, 100 μM guanosine diphosphate (GDP) were added to the synthesis reaction and incubated for 10 min at 37°C, followed by an addition of 3 pM [γ32P] adenosine triphosphate (ATP) (PerkinElmer) incubated for 45 min, 37°C. 32PppGpp was extracted from the reaction medium by centrifugation in Amicon 3K Centrifugal filter (Millipore) at 13 000 × g for 25 min. The hydrolase assays were performed by incubating for 10 min at 37°C (i) 2 μM of SpoT1–373 or SpoTΔACT (Figure 4A), (ii) 1 μM of SpoT with increasing concentrations of EIIANtr or EIIANtrH66E (5, 10 and 20 μM) (Figure 4B), (iii) 1 μM of SpoT with 20 μM of purified EIIANtr∼P (Supplementary Figure S4a) or (iv) 1 μM of SpoT with or without EINtrΔGAF, HPr and EIIANtr (5 μM each) (Figure 4C). Samples concentrations were determined by absorbance measurement at 280 nm. Then, 10 μl of 32PppGpp were added to the reaction mix to reach a final volume of 30 μl. The reaction was stopped by adding 2 μl of 12 M formic acid. Reaction products were separated by TLC by transferring 2 μl of the reaction medium onto a TLC PEI Cellulose F membrane (Millipore). Chromatography membranes were placed in 1 M of KH2PO4 buffer, pH 3.0, for 50 min at RT. The dried TLC membrane was placed in a Phosphor Screen plate (GE Healthcare) for 1 h and the Phosphor Screen revealed with a phosphoimager.
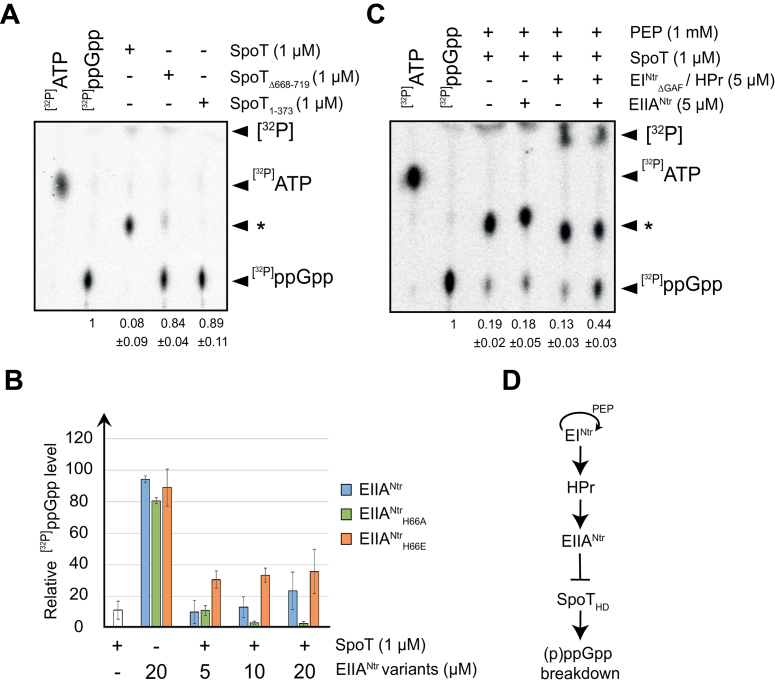
Figure 4.
EIIANtr∼P inhibits the hydrolase activity of SpoT in vitro in an ACT-dependent way. (A) The ACT domain of SpoT is required to hydrolyze ppGpp. Radiolabelled [32P]ppGpp incubated with 1 μM of SpoT, SpoTΔ668–719 or SpoT1–373 was separated by TLC. (B) Phosphomimetic variant of EIIANtr (EIIANtrH66E) inhibits the hydrolase activity of SpoT. Radiolabelled [32P]ppGpp incubated with 1 μM of SpoT in the presence of increasing concentrations (5, 10 and 20 μM) of unphosphorylated EIIANtr, phospho-dead EIIANtrH66A or phosphomimetic EIIANtrH66E variants was separated by TLC. [32P]ppGpp incubated with EIIANtr variants alone were added as controls. The data were normalized to the signal for [32P]ppGpp incubated alone without proteins (100%). (C) Phosphorylated EIIANtr protects ppGpp from hydrolysis by SpoT. Radiolabelled [32P]ppGpp incubated with 1 μM of SpoT in the presence of EIIANtr (5 μM) with or without EINtrΔGAF (5 μM) and HPr (5 μM) was separated by TLC. The star ‘*’ indicates a [32P]ppGpp intermediate degradation. [32P]ATP and [32P]ppGpp were used as references. Relative amounts of [32P]ppGpp are indicated below each lane of TLC. Error bars = SD, n ≥ 3 for (A-C). (D) Schematic representation of the PTSNtr pathway in C. crescentus showing that phosphorylated EIIANtr inhibits SpoT hydrolase activity.
Isothermal Titration Calorimetry (ITC) assay
To measure the interaction between the ACT domain of SpoT and EIIANtr∼P, EIIANtrH66E or EIIANtrH66A, purified proteins were concentrated by centrifugation in Amicon 3K Centrifugal filter (Millipore) at 4000 × g, placed in Slide-A-Lyzer 3500 MWCO Dialysis Cassettes (ThermoScientific) and dialyzed in 50 mM HEPES, 500 mM KCl, 500 mM NaCl, 2 mM MgCl2, 1 mM TCEP, 2% glycerol, 0.002% mellitic acid, Protease Inhibitor Cocktail (Roche), pH 7.5 during 24 h. Proteins were diluted to reach a final concentration of ∼110 μM (EIIANtr∼P, EIIANtrH66E and EIIANtrH66A) or 8 μM (SpoT ACT domain), degassed and equilibrated at titration temperature. Samples concentrations were determined by absorbance measurement at 280 nm. Isothermal Titration Calorimetry (ITC) measurements were performed with an Affinity ITC calorimeter (TA instruments) at 25°C, with a stirring rate of 75 rpm. A constant volume of 2 μl titrant was injected into the cell (177 μl) with an injection interval time of 250 s.
RESULTS
EIIANtr∼P interacts directly with the ACT domain of SpoT
We showed previously that only the phosphorylated version of C. crescentus EIIANtr (EIIANtr∼P) was able to interact with SpoT in a BTH assay (15). To map the domains of SpoT interacting with EIIANtr∼P, we used truncated versions of SpoT in a BTH assay (Figure 1A). We found that deleting the ACT domain (SpoTΔ668–719, referred to as SpoTΔACT throughout the manuscript) precluded the interaction with EIIANtr∼P as well as with the isolated ACT domain (ACT506–742), but not with full-length SpoT (Figure 1B and Supplementary Figure S1a). Moreover, the ACT domain alone was able to strongly interact with EIIANtr∼P (Figure 1B and Supplementary Figure S1a) as well as with itself (Supplementary Figure S1b). Together, these results suggest that the ACT domain is sufficient to mediate the interaction between SpoT and EIIANtr∼P.
Inactivating the ACT domain increases (p)ppGpp levels
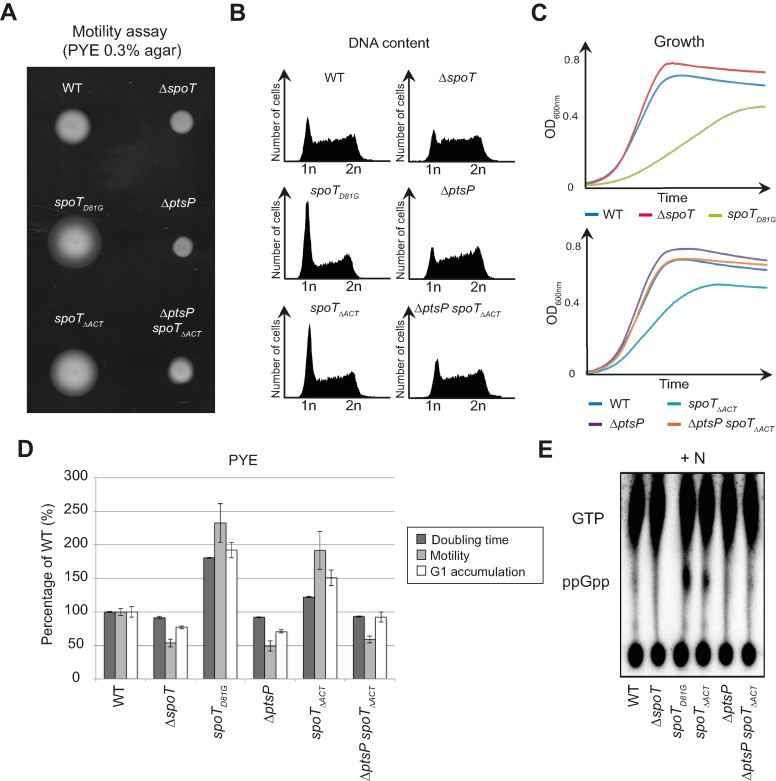
Disrupting the interaction between SpoT and EIIANtr∼P should release the hydrolase activity of SpoT, since strains that either did not express EIIANtr (ΔptsN) or expressed only the non-phosphorylated form of EIIANtr (ΔptsP, ΔptsH or ptsNH66A) increased (p)ppGpp hydrolysis in vivo (15). Accordingly, a C. crescentus strain expressing spoTΔACT as the only copy of spoT might phenocopy the ΔptsP strain by decreasing motility and shortening G1 lifetime (15). Surprisingly, we found that spoTΔACT cells phenocopied rather the hydrolase-dead mutant spoTD81G than ΔptsP (15). Indeed, the spoTΔACT mutation led to an increase of motility, an accumulation of G1/swarmer cells and a growth delay in PYE complex medium (Figure 2A–D). As for spoTD81G, these phenotypes might be due to a (p)ppGpp excess in spoTΔACT. In support of this, spoTΔACT cells accumulated (p)ppGpp without stress (Figure 2E). In addition, abolishing the synthetase activity in spoTΔACT cells, either by deleting ptsP (EINtr) or by incorporating a Y323A substitution into SpoT (spoTY323A ΔACT) known to specifically inactivate synthetase activity (15,21), suppressed all the phenotypes (Figure 2A–E and Supplementary Figure S2a).
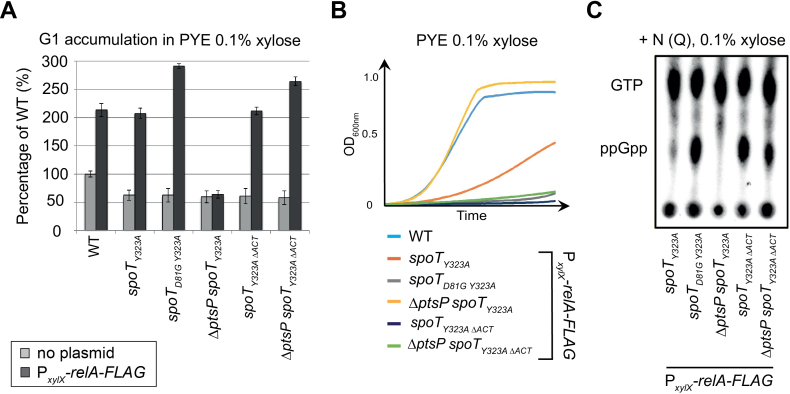
Figure 2.
Inactivation of the ACT domain of SpoT leads to (p)ppGpp accumulation, which consequently extends the G1/swarmer cell lifetime of C. crescentus. (A–D) Extension of the G1/swarmer lifetime in spoTΔACTcells is suppressed by inactivating ptsP (encoding EINtr). Motility (A), DNA content (B) and growth (C) were measured in WT (RH50), ΔspoT (RH1755), spoTD81G(RH1752), ΔptsP (RH1758), spoTΔACT (RH1476) and ΔptsP spoTΔACT(RH1478) strains grown in complex media PYE. (D) Data shown in a-c were normalized to the WT (100%). Error bars = SD, n = 3. (E) Inactivation of the ACT domain leads to (p)ppGpp accumulation. The intracellular levels of (p)ppGpp were evaluated by TLC after nucleotides extraction from the same strains grown in nitrogen-replete (+N) conditions.
We observed that SpoTΔACT and SpoTD81G protein levels in the corresponding mutant strains were slightly higher than the SpoT level in the wild-type strain (Supplementary Figure S2b,c). Nevertheless, upon nitrogen starvation, (p)ppGpp levels in spoTΔACT were slightly lower than in wild-type cells (Supplementary Figure S2d). The slight increase of SpoTΔACT and SpoTD81G levels is likely a result of the positive feedback loop of (p)ppGpp on spoT promoter. Indeed, PspoT displayed higher activity in strains accumulating (p)ppGpp (ptsPL83Q and PxylX::relA-FLAG) and lower activity in ppGpp0 strains (ΔptsP, ΔptsH and ΔptsN), so that SpoT protein levels varied according to (p)ppGpp levels (Supplementary Figure S2e-g). Together, our data suggest that ACT might be required for SpoT hydrolase activity.
The hydrolase activity of SpoT requires the ACT domain
To test whether ACT was required for (p)ppGpp hydrolysis, we first measured (p)ppGpp hydrolysis in vivo (15). To this end, we used C. crescentus strains in which (p)ppGpp (i) cannot be produced anymore by the endogenous SpoT since its synthetase activity has been inactivated with the Y323A mutation (15,21), but (ii) can be synthesized upon addition of xylose by a truncated version of the E. coli RelA (p)ppGpp synthetase expressed from the xylose-inducible promoter (PxylX::relA-FLAG) at the xylX locus (14). Thus, in these strains the only hydrolase activity capable to degrade (p)ppGpp produced by E. coli RelA was supplied by endogenous SpoT variants. Note that, as expected, all the strains harbouring the spoTY323A mutation but without PxylX::relA-FLAG had a G1 proportion lower than the WT, because they did not produce (p)ppGpp (Figure 3A). In agreement with our previous observations, inactivation of SpoT hydrolase in such a background (spoTD81G Y323A;PxylX::relA-FLAG) led to a strong (p)ppGpp accumulation, an extension of the G1 phase and a growth arrest (Figure 3 and (15)). In contrast, releasing SpoT hydrolase activity (ΔptsP spoTY323A;PxylX::relA-FLAG) led to undetectable levels of (p)ppGpp, a reduced G1 phase and an optimal growth (Figure 3 and (15)). Interestingly, deleting the ACT domain of SpoT (spoTY323A ΔACT;PxylX::relA-FLAG) led to a (p)ppGpp accumulation, a G1 proportion and a growth rate similar to the hydrolase-dead mutant (spoTD81G Y323A;PxylX::relA-FLAG) and this independently of the presence of ptsP (Figure 3 and Supplementary Figure S3).
These data strongly suggest that the ACT domain is strictly required in vivo for (p)ppGpp hydrolysis and that EIIANtr∼P inhibits this activity by interfering with the ACT domain of SpoT. To test these hypotheses, we performed in vitro ppGpp hydrolysis assays with the full-length enzyme and mutants lacking either the entire C-terminal regulatory domains (SpoT1–373) or only the ACT domain (SpoTΔ668–719). We observed that full-length SpoT could efficiently hydrolyze [32P]ppGpp but the deletion of the ACT domain in both ACT-deficient SpoT variants strongly affected the hydrolase activity. Indeed, radiolabelled [32P]ppGpp remained mostly intact in the presence of SpoTΔACT (84% ± 4%) or SpoT1–373 (89% ± 11%), whereas more than 90% of [32P]ppGpp were hydrolyzed by the full-length SpoT (Figure 4A). To check whether phosphorylated EIIANtr could modulate the hydrolase activity of SpoT, [32P]ppGpp hydrolysis was first measured in the presence of the WT (EIIANtr), a phospho-dead (EIIANtrH66A) or a phosphomimetic (EIIANtrH66E) version of EIIANtr. We found that EIIANtrH66E interfered with the SpoT hydrolase activity, in contrast to EIIANtrH66A and EIIANtr that had only a slight effect on the activity of the enzyme (Figure 4B). It is noteworthy that WT EIIANtr has a residual inhibition activity of SpoT at high concentration (20 μM). This dose-dependent effect was not observed with EIIANtrH66E (Figure 4B), suggesting that a certain amount of the EIIANtr population is phosphorylated in E. coli and remains stable after purification. Indeed, it has been shown that E. coli EIIANtr remains phosphorylated after purification (22,23) and Caulobacter EIIANtr can be phosphorylated in vivo in E. coli (15). Together these data suggest that phosphorylation of EIIANtr is required to inhibit (p)ppGpp hydrolysis by SpoT. To challenge this hypothesis, we performed in vitro hydrolase assays with a reconstituted PTSNtr system (PEP, EINtr, HPr and EIIANtr). Note that EIIANtr can be phosphorylated on His66 in this condition only in the presence of PEP, EINtr and HPr (data not shown). We found that more than 40% of [32P]ppGpp was protected from hydrolysis by SpoT when incubated with all the PTSNtr members whereas the same mix but lacking EIIANtr did not inhibit SpoT hydrolase activity (Figure 4C). Likewise, incubating SpoT with repurified EIIANtr∼P once phosphorylated in vitro interfered with [32P]ppGpp degradation in comparison to unphosphorylated EIIANtr (Supplementary Figure S4a). Moreover, phosphorylation of EIIANtr increased its affinity for the ACT domain of SpoT (Supplementary Figure S4b). This not only supports that phosphorylation enhances in vivo binding of EIIANtr to the ACT domain (Figure 1B and Supplementary Figure S1a), but also explains why SpoT hydrolase activity was not inhibited by non-phosphorylated EIIANtr. Altogether, these data suggest that EIIANtr∼P interacts with the ACT domain to inhibit hydrolase activity of SpoT (Figure 4D).
PTSNtr regulates (p)ppGpp accumulation in Sinorhizobium meliloti
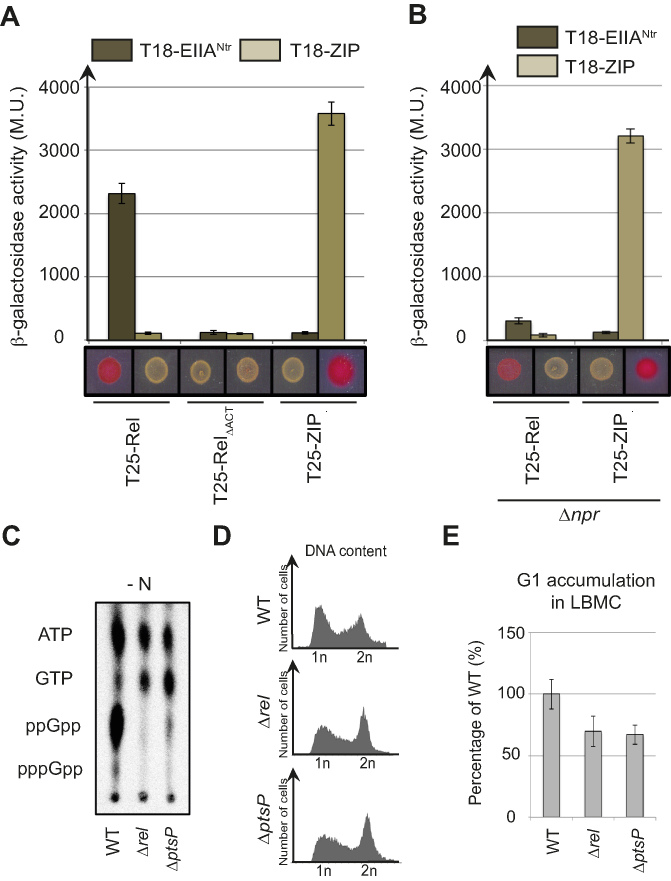
The inhibition of EINtr autophosphorylation by glutamine was first observed in the γ-proteobacterium E. coli (24) and the plant-associated α-proteobacterium S. meliloti (25). In addition, S. meliloti also accumulates (p)ppGpp in response to nitrogen starvation (16,17). This suggests that PTSNtr might also stimulate (p)ppGpp accumulation in S. meliloti in response to glutamine deprivation as shown for C. crescentus (15). To test this hypothesis, we checked whether the nitrogen-related EIIA component of S. meliloti (EIIANtr) was able to interact with the bifunctional (SD/HD) RSH of S. meliloti (Rel) in a BTH assay. As shown in Figure 5A, the full-length Rel fused to T25 (T25-Rel) interacted with EIIANtr fused to T18 (T18-EIIANtr). Similarly to C. crescentus, the deletion of ACT (T25-RelΔACT) abolished this interaction (Figure 5A). Another conserved feature is that phosphorylation of EIIANtr enhanced interaction with Rel. Indeed, we previously showed that (i) EIIANtr was phosphorylated in the BTH assay by the endogenous PTSNtr system of E. coli (EINtr and NPr) and (ii) the interaction between SpoT and EIIANtr was altered in an E. coli Δnpr background (15). Likewise, the interaction between S. meliloti T18-EIIANtr and T25-Rel was strongly diminished in a Δnpr background (Figure 5B), indicating that phosphorylation of EIIANtr also enhances the interaction with Rel.
Figure 5.
PTSNtr modulates (p)ppGpp accumulation and cell cycle progression in S. meliloti. (A and B) EIIANtr interacts with Rel in an ACT-dependent way. β-galactosidase assays were performed on (A) MG1655 cyaA::frt (RH785) or (B) MG1655 cyaA::frt Δnpr (RH2122) strains coexpressing T18- fused to ptsN or ZIP with T25- fused to rel, relΔACT or ZIP. Error bars = SD, n = 3. The same strains were spotted on MacConkey Agar Base plates supplemented with 1% maltose. Plates were incubated for 1 day at 30°C. The red colour indicates positive interactions. (C–E) A functional PTSNtr is required for (p)ppGpp accumulation in S. meliloti upon nitrogen starvation (-N) and for cell cycle progression in nitrogen-replete (+N) condition. (C) The intracellular levels of (p)ppGpp were evaluated by TLC after nucleotides extraction from WT (RH2000), Δrel (RH2327) and ΔptsP (RH2326) strains grown for 6 h without nitrogen source (-N). (D-E) DNA content (D) and G1 proportion (E) were measured in the same strains grown in complex media (LBMC). G1 proportions were normalized to the WT (100%). Error bars = SD, n = 3.
We constructed single in-frame deletion of ptsP (SMc02437) and rel (SMc02659) genes to test the PTSNtr-dependent accumulation of (p)ppGpp in S. meliloti in response to nitrogen deprivation. Upon nitrogen starvation (-N), neither Δrel nor ΔptsP cells accumulated (p)ppGpp in contrast to wild-type cells (Figure 5C). The increase of G1 proportion that results from the accumulation of (p)ppGpp upon nutrient limitation was previously used to synchronize S. meliloti (26). Thus, if PTSNtr regulates (p)ppGpp levels, we reasoned that the G1 proportion of Δrel and ΔptsP populations should be reduced. This is exactly what we observed, with a proportion of G1 cells in the ΔptsP and Δrel strains reduced in comparison to the wild-type strain (Figure 5D and E). In contrast, the ectopic production of (p)ppGpp in unstarved S. meliloti cells with an IPTG-inducible version of RelAEc strongly increased the proportion of G1 cells and led to growth arrest (Supplementary Figure S5). Altogether, these results support a conserved role of PTSNtr in regulating (p)ppGpp accumulation in response to nitrogen starvation as well as in the control of cell cycle progression in S. meliloti.
DISCUSSION
Two decades ago, mutational analysis of the spoT gene of E. coli suggested that the C-terminal end of SpoT including the ACT domain was an anchor point for regulators of the hydrolase activity of SpoT (27). Later, Mechold et al. reported that deleting the CTD of SpoT in Streptococcus equisimilis led to a strong inhibition of (p)ppGpp hydrolysis in vitro of ∼150-fold in comparison to the full-length enzyme (28). In agreement with these studies, our work shows that the ACT domain modulates SpoT hydrolase activity in C. crescentus. In a nitrogen-rich environment (+N), the ACT domain stimulates hydrolase activity, thereby limiting (p)ppGpp concentration. Upon nitrogen starvation (-N), the last component of the nitrogen-related PTS (PTSNtr), EIIANtr, is phosphorylated and binds to the ACT domain of SpoT. This interaction likely interferes with the ACT-dependent stimulation of SpoT hydrolase activity, thereby inhibiting (p)ppGpp hydrolysis (Figure 6). The recent RelA structures on stalled ribosomes highlighted the role of CTD in sensing nutrient availability (5–7). When bound to the ribosome, RelA adopts an extended conformation, which is thought to relieve the inhibitory effect of the CTD on its synthetase activity and enhances (p)ppGpp synthesis (29). This extended conformation seems to be favoured by specific interactions between the ribosome stalk, the A-site finger and the tRNAs with the CTD (7). Given the conserved domain architecture of the long RSH proteins, we propose that EIIANtr∼P modulates SpoT/Rel conformation to decrease its hydrolase activity (Figure 6). As ACT seems to interact with itself, it is tempting to speculate that dimerization of ACT induces a conformation that enhances the hydrolase activity. Oligomerization of long RSH has already been proposed to regulate their activity (29,30). For instance, mutations in the bifunctional Rel of Mycobacterium tuberculosis that lead to monomerization of Rel were shown to increase synthetase activity (30). Nevertheless, the SpoTΔACT variant can still homo-dimerize in a BTH assay (Figure 1 and Supplementary Figure S1), thereby ruling out the hypothesis that inactivation of hydrolase activity is simply due to monomerization of SpoT/Rel. Indeed, our data show that SpoT harbours at least two multimerization domains, (i) ACT at the C-terminal end and (ii) another one located in the N-terminal catalytic domains (Figure 1 and our unpublished data). Note that the N-terminal extremity of M. tuberculosis Rel harbouring the catalytic domains was already shown to homo-dimerize (31). In addition, the ACT alone can interact with full length SpoT but not with SpoTΔACT (Figure 1 and Supplementary Figure S1), showing that the ACT domain unlikely interferes directly with HD. Thus, the multimerization of C-terminal ACT might rather induce a conformational change of the catalytic domains located at the N-terminal extremity, favouring the hydrolase over the synthetase activity. In such a scenario, EIIANtr∼P might compete with ACT multimerization to preclude or interfere with an active conformational state favourable for (p)ppGpp hydrolysis. Whatever the mechanism(s) used, the role of the ACT domain in sustaining the hydrolase activity of long RSH enzymes might be conserved, since more than 88% of them harbour a C-terminal ACT domain (InterPro IPR002912).
Figure 6.
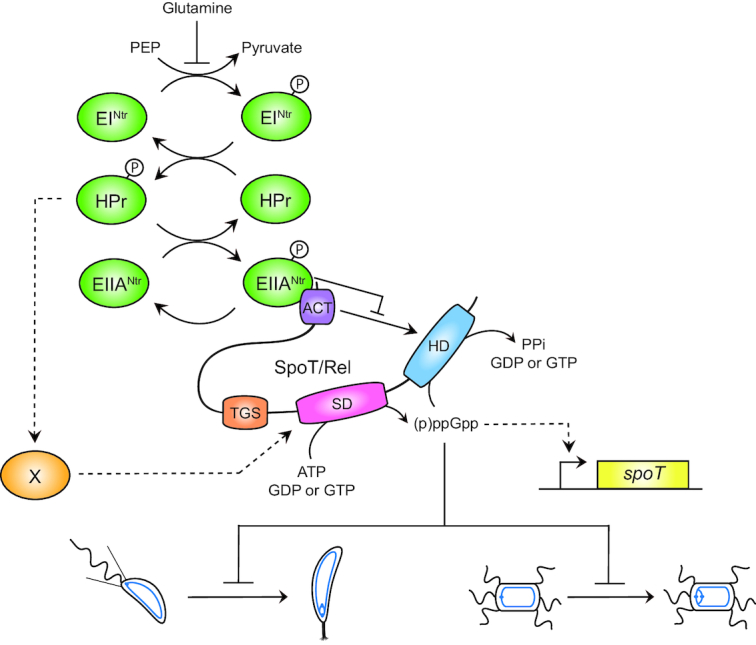
EIIANtr∼P binds the ACT domain of SpoT and inhibits its hydrolase activity. Upon nitrogen starvation (i.e. glutamine deprivation), EINtr phosphorylates its downstream components, HPr and EIIANtr. As a consequence, EIIANtr∼P interacts with ACT to likely inhibit its stimulating effect on the hydrolase activity of SpoT. This inhibition avoids to degrade (p)ppGpp produced by the SD, this latter being strongly stimulated by HPr∼P by an unknown mechanism. In addition, increasing the (p)ppGpp level promotes the accumulation of the SpoT protein by a positive feedback loop mechanism on transcription of the spoT gene. Ultimately, the burst of (p)ppGpp delays the G1-to-S transition of C. crescentus (left) and S. meliloti (right) cells.
The ACT domains have been shown to bind small molecules—mostly amino acids—and to regulate activity of the associated enzymatic domain (32). Recently, branched-chain amino acids (BCAAs)—valine and isoleucine—were found to bind the ACT domain of Rhodobacter capsulatus Rel (RelRc) and to stimulate (p)ppGpp hydrolysis (33). However, even though R. capsulatus is also an α-proteobacterium, BCAAs do not bind Caulobacter SpoT protein (33). Interestingly, we found that the interaction between Rel and the phosphorylated form of EIIANtr also occurs in an α-proteobacterium very close to R. capsulatus, Rhodobacter sphaeroides (Supplementary Figure S6). This suggests that the Rhodobacter genus could use BCAAs and PTSNtr to regulate (p)ppGpp hydrolysis in response to different metabolic cues. The ACT of E. coli RelA was also shown to be in close contact with the A-site finger of 23S ribosomal RNA (rRNA) (5,6). Although we cannot exclude the possibility that ACT of C. crescentus SpoT also binds other small molecules than BCAAs and/or rRNA, we show here that the ACT domain interacts with the regulatory protein (EIIANtr) to modulate its hydrolase activity. As the phosphorylation of EIIANtr that favoured interaction with ACT is inversely correlated with the glutamine concentration, this provides an elegant mechanism to tightly coordinate nutrient availability with the intracellular concentration of (p)ppGpp (15).
We also reported that SpoTΔACT is still able to increase (p)ppGpp concentration upon nitrogen starvation—although to a lesser extent in comparison to SpoT—by stimulating its synthetase activity in a PTSNtr-dependent way (Supplementary Figure S2d). Although the ACT domain might also be required for an optimal synthetase activity, the TGS domain seems be more important for synthetase activity. Indeed, the deletion of the CTD harbouring the TGS and the ACT domains completely abolished the ability of C. crescentus SpoT to produce (p)ppGpp upon nitrogen starvation (10). As we showed that PTSNtr is required for synthesizing (p)ppGpp in Caulobacter cells starved for nitrogen (15) (Figure 6), it will be interesting to test in vitro if the PTSNtr also modulates the synthetase activity of SpoT/Rel and if TGS and/or ACT domains are required for this regulation.
The hydrolase activity of E. coli SpoT was recently shown to be activated by the anti-σ70 factor (Rsd) via a direct interaction with the TGS domain (34). Intriguingly, this Rsd-dependent control of (p)ppGpp hydrolysis is also regulated by the PTS. Indeed, unphosphorylated HPr was previously shown to interact with Rsd in order to sequester it and prevent activation of SpoT hydrolase activity (34,35). Since HPr phosphorylation is determined by carbon source availability in E. coli, the authors suggested that Rsd could balance (p)ppGpp levels during a carbon source downshift (34). All these examples together with our work illustrate the importance of the CTD in the regulation of catalytic activities (hydrolase and synthetase) of SpoT/Rel enzymes.
Besides C. crescentus, another α-proteobacterium, S. meliloti, uses PTSNtr to sense nitrogen starvation and to stimulate (p)ppGpp accumulation (Figure 6). We found that EIIANtr interacts with Rel (Figure 5A) and that phosphorylation of EIIANtr promotes this interaction (Figure 5B). As glutamine also inhibits phosphorylation of EINtr in S. meliloti (25), glutamine deprivation likely leads to hyperphosphorylation of downstream PTSNtr components, favouring subsequent interaction between EIIANtr∼P and Rel. In support of that, we showed S. meliloti required EINtr protein to accumulate (p)ppGpp upon nitrogen starvation (Figure 5C), as previously shown for C. crescentus (15). Altogether, our data support that the PTSNtr-dependent control of Rel activities is another conserved feature in α-proteobacteria (36). But beyond α-proteobacteria, other bacterial phyla also use PTS proteins to determine (p)ppGpp levels accordingly to the metabolic status. As already mentioned, HPr can indirectly modulate the hydrolase activity of SpoT in the α-proteobacterium E. coli (34) whereas the unphosphorylated EIIANtr protein directly interacts with a RSH enzyme in the β-proteobacterium Ralstonia eutropha (37).
Similarly to C. crescentus (14,15), we found that (p)ppGpp accumulation also delays the G1-to-S transition in S. meliloti, (Figure 5D and E; Supplementary Figure S5). In fact, this feature has been used to synchronize a population of S. meliloti by transiently blocking bacteria starved for carbon and nitrogen in G1 phase of the cell cycle (26 ). The alarmone was previously shown to be critical for S. meliloti to establish a symbiotic relationship with its host plant (17). Indeed, (p)ppGpp strongly influences transcription of hundreds of genes, thereby affecting multiple aspects of symbiosis such as nodulation or succinoglycan production (16). Interestingly, secondary mutations in RNA polymerase suppressed all the defects of a (p)ppGpp0 strain, demonstrating that transcriptional control by the alarmone is critical for symbiosis (17). However, the (p)ppGpp-dependent control of the cell cycle might also be critical for symbiosis. Indeed, it has been shown that the intracellular pathogen Brucella abortus uses specific cell cycle stage to promote invasion of macrophages, with G1 cells being the predominant infectious bacteria (38). Therefore, asymmetric cell division might have been conserved in α-proteobacteria (36) for the ability of the invasive newly divided G1 cells to efficiently colonize new environments, and (p)ppGpp might systematically control this adaptive feature. The specific interference of (p)ppGpp with the G1-to-S transition of the cell cycle could thus be another conserved feature in α-proteobacteria.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Sean Crosson, Emanuele Biondi and Gabriele Klug for providing strains and/or plasmids. We thank the members of the BCcD team for critical reading of the manuscript and helpful discussions; Guy Houbeau at the Animal Care Facility of the University of Namur for immunizing rabbits with purified SpoT.
Author contributions: S.R. and R.H. conceived and designed the experiments. S.R. performed all the experiments except otherwise stated. J.C-M. purified the proteins for the biochemical assays and performed the in vitro hydrolase assays, the phosphorylation of EIIANtr and ITC assays (Figure 4 and Supplementary Figure S4). J.C. did the experiments for the reviewing and A.M. did the cloning and preliminary tests for proteins purification and in vitro phosphorylation assays. S.R., J.C-M., A.G-P. and R.H. analysed the data. S.R. and R.H. wrote the paper.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Fonds de la Recherche Scientifique - FNRS (F.R.S.-FNRS) [CDR J.0169.16 to R.H.]; [EQP U.N043.17F; WELBIO CR-2017S-03; PDR T.0066.18 to A.G-P.]; Programme ‘Actions de Recherche Concertée’ 2016–2021 from the ULB and the Fonds d’Encouragement à la Recherche ULB (FER-ULB to A.G-P.); FRIA (Fund for Research Training in Industry and Agriculture) fellowships from the F.R.S.-FNRS to S.R. and J.C-M. R.H. is a Research Associate of the F.R.S.-FNRS. Funding for open access charge: University of Namur.
Conflict of interest statement. None declared.
REFERENCES
- 1. Hallez R., Delaby M., Sanselicio S., Viollier P.H.. Hit the right spots: cell cycle control by phosphorylated guanosines in alphaproteobacteria. Nat. Rev. Microbiol. 2017; 15:137–148. [DOI] [PubMed] [Google Scholar]
- 2. Hauryliuk V., Atkinson G.C., Murakami K.S., Tenson T., Gerdes K.. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 2015; 13:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Potrykus K., Cashel M.. (p)ppGpp: still magical. Annu. Rev. Microbiol. 2008; 62:35–51. [DOI] [PubMed] [Google Scholar]
- 4. Haseltine W.A., Block R.. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc. Natl. Acad. Sci. U.S.A. 1973; 70:1564–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arenz S., Abdelshahid M., Sohmen D., Payoe R., Starosta A.L., Berninghausen O., Hauryliuk V., Beckmann R., Wilson D.N.. The stringent factor RelA adopts an open conformation on the ribosome to stimulate ppGpp synthesis. Nucleic Acids Res. 2016; 44:6471–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown A., Fernandez I.S., Gordiyenko Y., Ramakrishnan V.. Ribosome-dependent activation of stringent control. Nature. 2016; 534:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loveland A.B., Bah E., Madireddy R., Zhang Y., Brilot A.F., Grigorieff N., Korostelev A.A.. Ribosome*RelA structures reveal the mechanism of stringent response activation. Elife. 2016; 5:e17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Battesti A., Bouveret E.. Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol. Microbiol. 2006; 62:1048–1063. [DOI] [PubMed] [Google Scholar]
- 9. Atkinson G.C., Tenson T., Hauryliuk V.. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One. 2011; 6:e23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boutte C.C., Crosson S.. The complex logic of stringent response regulation in Caulobacter crescentus: starvation signalling in an oligotrophic environment. Mol. Microbiol. 2011; 80:695–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lesley J.A., Shapiro L.. SpoT regulates DnaA stability and initiation of DNA replication in carbon-starved Caulobacter crescentus. J. Bacteriol. 2008; 190:6867–6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Curtis P.D., Brun Y.V.. Getting in the loop: regulation of development in Caulobacter crescentus. Microbiol. Mol. Biol. Rev. 2010; 74:13–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiaverotti T.A., Parker G., Gallant J., Agabian N.. Conditions that trigger guanosine tetraphosphate accumulation in Caulobacter crescentus. J. Bacteriol. 1981; 145:1463–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonzalez D., Collier J.. Effects of (p)ppGpp on the progression of the cell cycle of Caulobacter crescentus. J. Bacteriol. 2014; 196:2514–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ronneau S., Petit K., De Bolle X., Hallez R.. Phosphotransferase-dependent accumulation of (p)ppGpp in response to glutamine deprivation in Caulobacter crescentus. Nat. Commun. 2016; 7:11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krol E., Becker A.. ppGpp in Sinorhizobium meliloti: biosynthesis in response to sudden nutritional downshifts and modulation of the transcriptome. Mol. Microbiol. 2011; 81:1233–1254. [DOI] [PubMed] [Google Scholar]
- 17. Wells D.H., Long S.R.. The Sinorhizobium meliloti stringent response affects multiple aspects of symbiosis. Mol. Microbiol. 2002; 43:1115–1127. [DOI] [PubMed] [Google Scholar]
- 18. Casadaban M.J., Cohen S.N.. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 1980; 138:179–207. [DOI] [PubMed] [Google Scholar]
- 19. Mendrygal K.E., Gonzalez J.E.. Environmental regulation of exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 2000; 182:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beaufay F., Coppine J., Mayard A., Laloux G., De Bolle X., Hallez R.. A NAD-dependent glutamate dehydrogenase coordinates metabolism with cell division in Caulobacter crescentus. EMBO J. 2015; 34:1786–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boutte C.C., Henry J.T., Crosson S.. ppGpp and polyphosphate modulate cell cycle progression in Caulobacter crescentus. J. Bacteriol. 2012; 194:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bahr T., Luttmann D., Marz W., Rak B., Gorke B.. Insight into bacterial phosphotransferase system-mediated signaling by interspecies transplantation of a transcriptional regulator. J. Bacteriol. 2011; 193:2013–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang G., Peterkofsky A., Keifer P.A., Li X.. NMR characterization of the Escherichia coli nitrogen regulatory protein IIANtr in solution and interaction with its partner protein, NPr. Protein Sci. 2005; 14:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee C.R., Park Y.H., Kim M., Kim Y.R., Park S., Peterkofsky A., Seok Y.J.. Reciprocal regulation of the autophosphorylation of enzyme INtr by glutamine and alpha-ketoglutarate in Escherichia coli. Mol. Microbiol. 2013; 88:473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goodwin R.A., Gage D.J.. Biochemical characterization of a nitrogen-type phosphotransferase system reveals that enzyme EI(Ntr) integrates carbon and nitrogen signaling in Sinorhizobium meliloti. J. Bacteriol. 2014; 196:1901–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Nisco N.J., Abo R.P., Wu C.M., Penterman J., Walker G.C.. Global analysis of cell cycle gene expression of the legume symbiont Sinorhizobium meliloti. Proc. Natl. Acad. Sci. U.S.A. 2014; 111:3217–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gentry D.R., Cashel M.. Mutational analysis of the Escherichia coli spoT gene identifies distinct but overlapping regions involved in ppGpp synthesis and degradation. Mol. Microbiol. 1996; 19:1373–1384. [DOI] [PubMed] [Google Scholar]
- 28. Mechold U., Murphy H., Brown L., Cashel M.. Intramolecular regulation of the opposing (p)ppGpp catalytic activities of Rel(Seq), the Rel/Spo enzyme from Streptococcus equisimilis. J. Bacteriol. 2002; 184:2878–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gropp M., Strausz Y., Gross M., Glaser G.. Regulation of Escherichia coli RelA requires oligomerization of the C-terminal domain. J. Bacteriol. 2001; 183:570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jain V., Saleem-Batcha R., China A., Chatterji D.. Molecular dissection of the mycobacterial stringent response protein Rel. Protein Sci. 2006; 15:1449–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singal B., Balakrishna A.M., Nartey W., Manimekalai M.S.S., Jeyakanthan J., Gruber G.. Crystallographic and solution structure of the N-terminal domain of the Rel protein from Mycobacterium tuberculosis. FEBS Lett. 2017; 591:2323–2337. [DOI] [PubMed] [Google Scholar]
- 32. Grant G.A. The ACT domain: a small molecule binding domain and its role as a common regulatory element. J. Biol. Chem. 2006; 281:33825–33829. [DOI] [PubMed] [Google Scholar]
- 33. Fang M., Bauer C.E.. Regulation of stringent factor by branched-chain amino acids. Proc. Natl. Acad. Sci. U.S.A. 2018; 115:6446–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee J.W., Park Y.H., Seok Y.J.. Rsd balances (p)ppGpp level by stimulating the hydrolase activity of SpoT during carbon source downshift in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2018; 115:E6845–E6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park Y.H., Lee C.R., Choe M., Seok Y.J.. HPr antagonizes the anti-sigma70 activity of Rsd in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:21142–21147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hallez R., Bellefontaine A.F., Letesson J.J., De Bolle X.. Morphological and functional asymmetry in alpha-proteobacteria. Trends Microbiol. 2004; 12:361–365. [DOI] [PubMed] [Google Scholar]
- 37. Karstens K., Zschiedrich C.P., Bowien B., Stulke J., Gorke B.. Phosphotransferase protein EIIANtr interacts with SpoT, a key enzyme of the stringent response, in Ralstonia eutropha H16. Microbiology. 2014; 160:711–722. [DOI] [PubMed] [Google Scholar]
- 38. Deghelt M., Mullier C., Sternon J.F., Francis N., Laloux G., Dotreppe D., Van der Henst C., Jacobs-Wagner C., Letesson J.J., De Bolle X.. G1-arrested newborn cells are the predominant infectious form of the pathogen Brucella abortus. Nat. Commun. 2014; 5:4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.