Abstract
A broadly general, three-component reaction strategy for the construction of compounds containing multiple heterocycles is described. Thermal benzyne formation (by the hexadehydro-Diels–Alder (HDDA) reaction) in the presence of tertiary cyclic amines and a protic nucleophile (HNu) gives, via ring-opening of intermediate ammonium ion/Nu– ion pairs, heterocyclic products. Many reactions are efficient even when the stoichiometric loading of the three reactants approaches unity. Use of HOSO2CF3 as the HNu gives ammonium triflate intermediates, which can then be ring opened by an even wider variety of nucleophiles.
Graphical Abstract
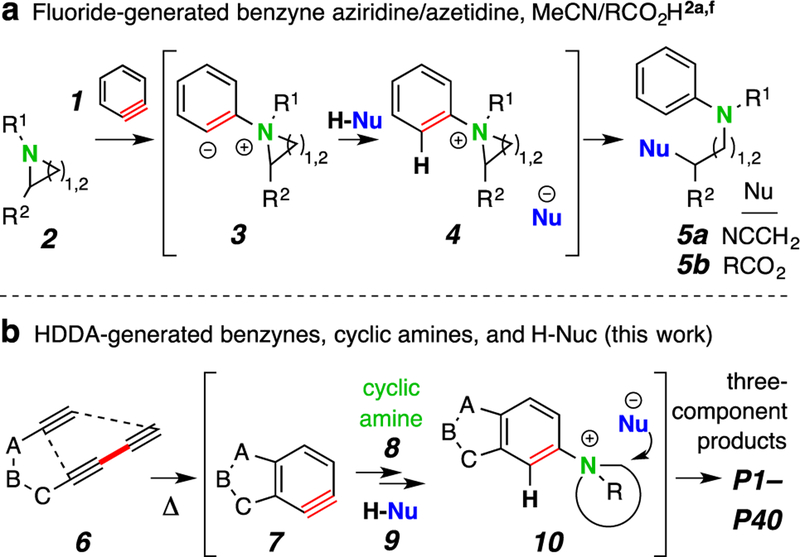
The rapid construction of complex products from simple starting materials has driven the development of numerous synthetic strategies. These include a wide variety of multicomponent reactions that allow for the modular generation of structural complexity and diversity.1 One example is initiated by the nucleophilic addition of tertiary amines to benzyne (1, Scheme 1a).2 This is thought to proceed by initial 1,3-zwitterion formation (cf. 3),3 which then engages either a carbonyl compound2c-e or a proton source (cf. 4).2a,b,f Cyclic amines, when strained [cf. aziridines and azetidines 2], lead to adducts 5 in the presence of acetonitrile2a or carboxylic acids2f in a process involving nucleophilic opening of the ion pair 4.
Scheme 1.
Three-component Reactions of Benzynes, Tertiary cyclic Amines, and Protic Nucleophiles (H-Nu)
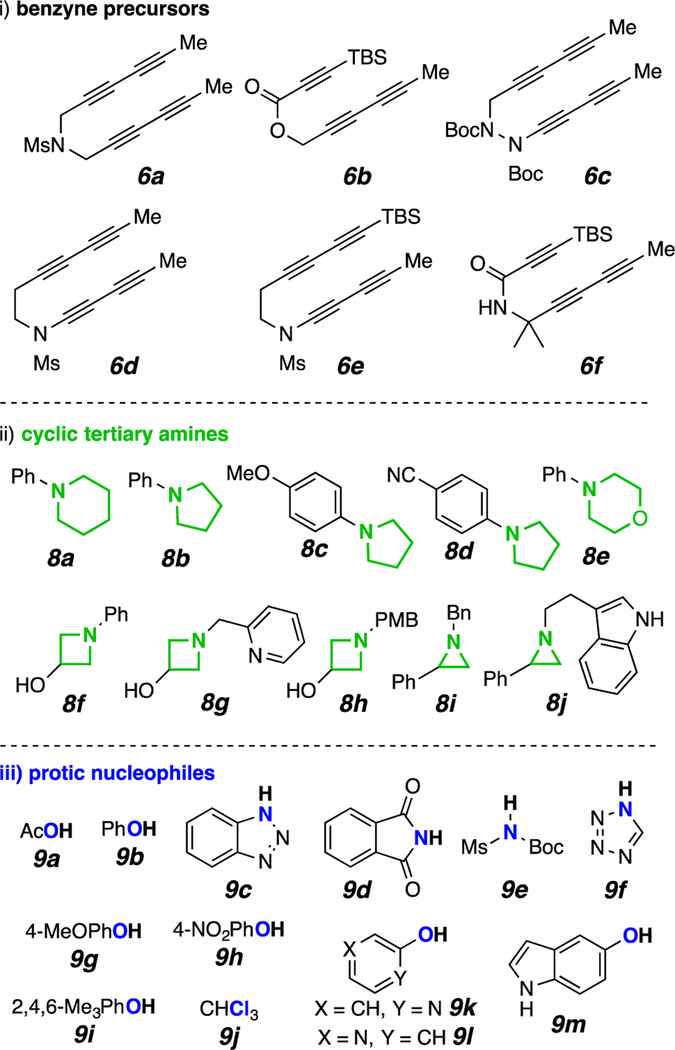
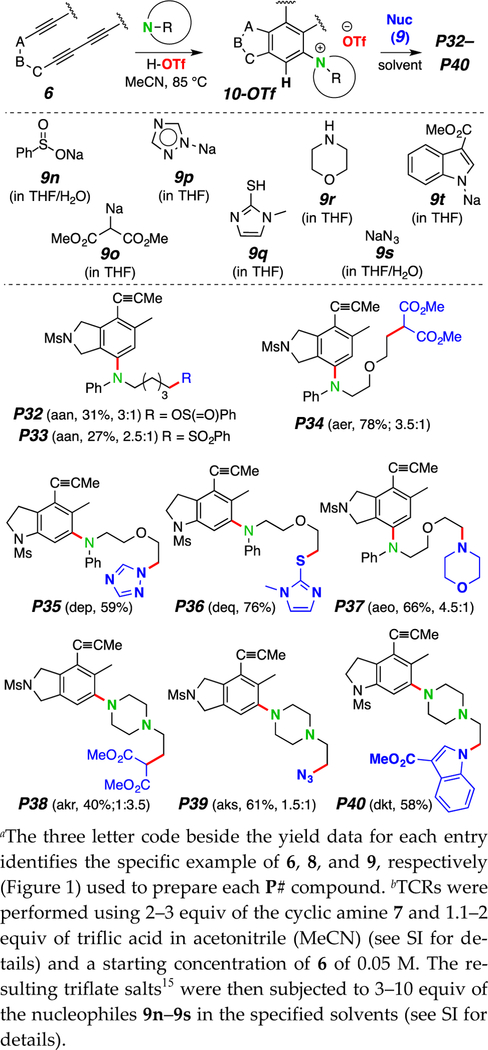
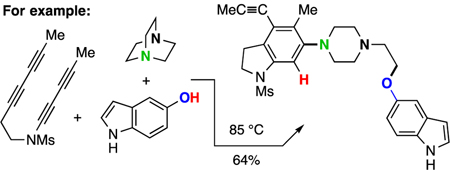
The thermal cycloisomerization of tethered tri- and tetra-ynes4 leads to benzynes under neutral conditions in a process we have termed the hexadehydro-Diels–Alder (HDDA) reaction.5 These can then participate in myriad novel trapping processes, including three-component reactions (TCRs) initiated by cyclic sulfide addition6 or alkaloidal natural products.7 We now report that a diverse array of multiheterocyclic products can be efficiently assembled in a single, thermally driven operation. Substrate 6 (Scheme 1b) cyclizes in a rate-limiting event to the benzyne 7 that, in the presence of a cyclic amine 8 and proton source 9, gives the ion pair 10, which ring-opens to the three-component product P#. There is considerable breadth in each of the three classes of reactants that readily participate: the benzyne precursors [6a-f, Figure 1, panel i)], the cyclic tertiary amines [8a-j, Figure 1, panel ii), non-bicyclic; and 8k-n, Figure 3, panel i), bicyclic], and the protic nucleophiles (9a-m, H–Nu, Figure 1, panel iii). We note that in the absence of a tertiary amine, most of the protic nucleophiles 9 are capable of engaging HDDA benzynes, indicating that the reaction rate of addition of the cyclic amine 8 is considerably faster than that of 9.
Figure 1.
The three classes of reactants [i)-iii)] used to prepare the TCR products P# in Figures 2–4.
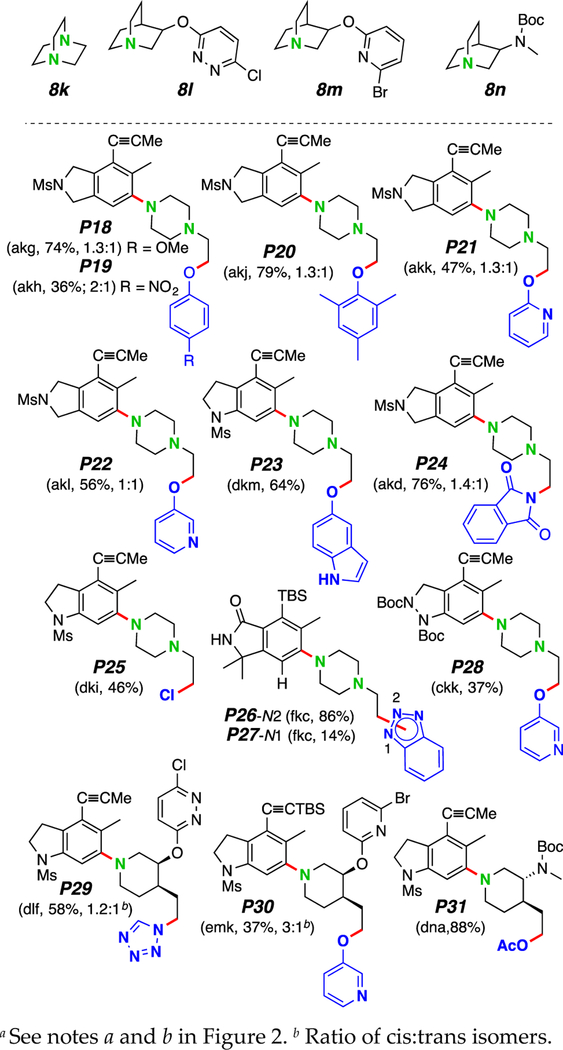
Figure 3.
Three-component reactions of HDDA-generated benzynes with bicyclic amines and protic nucleophilesa.
We did not undertake an exhaustive survey of every possible combination, but rather chose to explore a representative subset to display the versatility of this strategy. The tri- or tetrayne precursors 6 cyclize to produce the benzynes 7 with convenient half-lives of reaction of a few hours at temperatures ranging from 80–130 °C. One limitation is that triynes containing an ynoate or an ynone subunit gave variable results; presumably because these electron deficient alkynes reacted prematurely with some of the more nucleophilic tertiary amines.8 This could be mitigated by using ynoates and ynamides containing a bulky TBS group on the terminus of the conjugated alkyne (cf. 6b and 6f).
We describe here three categories of TCR. These differ in the use of: monocyclic tertiary amines (including N-arylpyrrolidines and -piperidines, Figure 2), bicyclic amines with a bridgehead nitrogen atom (Figure 3), and arylammonium triflates (10, Nu– = TfO–).
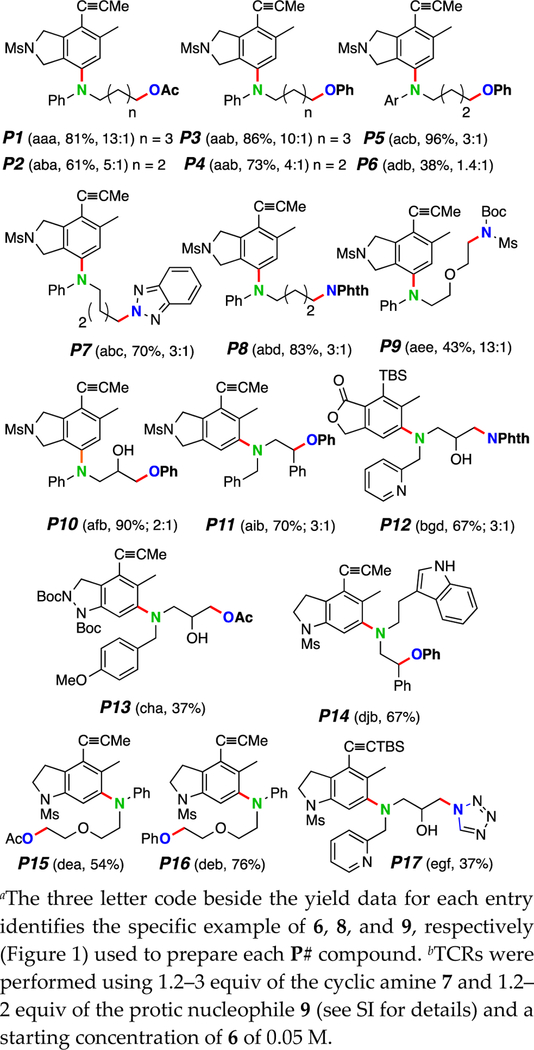
Figure 2.
Three-component reactions of HDDA-generated benzynes with monocyclic tertiary amines and protic nucleophilesa,b.
We have elected to identify the structures of each product of a TCR in this manuscript by a preceding “P” followed by a unique number, in sequence throughout. Thus, P1-P17 are the products in Figure 2. The three-letter code in parentheses indicates the first (poly-yne 6), second (amine 8), and third (nucleophile 9) components used to prepare each product. Each benzyne intermediate in this study was unsymmetrical; as such, each could react in two different ways with the nucleophilic amine. The major constitutional isomer of the product is shown (see supporting information (SI) for characterization of both isomers); the regioselectivity of the reaction is given in parentheses as the ratio of major to minor products. For example, P5 (acb, 96%, 3:1) arose from the TCR of 6a, 8c, and 9b to give a 3:1 ratio of isomers in 96% yield (of chromatographically purified material).
These TCRs of N-alkyl cyclic amines are limited to strained ring compounds since dealkylation of the exocyclic alkyl group is a seriously competitive process (see SI). In contrast, N-arylated piperidines, pyrrolidines, and morpholine (8a–8e) undergo efficient ring-opening to give the three-component adducts P1–P9, P15, and P16. Electron-deficient aniline derivatives were not as effective in capturing the benzyne (e.g., cf. yields of P6 vs. P5). N-Alkylazetidines and -aziridines (8g–8j) are competent TCR partners. Products P7–P9, P12, and P17 demonstrate successful trapping by a variety of nitrogenous nucleophiles; all other products in Figure 2 arose from the use of oxygen-based nucleophiles.
The benzynes from 6a and 6b often proceed with low levels of regioselectivity.7,9 In contrast the benzynes from 6c–6f give only a single regioisomer (P13–P17), a reflection of both predistortion of the benzyne ring10 and the added steric congestion adjacent to one of the two benzyne carbons. Various nitrogen-containing heterocycles are compatible with the process, including as a preexisting substituent on cyclic amine (cf. P12, P14, and P17) or as the protic nucleophile (cf. P7, P8, P12, and P17).
We next turned our attention to the use of the bicyclic amines 8k–8n (Figure 3), each containing a bridgehead nitrogen atom. These gave rise to aryl piperazines11 P18–P28 [each from DABCO (8k)] and aryl piperidines12 P29–P31, motifs often viewed as privileged structures in drug discovery efforts.13 As can easily be discerned from the structures in Figure 3, a considerably diverse array of multiheterocyclic products can be efficiently generated by this strategy. The stoichiometric ratio among the three substrates 6:8:9 (ca. 1:≤1.5:≤1.5) is particularly noteworthy, suggesting that any or all of the three participants could be of considerable structural complexity. Note that: i) many different classes of nitrogen-containing heterocycles are compatible with the process; ii) chloride can be introduced by performing the reaction in chloroform solution and in the absence of any additional proton source (P25; we have observed a similar chloride ion transfer from, presumably, Cl3C– in a recently reported mechanistic study3) iii) use of the quinuclidinol derivatives 8l–m allows for facile introduction of additional heteroatoms into the products (cf. P29–P31).
All of the single-step TCRs described in Figures 2 and 3 meet the following criteria: i) neither the amine or the protic nucleophile should react with the HDDA precursor faster than its rate of cyclization; ii) the tertiary amine should add to the benzyne faster than does the protic nucleophile; iii) the protic nucleophile (H-Nu) should be acidic enough to protonate the intermediate 1,3-zwitterion;14 and iv) the conjugate base of H-Nu should be sufficiently nucleophilic to ring open the aryl ammonium intermediate (cf. 10, Scheme 1).
We hypothesized that several of these limitations would be circumvented through a two-step process involving the formation and subsequent nucleophilic ring-opening of stoichiometric ammonium triflates 10-OTf (Figure 4).15 Indeed, if a benzyne 7 is produced from 6 in a solution containing both free tertiary amine and its ammonium triflate, the triflate salts 10-OTf are produced in stoichiometric fashion.16 For the examples shown in Figure 4, N-phenylpiperidine, N-phenylmorpholine, or DABCO was the amine component. The triflate was then exposed to a nucleophile to effect a 2-step, 3-component coupling. The examples in Figure 4 use one of 9n–9t as the nucleophile, but these by no means constitute a comprehensive set: i) sodium benzenesulfinate (9n) produced a mixture of sulfone P32 and sulfenic ester P33; ii) sodium amide salts 9p and 9t gave rise to the triazole P35 and indole P40, respectively; iii) a heterocyclic thiol (9q) led to the sulfide P36; iv) dimethyl sodiomalonate (9o) gave esters P34 and P38; v) morpholine (9r) produced the tertiary amine P37; and vi) sodium azide (9s) gave the azides P39. Through this protocol the scope of compatible nucleophiles is significantly broadened.
Figure 4.
The use of ammonium triflate intermediates allows for incorporation of an even broader range of nucleophiles.
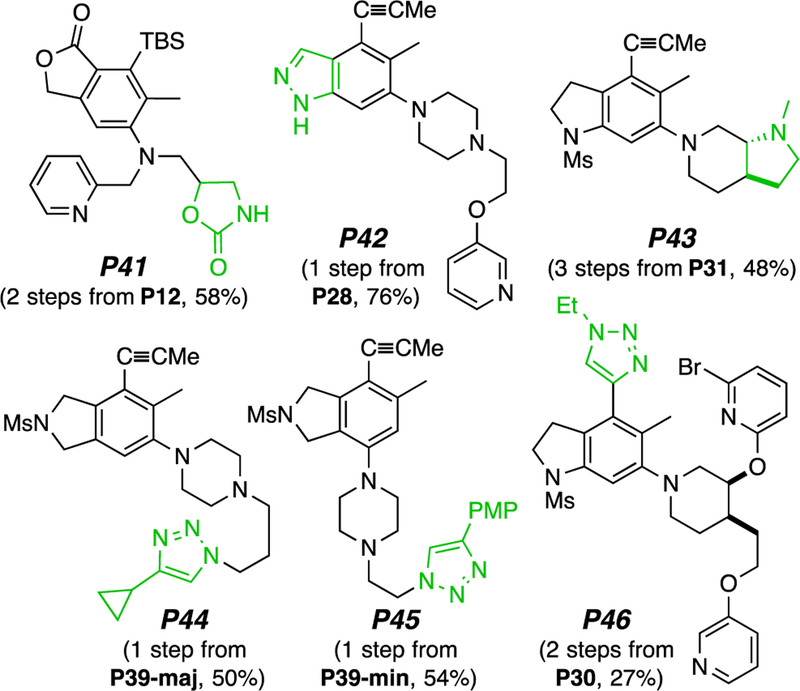
Nearly all of the types of products produced through the TCRs described here can, in principle, be further diversified to give a wide range of heterocycle-rich small molecules. We show examples in Figure 5. The newly introduced heterocycles (in green) include: oxazolidinone (P41), benzopyrazole (P42), pyrrolopiperidine (P43), and triazoles (P44–P46). Other similar transformations are easily imagined.
Figure 5.
Examples demonstrating diversification of some of the TCR products to produce multiheterocyclic adducts.
In conclusion, we have developed the three-component reaction of HDDA-generated benzynes, cyclic tertiary amines, and protic nucleophiles. Many amines productively engage the benzyne, including N-aryl and N-alkyl cyclic amines and bicyclic amines containing a bridgehead nitrogen atom (8a–n). Many protic nucleophiles are effective (9a–9s). The use of ammonium triflate intermediates increases the scope of compatible nucleophiles (Figure 4). Facile post-TCR modification allows the introduction of additional heterocycles (Figure 5). This new TCR strategy is quite general and has considerable potential for the rapid construction of multiheterocyclic compounds.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the U.S. Department of Health and Human Services, National Institute of General Medical Sciences (GM-65597). NMR spectral data were obtained with an instrument procured with a grant from the National Institutes of Health Shared Instrumentation Grant program (S10OD011952). We thank Mr. Juntian Zhang for performing the representative example of a one mmol-scale reaction (see SI).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Experimental procedures, characterization data, and copies of all 1H- and 13C-NMR spectra for all isolated compounds (single PDF). The Supporting Information is available free of charge on the ACS Publications website.
The authors have no competing financial interests.
REFERENCES
- 1.Multicomponent Reactions; Zhu J, Bienaymé H, Eds.; Wiley-VCH: Weinheim, FRG, 2005. [Google Scholar]
- 2.(a) Stephens D; Zhang Y; Cormier M; Chavez G; Arman H; Larionov OV Chem. Commun 2013, 49, 6558–6560. [DOI] [PubMed] [Google Scholar]; (b) Bhojgude SS; Baviskar DR; Gonnade RG; Biju AT Org. Lett 2015, 17, 6270–6273. [DOI] [PubMed] [Google Scholar]; (c) Roy T; Baviskar DR; Biju AT J. Org. Chem 2015, 80, 11131–11137. [DOI] [PubMed] [Google Scholar]; (d) Roy T; Thangaraj M; Gonnade RG; Biju AT Chem. Commun 2016, 52, 9044–9047. [DOI] [PubMed] [Google Scholar]; (e) Bhojgude SS; Roy T; Gonnade RG; Biju AT Org. Lett 2016, 18, 5424–5427. [DOI] [PubMed] [Google Scholar]; (f) Roy T; Bhojgude SS; Kaicharla T; Thangaraj M; Garai B; Biju AT Org. Chem. Front 2016, 3, 71–76. [Google Scholar]
- 3.Ross SP; Baire B; Hoye TR Org. Lett 2017, 19, 5705–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Bradley AZ; Johnson RP J. Am. Chem. Soc 1997, 119, 9917–9918. [Google Scholar]; (b) Miyawaki K; Suzuki R; Kawano T; Ueda I Tetrahedron Lett 1997, 38, 3943–3946. [Google Scholar]; (c) Tsui JA; Sterenberg BT Organometallics 2009, 28, 4906–4908. [Google Scholar]; (d) Yun SY; Wang K-P; Lee N-K; Mamidipalli P; Lee DJ Am. Chem. Soc 2013, 135, 4668–4671. [DOI] [PubMed] [Google Scholar]
- 5.(a) Hoye TR; Baire B; Niu D; Willoughby PH; Woods BP Nature 2012, 490, 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Baire B; Niu D; Willoughby PH; Woods BP; Hoye TR Nature Protocols 2013, 8, 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Diamond OJ; Marder TB Org. Chem. Front 2017, 4, 891–910. [Google Scholar]
- 6.Chen J; Palani V; Hoye TR J. Am. Chem. Soc 2016, 138, 4318–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross SP; Hoye TR Nature Chem 2017, 9, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Hong B-C; Shr Y-J; Wu J-L; Gupta AK; Lin K-J Org. Lett 2002, 4, 2249–2252. [DOI] [PubMed] [Google Scholar]; (b) Mizoguchi H; Watanabe R; Minami S; Oikawa H; Oguri H Org. Biomol. Chem 2015, 13, 5955–5963. [DOI] [PubMed] [Google Scholar]
- 9.Karmakar R; Yun SY; Wang K-P; Lee D Org. Lett 2014, 16, 6–9. [DOI] [PubMed] [Google Scholar]
- 10.(a) Garr AN; Luo D; Brown N; Cramer CJ; Buszek KR; VanderVelde D Org. Lett 2010, 12, 96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cheong PHY; Paton RS; Bronner SM; Im G-YJ; Garg NK; Houk KN J. Am. Chem. Soc 2010, 132, 1267–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H-J; Earley WG; Lewis RM; Srivastava RR; Zych AJ; Jenkins DM; Fairfax DJ Tetrahedron Lett 2007, 48, 3043–3046. [Google Scholar]
- 12.Axelsson O; Peters DJ Het. Chem 1997, 34, 461–463. [Google Scholar]
- 13.Welsch ME; Snyder SA; Stockwell BR Curr. Opin. Chem. Biol 2010, 14, 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.We carried out the following NMR experiment to verify that the newly incorporated, aromatic hydrogen atom in the benzenoid product originated from the protic nucleophile. Substrate 6a, amine 8a, and CH3COOD or CD3COOD were heated in benzene-d6. In each case product P1 was primarily (the solution at time zero showed evidence of a small amount of protium from H2O in the reactants and <100% labeling of the acetic acid) deuterated by integration of the (residual) aromatic proton resonance and reinforced by ESI-MS analysis.
- 15.In all cases the triflate salts were freed of solvent (CH3CN) prior to being used for the subsequent nucleophilic ring opening. To demonstrate additional parameters, we precipitated with ether and isolated by filtration the ammonium triflate that leads to P40. This solid material was spectroscopically characterized, although as an admixture with remaining DABCOH+•TfO–. This material was stored for over two years with no appreciable change in integrity.
- 16.(a) Cant AA; Bertrand GHV; Henderson JL; Roberts L; Greaney MF Angew. Chem. Int. Ed 2009, 48, 5199–5202. [DOI] [PubMed] [Google Scholar]; (b) Bhojgude SS; Kaicharla T; Biju AT Org. Lett 2013, 15, 5452–5455. [DOI] [PubMed] [Google Scholar]; (c) Hirsch M; Dhara S; Diesendruck CE Org. Lett 2016, 18, 980–983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.