Abstract
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder characterized by social communication deficits and the presence of restricted interests and repetitive behaviors. We have previously reported significant improvements in behavior, including increased social functioning, improved communication abilities, and decreased clinical symptoms in children with ASD, following treatment with a single infusion of autologous cord blood in a phase I open‐label trial. In the current study, we aimed to understand whether these improvements were associated with concurrent changes in brain structural connectivity. Twenty‐five 2‐ to 6‐year‐old children with ASD participated in this trial. Clinical outcome measures included the Vineland Adaptive Behavior Scales‐II Socialization Subscale, Expressive One‐Word Picture Vocabulary Test‐4, and the Clinical Global Impression‐Improvement Scale. Structural connectivity was measured at baseline and at 6 months in a subset of 19 children with 25‐direction diffusion tensor imaging and deterministic tractography. Behavioral improvements were associated with increased white matter connectivity in frontal, temporal, and subcortical regions (hippocampus and basal ganglia) that have been previously shown to show anatomical, connectivity, and functional abnormalities in ASD. The current results suggest that improvements in social communication skills and a reduction in symptoms in children with ASD following treatment with autologous cord blood infusion were associated with increased structural connectivity in brain networks supporting social, communication, and language abilities. stem cells translational medicine 2019;8:138&10
Keywords: Autism spectrum disorder, Autologous umbilical cord blood, White matter connectivity, Diffusion tensor imaging
Significance Statement.
In a phase I open‐label trial, improvements in social communication skills and a reduction in symptoms in children with autism spectrum disorder following treatment with autologous cord blood infusion were associated with increased connectivity in brain networks supporting social, communication, and language abilities.
Introduction
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder characterized by social communication deficits and the presence of restricted interests and repetitive behaviors 1. Current treatment approaches include behavioral interventions, occupational and speech therapies, and educational or vocational supports. Furthermore, medical treatments focus on ameliorating the associated comorbid symptoms, rather than improving core autism symptoms or influencing the underlying pathophysiology 2. As such, treatments that address the underlying pathophysiology of autism, and thus impact core symptoms, are greatly needed.
It is well established that individuals with ASD have different patterns of white matter development as compared to individuals without ASD. Specifically, studies following infant siblings at high risk for autism have provided evidence for altered global white matter structure by 6 months of age in those infants who later develop autism 3. This includes a differential trajectory of white matter development, characterized by an early pattern of increased fractional anisotropy (FA), a measure of white matter fiber tract microstructure, at 6 months of age followed by a slowing in white matter development. Other studies have shown decreased FA in older children and adults with autism 4, 5, 6. Importantly, this differential pattern of white matter development has been linked to autism symptom severity, both in the social‐communication domain 4 and in the repetitive behavior domain 7.
Although the exact mechanism underlying these neural changes remains unknown, a number of studies have linked altered immune responses during both the prenatal and the postnatal period to changes in brain development and increased risk for autism 8, 9, 10, 11, 12, 13. Prenatally, a number of studies have found a relationship between increased levels of maternal IgG autoantibodies with reactivity to fetal brain proteins and later autism diagnosis. The increased levels of maternal autoantibodies have been shown to alter neurogenesis in the developing brain 14, are associated with increased total cerebral volume in children with ASD 15, and have been linked to the severity of behavioral symptoms associated with ASD, such as increased irritability 16. Primate models of the maternal immune activation model have demonstrated an association between increased levels of maternal autoantibodies and both increased stereotypic behavior and decreased social interactions 17, 18.
Postnatally, circulating brain‐directed autoantibodies have also been found in individuals with ASD 19. In addition to increased levels of brain‐directed autoantibodies in autism, studies have demonstrated increased levels of neuroinflammation, as evidenced by higher numbers of activated astrocytes and microglia, in the brains of children with autism 13, 20. When these neuroimmune cells are activated, it results in the release of proinflammatory cytokines 20 These proinflammatory cytokines have been demonstrated to be increased in both individuals with autism and their mothers across a number of studies 8, 12, 13. Proinflammatory cytokines have been linked to a number of neuronal processes, including maintenance of long‐term potentiation, regulation of both neuron proliferation and death, and neurite outgrowth 12, 21. Higher levels of proinflammatory cytokines are associated with more stereotypic behaviors and increased difficulties with both social interactions and nonverbal communication in children with autism 21, 22, 23.
The mechanism by which neuroinflammation may lead to behavioral changes in individuals with autism remains unclear; however, there is evidence connecting neuroinflammation to changes in brain connectivity. Animal models of prenatal and postnatal inflammation have been linked to the developmental changes in the regulation of both excitatory and inhibitory connectivity between the medial prefrontal cortex and the amygdala 24. This is a key brain circuit involved in the regulation of social‐emotional behaviors, which have been implicated in ASD 25. In a recent set of studies by Buss and colleagues 26, 27, the cognitive and neurodevelopmental consequences of differential maternal inflammation during pregnancy were evaluated. Higher levels of maternal inflammation, as measured via elevated interleukin‐6 (IL‐6), were associated with increased volume of the amygdala and differential connectivity between the amygdala and brain regions important for a number of cognitive processes implicated in ASD 26. This is in line with previous studies of increased amygdala volume and differential connectivity of the amygdala in children with autism 25, 28, 29, 30, 31. Elevated IL‐6 has also been shown to be correlated with poorer cognitive development later in life, and this relationship was mediated by the changes in frontolimbic structural connectivity 27.
Given evidence for increased neuroinflammation and aberrant neuronal connectivity in individuals with ASD, therapeutic interventions that impact immune modulation and regulation of neural connectivity show promise in the treatment of ASD. Preclinical models have shown that umbilical cord blood contains effector cells that, through paracrine signaling, suppress inflammation and alter brain connectivity 32, 33. We have reported significant improvements in behavior, including increased social functioning, improved communication abilities, and decreased clinical symptoms, following treatment with a single infusion of autologous cord blood in a phase I open‐label trial for children with ASD 34. In the current study, we provide additional outcome information from this phase I trial. Specifically, results of a secondary biomarker outcome based on magnetic resonance imaging (MRI) obtained at baseline and 6 months after treatment. We aimed to understand whether the increased social functioning, improved communication abilities, and decreased clinical symptoms observed in the phase I trial were associated with concurrent changes in brain structural connectivity. We specifically hypothesized that behavioral improvement in core autism symptoms involving social and communication skills would be associated with increased white matter connectivity among frontal, temporal, and subcortical (amygdala, hippocampus, and basal ganglia) circuits that support social and communicative functions.
Methods
Study Design
The present study was conducted as part of a phase I open‐label trial of a single i.v. infusion of autologous umbilical cord blood in children with ASD who were 24–72 months of age at baseline. The methods of this trial have been described in detail elsewhere 34, 35, 36. In summary, the aims of the phase I trial were to evaluate safety of the treatment and to assess the feasibility and sensitivity to change of various primary and secondary outcome measures and biomarkers for use as endpoints in a future phase II randomized, placebo‐controlled clinical trial. This was a single‐site study involving 25 participants with ASD. Children with a confirmed diagnosis of ASD and a banked autologous umbilical cord blood unit of adequate size and quality participated in the trial.
All participants' caregivers completed a prestudy phone screening, and the research team reviewed medical records to assess eligibility. The umbilical cord blood unit was evaluated to determine whether it contained sufficient numbers of cells and had negative sterility culture results. The unit itself was shipped to Duke University prior to the participants' evaluations and infusions. Participants and their caregivers traveled to Duke University three times as part of their participation in the study. At their baseline visit, they were administered clinical evaluations and infusion of their cells. At 6 and 12 months postbaseline, participants returned for follow‐up clinical assessments. Additional caregiver interviews and questionnaires were collected at 3, 9, and 12 months postbaseline.
The trial was approved by the Duke Hospital Institutional Review Board and conducted under IND #15949. ClinicalTrials.gov identifier is NCT02176317.
Participants
Participants between 24 and 72 months of age who met criteria for a clinical diagnosis of ASD based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) were eligible for study inclusion in the phase I trial. The DSM‐5 diagnosis of ASD was established by expert clinicians and informed by the Autism Diagnostic Observation Scale‐2 (ADOS‐2) 37 and the autism diagnostic interview, revised 38. Additional inclusion criteria included (1) an intelligence quotient (IQ) of ≥35 on the Stanford‐Binet Intelligence Scales for Early Childhood, Fifth Edition 39 or Mullen Scales of Early Learning 40, (2) availability of autologous umbilical cord blood with a minimum total nucleated precryopreservation cell dose of ≥1 × 10−7 cells per kilogram of participant weight that met acceptance criteria with confirmed human leukocyte antigen matching, (3) stability on current medications for at least 2 months prior to the infusion, (4) ability to travel to Duke University three times (baseline and 6 and 12 months postbaseline), and (5) parents were English speaking. Exclusion criteria included (1) a history of prior cell therapy, (2) use of i.v. immunoglobulin or other anti‐inflammatory medications (with the exception of Nonsteroidal anti‐inflammatory drugs (NSAIDs)), (3) known genetic syndrome (e.g., fragile X) or other significant medical comorbidity, (4) obvious physical dysmorphology, (5) an uncontrolled seizure disorder, (6) significantly impaired renal or liver function, and/or (7) clinically significant abnormalities in complete blood count. Caregivers of eligible study participants provided written informed consent prior to screening and again at the baseline study visit.
Twenty‐three of the 25 participants (92%) completed baseline, 6‐month, and 12‐month visits. The present study focuses on the baseline and 6‐month visits. Nineteen participants provided high‐quality, artifact‐free data for the MRI at both baseline and 6‐month visits (17 males and 2 females). The 19 participants were between 27 and 71 months of age at their baseline visit, with a mean age of 53.95 months (SD ± 13.00). The mean nonverbal developmental quotient of study participants at the baseline visit was 58 (SD ± 22.81, median: 60, range: 22–95). The mean ADOS‐2 Severity Score across modules at the baseline visit was 8 (SD ± 1.39), indicating that the sample was in the severe range of ASD symptoms.
Clinical Measures
Vineland Adaptive Behavior Scales‐II Socialization Subscale
The Vineland Adaptive Behavior Scales‐II Socialization Subscale (VABS‐SS) 41 is a caregiver questionnaire that is used to assess children's adaptive behavior. VABS‐SS is a well‐standardized measure with strong reliability and validity, which yields an overall composite score, as well as subscale standard scores in the following domains: socialization, communication, daily living skills, and motor skills. The VABS‐SS was collected from the participant's primary caregiver at the baseline and 6‐ and 12‐month visits. The change in the VABS‐SS (6 months to baseline) was used to measure change in social behavior.
Clinical Global Impression Severity and Improvement Scales
The Clinical Global Impression Severity (CGI‐S) and Improvement (CGI‐I) Scales 42 are commonly used rating scales that rate the children's overall level of core ASD symptoms and related functioning and support requirements (CGI‐S), as well as the amount of improvement or worsening of overall core ASD symptoms in addition to related functioning and need for supports from the time of the previous CGI‐S rating (CGI‐I). The CGI‐I is a seven‐point scale indicating the degree of improvement or worsening of ASD symptoms relative to baseline. Based on all available information, each participant was rated as 1, very much improved; 2, much improved; 3, minimally improved; 4, no change; 5, minimally worse; 6, much worse; or 7, very much worse. Each participant was assigned a CGI‐I rating at the 6‐ and 12‐month visits, and each referenced the degree of improvement or worsening relative to baseline. Ratings were made by highly experienced researchers with expertise in ASD. In the current study, the 6‐month CGI‐I rating was used to measure change in behavior between baseline and 6‐month visits.
Expressive One‐Word Picture Vocabulary Test‐4
The Expressive One‐Word Picture Vocabulary Test 4 EOWPVT 43 is a clinician‐administered assessment, which measures an individual's ability to match a spoken word with an image of an object, action, or concept. The EOWPVT was administered to each child at the baseline and 6‐ and 12‐month visits. The change in the raw score (6 months to baseline) was used to measure change in expressive language.
Magnetic Resonance Imaging
Image Acquisition
MRI scanning was conducted on a 3.0T GE MR750 whole‐body 60‐cm bore MRI scanner (GE Healthcare, Waukesha, WI). Participants were sedated to reduce motion artifacts in the MRI. Sedation was patient specific and included a combination of dexmedetomidine, propofol, and/or midazolam.
Diffusion‐weighted images (DTI) were acquired using a 25‐direction gradient encoding scheme at b = 1,000 seconds/mm2 with three nondiffusion‐weighted images, an average (SD) echo time (TE) of 85 ms (2 ms), and a repetition time (TR) of 12,000 ms. An isotropic resolution of 2 mm3 was achieved using a 96 × 96 acquisition matrix in a field of view of 192 × 192 mm2 at a 2‐mm‐slice thickness. T1‐weighted images were obtained with an inversion‐prepared three‐dimensional fast spoiled‐gradient‐recalled pulse sequence with a TE of 2.7 ms, an inversion time of 450 ms, a TR of 7.2 ms, and a flip angle of 12°, at a 1 mm3 isotropic resolution.
Regions of Interest
Each participant's T1 image and the first nondiffusion‐weighted image (b0) of the DTI acquisition were skull stripped using the FMRIB Software Library (FSL) brain extraction tool 44, 45 (Fig. 1A). The T1 image was registered to the b0 image with an affine registration created using FMRIB Linear Image Registration Tool (FSL FLIRT) 46, 47 (Fig. 1B). Region of interest (ROI) parcellation was performed by warping the dilated UNC Pediatric Brain atlas (available publicly at http://www.nitrc.org/projects/unc_brain_atlas/) into each participant's T1 in diffusion image space via the advanced normalization tools toolkit 48, 49 (Fig. 1C). The parcellation results were visually inspected to confirm anatomical consistency. A total of 83 regions were defined for each participant, 41 gray matter regions in each hemisphere, and a single region encompassing the brainstem. FMRIB's automated segmentation tool was used to calculate whole brain white matter volume for each participant at both baseline and 6‐month visits 50.
Figure 1.
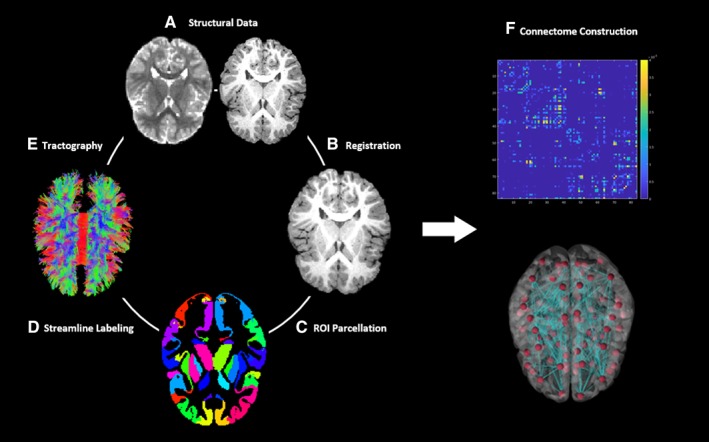
Overview of structural connectome creation. (A): Left, diffusion image; right, T1. (B) T1 registered in diffusion space. (C) Warp parcellated dilated UNC pediatric atlas to diffusion image space using advanced normalization tools. (D) Parcellated ROIs used to label tractography results. (E) DTI and deterministic tractography using the Connectome Mapper. (F) Visualization of connectome for a representative participant. Abbreviation: ROI, region of interest.
White Matter Tractography and Connectome Construction
Diffusion‐weighted images were inspected for data quality and motion corruption, resulting in 19 participants with data suitable for analysis. A standardized pipeline based on the Connectome Mapper was used to analyze participant data at both baseline and 6‐month visits (http://www.cmtk.org) 51, 52. DTI tensors were estimated and whole‐brain deterministic tractography was performed using the fiber assignment by continuous tracking streamline tracking algorithm 53 (Fig. 1E). Streamlines were spline filtered and removed if they were less than 20 mm or longer than 500 mm in length. Using the individual parcellation maps, streamlines were labeled by which ROIs contain their origination and termination points (Fig. 1D). Streamlines were considered orphan fibers and discarded if they did not begin and end in an ROI 54, 55.
Structural Connectome Analysis
Based on a priori hypotheses, changes in mutual connectivity within the frontal, temporal, and specified subcortical regions were calculated (6 months to baseline). The parcellated gray matter ROIs included in this analysis were defined as nodes. Edges were defined as the volume of voxels containing valid streamlines that originate and terminate within a pair of nodes. For each participant, edge volumes were calculated and normalized by whole‐brain white matter volume at both baseline and 6‐month visits. A connectivity matrix and network of a representative participant's nodes and edges are visualized using the BrainNet Viewer in Figure 1F (http://www.nitrc.org/projects/bnv/) 56. Mutual connectivity represents the singular edge connecting two nodes. Edges were included in the resulting mutual connectivity analysis if at least 15 of the 19 participants contained trackable streamlines.
Statistical Analysis
Associations between changes in frontal, temporal, and subcortical ROI connectivity and changes in behavior between baseline and 6‐month visits were examined using the Spearman rank correlations. The Spearman rank correlation is a nonparametric statistic that does not require data to be normally distributed. In order to reduce type 1 error, we only report findings in which the relationship between increased mutual connectivity and at least two of the three outcome measures were significant at p < .05.
Results
Table 1 and Figure 2 summarize the findings. Improvement across all three behavioral outcome measures (i.e., VABS‐SS, CGI‐I, and EOWPVT) was correlated with increased connectivity between the frontal pole and the globus pallidus (VABS‐SS: r[17] = .51, p < .05; CGI‐I: r[17] = −.6, p < .01; EOWPVT: r[17] = .48, p < .05) in the right hemisphere and between the fusiform and superior temporal cortex (VABS‐SS: r[17] = .47, p < .05; CGI‐I: r[17] = −.47, p < .05; EOWPVT: r[17] = .47, p < .05) in the left hemisphere. Improvement on both the VABS‐SS and the EOWPVT was associated with increased connectivity between the superior temporal cortex and the putamen in the left hemisphere (VABS‐SS: r[17] = .49, p < .05; EOWPVT: r[17] = .5, p < .05). Improvement in social abilities and overall clinical improvement, as measured with the VABS‐SS and the CGI‐I, was associated with increased connectivity between the inferior temporal and superior temporal cortex (VABS‐SS: r[17] = .6, p < .001; CGI‐I: r(17) = −.49, p < .05), the thalamus and the hippocampus (VABS‐SS: r[17] = .59, p < .01; CGI‐I: r[17] = −.48, p < .05), and the temporal pole and the globus pallidus (VABS‐SS: r[17] = .55, p < .05; CGI‐I: r[17] = −.52, p < .05), all in the left hemisphere. Improvement in communication abilities and overall clinical improvement, as measured with the EOWPVT and the CGI‐I, was associated with increased connectivity between the frontal and temporal poles (EOWPVT: r[17] = .64, p < .001; CGI‐I: r(17) = −.7, p < .001), the superior temporal cortex and the globus pallidus (EOWPVT: r[17] = .5, p < .05; CGI‐I: r[17] = −.5, p < .03), and the rostral middle frontal cortex and globus pallidus (EOWPVT: r[17] = .49, p < .05; CGI‐I: r[17] = −.55, p < .05) in the right hemisphere, as well as between the fusiform and middle temporal cortex in the left hemisphere (EOWPVT: r[17] = .46, p < .05; CGI‐I: r[17] = −.47, p < .05).
Table 1.
Significant correlations between increases in connectivity between pairs of brain regions and behavioral improvement in at least two out of three clinical outcome measures
| Vineland socialization | Expressive One Word Picture Vocabulary | Clinical Global Impression‐Improvement | |||||
|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | ||
| R frontal pole | R pallidum | .51 | .03 | .48 | .04 | −.60 | .006 |
| L fusiform | L sup temporal | .47 | .04 | .47 | .04 | −.47 | .04 |
| L superior temporal | L putamen | .49 | .03 | .50 | .03 | — | — |
| L inferior temporal | L superior temporal | .60 | .0065 | — | — | −.49 | .04 |
| L thalamus | L hippocampus | .59 | .009 | — | — | −.48 | .04 |
| L temporal pole | L pallidum | .55 | .02 | — | — | −.52 | .02 |
| R frontal pole | R temporal pole | — | — | .64 | .003 | −.70 | .0009 |
| R superior temporal | R pallidum | — | — | .50 | .03 | −.50 | .03 |
| R rostral middle frontal | R pallidum | — | — | .49 | .03 | −.55 | .02 |
| L fusiform | L middle temporal | — | — | .46 | .046 | −.47 | .04 |
Associations between changes in frontal, temporal, and subcortical region of interest connectivity and changes in behavior between baseline and 6 month visits were examined using the Spearman rank correlations. Higher scores on the Vineland socialization subscale and Expressive One‐Word Picture Vocabulary Test and lower scores on the Clinical Global Impression‐Improvement represent improved functioning.
Abbreviation: R, right hemisphere; L, Left hemisphere.
Figure 2.
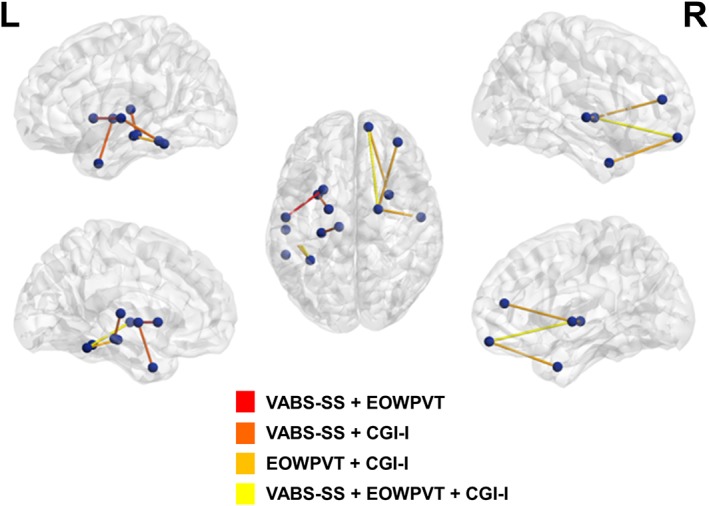
Mutual connectivity maps. Associations between changes in frontal, temporal, and subcortical white matter connectivity and changes in behavior following treatment with umbilical cord blood. Spheres represent region of interest node pairs. Tubes between spheres represent increased connectivity in edges between the nodes that is correlated with at least two outcome measures. Abbreviations: CGI‐I, Clinical Global Impression Improvement Scale; EOWPVT, Expressive One‐Word Picture Vocabulary Test; VABS, Vineland Adaptive Behavior Scale R, right hemisphere; L, Left hemisphere.
In order to investigate whether increased brain connectivity in regions that correlated with behavioral improvements were because of differences in participant baseline characteristics, we explored whether changes in mutual connections that showed a significant correlation with improvement were also correlated with age at baseline and child cognitive functioning level. We found that a subset of the identified connections showed a significant negative correlation with age at baseline (Table 2). Specifically, younger children had higher levels of increased mutual connectivity between the fusiform and superior temporal cortex (r[17] = −.56, p < .05), the thalamus and the hippocampus (r[17] = −.58, p < .01), and the temporal pole and the globus pallidus (r[17] = −.5, p < .05) in the left hemisphere. Age at baseline was also negatively correlated with increased connectivity between the superior temporal cortex and the globus pallidus in the right hemisphere (r[17] = −.52, p < .05). After running a Spearman partial correlation between white matter connectivity and behavioral improvements that controlled for age at baseline, it was found that higher levels of improvement on the VABS‐SS remained significantly associated with increased connectivity between the thalamus and hippocampus (r[17] = .54, p < .05) and the temporal pole and pallidum (r[17] = .49, p < .05) in the left hemisphere. No other relationships remained when accounting for age.
Table 2.
Correlations between increases in connectivity between pairs of brain regions after accounting for significant association with age at baseline
| Vineland socialization | Expressive One‐Word Vocabulary | Clinical Global Impression‐Improvement | Age | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | ||
| L fusiform | L superior temporal | .39 | .1 | .38 | .12 | −.33 | .18 | −.56 | .01 |
| L thalamus | L hippocampus | .54 | .02 | — | — | −.33 | .18 | −.58 | .009 |
| L temporal pole | L pallidum | .49 | .04 | — | — | −.41 | .09 | −.50 | .03 |
| R superior temporal | R pallidum | — | — | .43 | .08 | −.38 | .13 | −.52 | .02 |
Associations between changes in frontal, temporal, and subcortical region of interest connectivity and changes in behavior between baseline and 6 month visits were examined using the Spearman rank correlations with age at baseline included as a covariate. Higher scores on the Vineland socialization subscale and Expressive One‐Word Vocabulary Test and lower scores on the Clinical Global Impression‐Improvement represent improved functioning.
Abbreviation: —, no data.
Child cognitive functioning level (nonverbal IQ) was also found to be positively correlated with increased connectivity for a subset of connections (Table 3). Specifically, higher cognitive abilities were associated with increases in white matter connectivity between the superior temporal cortex and the putamen (r[17] = .5, p < .05) in the left hemisphere, the fusiform gyrus and middle temporal cortex (r[17] = .5, p < .05) in the left hemisphere, as well the frontal and temporal poles (r[17] = .58, p < .01) in the right hemisphere. After controlling for child cognitive functioning in our correlation analyses between white matter connectivity and behavioral improvements, the relationship between improvement in communication abilities and overall clinical improvement, as measured with the EOWPVT and the CGI‐I, and increased connectivity between the frontal and temporal pole (EOWPVT: r[17] = .52, p < .05; CGI‐I: r[17] = −.57, p < .05) in the right hemisphere remained significant.
Table 3.
Correlations between increases in connectivity between pairs of brain regions after accounting for significant association with child cognitive level
| Vineland socialization | Expressive One‐Word Vocabulary | Clinical Global Impression‐Improvement | Cognitive functioning | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | P | ||
| L superior temporal | L putamen | .34 | .17 | .35 | .15 | — | — | .50 | .03 |
| R frontal pole | R temporal pole | — | — | .52 | .03 | −.57 | .03 | .58 | .009 |
| L fusiform | L middle temporal | — | — | .30 | .23 | −.42 | .08 | .50 | .03 |
Associations between changes in frontal, temporal, and subcortical region of interest connectivity and changes in behavior between baseline and 6 month visits were examined using the Spearman rank correlations with nonverbal developmental quotient included as a covariate. Higher scores on the Vineland socialization subscale and Expressive One‐Word Vocabulary Test and lower scores on the Clinical Global Impression‐Improvement represent improved functioning.
Abbreviation: —, no data.
Finally, we also explored the hypothesis that an increased dose of cord blood cells was correlated with degree of change in connectivity and found that dose did not correlate with increases in connectivity (all p > .5).
Discussion
We previously published results showing improvements in social communication skills after a single infusion of autologous umbilical cord blood in young children with autism. The current study analyzed MRI biomarker outcomes in the same phase I clinical trial and explored the hypothesis that the behavioral improvements were associated with concurrent changes in white matter connectivity in frontal, temporal, and specific subcortical regions (amygdala, hippocampus, and basal ganglia) that have been previously been shown to show anatomical, connectivity, and functional abnormalities in ASD 57. Our results supported this hypothesis.
In the current study, we found that clinical improvement and increased social skills were associated with increased connectivity between the left thalamus and the hippocampus, which aligns with one of the primary limbic white matter pathways, the fornix 58. The limbic system is a critical hub in the brain for social and emotional processes. Alterations in the white matter pathways connecting the limbic system to itself, as well as to other parts of the brain, have been implicated in the pathophysiology of autism. The fornix more specifically plays an important role in the regulation of affective behavior, and decreased integrity of the left fornix has been linked to poorer social and communicative functioning in individuals with ASD 59.
Additionally, clinical and communication improvements were associated with increased mutual connectivity between the frontal and temporal poles in the right hemisphere. This white matter tract aligns with another major limbic tract, the right uncinate fasciculus. The right uncinate fasciculus has been implicated in emotion processing, memory, and language abilities 58. Structural integrity of the right uncinate fasciculus early in development has been linked to the development of joint‐attention abilities, which are critical for the development of social‐communicative abilities, including language development 60. Furthermore, a number of studies have reported decreased integrity of the right uncinate fasciculus in individuals with autism and have linked this with decreased social and communicative abilities 61, 62, 63, 64, 65.
Increased connectivity following treatment was also found between the fusiform gyrus and both the inferior and superior temporal cortices, in the left hemisphere. These connections lie along the path of the inferior longitudinal fasciculus, which is composed of both long and short fibers that connect visual processing areas to the temporal cortex and the limbic system 58. Decreased integrity of the left inferior longitudinal fasciculus has been reported in individuals with ASD in a number of studies 61, 62, 66, 67. The inferior longitudinal fasciculus is implicated in a number of functions that are dysregulated in autism, including face identification and perception, and language abilities 58. Increased connectivity in this pathway was associated with improvements across all measures.
We also found increases in connectivity between the basal ganglia, particularly the pallidum, and both the frontal and temporal cortices. This increased connectivity was associated with overall clinical improvement and improvement in social abilities as measured with both the VABS‐SS and CGI‐I. The basal ganglia have been linked to the selection and maintenance of goal‐directed behaviors 68. Interestingly, a circuit including the frontal cortex, the limbic system, and the pallidum has been demonstrated to be critical for pair‐bond formation and is part of the mesolimbic dopamine reward system 69. As such, it is likely involved in social motivation and social reward, both of which are decreased in individuals with autism. In support of this, the integrity of white matter surrounding the basal ganglia has been shown to correlate with ASD symptoms severity across all domains, including social, communication, and repetitive behaviors 63. Furthermore, previous research has found decreased connectivity between the superior temporal sulcus and a number of regions, including the putamen, in individuals with autism 70. In particular, these authors found that decreased connectivity in the superior temporal cortex and the putamen was associated with worse communication abilities. Consistent with this, we showed that increased connectivity between the superior temporal sulcus and the putamen was associated with improvement in social communication and language abilities. Notably, this increased connectivity was more commonly found within the left hemisphere and a failure of left hemisphere specialization for language in ASD has been supported by functional MRI and electroencephalography (EEG) studies 71, 72.
Although not directly tested in the current article, we have previously reported that treatment with cord blood was associated with increased time spent attending to an actor as compared to nonsocial aspects of a social scene using an eye‐tracking (ET) task 34. Furthermore, we have shown that attention to the actor measured via ET was strongly associated with caregiver‐reported outcome measures that are commonly used to assess social communication, including the VABS‐SS 36. As such, it is possible that cord blood imparts changes in the neural systems underlying social motivation and reward, which increases the child's attention to social information, allowing them to improve in their ability to perceive and discriminate faces, thus improving their social abilities.
There are several plausible explanations for our findings. The first explanation is that, in line with previously described preclinical models, treatment with umbilical cord blood decreases neural inflammation and this in turn promotes the development of white matter connectivity in neural networks important for social and communicative abilities. This is further supported by our previously reported finding of improvements in social attention as measured with ET, and normalization of spectral EEG features. Taken together, this suggests that cord blood treatment has broad impacts on both brain structure and function and that this is associated with improvements in social and communicative functioning.
An alternative explanation, however, is that the changes in white matter connectivity represent developmental changes that are occurring coincident with the development of social and communicative abilities common for this age range, independent of treatment with effects. We found correlations between both baseline age and child cognitive functioning and degree of increased connectivity in some, but not all, of the white matter pathways for which there were correlations with behavioral improvements. After controlling for baseline age or child cognitive level, degree of clinical improvement and increases in white matter connectivity remained for a subset of brain connections. Specifically, the relationship between degree of improvement based on overall ratings of clinical improvement (CGI‐I) and expressive language skills and increased mutual connectivity between the frontal and temporal poles in the right hemisphere remained significant. This is a promising finding given previous research implicating decreased integrity of the uncinate fasciculus, of which this connection is part, with increased symptom severity in individuals with autism. As this was a single‐arm open‐label trial, it is possible that our findings reflect correlated changes in behavioral and brain development. However, the current data provide targets for exploring brain‐related changes in future placebo‐controlled double‐blind trials, which are currently taking place.
Limitations
The current study must be considered in light of some limitations. First, because this was an exploratory analysis, we restricted our analyses to brain networks that have previously been implicated in autism. Furthermore, we only reported those findings where there was increased connectivity that correlated with at least two of our outcome measures. Previous work looking at brain changes in response to treatment with cord blood in children with cerebral palsy (CP) demonstrated strong correlations between increased whole brain white matter connectivity and improved functional abilities in children with CP 52. Although we did not see evidence of such a pattern in the current data, it is possible that other regions that we did not explore also showed changes in mutual connectivity. Future work looking at this may provide an even greater picture of the brain networks associated with behavioral improvement following treatment.
Additionally, although DTI is a commonly used metric for exploring the microstructure of white matter in individuals, the tractography methods used in the current article are not without limitations. Specifically, tractography assumes that white matter fibers are homogenously distributed within each voxel; however, it is well known that this is not always the case 73. Future research into the development of white matter tracts in relation to clinical treatments would benefit from the implementation of additional DTI technologies, which better able to resolve discrepancies due to the inhomogeneous nature of fibers within each voxel.
Conclusion
Significant improvements in social communication skills and a reduction in symptoms in children with ASD were described in a phase I, open‐label study after autologous cord blood infusion. The current results suggest that these improvements in behavior were associated with increased neuronal connectivity in limbic, frontal, temporal, and basal ganglia neural networks that have previously been implicated in the pathophysiology of autism. Given the heterogeneity inherent in ASD, it is important to identify biomarkers that may be useful for predicting and measuring treatment efficacy 35, 36. Understanding the mechanism of action by which autologous cord blood infusion may impact autism symptoms is the first step toward identifying such biomarker targets.
Author Contributions
K.L.H.C., S.M., C.T., and L.W.C.: data analysis and interpretation, manuscript writing, final approval of manuscript; L.F.: collection and/or assembly of data, manuscript writing, final approval of manuscript; J.S. and J.K., A.S., and G.D.: conception/design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
G.D. is on the Scientific Advisory Board and receives research funding from Janssen Research and Development. L.W.C. is a consultant for Roche Pharmaceuticals, LabCorps, Inc., and Akili, Inc., received research funding from PerkinElmer, and receives royalties from Guilford Press and University of Oxford Press. J.K. is Director of the Carolinas Cord Blood Bank and Medical Director of Cord: Use Cord Blood Bank. The other authors indicated no potential conflicts of interest.
Acknowledgments
This work would not have been possible without the participation of the participants and their families. We thank the Billi and Bernie Marcus Foundation (NCT02176317), PerkinElmer, Inc., and a Stylli Translational Neuroscience Award for their financial support for this study. We also thank the children who participated and their families, and the following staff members: Jennifer Baker, Colleen McLaughlin, Rebecca Durham, Barbara Waters‐Pick, Todd Calnan, Crystal Chiang, Kendyl Cole, Michelle Perry, Mallory Harris, Jennifer Newman, Katherine S. Davlantis, Elizabeth Paisley, Charlotte Stoute, and Elizabeth Sturdivant.
Authored by a member of IFATS
References
- 1. Diagnostic and Statistical Manual of Mental Disorders (5th ed.). 5th ed. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 2. Masi A, DeMayo MM, Glozier N et al. An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci Bull 2017;33:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piven J, Elison JT, Zylka MJ. Toward a conceptual framework for early brain and behavior development in autism. Mol Psychiatry 2017;22:1385–1,394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Solso S, Xu R, Proudfoot J et al. Diffusion tensor imaging provides evidence of possible axonal overconnectivity in frontal lobes in autism spectrum disorder toddlers. Biol Psychiatry 2016;79:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolff JJ, Gu H, Gerig G et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry 2012;169:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ouyang M, Cheng H, Mishra V et al. Atypical age‐dependent effects of autism on white matter microstructure in children of 2–7 years. Hum Brain Mapp 2016;37:819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolff JJ, Swanson MR, Elison JT et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Molecular Autism 2017;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meltzer A, Van de Water J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacology 2017;42:284–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones KL, Van de Water J. Maternal autoantibody related autism: Mechanisms and pathways. Mol Psychiatry 2018; E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McAllister AK. Immune contributions to cause and effect in autism spectrum disorder. Biol Psychiatry 2017;81:380–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Young AM, Chakrabarti B, Roberts D et al. From molecules to neural morphology: Understanding neuroinflammation in autism spectrum condition. Molecular Autism 2016;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashwood P, Wills S, Van de Water J. The immune response in autism: A new frontier for autism research. J Leukoc Biol 2006;80:1–15. [DOI] [PubMed] [Google Scholar]
- 13. Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry 2005;17:485–495. [DOI] [PubMed] [Google Scholar]
- 14. Braunschweig D, Krakowiak P, Duncanson P et al. Autism‐specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry 2013;3:e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nordahl CW, Braunschweig D, Iosif AM et al. Maternal autoantibodies are associated with abnormal brain enlargement in a subgroup of children with autism spectrum disorder. Brain Behav Immun 2013;30:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Braunschweig D, Duncanson P, Boyce R et al. Behavioral correlates of maternal antibody status among children with autism. J Autism Dev Disord 2012;42:1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bauman MD, Iosif AM, Ashwood P et al. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Transl Psychiatry 2013;3:e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin LA, Ashwood P, Braunschweig D et al. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun 2008;22:806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cabanlit M, Wills S, Goines P et al. Brain‐specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci 2007;1:92–103. [DOI] [PubMed] [Google Scholar]
- 20. McDougle CJ, Landino SM, Vahabzadeh A et al. Toward an immune‐mediated subtype of autism spectrum disorder. Brain Res 2015;1:72–92. [DOI] [PubMed] [Google Scholar]
- 21. Mead J, Ashwood P. Evidence supporting an altered immune response in ASD. Immunol Lett 2015;163:49–55. [DOI] [PubMed] [Google Scholar]
- 22. Careaga M, Rogers S, Hansen RL et al. Immune endophenotypes in children with autism spectrum disorder. Biol Psychiatry 2017;81:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ashwood P, Krakowiak P, Hertz‐Picciotto I et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 2011;25:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y, Missig G, Finger BC et al. Maternal and early postnatal immune activation produce dissociable effects on neurotransmission in mPFC‐amygdala circuits. J Neurosci 2018;38:3358–3,372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen MD, Li DD, Keown CL et al. Functional connectivity of the amygdala is disrupted in preschool‐aged children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2016;55:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Graham AM, Rasmussen JM, Rudolph MD et al. Maternal systemic interleukin‐6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiatry 2018;83:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rasmussen JM, Graham AM, Entringer S et al. Maternal Interleukin‐6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. NeuroImage 2019;185:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aylward EH, Minshew NJ, Goldstein G et al. MRI volumes of amygdala and hippocampus in non‐mentally retarded autistic adolescents and adults. Neurology 1999;53:2145–2150. [DOI] [PubMed] [Google Scholar]
- 29. Barnea‐Goraly N, Frazier TW, Piacenza L et al. A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog Neuropsychopharmacol Biol Psychiatry 2014;48:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Munson J, Dawson G, Abbott R et al. Amygdalar volume and behavioral development in autism. Arch Gen Psychiatry 2006;63:686–693. [DOI] [PubMed] [Google Scholar]
- 31. Schumann CM, Hamstra J, Goodlin‐Jones BL et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci 2004;24:6392–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bachstetter AD, Pabon MM, Cole MJ et al. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci 2008;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shahaduzzaman M, Golden JE, Green S et al. A single administration of human umbilical cord blood T cells produces long‐lasting effects in the aging hippocampus. Age (Dordr) 2013;35:2071–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dawson G, Sun JM, Davlantis KS et al. Autologous cord blood infusions are safe and feasible in young children with autism spectrum disorder: Results of a single‐center phase i open‐label trial. Stem Cells Translational Medicine 2017;6:1332–1,339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murias M, Major S, Compton S et al. Electrophysiological biomarkers predict clinical improvement in an open‐label trial assessing efficacy of autologous umbilical cord blood for treatment of autism. Stem Cells Translational Medicine 2018;7:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murias M, Major S, Davlantis K et al. Validation of eye‐tracking measures of social attention as a potential biomarker for autism clinical trials. Autism Res 2018;11:166–174. [DOI] [PubMed] [Google Scholar]
- 37. Lord C, Rutter M, DiLavore PC et al. Autism Diagnostic Observation Schedule: ADOS‐2. Los Angeles, CA: Western Psychological Services, 2012. [Google Scholar]
- 38. Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview‐Revised. Los Angeles, CA: Western Psychological Services, 2003. [Google Scholar]
- 39. Roid GH. Stanford‐Binet intelligence scales. Itasca, Il: Riverside Pub, 2003. [Google Scholar]
- 40. Mullen EM. Mullen Scales of Early Learning. AGS ed. Bloomington, MN: NCS Pearson Inc, 1995. [Google Scholar]
- 41. Sparrow S, Cicchetti D, Balla D. Vineland Adaptive Behavior Scales (Vineland II): Caregiver/Caregiver Rating Form. 2nd ed. Minneapolis, MN: NCS Pearson Inc, 2005. [Google Scholar]
- 42. Guy W, Bonato RR, Laboratory GWUB, et al., Manual for the ECDEU Assessment Battery. U.S. Department of Health, Education, and Welfare, National Institute of Mental Health, 1970. [Google Scholar]
- 43. Martin NA, Brownell R. Expressive One‐Word Picture Vocabulary Test (EOWPVT‐4). 4th ed. Novato, CA: Academic Therapy Publications Inc, 2011. [Google Scholar]
- 44. Jenkinson M, Pechaud M, Smith S. BET2: MR‐based estimation of brain, skull and scalp surfaces. Paper presented at: Eleventh Annual Meeting of The Organization for Human Brain Mapping; 2005; Toronto.
- 45. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jenkinson M, Bannister P, Brady M et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 47. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001;5:143–156. [DOI] [PubMed] [Google Scholar]
- 48. Avants BB, Tustison N, Song G. Advanced normalization tools (ANTS). Insight J 2009;2:1–35. [Google Scholar]
- 49. Avants BB, Tustison NJ, Song G et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011;54:2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation–maximization algorithm. IEEE Trans Med Imaging 2001;20:45–57. [DOI] [PubMed] [Google Scholar]
- 51. Gerhard S, Daducci A, Lemkaddem A et al. The connectome viewer toolkit: An open source framework to manage, analyze, and visualize connectomes. Front Neuroinform 2011;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Englander ZA, Sun J, Laura C et al. Brain structural connectivity increases concurrent with functional improvement: Evidence from diffusion tensor MRI in children with cerebral palsy during therapy. NeuroImage Clin 2015;7:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mori S, Crain BJ, Chacko VP et al. Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999;45:265–269. [DOI] [PubMed] [Google Scholar]
- 54. Daducci A, Gerhard S, Griffa A et al. The connectome mapper: An open‐source processing pipeline to map connectomes with MRI. PLoS One 2012;7:e48121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Englander ZA, Pizoli CE, Batrachenko A et al. Diffuse reduction of white matter connectivity in cerebral palsy with specific vulnerability of long range fiber tracts. NeuroImage Clin 2013;2:440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xia M, Wang J, He Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One 2013;8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: Age‐specific changes in anatomical pathology. Brain Res 2011;1:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008;44:1105–1132. [DOI] [PubMed] [Google Scholar]
- 59. Poustka L, Jennen‐Steinmetz C, Henze R et al. Fronto‐temporal disconnectivity and symptom severity in children with autism spectrum disorder. World J Biol Psychiatry 2012;13:269–280. [DOI] [PubMed] [Google Scholar]
- 60. Elison JT, Wolff JJ, Heimer DC et al. Frontolimbic neural circuitry at 6 months predicts individual differences in joint attention at 9 months. Dev Sci 2013;16:186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ameis SH, Catani M. Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex 2015;62:158–181. [DOI] [PubMed] [Google Scholar]
- 62. Cheon KA, Kim YS, Oh SH et al. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: A Diffusion Tensor Imaging study. Brain Res 2011;1:77–86. [DOI] [PubMed] [Google Scholar]
- 63. Cheung C, Chua SE, Cheung V et al. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J Child Psychol Psychiatry 2009;50:1102–1112. [DOI] [PubMed] [Google Scholar]
- 64. Kumar A, Sundaram SK, Sivaswamy L et al. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cerebral Cortex 2010;20:2103–2113. [DOI] [PubMed] [Google Scholar]
- 65. Sahyoun CP, Belliveau JW, Mody M. White matter integrity and pictorial reasoning in high‐functioning children with autism. Brain Cogn 2010;73:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bloemen OJ, Deeley Q, Sundram F et al. White matter integrity in Asperger syndrome: A preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Res 2010;3:203–213. [DOI] [PubMed] [Google Scholar]
- 67. Jou RJ, Jackowski AP, Papademetris X et al. Diffusion tensor imaging in autism spectrum disorders: Preliminary evidence of abnormal neural connectivity. Aust N Z J Psychiatry 2011;45:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fuccillo MV. Striatal circuits as a common node for autism pathophysiology. Front Neurosci 2016;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci 2004;7:1048–1054. [DOI] [PubMed] [Google Scholar]
- 70. Abrams DA, Lynch CJ, Cheng KM et al. Underconnectivity between voice‐selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A 2013;110:12060–12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Eyler LT, Pierce K, Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain 2012;135:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yau SH, Brock J, McArthur G. The relationship between spoken language and speech and nonspeech processing in children with autism: A magnetic event‐related field study. Dev Sci 2016;19:834–852. [DOI] [PubMed] [Google Scholar]
- 73. Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. Neuroimage 2013;73:239–254. [DOI] [PubMed] [Google Scholar]


