Abstract
Hearing loss is the most prevalent sensory disability worldwide and may be caused by age, drugs or exposure to excessive noise. We have previously developed a minimally-invasive nanohydrogel drug delivery system that successfully delivers nanoparticles into the inner ear. We have substantially extended this technique by functionalizing the nanoparticles and introducing a targeting peptide which recognizes prestin, a transmembrane electromotile protein uniquely expressed in outer hair cells (OHCs) of the inner ear. We demonstrate the successful delivery of molecules and plasmids specifically to OHCs. When compared to untargeted nanoparticles, the delivery of a c-Jun N-terminal kinase (JNK) inhibitor, D-JNKi-1, to OHCs by targeted nanoparticles improved protection from noise induced hearing loss (NIHL). This is the first demonstration of a protection from NIHL using a novel safe and controllable delivery system which is minimally-invasive to the inner ear and, as such, is an extremely appealing technique for use in many clinical applications.
Keywords: Minimally-invasive inner ear biomaterial, delivery platform, Targeted multifunctional nanoparticles, Prestin, Outer hair cell, Noise exposure, c-Jun N-terminal kinase (JNK) pathway
1. Introduction
Hearing loss (HL) represents the most prevalent sensory disability worldwide, affecting over 360 million people [1]. From 2000 to 2015, the number of hearing impaired people in the US doubled to nearly 50 million (Hearing Health Foundation), predominately because of an aging population and excessive noise exposure at work or during recreational activities. Diffculties for patients with HL can go well beyond just issues with hearing, negatively affecting work productivity, health-related quality of life, and emotional status. The costs of increased needs and diminished autonomy associated with HL are shared by society with the costs associated with noise-induced (NI)HL are estimated to be in the billions of dollars [2].
Currently, sensorineural (SN)HL, which accounts for the majority of HL, has no effective, non-invasive options available for treatment: the only therapies offered are hearing aids and cochlear implants, whose effectiveness in patients is highly variable. Thus, novel therapies using less invasive techniques would dramatically improve patient care. There have been several regimens under investigation that deliver therapeutic payloads (e.g., antioxidants, drugs, genetic material) to the inner ear which have been shown to be somewhat effective in preventing or reducing HL [3]. There are, however, two major barriers to the application of such treatments: the inner ear anatomical restrictions and the lack of targeting, resulting in undesired side effects.
Access to the inner ear by therapeutic biomaterials is restricted by its anatomical structure and the existence of the blood-inner ear barrier. This results in the systemic application of drugs for inner ear treatment being ineffcient: there may be off target side effects, particularly as high concentrations of drugs may be required in order to attain a therapeutic level in the inner ear. The use of intratympanic injection, as a local approach for drug delivery, circumvents off target systemic side effects but cannot control the duration and concentration of drug application and often causes inconsistent therapeutic outcomes. Thus, it is necessary to develop local delivery systems offering sustainable, controlled release of the drug or biomaterial of interest. In order to improve such delivery across the round window membrane (RWM) of the middle ear, we have previously established a novel, stable, safe, regulated and controllable chitosan glycerophosphate (CGP)-hydrogel system that allows the reliable delivery of any therapeutic material. Using this system, we successfully delivered gentamicin, dexamethasone and biomaterials into the inner ear in a minimally-invasive fashion [4,5].
The GCP-hydrogel delivery system is highly versatile. Variations in the proportions of its components result in the hydrogel being able to transform from a liquid to a solid at a wide range of temperatures. We determined developed a GCP-hydrogel that was a liquid at room temperature and a semi-solid gel at 37 °C. This allowed direct application of the liquid hydrogel into the small anatomical space of the round window niche (RWN) in the middle ear and the technique can easily be applied to the small and irregularly shaped ear anatomies of a variety of animals and humans. Once in the RWN, the temperature of the hydrogel gradually reaches body temperature and becomes a gel that attaches to the RWM. This transition allows the hydrogel to withstand the constant shear forces that flow through the Eustachian tube and enabled the payloads to be delivered solely into the inner ear. Our previous studies clearly demonstrated that biomaterials can be slowly and steadily released from the CGP-hydrogel into the cochlear perilymph through the RWM.
This delivery system is ideal for individualized treatment paradigms due to its capability to apply differing concentrations of therapeutic biomaterials consistently over a defined period and to terminate drug application at any time. This local drug delivery system can be regulated by chitosanase, which hydrolyzes the chitosan chains in the gel via a glycolic reaction, resulting in the surface degradation of the hydrogel [6]. The resulting sugars and water, together with any payload, are then rapidly washed away down the Eustachian tube. Thus, the delivery of therapeutic material system can, if required, be terminated. More recently, we incorporated liposomal nanoparticles or multifunctional nanoparticles (MFNPs), capable of encapsulating virtually any biologic payload, into the hydrogel. These proved to be more effcacious in delivering biomaterials into the inner ear space [7].
The pathogenesis of many causes of SNHL occurs in specific cell types in the inner ear, such as outer hair cells (OHCs) or spiral ganglion neurons, and it would be ideal to apply therapeutic materials to only those affected cells [8]. For instance, collateral damage caused by the off-target effects of gene delivery is potentially toxic to patients and currently diffcult to avoid. The application of anti-apoptosis agents, such as JNK inhibitors or caspase inhibitors, has been demonstrated to limit OHC death following an acute insult [9–12]. Realistic usage of these therapies in a clinical setting, however, would require localized, cell-specific application of these agents to maximize therapeutic effects and reduce potential toxicity.
In this study, we delivered MFNPs into the inner ear fluids that were targeted to specific cell types in the cochlea (Fig. 1). We targeted the electromotile protein, prestin, which is exclusively expressed in the membranes of OHCs of the inner ear [13], by conjugating a peptide that recognized prestin to the outer surface of the MFNPs. We then demonstrated that the targeted MFNPs (tMFNPs) were preferentially delivered to the OHCs. Finally, we further incorporated a payload of the c- Jun N-terminal kinase (JNK) inhibitor, D-JNKi-1, to the tMFNPs and successfully utilized these tMFNPs to deliver the inhibitor to the OHCs partially protecting the animal model from NIHL. The present study shows that the targeted nanohydrogel delivery system has the potential to be used a platform for delivery of drugs and biomaterials for the treatment of a wide variety of inner ear diseases.
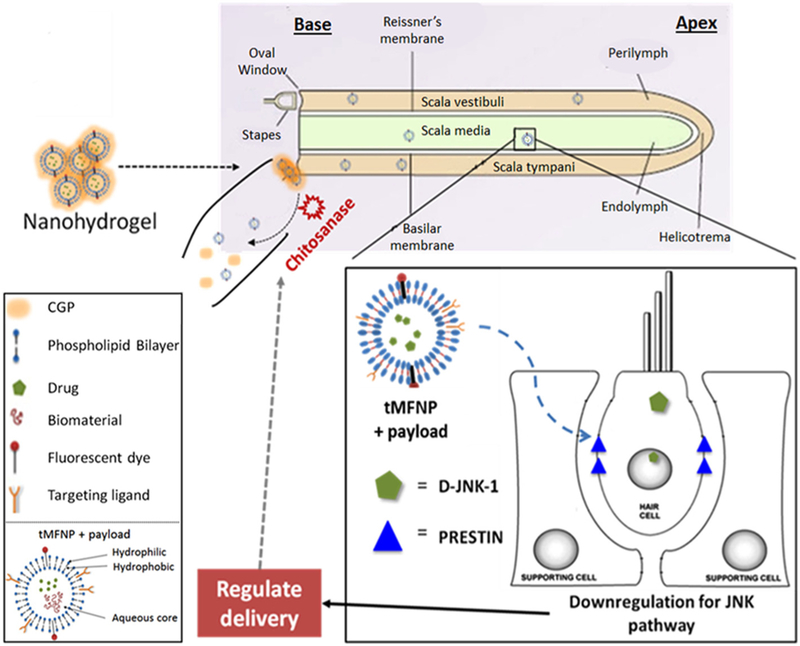
Fig. 1.
Overview of the targeted multifunctional nanoparticle (tMFNP) chitosan glycerophosphate (CGP)-hydrogel delivery platform. The tMFNP comprises an outer phospholipid bilayer (containing a targeting ligand and a fluorescent dye for detection purposes), which encompasses encloses an aqueous core containing drugs and/or biomaterials. The liquid nanohydrogel containing tMFNP with a drug or biomaterial payload is applied into the round window niche of the inner ear. It transforms into a semi-solid gel attached to the round window membrane and releases the tMFNPs into the cochlea. The tMFNPs are then able to diffuse throughout the cochlea. As an example, we highlight the role of a MFNP possessing a targeting ligand which recognizes prestin, a transmembrane protein expressed exclusively in outer hair cells and carrying a payload of D JNKi 1, a JNK pathway inhibitor. While we use this combination in our experiments, any drug or biomaterial can delivered to any targeted cells using this nanohydrogel platform.
2. Methods
2.1. Targeting peptide design and synthesis
We utilized a peptide reported to target extracellular domains of the OHC-specific protein, prestin [14]. We modified the design of this peptide by adding a further eight amino acids to the C-terminal end, GGSCGGSK(N3). The modification enable the peptide could be conjugated to other moieties either through its terminal azide (N3) group or by the cysteine residue. The GGS sequences act as linkers and spacers to minimize any potential steric hindrance which could prevent access to the reactive cysteine or N3 groups. This peptide was given the designation PrTP1 (Prestin Targeting Peptide 1) and was synthesized by GenScript (Piscataway, NJ, USA). The amino acid sequence for PrTP1 is: LSTHTTESRSMVGGSCGGS[Lys(N3)].
2.2. Peptide conjugated fluorescent nanoparticle preparation, characterization, and purification
The MFNPs were prepared using a modification to the thin-film hydration technique previously described [7]. Briefly, 55 mol% hydrogenated soy phosphatidylcholine (HSPC)/39.9 mol% cholesterol/ 4 mol% 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] (DSPE-PEG[2000])/1 mol% 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[dibenzocyclooctyl(polyethylene glycol)-2000](ammonium salt) (DSPE-PEG[2000]-DBCO)/0.1 mol% 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl)(ammonium salt) (18:1 Liss Rhod PE) or 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(Cyanine 5) (18:1 Cy5 PE) were dissolved and mixed in chloroform and dried by rotary evaporation under a N2 flow for 4 h to remove solvent and yield a thin lipid film. All lipids and the mini-extruder were obtained from Avanti Polar Lipids (Alabaster, AL, USA). The lipids were hydrated with PBS and incubated at 60 °C for 30 min, followed by sonication for 30 min. The c-Jun N-terminal kinase inhibitor, D-JNKi-1was added to the MFNP components in the PBS hydration step. The samples were then extruded at least 21 times using a mini-extruder with a 0.2 μm membrane at 65 °C. The targeting peptide was added in a 1.25 × molar excess of the total DSPE-PEG[2000]-DBCO concentration and stirred at room temperature for 24 h. The MFNPs were purified using a PD-10 column (GE Healthcare, Piscataway, NJ, USA), concentrated at 14,000 g using a Amicon 50k filter unit (EMD Millipore, Billerica MA USA) and their size analyzed using a Zetasizer Nano range dynamic light scattering (DLS) instrument (Malvern Instruments, Malvern, UK). The eluted samples were kept at 4 °C in the dark until use.
2.3. Determination of peptide:DBCO conjugation ratio
We developed a method of determining peptide conjugation to MFNPs for peptides that do not possess a fluorescent moiety. From the MFNP composition above, the DBCO-rhodamine ratio is 10:1. The MFNP comprises a lipid bilayer thus, for a 90 nm diameter MFNP and assumed bilayer thickness of 5 nm (http://www.liposomes.org/2009/01/number-of-lipid-molecules-per-liposome.html), the outer DBCO:rhodamine ratio is 5.6. To confirm this assumption, we incubated unconjugated MFNPs for 24 h at 25 °C with a 1.25-fold excess of the fluorescent compound, pyrene azide (N3), to DBCO groups in the MFNP. We determined the absorption measurements at 339 nm (pyrene) and 568 nm (rhodamine) using a DU 730 Life Science UV/Vis spectrophotometer (Beckmann Coulter, Indianapolis, IN USA) and calculated their concentrations by using molar absorptivity values, determined from standard curves, of 10,100 and 53,900 M−1 cm−1, respectively. We determined the pyrene:rhodamine concentration ratio as 5.9 ± 0.58 (n = 5). This closely correlates with our assumed value of 5.6 and so we concluded that all the available external DBCO groups were bound by pyrene within 24 h. We used this to indirectly determine the peptide:rhodamine ratio after conjugation of peptide and MFNP (see above), the peptide-MFNP complex was then exposed to pyrene-N3 for a further 24 h which bound to the remaining available DBCO groups. By comparing concentration ratios for this sample to the pyrene-N3 only ratio (see above), we were able to calculate the peptide:DBCO conjugation ratio.
2.4. Cell culture, transfection and flow cytometry
For the cell line experiments, we utilized both House Ear Institute- Organ of Corti 1 (HEI-OC1) cells (for methodology, see [15]) and a CHO cell line expressing prestin (prestin-CHO). In the HEI-OC1 cell experiments we incubated cells with PrTP1-conjugated MFNPs and unconjugated MFNPs for 48 h at 37 °C and 10% CO2. After washing four times with PBS, cells were imaged (Eclipse TS100 microscope, Nikon Instruments, Melville NY, USA). IN the CHO experiments, we applied various concentrations of PrTP1 conjugated with a fluorophore for 1 h at 37 °C and analyzed cell fluorescence using flow cytometry. Flow cytometry was performed in the University of Pennsylvania Flow Cytometry Core using a LSRII flow cytometer (BD Biosciences, San Jose CA, USA) and data recorded with DiVa 6.1.3 software (BD Biosciences). Data was analyzed using FCSalyzer-α freeware (Sven Mostböck, Vienna, Austria).
2.5. Hydrogel preparation and application
The CGP-hydrogel was freshly prepared essentially as previously described [6]. Briefly, a 2% w/v solution of ultrapure chitosan 95/1000 (Heppe Medical Chitosan GmbH, Halle Germany) in 0.1 M HCl was prepared by vortexing overnight at room temperature. This solution was then kept at 4 °C until use. The thermosensitive hydrogel was prepared by adding a 55% (w/v) aqueous solution of glycero-2-phosphate (GP) (EMD Millipore, Billerica, MA, USA) dropwise while stirring by hand until the pH of the solution reached 7.2 ± 0.1. The addition of GP resulted in crosslinking with the hydrogel, forming a highly viscous thermosensitive chitosan-GP (CGP)-hydrogel complex. The complex was kept on ice until use within 2 h of preparation. The final concentrations of chitosan and GP in the CGP-hydrogel were 1.8% (w/v) and 5% (w/v), respectively.
Female CBA/J mice (Envigo, East Millstone NJ, USA), 6–10 weeks, weighing 19–25 g were used in the in vivo experiments. All animal experiments were carried out in accordance with the University of Pennsylvania Animal Care and Use Committee and the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). Anesthesia was achieved by intraperitoneal injection of a ketamine/xylazine (100 mg kg−1/ 10 mg kg−1) cocktail as described in our previous studies [4,5]. After anesthesia was achieved, a retroauricular incision was made to approach the temporal bone in the left ear. Using a microsurgical approach, a hole was drilled through the bulla with a 1-mm diamond burr to expose the RWN. Where appropriate, 0.2 μl CGP-hydrogel containing targeted or untargeted MFNPs was carefully applied to the RWN using a custom-made flame pulled glass electrode. The incision was closed with 4–0 Sofsilk suture (Covidien, Mansfield MA) and mice were allowed to recover while the hydrogel continued to solidify. The ability of the targeting peptide to facilitate MFNP binding to outer hair cells was investigated: after 24 h and 48 h, mice were euthanized and their fixed in 4% paraformaldehyde. The cochleae were removed, stained with DAPI and then imaged. For behavioral studies (auditory brainstem recordings, see below), the following were applied to the RWM using hydrogel: targeted and untargeted D-JNKi-1 containing MFNPs, targeted empty MFNPs and hydrogel only.
2.6. Perilymph harvesting
Cy5-conjugated MFNPs were prepared and applied to the RWM as described above. After 2 days, mice were euthanized and the cochleae (one treated with MFNPs and one untreated) immediately dissected. A small hole was made in the RWM using a custom-made borosilicate needle and approximately 0.3 μl perilymph solution extracted using this needle and an attached 1 ml syringe. The sample was transferred to a centrifuge tube, diluted approximately 1:10 in PBS and stored at 4 °C. The sample volume was then increased to 50 μl and the sample’s fluorescence was recorded using an Infinite M1000 plate reader and Magellan software (Tecan Group Ltd., Männedorf, Switzerland). The approximate concentration of MFNPs in the sample was then calculated by comparing its fluorescence to standard curve consisting of a series of Cy5-conjugated MFNPs concentrations.
2.7. Tissue harvesting, preparation and visualization
At designated time points, the mice were euthanized, and the cochleae on both sides of the mice were immediately dissected and fixed as previously described [6]. Some of the fixed cochleae were further stained for p-c-Jun and caspase-9, representing genes that are upregulated during apoptosis through extrinsic and intrinsic pathways, respectively. Small holes were made at the apex and base of the bony cochlea to allow free flow of fluid through the cochleae. 1% bovine serum albumin (BSA) in PBS was applied to the apex and allowed to fill the cochleae. The cochleae were then placed in a 1.5 ml centrifuge tube completely filled with 1% BSA and slowly rocked for 2 h. The cochleae were washed 3 times in PBS for 5 min each and then treated, using the same procedure as for the 1% BSA step, with p-c-Jun antibody (KM1) conjugated to Alexa Fluor 488 and caspase-9 antibody (96.1.23) conjugated to Alexa Fluor 647 (Santa Cruz Biotechnology, Inc., Dallas TX; both diluted 1:200 in 1% BSA) and rocked overnight at 4 °C. Finally, the cochleae were washed with PBS.
All tissues were kept in the fixative at 4 °C in the dark for at least 24 h and processed by the surface preparation technique [16,17]. The membranous cochlea was decalcified in 0.5 M EDTA, pH 8 for 1 h at room temperature. The tissues were then washed with PBS and mounted on slides. Fluorescence microscopy images were captured using an Axio Observer.Z1 motorized microscope system and Zen 2.3 software (Zeiss, Thornwood NY, USA), and analyzed using ImageJ (National Institute of Health, Bethesda, MD, USA).
2.8. Acoustic trauma
Two days after surgical application of the hydrogel (see above), the mice were subjected to acoustic trauma. Mice were placed in individual compartments of a custom made circular wire mesh cage (diameter 28 cm, height 15 cm, 6 compartments). The cage was placed on a slowly rotating turntable in a reverberant wooden box with a heavy duty speaker (PDBT68; Pyle Audio, Brooklyn NY) centrally located immediately above the cage. The mice were exposed for 4 h to broadband white noise (1382 Random Noise Generator; General Radio Company, Concord MA) at 115–120 dB for frequencies between 6.3 kHz and 20 kHz using a GEQ 131 31 band graphic equalizer (Nady, Emeryville CA) and an A205 dual high resolution power amplifier (SAE, Seattle WA).
2.9. Auditory brainstem response recordings
CBA/J mice were anesthetized as described above. Auditory brainstem responses (ABRs) were recorded in response to clicks and tones (4, 8, 16, 24 and 32 kHz) between 100 dB and 20 dB in 5 dB increments using a RZ6 signal processor and BioSig software (TDT, Alachua FL). ABRs were performed at various time-points: control, pre-noise (2 days post surgery), 1, 3, 7 and 14 days after noise. Pre-noise values were used as the baseline for threshold shifts and mice were excluded from the study if the threshold shifts between control and pre-noise, i.e., caused by surgery, exceeded 20 dB.
2.10. Statistics
All statistics were obtained using Student’s t-test and errors represent standard errors of the mean.
3. Results and discussion
3.1. Functionalization of MFNPs and incorporation of targeting moieties
Previously, we developed a novel method to deliver drugs to the inner ear minimally-invasively using MFNPs. This method, however, had no targeting function. In order to deliver therapeutic agents to specific targeted inner ear cell types, we developed a novel delivery system by replacing 1 mol% (20%) of the DSPE-PEG[2000] with DSPE-PEG[2000]-DBCO (see Methods). The incorporation of a reactive DBCO group into the MFNP allowed conjugation with any targeting molecule containing an azide (N3) group through copper-free click chemistry. We confirmed that this modification did not affect the stability of the MFNPs: using dynamic light scattering, the average diameter of MFNPs was unchanged at 86.7 ± 5.30 nm (n = 9). The protocol outlined in Fig. 2A (see Methods) was devised to demonstrate the degree of conjugation between targeting moieties and DBCO groups. We determined a peptide:DBCO conjugation ratio of 49 ± 6.7% (n = 6) and concluded that functionalized peptides successfully conjugated approximately half of the available DBCO groups in the MFNPs over a 24 h incubation period.
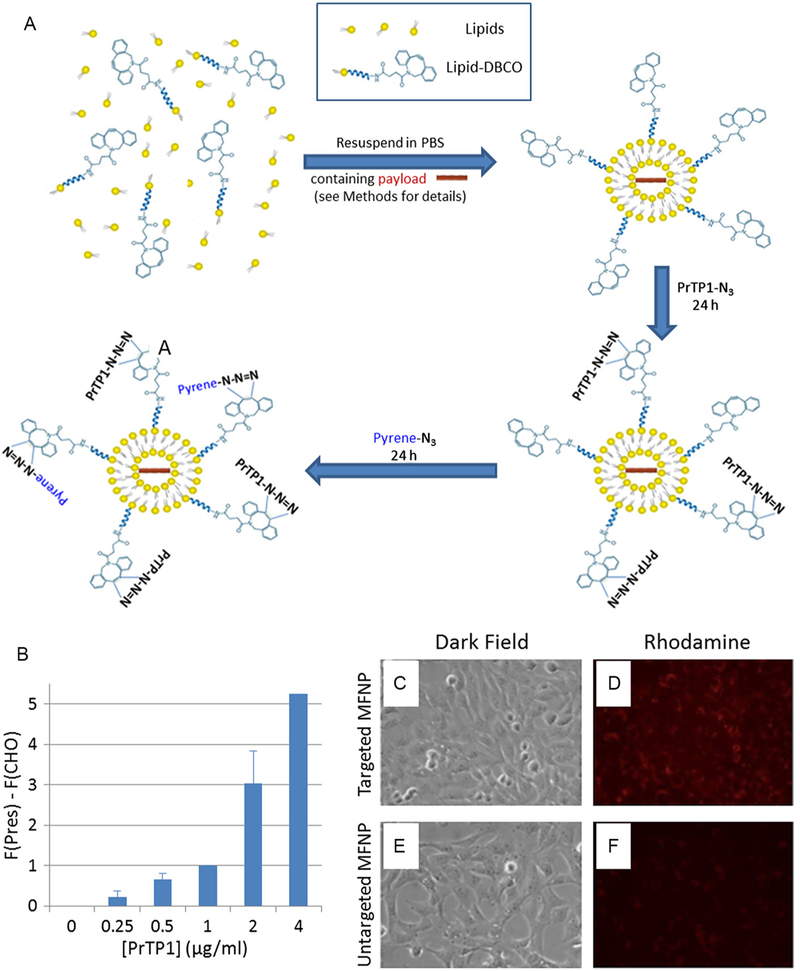
Fig. 2.
(A) Schematic representation of the determination of the conjugation ratio. The MFNP components dissolved in chloroform were mixed before the chloroform was removed by nitrogen gas and vacuum. The resulting constituents were resuspended in PBS (containing a payload where necessary) and treated as described in the Methods to form MFNPs. Targeted MFNPs were produced by incubating untargeted MFNPs with a 1.25-excess of PrTP1 for 24 h. The targeted MFNPs were then further incubated with pyrene-N3 for 24 h to determine the degree of peptide conjugation. By comparing the pyrene:rhodamine concentration ratio in these samples and those from samples only incubated in pyrene, we deduced a PrTP1:DBCO conjugation ratio of 49 ± 2.7% (n = 6, see Methods for more details). (B) The ability of the PrTP1 peptide to bind to gerbil prestin (gPrestin) expressed in CHO cells. A concentration-dependent increase in the fluorescent signal was observed when the Prestin-CHO cells were incubated with differing concentrations of the fluorescent PrTP1. F (gPres) = average fluorescence for gerbil prestin-expressing CHO cells. F(CHO) = average auto- fluorescence from untransfected CHO cells. Data normalized to the 1 μg/ml response. PrTP concentration [PrTP1] refers to the peptide concentration exclusive of any attached fluorescent probe. Number of replicates: 0, 0.25, 1 μg/ml: n = 4; 0.5, 2 μg/ml: n = 3; 4 μg/ml: n = 2. (C–F) PrTP1-targeted MFNPs demonstrate increased binding and fusion with HEI-OC1 cells. Cells were incubated for 48 h with targeted (C, D) and untargeted (E, F) MFNPs. (C, E) Dark field, (D, F) rhodamine.
One obstacle to targeting specific cell types is the identification of a unique constituent of the cells to target. One such unique inner ear component is the electromotile protein, prestin, which is exclusively expressed in OHCs [13]. We identified from the literature a novel peptide that binds to some extracellular domains of the transmembrane electromotile protein, prestin [14]. The peptide was then functionalized by incorporating a C-terminus Lys-N3 moiety and denoted as Prestin Targeting Peptide 1 (PrTP1).
3.2. PrTP1 targets cells expressing prestin
We studied the ability of PrTP1 to target prestin using 2 techniques: firstly, we used a stable prestin-CHO (Chinese Hamster Ovary) cell line to confirm the ability of PrTP1 conjugated to a fluorophore to bind to prestin-CHO cells. We applied various concentrations (125–4000 ng/ml PrTP1 content) of PrTP1 and analyzed cell fluorescence using flow cytometry (Fig. 2B). After selecting a population of cells which excluded cell debris or cell aggregates, we measured a concentration-dependent increase in fluorescence from Prestin-expressing CHO cells compared to untransfected CHO cells, demonstrating that PrTP1 bound to prestin.
Secondly, we investigated the binding effciency of tMFNPs to HEI-OC1 cells (Fig. 2C–F). We hypothesized that the use of PrTP1 conjugated to MFNPs would increase the binding effciency of the MFNPs to OHCs. The uptake of the MFNPs by these cells was significantly higher for tMFNPs (Fig. 2D) than with untargeted MFNPs (Fig. 2F), suggesting that PrTP1 increases binding effciency of the MFNP to these cells.
3.3. MFNPs are delivered to inner ear fluids using nanohydrogel system in vivo
Having confirmed that PrTP1 targets prestin expressed in HEI-OC1 and prestin-CHO cells, we use our previously published nanohydrogel technique to apply the tMFNPs and untargeted MFNPs to the cochleae of adult mice. We confirmed that this method successfully delivered MFNPs by analyzing fluorescence from MFNPs in the perilymph 2 days after treatment. MFNP concentrations of 0.06 and 0.14 nM were detected in perilymph 2 samples that had been diluted 500-fold. This indicated that the MFNP concentration within the perilymph was approximately 50 nM. This represents around 5% of the concentration in the hydrogel applied to the RWM.
3.4. Targeted MFNPs bind to OHCs in vivo
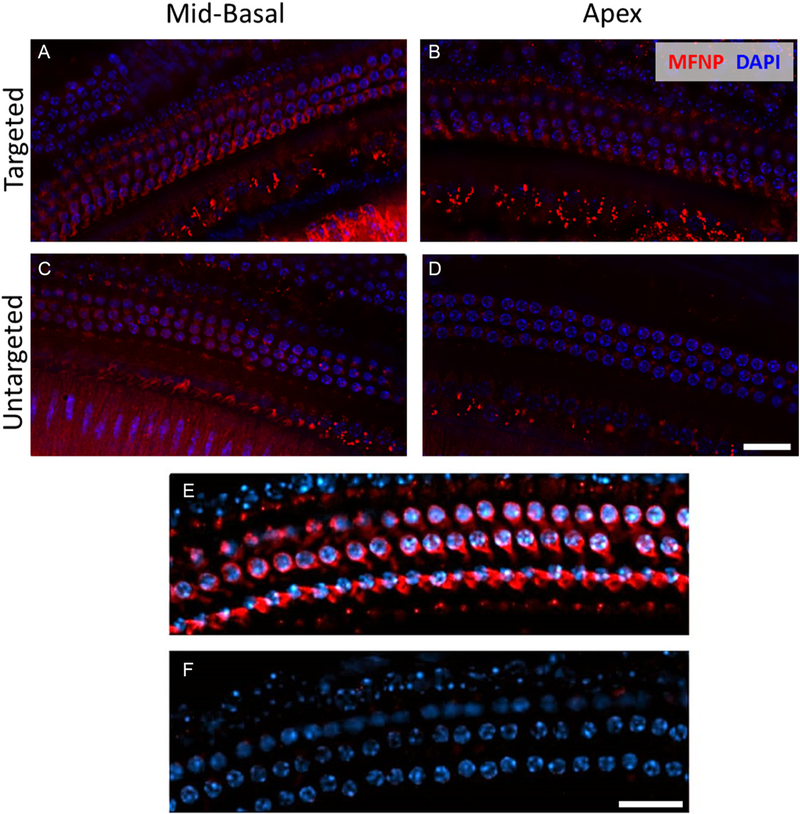
We studied the ability of tMFNPs to bind to OHCs in vivo using fluorescence microscopy. Rhodamine (representing the MFNP) was present in OHCs located in the mid-basal region of the cochlea for both tMFNPs and untargeted MFNPs (Fig. 3A,C). In contrast, rhodamine was observed in the apical region (Fig. 3B) when tMFNPs were present, but was absent when untargeted MFNPs were used(Fig. 3D). Thus, the use of tMFNPs enhances the delivery of the MFNPs to the apical region, which is critical for speech perception in humans. This is finding is potentially highly significant as this region has proved to be hard to reach using the existing methods of drug delivery. The red coloration of the OHC membranes (Fig. 3E), indicates the presence of bound tMFNPs. This coloration was absent in untreated samples (Fig. 3F), demonstrating that the tMFNPs were associated with the cell membrane.
Fig. 3.
The targeting peptide PrTP1 enhances MFNPs binding to OHCs in vivo. Images from the mid basal (A,C) and apical (B,D) regions of adult fixed cochleae for targeted MFNP (A,B) and untargeted MFNP (C,D) 2 days after hydrogel administration. Cyan represents DAPI and red represents MFNPs. (E,F) Exemplar high magnification confocal images of the organ of Corti clearly showing 3 rows of OHCs. (E) Nanohydrogel with PrTP1-conjugated rhodamine-containing MFNPs was applied to the round window niche of the middle ear and left for 24 h. Scale bars, 20 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. tMFNPs deliver payload to OHCs in vivo
We delivered tMFNPs containing a plasmid encoding TdTomato (TdT) and 5(6)-carboxyfluorescein (CF) using the hydrogel technique, (Fig. 4). After 5 days, the mice were euthanized and the cochleae removed, fixed and the organs of Corti dissected. Fig. 4 (D,F) demonstrate that an increase in fluorescence signal of the green (CF), (D) and red (TdT), (F) was observed in the treated cochleae. However, the untreated control cochleae showed no fluorescence for CF (C) or TdT (E), other than the autofluorescence from supporting cells. This indicates that the MFNPs bound to OHCs, released their payload into the cytoplasm and, in the case of the plasmid, the TdTomato protein was expressed by the cell. This clearly demonstrates that tMFNPs can transport payloads into specific cell types then release the payloads into the cell which can subsequently be used or translated by the cells. This is consistent with work in the mouse eye where liposomes were used to deliver plasmid to ocular cells resulting in mRNA expression levels 30–50% of those observed for AAV infection [18].
Fig. 4.
Targeted MFNPs containing 5(6) carboxyfluorescein (CF) (Green) and TdTomato (TdT) (Red) plasmid was given in vivo, to delivere both payloads to OHCs. After 5 days, the mice were euthanized. Scale bar, 20 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. tMFNPs with a D-JNKi-1 payload protect from acoustic trauma
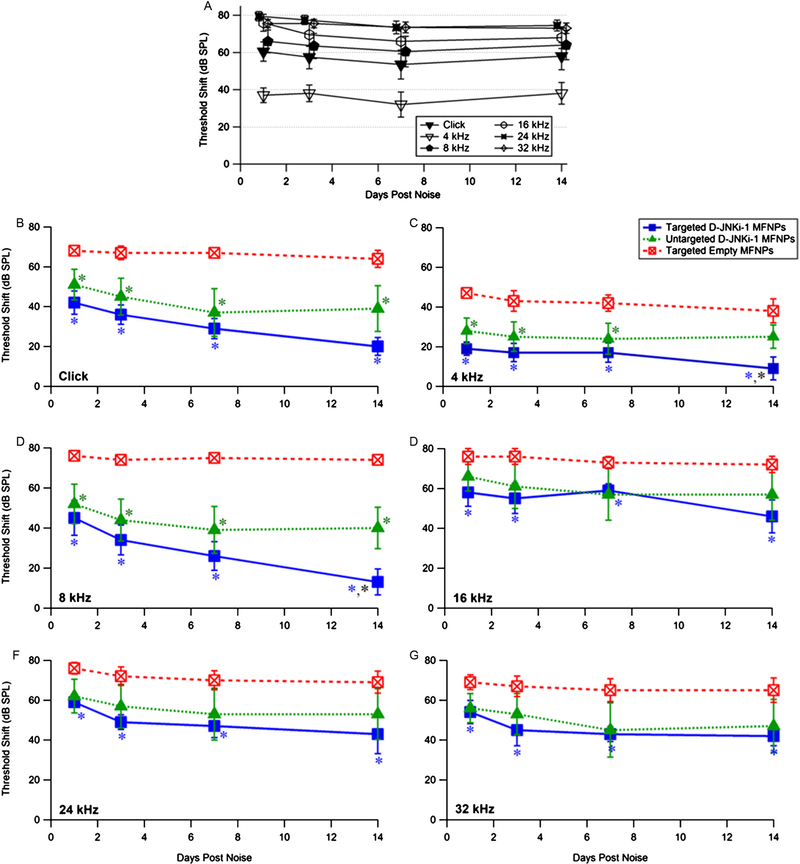
Having determined that PrTP1 can target MFNPs and deliver payloads to OHCs in vivo, the ability of these tMFNPs to deliver a JNK-inhibitor peptide, D-JNKi-1, to the inner ear and protect OHCs from NIHL was investigated. This agent was chosen because D-JNKi-1 had previously been demonstrated to rescue HL induced by noise in guinea pigs [11]. We compared the data obtained with targeted D-JNKi-1 containing MFNPs with those obtained with untargeted D-JNKi-1 containing MFNPs, and empty tMFNPs controls. tMFNPs containing D-JNKi-1 protected from noise exposure significantly more than controls at all time points and frequencies (Fig. 5B–G P < 0.05; *). In contrast, untargeted D-JNKi-1 were only significantly different from empty MFNPs for clicks, and at 4 and 8 kHz frequencies (B–D; P < 0.05, *). Additionally, the hearing threshold for D-JNKi-1 tMFNPs in response to clicks, 4 kHz and 8 kHz stimulations was improved compared to untargeted D-JNKi-1 MFNPs, with significance noted at 14 d post noise at 4 and 8 kHz (Fig. 5C,D). This is consistent with our observations that more tMFNPs binding was observed to apical OHCs than with untargeted MFNPs (Fig. 3B,D). The D-JNKi-1 tMFNPs consistently produced significant improvements in hearing threshold shift compared to empty tMFNPs, in contrast to the higher variability in results obtained with untargeted d-JNKi-1 MFNPs (compare targeted vs. untargeted error bars in Fig. 5B–G). Both targeted and untargeted D-JNKi-1-MFNPs provide similar partial protection at 16, 24 and 32 kHz (E–G).
Fig. 5.
(A) Acoustic trauma paradigm produces a permanent stable threshold shift over 14 days (n = 10). Mice were exposed to noise for 4 h at 115–120 dB as described (see Methods). Data for4, 8, 24 and 32 kHz are offset for clarity. Pretreatment 2 d prior to noise exposure with D-JNKi-1 imparts partial protection from acoustic trauma in mice up to 14 d post noise at most frequencies tested. Hearing threshold shifts for targeted empty (red), targeted D-JNKi-1 (blue) and untargeted D-JNKi-1 (green) MFNPs are shown for responses to (B) Click, (C) 4, (D) 8, (E) 16, (F) 24 and (G) 32 kHz. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The present study demonstrates partial recovery of hearing from NIHL, which contrasts with the complete recovery, described previously [11]. In that publication, the authors applied a brief 15 min 130 dB 6 kHz pure tone to guinea pigs, which resulted in permanent threshold shifts of only approximately 10 dB at 4 kHz and above 20 kHz, with maximal shifts of 30–35 dB at 6–12 kHz [11]. Our paradigm (4 h, 6.3 kHz–20 kHz, 115–120 dB) produces much more dramatic permanent threshold shifts (40 dB at 4 kHz and 65–75 kHz for 8–32 kHz). Thus, when comparing changes in threshold shift between untreated and D-JNKi-1 treated mice, our data are more compelling: while Wang and colleagues observed full recovery of hearing (i.e., threshold shift = 0 dB) at all frequencies at 15 d [11]. This represents threshold changes of only approximately 10–35 dB. We consistently observed greater changes in threshold shifts after 14 days at all frequencies studied, with the most dramatic changes at 8 kHz (approximately 60 dB improvement). Thus, application of tMFNPs containing d-JNKi-1 using a minimally-invasive hydrogel system produces a more desirable outcome in hearing improvement than D-JNKi-1 applied using a highly invasive minipump system [11].
3.7. Acoustic Trauma activates an extrinsic but not an intrinsic apoptotic pathway
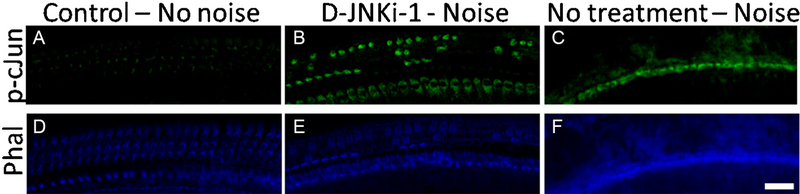
D-JNKi-1 partially protected mice from NIHL. We confirmed by immunofluorescence that the JNK pathway was indeed activated by our noise paradigm. Five days after noise exposure, while p-c-Jun (a phosphorylated protein encoded by JNK) was not activated in unexposed cochleae (Fig. 6A), the presence of p-c-Jun was clearly visible in D-JNKi-1 treated cochleae (Fig. 6B) but was essentially absent from untreated noise exposed cochleae (Fig. 6C). This is consistent with c- Jun phosphorylation being activated by non-traumatic noise exposure, indicating p-c-Jun is a marker for cochlear stress, rather than hair cell apoptosis [19]. This stress ultimately may lead to hair cell death: in the unprotected exposed cochleae, the lack of phalloidin staining (Fig. 6F), compared to control or D-JNKi-1 treated cochleae (Fig. 6D,E), demonstrates substantial OHC loss. In contrast, D-JNKi-1-treated exposed cochleae showed stress but little OHC death. Thus, D-JNKi-1 clearly inhibited apoptosis and protected OHCs.
Fig. 6.
The JNK pathway is activated by noise exposure in hair cells. Cochleae were stained for p-c- Jun (green, A–C) and phalloidin (blue, D–F). While there was no p-c-Jun staining observed in untreated, unexposed cochleae (A), some was observed in noise exposed, D-JNKi-1 treated cochleae (B) and only non-specific binding observed in noise-exposed, untreated cochleae (C). This can be explained by comparing phalloidin staining, which demonstrates the presence of hair cells. The 3 rows of OHCs were present in control cochleae (D), individual OHCs were missing in the exposed, D-JNKi-1 treated cochlea (E) and completely absent in the exposed, untreated cochleae (F). Thus, noise exposure resulted in most OHCs being destroyed in the untreated mice, whereas some OHCs were preserved by D-JNKi-1. Scale bar, 20 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Our minimally-invasive system delivered tMFNPs with a D-JNKi-1 payload to the inner ear and protected from NIHL to a greater extent than previously described for more invasive procedures. The remaining threshold shifts after treatment may be a result of other potential hair cell apoptotic pathways, independent of the c-Jun pathway, which may have been activated by our noise exposure protocol and hence would not have been inhibited by our application of D-JNKi-1 (see [20] for a review of the various pathways in hair cells). For example, noise exposure has been demonstrated to activate the intrinsic reactive oxygen species (ROS) pathway, which is unaffected by D-JNKi-1 inhibition of the JNK pathway (for a review, see [21]) and thus would be expected to be a contributing factor to NIHL.
4. Conclusions
Sensorineural hearing loss is a significant clinical problem with currently no effective means of prevention or treatment. Delivering drugs and biomaterials to the inner ear is challenging because of its anatomical restrictions and the presence of the blood-inner ear barrier, which limit the use of systemic drugs for this purpose. Our previous research has demonstrated that application of a stable, regulated MFNP-containing hydrogel onto the round window membrane allows for the controlled and sustained delivery of therapeutic agents to the inner ear structures. The MFNPs can stably encapsulate their payload under physiological conditions and so are ideal to deliver any biomaterial to the inner ear. Once these nanoparticles entered the perilymph, however, there were no mechanisms in place to control binding to specific inner ear cell types. We have overcome this issue by using a targeting peptide.
We have demonstrated successful delivery of PrTP1-conjugated multifunctional nanoparticles to the inner ear using a minimally-invasive nanohydrogel delivery system. This system allowed us to successfully deliver a JNK pathway inhibitor, D-JNKi-1, which attenuated hearing loss caused by acoustic trauma. Additionally, we were able to deliver more tMFNPs with payloads to specific cell types at the cochlear apex than untargeted MFNPs, opening the possibility of therapeutic treatments at critical speech perception frequencies. The potential to deliver biomaterials to specific inner ear cell types at speech-relevant frequencies, thereby reducing potential side effects caused by untargeted delivery systems, and protect from acoustic trauma is a very exciting development. This targeted nanohydrogel delivery system is expected to form the basis of a treatment platform for future clinical applications and has the potential to produce a dramatic improvement in therapeutic outcomes for many inner ear diseases.
Acknowledgements
We thank Dr. James Saunders for his assistance with the noise exposure procedure, Dr. Rende Gu (Sound Pharmaceuticals Inc., Seattle WA) for assistance with inner ear morphology, Dr. Qiang Song for technical assistance and the University of Pennsylvania Microscopy Core.
Funding
This work was supported by the National Institute for Deafness and Communication Disorders (NIDCD R01 DC014464, 2015). NIDCD had no involvement in study design, the collection, analysis and interpretation of data, writing of this report or the decision to submit this article for publication.
Abbreviations:
- HL
Hearing loss
- SNHL
Sensorineural hearing loss
- NIHL
Noise-induced hearing loss
- CGP
Chitosan glycerophosphate
- RWM
Round window membrane
- RWN
Round window niche
- PrTP
Prestin targeting peptide 1
- (t)MFNP
Targeted multifunctional nanoparticle
- OHC
Outer hair cell
- JNK
c-Jun N-terminal kinase
Footnotes
Declarations of interest
None.
References
- [1].http://www.who.int/mediacentre/factsheets/fs300/en/, (2017), Accessed date: 23 October 2017.
- [2].Ruben RJ, Laryngoscope 110 (2000) 241–245. [DOI] [PubMed] [Google Scholar]
- [3].Huth ME, Ricci AJ, Cheng AG, Int. J. Otolaryngol 2011 (2011) 937861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Paulson DP, Abuzeid W, Jiang H, Oe T, O’Malley BW, Li D, Laryngoscope 118 (2008) 706–711. [DOI] [PubMed] [Google Scholar]
- [5].Xu L, Heldrich J, Wang H, Yamashita T, Miyamoto S, Li A, Uboh CE, You Y, Bigelow D, Ruckenstein M, O’Malley B, Li D, Otol. Neurotol 31 (2010) 1115–1121. [DOI] [PubMed] [Google Scholar]
- [6].Lajud SA, Han Z, Chi FL, Gu R, Nagda DA, Bezpalko O, Sanyal S, Bur A, Han Z, O’Malley BW Jr., Li D, J. Control. Release 166 (2013) 268–276. [DOI] [PubMed] [Google Scholar]
- [7].Lajud SA, Nagda DA, Qiao P, Tanaka N, Civantos A, Gu R, Cheng Z, Tsourkas A, O’Malley BW Jr., Li D, Otol. Neurotol 36 (2015) 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pinyon JL, Tadros SF, Froud KE, Wong ACY, Tompson IT, Crawford EN, Ko M, Morris R, Klugmann M, Housley GD, Sci. Transl. Med 6 (2014) 233ra254. [DOI] [PubMed] [Google Scholar]
- [9].Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto AM, Walton KM, Ylikoski J, Neurosci J. 20 (2000) 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Suckfuell M, Canis M, Strieth S, Scherer H, Haisch A, Acta Otolaryngol 127 (2007) 938–942. [DOI] [PubMed] [Google Scholar]
- [11].Wang J, Ruel J, Ladrech S, Bonny C, van de Water TR, Puel JL, Mol. Pharmacol 71 (2007) 654–666. [DOI] [PubMed] [Google Scholar]
- [12].Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U, Hear. Res 163 (2002) 71–81. [DOI] [PubMed] [Google Scholar]
- [13].Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P, Nature 405 (2000) 149–155. [DOI] [PubMed] [Google Scholar]
- [14].Surovtseva EV, Johnston AH, Zhang W, Zhang Y, Kim A, Murakoshi M, Wada H, Newman TA, Zou J, Pyykko I, Int. J. Pharm 424 (2012) 121–127. [DOI] [PubMed] [Google Scholar]
- [15].Kalinec GM, Park C, Thein P, Kalinec F, J. Vis. Exp 115 (2016) e54425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].O’Malley BW Jr., Li D, Turner DS, Hear. Res 88 (1995) 181–189. [DOI] [PubMed] [Google Scholar]
- [17].Xiang M, Gan L, Li D, Chen ZY, Zhou L, O’Malley BW Jr., Klein W, Nathans J, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 9445–9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Han Z, Conley SM, Makkia R, Guo J, Cooper MJ, Naash MI, PLoS One 7 (2012) e52189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Anttonen T, Herranen A, Virkkala J, Kirjavainen A, Elomaa P, Laos M, Liang X, Ylikoski J, Behrens A, Pirvola U, eNeuro 3 (2016) 1–17 e0047–16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dinh CT, Goncalves S, Bas E, Van De Water TR, Zine A, Front. Cell. Neurosci 9 (2015) 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Henderson D, Bielefeld EC, Harris KC, Hu BH, Ear Hear 27 (2006) 1–19. [DOI] [PubMed] [Google Scholar]