A screen of microbial culture broths led by Akira Endo in the 1970’s led to the discovery of compactin, a competitive inhibitor of the rate-limiting enzyme in cholesterol synthesis HMG-CoA reductase (HMGCR). Subsequent development of closely related compounds gave rise to statin medications. Numerous clinical trials of statin therapy demonstrate robust reductions in both low-density lipoprotein cholesterol (LDL-C) and cardiovascular events, reinforcing their role as the pharmacologic backbone of current hyperlipidemia treatment strategies.
These striking clinical trial results led researchers to seek evidence for pleiotropic cholesterol-independent effects of statins; the impact of statins on metrics of inflammation, endothelial dysfunction, oxidative stress, and immunomodulation was carefully examined. Favorable results from these studies gave rise to the “statin exceptionalism” hypothesis, the notion that statins exert clinical gain beyond that which would be predicted by the LDL-C reduction achieved. Recent advances in the realms of both clinical trials and human genetics provide an opportunity to test this hypothesis.
Clinical Trials.
From a clinical trial standpoint, the results of trials involving LDL-C reduction via two distinct mechanisms were reported in the past year. Ezetemibe is an inhibitor of cholesterol absorption via the Niemann-Pick C1-like 1 (NPC1L1) protein. The drug demonstrated modest but significant reductions in both LDL-C and cardiovascular events (12.8 mg/dl and 7.2% respectively) when added to simvastatin in patients following an acute coronary syndrome in the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT).1 Secondly, combined analyses of short-term studies implementing monoclonal antibodies that inhibit proprotein convertase subtilisin-kinexin 9 (PCSK9) were recently reported. PCSK9 inhibition lowers LDL-C by preventing the degradation of hepatic LDL receptors. Two different injectable compounds, alirocumab and evolocumab, led to dramatic reductions in both LDL-C (74 and 71 mg/dl respectively) and major cardiovascular events (risk reductions of 48% and 53% respectively) in post hoc analyses from the Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG TERM) and Open-Label Study of Long Term Evaluation against LDL-Cholesterol (OSLER) investigators.2,3 Critical additional trials powered for cardiovascular event and safety analyses are underway.
These new data allow for direct comparisons to now classic analyses from the Cholesterol Treatment Trialists’ Collaboration demonstrating a strong correlation between the LDL-C reduction noted after one-year of statin therapy in 14 randomized trials and the observed cardiovascular risk reduction.4 In contrast to the statin exceptionalism hypothesis, the recent NPC1L1- and PCSK9-based interventions demonstrate remarkable concordance with the expected cardiovascular benefit derived from statin trials (Figure 1A).
Figure 1.
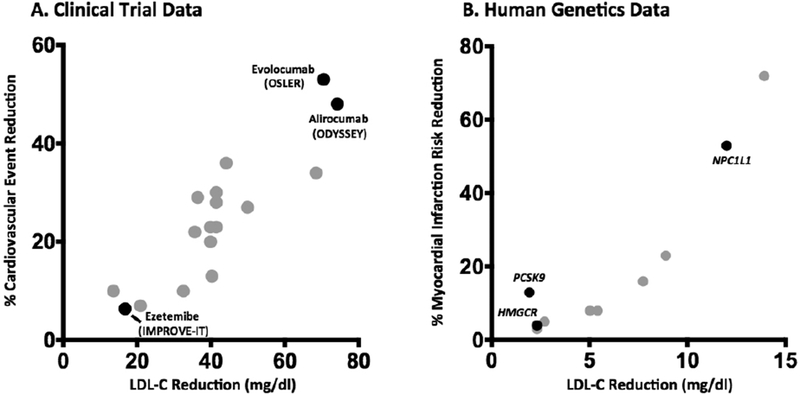
Relationship between pharmacologic and genetically mediated LDL-C reduction and vascular risk. A. The absolute LDL-C reduction achieved after roughly one year of therapy in a given trial is plotted against the percent reduction in cardiovascular event rate achieved. Gray points represent statin trials while labeled black points represent non-statin therapeutics (Refs 1–4). B. The absolute LDL-C reduction per inherited allele of a given polymorphism is plotted against the prospectively determined reduction in risk of myocardial infarction; NPC1L1 data point represents the impact of a combination of inactivating mutations on risk of coronary disease (Refs 5–6).
Human Genetics.
Rapid advances in DNA genotyping and sequencing technology have facilitated large-scale human genetics studies for numerous traits, including LDL-C. Such analyses permit the unbiased discovery of genes associated with natural LDL-C variation and subsequent interrogation of the effects of these variants on cardiovascular risk. Furthermore, because these variants are distributed in a random fashion at time of inception, this approach is less susceptible to the confounding that makes assessment of causality difficult using observational epidemiology approaches. One such analysis demonstrated that genetic variants related to circulating HDL-C levels have minimal relationship to coronary disease, calling into question the causal nature of the inverse relationship between circulating HDL-C and vascular events.5
By contrast, genetic variants linked to decreased LDL-C levels have demonstrated a fairly uniform relationship to decreased atherosclerotic risk. For example, individuals harboring a common variant at the HMGCR locus have a 2.3 mg/dl lower LDL-C level and a 4% coronary risk reduction.5 Importantly, ten other loci demonstrate similarly robust relationships with LDL-C and coronary risk, including those at the NPC1L1 and PCSK9 genes whose products are targeted in the trials discussed above.5–6 Because these variants affect LDL-C via mechanisms distinct from HMGCR, they offer a second key test of the exceptionalist hypothesis. Specifically, if HMGCR modulation is unique in its atheroprotective properties, its impact on coronary disease should exceed the observed cholesterol reduction. We see no evidence of this; a linear relationship between LDL-C reduction and coronary disease is again noted (Figure 1B). Comparison of the clinical trial and genetic data suggests that risk reduction for any given LDL-C change is magnified via the genetically-mediated lifelong reduction in cumulative exposure (“LDL-years”) as opposed to pharmacologic reductions restricted to the relatively short trial intervention and follow-up.
Implications.
The data described serve to increase optimism for the utility of genotype-phenotype studies in confirming causal relationships and predicting the impact of pharmacologic modulation of a putative drug target. For the HMGCR, NPC1L1, and PCSK9 loci, the benefit of inhibition predicted by human genetics was realized in large-scale clinical trials. Importantly, these analyses can be useful in screening for potential on-target safety signals in addition to efficacy. For example, a HMGCR polymorphism linked to decreased LDL-C is also associated with a modest increase in plasma glucose and risk of diabetes; this observation is strikingly consistent with the somewhat increased risk of diabetes observed in statin-based clinical trials.7
Although the benefits of statins appear largely captured by reductions in circulating cholesterol at the population level, we cannot rule out clinically relevant pleiotropy in specific patient subgroups or disease states. We nevertheless would urge caution in the use of statin for non-cardiovascular clinical benefit in the absence of robust trial evidence.
Moving forward, statins will remain the gold standard for a medication class that is safe, efficacious, and cost-effective in cardioprevention; they just might be less exceptional than once thought. We believe that a revision of the statin exceptionalism hypothesis should be met with enthusiasm, providing hope that moving beyond HMGCR inhibition will allow for additional risk attenuation.
Footnotes
Disclosures
Dr. Khera has served as a consultant for Merck. Dr. Kathiresan has received research grants from Merck, Bayer, and Aegerion; has served on scientific advisory boards for Catabasis, Regeneron Genetics Center, Merck, and Celera; holds equity in San Therapeutics and Catabasis; and has served as a consultant for Novartis, Aegerion, Bristol-Myers Squibb, Sanofi, AstraZeneca, and Alnylam.
References
- 1.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372(25):2387–97. [DOI] [PubMed] [Google Scholar]
- 2.Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99. [DOI] [PubMed] [Google Scholar]
- 3.Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–9. [DOI] [PubMed] [Google Scholar]
- 4.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78. [DOI] [PubMed] [Google Scholar]
- 5.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myocardial Infarction Genetics Consortium Investigators. Inactivating mutations in NPC1L1 and protection from coronry heart disease. N Engl J Med. 2014;371(22):2072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swerdlow DI, Preiss D, Kuchenbaecker KB, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385(9965):351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


