Abstract
The semantic variant of primary progressive aphasia (PPA-S) is diagnosed based on impaired single-word comprehension, but nonverbal impairments in face and object recognition can also be present, particularly in later disease stages. PPA-S is associated with focal atrophy in the left anterior temporal lobe (ATL), often accompanied by a lesser degree of atrophy in the right ATL. According to a dual-route account, the left ATL is critical for verbal access to conceptual knowledge while nonverbal access to conceptual knowledge depends upon the integrity of right ATL. Consistent with this view, single-word comprehension deficits in PPA-S have consistently been linked to the degree of atrophy in left ATL. In the current study we examined object processing and cortical thickness in 19 patients diagnosed with PPA-S, to evaluate the hypothesis that nonverbal object impairments would instead be determined by the amount of atrophy in the right ATL. All patients demonstrated inability to access conceptual knowledge on standardized tests with word stimuli: they were unable to match spoken words with their corresponding pictures on the Peabody Picture Vocabulary Test. Only a minority of patients, however, performed abnormally on an experimental thematic verification task, which requires judgments as to whether pairs of object pictures are thematically-associated, and does not rely on auditory or visual word input. The entire PPA-S group showed cortical thinning in left ATL, but atrophy in right ATL was more prominent in the subgroup with low verification scores. Thematic verification scores were correlated with cortical thickness in the right rather than left ATL, an asymmetric mapping which persisted when controlling for the degree of atrophy in the contralateral hemisphere. These results are consistent with a dual-route account of conceptual knowledge: breakdown of the verbal left hemispheric route produces an aphasic syndrome, which is only accompanied by visual object processing impairments when the nonverbal right hemispheric route is also compromised
Keywords: Anterior temporal lobe, Progressive aphasia, Semantic dementia, Agnosia, Object recognition
1. INTRODUCTION
The anterior temporal lobe (ATL) was ignored in classic accounts of language and cognition (Mesulam et al., 2015), in part because it is rarely the center of stroke lesions. This has changed dramatically through the study of patients with focal neurodegeneration in ATL, most frequently caused by TDP-43 proteinopathy and Pick’s disease (Mesulam et al., 2014a, 2014b; Rohrer et al., 2011). The most consistent symptoms shown by such patients are inability to name objects (anomia) and loss of single-word comprehension, but nonverbal impairments in object and face recognition are also common (Mesulam et al., 2014a, 2014b). Collectively, these symptoms suggest a fundamental problem in employing conceptual/semantic knowledge, so this syndrome is either referred to as the semantic variant of primary progressive aphasia (PPA-S) or as semantic dementia (SD) (Gorno- Tempini et al., 2004, 2011; Mesulam et al., 2003, 2014a, 2014b; Snowden et al., 1989). PPA-S and SD may be distinguished based on the prominence of nonverbal impairments (being more central to the latter) (Adlam et al., 2006; Mesulam et al., 2003), but they are also often treated interchangeably (see (Gorno-Tempini et al., 2011)). Regardless of these changes in nosology, such patients provide a valuable opportunity to study how conceptual knowledge is arranged in the human brain.
Warrington (1975) provided the first modern description of SD. She identified three patients with neurodegenerative etiology, all of whom showed no visuoperceptual impairments in viewing geometric shapes, common objects, or faces. Warrington assessed their ability to access conceptual knowledge by asking them to define (describe aloud) pictures of common objects (e.g. animals), to define the spoken names of those same objects, and to match spoken names with pictures on the Peabody Picture Vocabulary Test (PPVT) (Dunn, 2007). All three patients showed impairments on the PPVT and word definitions, and two of three patients produced abnormal picture definitions, thus presenting with a mixture of verbal and nonverbal conceptual deficits.
The standard neurological diagnoses for these patients would have been concurrent Wernicke-like aphasia (Wernicke, 1874) and associative agnosia (Lissauer, 1890), attributable to dysfunction in the left perisylvian language network and the ventral visual stream, respectively (Geschwind, 1965). Warrington offered an alternative mechanism based on a construct that was emerging at that time in the field of cognitive psychology: semantic memory (Tulving, 1972). Difficulties with both word and picture stimuli were parsimoniously explained as commonly stemming from damage to a unitary long-term memory system, which was dedicated to the storage of conceptual knowledge. The proposed storage mechanism rendered the route of access immaterial (verbal, nonverbal), as each pathway eventually leads to the same damaged knowledge system (i.e. a unitary system). Warrington’s semantic memory framework was widely adopted afterwards (Snowden et al., 1989). Morphometric magnetic resonance imaging (MRI) eventually implicated the anterior portion of the temporal lobes as the common denominator in virtually every case of SD (Mummery et al., 2000, 1999; Rosen et al., 2002), with some degree of atrophy usually being present in the ATLs in both hemispheres. Based on this evidence, Warrington’s unitary storehouse was proposed to be localized to ATL (Adlam et al., 2006; Rogers et al., 2004), which has been characterized as the brain’s primary “semantic hub” (Lambon Ralph, 2014; Patterson et al., 2007).
Despite favoring a unitary account of conceptual impairments, in her initial report Warrington (1975) noted that the “correspondence of the deficits for words and for visual objects is far from complete”. For example, while patient E.M. successfully defined 93% of the picture stimuli in that study, she was only able to define 65% of the corresponding word stimuli. Similar verbal/nonverbal splits in performance are now known to be common in SD (Bozeat et al., 2000; Lambon Ralph et al., 1999), and are perhaps most clearly demonstrated in the related and overlapping syndrome of PPA-S.
PPA-S has historically been described and diagnosed as an aphasic syndrome rather than as a disorder of semantic memory (Gorno- Tempini et al., 2011; Mesulam et al., 2009a; Mesulam, 1982). In its initial stages atrophy is often confined to ATL in the left hemisphere, as evidenced by a consecutive case-series analysis where 8 out of 11 early- stage PPA-S patients showed left unilateral rather than bilateral atrophy according to cortical thickness analysis (Mesulam et al., 2013). Patients in that study and others (Mesulam et al., 2009a) produced fewer specific details when defining nouns than when defining pictures of those same items. PPA-S patients are also slower and less accurate when matching noun stimuli, and generate lower amplitude N400 potentials in response to nouns, compared to judgments of picture stimuli, and these verbal abnormalities correlate with cortical thickness in left ATL (Hurley et al., 2012). Likewise, patient scores on the PPVT, which is commonly used to assess single-word comprehension, are correlated with cortical thickness in left ATL (Rogalski et al., 2011a). In summary, verbal impairments often outweigh nonverbal impairments in PPA-S and SD, and critical substrates of verbal comprehension have been localized to the left rather than right ATL.
Discrepancies in performance based on route of access conflict with a basic unitary storehouse model, which have prompted a variety of elaborations to that model and the development of alternative accounts. According to a dual-route account of ATL functionality, ATL is part of a verbal route to conceptual knowledge in the left hemisphere, and partof a nonverbal route to conceptual knowledge in the right hemisphere (Gefen et al., 2013; Hurley et al., 2012; Mesulam et al., 2013; Nilakantan et al., 2017). The dual-route account thus describes a functional asymmetry in ATL: the left ATL is critical for conceptual access via word form input (written or spoken), while the right ATL mediates access via nonverbal object input (e.g. visual images).
Selective disruption of the verbal route has been well-characterized in PPA-S, and many theories and descriptions of ATL now include provisions for left-hemispheric language specialization (Gainotti, 2014; Lambon Ralph, 2014; Lambon Ralph et al., 2001; Mesulam et al., 2013). Fewer attempts have been made to localize the nonverbal route, but the results from several studies suggest that right ATL atrophy is particularly disruptive to recognition of facial images. Whereas left ATL lesions are associated with failure to describe celebrities when provided with their names, right-hemispheric lesions are associated with failure to describe those same individuals based on pictures of their faces (Evans et al., 1995; Gainotti, 2007; Gainotti et al., 2003, 2010; Gefen et al., 2013; Snowden et al., 2004, 2012; Tranel, 2006). These studies support the hypothesized role of right ATL in nonverbal access, but some have argued that recognition of specific persons depends on different neural machinery compared to recognition of “common object” stimuli such as animals or tools (McMullen et al., 2000; Pitcher et al., 2009; Thompson et al., 2004; Wallis, 2013).
The Pyramids and Palm Trees Test (PPT) is commonly employed to assess conceptual knowledge of common objects, where a cue picture must be matched to a thematically associated target picture rather than a foil (Howard and Patterson, 1992).1 The verbal version of the PPT includes a comparable test with written words rather than pictured objects. At least one of the aforementioned studies of person knowledge found correlations between famous face recognition and the picture version of the PPT, and between famous name recognition and the word version of the PPT, but no formal analyses were conducted in that study to directly demonstrate a relationship between PPT scores and leftward vs rightward ATL atrophy (Snowden et al., 2004). Another study including larger samples of both leftward and rightward patients found no differences between the word and picture versions of the PPT in either subgroup (Thompson et al., 2003). Butler et al. (2009) examined correlations between PPT scores and atrophy (via voxel-based morphometry) in a large sample of degenerative patients including SD and other syndromes. Word and picture scores were both correlated with atrophy in both the left and right ATLs. When picture scores were included as a covariate, however, there was a specific association between word scores and left (but not right) ATL atrophy. Likewise, when word scores were included as a covariate, picture scores were selectively correlated with atrophy in right ATL. Further clinic anatomic investigations are thus needed to help clarify what role the ATLs in each hemisphere play in nonverbal object processing.
There are two inherent methodological challenges when attempting to assess nonverbal object processing, and previous studies may be critiqued on both points. Firstly, it is extremely difficult to eliminate verbal components from ostensible “nonverbal” tests. For example, patients are often asked to define pictures aloud, and although this ensures that the stimuli themselves are nonverbal, the subsequent production of definitions depends upon speech output, and may thus encourage verbal mediation. When patients with aphasia or motor speech impairments provide imprecise definitions this may partially reflect difficulties with word finding and/or other aspects of language production rather than corruption of conceptual knowledge.
The second inherent challenge is to disentangle conceptual from perceptual stages of object processing. Receptive fields of neurons in the occipitotemporal ventral visual stream become increasingly complex as the visual signal is passed forward rostrally (Logothetis and Sheinberg, 1996; Palmeri and Gauthier, 2004), and convergent lines of evidence (axonal tracing, functional and structural connectivity) all suggest that ATL is the terminus of this visual “stream of complexity” (Binney et al., 2012; Fan et al., 2013; Kondo et al., 2003; Moran et al., 1987; Pascual et al., 2015). According to serial and cascading models of object recognition, low-level visuoperception therefore takes place in posterior areas and is a prerequisite to subsequent conceptual processing in anterior components of the stream (DiCarlo et al., 2012; Humphreys et al., 1999; Martin, 2007; Palmeri and Gauthier, 2004). Object misrecognition can be grounded in either of these stages, as occurs in apperceptive (perceptual-based) versus associative (conceptual-based) variants of agnosia (Bauer, 2006; Lissauer, 1890). Warrington’s (1975) patients were well able to discriminate between basic geometric shapes and to match facial photographs of the same individuals from different viewpoints, inconsistent with an apperceptive impairment. Instead, ATL atrophy in SD/PPA-S seems to typically result in conceptual/associative object impairments (Adlam et al., 2006; Bozeat et al., 2002, 2003), but it remains important to explicitly test for and rule out apperception.
Conversely, in cases where visuoperception is spared, individuals with associative agnosia may employ perceptually-grounded strategies such as inference and affordance (Gibson, 1979) to circumlocute conceptual failures. For example, when trying to match a picture cue (e.g. hand) with one of two picture probes (e.g. glove or shoe), a patient may fail to access conceptual knowledge of all three items, yet still correctly select the target because its shape is complementary with the cue (e.g. both hand and glove have five elongations while the terminus of a shoe is undifferentiated). These strategies are unavailable for word stimuli (whose form are perceptually arbitrary), and have been described as granting object images “privileged accessibility” (Caramazza et al., 1990; Lambon Ralph et al., 1999), presenting additional challenges for disentangling perceptual from conceptual stages of object processing during testing.
In the current study, participants with PPA-S and controls completed a nonverbal test of conceptual knowledge designed to address these challenges. The thematic verification task involves neither word stimuli nor oral responses, minimizing the potential for verbal mediation. Pictures of common objects were presented in pairs, and participants judged by button press whether the objects share a thematic association. Pairs were picked that shared minimal physical resemblance, preventing strategies such as perceptual inference and affordance, and thus requiring access of conceptual knowledge.
Verification (“yes” or “no”) judgments were required in the the matic task, rather than the common approach of matching a cue item with an associate embedded amongst foils, in order to prevent certain strategies that are available while matching. Specifically, during matching tasks the participant is armed with the fact that one and only one of the probes is a thematic associate, and can employ the process of elimination and process of comparison in executing a probabilistic judgment. As an example of the former, in tests where a cue item (e.g. wedge of cheese) must be matched to one of two probes (e.g. a mouse vs a chicken), a patient with object processing impairments may be completely unable to recognize the target (e.g. the mouse), yet still correctly select the target based on successful recognition and elimination of the foil (e.g. the chicken is recognized and conceptual access yields the knowledge that chickens don’t eat cheese). Similarly, patients with object impairments may fail to recognize both probes, yet still select the target through the process of comparison. In addition to perceptual inference and affordance, generic categorical knowledge (the object is a mammal) is often available in SD/PPA-S, even when item-specific knowledge has deteriorated (the object is a mouse, and mice eat cheese) (Hodges et al., 1995; Hurley et al., 2012; Lambon Ralph and Patterson, 2008; Mesulam et al., 2009a). Generic-level knowledge may be sufficient to allow for process of comparison between probes, for example to correctly select a mammal (mouse) as being more likely to eat cheese than a bird (chicken), even though neither item was specifically identified. By removing these comparative elements intrinsic to matching, verification further compels participants to retrieve associations between specific objects in long-term memory.
A shapes verification task was administered alongside the thematic verification task, in order to ensure that abnormal performance in the thematic task was not driven by visuoperceptual dysfunction. Identification of the shape and form of objects can be thought of as an intermediate step in visual processing, after low-level discrimination of contrast contours and line orientation, but prior to late-stage identification of featural configurations and whole-objects (DiCarlo et al., 2012; Hoffman and Logothetis, 2009; Humphreys et al., 1999). These steps of middle-vision fail in apperceptive variants of agnosia which have been variously referred to as “visual form agnosia” (Benson and Greenberg, 1969), “pseudoagnosia” (Warrington, 1985), “shape agnosia” (Humphreys and Riddoch, 1987), and “dysmorphosia” (Milner et al., 1991), and are linked to lesions in posterior temporal and occipital components of the ventral visual stream (Grossman et al., 1997; Karnath et al., 2009; Milner et al., 1991). The shapes verification task was designed to rule out such intermediate impairments in vision, by presenting participants with pairs of simple geometric shapes, and requiring them to press one button when the shapes are identical and another when the shapes differ. Both the thematic and shapes verification tasks were computerized, allowing for assessment of reaction times in addition to accuracy.
Our hypotheses for this study were derived from the dual-route account of ATL functionality. Based on previous experience, we expected consecutive patient recruitment to yield a series of PPA-S patients who all have L-ATL atrophy, but only some of whom show bilateral atrophy (Mesulam et al., 2013; Rogalski et al., 2011b). Single-word comprehension deficits are the core diagnostic criterion for PPA-S (Gorno-Tempini et al., 2011), as measured in the current study via abnormal scores on the PPVT (spoken word to picture matching). In terms of the dual-route account, this indicates that the verbal left- hemispheric route is compromised in all patients. The key predictions in the current study are with respect to the experimental thematic and shapes verification tasks. According to a dual-route account, the nonverbal route to conceptual access will only be blocked in the minority of patients with right-hemispheric (i.e. bilateral) ATL atrophy. As such, we predicted that thematic verification scores would be associated with the integrity of right rather than left ATL, as assessed by cortical thickness analysis. If instead no hemispheric-specific associations between atrophy and thematic scores are found, this would suggest that both ATLs play a similar role in conceptual knowledge, and that the aggregate amount of atrophy across both is the determinative factor.
Finally, we predicted that although a minority of PPA-S patients would show abnormal thematic verification scores, no patients would show deficits on the shapes verification task. If ATL represents the terminus of the perceptual-to-conceptual visual stream of complexity, any object processing impairments associated with ATL atrophy should be associative rather than apperceptive in nature, and should thus manifest as abnormalities in the thematic rather than shapes verification task. Alternatively, if PPA-S patients show abnormal performance on both tasks, this would suggest a mixture of perceptual and conceptual object impairments, or miscoordination between these processing stages (Ikeda et al., 2006).
2. Methods
2.1. Participants
All participants were consecutively recruited from an ongoing longitudinal investigation of PPA. Nineteen PPA-S patients (9 female) and 32 healthy controls (16 female) completed the study, all righthanded native English-speakers. A clinical diagnosis of PPA was assigned (author M.M.M.) based on the presence of salient language impairment caused by neurodegenerative disease, according to established criteria (Gorno-Tempini et al., 2011; Mesulam et al., 2012). In fulfillment of these criteria, language impairments were clinically judged to be more salient than object recognition impairments at the time of initial diagnosis. Clinical diagnosis of an aphasic syndrome was quantitatively confirmed by administration of the Western Aphasia Battery (Kertesz, 1982). Subtyping of the PPA-S variant was based on the presence of anomia, as measured by confrontation naming on the Boston Naming Test (Kaplan et al., 1983), and by the presence of single-word comprehension deficits, as measured in the PPVT by requiring participants to match a spoken noun to one of four pictures. A PPVT index was derived from the average of 36 items (questions 157–192), which has been extensively validated by our group as being sensitive to single-word comprehension deficits in PPA (Mesulam et al., 2009b, 2012; Rogalski et al., 2011a). The Benton Facial Recognition Test (Benton, 1994), which requires identity matching of faces photographed from different angles, was included as a rigorous test of object visuoperception free of conceptual demands.
2.2. Experimental tasks
Two computerized experimental tasks were given (Fig. 1). On the shapes verification task participants were shown pairs of geometric line drawings, presented side by side horizontally. Participants were instructed to press one of two buttons “to decide if the two pictures are the same shapes, or if they are different shapes”. Stimuli for the shapes task were 20 monochromatic line drawings, composed of triangles (n = 2), quadrilaterals (n = 4), other polygons (n = 6), non-polygonal 2D shapes (n = 4, e.g. circle, moon), and 3D shapes (n = 4, e.g. cube, cylinder). The shapes task contained 80 total trials, evenly divided into matching (shape paired with an identical copy) and mismatched trials (two different shapes).
Fig. 1. Schematic of the experimental design.

The shapes verification task requires participants to judge whether pairs of geometric line drawings are identical or different. The thematic verification task requires participants to judge whether pairs of objects are commonly associated with one another.
On the thematic verification task participants were shown pairs of object pictures, and were asked to decide by button press whether the objects “go together, can be used together in some way, or are seen together”. Thus, unlike the shapes task, the thematic task required participants to draw upon learned associations in long-term memory (i.e. access to conceptual knowledge). Stimuli for the thematic task were color photographs of objects collected from internet image searches. Objects within each image were cropped to remove any features in the background (when applicable), so that all objects were ultimately presented upon a white background. Forty picture “targets” were collected, half depicting animals and the other half depicting tools. These targets were each presented twice; once with a picture “probe” that was a thematic associate (matching trials), and once with an unrelated foil (mismatched trials), for a total of 80 trials. By necessity the thematic associates and unrelated foils represented a potpourri of many object categories, including food (carrot for rabbit), plants (tree for axe), household objects (bowl for whisk), etc. Animal targets were always paired with thematic associates from a different category (mouse- cheese), and likewise always paired with unrelated foils from a different category (mouse-watch). Tool targets were paired with a mixture of same and different-category associates, and likewise paired with a mixture of same and different-category foils. In this way we hoped to prevent participants from establishing inferences based on stimulus category, which could influence task performance. Thematic associates and foils were balanced for visual complexity as estimated by a compression ratio (t78 = 0.22; p = .83), calculated as each image’s uncompressed file size over compressed file size in Graphic Interchange Format (Palumbo et al., 2014). A full list of the stimuli is provided in Supplementary Table 1.
The shapes task was a later addition to the experimental battery, so was only available for 14/19 PPA-S patients and 18/32 controls. Trials where the control participants performed with less than 85% accuracy were excluded from analysis. This included 5/80 trials on the shapes verification task, and 10/80 trials on the thematic verification task (indicated in Supplementary Table 1).
2.3. Neuroimaging
Structural magnetic resonance images (MRI) were acquired at Northwestern University’s Center for Translational Imaging using a Siemens TIM Trio 3 T scanner. A T1-weighted 3D MPRAGE sequence was used (repetition time, 2300 ms; echo time, 2.91 ms; flip angle, 9°; field of view, 256 mm; slice thickness 1 mm). Cortical thickness analyses were conducted using FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/). After registering the cortical surface into a native vertex-based space, registration errors were manually corrected and the surface was reconstructed in an iterative fashion until all errors were resolved (Segonne et al., 2007). Cortical thickness was then calculated as the distance between the gray matter/white matter boundary and the pial surface, resulting in thickness estimates at each vertex. Patient thickness values at each cortical vertex were contrasted, via a general linear model, against values from a normative group of 35 previously described healthy older adults with similar demographic properties to the patients (Rogalski et al., 2014). The normative group included 26 out of the 32 control participants in the current study, and is described in more detail elsewhere (Rogalski et al., 2014). Average cortical thickness was also extracted from ATL regions of interest (ROI), using the Desikan et al. (2006) atlas temporal pole label for each hemisphere, which include a small roughly circular area (4.0 cm average diameter) at the apex (or “tip”) of the temporal poles in each hemisphere.
2.4. Statistical analysis
Data points greater than two standard deviations from the group mean were winsorized to reduce the influence of outliers (Dixon, 1960). All inferential tests were conducted two-tailed. Behavioral data, including demographic measurements, neuropsychological test scores, and values from the experimental shapes and thematic verification tasks, were compared between-groups with independent samples t-tests with degrees of freedom corrected for unequal variance. Whole-brain cortical thickness tests (general linear models) were thresholded via a false discovery rate of 0.0001 to correct for multiple comparisons. This threshold has been shown to reliably detect areas of peak atrophy in PPA (Rogalski et al., 2016).
3. Results
3.1. Demographic and neuropsychological testing
Demographic and neuropsychological characteristics of each group are shown in Table 1. The groups had similar age (t37. 2 = 0.14, p = .89) and education (t32. 3 = 0.37, p = .71). PPA-S patients showed lower Aphasia Quotients (summary scores representing overall severity of aphasia) on the Western Aphasia Battery (vs controls t18.2 = 8.1, p < .001), as evidence that they met standardized research criteria for an aphasia. The PPA-S group showed lower scores on the Boston Naming Test (vs controls t18.5 = 21.5, p < .001) and the PPVT (vs controls t18.2 = 9.5, p < .001), thus fulfilling the subtype criteria for anomia and noun comprehension impairments, respectively. Performance was comparable to controls, however, on the Benton Facial Recognition test (vs controls t40.2 = 0.5, p = .62)
Table 1.
Demographic and neuropsychological profiles of the control and PPA-S groups.
| Measure (Max Score) | Control | PPA-S |
|---|---|---|
| Age (years) | 62.5 (6.1) | 62.2 (6.3) |
| Gender (M/F) | 16/16 | 10/9 |
| Education (years) | 15.9 (2.4) | 15.6 (2.9) |
| Symptom duration (years) | n/a | 4.1 (2.0) |
| Aphasia Quotient (100) | 99.8 (0.5) | 83.2 (9.0)* |
| Boston Naming Test (60) | 58.7 (1.4) | 11.8 (9.4)* |
| PPVT (36) | 35.5 (0.7) | 18.9 (7.6)* |
| Facial Recognition (54) | 46.6 (4.1) | 46.0 (3.5) |
Values are shown mean (SD).
Significant differences between groups (p < .05).
3.2. Behavior on the experimental tasks
Reaction times of PPA-S patients and controls on the shapes and thematic verification tasks are shown in Fig. 2A. PPA-S patients were somewhat slower to respond than controls on the shapes task (PPA M ± SD = 987 ± 164 ms; control M ± SD = 890 ± 115 ms), and this group difference approached significance (t22.4 = 1.9, p = .07). The PPA-S group was significantly slower than controls on the thematic task (PPA M ± SD = 1955 ±371 ms; control M ± SD = 1381 ± 207 ms) (t24.8 = 6.2, p < .001). Both groups showed longer reaction times on the conceptually-demanding thematic task than on the shapes task, but the difference between the two tasks was disproportionate in the PPA-S group, according to the interaction term in a 2×2 mixed-model ANOVA (F(1,30) = 33.6, p < .001).
Fig. 2. Behavior in the experimental tasks.
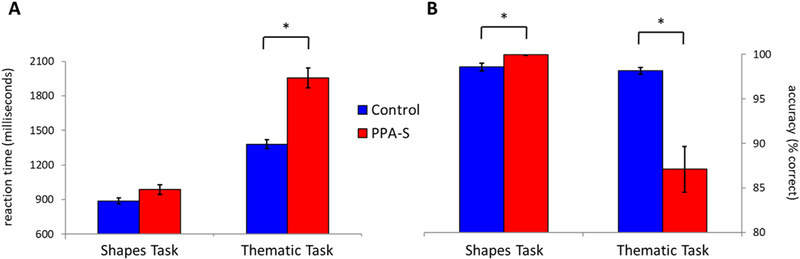
(A) The PPA-S group was slower to respond in the thematic but not the shapes verification task, compared to the control group. (B) The PPA-S group was less accurate in the thematic task but slightly more accurate in the shapes task. Standard error bars are shown around each mean. *: Significant differences between groups (p < .05).
Accuracies of PPA-S patients and controls on the shapes and the matic verification tasks are shown in Fig. 2B. Both groups were highly accurate in performing the shapes task (M ± SD Controls = 98.6 ± 1.8%; PPA-S = 99.9 ± 0.2%), with the PPA-S group showing a small but statistically significant increase compared to controls (t17.6 = 3.1, p = .006). Controls were also highly accurate in performing the thematic task (M ± SD = 98.2 ± 2.1%), but the PPA-S group showed considerably poorer performance (M ± SD = 87.1 ± 11.2%) (t18. 8 =4.3, p < .001). Thematic performance in the PPA-S group was not only lower on average, but was more variable across participants; the standard deviation in the PPA-S group was more than four times greater than in the control group, and Levene’s test for inequality of variance (between-groups) was significant (p < .001).
3.3. Characteristics of higher and lower performing patients
Thematic performance was highly variable in the PPA-S group (Fig. 2B), suggesting that some patients were successful while other patients were unsuccessful in making their judgments. In order to explore this possibility the PPA-S group was split into two subgroups based on accuracy on the thematic verification task. Twelve patients performed above the group average (87.1%) and seven patients performed below the group average, suggesting that the latter were driving differences from controls. Demographic and neuropsychological characteristics of these “higher performing” and “lower performing” subgroups are shown in Table 2. The subgroups were matched on demographic characteristics (age t11.1 = 1.2, p = .25; years of education t16. 3 = 0.009, p = .99; symptom duration t17.0 = 0.31, p = .80) and on visuoperceptual function (shapes verification task t4 = 1.0, p = .37; Benton Facial Recognition t16. 0 = 1.2, p = .23). The lower performing patients were less accurate on the thematic task (75.1 ± 10%) compared to higher performing patients (94.1 ± 2.6; t6.5 = 4.9, p = .002), and also compared to controls (98.2 ± 2.1%; t6.1 = 6.1, p = .001). Although the higher performing subgroup was only 4.1% less accurate than controls on average, this difference was significant (t16.9 = 4.9, p < .001).
Table 2.
Demographic and neuropsychological profiles of each PPA-S subgroup.
| Measure (max score) | Higher performing PPA-S subgroup |
Lower performing PPA- S subgroup |
|---|---|---|
| Age (years) | 63.6 (5.8) | 60.0 (6.8) |
| Gender (M/F) | 8/4 | 2/5 |
| Education (years) | 15.6 (3.3) | 15.6 (2.3) |
| Symptom duration (years) | 4.0 (2.4) | 4.2 (1.3) |
| Aphasia Quotient (100) | 87.6 (5.9) | 75.6 (8.4)* |
| Boston Naming Test (60) | 15.6 (9.9) | 5.4 (3.5)* |
| PPVT (36) | 21.9 (7.2) | 13.9 (5.6)* |
| Facial Recognition (54) | 46.7 (4.0) | 44.9 (2.4) |
| Shapes Verification Task (100) | 100.0 (0.0) | 99.8 (0.4) |
| Thematic Verification Task (100) | 94.1 (2.6) | 75.1 (10.0)* |
Values are shown mean (SD). The PPA-S sample was split into those performing above versus below the group average on the thematic verification task.
Significant differences between subgroups (p < .05).
The lower performing subgroup had a more severe Aphasia Quotient on the Western Aphasia Battery (t9.6 = 3.3, p = .008), greater anomia according to the Boston Naming Test (t14.9 = 3.2, p = .006), and more severe single-word comprehension deficits on the PPVT (t15.3 = 2.7, p = .02). This could raise concern that subgroup differences in nonverbal competency (as indexed by thematic verification scores) are confounded by a greater degree of verbal impairment (aphasia severity) in the low performing subgroup. An ANCOVA was performed contrasting thematic verification scores across subgroups (modeled as a fixed factor), while partialling out Aphasia Quotients, Boston Naming Test scores, and PPVT scores (each modeled as a covariate). The main effect of subgroup was significant (F(1,14) = 13.1, p = .003), suggesting that differences in thematic verification accuracy between subgroups are not mediated by verbal competency.
Atrophy patterns for the lower performing (n = 7) and higher performing (n = 12) subgroups were assessed separately using a vertex- based cortical thickness analysis by hemisphere. Significant areas of thinning (false discovery rate of 0.0001) relative to a normative sample of control thickness values are displayed in Fig. 3. Both the higher and lower performing subgroups showed extensive atrophy throughout most of the left temporal lobe, with the exception of primary auditory cortex. Atrophy was also present in left insular and orbitofrontal cortex. The most salient differences between subgroups, however, were in the right hemisphere, where lower performing patients showed extensive atrophy in ATL. In contrast, the higher performing subgroup showed no significant right-hemispheric atrophy at this threshold.
Fig. 3. Cortical thickness in subgroups of PPA-S patients with higher and lower thematic performance.
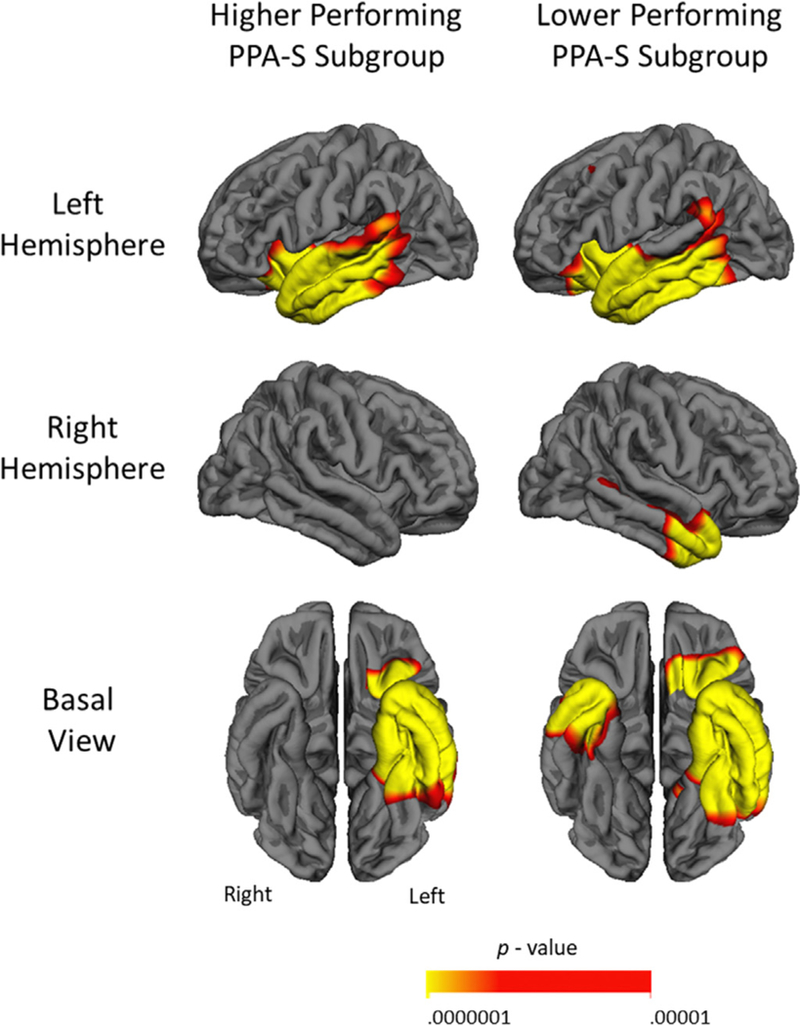
Areas in red-yellow indicating significantly thinner cortex in each compared to controls. Both subgroups show extensive left temporal atrophy, but only lower thematic performers show significant right ATL atrophy at a false discovery rate of 0.0001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
It is important to note, however, that failure to detect thinning at a given threshold does not indicate total absence of atrophy; in the case of PPA subthreshold thinning is usually present in additional areas, which tends to become significant later in the course of disease (Rogalski et al., 2011b). We chose a false discovery rate of 0.0001 as this threshold reliably showed areas of peak atrophy in previous investigations (Rogalski et al., 2016), but less stringent thresholds are commonly reported. When we regenerated each subgroup thickness map at a more lenient false discovery rate of 0.001, the higher performing subgroup showed additional thinning in a lateral section of the right temporal pole, and the lower performing subgroup showed additional thinning in the left insula and right middle temporal gyrus and sulcus (Supplementary Fig. 1). When the thickness maps from the two subgroups were directly contrasted no differences survived false discovery rate correction, likely representing type II error due to low sample size.
3.4. ROI analyses
An additional set of ROI-based analyses were conducted in the PPA- S group. As shown in Fig. 4A, cortical thickness in the right ATL ROI differed between the lower and higher performing subgroups (t2.9 = 2.9, p = .02), but not in the left ATL ROI (t14.5 = 0.25, p = .81). Correlations between thematic verification task scores and ROI thickness were then examined in the entire PPA-S group (regardless of subgroup membership; n = 19), in order to quantify any hemispheric asymmetries in the relationship between ATL atrophy and thematic (nonverbal) performance. As shown in Fig. 4B, thematic verification scores were significantly correlated with thickness in the right ATL ROI (r(19) = 0.57, p = .01), but not with the left ATL (r(19) = 0.26, p = .28).
Fig. 4. Relationships between thematic performance and thickness in ATL ROI.
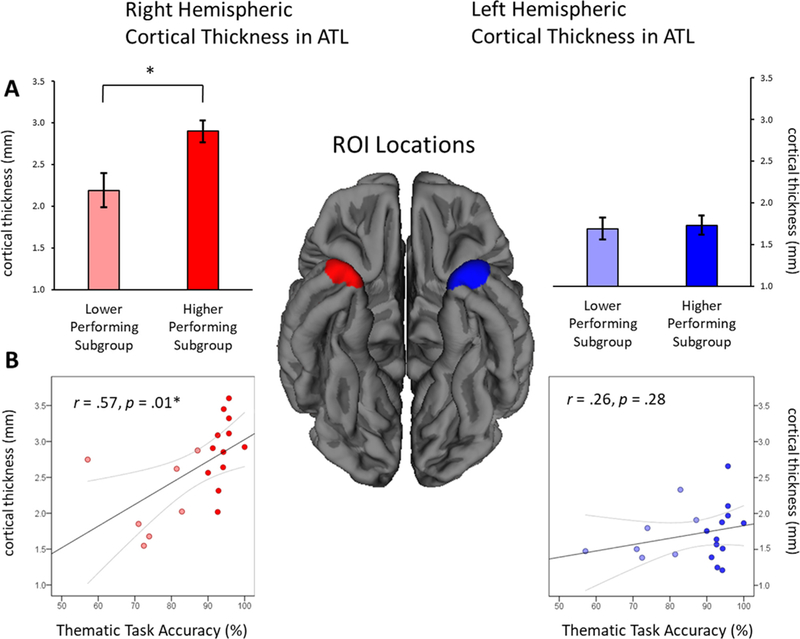
Locations of the ATL ROI in each hemisphere are shown at center. (A) Average cortical thickness in each ROI is shown for subgroups of PPA-S patients performing lower and higher on the thematic verification task. (B) Correlation scatterplots between ROI thickness and accuracy of thematic verification, with each dot representing values from a single PPA-S participant. ROI = region of interest. *: Statistically significant (p < .05).
Atrophy in PPA-S tends to co-progress in both hemispheres (Rogalski et al., 2014), however, leaving open the possibility that an apparent relationship between right ATL atrophy and thematic scores may be confounded by greater atrophy in left ATL as well. Partial correlations were performed in order to address this issue, this time controlling for thickness in the contralateral ROI. Partial correlations replicated the same pattern of results: thematic scores were correlated with thickness in the right (r(16) = 0.54, p = .02) but not left ATL (r(16) = 0.16, p = .54), after partialling out any variance shared with contralateral thickness.
4. Discussion
4.1. Summary of findings
The goal of this study was to examine how ATL atrophy in each hemisphere affects nonverbal access to conceptual knowledge. Our primary innovation was the development of a thematic verification task which resolved several limitations of previous nonverbal assessments. A dissociation was revealed between a standardized verbal test of spoken noun comprehension (PPVT) and the thematic verification task: whereas all PPA-S patients showed impairments on the former, only a subgroup of patients with prominent right ATL atrophy showed impairments on the latter. This subgroup showed intact performance on the shapes verification task and on Benton Facial Recognition, suggesting their object impairments were associative rather than apperceptive in nature. Thematic scores were correlated with right rather than left-hemispheric atrophy in ATL, and this asymmetric relationship persisted when controlling for atrophy in the contralateral hemisphere. The observed behavioral dissociations and asymmetric hemispheric mappings are consistent with a dual-route account, in which ATL is part of a verbal route for conceptual access in the left hemisphere, and part of a nonverbal route for conceptual access in the right hemisphere.
4.2. Nonverbal object assessments
Unlike many object-based assessments, the thematic verification task has no explicitly verbal components, involving only picture stimuli and button-press responses. We do not claim that this prevents verbal mediation, but rather enables the task to be completed via a nonverbal route when necessary. As proposed by Paivio (1986), the thematic relationships evoked by our task can be dual-coded as associations with visual imagery or with lexical representations. Typical adults flexibly draw upon both sorts of associations, and when neuropathology compromises one route (e.g. the verbal route in aphasia) dual-coding provides an alternative route to conceptual knowledge. As phrased by one of the current authors (M.M.M.), “even patients with severe classic Wernicke’s aphasia run away from snakes”. Humans would not be alone in employing a nonverbal, image-based route to access conceptual knowledge: non-human species also readily acquire thematic associations between objects in the laboratory (Vaughan, 1988) and in the wild (Seed and Byrne, 2010).
More than a third of the PPA-S sample showed poor performance on the thematic verification task, demonstrating inability to access knowledge via a nonverbal route. These same patients performed quite well, however, on the shapes verification task, precluding an intermediate-stage apperceptive impairment as occurs in visual form agnosia (Effron, 1968; Karnath et al., 2009; McMullen et al., 2000; Milner et al., 1991; Warrington, 1985). Patients completed the shapes task with slightly greater accuracy than controls, while maintaining equivalent speed, consistent with reports of visuoperceptual enhancement rather than dysfunction in the SD literature (Green and Patterson, 2009; Viskontas et al., 2011). Although the geometric stimuli in the shapes task were not as visually complex as the common object stimuli in the thematic task, patient performance was also comparable to that of controls on the Benton Facial Recognition Test, which includes more perceptually complex facial features. The object processing impairments observed in this study therefore appear to have a conceptual rather than perceptual basis, as occurs in associative visual agnosia (Lissauer, 1890). In contrast, Ikeda and colleagues report that patients with SD have difficulty matching pictures of common objects from different views or from different exemplars, and that these difficulties are aggravated in patients with substantial atrophy in right ATL (Ikeda et al., 2006). They argue that such tests tap into perceptual-conceptual interactions in object processing. We were unable to address this possibility in the current study as our battery did not include comparable tests with common object stimuli (as opposed to geometric shapes and faces). By including such tests, future studies may be able to determine whether atrophy in ATL affects higher-level visuoperception and perceptual/conceptual interactions.
4.3. Asymmetric functionality of ATL
Cortical thickness analyses consistently showed that thematic performance depended upon the integrity of the right ATL. As expected, all PPA-S patients had extensive atrophy throughout the left temporal lobe, excluding only auditory cortex. When PPA-S patients were sorted into subgroups according to thematic verification scores, only the subgroup with low thematic performance showed additional right hemispheric atrophy in ATL at a stringent false discovery rate of 0.0001. When the false discovery rate was lowered to 0.001, additional right temporopolar atrophy emerged in the higher performing subgroup as well, suggesting that nonverbal impairments only emerge when right-hemispheric atrophy reaches a certain degree of prominence. Correlational analyses further related poor thematic performance to the integrity of right rather than left ATL.
The key anatomic signature of nonverbal impairments in PPA-S therefore appears to be in the hemispheric distribution of atrophy in ATL, with impairments only emerging when ATL atrophy is prominent in the right hemisphere. This complements previous studies linking facial recognition impairments to right hemispheric or bilateral atrophy in ATL (Evans et al., 1995; Gainotti, 2007; Gainotti et al., 2003, 2010; Gefen et al., 2013; Snowden et al., 2004, 2012; Thompson et al., 2003). Although some areas may be selectively involved in face versus common object recognition (McMullen et al., 2000; Pitcher et al., 2009; Thompson et al., 2004; Wallis, 2013), conceptual judgments of both types of stimuli are inter-correlated in SD (Snowden et al., 2004). The current study provides further evidence that the nonverbal route through right ATL supports knowledge of common objects in addition to faces, as predicted by the dual-route account. These findings are consistent with those from Butler et al. (2009), who reported preferential linkage between the picture version of the PPT and right ATL atrophy, when word scores were co-varied out in analysis. Unlike previous studies, the current results were obtained after controlling for the degree of contralateral atrophy, which is important as ATL atrophy tends to co-progress in both hemispheres throughout the course of PPA- S (Rogalski et al., 2014).
These findings fit with an emerging motif in the literature: not all parts of ATL are created equal, certain areas within ATL are functionally specialized, and these areas are arranged along gradients that reflect their patterns of connectivity with various cortical networks (e.g.s. auditory, visual, language) (Binney et al., 2012; Hurley et al., 2015; Jackson et al., 2016; Papinutto et al., 2016; Pascual et al., 2015). The asymmetric functionality described in dual-route theory fits comfortably within this framework.
4.4. Future directions
Much work is needed to further characterize the nonverbal route for conceptual access. It remains unclear whether the nonverbal route is exclusive to ATL in the right hemisphere or exists in both hemispheres (Schapiro et al., 2013). Some aspects of vision are right-lateralized in posterior components of the ventral stream (Bouvier and Engel, 2006; Koutstaal et al., 2001; Simons et al., 2003), leading to the hypothesis that ipsilateral connections with those posterior regions grants the right ATL a privileged role in object processing compared to its left hemispheric homolog (Ikeda et al., 2006). The current results do not address whether the nonverbal route is bilateral or exclusive to the right hemisphere, as all patients with atrophy in right ATL also had left- hemispheric (i.e. bilateral) atrophy. Although atrophy in PPA-S is often bilateral, cases where atrophy is largely unilateral (at a given threshold) are also common, particularly in early disease stages. A previous study employing rigorous quantitative imaging methods (cortical thickness) showed that circumscribed left unilateral ATL atrophy is sufficient to create noun comprehension impairments (Mesulam et al., 2013). Comparable studies are necessary to determine whether unilateral atrophy in right ATL atrophy is sufficient to block the nonverbal route (versus the alternative that both ATLs would need to be compromised).
Nonverbal task correlations in the current study were obtained using a priori temporopolar ROIs, which were confined to the distal apex of ATL, sometimes referred to as the “temporal tip” (Mesulam et al., 2013). This area was atrophic in all patients (in both subgroups). We should note, however, that our ROI selection was largely one of convenience, being the only ROI that is specific to ATL in the Desikan et al. (2006) FreeSurfer atlas. Other areas within the wider ATL region undoubtedly contribute to object recognition. Ventral aspects of ATL, in particular, appear to share selective functional and structural connections with posterior components of the inferotemporal object recognition network (Binney et al., 2012; Jackson et al., 2016; Papinutto et al., 2016; Pascual et al., 2015). Future studies may further clarify which subregions within ATL contribute to nonverbal versus verbal impairments, and establish the hemispheric specificity of those relationships.
In this study we found that atrophy in right ATL impaired ability to access knowledge of visual object images. Evidence continues to accumulate, however, that right ATL atrophy also affects recognition of auditory object sounds (Bozeat et al., 2000; Golden et al., 2015; Goll et al., 2010), and even olfactory object recognition (Magerova et al., 2014; Olofsson et al., 2013; Piwnica-Worms et al., 2010; Rami et al., 2007). As such, our description of “dual-routes” in ATL is almost certainly incomplete, as ATL likely participates in several modality-specific nonverbal routes. Future studies are needed to establish the routes through which ATL participates in non-visual modalities of object recognition, and to localize these routes to specific hemispheres and subregions within ATL.
More work also is needed to clarify the mechanisms by which ATL contributes to nonverbal knowledge. Warrington (1975) described the syndrome that would become known as SD as reflecting damage to a unitary storehouse. The current results could be interpreted as supporting a multiple-storehouse view instead, localizing Paivio’s (1986) theoretical verbal and nonverbal knowledge stores to the left and right ATL, respectively. Alternatively, Warrington (1975) also raised the possibility of separate verbal and nonverbal routes of input to the same unitary knowledge store (as a way of explaining greater verbal impairment in patient E.M.), in which case the current results localize these access gateways to separate hemispheres. As yet a third possibility, separate access points may lead to separate verbal and nonverbal storehouses. This study, motivated by the dual route framework, simply demonstrates that the hemispheric distribution of atrophy in ATL affects which sorts of stimuli patients are able to recognize. The current results do not distinguish between disorders of access versus storage, and they do not establish the number of concept storehouses. Resolving those theoretical issues would lead to a deeper understanding of how knowledge is organized in the human brain.
The current results add to previous studies demonstrating that some but not all PPA-S patients have significant nonverbal impairments in object recognition (Mesulam et al., 2009a, 2013; Warrington, 1975), and localize those nonverbal impairments to ATL in the right hemisphere. This helps to explain the historical differences in the way PPA-S and SD were characterized (with the latter placing greater emphasis on object recognition impairments) (Adlam et al., 2006; Mesulam et al., 2003), while also supporting the current consensus framework in which the two syndromes overlap (Gorno-Tempini et al., 2011). PPA-S and SD could be considered as falling along a single phenotypic continuum, resulting from the same underlying neuropathology, with placement along that continuum depending on the hemispheric distribution of anterior temporal atrophy. Patients with initially isolated left-hemispheric atrophy and language symptoms may go on to develop object recognition impairments as atrophy becomes more prominent in the right hemisphere. Longitudinal investigations including detailed nonverbal assessments could help to confirm this possibility.
Supplementary Material
Acknowledgements
We would like to thank all the patients who took part in this study. We also thank Borna Bonakdarpour, Soojin Cho-Reyes, Jim Kloet, Charis Price, and Christina Wieneke for help with stimulus development and data collection, and Brittany Lapin for guidance on statistical analysis.
Funding
This work was supported by NIH/NIA P30 AG13854, NIH/NIDCD R01 DC008552, NIH/NINDS R01 NS075075.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.10167j.neuropsychologia.2018.05.019
Footnotes
Declarations of interest
None.
As defined by Estes and colleagues, a thematic relationship is “a temporal, spatial, causal, or functional relation between things that perform complementary roles in the same scenario or event” (Estes et al., 2011).
References
- Adlam AL, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J, Hodges JR, 2006. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain 129, 3066–3080. [DOI] [PubMed] [Google Scholar]
- Bauer RM, 2006. The agnosias In: Snyder PJ, Nussbaum PD, Robins DL (Eds.), Clinical Neuropsychology: A Pocket Handbook for Assessment. American Psychological Association, Washington, pp. 508–533. [Google Scholar]
- Benson DF, Greenberg JP, 1969. Visual form agnosia. A specific defect in visual discrimination. Arch. Neurol. 20, 82–89. [DOI] [PubMed] [Google Scholar]
- Benton AL, 1994. Contributions to Neuropsychological Assessment: A Clinical Manual, 2nd ed. Oxford University Press, New York. [Google Scholar]
- Binney RJ, Parker GJ, Lambon Ralph MA, 2012. Convergent connectivity and graded specialization in the rostral human temporal lobe as revealed by diffusion- weighted imaging probabilistic tractography. J. Cogn. Neurosci. 24, 1998–2014. [DOI] [PubMed] [Google Scholar]
- Bouvier SE, Engel SA, 2006. Behavioral deficits and cortical damage loci in cerebral achromatopsia. Cereb. Cortex 16, 183–191. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR, 2000. Non-verbal semantic impairment in semantic dementia. Neuropsychologia 38, 1207–1215. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Hodges JR, 2002. When objects lose their meaning: what happens to their use? Cogn. Affect. Behav. Neurosci. 2, 236–251. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Ralph MA, Graham KS, Patterson K, Wilkin H, Rowland J, Rogers TT, Hodges JR, 2003. A duck with four legs: investigating the structure of conceptual knowledge using picture drawing in semantic dementia. Cogn. Neuropsychol. 20, 27–47. [DOI] [PubMed] [Google Scholar]
- Butler CR, Brambati SM, Miller BL, Gorno-Tempini ML, 2009. The neural corre-lates of verbal and nonverbal semantic processing deficits in neurodegenerative disease. Cogn. Behav. Neurol. 22, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A, Hillis AE, Rapp BC, Romani C, 1990. The multiple semantics hypothesis: multiple confusions? Cogn. Neuropsychol. 7, 161–189. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- DiCarlo JJ, Zoccolan D, Rust NC, 2012. How does the brain solve visual object re-cognition? Neuron 73, 415–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ, 1960. Simplified estimation from censored normal samples. Ann. Math. Stat.18,. 413–426. [Google Scholar]
- Dunn LM, 2007. Peabody Picture Vocabulary Test PPVT-4. S l: Pearson Assessments. [Google Scholar]
- Effron R, 1968. What is perception? In: Cohen RS, Wartofsky M (Eds.), Boston Studies in the Philosophy of Science 4. Humanities, New York, pp. 137–173. [Google Scholar]
- Estes Z, Golonka S, Jones LL, 2011. Thematic thinking: the apprehension and consequences of thematic relations In: Ross BH, Ross BH (Eds.), The Psychology of Learning and Motivation: Advances in Research and Theory Vol. 54 Elsevier Academic Press, San Diego, CA, US, pp. 249–294. [Google Scholar]
- Evans JJ, Heggs AJ, Antoun N, Hodges JR, 1995. Progressive prosopagnosia associated with selective right temporal lobe atrophy. A new syndrome? Brain 118 (Pt 1), 1–13. [DOI] [PubMed] [Google Scholar]
- Fan L, Wang J, Zhang Y, Han W, Yu C, Jiang T, 2013. Connectivity-based par- cellation of the human temporal pole using diffusion tensor imaging. Cereb. Cortex. [DOI] [PubMed] [Google Scholar]
- Gainotti G, 2007. Different patterns of famous people recognition disorders in patients with right and left anterior temporal lesions: a systematic review. Neuropsychologia 45, 1591–1607. [DOI] [PubMed] [Google Scholar]
- Gainotti G, 2014. Why are the right and left hemisphere conceptual representations different? Behav. Neurol. 2014, 603134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G, Barbier A, Marra C, 2003. Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain 126, 792–803. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Ferraccioli M, Marra C, 2010. The relation between person identity nodes, familiarity judgment and biographical information. Evidence from two patients with right and left anterior temporal atrophy. Brain Res. 1307, 103–114. [DOI] [PubMed] [Google Scholar]
- Gefen T, Wieneke C, Martersteck A, Whitney K, Weintraub S, Mesulam M-M, Rogalski E, 2013. Naming vs knowing faces in primary progressive aphasia: a tale of 2 hemispheres. Neurology 81, 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, 1965. Disconnexion syndromes in animals and man. I. Brain 88, 237–294. [DOI] [PubMed] [Google Scholar]
- Gibson JJ, 1979. The Ecological Approach to Visual Perception. Houghton Mifflin, Boston. [Google Scholar]
- Golden HL, Downey LE, Fletcher PD, Mahoney CJ, Schott JM, Mummery CJ, Crutch SJ, Warren JD, 2015. Identification of environmental sounds and melodies in syndromes of anterior temporal lobe degeneration. J. Neurol. Sci. 352, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll JC, Crutch SJ, Loo JH, Rohrer JD, Frost C, Bamiou DE, Warren JD, 2010. Non-verbal sound processing in the primary progressive aphasias. Brain 133, 272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL, 2004. Cognition and anatomy in three variants of primary progressive aphasia. Ann. Neurol. 55, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam M-M, Grossman M, 2011. Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green HA, Patterson K, 2009. Jigsaws-a preserved ability in semantic dementia. Neuropsychologia 47, 569–576. [DOI] [PubMed] [Google Scholar]
- Grossman M, Galetta S, D’Esposito M, 1997. Object recognition difficulty in visual apperceptive agnosia. Brain Cogn. 33, 306–342. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham N, Patterson K, 1995. Charting the progression in semantic dementia: implications for the organisation of semantic memory. Memory 3, 463–495. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, Logothetis NK, 2009. Cortical mechanisms of sensory learning and object recognition. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 364, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, Patterson KE, 1992. The Pyramids and Palm Trees Test. Thames Valley Company, Bury St. Edmunds. [Google Scholar]
- Humphreys GW, Price CJ, Riddoch MJ, 1999. From objects to names: a cognitive neuroscience approach. Psychol. Res./Psychol. Forsch. 62, 118–130. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Riddoch MJ, 1987. The fractionization of Visual Agnosia. Lawrence Erlbaum, London. [Google Scholar]
- Hurley RS, Bonakdarpour B, Wang X, Mesulam M-M, 2015. Asymmetric connectivity between the anterior temporal lobe and the language network. J. Cogn. Neurosci. 27, 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RS, Paller KA, Rogalski EJ, Mesulam M-M, 2012. Neural mechanisms of object naming and word comprehension in primary progressive aphasia. J. Neurosci. 32, 4848–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Patterson K, Graham KS, Ralph MA, Hodges JR, 2006. A horse of a different colour: do patients with semantic dementia recognise different versions of the same object as the same? Neuropsychologia 44, 566–575. [DOI] [PubMed] [Google Scholar]
- Jackson RL, Hoffman P, Pobric G, Lambon Ralph MA, 2016. The semantic network at work and rest: differential connectivity of anterior temporal lobe subregions. J. Neurosci. 36, 1490–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S, 1983. Boston Naming Test. Lea & Febiger, Philadelphia. [Google Scholar]
- Karnath HO, Ruter J, Mandler A, Himmelbach M, 2009. The anatomy of object recognition-visual form agnosia caused by medial occipitotemporal stroke. J. Neurosci. 29, 5854–5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, 1982. The Western Aphasia Battery. Grune & Stratton, New York; London. [Google Scholar]
- Kondo H, Saleem KS, Price JL, 2003. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol. 465, 499–523. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL, 2001. Perceptual specificity in visual object priming: functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia 39, 184–199. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, 2014. Neurocognitive insights on conceptual knowledge and its breakdown. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 369, 20120392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Graham KS, Patterson K, Hodges JR, 1999. Is a picture worth a thousand words? Evidence from concept definitions by patients with semantic dementia. Brain Lang. 70, 309–335. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR, 2001. No right to speak? The relationship between object naming and semantic impairment: neuropsychological evidence and a computational model. J. Cogn. Neurosci. 13, 341–356. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K, 2008. Generalization and differentiation in semantic memory: insights from semantic dementia. Ann. N. Y. Acad. Sci. 1124, 61–76. [DOI] [PubMed] [Google Scholar]
- Lissauer H, 1890. Ein fall von seelenblindheit nebst einem beitrag zur theorie derselben. Arch. fur Psychiatr. und Nervenkrankh. 21, 222–270. [Google Scholar]
- Logothetis NK, Sheinberg DL, 1996. Visual object recognition. Annu. Rev. Neurosci.19 577–621. [DOI] [PubMed] [Google Scholar]
- Magerova H, Vyhnalek M, Laczo J, Andel R, Rektorova I, Kadlecova A, Bojar M, Hort J, 2014. Odor identification in frontotemporal lobar degeneration subtypes. Am. J. Alzheimers Dis. Other Demen 29, 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, 2007. The representation of object concepts in the brain. Annu. Rev. Psychol. 58, 25–45. [DOI] [PubMed] [Google Scholar]
- McMullen PA, Fisk JD, Phillips SJ, Maloney WJ, 2000. Apperceptive agnosia and face recognition. Neurocase 6, 403–414. [Google Scholar]
- Mesulam M-M, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH, 2014a. Asymmetry and heterogeneity of Alzheimer and frontotemporal pathology in primary progressive aphasia. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, Weintraub S, 2009a. Neurology of anomia in the semantic variant of primary progressive aphasia. Brain 132, 2553–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S, 2009b. Quantitative template for subtyping primary progressive aphasia. Arch. Neurol. 66, 1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, 1982. Slowly progressive aphasia without generalized dementia. Ann. Neurol. 11, 592–598. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Grossman M, Hillis A, Kertesz A, Weintraub S, 2003. The core and halo of primary progressive aphasia and semantic dementia. Ann. Neurol. 54 (Suppl 5), S11–S14. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Rogalski EJ, Wieneke C, Hurley RS, Geula C, Bigio EH, Thompson CK, Weintraub S, 2014b. Primary progressive aphasia and the evolving neurology of the language network. Nat. Rev. Neurol. 10, 554–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Thompson CK, Weintraub S, Rogalski EJ, 2015. The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain 138, 2423–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, Rogalski EJ, 2013. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain 136, 601–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Wieneke C, Thompson C, Rogalski E, Weintraub S, 2012. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain 135, 1537–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner AD, Perrett DI, Johnston RS, Benson PJ, Jordan TR, Heeley DW, Bettucci D, Mortara F, Mutani R, Terazzi E, et al. , 1991. Perceptionand action in ‘visual form agnosia’. Brain 114 (Pt 1B), 405–428. [DOI] [PubMed] [Google Scholar]
- Moran MA, Mufson EJ, Mesulam M-M, 1987. Neural inputs into the temporopolar cortex of the rhesus monkey. J. Comp. Neurol. 256, 88–103. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR, 2000. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann. Neurol. 47, 36–45. [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Wise RJ, Vandenberghe R, Price CJ, Hodges JR, 1999. Disrupted temporal lobe connections in semantic dementia. Brain 122 (Pt 1), 61–73. [DOI] [PubMed] [Google Scholar]
- Nilakantan AS, Voss JL, Weintraub S, Mesulam M-M, Rogalski EJ, 2017. Selective verbal recognition memory impairments are associated with atrophy of the language network in non-semantic variants of primary progressive aphasia. Neuropsychologia 100, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Rogalski E, Harrison T, Mesulam M-M, Gottfried JA, 2013. A cortical pathway to olfactory naming: evidence from primary progressive aphasia. Brain 136, 1245–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paivio A, 1986. Mental Representations: A Dual Coding Approach. Oxford University Press, New York. [Google Scholar]
- Palmeri TJ, Gauthier I, 2004. Visual object understanding. Nat. Rev. Neurosci. 5, 291–303. [DOI] [PubMed] [Google Scholar]
- Palumbo L, Ogden R, Makin AD, Bertamini M, 2014. Examining visual complexity and its influence on perceived duration. J. Vis. 14, 3. [DOI] [PubMed] [Google Scholar]
- Papinutto N, Galantucci S, Mandelli ML, Gesierich B, Jovicich J, Caverzasi E, Henry RG, Seeley WW, Miller BL, Shapiro KA, Gorno-Tempini ML, 2016. Structural connectivity of the human anterior temporal lobe: a diffusion magnetic resonance imaging study. Hum. Brain Mapp. 37, 2210–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual B, Masdeu JC, Hollenbeck M, Makris N, Insausti R, Ding SL, Dickerson BC, 2015. Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cereb. Cortex 25, 680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT, 2007. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 8, 976–987. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Charles L, Devlin JT, Walsh V, Duchaine B, 2009. Triple dissociation of faces, bodies, and objects in extrastriate cortex. Curr. Biol. 19, 319–324. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms KE, Omar R, Hailstone JC, Warren JD, 2010. Flavour processing in semantic dementia. Cortex 46, 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami L, Loy CT, Hailstone J, Warren JD, 2007. Odour identification in fronto- temporal lobar degeneration. J. Neurol. 254, 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, Mesulam M-M, 2011a. Anatomy of language impairments in primary progressive aphasia. J. Neurosci. 31, 3344–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam M-M, 2011b. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology 76, 1804–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Martersteck A, Rademaker A, Wieneke C, Weintraub S, Mesulam M-M, 2014. Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology 83, 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Sridhar J, Rader B, Martersteck A, Chen K, Cobia D, Thompson CK, Weintraub S, Bigio EH, Mesulam M-M, 2016. Aphasic variant of Alzheimer disease: clinical, anatomic, and genetic features. Neurology 87, 1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K, 2004. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol. Rev. 111, 205–235. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, Beck J, Hardy J, de Silva R, Warrington E, Troakes C, Al-Sarraj S, King A, Borroni B, Clarkson MJ, Ourselin S, Holton JL, Fox NC, Revesz T, Rossor MN, Warren JD, 2011. Clinical and neuroanatomical signatures of tissue pathology in fronto- temporal lobar degeneration. Brain 134, 2565–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL, 2002. Patterns of brain atrophy in fronto- temporal dementia and semantic dementia. Neurology 58, 198–208. [DOI] [PubMed] [Google Scholar]
- Schapiro AC, McClelland JL, Welbourne SR, Rogers TT, Lambon Ralph MA, 2013. Why bilateral damage is worse than unilateral damage to the brain. J. Cogn. Neurosci. 25, 2107–2123. [DOI] [PubMed] [Google Scholar]
- Seed A, Byrne R, 2010. Animal tool-use. Curr. Biol. 20, R1032–R1039. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B, 2007. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med. Imaging 26, 518–529. [DOI] [PubMed] [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL, 2003. Neural mechanisms of visual object priming: evidence for perceptual and semantic distinctions in fusiform cortex. NeuroImage 19, 613–626. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D, 1989. Semantic dementia: a form of circumscribed cerebral atrophy. Behav. Neurol. 2, 167–182. [Google Scholar]
- Snowden JS, Thompson JC, Neary D, 2004. Knowledge of famous faces and names in semantic dementia. Brain 127, 860–872. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, Neary D, 2012. Famous people knowledge and the right and left temporal lobes. Behav. Neurol. 25, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Graham KS, Williams G, Patterson K, Kapur N, Hodges JR, 2004. Dissociating person-specific from general semantic knowledge: roles of the left and right temporal lobes. Neuropsychologia 42, 359–370. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Patterson K, Hodges JR, 2003. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology 61, 1196–1203. [DOI] [PubMed] [Google Scholar]
- Tranel D, 2006. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology 20, 1–10. [DOI] [PubMed] [Google Scholar]
- Tulving E, 1972. Episodic and semantic memory In: Tulving E, Donaldson W (Eds.), Organization of Memory. Academic Press, New York, NY, pp. 382–403. [Google Scholar]
- Vaughan W, 1988. Formation of equivalence sets in pigeons. J. Exp. Psychol.: Anim. Behav. Process. 14. [Google Scholar]
- Viskontas IV, Boxer AL, Fesenko J, Matlin A, Heuer HW, Mirsky J, Miller BL, 2011. Visual search patterns in semantic dementia show paradoxical facilitation of binding processes. Neuropsychologia 49, 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis G, 2013. Toward a unified model of face and object recognition in the human visual system. Front. Psychol. 4, 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, 1975. The selective impairment of semantic memory. Q. J. Exp. Psychol. 27, 635–657. [DOI] [PubMed] [Google Scholar]
- Warrington EK, 1985. Visual deficits associated with occipital lobe lesions in man. Exp. Brain Res. Supplement 11, 247–261. [Google Scholar]
- Wernicke C, 1874. Der aphasische Symptomencomplex; eine psychologische Studie auf anatomischer Basis. Cohn & Weigert, Breslau. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


