Abstract
Background:
Spontaneous preterm labor leading to preterm birth is a significant obstetric problem leading to neonatal morbidity and mortality. Current tocolytics are not completely effective and novel targets may afford a therapeutic benefit.
Objective:
To determine whether the anoctamin (ANO) family, including the calcium-activated chloride channel ANO1, is present in pregnant human uterine smooth muscle (USM) and whether pharmacological and genetic modulation of ANO1 modulates USM contraction.
Methods:
Reverse transcription-polymerase chain reaction (RT-PCR), quantitative RT-PCR, and immunohistochemical staining were done to determine which members of the ANO family are expressed in human USM. Uterine smooth muscle strips were studied in an organ bath to determine whether ANO1 antagonists inhibit oxytocin-induced USM contractions. Anoctamin 1 small interfering RNA (siRNA) knockdown was performed to determine its effect on filamentous-/globular (F/G)-actin ratio, a measurement of actin polymerization’s role in promoting smooth muscle contraction.
Results:
Messenger RNA (mRNA) encoding all members of the ANO family (except ANO7) are expressed in pregnant USM tissue. Anoctamin 1 mRNA expression was decreased 15.2-fold in pregnant USM compared to nonpregnant. Anoctamin 1 protein is expressed in pregnant human USM tissue. Functional organ bath studies with pregnant human USM tissue demonstrated that the ANO1 antagonist benzbromarone attenuates the force and frequency of oxytocin-induced contractions. In human USM cells, siRNA knockdown of ANO1 decreases F-/G-actin ratios.
Conclusion:
Multiple members of the ANO family, including the calcium-activated chloride channel ANO1, are expressed in human USM. Antagonism of ANO1 by pharmacological inhibition and genetic knockdown leads to an attenuation of contraction in pregnant human USM. Anoctamin 1 is a potentially novel target for tocolysis.
Keywords: ANO1, benzbromarone, calcium-activated chloride channel, TMEM16A, uterine smooth muscle
Introduction
Spontaneous preterm birth (sPTB) remains an obstetric dilemma associated with increased risk of neonatal death and lifelong complications.1 Unfortunately, sPTB rates have not significantly declined in the last decade, in part because effective therapeutic options for thwarting preterm labor (PTL) remain limited. Although 17-hydroxyprogesterone caproate treatment in women with a history of sPTB has shown clinical benefit for prevention of sPTB, efficacy is also limited.2 In addition, once spontaneous PTL (sPTL) has begun, conventional tocolytics such as nifedipine, terbutaline, or magnesium have limited effectiveness beyond 48 hours of treatment.3 In order to decrease rates of sPTB occurring secondary to sPTL, it is imperative that we identify novel targets that contribute to uterine smooth muscle cell (USMC) excitation and activation (key processes that are thought to contribute to the development of sPTL).
Uterine smooth muscle cell activation leading to coordinated uterine contractions is a complex, multifaceted process that involves alterations in USMC contractile agonist receptors, changes in ion channel composition, and increases in gap junctions (channels that transmit action potentials [APs] between cells).4 These changes allow the uterus to transform from a quiescent muscle bed with a low intrinsic excitability into a more responsive contractile USM phenotype that displays high intrinsic excitability.5–9
It is understood that a critical component of this process involves interactions between several USMC ion channels to promote changes in membrane potential. In particular, when the net effect of USMC ion channel effects allows for a shift to a more depolarized potential, it allows for recruitment and activation of voltage-dependent ion channels (eg, voltage-gated calcium channels [VGCCs]) to augment intracellular calcium entry and promote a widespread AP wave into adjoining myometrial cells. Although conventional tocolytics such as nifedipine and magnesium have targeted the VGCC to limit its role in promoting calcium flux and AP generation, the clinical efficacy of these drugs has been disappointing. Several potential factors (excessive hypotension, drug tolerance, and mechanistic redundancy through other calcium-permeable channels such as TRPV4 (transient receptor potential cation channel subfamily V member 4))10 may contribute to the observed inadequacy of VGCC blockade successfully terminating sPTL in clinical practice.
However, it should be emphasized that since these channels are voltage-gated—they require that a depolarizing threshold be met in order to activate them. During mid-gestation (and up to the onset of labor), USM in mammals undergoes a gestation-dependent hyperpolarization (known to be mediated by differential expression in potassium channels).11–13 As term and labor approaches, resting membrane potential in USM will depolarize closer to AP threshold, but the underlying ionic conductance that initiates this change remains unknown. Understanding which ion channels provide the antecedent excitability to drive this process may be a key to improved tocolysis. One group of channels proposed to be important for this antecedent depolarizing drive (but not well characterized in the human uterus) are calcium-activated chloride channels (CaCCs). We have shown that blockade of a subset of these channels (specifically anoctamin 1 [ANO1] and ANO2) from the ANO family (aka TMEM16) reduces pro-contractile depolarizing membrane currents and results in relaxation of murine USM contraction.14 To date, however, the role of ANOs in the late gestation human USM contractility has not been evaluated. Our goal in this study was to determine the expression profiles of the ANO family in human USM and determine whether blocking ANO1/2 can suppress human USM contractility and pacing frequency. Knowledge from this study may identify specific CaCCs as novel tocolytic targets to more effectively treat PTL and subsequent sPTB.
Methods
Reagents/Chemicals
All reagents were purchased from Sigma (St. Louis, Missouri) unless stated otherwise. Benzbromarone was dissolved in dimethyl sulfoxide (DMSO).
Human USM Specimens
In accordance with an institutional review board (IRB)-approved protocol (#AAAL4005), de-identified fresh human uterine tissue was obtained from the superior margin of the uterine incision following elective cesarean delivery (>38-40 weeks of gestation). All samples are from nonlaboring patients. Additionally, using an IRB-approved protocol (#AAAI0337), fresh nonpregnant myometrium was obtained following hysterectomy for benign gynecologic indications in women of similar age (only non-fibroid tissue was harvested) for quantitative reverse transcription-polymerase chain reaction (qRT-PCR) studies. In all cases, tissue was immediately placed in cold sterile Hank's Balanced Salt Solution on ice. Tissue from pregnant myometrium was also processed to establish primary USM cell lines or generate smooth muscle strips for immunohistochemistry (IHC) and/or organ bath studies.
Cell Culture
Primary USM cell cultures were established by enzymatic dissociation of fresh human myometrium, utilizing the Worthington Papain tissue dissociation. Briefly, USM was bluntly dissected, minced, and enzymatically dissociated using papain and collagenase. Following an ovamucoid/albumin separation, isolated USM cells were seeded into a 75 cm2 culture flask. To maintain primary phenotype, experiments were restricted to less than 6 passages in culture. Contractile phenotype was previously assessed by immunohistochemical staining for smooth muscle heavy chain and calcium release in response to contractile agonists (oxytocin, data not shown) human telomerase reverse transcriptase-immortalized human USM cells were a gift from Dr Darlene Dixon (NIH, Bethesda, Maryland).15 Uterine smooth muscle cells were grown in Smooth Muscle Growth Medium-2 medium with manufacturer’s recommended additives (Lonza, Walkersville, Maryland).
Assessment of ANO Family Messenger RNA Expression
Surveillance RT-PCR of ANO family members
RNA was extracted and reverse transcribed as described previously.16 Briefly, total RNA was extracted from human uterine cultured cells and grossly dissected myometrium using TRIzol (Ambion, Austin, Texas). Total RNA from human liver (Clontech, Mountain View, California) was used as a positive control. Using the Super Script VILO complementary DNA (cDNA) synthesis kit (Invitrogen, Carlsbad, California), 2 μg of RNA was reverse transcribed in a 20 μL reaction, which was then diluted 5-fold. Polymerase chain reaction was performed with 5 μL cDNA using the Advantage 2 PCR Kit (Clontech) on an MJ Research PTC-200 Peltier thermal cycler (Bio-Rad, Hercules, California). Forward and reverse primers specific for 10 members of the ANO family (ANO 1-10) were utilized (Table 1).16 All cDNA samples were initially denatured at 94°C for 30 seconds, and optimal annealing temperatures for each primer set were established and utilized as described previously for each ANO family member.16 Polymerase chain reaction products were electrophoresed and visualized.
Table 1.
Primer Sequences Used for Surveillance Reverse Transcription-Polymerase Chain Reaction (RT-PCR) of ANO Family.
| Target | Human Accession Numbers | Sequence: 5′- to -3′ | Expected PCR Base Pair Size | |
|---|---|---|---|---|
| ANO1 | NM_018043 | F R |
GAGCCGCCGTGGTCGGAAAA GGGAGAGGGTGCCCATCGGT | 416 |
| ANO2 | NM_020373 | F R |
GAGCTGAGACGGCCGGATGC CCCCACCTCCTGGGCTCCTC | 556 |
| ANO3 | NM_031418 | F R |
TGCCAGCCCGAGCAACTGAC TTTGGAACTCCAGGGCGGGC | 290 |
| ANO4 | NM_178826 | F R |
TGGGGAGGGGGAGGACCAGT CTGGGCGGCTTGCCACACTT | 390 |
| ANO5 | NM_213599 | F R |
AGTGAGCAGAGCCTGAGCAGC GGAGTGTGCTTAGGGCGGGG | 413 |
| ANO6 | NM_001025356 | F R |
CCCCTGAGCCCAAGCGACAC ATGCGGCTTCTGGTGGCTGG | 755 |
| ANO7 | NM_001001891 | F R |
AGTGGTGGGCACACTGGTGTT GCGCTGGAGAGCAGCCAGAAA | 141 |
| ANO8 | NM_020959 | F R |
GCCAAGCAGGGAGAAGCACTCCACAA ACCCATGACTTCATGAGGCGGTTCAGAATA | 143 |
| ANO9 | NM_001012302 | F R |
AAGAAGACGTGGGCGCGGTG GGAGGCCAGGACGCGGTAGA | 597 |
| ANO10 | NM_018075 | F R |
GTGGAGCACGCACTCCTGGC CCGAGCCAAGCCACTGCGAA | 373 |
Abbreviations: ANO, anoctamin; F, forward; R, reverse.
Differential qRT-PCR expression of ANO family members between pregnant and nonpregnant human myometrium
To analyze which ANO family members are differentially expressed in myometrium from nonpregnant and pregnant participants, we performed qRT-PCR. Relative expression of the different isoforms in myometrium from nonpregnant and pregnant participants was determined using Applied Biosystems PowerUp SYBR Green PCR master mix as previously described.17 Briefly, RNA was extracted and reverse transcribed as above. To prevent RNA degradation influencing our results, RNA integrity number (RIN) was measured for each sample with an Agilent 2100 Bioanalyzer, and only samples with RIN > 7 were used. Primers for qRT-PCR were designed for each ANO family member (Table 2). Quantitative PCR was performed on Mx3000p (Agilent Technologies, Santa Clara, California). Amplification plots and Ct values were obtained and analyzed with Mx3000p software. To confirm analysis was directed only to the appropriate single PCR product, melt curves were performed, and PCR products were electrophoresed and visualized. Delta Ct (ΔCt) was calculated as: Ct ANO1 − Ct Glyceraldehyde-3-phosphate dehydrogenase, housekeeping gene). Delta delta Ct (ΔCt) was calculated as: ΔCt (pregnant) −ΔCt (nonpregnant). Fold change was calculated 2(ΔΔCt).
Table 2.
Primer Sequences Used for Differential Quantitative Reverse Transcription-Polymerase Chain Reaction (QRT-PCR) of ANO Family Between Pregnant and Nonpregnant Myometrium.
| Target | Human Accession Numbers | Sequence: 5′- to -3′ | Expected PCR Base Pair Size | |
|---|---|---|---|---|
| ANO1 | NR_030691 | F R |
GGCTTTCCTGCTGAAGTTTG TTCAGGTAGCGGATGAGCTT | 225 |
| ANO2 | NM_001278596 | F R |
TCATGCATACCTGATTCCA ACTCCGTCGTGTTTGTTTCC | 202 |
| ANO3 | NM_001313726 | F R |
TCATCGCATACCTGATTCCA TCCGTTGTTGTTGCAAATGT | 120 |
| ANO4 | NM_001286616 | F R |
CTTTCGCTGCCTTTAAGTGG TCAGAAGCAGGGCAACTTTT | 132 |
| ANO5 | NM_213599 | F R |
AAGGAAGAGCAGGCCCTTAG AGCTGGTCTCTCTGCTGCTC | 263 |
| ANO6 | NM_001025356 | F R |
TCATGGGAGGAAAAGCAATC AAGGTGACGAACCCAAACTG | 205 |
| ANO9 | NM_001012302 | F R |
TTCTCCGAGCAGTTCTGGTT CCTTCCATGCCACATCTTCT | 183 |
| ANO10 | NM_018075 | F R |
CTAACTGTGCGCTGATTGGA GCTTGAGTGCCTCCAAAGAC | 204 |
Abbreviations: ANO, anoctamin; F, forward; R, reverse.
Immunohistochemistry
Human myometrial smooth muscle strips were fixed in 4% paraformaldehyde (4°C overnight), incubated in 30% sucrose for 24 hours, frozen, and then sectioned into 6 μm sections. Sections were washed in phosphate-buffered saline (PBS), incubated with 0.1% Triton X-100 for 10 minutes, blocked with 15% goat serum and incubated overnight at 4°C in primary antisera. Primary antibodies/stains used were (1) anti-TMEM16A (transmembrane member 16A) antibodies (rabbit, monoclonal; Abcam no ab64085, 1:100 and rabbit, polyclonal; Alomone labs Cat# ACL-011, 1:100) and (2) rhodamine phalloidin (1:200; Life Technologies, Grand Island, NY). The secondary antibody for ANO1/TMEM16A antibodies consisted of fluorescein isothiocyanate-conjugated goat anti-rabbit Immunoglobulin G (green, 1:400 dilution). Nuclear staining was achieved with 4′,6-diamidino-2-phenylindole (DAPI) stain (#H-1500; Vector laboratories, Burlingame, California). Negative controls were performed by omitting primary antibody. All immunofluorescence experiments were repeated at least 3 times. Images were acquired with confocal microscopy using NIS software version 4.10 (Nikon Eclipse, Japan).
Functional organ bath force recordings
Freshly obtained late gestation myometrium was finely dissected into 4 × 6 mm2 strips and attached inferiorly to a fixed tissue hook in a 16-mL bath (Radnoti Glass Technology, Monrovia, California) and superiorly to a Grass FT03 force transducer (Grass Telefactor, West Warwick, Rhode Island) by silk thread. BioPac hardware and Acknowledge 3.7.3 software (Biopac Systems, Goleta, California) continuously recorded muscle force. Uterine strips were equilibrated under 1 g of tension for 1 hour in a modified Krebs-Henseleit buffer (concentration in mM: NaCl 112.0, KCl 5.0, CaCl2 2.5, MgSO4 1.2, NaHCO3 25.0, NaH2PO4 1.0, d-glucose 11.5) continuously bubbled with 95% O2 and 5% CO2. Following contractile stimulation with oxytocin (0.5 μM), strips (9 different patients) were allowed to equilibrate to an increased baseline contractility for 30 minutes, after which they were treated with varying concentrations of benzbromarone (1-500 μM), a known ANO1 antagonist.18 To avoid significant variability in contraction frequency and minimize significant decay in force as a function of time, an EC85 (85% effective concentration) dose of oxytocin (0.5 μM) was employed to stimulate forceful rhythmic contractions (as shown by other investigators).19 Force tracings were analyzed for differences in force and frequency of contractions. The integral change in force was measured over 60 minutes and calculated as a percentage of reduction in integral force (gram × seconds) from baseline oxytocin-induced contractility. Percent reduction in integral force was also plotted using a variable slope sigmoidal dose–response curve (Y = bottom + [top − bottom]/[1 + 10∩[[log EC50-X] × HillSlope]]). Baseline contraction frequency was assessed following oxytocin over 30 minutes, and subsequent contraction frequency was measured after varying doses of benzbromarone (1-500 μM) following 2 sequential 30-minute time intervals. Percentage frequency compared to baseline was then plotted at each concentration of benzbromarone at 30 and 60 minutes. Linear regression analysis and compiled slopes of plots were analyzed between groups using ANOVA (analysis of variance).
Filamentous-Actin:G-Actin Ratio Measurements
To assess the potential relationship of ANO1-mediated effects on actin changes associated with contractile changes, we measured changes in F-Actin:G-Actin (filamentous/globular [F/G]) ratio between nontargeting control siRNA (scrambled)-treated cells and ANO1 siRNA-treated cells (ON-TARGET plus SMART pool Human ANO1; Dharmacon, Lafayette, Colorado). To include proper assay controls, we also ran in parallel human USM cells receiving no treatment and cells just receiving the lipid vehicle (with no siRNA). Transfection of siRNAs was performed at 100 nM with DharmaFECT1. Uterine smooth muscle cells were plated on 8-well chamber slides and exposed to vehicle or 1 μM oxytocin for 10 minutes after 72 hours siRNA knockdown. After 10 minutes, the cells were fixed and agonist action terminated by the addition of 4% fresh paraformaldehyde in PBS for 15 minutes. After 3 washes with PBS, cells were permeabilized with 0.1% Triton X-100 in PBS for 5 minutes. Cells were next pretreated with blocking solution (1%-0.1% Triton X-100 in PBS) for 15 minutes. Cells were simultaneously stained with rhodamine-conjugated phalloidin (Molecular Probes R415; ThermoFisher Scientific, Waltham, Massachusetts) and Alexa 488–conjugated DNase I (Molecular Probes D12371; ThermoFisher Scientific) in 1% bovine serum albumin in PBS to localize pools of F-actin and monomeric or G-actin, respectively. The cell staining was performed for 20 minutes in the dark at room temperature. The wells were washed 3 times with PBS, and a coverslip was mounted on the slide with the mounting medium Vectashield H-1000 (Vector, Burlingame, California) to prevent rapid photobleaching. Incubation, fixation, and staining were performed in parallel for all wells on a slide. Fluorescent intensities were recorded within each microscopic field, quantified using Image J software (NIH), and recorded as ratios, as previously described.20,21
Statistical analysis
Unless otherwise stated, data were analyzed using one-way ANOVA with the Bonferroni correction for multiple comparisons or unpaired, 2-tailed t tests (for less than 3 grouped observations) where appropriate. Data are expressed as mean ± SE with P < .05 taken as statistically significant.
Results
Expression of Messenger RNA Encoding the ANO Family in Human USM Tissue and Cell Culture
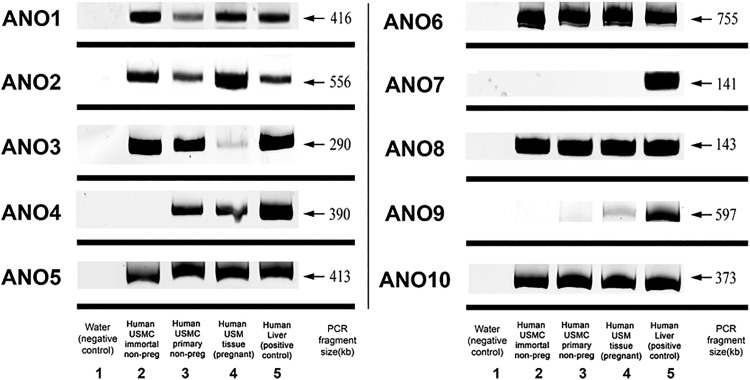
Human USM tissue and cell culture models were first surveyed for expression of messenger RNA (mRNA) encoding different members of the ANO family (Figure 1). mRNA encoding all members of the ANO family except ANO7 was expressed in pregnant human USM tissue. Anoctamin 7 was also not expressed in both immortalized and primary USMC in culture. Primary USMC in culture expressed ANO 1-6, 8, and 10, differing only from pregnant tissue by the lack of expression of ANO9. Immortalized USMC expressed ANO1-3, 5-6, 8, and 10, lacking ANO4 when compared to primary USMC.
Figure 1.
Anoctamin (ANO) messenger RNA (mRNA) expression in human uterine smooth muscle (USM). Representative gel image of 10 members of the (ANO; TMEM16) family. All members except ANO7 were expressed in pregnant human USM tissue. Immortalized human USMCs expressed ANO1-3, 5-6, 8, and 10, whereas primary human USMCs expressed ANO 1-6, 8, and 10. All negative controls demonstrated no bands and all members were detected in positive control (human liver).
Differential qRT-PCR Expression of ANO Family Members Between Pregnant and Nonpregnant Myometrium
After establishing that the ANO family is expressed in human USM tissue and cell culture lines, we then explored whether the expression of the ANO family changed with pregnancy. For this quantitative evaluation, we used pregnant and nonpregnant USM tissue from women of similar age. Relative comparisons of mRNA expression levels between pregnant and nonpregnant myometrium revealed significant differences for only the ANO1 isoform. Our data reveal that ANO1 mRNA expression is decreased in pregnant term myometrium as compared to nonpregnant myometrium by 15.2-fold (nonpregnant ΔCt 7.1 ± 0.5, pregnant ΔCt 11.0 ± 1.0, n = 4, P = .01; Table 3). Other ANO family members were not found to have a significant change in expression between nonpregnant and pregnant USM tissue (Table 3).
Table 3.
Quantitative Reverse Transcription-Polymerase Chain Reaction (QRT-PCR) Results for Anoctamin Family Comparing Nonpregnant and Pregnant Myometrium.
| Average Ct Value | Average ΔCt Value | Fold Change | P Value | |||
|---|---|---|---|---|---|---|
| Nonpregnant | Pregnant | Nonpregnant | Pregnant | Pregnant Versus Nonpregnant | ||
| ANO1 | 27.2 ± 0.2 | 31.2 ± 1.0 | 7.1 ± 0.5 | 11.0 ± 1.0 | −15.2 | a(.01) |
| ANO2 | 27.9 ± 0.6 | 30.1 ± 0.7 | 7.8 ± 0.8 | 10.0 ± 0.8 | −3.3 | ns (.11) |
| ANO3 | 30.6 ± 0.3 | 31.7 ± 1.3 | 10.6 ± 0.2 | 11.5 ± 1.3 | −1.9 | ns (.51) |
| ANO4 | 33.8 ± 0.4 | 33.1 ± 0.5 | 13.6 ± 0.1 | 13.0 ± 0.5 | 0.5 | ns (.35) |
| ANO5 | 30.2 ± 0.3 | 31.7 ± 1.1 | 10.1 ± 0.2 | 11.4 ± 1.1 | −2.6 | ns (.27) |
| ANO6 | 28.9 ± 0.7 | 28.4 ± 0.3 | 7.0 ± 0.9 | 7.2 ± 0.6 | −1.1 | ns (.83) |
| ANO9 | 27.8 ± 1.4 | 29.8 ± 1.3 | 7.8 ± 1.5 | 10.5 ± 2.7 | −1.8 | ns (.41) |
| ANO10 | 25.9 ± 0.3 | 25.8 ± 0.9 | 6.0 ± 0.3 | 4.7 ± 1.6 | 0.4 | ns (.46) |
Abbreviations: ANO, anoctamin; ns, not significant; ΔCt, delta Ct.
Bold indicates statistically significant.
Anoctamin 1 Protein Is Expressed in Pregnant Human USM Tissue
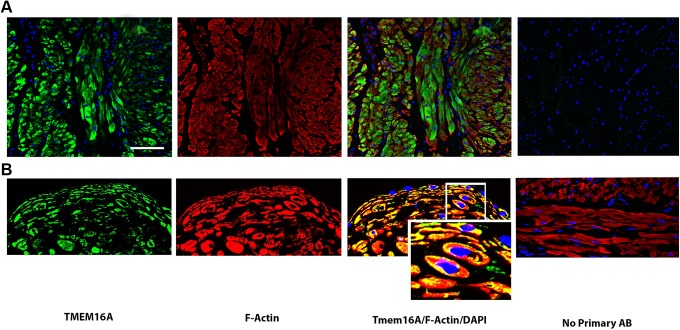
Since ANO1 (or TMEM16A) is known to be an important CaCC for contraction and relaxation in other types of smooth muscle,22,23 we also assessed protein expression of ANO1 in the human uterus. Immunofluorescence demonstrated abundant expression of ANO1 (green) in late gestation throughout human USM (Figure 2). Two different antibodies were utilized (Figure 2A and B). The tissue was also co-stained with α-actin (red) to identify smooth muscle.
Figure 2.
Detection of anoctamin 1 (ANO1; TMEM16A) on intact pregnant human uterine smooth muscle. Representative images (A and B) from intact late gestation pregnant human uterine smooth muscle sections (2 distinct patients with 2 distinct antibodies) demonstrating expression of ANO1. Confocal microscopy images employing single, double, and triple staining with immunofluorescence labeling of antibodies directed against ANO1/TMEM16A (green), smooth muscle actin (red), and/or the nuclear counterstain 4′,6-diamidino-2-phenylindole (DAPI) (blue). A, Calibration bar represent 60 μm. B, Colocalization with cellular distribution of ANO1 is highlighted in the third column (×550).
Pharmacological ANO1 Antagonism Mediates Reductions in Oxytocin-Induced Contractile Force and Frequency
After confirming expression in pregnant USM, we next sought to determine whether pharmacological antagonism of ANO1 could induce relaxation. Following an EC85 dose of oxytocin (0.5 μM), subsequent treatment with varying concentrations of benzbromarone (1-500 μM) demonstrates a dose-dependent reduction in force and frequency in human late gestation myometrial strips precontracted with oxytocin (Figure 3B). The percentage reduction in integral force was plotted on a sigmoidal dose–response curve to calculate the IC50 (half maximal inhibitory concentration) of benzbromarone as 45 μM (Figure 3C). Additionally, we observed a significant reduction in the percentage integral force starting at 25 μM benzbromarone (65.4 ± 3.5%, n = 7, P < .001; Figure 3D). To determine benzbromarone’s effect on frequency of contraction, frequency was measured over two 30-minute time intervals and calculated as a change in percentage of baseline frequency. The percentage of baseline frequency was plotted at different concentrations of benzbromarone (Figure 3E). Slopes of regression lines were plotted, and a significant reduction in contraction frequency was seen at 10 μM (n = 5-8, P < .05; Figure 3F). Samples for these studies were obtained from 7 distinct patients, with an n of 5 to 7 strips tested at each concentration or vehicle control.
Figure 3.
Anoctamin 1 (ANO1) antagonism leads to a reduction in human uterine smooth muscle (USM) contractility. A, Experimental design outline of functional organ bath recordings. B, Representative tracings of oxytocin-induced (EC85) contractions in human USM with treatment of vehicle (0.1% dimethyl sulfoxide (DMSO)), 50 μM benzbromarone, and 500 μM benzbromarone. C, Sigmoidal dose–response curve demonstrating the percent reduction in integral force (gram × seconds) at different concentrations of benzbromarone (7 patients; with n = 5 to 7 strips at each concentration tested). IC50 of benzbromarone on reduction in force from an EC85 oxytocin-induced contraction of human USM is 45 μM. D, Benzbromarone significantly decreases integral force of oxytocin-induced (EC85) human USM contractions in a dose-dependent manner. The lowest effective dose of benzbromarone was 25 μM (***P < .001; ns, not significant). E, Percentage changes in frequency measured at 2 intervals (30 and 60 minutes) is decreased by benzbromarone in a concentration-dependent manner (0-500 μM). F, Slopes of tracings from (E) were calculated and compiled. Benzbromarone decreases oxytocin-induced USM contractile frequency in a dose-dependent manner. The lowest effective concentration of benzbromarone on frequency was 10 μM. (*P < .05, ***P < .001).
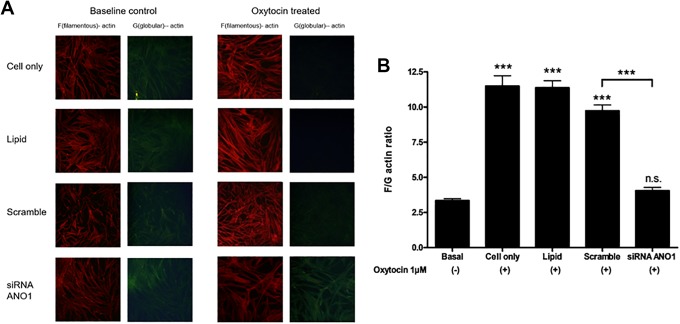
Anoctamin 1 siRNA Knockdown Decreases the F-/G-Actin Ratio in Human USMCs
Given the inherent challenge in demonstrating specificity of ANO1 targeting with pharmacological agents, we expanded our studies to include siRNA knockdown of ANO1 in human USM cells and measured its effect on F-/G-actin ratio, a marker of actin polymerization. Oxytocin treatment increased F-actin (Figure 4A). Filamentous-/globular-actin ratio was increased from 3.4 ± 1.5 (basal) to 11.5 ± 0.7 (cell only; n = 6, P < .001) with oxytocin treatment (Figure 4B). After siRNA knockdown of ANO1, F-/G-actin did not increase significantly with oxytocin treatment (4.1 ± 0.2, n = 6, not significant from basal). Both lipid and scramble siRNA control show a significant difference in the F-/G-actin ratio from siRNA knockdown of ANO1 (n = 6, P < .001).
Figure 4.
The filamentous (F)-actin to globular (G)-actin ratio in cultured human uterine smooth muscle cells (USMCs). A, Representative fluorescence microscopy images, demonstrating differences in F- and G-actin staining in cells with no treatment control (cell only), lipid vehicle-treated control (lipid), nonspecific, scrambled siRNA treatment control (scramble) and anoctamin 1 (ANO1) siRNA knockdown (ANO1), under baseline conditions (baseline) and oxytocin treatment. B, In USMCs, oxytocin markedly increased the F-actin:G-actin (F/G) ratio consistent with increased F-actin polymerization (pro-contractile effect). The ratio was significantly decreased by ANO1 siRNA knockdown (pro-relaxant effect). All cultured USMC control groups (cell only, lipid, and scrambled siRNA) of siRNA ANO1 knockdown showed a significantly decreased F/G ratio at baseline, no transfection cells (cell only), DharmaFECT 1-treated cells (lipid) or scramble siRNA transfection cells (scramble), respectively (n = 6 experiments compiled, ***P < .001).
Comment
In this study, we report on the expression profile of the ANO family in human myometrium and the functional significance of the CaCC members in this family (ANO1/2). We also demonstrate that ANO1 is the only ANO mRNA isoform differentially expressed between pregnant (late gestation, nonlaboring) and nonpregnant myometrium. We pair these findings with experimental observations that pharmacologic antagonism of ANO1/2 results in functional reductions in late gestation pregnant human USM contractility ex vivo (reducing both frequency and force). Finally, we show that highly selective and specific targeting of ANO1 (by siRNA silencing) results in a concomitant reduction in F-/G-actin ratio (a well-established marker for actin polymerization).
The ultimate goals of these studies are to reveal a potential role that ANO1/2 CaCCs play in human myometrial contractility and lay an experimental foundation to determine whether targeting these channels may be a viable and novel approach for tocolysis of PTL. Although PTL is a complex process that can originate from distinct pathogenic etiologies, premature uterine contractions remain a fundamental component of this disease. The organization of phasic uterine contractions is classically thought to depend on 2 processes: an initial intracellular calcium wave caused by membrane depolarization leading to an AP and propagation of this AP facilitated by elevations in intercellular calcium.24 The initial intracellular calcium wave within the cell results from an increase in calcium from both extracellular calcium and intracellular calcium stores including stores in the sarcoplasmic reticulum that promote an AP. This AP is then propagated by elevations in intercellular calcium. Intercellular calcium waves cross cell boundaries and allow a tissue level of communication or a recruitment of multiple myocytes to propagate a contraction. Intercellular calcium waves are complex and may involve communication through gap junctions between myocytes and paracrine signaling involving many signaling molecules such as prostaglandins.24,25 Although the role of calcium in promoting myometrial contractility has been well established, the ionic contributions leading up to AP generation and ionic modulation of intracellular calcium dynamics remain incompletely understood. Nevertheless, it is believed that ion channel modulation can control myometrial calcium flux and thereby facilitate USM tocolysis.26
Indeed, CaCCs have been proposed as an important contributor to myometrial AP generation since they have been shown to shift resting membrane potentials in other tissues from a relatively negative potential to a more positive threshold capable of triggering activation of VGCCs.4 As such, the ion channel(s) responsible for this depolarizing drive are thought to be critical for establishing contractile frequency and enhancing myometrial excitability. However, the molecular identity of the channel(s) responsible for generating the depolarizing current that shifts human USM resting membrane potential toward the AP threshold has been enigmatic.12 Evidence for a role of CaCCs in myometrial contractility was first provided in studies in the rat, which describes a prominent inward (depolarizing) current that is activated by Ca2+ entry and plays a functionally important role by contributing to membrane potential and firing frequency in myometrial cells. Although these investigators did not identify the actual ion channels responsible for their observations, they did demonstrate that ubiquitous chloride channel blockade resulted in a significant reduction in the frequency of spontaneous contractions and inhibited oxytocin-induced contractions and Ca2+ transients. In alignment with these observations, our lab has previously shown in murine USMC that ANO1/2 channel activity provides CaCC depolarizing currents and that antagonism of these channels can suppress spontaneous USM contractions in mice.14 The current studies sought to determine whether ANO1/2 is present and potentially responsible for similar CaCC activity in human myometrium.
To do this, we first examined the mRNA expression profile of the entire ANO family (of which ANO1 and ANO2 are considered the only ion channels with CaCC characteristics).22,27 We found qualitative expression of all ANO members in pregnant myometrium with the exception of ANO7; however, only ANO1 showed differential expression following qRT-PCR analysis between late gestation pregnant (nonlaboring) myometrium and nonpregnant myometrial samples. Given experimental observations in ex vivo organ bath that demonstrate higher spontaneous contraction frequency in nonpregnant versus pregnant myometrium, our observation that relative ANO1 expression is reduced in late gestation myometrium is consistent with ANO1, participating in driving human myometrial contractile frequency. Our qRTPCR data illustrated a reduction in ANO1 expression in pregnant (nonlaboring) myometrium, which is consistent with the need for the gravid uterus to remain in a relatively quiescent state until labor commences. Although we did profile the other members of this family and demonstrate that all of them (except ANO7) are expressed (at the mRNA level), they are thought to possess other activities (ie, scramblases)27–30 and are not known to contribute to myometrial excitability in the classical ion channel sense. Although these other targets may play important roles in myometrial biology, we chose to focus on the member that displayed differential expression as a function of pregnancy (ANO1) in our functional studies. One limitation of our study is that we did not quantify expression profiles of the ANO family with respect to spontaneous labor. However, although results from published transcriptomes of laboring versus nonlaboring late gestation human myometrium reveal no changes in expression, they do show ample and relatively abundant expression of ANO1 (when compared to other expression levels of other “important” myometrial ion channel members).31 It should be emphasized that our results are in close agreement with the ANO results within this transcriptome data previously published. Although the methodologies are different, we also observed abundant expression for mRNA coding ANO 10, 8, 6, and 1 transcripts (with ANO2 expression present but less abundant). Given this agreement between our findings, it would suggest that the ANO members with significant biological expression are most likely to be limited to ANO 1, 6, 8, and 10.
We also demonstrate immunohistochemical evidence for ANO1/2 in late gestation intact myometrium using 2 commercially available antibodies. Indeed, the antibodies we chose to employ in our studies have been utilized to demonstrate ANO1 in the literature.32–42 Nevertheless, we do recognize that not all commercially available ANO1 antibodies provide convincing staining for “human” USM ANO1/2. Whether this is due to epitope interference due to posttranslational glycosylation (ANO1 is known to undergo)43 or loss of antibody recognition due to USM-specific splice variant forms of ANO1 (there are at least 4 known splice variants in other tissues)22,44–47 is unclear. Nevertheless, we provide the first report on ANO 1/2 CaCC protein expression in human myometrium using 2 distinct commercially available antibodies and also provide novel evidence that ANO1 protein expression in tissue is not limited to the cell membrane. While our confocal images of intact myometrium show IHC features consistent with intracellular ER/SR (endoplasmic/sarcoplasmic reticulum) expression further investigation is underway to determine whether distinct extracellular and intracellular expression of ANO1 (both membrane and SR) is present and functionally relevant to myometrial contractile pathways.
We next sought to establish the functional relevance of the ANO1/2 CaCC blockade on late gestation human USM. Since a primary goal of the study was to establish that targeting ANO1/2 channels represents a novel tocolytic strategy, we performed dose response studies utilizing benzbromarone on precontracted human USM and determined IC50 measurements measuring at both contractile force and frequency. Although the study of CaCCs has been historically hampered by a lack of specific pharmacologic antagonists, following the identification of ANO1 and 2 in 2008,48,49 more selective inhibitors for CaCCs have been developed. In particular, Huang et al first identified benzbromarone as a potent ANO1 (TMEM16A)-specific antagonist, utilizing high throughput screening.18 Other CaCC inhibitors such as tannic acid,50 T16Ainh-A01,50 MONNA,51 and B2552 have also been identified. However, Huang et al found benzbromarone to be a more potent ANO1 blocker than ubiquitous chloride channel blockers (niflumic acid and NPPB (5-nitro-2-(3-phenylpropylamino)benzoic acid)) and demonstrated benzbromarone effectively blocks ANO1 and 2 but not CFTR (cystic fibrosis transmembrane conductance regulator).18 We chose to utilize benzbromarone in these studies due to its potency, specificity, and its effectiveness in other smooth muscle relaxation studies targeting ANO1.53 Although we did not study all classes of ANO1 antagonists side by side for relative potency profiles, we do believe this approach would be valuable for tocolytic development in the future.
Although many studies often assess functional inhibition of force from an EC50 dose of oxytocin, we purposely used the more robust contractility associated with an EC85 dose of oxytocin. This oxytocin dose demonstrates more stability of contraction force over time, less variability in frequency of contractions as a function of time, and results in reduced fusiform contractions which can complicate frequency analysis. As a result, we are showing significant tocolysis from very robust contractions, both from a force perspective and a frequency perspective.
To support the pharmacological data and confirm the role of ANO1 on USM contractile signaling molecules, we employed siRNA knockdown in human USMCs. We demonstrate that after siRNA-mediated knockdown of ANO1, human uterine cells that are stimulated with oxytocin show a significant reduction in F-/G-actin ratio compared to basal cell and scrambled siRNA controls treated with oxytocin. Although the methodology employs quantitative densitometry, we did include representative pictures in Figure 4A to accompany the compiled graphs shown in Figure 4B. Essentially, with contractile agonist administration, there is a shift in the pool of actin—with a relative reduction in G-actin (seen as green) and a relative increase in F-actin (seen as red). We had 4 treatment groups (3 control groups and 1 siRNA knockdown of ANO1 group) that were used to assess ANO1 knockdown versus knockdown vehicle effects. In all groups exposed to oxytocin except the group in which ANO1 is selectively knocked down (siRNA), we see a reduction in green and an enhancement in red staining—consistent with oxytocin-induced changes in actin pools that accompany contractility. Since increased F-actin formation is a well-established marker for cytoskeletal changes that accompany an increase in contractility,20,21 these findings show inhibition of ANO1 activity that can reduce myometrial cell actin polymerization. These studies complement our functional studies utilizing benzbromarone, demonstrating that ANO1 activity promotes USM contraction and that antagonizing this CaCC channel specifically reduces myometrial contractile signaling. In summary, we provide evidence that future drug development focused on ANO1-specific blockade holds promise as a potential new tocolytic target.
Footnotes
Authors’ Note: This research was presented at Columbia University College of Physicians and Surgeons, Columbia University Medical Center, New York city, New York.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants 1R01HD082251 (GG), 1K08HL132203 (JD), K08HD088758 (JV), and the Louis V. Gerstner Jr. Scholars Program (GG, JD).
References
- 1. Center for Disease Control and Prevention: Preterm Birth, Published and updated November 27, 2017. https://www.cdc.gov/reproductivehealth/MaternalInfantHealth/PretermBirth.htm. (accessed February 2018).
- 2. Meis PJ. The role of 17 alpha-hydroxyprogesterone caproate in the prevention of preterm birth. Womens Health (Lond). 2006;2(6):819–824. [DOI] [PubMed] [Google Scholar]
- 3. Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta-analysis. BMJ. 2012;345:e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shmygol A, Blanks AM, Bru-Mercier G, Gullam JE, Thornton S. Control of uterine Ca2+ by membrane voltage: toward understanding the excitation-contraction coupling in human myometrium. Ann N Y Acad Sci. 2007;1101:97–109. [DOI] [PubMed] [Google Scholar]
- 5. Garfield RE, Sims S, Daniel EE. Gap junctions: their presence and necessity in myometrium during parturition. Science. 1977;198(4320):958–960. [DOI] [PubMed] [Google Scholar]
- 6. Fuchs AR, Fuchs F, Husslein P, Soloff MS, Fernstrom MJ. Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science. 1982;215(4538):1396–1398. [DOI] [PubMed] [Google Scholar]
- 7. Slater DM, Berger LC, Newton R, Moore GE, Bennett PR. Expression of cyclooxygenase types 1 and 2 in human fetal membranes at term. Am J Obstet Gynecol. 1995;172(1 pt 1):77–82. [DOI] [PubMed] [Google Scholar]
- 8. Maner WL, Garfield RE. Identification of human term and preterm labor using artificial neural networks on uterine electromyography data. Ann Biomed Eng. 2007;35(3):465–473. [DOI] [PubMed] [Google Scholar]
- 9. Garfield RE, Maner WL. Physiology and electrical activity of uterine contractions. Semin Cell Dev Biol. 2007;18(3):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ying L, Becard M, Lyell D, et al. The transient receptor potential vanilloid 4 channel modulates uterine tone during pregnancy. Sci Transl Med. 2015;7(319):319ra204. [DOI] [PubMed] [Google Scholar]
- 11. McCloskey C, Rada C, Bailey E, et al. : The inwardly rectifying K+ channel KIR7.1 controls uterine excitability throughout pregnancy. EMBO Mol Med. 2014;6(9):1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brainard AM, Korovkina VP, England SK. Potassium channels and uterine function. Semin Cell Dev Biol. 2007;18(3):332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lorca RA, Prabagaran M, England SK. Functional insights into modulation of BKCa channel activity to alter myometrial contractility. Front Physiol. 2014;5:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernstein K, Vink JY, Fu XW, et al. Calcium-activated chloride channels anoctamin 1 and 2 promote murine uterine smooth muscle contractility. Am J Obstet Gynecol. 2014;211(6):688 e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carney SA, Tahara H, Swartz CD, et al. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest. 2002;82(6):719–728. [DOI] [PubMed] [Google Scholar]
- 16. Gallos G, Remy KE, Danielsson J, et al. Functional expression of the TMEM16 family of calcium-activated chloride channels in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2013;305(9):L625–L634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gallos G, Townsend E, Yim P, et al. Airway epithelium is a predominant source of endogenous airway GABA and contributes to relaxation of airway smooth muscle tone. Am J Physiol Lung Cell Mol Physiol. 2013;304(3):L191–L197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang F, Zhang H, Wu M, et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci U S A. 2012;109(40):16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balki M, Erik-Soussi M, Kingdom J, Carvalho JC. Comparative efficacy of uterotonic agents: in vitro contractions in isolated myometrial strips of labouring and non-labouring women. Can J Anaesth. 2014;61(9):808–818. [DOI] [PubMed] [Google Scholar]
- 20. Hirshman CA, Zhu D, Pertel T, Panettieri RA, Emala CW. Isoproterenol induces actin depolymerization in human airway smooth muscle cells via activation of an Src kinase and GS. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L924–L931. [DOI] [PubMed] [Google Scholar]
- 21. Mikami M, Zhang Y, Danielsson J, et al. Impaired relaxation of airway smooth muscle in mice lacking the actin-binding protein gelsolin. Am J Respir Cell Mol Biol. 2017;56(5):628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins). Physiol Rev. 2014;94(2):419–459. [DOI] [PubMed] [Google Scholar]
- 23. Huang F, Rock JR, Harfe BD, et al. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci U S A. 2009;106(50):21413–21418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Young RC. Tissue-level signaling and control of uterine contractility: the action potential-calcium wave hypothesis. J Soc Gynecol Investig. 2000;7(3):146–152. [DOI] [PubMed] [Google Scholar]
- 25. Young RC, Schumann R, Zhang P. The signaling mechanisms of long distance intercellular calcium waves (far waves) in cultured human uterine myocytes. J Muscle Res Cell Motil. 2002;23(4):279–284. [DOI] [PubMed] [Google Scholar]
- 26. Sanborn BM. Relationship of ion channel activity to control of myometrial calcium. J Soc Gynecol Investig. 2000;7(1):4–11. [DOI] [PubMed] [Google Scholar]
- 27. Picollo A, Malvezzi M, Accardi A. TMEM16 proteins: unknown structure and confusing functions. J Mol Biol. 2015;427(1):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu K, Whitlock JM, Lee K, Ortlund EA, Cui YY, Hartzell HC. Identification of a lipid scrambling domain in ANO6/TMEM16F. Elife. 2015;4:e06901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oh U, Jung J. Cellular functions of TMEM16/anoctamin. Pflugers Arch. 2016;468(3):443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bethel NP, Grabe M. Atomistic insight into lipid translocation by a TMEM16 scramblase. Proc Natl Acad Sci U S A. 2016;113(49):14049–14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan YW, van den Berg HA, Moore JD, Quenby S, Blanks AM. Assessment of myometrial transcriptome changes associated with spontaneous human labour by high-throughput RNA-seq. Exp Physiol. 2014;99(3):510–524. [DOI] [PubMed] [Google Scholar]
- 32. Nakazawa MS, Eisinger-Mathason TS, Sadri N, et al. Epigenetic re-expression of HIF-2alpha suppresses soft tissue sarcoma growth. Nat Commun. 2016;7:10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caci E, Scudieri P, Di Carlo E, et al. Upregulation of TMEM16A protein in bronchial epithelial cells by bacterial pyocyanin. PLoS One. 2015;10(6):e0131775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bill A, Hall ML, Borawski J, et al. Small molecule-facilitated degradation of ANO1 protein: a new targeting approach for anticancer therapeutics. J Biol Chem. 2014;289(16):11029–11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Britschgi A, Bill A, Brinkhaus H, et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci U S A. 2013;110(11):E1026–E1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hwang SJ, Blair PJ, Britton FC, et al. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587(pt 20):4887–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thomas-Gatewood C, Neeb ZP, Bulley S, et al. TMEM16A channels generate Ca(2)(+)-activated Cl(-) currents in cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2011;301(5):H1819–H1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cho H, Yang YD, Lee J, et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci. 2012;15(7):1015–1021. [DOI] [PubMed] [Google Scholar]
- 39. Sanders KM, Zhu MH, Britton F, Koh SD, Ward SM. Anoctamins and gastrointestinal smooth muscle excitability. Exp Physiol. 2012;97(2):200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. He Q, Halm ST, Zhang J, Halm DR. Activation of the basolateral membrane Cl- conductance essential for electrogenic K+ secretion suppresses electrogenic Cl- secretion. Exp Physiol. 2011;96(3):305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhu MH, Kim TW, Ro S, et al. A Ca(2+)-activated Cl(-) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587(pt 20):4905–4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pineda-Farias JB, Barragan-Iglesias P, Loeza-Alcocer E, et al. Role of anoctamin-1 and bestrophin-1 in spinal nerve ligation-induced neuropathic pain in rats. Mol Pain. 2015;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang B, Li C, Huai R, Qu Z. Overexpression of ANO1/TMEM16A, an arterial Ca2+-activated Cl- channel, contributes to spontaneous hypertension. J Mol Cell Cardiol. 2015;82:22–32. [DOI] [PubMed] [Google Scholar]
- 44. Ferrera L, Caputo A, Ubby I, et al. Regulation of TMEM16A chloride channel properties by alternative splicing. J Biol Chem. 2009;284(48):33360–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mazzone A, Bernard CE, Strege PR, et al. Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem. 2011;286(15):13393–13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O’Driscoll KE, Pipe RA, Britton FC. Increased complexity of Tmem16a/anoctamin 1 transcript alternative splicing. BMC Mol Biol. 2011;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ubby I, Bussani E, Colonna A, et al. TMEM16A alternative splicing coordination in breast cancer. Mol Cancer. 2013;12:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Caputo A, Caci E, Ferrera L, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–594. [DOI] [PubMed] [Google Scholar]
- 49. Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134(6):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011;286(3):2365–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oh SJ, Hwang SJ, Jung J, et al. MONNA, a potent and selective blocker for transmembrane protein with unknown function 16/anoctamin-1. Mol Pharmacol. 2013;84(5):726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kumar S, Namkung W, Verkman AS, Sharma PK. Novel 5-substituted benzyloxy-2-arylbenzofuran-3-carboxylic acids as calcium activated chloride channel inhibitors. Bioorg Med Chem. 2012. 20(14):4237–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Danielsson J, Perez-Zoghbi J, Bernstein K, et al. Antagonists of the TMEM16A calcium-activated chloride channel modulate airway smooth muscle tone and intracellular calcium. Anesthesiology. 2015;123(3):569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]