Abstract
Uterine fibroids are benign uterine smooth muscle tumors that are present in up to 8 out of 10 women by the age of 50. Many of these women experience symptoms such as heavy and irregular menstrual bleeding, early pregnancy loss, and infertility. Traditionally believed to be inert masses, fibroids are now known to influence endometrial function at the molecular level. We present a comprehensive review of published studies on the effect of uterine fibroids on endometrial function. Our goal was to explore the current knowledge about how uterine fibroids interact with the endometrium and how these interactions influence clinical symptoms. Our review shows that submucosal fibroids produce a blunted decidualization response with decreased release of cytokines critical for implantation such as leukocyte inhibitory factor and cell adhesion molecules. Furthermore, fibroids alter the expression of genes relevant for implantation, such as bone morphogenetic protein receptor type II, glycodelin, among others. With regard to heavy menstrual bleeding, fibroids significantly alter the production of vasoconstrictors in the endometrium, leading to increased menstrual blood loss. Fibroids also increase the production of angiogenic factors such as basic fibroblast growth factor and reduce the production of coagulation factors resulting in heavy menses. Understanding the crosstalk between uterine fibroids and the endometrium will provide key insights into implantation and menstrual biology and drive the development of new and innovative therapeutic options for the management of symptoms in women with uterine fibroids.
Keywords: uterine fibroids, endometrium, endometrial stromal cells, leiomyoma, implantation, menstrual bleeding, infertility, bone morphogenetic receptor type II (BMPR-2), recurrent pregnancy loss
Introduction
Uterine fibroids are the most common gynecologic tumor, present in up to 80% of all women by the age of 50.1 While most uterine fibroids do not cause symptoms, some women can experience severe symptoms that significantly impact their quality of life. Fibroid symptoms include heavy and irregular menstrual bleeding with accompanying anemia, pelvic pain, dysmenorrhea, dyspareunia, increased urinary frequency, infertility, early pregnancy loss, among others.2,3 Fibroids are the leading indication for hysterectomy in the United States and account for up to US$34.4 billion dollars annually in health-care costs.4
The effects of fibroids on fertility were formerly believed to be exclusively as a result of their size; however, this perspective has changed as our understanding of fibroid pathogenesis at the molecular level has broadened. Fibroids influence endometrial gene expression through paracrine interactions. Additionally, the effect of fibroids on the endometrium is global and not localized to the endometrium overlying the fibroid itself.5 We conducted a review of the literature to evaluate and discuss what is currently known about how uterine fibroids interact with the endometrium and how these interactions lead to clinical symptoms, specifically infertility, miscarriage, and heavy menstrual bleeding.
Methods
We performed a comprehensive review of the literature on uterine fibroids, the influences they exert on endometrial function, and the potential mechanisms through which these lead to the impaired implantation. PubMed and Google Scholar websites were used to identify relevant articles. Search terms such as “uterine fibroids,” “leiomyoma,” and “endometrium” were used in combination with “implantation,” “heavy menstrual bleeding,” “irregular menses,” “recurrent pregnancy loss,” “miscarriage,” “early pregnancy loss,” “infertility,” “subfertility,” and “fertility outcomes.” References from these articles were used to identify additional sources.
Only reports written in English were included in the literature review. We placed no restrictions on year of publication; we included all publications from the earliest database dates until March 2017. We described and expanded on what is currently known about the relationship between uterine fibroids and the endometrium as it pertains to fertility and menstrual bleeding.
Results
Cellular Origins of Uterine Fibroids
Uterine fibroids are monoclonal tumors believed to arise from a single fibroid stem cell within the myometrium.6 Three cell populations have been identified in uterine fibroids: fully differentiated fibroid smooth muscle cells, a cell population with intermediate characteristics, and fibroid stem cells.7 Both myometrium and fibroid tissue have side population cells that possess cell surface markers characteristic of stem cells.7,8 Fibroid stem cells are critical for fibroid growth and expansion. In fact, in a murine model, tumors composed only of fully differentiated or intermediate populations of fibroid cells demonstrate significantly slower growth rates than those tumors composed of fibroid stem cells.7
It appears that fibroid stem cells occur as a result of a genetic hit to a myometrial stem cell, such as point mutations in the mediator complex subunit 12 (MED12) gene or chromosomal rearrangements that affect the expression of the high-mobility group AT-hook 2 (HMGA2) gene.6 Chromosomal rearrangements involving HMGA2 on the long arm of chromosome 12 are believed to play a role in the induction of fibroid stem cells and fibroid tumorigenesis, especially in larger tumors.6,9 Additionally, some fibroid stem cells possess MED12 mutations that have not been identified in the myometrial stem cell population.10 Introduction of a MED12 mutation in murine uterine tissue has been shown to give rise to fibroid-like tumor formation.11 These findings suggest that a genetic hit may be important for the initiation of fibroid tumors and their growth. Ethnicity and environmental factors are believed to play a role in tumorigenesis. Endocrine-disrupting chemicals (EDCs) have been shown to interfere with growth and differentiation in different stem cell types. Recent studies suggest that exposure to EDCs may lead to genetic alterations in stem cells, which may be important in fibroid tumorigenesis.12-14 With regard to ethnicity, studies reveal that the number of tumor-initiating myometrial stem cells is directly correlated with the likelihood of developing uterine fibroids, with the highest number being present in African American women with uterine fibroids and the lowest in Caucasian women without uterine fibroids.15
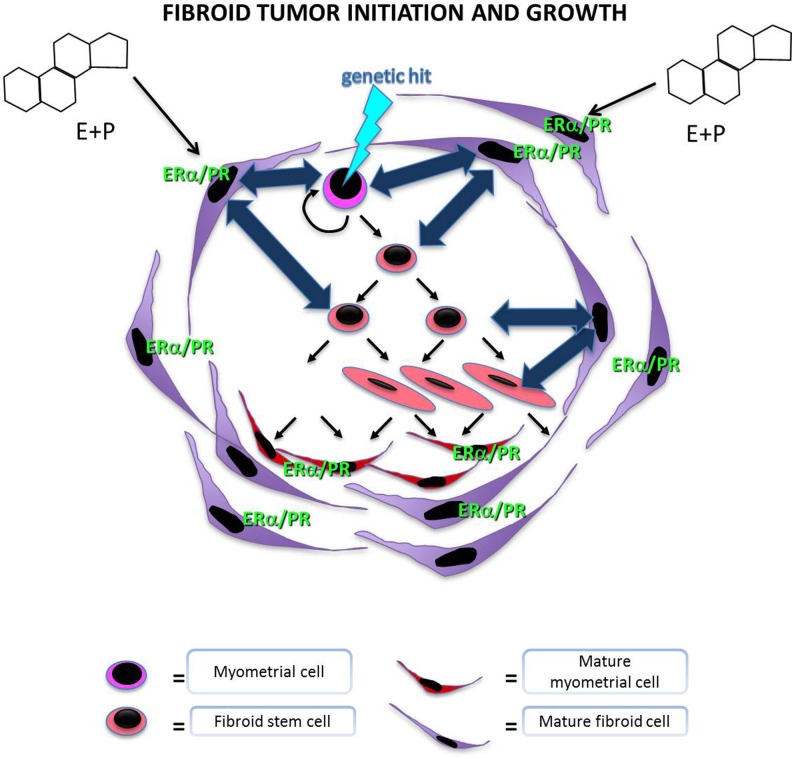
Fibroids are hormonally responsive tumors. Mature fibroid cells possess estrogen receptors, and estradiol is associated with increased proliferation of uterine fibroid smooth muscle cells.16,17 Uterine fibroids not only respond to systemic steroids but also to local steroids biosynthesized by aromatase within the fibroid itself.18 Despite the hormonally dependent nature of fibroids, fibroid stem cells express low levels of estrogen and progesterone receptors, suggesting that steroid hormones utilize a paracrine mechanism to exert their tropic effects on fibroid stem cells (Figure 1).
Figure 1.
Illustration of stem cell populations in the myometrium and fibroid tissue. Stem cells are self-renewing and are involved in the proliferation of both normal myometrium and fibroid tissue. It is thought that a genetic hit, such as a mutation in the MED12 gene, can lead to the transformation of a myometrial stem cell into a fibroid stem cell. Fibroids are hormone-responsive tissues. However, fibroid stem cells, which are mainly responsible for proliferation and fibroid growth, are devoid of estrogen and progesterone receptors. Thus, stem cell replication and growth is likely regulated via paracrine signals, which lead to fibroid growth. ERα indicates estrogen receptor alpha; PR, progesterone receptor.
An important signaling pathway implicated in promoting fibroid growth is the wingless-type Mouse Mammary Tumor Virus (MMTV) integration site Wingless Type (WNT)/β-catenin pathway.19 Because β-catenin targets the MED12 subunit, mutations in the MED12 gene can lead to alterations in the interactions between MED12 and β-catenin leading to inhibition of β-catenin transactivation in response to WNT signaling.20 In the WNT/β-catenin pathway, secreted WNT proteins bind to frizzled family cell surface receptors, leading to decreased β-catenin degradation in the cytoplasm and a subsequent increase in nuclear β-catenin.2,21 In the murine model, increased β-catenin level seen with increasing parity is correlated with the number of fibroid-like tumors present in the uteri of such mice, which exhibit both histologic and molecular characteristics of fibroids.22 However, in this study, it was unclear whether the increased β-catenin level or the increased parity of these mice is the primary driver of the increase in fibroid-like tumors. Recent data show that MED12 knockdown in human fibroid cells leads to decreased cell proliferation via downregulation of the WNT/β-catenin signaling pathway.23
Additionally, activation of the WNT/β-catenin pathway leads to increased levels of transforming growth factor β3 (TGF-β3). Fibroid cells secrete markedly elevated levels of TGF-β3 in a steroid-responsive manner when compared to myometrial cells.24 Transforming growth factor β3 has also been shown to play a key role in cell proliferation and deposition of extracellular matrix.22 Taylor and colleagues have demonstrated that TGF-β3 secreted by fibroid cells exerts paracrine effects on endometrial stromal cells (ESCs) and epithelial cells.5,25,26
Clinical Fertility Outcomes in the Presence of Uterine Fibroids
One in every 10 women seeking fertility treatment has uterine fibroids.27-29 The effect of uterine fibroids on infertility is largely dependent on the location of the fibroid, with submucosal and intramural fibroids having the most significant impact.
Submucosal fibroids
Submucosal fibroids, which impinge into the uterine cavity, have been associated with impaired reproductive outcomes. In 2008, Klatsky et al performed a systematic review showing that women with submucosal fibroids had lower implantation rates (3.0%-11.5% vs 14%-30%) and a higher incidence of early pregnancy loss (47% vs 22%) compared to women without fibroids.30-33 A meta-analysis by Pritts et al found that women with submucosal fibroids had significantly lower implantation rates, pregnancy rates, ongoing pregnancies, and live birth rates. In this meta-analysis, submucosal fibroids were associated with an increased risk of spontaneous abortion.34 Although most of these data are from retrospective or prospective cohort studies, the consensus is to surgically remove submucosal fibroids in a woman who is actively pursuing pregnancy, regardless of other symptoms.
Intramural fibroids
Fibroids located within the wall of the myometrium are known as intramural fibroids. The data on the relationship between intramural fibroids and infertility are inconclusive at best. A meta-analysis by Pritts et al found higher rates of spontaneous abortions and significantly lower rates of implantation, ongoing pregnancies, and live births in women with intramural fibroids.34 In 2017, Christopoulos et al showed decreased pregnancy rates after in vitro fertilization (IVF) in women with noncavity-distorting fibroids. Sagi-Dain and colleagues observed a similar trend in recipients of donor oocytes with uterine fibroids.35,36 However, in this study, oocyte recipients with intramural fibroids received a significant lower percent of good quality embryos and this was not controlled for in the results. However, other studies show data to the contrary. Klatsky et al also studied pregnancy rates in recipients of donor oocytes and noted no difference in implantation or clinical pregnancy rates between women with and without uterine fibroids.37 Additionally, the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation clinical trial by the Reproductive Medicine Network showed no difference in conception and live birth rates in women with noncavity-distorting intramural fibroids.38 Given the conflicting data, there is still some debate about the clinical effect of noncavity-distorting intramural fibroids. Current data suggest that if a clinical effect is present, it may be unmasked by and as a result more clinically relevant for IVF cycles than with ovarian stimulation and intrauterine insemination.
In addition to the controversy on fibroid location, there is still some debate as to whether the degree of the detrimental effect of uterine fibroids’ endometrial function correlates with the size of the fibroid. A 2008 meta-analysis by Pritts et al both show no difference in effect due to fibroid size or number on outcomes.34 However, fibroid size was only reported by 5 of the studies included in this meta-analysis.
The effect of myomectomy
These observations, specifically in the case of submucosal fibroids, raise the question of whether myomectomy leads to an improvement in fertility and early pregnancy outcomes. A 2013 Cochrane review concluded that hysteroscopic myomectomy improves clinical pregnancy rates with timed intercourse from a baseline of 21% to 39%.39 Although these findings suggest that hysteroscopic myomectomy provides a clinical benefit in the presence of a submucosal fibroid, more data from randomized controlled trials with larger populations are needed to better understand the effect of hysteroscopic myomectomy on endometrial function and implantation.
Effect of Uterine Fibroids on the Endometrium and Implantation
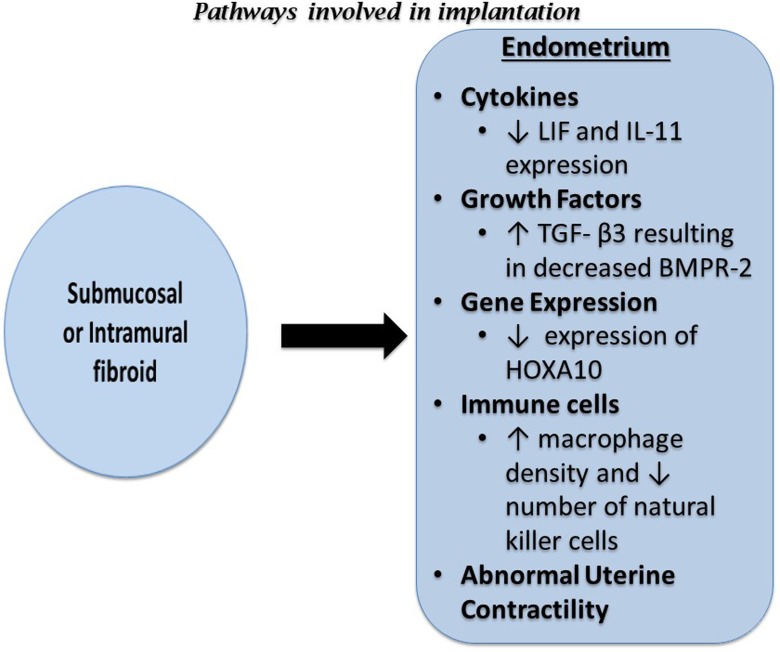
The narrow time period during which the endometrium is receptive to implantation of the embryo is known as the window of implantation (WOI). The WOI occurs between 7 and 10 days following the luteinizing hormone surge and it is when the endometrium prepares for the attachment of the blastocyst.40 The steps necessary for successful implantation are apposition, adhesion, and invasion. A complex series of interactions between various processes are necessary for these steps to occur and any aberrations can result in recurrent implantation failure, early pregnancy loss, or infertility. The effects of uterine fibroids on implantation are summarized in Figure 2 and described in detail below.
Figure 2.
Diagram summarizing the effects of submucosal and intramural fibroids on implantation. BMP2 indicates bone morphogenetic protein 2; HOXA10, Homeobox A10; IL-1β, interleukin 1 beta; IL-11, interleukin 11; LIF, leukemia inhibitory factor; TGF-β3, transforming growth factor beta 3; VEGF, vascular endothelial growth factor.
Cell adhesions molecules, homeobox genes, and other gene expression
Transcription factors known as homeobox genes, specifically homeobox A10 (HOXA10) and homeobox A11 (HOXA11), are expressed in the female reproductive system and are important for implantation.41 In mice, HOXA10 expression increased during the WOI. Knockout mice for HOXA10 are infertile due to implantation failure, specifically embryos from HOXA10 knockout mice are able to grow normally in wild-type mice, demonstrating that the defect lies with endometrial receptivity and not with the embryos themselves.42
The HOXA10 expression is decreased in the endometrium of women with submucosal fibroids. This decrease in HOXA10 expression is most prominent in the endometrium overlying the submucosal fibroid but is also observed throughout the endometrium.5
Decreased expression of HOXA10 and the cell adhesion molecule E-cadherin have been described in the endometrium of women with noncavity-distorting intramural uterine fibroids during the WOI.43 In fact, 68.8% of women with fibroids have low mid-secretory phase HOXA10 protein expression.44 Furthermore, it appears that this decrease in HOXA10 expression reverses following myomectomy. Interestingly, the same study failed to show any improvement in HOXA10 expression following myomectomy for submucosal fibroids.45 Bone morphogenetic protein type II (BMP2) mediates HOXA10 expression; thus, increased endometrial resistance to BMP2 may contribute to the low HOXA10 expression in the endometrium of these patients25 (Figure 2).
Ben-Nagi and colleagues evaluated levels of glycodelin, osteopontin, interleukin (IL) 6, IL-10, and tumor necrosis factor (TNF) α in uterine flushings of women with and without submucosal fibroids during the WOI. They found lower levels of glycodelin and IL-10 in uterine flushings from the mid-luteal endometrium of women with uterine fibroids and no differences in osteopontin, IL-6, and TNFα compared to women without fibroids.46 However, this was a study of uterine flushings and the accuracy of the correlation between uterine flushings and secretions from ESCs is unclear.
Horcajadas et al performed gene expression analysis on endometrial tissue from women with or without intramural uterine fibroids during the WOI. They identified 3 genes that are dysregulated in women with intramural fibroids >5 cm compared to controls: glycodelin and aldehyde dehydrogenase 3 family member B2.47 Glycodelin was dysregulated in women with intramural fibroids <5 cm. This suggests that larger fibroids may have a more profound effect on endometrial gene expression; however, additional studies are needed to better elucidate this point.
Cytokines
The rise in progesterone following ovulation is responsible for decidualization of the endometrium, which is marked by increasing amounts of prostaglandins and vascular endothelial growth factor (VEGF).48 These prostaglandins and VEGF increase vessel permeability in endometrial blood vessels allowing for extravasation of polymorphonuclear cells, which also produce cytokines important for implantation, including leukocyte inhibitory factor (LIF).
Another effect of progesterone and estrogen on ESCs is the secretion of decidual markers such as prolactin and insulin-like growth factor-binding protein 1, which are associated with IL-11.49,50 IL-11 is essential for implantation.51 Both LIF and IL-11 are pleiotropic cytokines belonging to the IL-6 family and have been noted to be essential for embryo implantation in the murine model. Both LIF and IL-11 bind to ligand-specific receptors, LIFR and IL-11R, and share the same signal transduction target, gp130. The gp130 signaling pathway is important for embryo implantation,41,52 with inactivation of gp130 in a murine model resulting in implantation failure.53
The LIF-deficient mice show a complete failure of implantation due to defective decidualization. Interestingly, embryos from LIF-deficient mice are unable to implant in the endometrium of LIF-deficient mice, but they are able to implant in the endometrium of wild-type mice.54 In humans, LIF expression increases in the luteal phase and peaks during the implantation window; however, in the presence of submucosal fibroids, the luteal phase increase in LIF protein expression is blunted.55 Clinically, deregulation of LIF production in the secretory endometrium has been associated with unexplained infertility and recurrent abortions.56
Interleukin 11 is essential for sustained decidualization. The IL-11-deficient mice are able to begin decidualization but cannot sustain or complete the decidual response, thus leading to pregnancy loss by day 8.51,57 In humans, IL-11 plays a role in the regulation of trophoblast invasion, and low levels of IL-11 are associated with decreased numbers of uterine natural killer (NK) cells in the secretory endometrium.58,59 The production of IL-11 is decreased during the WOI in the presence of submucosal fibroids.55 Because of its known role in trophoblast invasion and decidualization, reduction in IL-11 may lead to defective implantation; however, further study is needed to determine the clinical correlation.
Growth factors
Progesterone induces the secretion of BMP2 and its downstream target wingless-type MMTV integration site family, member 4 (WNT4) by ESCs.60 This occurs via decidual signals from TGF-β3 family proteins such as heparin-binding epidermal growth factor.61 The endometrium in BMP2-deficient mice is unable to undergo decidual differentiation due to the absence of BMP2 production.62,63 Furthermore, although embryo attachment is possible in BMP2-deficient mice, impaired decidual differentiation leads to defective implantation and pregnancy loss.60,63 When exposed to progesterone, WNT4 knockout mice have defective implantation as a result of impaired ESC survival and decidualization.64 The activation of BMP2 in response to progesterone appears to be necessary for WNT4 activation and subsequently implantation.
In humans, BMP2 resistance is one of the proposed mechanisms by which submucosal fibroids impair implantation. Submucosal fibroids secrete high levels of TGF-β3, which downregulates BMP receptor type II expression in ESC and subsequently leads to ESC resistance to BMP2.25 This resistance to BMP2 negatively affects cell proliferation and differentiation, causing impaired decidualization and implantation site formation.63 Given the essential role of BMP2 and its downstream targets in decidualization and successful implantation, endometrial resistance to BMP2 in the presence of uterine fibroids has the potential to result in suboptimal decidualization and defective implantation. Clinically, this may manifest as a higher incidence of spontaneous abortions and a lower rate of implantation.
Immune cells
The progesterone-dependent increase in VEGF and prostaglandin secretion seen with decidualization promotes extravasation of immune cells into the endometrium. These cells consist mainly of macrophages and NK cells.65 Macrophages produce cytokines, such as LIF, which as described above are essential for implantation.58,66 Furthermore, macrophages play an integral role in trophoblast invasion and placental development.67
The NK cells are the principal immune cells present during the WOI and are important for immune tolerance, angiogenesis, trophoblast migration, and invasion.68 The NK cells produce pro-angiogenic factors such as VEGF and placental growth factor, which regulate maternal–uterine vasculature remodeling and trophoblast invasion.69,70 Mice deficient in NK cells are still fertile, but their pregnancies are marked by fetal loss, severe intrauterine growth restriction, and preeclampsia.71
Mid-secretory endometrium of women with uterine fibroids compared to women without fibroids show an increase in macrophage density and a decrease in the density of NK cells72 (Figure 2). These abnormalities in macrophage and NK cell density result in altered endometrial function and may impede endometrial receptivity to implantation.
Mechanical stretch, uterine wall contractility, and implantation
Uterine fibroids can place tremendous stress and stretch on the nearby myometrium and overlying endometrium, proportionate to the size and location of the fibroid. This increase in uterine stretch results in abnormal gene expression.73-75
These abnormalities in gene expression, together with the physical presence of fibroids, contribute to impaired uterine contractility. Recent studies have implicated uterine contractility in implantation. Abnormal uterine contractions and peristalsis during the mid-luteal phase have been observed on cine magnetic resonance imaging of women with uterine fibroids.76 Yoshino et al further described lower pregnancy rates in women with intramural fibroids and a higher frequency of uterine peristalsis during the WOI. In that study, 10 of the 29 women with intramural fibroids in the low-frequency peristalsis group achieved pregnancy compared to none of the 22 women in the high-frequency peristalsis group.77 Although it was a small study, the data suggest that abnormal uterine peristalsis may play a role in implantation and pregnancy outcomes in women with intramural fibroids. However, additional larger studies are needed before these clinical relevance of these data can be determined.
Heavy Menstrual Bleeding and Dysmenorrhea Associated With Uterine Fibroids
Abnormal uterine bleeding is one of the most common symptoms in women with uterine fibroids. Normal menses occurs every 24 to 35 days. The American Congress of Obstetrics and Gynecology (ACOG) defines heavy menstrual bleeding as diagnosed when bleeding exceeds 80 mL, however, for clinical purposes, any level of menstrual bleeding which causes distress to the patient is managed as heavy menstrual bleeding.78 The quantity of bleeding experienced with each menses depends on a complex interplay of vasoconstriction, angiogenesis, and coagulation. The most common type of abnormal uterine bleeding observed with fibroids is excessive menstrual bleeding that is frequently accompanied by dysmenorrhea.2
Endothelin-1 (ET-1) and prostaglandin F2alpha (PGF2α) are the 2 most important vasoconstrictors involved in menstruation.79 Endothelin-1 is a potent vasoconstrictor that stimulates mitogenesis and myometrial contraction.80 In the endometrium, ET-1 plays a role in spiral arteriole vasoconstriction and thus blood flow. Significantly higher levels of ET-1 are expressed in the endometrium compared to the myometrium and fibroid tissue. Endothelin-1 exerts its effects via its receptors ETA-R and ETB-R. Higher levels of ETA-R are found in fibroid tissue relative to the myometrium, but the opposite is observed for ETB-R. The alterations in receptor levels suggest that ET function is aberrant in the presence of uterine fibroids.81 The altered myometrial expression of ETA-R and ETB-R may result in abnormal uterine contractions leading to defective vasoconstriction and increased menstrual blood flow, especially in the setting of intramural fibroids. Consistent with these data, the endometrial stroma in women with fibroids and heavy menstrual bleeding have been shown to have dilated endometrial stromal venous spaces compared to normal controls. This supports the idea that defective vasoconstriction is one of the mechanisms by which heavy menstrual bleeding occurs.82 Prostaglandin F2alpha receptors are present in normal myometrium and regulate uterine contractions. Uterine fibroids have increased PGF2α production, which is accompanied by disordered uterine contraction and may play a role in the greater menstrual blood loss observed in women with uterine fibroids.65
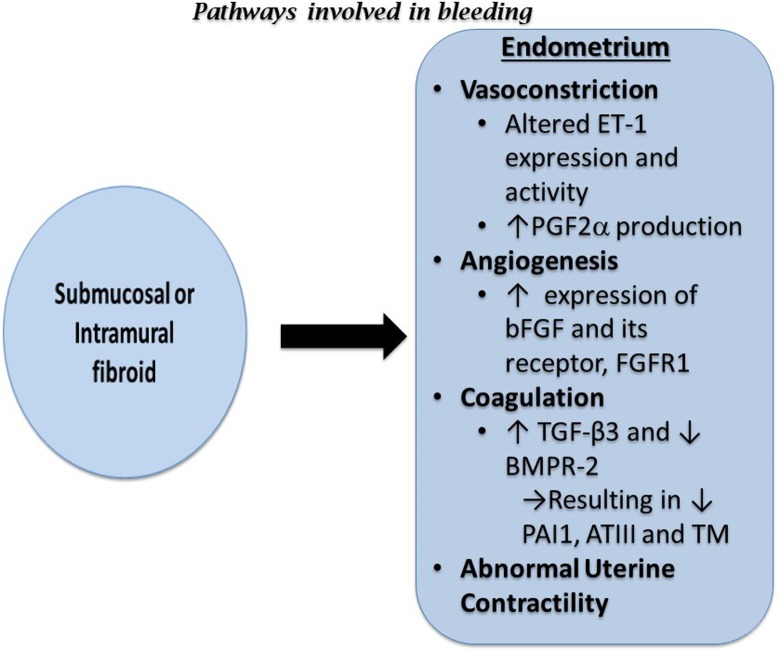
When compared to normal endometrium, fibroids overexpress basic fibroblast growth factor (bFGF), an important regulator of angiogenesis.83 Concurrently, the endometrial stroma of women with uterine fibroids expresses increased levels of basic fibroblast growth factor receptor 1 (FGFR1).83 The combined increase in the expression of bFGF and FGFR1 may play a role in abnormal angiogenesis and excess bleeding during menses observed in women with uterine fibroids (Figure 3).
Figure 3.
Diagram summarizing the effects of submucosal and intramural fibroids on bleeding. ATIII indicates antithrombin III; bFGF, basic fibroblast growth factor; BMPR-2, bone morphogenetic receptor type II; ET-1, endothelin-1; FGFR1, basic fibroblast growth factor receptor 1; PAI1, plasminogen activator inhibitor 1; PGF2α, prostaglandin F 2-alpha; TGF-β3, transforming growth factor beta 3; TM, thrombomodulin.
As described previously, fibroids secrete TGF-β3. Increased TGF-β3 secretion impedes production of coagulation and thrombosis factors, such as thrombomodulin, antithrombin III, and plasminogen activator inhibitor 1. Therefore, disproportionately higher levels of TGF-β3 secreted by fibroids inhibit expression of genes related to fibrinolytic and anticoagulant activity, which results in heavy menstrual bleeding (Figure 3).
Conclusion
Our understanding of the intricate communication between uterine fibroids and the endometrium continues to grow. Although a clear link exists between uterine fibroids and heavy menstrual bleeding, a causative relationship between uterine fibroids and fertility is less clear given that both conditions are relatively common. There is consensus that submucosal fibroids, which distort the uterine cavity, are associated with infertility and early pregnancy loss and should be removed in patients with infertility. In contrast, the clinical significance of intramural fibroids remains controversial.
Submucosal and intramural fibroids both exert significant effects on endometrial gene expression and function. The downstream effects of excessive TGF-β3 secretion from uterine fibroids influence the entire endometrium. This leads to decreased production of transcription factors necessary for implantation during the WOI and aberrant production of coagulation factors during menses. Fibroids also exert their effect on the endometrium through altered gene expression and changes to the immune environment and vasoconstrictive factors.
Despite the significant strides that have been made in this field in recent years, further study is warranted to better understand the crosstalk between uterine fibroids and the endometrium. Such knowledge has the potential to lead to new therapeutic options for the management of symptomatic uterine fibroids.
Footnotes
Authors’ Note: DEI designed the review, performed the literature search, and wrote the manuscript. SEB designed the manuscript, supervised and performed revisions, and critically discussed and reviewed the complete manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NIH grants, POI-HD57877 and R37-HD38691 (to S.E.B) and the Friends of Prentice (to D.E.I).
References
- 1. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. [DOI] [PubMed] [Google Scholar]
- 2. ACOG Practice Bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400. [DOI] [PubMed] [Google Scholar]
- 3. Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health. 2012;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344–1355. [DOI] [PubMed] [Google Scholar]
- 7. Yin P, Ono M, Moravek MB, et al. Human uterine leiomyoma stem/progenitor cells expressing CD34 and CD49b initiate tumors in vivo. J Clin Endocrinol Metab. 2015;100(4):E601–E606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ono M, Maruyama T, Masuda H, et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci U S A. 2007;104(47):18700–18705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mas A, Cervello I, Fernandez-Alvarez A, et al. Overexpression of the truncated form of high mobility group A proteins (HMGA2) in human myometrial cells induces leiomyoma-like tissue formation. Mol Hum Reprod. 2015;21(4):330–338. [DOI] [PubMed] [Google Scholar]
- 10. Ono M, Qiang W, Serna VA, et al. Role of stem cells in human uterine leiomyoma growth. PLoS One. 2012;7(5):e36935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mittal P, Shin YH, Yatsenko SA, Castro CA, Surti U, Rajkovic A. Med12 gain-of-function mutation causes leiomyomas and genomic instability. J Clin Invest. 2015;125(8):3280–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang Q, Diamond MP, Al-Hendy A. Early life adverse environmental exposures increase the risk of uterine fibroid development: role of epigenetic regulation. Front Pharmacol. 2016;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katz TA, Yang Q, Trevino LS, Walker CL, Al-Hendy A. Endocrine-disrupting chemicals and uterine fibroids. Fertil Steril. 2016;106(4):967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Q, Diamond MP, Al-Hendy A. Converting of myometrial stem cells to tumor-initiating cells: mechanism of uterine fibroid development. Cell Stem Cells Regen Med. 2016;2(1) PMID: 28042616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mas A, Stone L, O’Connor PM, et al. Developmental exposure to endocrine disruptors expands murine myometrial stem cell compartment as a prerequisite to leiomyoma tumorigenesis. Stem Cells. 2017;35(3):666–678. [DOI] [PubMed] [Google Scholar]
- 16. Andersen J, DyReyes VM, Barbieri RL, Coachman DM, Miksicek RJ. Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. J Soc Gynecol Investig. 1995;2(3):542–551. [DOI] [PubMed] [Google Scholar]
- 17. Pedeutour F, Quade BJ, Weremowicz S, Dal Cin P, Ali S, Morton CC. Localization and expression of the human estrogen receptor beta gene in uterine leiomyomata. Genes Chromosomes Cancer. 1998;23(4):361–366. [DOI] [PubMed] [Google Scholar]
- 18. Bulun SE, Simpson ER, Word RA. Expression of the CYP19 gene and its product aromatase cytochrome P450 in human uterine leiomyoma tissues and cells in culture. J Clin Endocrinol Metab. 1994;78(3):736–743. [DOI] [PubMed] [Google Scholar]
- 19. Rocha PP, Scholze M, Bleiss W, Schrewe H. Med12 is essential for early mouse development and for canonical WNT and WNT/PCP signaling. Development. 2010;137(16):2723–2731. [DOI] [PubMed] [Google Scholar]
- 20. Kim S, Xu X, Hecht A, Boyer TG. Mediator is a transducer of WNT/beta-catenin signaling. J Biol Chem. 2006;281(20):14066–14075. [DOI] [PubMed] [Google Scholar]
- 21. Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: regulation of WNT target gene activation. Nat Rev Mol Cell Biol. 2009;10(4):276–286. [DOI] [PubMed] [Google Scholar]
- 22. Tanwar PS, Lee HJ, Zhang L, et al. Constitutive activation of beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81(3):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al-Hendy A, Laknaur A, Diamond MP, Ismail N, Boyer TG, Halder SK. Silencing Med12 gene reduces proliferation of human leiomyoma cells mediated via WNT/beta-catenin signaling pathway. Endocrinology. 2017;158(3):592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arici A, Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73(5):1006–1011. [DOI] [PubMed] [Google Scholar]
- 25. Doherty LF, Taylor HS. Leiomyoma-derived transforming growth factor-beta impairs bone morphogenetic protein-2-mediated endometrial receptivity. Fertil Steril. 2015;103(3):845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sinclair DC, Mastroyannis A, Taylor HS. Leiomyoma simultaneously impair endometrial BMP-2-mediated decidualization and anticoagulant expression through secretion of TGF-beta3. J Clin Endocrinol Metab. 2011;96(2):412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cook H, Ezzati M, Segars JH, McCarthy K. The impact of uterine leiomyomas on reproductive outcomes. Minerva Ginecol. 2010;62(3):225–236. [PMC free article] [PubMed] [Google Scholar]
- 28. Guo XC, Segars JH. The impact and management of fibroids for fertility: an evidence-based approach. Obstet Gynecol Clin North Am. 2012;39(4):521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Surrey ES, Minjarez DA, Stevens JM, Schoolcraft WB. Effect of myomectomy on the outcome of assisted reproductive technologies. Fertil Steril. 2005;83(5):1473–1479. [DOI] [PubMed] [Google Scholar]
- 30. Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008;198(4):357–366. [DOI] [PubMed] [Google Scholar]
- 31. Casini ML, Rossi F, Agostini R, Unfer V. Effects of the position of fibroids on fertility. Gynecol Endocrinol. 2006;22(2):106–109. [DOI] [PubMed] [Google Scholar]
- 32. Eldar-Geva T, Meagher S, Healy DL, MacLachlan V, Breheny S, Wood C. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril. 1998;70(4):687–691. [DOI] [PubMed] [Google Scholar]
- 33. Farhi J, Ashkenazi J, Feldberg D, Dicker D, Orvieto R, Ben Rafael Z. Effect of uterine leiomyomata on the results of in-vitro fertilization treatment. Hum Reprod. 1995;10(10):2576–2578. [DOI] [PubMed] [Google Scholar]
- 34. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–1223. [DOI] [PubMed] [Google Scholar]
- 35. Christopoulos G, Vlismas A, Salim R, Islam R, Trew G, Lavery S. Fibroids that do not distort the uterine cavity and IVF success rates: an observational study using extensive matching criteria. BJOG. 2017;124(4):615–621. [DOI] [PubMed] [Google Scholar]
- 36. Sagi-Dain L, Ojha K, Bider D, et al. Pregnancy outcomes in oocyte recipients with fibroids not impinging uterine cavity. Arch Gynecol Obstet. 2017;295(2):497–502. [DOI] [PubMed] [Google Scholar]
- 37. Klatsky PC, Lane DE, Ryan IP, Fujimoto VY. The effect of fibroids without cavity involvement on ART outcomes independent of ovarian age. Hum Reprod. 2007;22(2):521–526. [DOI] [PubMed] [Google Scholar]
- 38. Styer AK, Jin S, Liu D, et al. Association of uterine fibroids and pregnancy outcomes after ovarian stimulation-intrauterine insemination for unexplained infertility. Fertil Steril. 2017;107(3):756–62.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bosteels J, Kasius J, Weyers S, Broekmans FJ, Mol BW, D’Hooghe TM. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev. 2013;(1):CD009461 PMID: 23440838. [DOI] [PubMed] [Google Scholar]
- 40. Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12(6):731–746. [DOI] [PubMed] [Google Scholar]
- 41. Dey SK, Lim H, Das SK, et al. Molecular cues to implantation. Endocr Rev. 2004;25(3):341–373. [DOI] [PubMed] [Google Scholar]
- 42. Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in HOXA10-deficient mice. Nature. 1995;374(6521):460–463. [DOI] [PubMed] [Google Scholar]
- 43. Makker A, Goel MM, Nigam D, et al. Endometrial expression of homeobox genes and cell adhesion molecules in infertile women with intramural fibroids during window of implantation. Reprod Sci. 2017;24(3):435–444. [DOI] [PubMed] [Google Scholar]
- 44. Matsuzaki S, Canis M, Darcha C, Pouly JL, Mage G. HOXA-10 expression in the mid-secretory endometrium of infertile patients with either endometriosis, uterine fibromas or unexplained infertility. Hum Reprod. 2009;24(12):3180–3187. [DOI] [PubMed] [Google Scholar]
- 45. Unlu C, Celik O, Celik N, Otlu B. Expression of endometrial receptivity genes increase after myomectomy of intramural leiomyomas not distorting the endometrial cavity. Reprod Sci. 2016;23(1):31–41. [DOI] [PubMed] [Google Scholar]
- 46. Ben-Nagi J, Miell J, Mavrelos D, Naftalin J, Lee C, Jurkovic D. Endometrial implantation factors in women with submucous uterine fibroids. Reprod Biomed Online. 2010;21(5):610–615. [DOI] [PubMed] [Google Scholar]
- 47. Horcajadas JA, Goyri E, Higon MA, et al. Endometrial receptivity and implantation are not affected by the presence of uterine intramural leiomyomas: a clinical and functional genomics analysis. J Clin Endocrinol Metab. 2008;93(9):3490–3498. [DOI] [PubMed] [Google Scholar]
- 48. Fritz MA, Speroff L. Clinical Gynecologic Endocrinology and Infertility. 8th ed Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 49. Dimitriadis E, Stoikos C, Baca M, Fairlie WD, McCoubrie JE, Salamonsen LA. Relaxin and prostaglandin E(2) regulate interleukin 11 during human endometrial stromal cell decidualization. J Clin Endocrinol Metab. 2005;90(6):3458–3465. [DOI] [PubMed] [Google Scholar]
- 50. Karpovich N, Klemmt P, Hwang JH, et al. The production of interleukin-11 and decidualization are compromised in endometrial stromal cells derived from patients with infertility. J Clin Endocrinol Metab. 2005;90(3):1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905. [DOI] [PubMed] [Google Scholar]
- 52. Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76(2):253–262. [DOI] [PubMed] [Google Scholar]
- 53. Ernst M, Inglese M, Waring P, et al. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J Exp Med. 2001;194(2):189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stewart CL, Kaspar P, Brunet LJ, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359(6390):76–79. [DOI] [PubMed] [Google Scholar]
- 55. Hasegawa E, Ito H, Hasegawa F, et al. Expression of leukemia inhibitory factor in the endometrium in abnormal uterine cavities during the implantation window. Fertil Steril. 2012;97(4):953–958. [DOI] [PubMed] [Google Scholar]
- 56. Hambartsoumian E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am J Reprod Immunol. 1998;39(2):137–143. [DOI] [PubMed] [Google Scholar]
- 57. Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4(3):303–308. [DOI] [PubMed] [Google Scholar]
- 58. Zenclussen AC, Hammerling GJ. Cellular regulation of the uterine microenvironment that enables embryo implantation. Front Immunol. 2015;6:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Paiva P, Salamonsen LA, Manuelpillai U, Dimitriadis E. Interleukin 11 inhibits human trophoblast invasion indicating a likely role in the decidual restraint of trophoblast invasion during placentation. Biol Reprod. 2009;80(2):302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li Q, Kannan A, Das A, et al. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154(1):446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Paria BC, Ma W, Tan J, et al. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci U S A. 2001;98(3):1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li Q, Kannan A, Wang W, et al. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving WNT4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007;282(43):31725–31732. [DOI] [PubMed] [Google Scholar]
- 63. Lee KY, Jeong JW, Wang J, et al. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27(15):5468–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Franco HL, Dai D, Lee KY, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25(4):1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Miura S, Khan KN, Kitajima M, et al. Differential infiltration of macrophages and prostaglandin production by different uterine leiomyomas. Hum Reprod. 2006;21(10):2545–2554. [DOI] [PubMed] [Google Scholar]
- 66. Jensen AL, Collins J, Shipman EP, Wira CR, Guyre PM, Pioli PA. A subset of human uterine endometrial macrophages is alternatively activated. Am J Reprod Immunol. 2012;68(5):374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Helige C, Ahammer H, Moser G, et al. Distribution of decidual natural killer cells and macrophages in the neighbourhood of the trophoblast invasion front: a quantitative evaluation. Hum Reprod. 2014;29(1):8–17. [DOI] [PubMed] [Google Scholar]
- 68. Lee SK, Kim CJ, Kim DJ, Kang JH. Immune cells in the female reproductive tract. Immune Netw. 2015;15(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tayade C, Hilchie D, He H, et al. Genetic deletion of placenta growth factor in mice alters uterine NK cells. J Immunol. 2007;178(7):4267–4275. [DOI] [PubMed] [Google Scholar]
- 70. Wang C, Umesaki N, Nakamura H, et al. Expression of vascular endothelial growth factor by granulated metrial gland cells in pregnant murine uteri. Cell Tissue Res. 2000;300(2):285–293. [DOI] [PubMed] [Google Scholar]
- 71. King A. Uterine leukocytes and decidualization. Hum Reprod Update. 2000;6(1):28–36. [DOI] [PubMed] [Google Scholar]
- 72. Kitaya K, Yasuo T. Leukocyte density and composition in human cycling endometrium with uterine fibroids. Hum Immunol. 2010;71(2):158–163. [DOI] [PubMed] [Google Scholar]
- 73. Rogers R, Norian J, Malik M, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198(4):474.e1-e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Payson M, Malik M, Siti-Nur Morris S, Segars JH, Chason R, Catherino WH. Activating transcription factor 3 gene expression suggests that tissue stress plays a role in leiomyoma development. Fertil Steril. 2009;92(2):748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Norian JM, Owen CM, Taboas J, et al. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Orisaka M, Kurokawa T, Shukunami K, et al. A comparison of uterine peristalsis in women with normal uteri and uterine leiomyoma by cine magnetic resonance imaging. Eur J Obstet Gynecol Reprod Biol. 2007;135(1):111–115. [DOI] [PubMed] [Google Scholar]
- 77. Yoshino O, Hayashi T, Osuga Y, et al. Decreased pregnancy rate is linked to abnormal uterine peristalsis caused by intramural fibroids. Hum Reprod. 2010;25(10):2475–2479. [DOI] [PubMed] [Google Scholar]
- 78. National Institute for Health and Clinical Excellence. Heavy Menstrual Bleeding: Assessment and Management. NICE Guidelines (CG44) 2007. Available at: https://www.nice.org.uk/guidance/cg44 [PubMed] [Google Scholar]
- 79. Maybin JA, Critchley HO. Menstrual physiology: implications for endometrial pathology and beyond. Hum Reprod Update. 2015;21(6):748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Masaki T. Endothelins: homeostatic and compensatory actions in the circulatory and endocrine systems. Endocr Rev. 1993;14(3):256–268. [DOI] [PubMed] [Google Scholar]
- 81. Pekonen F, Nyman T, Rutanen EM. Differential expression of mRNAs for endothelin-related proteins in human endometrium, myometrium and leiomyoma. Mol Cell Endocrinol. 1994;103(1-2):165–170. [DOI] [PubMed] [Google Scholar]
- 82. Farrer-Brown G, Beilby JO, Tarbit MH. Venous changes in the endometrium of myomatous uteri. Obstet Gynecol. 1971;38(5):743–751. [PubMed] [Google Scholar]
- 83. Anania CA, Stewart EA, Quade BJ, Hill JA, Nowak RA. Expression of the fibroblast growth factor receptor in women with leiomyomas and abnormal uterine bleeding. Mol Hum Reprod. 1997;3(8):685–691. [DOI] [PubMed] [Google Scholar]