Abstract
Introduction:
Chemotherapy is the most commonly used modality to treat human cancers; however, in many cases it causes irreversible ovarian failure. In this work, we plan to evaluate the restorative function of human bone marrow mesenchymal stem cells (BMSCs) in a chemotherapy-induced ovarian failure mouse model.
Methods:
Acclimatized 4 to 6 week-old female mice (C57BL/6) were assigned randomly to a vehicle-treated control group (group 1), chemotherapy-treated group followed by vehicle alone (group 2), or chemotherapy-treated group followed by stem cell intraovarian injection (group 3). Outcomes were evaluated using immunohistochemistry (IHC), serum hormonal assays, and estrous cycle monitoring and breeding potential.
Results:
Post BMSCs administration, group 3 promptly showed detectable vaginal smears with estrogenic changes. Increase in total body weight, ovarian weight, and weight of estrogen-responsive organs (uterus and liver) was observed at 2 weeks and continued to end of the experiment. Hematoxylin and Eosin histological evaluation of the ovaries demonstrated a higher mean follicle count in group 3 than in group 2. Group 3 had lower follicle-stimulating hormone (FSH) levels (P = .03) and higher anti-Müllerian hormone serum (AMH) levels (P = .0005) than group 2. The IHC analysis demonstrated higher expression of AMH, FSH receptor, inhibin A, and inhibin B in growing follicles of group 3 versus group 2. Tracking studies demonstrated that human BMSCs evenly repopulated the growing follicles in treated ovaries. Importantly, breeding data showed significant increases in the pregnancies numbers, 2 pregnancies in group 1 and 12 in group 3 (P = .02).
Conclusions:
Intraovarian administered BMSCs are able to restore ovarian hormone production and reactivate folliculogenesis in chemotherapy-induced ovarian failure mouse model.
Keywords: ovarian failure, stem cells, chemotherapy, infertility
Introduction
Premature ovarian failure (POF)—or primary ovarian insufficiency—refers to normal ovarian function loss before age 40. In a normal karyotype female, patient with POF is usually presented by amenorrhea and infertility, with elevated serum levels of follicle-stimulating hormone (FSH) and decreased anti-Müllerian hormone (AMH) and serum levels of estrogen.1 At the present time, no effective treatment for POF-related infertility and alternatives such as the use of egg donations are prohibitively expensive and ethically unacceptable to some couples.2
Approximately 600 000 women in the United States are diagnosed with cancer each year, of whom 8% are of prereproductive age.3 Consistent with the Surveillance, Epidemiology, and End Results (SEER) Cancer Statistics Review, 1975-2011, in the United States, the prevalence of cancer among females aged 20 to 29 years was 88 223; in those aged 30 to 39 years was 226 162, and in those aged 40 to 49 years was 638 879.4
Chemotherapy (CTX) is the most common treatment modality used in patients with cancer followed by surgery, radiotherapy, and other more specialized treatments.5 Premature ovarian failure is a common long-term consequence of CTX and radiotherapy. Both treatment modalities pose a significant risk of gonadal damage, with the severity of damage linked directly to dose and age at the time of treatment.5 Anticancer therapeutic agents such as cyclophosphamide and busulfan are alkylating agents5 typically associated with gonadal damage.6 These chemotherapeutic agents have been shown to destroy ovarian follicles.7 Studies have shown that subsequent to CTX treatment, female mice demonstrated elevated levels of FSH, decreased estrogen levels, and sterility. These observations were attributed to the absence of folliculogenesis and small nonfunctional ovaries.7 Accordingly, this CTX-induced murine ovarian failure model is currently the most accepted preclinical model for research in this field, albeit the differences between mice and human ovaries in structure, cell distribution, and hormonal cyclicity.5
The American Society of Clinical Oncology and the American Society for Reproductive Medicine have issued guidelines stipulating that treatment of cancer had potential adverse effect on fertility and recommend that fertility preservation options be presented to patients during the initial stages of treatment.8 Existing attitudes aimed at preserving fertility in female cancer survivors include protection of ovarian follicles with gonadotropin-releasing hormone agonist (GnRH-a) at the time of CTX administration; ovarian tissue, oocyte, or embryo cryopreservation prior to CTX, followed by reimplantation; or utilization of assisted reproductive techniques (ART).9 Unfortunately, these procedures have several important limitations such as inconsistent outcomes, the need for invasive surgical procedures, or prolonged ovulation induction accompanied by subsequent ART. Although these remarkable advances in the field of fertility preservation have provided hope to many cancer survivors who desire future fertility, they may or may not be viable options for certain types of malignancies.10 Moreover, some of these procedures are not applicable to preserve the fertility of young prepubertal girls. Of note, many of the advanced technology required for these options are not readily available, globally. Clearly, innovative approaches are needed for fertility preservation in prepubertal and reproductive-age female cancer survivors as well as for treatment of infertility associated with idiopathic POF and other types of POF.
Currently, controversy centers around which specific cell types within the ovarian follicles are affected by CTX.11 Some studies report that chemotherapeutic agents appear to directly target oocytes in primordial and primary follicles by apoptotic destruction.9 However, this is in stark contrast to evidence which shows that cells at rest (oocytes) are less sensitive to the cytotoxic effects of alkylating agents than rapidly dividing cells (granulosa cells), making steroidogenic cells of the follicle the likely target for CTX.12 Therefore, it is highly unlikely that postnatal oocytes which are at the dictyate state of arrest and do not undergo mitosis are susceptible to apoptotic destruction by alkylating agents used for treatment of cancer.10,12 We hypothesize that somatic cells (granulosa cells, theca cells, or both) rather than oocytes are the CTX-sensitive cells and are, therefore, more susceptible to the effects of cancer therapeutic agents.
Human bone marrow mesenchymal stem cells (BMSCs) transplantation has been cited in more than 344 clinical trials for the treatment of a diversity of diseases all over the world. Multiple studies of transplanted stem cells into a specific microenvironment showed their stimulation by the niche. This microenvironmental stimuli in turn triggers the release of cell growth factors that encourage the regeneration of the surrounding tissue, and the stem cells themselves may also be induced to turn into specific cells which then conform to the microenvironment.8,13,14
The mesenchymal approach has many advantages including accessibility, versatility, and their restorative ability with respect to various health disorders.13 It goes without saying that the use of stem cell transplantation in the treatment of human disease must be harmless and effective. Great interest has developed around the utility of MSCs due to 2 highly attractive features—they are free of both ethical concerns and teratoma formations when compared to embryonic stem cells and induced pluripotent stem cells.13
Although the rigorous mechanism is not entirely understood; mesenchymal stem cells (MSCs) can be used in allogeneic transplantations without immunosuppressive therapy due to their ability to escape immune detection (immune-privileged status).15 These MSCs express MHC class 1 but lack expression of MHC class 2 tissue antigenicity.16 Ironically, data suggest that this immunosuppressive effect is not specifically endowed to MSCs alone.17 Furthermore, these MSCs secrete a plethora of immune-modulating cytokines such as interleukin 10 and transforming growth factor β (TGF- β), which create an immune-suppressed zone around the area of MSCs implantation.18 On the contrary, the administration of MSCs has been shown to improve the clinical symptoms of graft-versus-host disease in clinical trials.19 Our group along with other researchers has demonstrated that spectrum of reproductive dysfunction can be treated by allogeneic BMSCs.5,20
Bone marrow transplantation (BMT) was used as rescue of fertility of adult female mice treated with CTX. They showed that recipient germ cells were the source of all offspring.5 The BMT experiments followed by detailed gene expression studies suggests that BMT rescues fertility by either supporting reinstitution of recipient oogenesis or reviving existing oocytes from CTX. Johnson et al’s work clearly demonstrated that BMT did not generate de novo germ cells (eggs) from implanted (donor) tissues.5
Up to date, various other mesenchymal phenotypes, such as umbilical cord MSCs, adipose-derived MSCs, and amniotic fluid MSCs, have been described as having therapeutic potential for CTX-induced ovarian damage.2,21–26 On both sides, preclinical animal models and human clinical trials, bone marrow has been the widely used source for MSCs.14 The BMSCs have shown great promise in the dawn of a new era within the field of regenerative medicine including reproductive dysfunction.13
Herein, our study is designed to determine whether surface marker–specific BMSCs from a young human female donor can be grafted (xenogeneic) into the ovaries of a CTX-induced ovarian failure immune-competent animal model to restore estrogen production, promote folliculogenesis, and treat infertility.
Materials and Methods
Animals
Adult female C57BL6 mice, 4 to 6 weeks of age, weighing between 20 and 25 g, were purchased from Charles River Co (Wilmington, Massachusetts). Animals were housed in groups of 6 within polyethylene cages and allowed to acclimatize to the animal facility environment for at least 1 week prior to study initiation. Animals were maintained in an environmentally controlled room with 22°C with a humidity range of 50% to 60%, 12-hour dark–12-hour light cycles (lights on at 6:00 am). Animals had free and continuous access to water and commercial pelleted mouse chow.
Augusta University Institutional Animal Care and Use Committee approved all animal procedures that were performed in accordance with the National Research Council Guide for Care and Use of Laboratory Animals. GRU Institutional Review Board (IRB) also granted the approval for the use of human cells. All surgeries were performed under isoflurane inhalation anesthesia, and mice were placed under a red heat lamp to maintain body temperature until recovery.
Animal Procedures
This study encompasses 2 parallel experiments: (1) treatment experiment, and (2) breeding experiment (Supplemental Figure 1).
The Treatment Experiment
At random, acclimatized mice (N = 6/group) were assigned to a control group (group 1); a chemotherapy group with sham stem cell treatment (group 2); or a stem cell-based treatment after chemotherapy group (group 3). Group 1 was subjected to bilateral intraovarian administration of 10 µL phosphate-buffered saline/ovary via laparotomy. Chemotherapy (CTX) combination treatment consisted of a single intraperitoneal injection of busulfan (12 mg/kg) and cyclophosphamide (70 mg/kg), both dissolved in saline as described previously and confirmed by the manufacturer (Sigma Aldrich, USA).27 This combination is hereafter referred to as CTX. Seven days post-CTX injection, mice in groups 2 and 3 were subjected to bilateral intraovarian administration of 10 µL phosphate-buffered saline via laparotomy or 10 µL of BMSCs suspension (5 × 105 cells) in each ovary, respectively. Dosing of BMSCs was determined based on our prior published work28 and related literature.14,27,29 A 10-μL G30 Hamilton syringe (Harvard apparatus, MA, USA) was used for stem cell injections; the fascia and skin were closed using vicryl 3 (ETHICON USA) zero sutures. Aseptic procedures were used at all times.
The Breeding Experiment
At random, acclimatized mice (N = 6/group) were assigned to a control group (group 1”), sham chemotherapy group (group 2”), or stem cell-based treatment after chemotherapy (group 3”) as aforementioned; however, in this experiment, animal cohabitation was initiated 1 week after surgical recovery with age- and strain-matched breeder males at a ratio of 2 females:1 male (Hareem theory). Fruitful mating was determined by the presence of plugs in the vaginal os of females. All pups were gathered, counted, and examined closely for weight as well as any evident congenital anomalies or abnormal physical findings.
Ovarian administration of BMSCs
The BMSCs were purchased from LONZA INC., Rochester, New York (catalogue number PT-2501). Cells were purchased at passage 2 and plated in vitro per the manufacturer’s instructions for 1 passage to confirm viability. Cells were collected at room temperature and suspended in 10 µL of phosphate-buffered saline. Cells were immediately injected into both ovaries (right and left) of each animal in group 3 at a concentration of 5 × 105 cells per ovary.
These cells were obtained from a 21-year-old healthy African American female donor according to the manufacturer’s (Lonza) certificate of analysis. The surface marker profile of these cells is as follows: CD105 positive, CD166 positive, CD90 positive, and CD73 positive as well as CD14 negative, CD34 negative, CD45 negative, human leukocyte antigen–antigen D related negative, and CD 19 negative.
Experiment timeline
Time points were set at 2, 4, 6, and 8 weeks from the day of surgery. At each time point, animals (N = 6/group) were weighed, sequential blood samples were collected, and hormonal assays were conducted. Moreover, 1 animal from each group was killed, and ovarian tissues were excised from each animal (N = 6/group) and fixed in 4% buffered formalin for 24 hours prior to storage (in 70% ethanol) until paraffin blocked and sectioned. Ovarian tissue sections (5-µm thick) were subsequently subjected to hematoxylin and eosin (H&E) to assess the distribution of ovarian follicular developmental stage and antral follicle size. Sections (3 per animal for each antibody) were also subjected to immunohistochemical (IHC) staining to assess ovarian functional activities via the expression of follicular-stimulating hormone receptor (FSHR), inhibin A, inhibin B, and AMH. The following antibodies were used:
| Antibody | Company | catalogue number |
|---|---|---|
| Rabbit anti-human AMH (MIS)(H-300) | Santa Cruz Technology (Santa Cruz, California) IF(1:200) | SC28912 |
| Rabbit anti-human FSHR (H-190) | Santa Cruz Technology IF(1:200) | SC13935 |
| Rabbit anti-human Inhibin A (H-134) | Santa Cruz Technology IF(1:200) | SC-50288 |
| Rabbit anti-human Inhibin B (H-120) | Santa Cruz Technology IF(1:200) | SC-30146 |
Daily vaginal smear and total body weight
Mice in all groups (N = 6/group) were weighed weekly and subjected to daily vaginal swabs for 21 days to determine CTX’s effect on stages and overall length of the estrous cycle. Rugh et al.30 described characteristic features of specific estrous cycle phases as follows: 1—diestrus, smear mostly contains leukocytes; 2—proestrus, contains equal numbers of both leukocytes and elongated nuclear epithelium in thin smear; 3—estrus, contains only large cornified epithelial cells; and 4—metestrus, contains equal numbers of both leukocytes and elongated nuclear epithelium in thick smear. Circulating levels of progesterone rise during metestrus and diestrus and then declined from proestrus to estrus; however, estradiol (E2) circulating levels peaked prior to ovulation (at estrus) in the early hours of the night.31,32
Blood collection for hormonal assays
Blood samples from all groups (N = 6/group) were collected using the retro-orbital technique with sample volume averaging 200 µL/animal. Samples were placed on ice until centrifugation at 4°C at 1500×g for 10 minutes. Sera were harvested and stored frozen at −80°C until analyzed for ovarian hormonal assay profile (E2, AMH, and FSH).
Hormone assays
All animals (N = 6/group) were subjected to blood sampling at baseline before CTX and 1 week before surgery (to avoid the risk of hypovolemia)—then at each time point (2, 4, 6, and 8 weeks from the day of surgery). Serum FSH, AMH, and E2 levels were measured without dilution factor by “The University of Virginia’s Center for Research in Reproduction Ligand Assay and Analysis Core” which is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934
For the detection of E2 in mouse serum, E2 was measured by enzyme-linked immunosorbent assay (ELISA; Rodent Estradiol ELISA; CalBiotech, Spring Vally, California). Assay precision was 6.1% (intra-assay) and 8.9% (interassay). Functional sensitivity was 3.0 pg/mL. The FSH was assayed by RIA using reagents provided by the National Hormone and Peptide Program and Dr A. F. Parlow, as described previously.33 Assay precision was 6.9% (intra-assay) and 9.4% (interassay). Functional sensitivity was 3.0 ng/mL.
Mouse serum samples were measured for AMH using a commercial ELISA kit (Rat/Mouse AMH ELISA; Ansh Labs, Webster, Texas). Assay precision was 3.6% (intra-assay) and 8.5% (interassay). Functional sensitivity was 0.28 ng/mL.
Ovarian histological studies
Animals were killed (CO2 asphyxiation in accordance with Augusta University [AU] animal facility protocols) from each group at 2, 4, 6, and 8 week time points after surgery. Organ samples (lung, heart, liver, spleen, uterus, ovaries, cervix, and vagina) were dissected, weighed, and stored at −80°C until additional processing. All organs were weighed by a technician who was unaware of group assignment. Mean organ weight was used for comparisons and normalization. Formalin (10%) was used to fix tissues overnight and embedded in paraffin for further studies. After the fifth cut of 5-µm-thick sections, tissues were stained with H&E for histological examination by light microscopic. In the case of the ovaries, the fifth cut was chosen to count the follicles number and to evaluate follicular development, as described previously.28
Sections of the ovaries (5-µm thick) from controls, CTX, and treated mice were subjected to IHC staining in AU’s IHC Core laboratory. Sections were stained for FSHR, inhibin A, inhibin B, and AMH to evaluate ovarian function activities within the 3 groups. Images of ovarian sections were acquired with an Icore Axioplane 2 Nikon TE2000-E inverted microscope, using a 10×, 20×, and 40× objective with numerical aperture (NA) 0.30 and 0.75 NA, respectively. Semiquantitative analysis of mean intensities of controls, CTX, and the treatment group for FSHR, inhibin A, inhibin B, and AMH-stained sections were performed. Regions of interest (ROIs) 4 to 6 were outlined on images obtained from each group. Background intensity was subtracted from the ROI, and an intensity threshold for all images analyzed was set and kept constant. By dividing the mean intensity units by the area of outlined regions, the mean intensity/µm2 was calculated.
Tracking BMSCs by IHC staining
To track human cells within the mouse ovarian tissue, we used human-specific antivimentin Ab (Abcam, Cambridge, Massachusetts) catalogue number (ab8069). This antibody, as listed in the certificate of analysis and confirmed with the manufacturer, does not react with mouse tissue and is human specific. Cells were fixed for 10 minutes with 4% formaldehyde, the 5-minute permeabilization with 0.1% Triton X-100, and then blocked with 1% BSA/10% normal goat serum/0.3 mol/L glycine in 0.1% phosphate-buffered saline-Tween for 1 hour. Then, the cells were incubated overnight at +4°C with ab8069 at 1/100 dilution. 4′, 6-diamidino-2-phenylindole (DAPI) (shown in blue) was used to label nuclear DNA, and a confocal microscope (TCS SP8, Leica-Microsystems, IL, USA) was used for imaging .
Statistical Analysis
All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, North Carolina) assuming an overall significance level of .05. Bonferroni method for multiple comparisons was used to adjust for multiple comparisons where appropriate.
Results
Vaginal Smear Changes
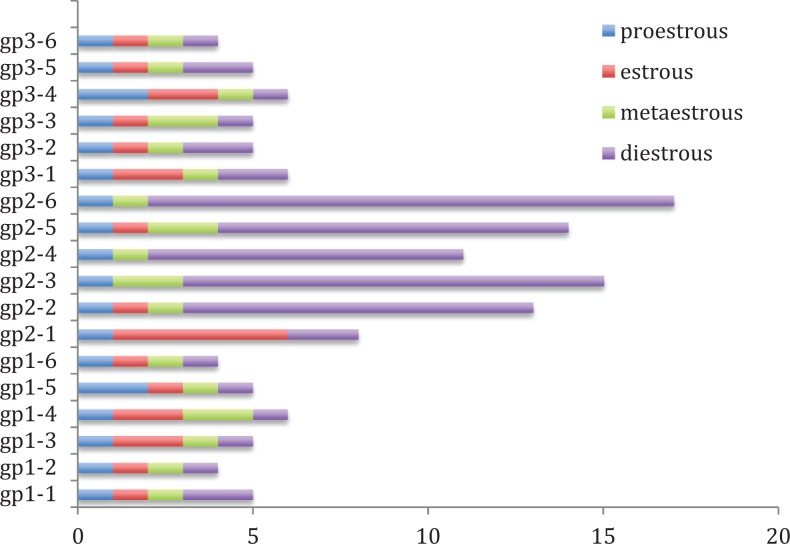
Mice (N = 6) treated with CTX showed an increase in the length of estrous cycle—from the physiological 4-day cycle observed among control mice (N = 6) to an 18 to 21 day cycle. The estrous cycle among CTX-treated mice was almost totally arrested at the diestrus phase, although we noticed arrested cyclicity in other stages as well such as proestrus, estrus, and metasterus. (Figure 1) A week after intraovarian administration of BMSCs, treated animals (N = 6) started to display vaginal smears changes, which were not as regular as normal animals. The mean number of diestrus days or total cycle days did not differ significantly between groups 1 and 3 (P = .6252 and .7998, respectively); also the cycle frequency in group 3 was in average 4 to 5 new cycles which were similar to group 1. On the other hand, group 3 differed significantly in the mean number of days in the diestrus cycle and the mean number of days for the total cycle when compared to group 2 (Figure 1, P < .0001).
Figure 1.
Mean cycle length (days) by group. Groups 1 and 3 differed significantly in the mean number of days in the diestrous cycle and the mean number of days for the total cycle than group 2 (P < .0001).
Total body weight and organ weight changes
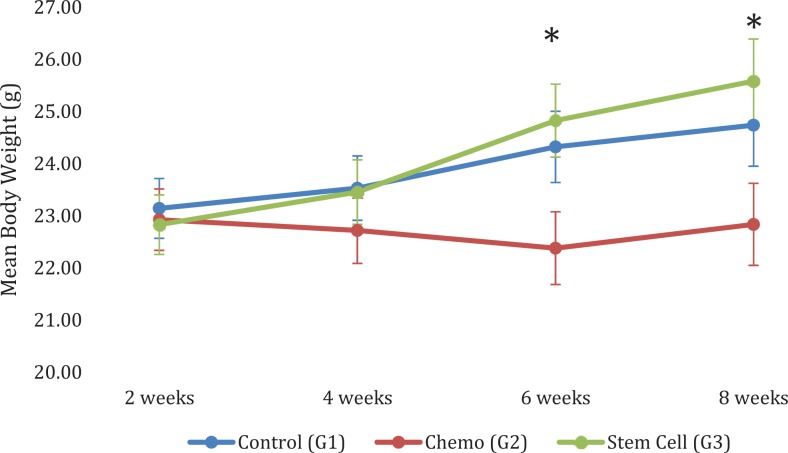
Mean body weight tended to increase with time in groups 1 and 3, whereas mean body weight remained relatively the same or slightly decreased over time in group 2 as shown in Figure 2. Groups 1 and 3 (N = 6/group) had a significantly higher mean body weight than group 2 (P < .0001; Figure 2).
Figure 2.
Mean body weight by group and time. Group 2, the trend was such that body weight remained relatively constant over time posttreatment. Group 3, significant increases in body weight of 1.99 g from 2 to 6 weeks (P < .0001), 2.75 g from 2 to 8 weeks (P < .0001), 1.37 g from 4 to 6 weeks (P = .0005), and 2.13 g from 4 to 8 weeks (P < .0001). At 6- and 8-week time points, group 3 had significantly higher mean body weight than group 2 (P < .0001). N = 6 at 2 weeks, N = 5 at 4 weeks, N = 4 at 6 weeks, and N = 3 at 8 weeks for each group.
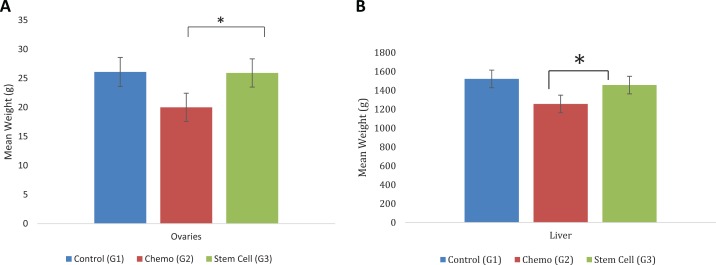
Estrogen responsive organs demonstrated remarkable increases in weight at all time points of the experiment, as shown in Figure 3. Statistical analysis indicated that there were significant differences among the groups (group 1 and 3 statistically higher than group 2) in the mean weight for the ovaries (P = .0030), uterus (P = .0015), kidneys (P = .0001), and liver (P = .0018), while there were no significant differences among the groups in the mean weight of spleen (P = .1106), vagina (P = .66), or lungs (P = .1778; Supplemental data; Figures 2 and 3).
Figure 3.
A, Total (left + right) ovaries weight by group. Group 3 had significantly higher mean total ovaries weight than group 2 by 6.09 g (P = .0022); there was no significant difference in mean total ovary weight between groups 1 and 3 (P = .92). B, Mean liver weight by group. Group 3 had significantly higher mean liver weight over time (N = 6 /group) than group 2 by 264.98 g (P = .0006); there was not a significant difference in liver weight between groups 1 and 3 (P = .31).
Hormonal changes
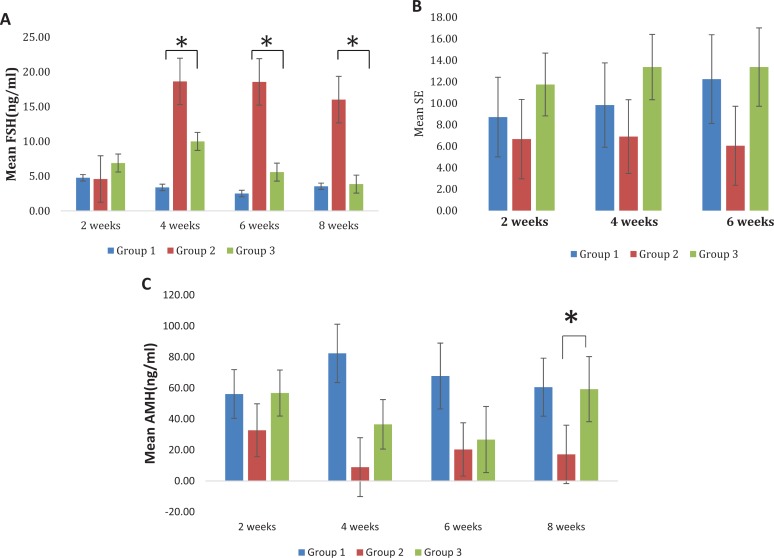
Within groups 1 and 3, there were no significant changes in FSH over time (P > .05). Within group 2, FSH levels were significantly increased and remained elevated from 2 weeks up to 8 weeks posttreatment (end of experiment; P < .001; Figure 4A). At the 4, 6, and 8 week time points, group 3 had significantly lower FSH levels than group 2 (P <.05; Figure 4A).
Figure 4.
A, Mean follicle-stimulating hormone (FSH) levels by group. At 4, 6, and 8 weeks posttreatment, group 3 had significantly lower FSH than group 2 (P = .0338, .0165, and .0034), respectively. B, Mean serum estradiol by group. Group 3 had higher mean serum estradiol than group 2 at 4 weeks (P = .0066) and at 6 weeks (P = .0064). C, Mean anti-Müllerian hormone (AMH) by group. At 4-week time point group 1 had significantly higher AMH than the group 2 (P < .0001) and group 3 (P = .0005). At 8 weeks posttreatment, and groups 1 and 3 had significantly higher AMH than group 2 (P = .0042), and the groups 1 and 3 did not have significantly different AMH levels (P = .9287). Within group 2, there were no significant differences in AMH over time (all P > .05).
In group 2, E2 was lower than normal. We observed an increase in the E2 serum level in group 3 when compared to group 2. Group 3 had a higher mean E2 serum level when compared to group 2 at 4 and 6 week time points (P = .0066 and P = .0064 respectively; Figure 4B).
Serum level of AMH was permanently low in group 2; however, a steady increase was observed in group 3 to reach a range comparable to normal levels within group 1 by week 8 post-cell administration. At the 4-week time point, groups 1 and 3 had significantly higher AMH serum levels when compared to group 2 (P < .0001; Figure 4C).
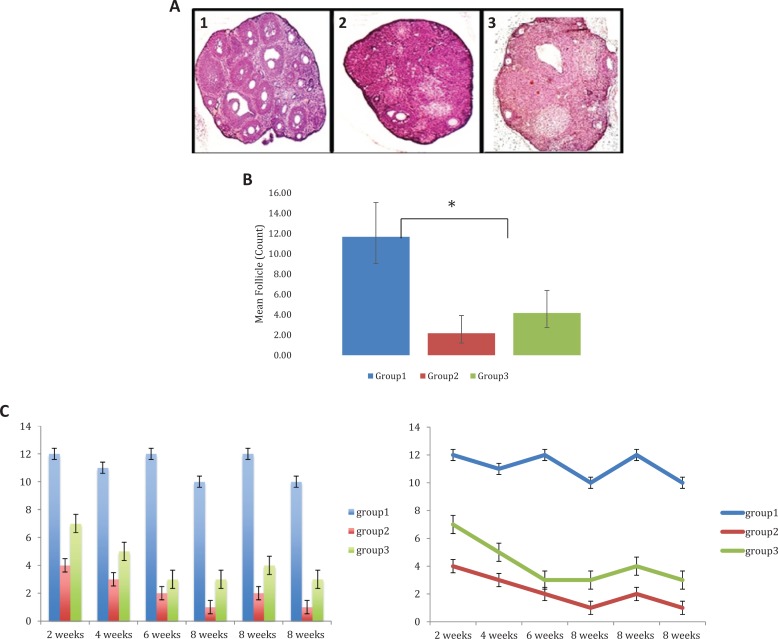
Follicular growth
Group 2 had an overall reduction in ovary size and failed to develop antral follicles. The fifth section cut was used for follicle count in all the groups. On average, 4 to 5 follicles/ovary/animal (N = 6) were observed in group 3, while 11 to 12 follicles/ovary/animal (N = 6) were observed in group 1, and 2 to 3 follicles/ovary/animal (N = 6) observed in group 2 (Figure 5A1). The generalized linear effects model had a significant group effect (P < .0001) which means that the mean follicle count differs among groups. Furthermore, the mean follicle count in group 3 was about 1.92 times greater than group 2 (Figure 5A2). A larger sample size is likely needed to realize such outcome effect.
Figure 5.
A, Hematoxylin and eosin staining of the ovarian tissues ×10. Hematoxylin and eosin staining of the ovarian tissue at 4-week timepoint. A1, Group 1 represents normal ovarian tissue. A2, Group 2 had an overall reduction in ovary size and failed to develop antral follicles. A3, Group 3 showed an increase in the number of follicles over time. B, Mean follicle count by group. Mean follicle count differs among groups (P < .0001). The mean follicle count in group 1 was 5.4 times larger than group 2 and 1.8 times larger than group 3. The mean follicle count in group 3 was 1.92 times greater than group 2; however, this difference was not statistically significant. C, Follicle count over time by group. Same data from 5A2 illustrating follicular densities (the fifth cut section for the ovary) for each animal at each time point. N = 1 at time point 2, 4, and 6 weeks; N = 3 at 8-week time point. D, Follicle-stimulating hormone receptor (FSHR) expression in recipient mouse ovaries. (A) FSHR were detected in group 1. (B) FSHR was not detected in recipient ovaries of group 2 (without stem cells). (C) FSHR were detected in recipient ovaries of group 3 two months after stem cells injection. Scale bars = 40 μm. anti-Müllerian hormone (AMH) expression in recipient mouse ovaries. (D) AMH was expressed in all granulosa cells of primary, preantral, and small antral follicles in normal ovaries. (E) AMH expression disappeared in stromal and atretic follicles in recipients’ ovaries without stem cells injection 2 months after chemotherapy. (F) AMH expression patterns are strong in recipient ovaries 2 months after stem cells injection; data are consistent with that from control normal ovaries. Scale bars = 40 μm. Inhibin A expression in recipient mouse ovaries. (G) Inhibin A was detected in group 1 (control). (H) Inhibin A was not detected in recipient ovaries of group 2 (without stem cells). (I) Inhibin A was detected in recipient ovaries of group 3 two months after stem cells injection. Scale bars = 40 μm. Inhibin B expression in recipient mouse ovaries (J) Inhibin B was detected in group 1(control). (K) Inhibin B was not detected in recipient ovaries of group 2 (without stem cells). (L) Inhibin B was detected in recipient ovaries of group 3 two months after stem cells injection. Scale bars = 40 μm.
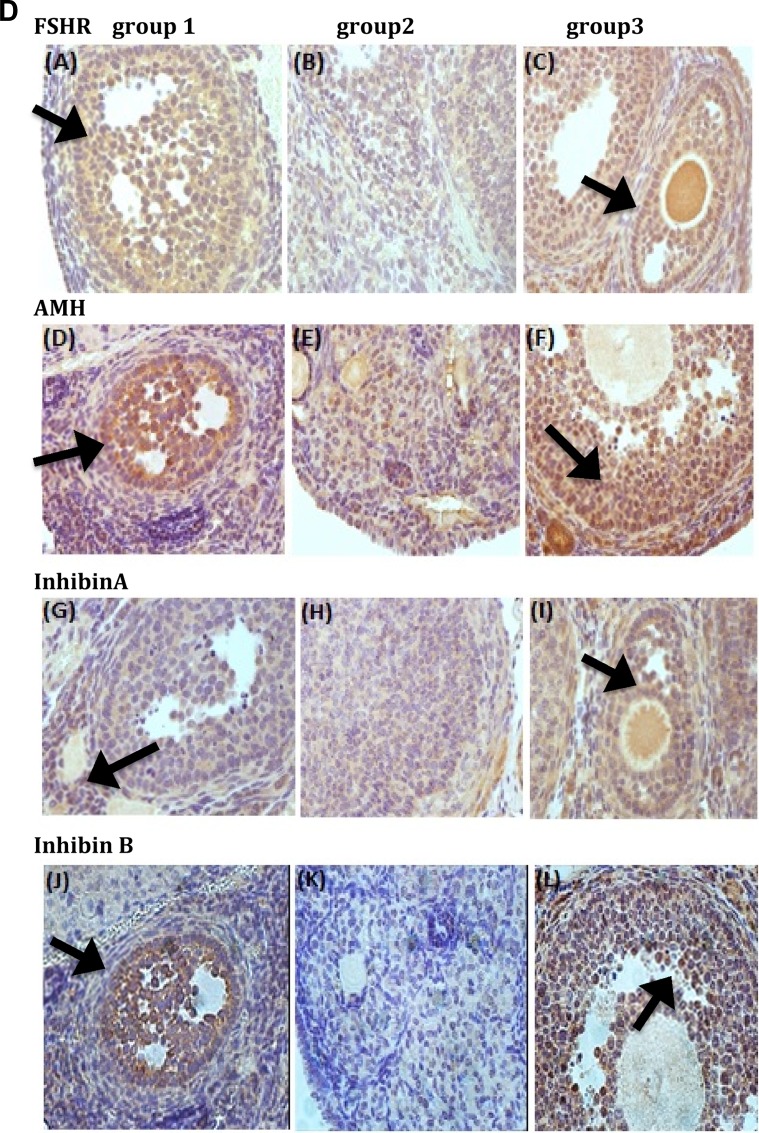
Ovarian expression profile of FSHR, AMH, and Inhibin (A and B)
Immunohistochemistry (Figure 5B) was employed to evaluate the expression of several markers of folliculogenesis. As shown in Figure 5B, groups 1 and 3 showed significantly higher FSHR expression than group 2 (P < .0001). Groups 1 and 3 also had significantly higher AMH expression than group 2 (P < .0001). Groups 1 and 3 had significantly higher inhibin A expression than group 2 (P < .0001). Groups 1 and 3 had significantly higher inhibin B expression than group 2 (P = .0005 and P = .0002, respectively). These results clearly demonstrate enhanced folliculogenesis after administration of BMSCs (Supplemental data; Figure 4).
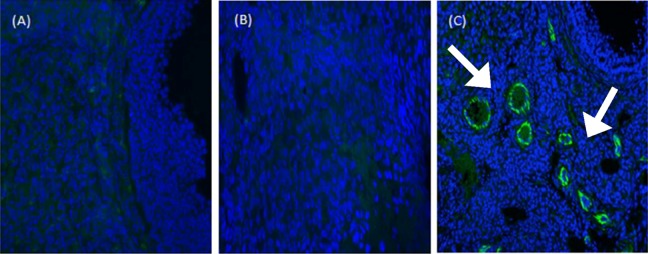
Detecting BMSCs in vivo engraftment by IHC
Vimentin are classified as class-III intermediate filaments, which are retrieved from human mesenchymal cells and other nonepithelial cells. It is mentioned that it attaches to the endoplasmic reticulum, nucleus, and mitochondria.34 Following the administration of human BMSCs, ovaries displayed Vimentin-positivity originating from human BMSCs starting from the 2-week time point to 8 weeks (end of experiment). Interestingly, the number of BMSCs was distributed mostly around the growing follicles in the ovarian cortex (Figure 6).
Figure 6.
Detecting bone marrow mesenchymal stem cells (BMSCs) in vivo. Grafted cells detected by fluorescent immunochemistry against human Vimentin antigens. (A) Negative control group with no positive Vimentin signal. (B) Human-specific cytoskeleton antigen expression (Vimentin) was negative in recipient ovaries of chemotherapy group without stem cells transplantation. (C) Human anti-Vimentin antibody (white arrows) was expressed in ovary stroma in recipient ovaries 2 months after stem cells implantation. Scale bars = 40 μm.
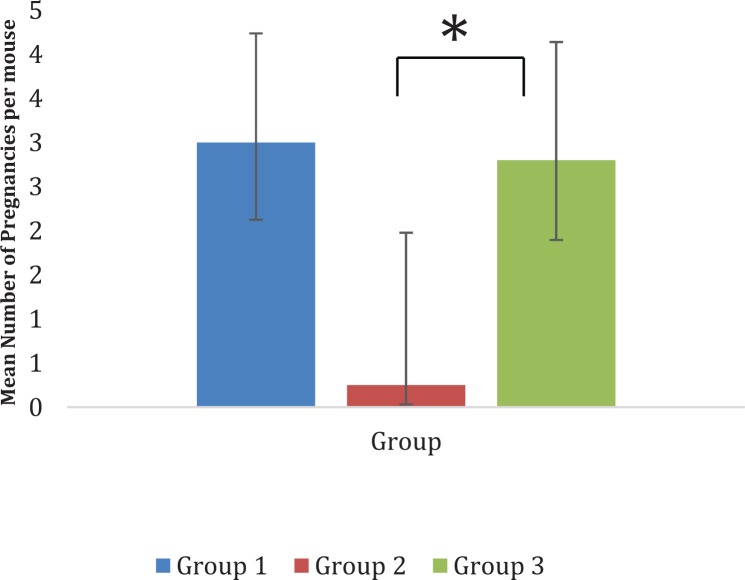
Breeding experiments
All mice in groups 1 and 3 became pregnant at least once, demonstrating a mating rate of 100%, while the mating rate in group 2 was 25% during the 3-month breeding test period. No significant difference was observed in the number of mating days between groups 1 and 3 (P = .5100).
Intraovarian BMSCs administration enhances fertility in CTX-treated mice
Mice within group 1 exhibited normal fertility as evidenced by the presence of a mating plug in each animals’ vaginal Os, within 7 days of cohabitation with breeder males (2 weeks from surgery date). Evidence of mating in group 2 was observed 2 weeks from the cohabitation date (3 weeks postsurgery and injection with vehicle). The majority of female mice in this group showed no mating at all (no pups). Mice in group 3 were mated within 1 week of BMSCs implantation. Interestingly, BMSCs treatment mating levels were higher than those in group 2 albeit lower than those of group 1. Moreover, consistent with the number of follicles produced, the mean number of pups per pregnancy was higher in group 1 when compared to group 3 (95% confidence interval = 1.19, 2.93, P = .0098). Mice in group 2 had only 1 pregnancy during the whole experiment resulting in the live birth of 2 pups within the 12-week period, while mice in group 3 had a total of 12 pregnancies during the same time period resulting in the live birth of 33 pups. During same experimental period, mice in group 1 produced a total of 74 pups. All pups in group 3 appeared physically normal with no visible malformations in comparison to group 1 pups; however, the 2 pups observed in group 2 were physically abnormal with decreased total body weight and 1 succumbed to death shortly after birth (Figure 7 and Supplemental Figure 5).
Figure 7.
Mean number of pregnancies. Pairwise comparisons suggest that the mean number of pregnancies were 12 times greater for group 1 than group 2 (P = .0223) and 11.2 times greater for group 3 versus group 2 (P = .0263); groups 1 and 3 did not have a different mean number of pregnancies (P = .7867). A larger sample is likely to show significant group differences.
Discussion
We present here ovarian administration of human BMSCs as an option to preserve female fertility for post-CTX cancer survivors. This is a novel approach with many potential advantages—minimally invasive, cost affordable, and widely applicable, especially for young patients with limited alternative choices. This notion has been perpetuated by others and confirmed by our current work.4,13
The chemotherapy-induced ovarian failure mouse model used in this work is biologically representative of human POF, as female mice when injected with CTX consistently have high FSH serum levels, low E2 levels, and are virtually reproductively infertile.
The busulfan/cyclophosphamide CTX experiment was designed to ablate vastly proliferating cells by cross-linkage the DNA.35 This cross-linkage affects the mitochondria and activates the apoptotic pathway.36 Consequently, it is believed broadly that chemotherapeutic drugs may immensly eliminate granulosa cells11 that are required for follicular development and oocyte survival.37
Folliculogenesis requires a carefully orchestrated cross talk between germ cells and surrounding somatic cells; however, CTX could destroy these ovarian niche interactions, reduce granulosa cell function, and, ultimately, provoke ovarian toxicity and function failure.38
Stem cells provide immeasurable possibilities as cell therapy to treat degenerative diseases; moreover, their ability to secrete several bioactive factors that modulate surrounding cells which ascribed to their inherent ability to differentiate into a variety of cell phenotypes.22
Our previous studies, along with others, have demonstrated that BMSCs can restore ovarian function and reactivate dormant folliculogenesis in several rodent models of ovarian dysfunction.5,28 We have previously shown that bone marrow nucleated cells intravenously delivered into the FSHR knocked out female mice (FORKO) and restored ovarian steroidogenesis.28 Specifically in that work, we demonstrated that this treatment restored FSHR expression in the ovaries, highly suggesting that administered cells did in fact transdifferentiate into a granulosa cell-like structures.28 It has also been shown that BMSCs can cause recovery of ovarian structure and function, injured by cyclophosphamide.8,39
Herein we have demonstrated that MSCs extracted from the human bone marrow of a healthy young female did efficiently graft into a preclinical chemotherapy-induced ovarian failure immune-competent mouse model (CTX POF). Treated animals displayed estrogenic changes in daily vaginal smears after mesenchymal stem cell administration, while control animals’ vaginal smears persisted unchanged. Reproductive organs and estrogen-responsive organ (liver) showed a noteworthy increase in weight at all experiment time points. There were no significant differences in ovary weight between groups 1 and 3, but they both had significantly higher mean total ovary weight than group 2 (P < .003). A trend of increased total number of follicles in treated animals was also observed at 2, 4, 6, and 8 week time points post-BMSCs administration. Increased E2 and AMH production as well as decreased serum FSH levels were also observed. Most importantly, there was observed “rescue” of the infertility phenotype in treated mice versus untreated group. These hormonal changes were consistently reflected in the reproductive physiology of this animal model.
In this study, the revived ovarian function in the form of observed hormonal pattern in stem cell-treated mice and resumed folliculogenesis occur in agreement with other several investigators demonstration of the mechanisms and robust regenerative abilities of BMSCs and other types of MSCs, that is, adipose-derived, menstrual blood-derived, skin-derived, and umbilical cord-derived MSCs.8,14,26,27,29,39
The mechanisms by which MSCs employ their reparative and immunomodulatory effects are work in progress which may be linked to multiple mechanisms mediated by both soluble factors and cell contact. In addition, it is possible that immune cells and MSCs interactions in the tissue’s microenvironment might modify MSCs function and justify MSCs-based therapy improved outcomes.40
The MSCs are able to home into injured tissues and restore local function after in vivo injection. The homing mechanism could be attributed to the growth factors secretion and chemokines, and the expression of extracellular matrix receptors on the MSCs cell surface, resulting in interactions such as the CXCR-4/SDF-1 and CD44/hyaluronic acid interactions. These cells can promote the regeneration/repair of injured tissue through the secretion of several molecules in a paracrine mechanism: a mechanism associated with antiapoptotic (eg, vascular endothelial growth factor and TGF-β), proangiogenic, immunomodulatory (eg, interleukin 10 and interleukin 6), and antifibrotic properties.17
In our work, follicle counts conducted after CTX treatment revealed vastly reduced primordial, primary, and secondary follicles, whereas BMSCs administration in the ovaries reconstituted the follicle reserve. No evidence exists to suggest that BMSCs can differentiate into oocytes in recipient mice. Herein, the number of primordial and growing follicles after BMSCs administration were not equal to the numbers found in normal female mice of the same age. This observation may explain why BMSCs administration did not completely restore fertility in CTX-induced sterilized mice to normal levels (normal mice in group 1, not exposed to CTX).
Our data have also shown higher FSHR staining in group 3 versus group 2, suggesting that there was an increase in the number of granulosa cells, as these are the only cells in the female ovary that express FSHR. The BMSCs actually differentiated into new granulosa cells or secreted trophic factors from BMSCs which then revived the partially damaged endogenous mouse granulosa cells.
Vimentin staining (indicative of the existence of human BMSCs) showed a circular distribution of such cells around the growing follicle in the ovarian stroma, seemingly in support of the latter option.
During the course of this study, we administered human BMSCs, derived from the human bone marrow of a 21-year-old African American female, into the ovaries of a POF-CTX mice model, suggesting that stem cells from a human source can successfully function xenogeneically; hence, it is not a far-reaching concept that they will likely act as well or better in the CTX-damaged allogeneic human ovary considering their immune-privileged status.15
The idea of using the allogeneic versus autologous stem cells presents several advantages. First, it eliminates the possibility of reintroducing malignant cells from the original cancer that existed in the cancer survivor. Clearly, this possibility depends on cancer type and stage, yet it is a realistic concern, especially in certain types of malignancy such as leukemia and gastrointestinal cancers.41,42 Second, the concept that if the patient’s own MSCs were able to do the task, they should have done so without the need for readministration (the homing effect of stem cells).
Previous studies have shown that donor’s age has an effect on the proliferation capacity of MSCs isolated from bone marrow which correlated with decreased clonogenicity.43 Further understanding of the molecular mechanisms involved in this process will help to improve the clinical application of MSCs. In our case, our donor was 21 years of age. It remains to be seen as to whether the observed restorative effects in this study can be generalized to other donors of varying age and more ethnically diverse groups.
In a review of the literature, there was no clear consensus concerning the male versus female origin; however, we elected to use a female donor, as we intend to translate this approach into a subsequent clinical translational trial in patients with POF and, therefore, deemed it appropriate to use female donors. We concede to the notion that the restorative ability of these BMSCs are likely due to the secretion of various trophic factors and/or differentiating into ovarian somatic cells, not germ cells; therefore, the gender of the donor is likely less critical.
Ovarian granulosa cells are the key element to sustain follicle survival. The BMSCs favorable effects on CTX-damaged ovaries is resulting primarily from the paracrine secretion of particular beneficial secreted growth factors that support ovarian follicle survival. Taken together, it is likely that the interaction between BMSCs and granulosa cells has the upper hand in this effect. Granulosa cells and BMSCs in vitro cocultured system establishment for screening candidate factors should be performed in the future to investigate this role.
One limitation of this study lies in the use of an animal model, which may not accurately represent the human situation. For example, follicular atresia induced by CTX peaked within the first week after CTX and unceased to reduce the primordial follicle pool. An attenuated therapeutic effect with time is quite reasonable which may raise the issue of needing multiple/repeated injections for a more lasting effect. This is especially true in the human situation, where the length of the ovarian cycle (28-30 days) differs dramatically from that of mice (4 days). We performed BMSCs administration 1 week post-CTX. This period was observed as an optimal time frame to evaluate BMSCs therapeutic effect in POF due to serious impairment of the ovaries, particularly when compared to healthy controls.
Undoubtedly, our preliminary animal model could be translated to human trials in the near future. Three major limitations for the human application may halt the progress of this work: First, how to inject the cells in human ovaries;second the significant cost to isolate and purify these cells; and moreover, BMSCs potential risks, efficiency, or stability. Collectively, further studies are needed.
The MSCs proliferative capacity in vitro is limited, and its attainment is partially invasive44; thus, another cell source could be considered for the clinical application of BMSCs in the treatment of POF. In the future, we intend to pursue this further using umbilical cord blood mesenchymal stem cells as well as other MSCs in comparative studies.
Conclusion
It is conceivable that human BMSCs could work as a potential treatment modality that either rescues follicles undergoing early atresia or quiescent primordial follicles from the adverse effects of CTX in the ovarian microenvironment. The potential for clinical application is clearly evident. Millions of women undertake fertility treatments each year due to age-related or nonage-related loss of oocytes. In young women who are rendered sterile worldwide especially by CTX, those treatments offer only uncertain improvements in their fertility. If oocytes remain viable, albeit dormant, in these women, there is potential to reestablish fertility using a stem cell administration approach for the revival of folliculogenesis. This evaluation of the regenerative use of BMSCs gives hope to women affected by POF and direct us to enquire if fertility restoration using BMSCs is a viable therapeutic option for these patients.
Supplementary Material
Acknowledgments
We appreciate Walidah Walker, MPH Grant Development Specialist, Department of Obstetrics & Gynecology, Medical College of Georgia, Georgia Regents University for her excellent editing and expertise.
Footnotes
Authors’ Note: A.A.H. was the principal investigator in this study, and all parts of the study were conducted under his leadership. S.A.M served as the main researcher in this study and therefore planned the study design, prepared the animal model and performed surgeries, collected data, evaluated results, and wrote the draft manuscript. S.M.S assisted in all animal handling during the course of experiments (vaginal swab, blood collection, and surgery) and conducted the evaluation of all histopathology results. S.B and M.A participated in surgical procedures. W.D.H and N.I helped drafting the manuscript, gave approval of the final version to be published, and agreed to be accountable for all aspects of the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by Augusta University Startup package, (Ayman Al-Hendy) and the core lab, which is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934. We also appreciate the core lab which is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934.
Supplemental material: Supplementary material for this article is available online.
References
- 1. Beck-Peccoz P, Persani L. Premature ovarian failure. Orphanet J Rare Dis. 2006;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xiao GY, Liu IH, Cheng CC, et al. Amniotic fluid stem cells prevent follicle atresia and rescue fertility of mice with premature ovarian failure induced by chemotherapy. PloS One. 2014;9(9):e106538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970-2002. JAMA. 2005;294(10):1255–1259. [DOI] [PubMed] [Google Scholar]
- 4. Howlader N, Noone AM, Krapcho M, et al., eds. SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]
- 5. Lee HJ, Selesniemi K, Niikura Y, et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25(22):3198–3204. [DOI] [PubMed] [Google Scholar]
- 6. Averette HE, Boike GM, Jarrell MA. Effects of cancer chemotherapy on gonadal function and reproductive capacity. CA Cancer J Clin. 1990;40(4):199–209. [DOI] [PubMed] [Google Scholar]
- 7. Plowchalk DR, Mattison DR. Reproductive toxicity of cyclophosphamide in the C57BL/6N mouse: 1. Effects on ovarian structure and function. Reprod Toxicol. 1992;6(5):411–421. [DOI] [PubMed] [Google Scholar]
- 8. Abd-Allah SH, Shalaby SM, Pasha HF, et al. Mechanistic action of mesenchymal stem cell injection in the treatment of chemically induced ovarian failure in rabbits. Cytotherapy. 2013;15(1):64–75. [DOI] [PubMed] [Google Scholar]
- 9. Ethics Committee of American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2013;100(5):1224–1231. [DOI] [PubMed] [Google Scholar]
- 10. Blumenfeld Z, Avivi I, Ritter M, Rowe JM. Preservation of fertility and ovarian function and minimizing chemotherapy-induced gonadotoxicity in young women. J Soc Gynecol Investig. 1999;6(5):229–239. [DOI] [PubMed] [Google Scholar]
- 11. Desmeules P, Devine PJ. Characterizing the ovotoxicity of cyclophosphamide metabolites on cultured mouse ovaries. Toxicol Sci. 2006;90(2):500–509. [DOI] [PubMed] [Google Scholar]
- 12. Blumenfeld Z. Gynaecologic concerns for young women exposed to gonadotoxic chemotherapy. Curr Opin Obstet Gynecol. 2003;15(5):359–370. [DOI] [PubMed] [Google Scholar]
- 13. Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta pharmacol Sin. 2013;34(6):747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fu X, He Y, Xie C, Liu W. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy. 2008;10(4):353–363. [DOI] [PubMed] [Google Scholar]
- 15. Kimbrel EA, Kouris NA, Yavanian GJ, et al. Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cells Dev. 2014;23(14):1611–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–896. [DOI] [PubMed] [Google Scholar]
- 17. English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell stem cell. 2010;7(4):431–442. [DOI] [PubMed] [Google Scholar]
- 18. Klyushnenkova E, Mosca JD, Zernetkina V, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12(1):47–57. [DOI] [PubMed] [Google Scholar]
- 19. Le Blanc K, Frassoni F, Ball L, et al. ; Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. [DOI] [PubMed] [Google Scholar]
- 20. Selesniemi K, Lee HJ, Niikura T, Tilly JL. Young adult donor bone marrow infusions into female mice postpone age-related reproductive failure and improve offspring survival. Aging. 2009;1(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun M, Wang S, Li Y, Yu L, Gu F, Wang C, Yao Y. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther. 2013;4(4):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang S, Yu L, Sun M, et al. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. BioMed Res Int. 2013;2013:690491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lai D, Wang F, Chen Y, Wang L, Wang Y, Cheng W. Human amniotic fluid stem cells have a potential to recover ovarian function in mice with chemotherapy-induced sterility. BMC Dev Biol. 2013;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu T, Huang Y, Guo L, Cheng W, Zou G. CD44+/CD105+ human amniotic fluid mesenchymal stem cells survive and proliferate in the ovary long-term in a mouse model of chemotherapy-induced premature ovarian failure. Int J Med Sci. 2012;9(7):592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang F, Wang L, Yao X, Lai D, Guo L. Human amniotic epithelial cells can differentiate into granulosa cells and restore folliculogenesis in a mouse model of chemotherapy-induced premature ovarian failure. Stem Cell Res Ther. 2013;4(5):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takehara Y, Yabuuchi A, Ezoe K, et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Investig. 2013;93(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu T, Huang Y, Zhang J, et al. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev. 2014;23(13):1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghadami M, El-Demerdash E, Salama SA, et al. Toward gene therapy of premature ovarian failure: intraovarian injection of adenovirus expressing human FSH receptor restores folliculogenesis in FSHR(-/-) FORKO mice. Mol Hum Reprod. 2010;16(4):241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lai D, Wang F, Dong Z, Zhang Q. Skin-derived mesenchymal stem cells help restore function to ovaries in a premature ovarian failure mouse model. PloS One. 2014;9(5):e98749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rugh R, Ginns EI, Ho HS, Leach WM. Responses of the mouse to microwave radiation during estrous cycle and pregnancy. Radiat Res. 1975;62(2):225–241. [PubMed] [Google Scholar]
- 31. Walmer DK, Wrona MA, Hughes CL, Nelson KG. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology. 1992;131(3):1458–1466. [DOI] [PubMed] [Google Scholar]
- 32. Fata JE, Chaudhary V, Khokha R. Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17beta-estradiol during the estrous cycle. Biol Reprod. 2001;65(3):680–688. [DOI] [PubMed] [Google Scholar]
- 33. Gay VL, Midgley AR, Jr, Niswender GD. Patterns of gonadotrophin secretion associated with ovulation. Fed Proc. 1970;29(6):1880–1887. [PubMed] [Google Scholar]
- 34. Liu Y, Du F, Zhao Q, Jin J, Ma X, Li H. Acquisition of 5-fluorouracil resistance induces epithelial-mesenchymal transitions through the Hedgehog signaling pathway in HCT-8 colon cancer cells. Oncol Lett. 2015;9(6):2675–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8(3):193–204. [DOI] [PubMed] [Google Scholar]
- 36. Zhao XJ, Huang YH, Yu YC, Xin XY. GnRH antagonist cetrorelix inhibits mitochondria-dependent apoptosis triggered by chemotherapy in granulosa cells of rats. Gynecol Oncol. 2010;118(1):69–75. [DOI] [PubMed] [Google Scholar]
- 37. Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296(5576):2178–2180. [DOI] [PubMed] [Google Scholar]
- 38. Sklar C. Reproductive physiology and treatment-related loss of sex hormone production. Med Pediatr Oncol. 1999;33(1):2–8. [DOI] [PubMed] [Google Scholar]
- 39. Kilic S, Pinarli F, Ozogul C, Tasdemir N, Naz Sarac G, Delibasi T. Protection from cyclophosphamide-induced ovarian damage with bone marrow-derived mesenchymal stem cells during puberty. Gynecol Endocrinol. 2014;30(2):135–140. [DOI] [PubMed] [Google Scholar]
- 40. Cipriani P, Ruscitti P, Di Benedetto P, et al. Mesenchymal stromal cells and rheumatic diseases: new tools from pathogenesis to regenerative therapies. Cytotherapy. 2015;17(7):832–849. [DOI] [PubMed] [Google Scholar]
- 41. Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2012;30(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sonmezer M, Shamonki MI, Oktay K. Ovarian tissue cryopreservation: benefits and risks. Cell Tissue Res. 2005;322(1):125–132. [DOI] [PubMed] [Google Scholar]
- 43. Li Y, Charif N, Mainard D, Bensoussan D, Stoltz J-F, de Isla N. Donor’s age dependent proliferation decrease of human bone marrow mesenchymal stem cells is linked to diminished clonogenicity. Biomed Mater Eng. 2014;24(1 suppl):47–52. [DOI] [PubMed] [Google Scholar]
- 44. Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22(5):675–682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.