Abstract
Objective
To determine whether hypertensive disorders of pregnancy (HDP) increased long-term stroke risk in women in the California Teachers Study (CTS), a prospective cohort study, and whether aspirin or statin use modified this risk.
Methods
CTS participants ≤60 years of age at the time of enrollment in 1995 were followed up prospectively for validated stroke outcomes obtained via linkage with California hospital records through December 31, 2015. We calculated unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (95% CIs) for the primary outcomes of all stroke and stroke before 60 years of age among those with and without a history of HDP. We tested for interactions (p < 0.2) and performed stratified analyses to assess the risk of the primary outcomes in women with and without self-reported use of aspirin or statins.
Results
Of 83,749 women included in the analysis, 4,070 (4.9%) had HDP. Women with prior HDP had increased risk of all stroke (adjusted HR 1.3, 95% CI 1.2–1.4) but no increased risk of stroke before age 60 (adjusted HR 1.2, 95% CI 0.9–1.7). There was an interaction (p = 0.18) between aspirin use and HDP history on risk of stroke before age 60: nonusers of aspirin had higher risk (adjusted HR 1.5, 95% CI 1.0–2.1) while aspirin users did not (adjusted HR 0.8, 95% CI 0.4–1.7). This effect was not seen with statins.
Conclusions
After controlling for comorbid conditions, women with prior HDP had increased long-term stroke risk, which was reduced by aspirin use. Randomized trials may be needed to assess whether long-term aspirin use could benefit selected women with a history of HDP.

Preeclampsia, a multisystem disorder characterized by hypertension and widespread endothelial dysfunction during pregnancy, occurs in 3% to 8% of all pregnancies.1 Preeclampsia and other hypertensive disorders of pregnancy (HDP) are associated with increased long-term cardiovascular risk, including stroke,2 a leading cause of death and disability in women in the United States.3 Preeclampsia may have unique effects on the cerebral vasculature, as evidenced by the elevated risk of peripartum stroke in this group4 and increased white matter lesions in women with a history of preeclampsia.5 While preeclampsia rates in most developed countries have been stable or declining, preeclampsia rates are rapidly rising in the United States,6,7 making non-US prospective cohort studies less generalizable to the US population.8 Some studies have suggested that strokes may occur at a younger age in women with a history of preeclampsia.9–13
Aspirin treatment during pregnancy reduces preeclampsia incidence among high-risk women.14 However, aspirin is typically discontinued after delivery. Whether continued aspirin use after pregnancy is beneficial in women with a history of preeclampsia is unknown. Guidelines for estimating 10-year cardiovascular risk, including stroke, to inform primary prevention with aspirin or lipid-lowering therapy do not include history of preeclampsia in risk calculations,15 despite preeclampsia being identified as a sex-specific risk factor for stroke by the American Heart Association/American Stroke Association.16
The California Teachers Study (CTS) has followed up a large cohort of female teachers continuously since 1995 to 1996. We hypothesized that history of HDP would independently increase the risk of stroke in women in the CTS. We further hypothesized that use of aspirin or statins would modify this risk.
Methods
Study design and population
Study participants were recruited in 1995 to 1996 by mailing questionnaires to all female active and recently retired members of the California State Teachers Retirement System. Women who returned a self-administered questionnaire (n = 133,479, median age at enrollment 53 years, age range 22–104 years) have been followed up prospectively since then, with 5 subsequent self-administered questionnaires and additional outcomes data obtained through linkage with the California Office of Statewide Health Planning and Development hospitalization discharge database via probabilistic record linkage based on Social Security number, date of birth, sex, and race/ethnicity. This process has previously been described.17 Briefly, the status of California residence is monitored through annual mailings, responses from participants, and routine record linkages with the US Postal Service National Change of Address database and the California Department of Motor Vehicles. The date and cause of death are ascertained through linkage with the California State Mortality Files, the Social Security Administration Death Master File, and the National Death Index. The California Health and Safety Code requires that all hospitals provide discharge data, with the exception of 11 state mental health and developmental hospitals. Data from the hospitals are required to meet data error tolerance levels of 0.1% for sex and date of birth. Social Security numbers in the CTS are 99% complete and were validated with a checking algorithm excluding numbers outside the possible range. All women ≤60 years of age at time of enrollment who did not report a prior stroke on the baseline questionnaire were included in this analysis, with outcomes available through December 31, 2015. Women >60 years of age at the time of study enrollment were excluded because of concern that participant recall of preeclampsia could be less reliable.18 Participants were censored at the time of death, move away from the state of California, or voluntary withdrawal from the study. Women who did not complete all questionnaires but for whom contact information was established and who remained in California were considered to still be enrolled in the study because the CTS continues to collect outcomes.
Exposures of interest
The primary exposure was HDP, defined as self-reported preeclampsia or hospital-diagnosed HDP, including gestational hypertension, preeclampsia, and eclampsia. In 1997 to 1998, the second questionnaire asked, “Considering ALL your pregnancies, were you ever diagnosed with pre-eclampsia? (Note: pre-eclampsia is a condition that can occur during the second half of a pregnancy and is marked by elevated blood pressure, protein in the urine, and fluid retention by the mother.)” Women who responded “yes” or were admitted to a hospital with an ICD-9 diagnostic code for a HDP (642.3x, 642.4x, 642.5x, 642.6x, 642.7x, or 642.9x) were considered to have had HDP (appendix available from Dryad, doi.org/10.5061/dryad.71b0hr3). Hospital admissions data were available from January 1, 1991, to December 31, 2015. To assess sensitivity of self-reported preeclampsia diagnoses in our study cohort, we correlated self-reported preeclampsia with prior HDP admissions. Women who had hospital-diagnosed HDP concurrent with or after stroke were not considered to have HDP exposure unless they had a separate, prior episode of HDP before the stroke. Because the date of preeclampsia diagnosis was not available for the majority of women with self-reported preeclampsia, the exposure was treated as a binary variable (any HDP/no HDP). Women who did not report a history of preeclampsia and had no admissions for HDP were assumed to have no history of HDP. All women who reported a prior stroke at the time of study enrollment were excluded from the analysis because we were unable to verify the date of HDP in relation to the date of stroke.
Additional variables of interest included age, race/ethnicity, and self-reported stroke risk factors, including hypertension, diabetes mellitus, obesity (defined as baseline body mass index of ≥30 kg/m2), migraine, and tobacco use (defined as history of smoking reported on any questionnaire before stroke). Women were considered regular aspirin users if they reported taking aspirin at least 3 times weekly for at least 1 year on questionnaire 1 (1995–1996), 4 (2005–2006), or 5 (2012–2013), the 3 questionnaires on which participants were asked to report aspirin use. Women were considered regular statin users if they reported using a statin daily for at least 2 months on questionnaire 4 (2005–2006) or 5 (2012–2013), the 2 questionnaires on which participants were asked to report statin use. Only medication use before stroke diagnosis (primary prevention) was considered.
Outcomes and diagnostic code validation
We prespecified 2 primary outcomes: any stroke after study enrollment (all stroke) and stroke before age 60. The cutoff of stroke before age 60 was based on the higher prevalence of stroke in US women compared to men in this age group in the National Health and Nutrition Examination Survey,19 suggesting a larger role for sex-specific stroke risk factors in younger women. Stroke was defined as hospital admission or death due to acute ischemic stroke, TIA, cerebral venous thrombosis, cervical artery dissection, acute cerebrovascular disease during the puerperium, or nontraumatic intracerebral or subarachnoid hemorrhage without concurrent HDP (appendix available from Dryad, doi.org/10.5061/dryad.71b0hr3). Secondary outcomes included development of hypertension after study enrollment and a combined outcome of stroke, myocardial infarction, or death resulting from any cause.
Validation of hospitalization discharge codes in California for stroke and TIAs among women in the CTS cohort
Primary and secondary hospital discharge codes were identified from the Office of Statewide Health Planning and Development linkage database with the use of ICD-9 codes. Stroke was defined as a hospitalization with an ICD-9 code of 430, 431, 432.x, 433.x1, 434.xx (excluding 434.x0), 436, 437.1, or 437.9. TIA was defined as a hospitalization with an ICD-9 code of 435. We selected ≈200 women diagnosed with incident stroke or TIA with a sampling distribution of ICD-9 codes equivalent to that of the entire group. Two trained interviewers contacted selected participants (or relatives for those too ill to participate or deceased) and obtained consent for review of medical records. For women with >1 stroke event, we targeted records for the first event, although records from the subsequent event were evaluated when obtained, resulting in a total of 239 records, comprising 212 incident and 27 recurrent stroke or TIAs.
Medical record review, following a standardized protocol, was the definitive diagnosis against which hospitalization codes were assessed. Two neurologists (K.L., M.S.V.E.) independently reviewed medical records. A third neurologist (D.W.), who was blinded to the 2 reviewers' decisions, provided adjudication in cases of discrepancy. An event was classified as a stroke if it was due to rapid onset of a persistent focal neurologic deficit attributable to an obstruction or rupture of the vascular system and not known to be secondary to brain trauma, tumor, infection, or other cause. The deficit must have lasted >24 hours or have been associated with a demonstrable lesion on computed tomography or MRI compatible with acute stroke. An event was classified as a TIA if it resulted from ≥1 episodes of focal neurologic deficit lasting >30 seconds but no longer than 24 hours. Rapid evolution of the symptoms to the maximal deficit must have occurred in <5 minutes with subsequent complete resolution without head trauma immediately before onset of the neurologic event. The final algorithm for defining stroke by ICD-9 codes in the CTS cohort excluded ICD-9 codes 437 (other and ill-defined cerebrovascular disease) and 432.1 (subdural hemorrhage) and restricted the definition of stroke and TIA to incident events recorded in the primary position. Positive predictive values for overall stroke and stroke subtypes and κ statistics for agreement between neurologists are reported (appendix available from Dryad, doi.org/10.5061/dryad.71b0hr3). Diagnostic codes for cerebral venous thrombosis, cervical artery dissection, and acute cerebrovascular disease during the puerperium have previously been validated in other cohorts.20–23
Institutional approvals
Permission to conduct this analysis was obtained from the Institutional Review boards of City of Hope National Medical Center and Columbia University Medical Center. Stroke outcomes validation was carried out in compliance with the Helsinki Declaration and approved by the Institutional Review boards at each study center, namely the City of Hope, the University of Southern California, the Cancer Prevention Institute of California, and the University of California at Irvine, and by the California State Committee for the Protection of Human Subjects, in accordance with assurances filed with and approved by the US Department of Health and Human Services. All study participants provided written informed consent to participate in the study.
Data availability statement
Deidentified data from the CTS are housed in an online secure data warehouse maintained by the City of Hope in collaboration with the Supercomputing Center of the University of California, San Diego. Data and statistical code used for the current analysis are available to other investigators on request to the CTS.
Statistical analysis
Kaplan-Meier survival analyses were calculated for time to stroke stratified by history of HDP. Date of study enrollment was considered the start of follow-up time for each participant. We used a complete case assessment and did not account for missing data because the proportion of missing data for our variables of interest was <10%. We used Cox proportional hazards models to calculate unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (95% CIs) for all stroke and stroke before age 60 among women with and without a history of HDP. The proportional hazards assumption was checked and was met. We tested for interactions between HDP and self-reported use of aspirin or statins. A value of p=0.2 was considered significant for a possible interaction, consistent with a higher threshold for statistical significance for assessing interactions, with acceptance of a higher likelihood of type 1 error.24,25 We created stratified models to assess risk of stroke in women with and without self-reported use of aspirin or statins. Because HDP can occur only during pregnancy and because of concern that pregnancy itself, rather than HDP, could influence the risk of future stroke, prespecified subgroup analyses were performed including only parous women in the non-HDP group. We performed sensitivity analyses excluding TIA and the stroke outcome codes that were not specifically validated in our cohort. The secondary outcome of hypertension was assessed via self-reported hypertension on questionnaire 5 among women with no self-reported baseline hypertension on questionnaire 1. Because we did not know the date of hypertension diagnosis, we used logistic regression to estimate adjusted odds ratios (ORs) and 95% CIs for the development of new hypertension in women with and without history of HDP.
Results
Baseline characteristics
Of the 83,749 women who met our inclusion criteria, 4,070 (4.9%) had a history of HDP (figure 1). Baseline demographics and self-reported comorbid conditions of the study sample are shown in table 1. Age distribution of the study sample at study entry and last follow-up is shown in figure 2. Of the 83,749 women, 11,734 were lost to follow-up because of moving away from the state of California, voluntary withdrawal from the CTS, or inability to establish contact information, giving a total follow-up time of 1,519,906 woman-years. Mean follow-up time was 18.1 years (SD 4.5 years) and did not differ by exposure groups (p = 0.43).
Figure 1. CONSORT diagram, study enrollment.
Women were classified as having a history of preeclampsia if they were admitted to a hospital with a diagnosis of preeclampsia or reported a history of preeclampsia on the second questionnaire in 1997 to 1998. Hospital admission data were available from January 1, 1991, through December 31, 2015. CONSORT = Consolidated Standards in Reporting Trials; CTS = California Teachers Study.
Table 1.
Characteristics of women enrolled at ≤60 years of age in the CTS with and without HDP (n = 83,749)
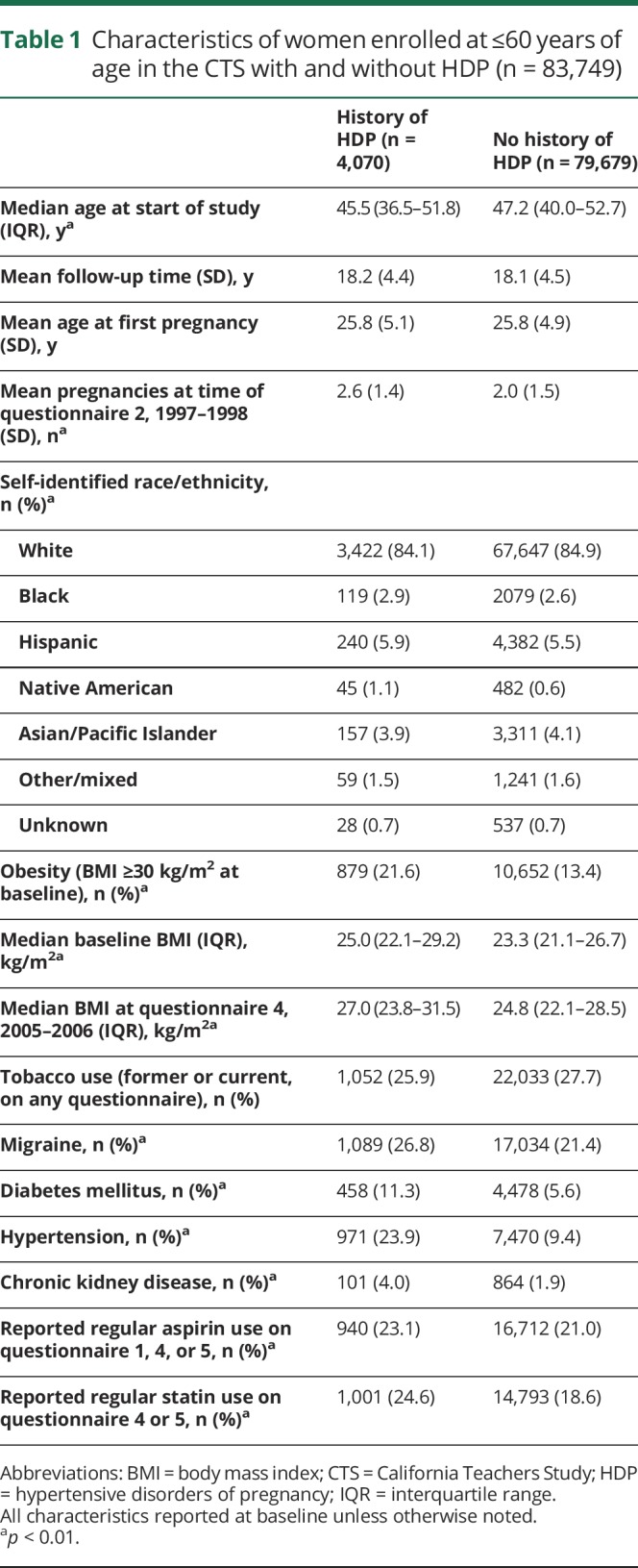
Figure 2. Age distribution of women included the analysis in 1995 (study enrollment) and 2015 (most recent follow-up data) in the CTS.
Age distribution of eligible study participants (n = 83,749) in 1995 at the time of enrollment and 2015 at the time of most recent follow-up. Participants were included in the analysis if they were ≤60 years of age at the time of study enrollment and had no baseline reported history of stroke. CTS = California Teachers Study.
Compared to women without prior HDP, women with prior HDP had more baseline self-reported vascular risk factors. Among women with HDP, 972 had hospital-diagnosed HDP and 3,361 had self-reported history of preeclampsia on questionnaire 2, with 262 women having both self-reported preeclampsia and hospital-diagnosed HDP. Women with self-reported preeclampsia were older at the time of study entry compared with women with hospital-diagnosed HDP, had higher parity, and had more vascular risk factors (table 2). Of 396 women who had hospital-diagnosed HDP before questionnaire 2, 249 (62.9%) reported preeclampsia on the questionnaire. Of 38 women who had hospital-diagnosed severe preeclampsia or eclampsia, 32 (84.2%) reported it. Compared with the 147 women who failed to report their diagnosis, self-reporters had higher proportions of severe preeclampsia or eclampsia (32 of 249 [12.9%] self-reporters vs 6 of 147 [4.1%] nonreporters, p = 0.004).
Table 2.
Characteristics of women with hospital-diagnosed HDP vs self-reported preeclampsia
Women in our sample who reported aspirin use were older, had a higher proportion of self-identified white race, and had more vascular risk factors than nonusers of aspirin. A higher proportion of aspirin users also reported regular statin use (4,761 of 17,652 aspirin users [27.0%] vs 11,033 of 66,097 nonusers of aspirin [16.7%], p < 0.0001). Similarly, statin users were older, had more vascular risk factors, and were more likely to report aspirin use (appendix available from Dryad, doi.org/10.5061/dryad.71b0hr3).
Primary outcomes
All stroke
There were 123 admissions for stroke in the HDP group (3.0%, event rate 166 per 100,000 woman-years) compared with 1823 (2.3%, 127 per 100,000 woman-years) in the non-HDP group (p = 0.002). Event subtypes are detailed in table 3. Risk of all stroke was higher in the HDP group (unadjusted HR 1.3, 95% CI 1.1–1.6) (table 4). Adjusting for age, race/ethnicity, tobacco use, migraine, hypertension, obesity, and diabetes mellitus did not alter the results (adjusted HR 1.3, 95% CI 1.2–1.4). Kaplan-Meier stroke-free survival curves for women with and without a history of HDP are shown in figure 3.
Table 3.
Cerebrovascular event subtypes in women enrolled at ≤60 years of age in the CTS
Table 4.
Risk of stroke in women with vs without history of HDP stratified by reported medication use

Figure 3. Event-free survival curves for women in the CTS ≤60 years of age at time of study entry, with and without history of HDP.

Kaplan-Meier product-limit survival estimate with 95% Hall-Wellner bands for women with (red) and without (blue) a history of self-reported or hospital-diagnosed hypertensive disorders of pregnancy (HDP). Participants were censored at the time of death, move away from the state of California, or voluntary withdrawal from the study. CTS = California Teachers Study.
There was no interaction between HDP and aspirin use for overall stroke risk (p = 0.49). In secondary stratified analyses, we found that the risk of all stroke was elevated in women with a history of HDP who were not taking aspirin regularly (adjusted HR 1.3, 95% CI 1.2–1.4); women with a history of HDP who were taking aspirin regularly remained at increased risk (adjusted HR 1.2, 95% CI 1.0–1.4). There was no increased risk of hemorrhagic stroke in women who were taking aspirin. We found no interaction between statin use and history of HDP for all stroke risk (p = 0.69).
Stroke before age 60
Risk of stroke before age 60 was higher in women with HDP history (event rate 51.3 per 100,000 woman-years) compared to those without HDP history (event rate 34.3/100,000 woman-years) in unadjusted models (unadjusted HR 1.5, 95% CI 1.1–2.1). After adjustment for age, race/ethnicity, tobacco use, migraine, hypertension, obesity, and diabetes mellitus, there was no increase in risk of stroke before age 60 in the HDP group (adjusted HR 1.2, 95% CI 0.9–1.7). There was an interaction between HDP and self-reported aspirin use on the risk of stroke before age 60 (p = 0.18). Stratified analyses showed an increased effect size in women who were not taking aspirin regularly (adjusted HR 1.5, 95% CI 1.0–2.1). HDP did not increase risk of stroke before age 60 in women who were taking aspirin (adjusted HR 0.8, 95% CI 0.4–1.7). We found no interaction between statin use and history of HDP on the risk of stroke before age 60 (p = 0.68).
Secondary outcomes
Hypertension
Self-reported hypertension was more prevalent in the HDP group (971 of 4,070, 23.9%) at the time of the first questionnaire compared with the non-HDP group (7,470 of 79,679, 9.4%) (p < 0.0001). Among the 75,308 women who did not report hypertension on questionnaire 1, a total of 31,615 women did not complete questionnaire 5. Of these, 2,811 had died; 5,002 had moved out of the state of California, had withdrawn from the study, or had contact information that was could not be verified; and the rest remained in the study with outcomes being tracked through the California State database. There was no difference between the HDP and non-HDP groups in follow-up time: mean follow-up time was 18.2 years in the unexposed group and 18.3 years in the exposed group (p = 0.29). Among those women who did not report baseline hypertension and completed questionnaire 5 (2012–2013) (n = 43,693), women with a history of HDP had increased odds of developing hypertension by the time of questionnaire 5 (adjusted OR 1.7, 95% CI 1.6–1.9). The odds were higher in women who were <40 years of age at the time of study entry (adjusted OR 2.5, 95% CI 2.0–3.2). Models were adjusted for age and self-reported race/ethnicity.
Combined stroke, myocardial infarction, or death
Women with a history of HDP had increased risk of the combined outcome of stroke, myocardial infarction, or death resulting from any cause (unadjusted HR 1.1, 95% CI 1.0–1.2; adjusted HR 1.2, 95% CI 1.0–1.3). Models were adjusted for age, race/ethnicity, tobacco use, obesity, diabetes mellitus, and hypertension.
Subgroup and sensitivity analyses
When the sample was restricted to women who reported at least 1 pregnancy on any questionnaire (n = 67,185), HDP increased the risk of all stroke (unadjusted HR 1.4, 95% CI 1.1–1.6; adjusted HR 1.3, 95% CI 1.1–1.6) and of stroke before age 60 (unadjusted HR 1.6, 95% CI 1.1–2.2; adjusted HR 1.3, 95% CI 0.9–1.8). Stratified by aspirin use, the results of this subgroup analysis were similar to the entire cohort (appendix available from Dryad, doi.org/10.5061/dryad.71b0hr3). A sensitivity analysis excluding patients with diagnostic codes for TIA, cervical artery dissections, cerebral venous thrombosis and pregnancy-associated stroke again showed results similar to the overall analysis (appendix available from Dryad, doi.org/10.5061/dryad.71b0hr3).
Discussion
In our analysis of 83,749 initially stroke-free women in the CTS, we found that a history of HDP increased women's risk of future stroke, even after adjusting for their increased prevalence of vascular risk factors. Furthermore, we found that while use of aspirin had a moderate effect on overall risk reduction for stroke, it appeared to have a stronger effect on reducing risk of stroke before age 60 in younger and middle-aged women without increasing hemorrhagic stroke risk. We did not find a similarly beneficial effect of statins in this population. Prior studies have shown increased long-term risk of stroke and cardiovascular disease in women with a history of HDP.2 Our study in a large cohort in a US population raises the possibility that primary preventive treatment with aspirin after a pregnancy complicated by HDP might reduce future stroke risk in this population.
Multiple studies have demonstrated that low-dose aspirin taken early in pregnancy reduces the risk of preeclampsia,14,26 leading the American College of Obstetrics and Gynecology to recommend its use in women at high risk for developing preeclampsia (such as women with a history of recurrent prior preeclampsia or preeclampsia with preterm delivery).27 Proposed mechanisms include antithrombotic effects and the anti-inflammatory and antioxidant properties of aspirin.28 However, our understanding of the effects of aspirin on the causal pathway of preeclampsia is limited.29 The complex pathophysiology of preeclampsia is still incompletely understood but involves abnormal placentation leading to placental hypoxia, imbalance of proangiogenic and antiangiogenic factors, immune dysregulation, and a cascade of cytokine-mediated inflammatory effects.29–31 Oxidative stress and persistent inflammation may play a role in the development of long-term stroke risk after preeclampsia.31 Both acute and chronic inflammation increases the risk of cerebrovascular disease.32 Preeclampsia, an inflammatory condition, likewise has severe effects on the cerebral vasculature. In the short term, preeclampsia increases the risk of peripartum stroke 5- to 6-fold.33,34 Preeclampsia leads to disruption of cerebral autoregulation and alterations in blood-brain barrier function, which may lead to elevated risk of peripartum and postpartum ischemic stroke, postpartum angiopathy, and intracerebral hemorrhage.35 While preeclampsia has been proposed as a “failed stress test,” indicating underlying high risk for cardiovascular disease,36 other research suggests that preeclampsia itself may permanently alter endothelial function, leading to hypertension and atherosclerotic disease37 and amplifying the effects of those risk factors through multiplicative interaction.38 It is biologically plausible that the anti-inflammatory, antioxidant, and antithrombotic effects of aspirin use could be beneficial for long-term cerebrovascular protection in women with a history of preeclampsia and other HDP. In addition, the lack of evidence in our study for statin efficacy should not be interpreted as evidence of inefficacy; statins also have shown promise in the prevention and treatment of preeclampsia,39 and our results may have been biased by indication (i.e., women in our study at highest risk of stroke may have been more likely to be prescribed statins). Furthermore, both aspirin and statin use may have been relatively brief in this younger population, making it harder to detect an effect and biasing our results to the null hypothesis.
The clinical and public health implications of increased risk of stroke in younger women are of particular importance because younger stroke patients, particularly women, suffer grave long-term consequences in terms of years of disability and lost capacity to work, despite having better functional outcomes than older adults in the short term.40 Stroke is the fourth leading cause of death41 and a leading cause of disability in US women.42,43 Simple, inexpensive primary preventive interventions could have major consequences in this high-risk group. The postpartum period is a time of high engagement with the medical system, representing an opportunity for primary intervention. HDP history remains underrecognized as a risk factor by many physicians,44 leading to missed opportunities for early counseling. Furthermore, HDP history is not included in cardiovascular 10-year risk calculators, which are commonly used by primary care physicians to decide whether to recommend aspirin or statin treatment.45
This is particularly relevant in the United States, where preeclampsia rates are rapidly rising, for unclear reasons.6,7 Both the increased prevalence of obesity and delayed childbearing have been proposed as explanations,46 but neither of these is unique to the United States. Racial disparities may be a culprit; preeclampsia rates in the United States are higher in black women than white women, and black women have higher rates of preeclampsia-associated severe complications.8 Primary prevention strategies, in turn, may have even greater potential for benefit in black women with preeclampsia.
Strengths of our study include its prospective design and the granularity of the data, including medication use, often missing from large administrative datasets. The CTS is a long-running cohort study of women in the United States and is unusual in that it enrolled a large number of young women and included detailed reproductive history. Such information is often lacking in cohort studies aimed at cerebrovascular outcomes because stroke is typically thought of as a disease of the elderly, and preeclampsia has been underrecognized as a long-term cardiovascular and stroke risk factor. Another strength of our study is the rigorous validation of stroke outcomes in the CTS cohort.
A limitation of our study is the small number of stroke outcomes in both groups, particularly among younger women, limiting the power of the study to detect interactions. We used a threshold of p < 0.2 for significance for a statistical interaction, recognizing that this approach has generated debate24,25 but can boost the power of a study to detect biologically plausible, carefully preselected interactions. This approach can result in increased type 1 errors. However, our stratified analyses suggested effect modification.
An additional limitation is the inclusion of TIA in the outcome variable, a less specific diagnostic code by our own validation study. However, this would lead to nondifferential misclassification, more likely to bias our results toward the null than otherwise, and our sensitivity analysis excluding TIA and other less well-validated diagnostic codes showed similar results. Survivor bias may have affected our results in that women with a history of HDP may have been more likely to have strokes or die before study entry and thus be excluded from the analysis, again biasing our results toward the null. Some cervical artery dissections may not have resulted in stroke; because all of the dissections occurred in the unexposed group, this may have biased our results to the null.
The heterogeneity of HDP diagnoses is an important limitation of our study because women with more severe forms of preeclampsia have worse long-term outcomes.12 For most women, the history of preeclampsia was ascertained by self-report, which may have introduced additional misclassification. A systematic review found that maternal recall of HDP was >90% specific, regardless of the length of the recall period.18 Thus, while the sensitivity of maternal preeclampsia recall may have been limited in our study, this would likely have biased our results to the null. We included women with admissions for gestational hypertension in the HDP group because diagnostic codes for gestational hypertension and mild preeclampsia have been shown to be virtually interchangeable when applied to inpatients, with high rates of misclassification in both directions.47 Furthermore, preeclampsia is regarded as a disease along a continuum, with gestational hypertension often progressing to preeclampsia.48 Clinical confirmation of the preeclampsia diagnoses in the CTS was not feasible; thus, for the purposes of this hypothesis-generating study, we chose an inclusive definition of HDP, using either self-report of preeclampsia (which has been shown to be highly specific18 and made up the majority of our cases) or hospital diagnosis of any HDP (less specific but more sensitive).49 This approach may have resulted in misclassification.
An important limitation is that this cohort of teachers may not be generalizable to the broader US population. For example, women who enrolled in the CTS were predominantly white and were more likely to have delayed childbearing to a later age than women in the general California population. While the prevalence of HDP in this cohort is similar to that in the general population, rates of preeclampsia have been shown to be considerably higher in blacks, with an incidence ratio of 1.5 for severe preeclampsia and eclampsia and >3-fold higher incidence ratio of “superimposed” preeclampsia (preeclampsia with preexisting chronic hypertension).50 Thus, our results should be confirmed in a cohort that better reflects the geographic, socioeconomic, racial, and ethnic diversity of the United States; should be interpreted cautiously; and should be considered hypothesis generating.
These data support that women with a history of HDP are more likely than those without this history to develop future stroke. The risk of stroke before age 60, but not overall stroke, was mitigated in women who were taking aspirin. History of HDP should be considered an important risk factor for future stroke, and some women with this history may warrant primary preventive treatment with aspirin even in the absence of additional vascular risk factors. More research is needed to better understand the mechanisms by which preeclampsia and related disorders increase stroke risk, and randomized controlled trials may be warranted to establish the efficacy of aspirin for the primary prevention of stroke in selected women with a history of HDP.
Glossary
- CI
confidence interval
- CTS
California Teachers Study
- HDP
hypertensive disorders of pregnancy
- HR
hazard ratio
- ICD-9
International Classification of Diseases, 9th Revision
- OR
odds ratio
Footnotes
Editorial page 159
Podcast: NPub.org/1ungrh
Author contributions
E.C. Miller: study concept and design, data analysis and interpretation, drafting and revision of manuscript. A.K. Boehme: data analysis and interpretation, statistical analysis. N.T. Chung: major role in data acquisition, data analysis and interpretation. S.S. Wang: major role in data acquisition, data analysis and interpretation, and critical revision of manuscript for important intellectual content. J.V. Lacey: major role in data acquisition, critical revision of manuscript for important intellectual content. K. Lakshminarayan: major role in data acquisition, data analysis and interpretation. C. Zhong: major role in data acquisition. D. Woo: major role in data acquisition, data analysis and interpretation. N.A. Bello and R. Wapner: critical revision of manuscript for important intellectual content. M.S.V. Elkind: major role in data analysis and interpretation, critical revision of manuscript for important intellectual content. J.Z. Willey: study supervision, critical revision of manuscript for important intellectual content.
Study funding
Dr. Miller received research support from the NIH National Institute of Neurological Disorders and Stroke StrokeNet Training Core (U10NS08672805) and the NIH National Center for Advancing Translational Sciences 5KL2TR001874-02. Dr. Boehme received support through NIH National Institute of Neurological Disorders and Stroke R03 NS101417 and NIH National Institute of Minority Health and Health Disparities R21 MD012451. Dr. Bello is supported by the NIH National Center for Advancing Translational Sciences 5KL2TR001874-02 and the American College of Cardiology. Dr. Wapner receives support from the NuMoM2b-Heart Health Study (NIH National Heart, Lung, and Blood Institute 5U10HL119992-03) for work related to long-term cardiovascular outcomes after pregnancy. Stroke validation in the CTS was supported by the NIH National Institute of Neurological Disorders and Stroke to Dr. Wang (R21NS075608), the National Cancer Institute (R01CA077398, K05CA136967), and the Beckman Research Institute of the City of Hope. The CTS data infrastructure that makes analyses possible is supported by the NIH National Cancer Institute to Dr. Lacey (U01CA188277).
Disclosure
E.C. Miller, A.K. Boehme, N.T. Chung, J.V. Lacey, S.S. Wang, K. Lakshminarayan, C. Zhong, D. Woo, N.A. Bello, and R. Wapner report no disclosures relevant to the manuscript. M.S.V. Elkind discloses receiving research support from the BMS-Pfizer Alliance for Eliquis, royalties from UpToDate, and personal compensation for expert testimony from Merck/Organon in litigation related to Nuvaring and stroke and from Auxilium related to testosterone and stroke. J.Z. Willey reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol 2013;170:1–7. [DOI] [PubMed] [Google Scholar]
- 2.Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2017;10:e003497. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics–2018 update: a report from the American Heart Association. Circulation Epub 2018 Jan 31. [DOI] [PubMed]
- 4.Yoshida K, Takahashi JC, Takenobu Y, Suzuki N, Ogawa A, Miyamoto S. Strokes associated with pregnancy and puerperium: a nationwide study by the Japan Stroke Society. Stroke 2017;48:276–282. [DOI] [PubMed] [Google Scholar]
- 5.Aukes AM, De Groot JC, Wiegman MJ, Aarnoudse JG, Sanwikarja GS, Zeeman GG. Long-term cerebral imaging after pre-eclampsia. BJOG 2012;119:1117–1122. [DOI] [PubMed] [Google Scholar]
- 6.Kuklina EV, Tong X, Bansil P, George MG, Callaghan WM. Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007: reasons for concern? Stroke 2011;42:2564–2570. [DOI] [PubMed] [Google Scholar]
- 7.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahul S, Tung A, Minhaj M, et al. Racial disparities in comorbidities, complications, and maternal and fetal outcomes in women with preeclampsia/eclampsia. Hypertens Pregnancy 2015;34:506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DW, Dueker N, Jamieson DJ, et al. Preeclampsia and the risk of ischemic stroke among young women: results from the Stroke Prevention in Young Women Study. Stroke 2006;37:1055–1059. [DOI] [PubMed] [Google Scholar]
- 10.Canoy D, Cairns BJ, Balkwill A, et al. Hypertension in pregnancy and risk of coronary heart disease and stroke: a prospective study in a large UK cohort. Int J Cardiol 2016;222:1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanz LJ, Stuart JJ, Williams PL, et al. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. Circulation 2017;135:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the Child Health and Development Studies cohort. Hypertension 2010;56:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Ami S, Oron G, Ben-Haroush A, Blickstein D, Hod M, Bar J. Primary atherothrombotic occlusive vascular events in premenopausal women with history of adverse pregnancy outcome. Thromb Res 2010;125:124–127. [DOI] [PubMed] [Google Scholar]
- 14.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017;377:613–622. [DOI] [PubMed] [Google Scholar]
- 15.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 16.Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prescott J, Lu Y, Chang ET, et al. Reproductive factors and non-Hodgkin lymphoma risk in the California Teachers Study. PLoS One 2009;4:e8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuart JJ, Bairey Merz CN, Berga SL, et al. Maternal recall of hypertensive disorders in pregnancy: a systematic review. J Womens Health 2013;22:37–47. [DOI] [PubMed] [Google Scholar]
- 19.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 20.Wasay M, Bakshi R, Bobustuc G, et al. Cerebral venous thrombosis: analysis of a multicenter cohort from the United States. J Stroke Cerebrovasc Dis 2008;17:49–54. [DOI] [PubMed] [Google Scholar]
- 21.Qureshi AI, Chaudhry SA, Hassan AE, et al. Thrombolytic treatment of patients with acute ischemic stroke related to underlying arterial dissection in the United States. Arch Neurol 2011;68:1536–1542. [DOI] [PubMed] [Google Scholar]
- 22.Morris NA, Merkler AE, Gialdini G, Kamel H. Timing of incident stroke risk after cervical artery dissection presenting without ischemia. Stroke 2017;48:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigakis MJG, Leffert LR, Mirzakhani H, et al. The validity of discharge billing codes reflecting severe maternal morbidity. Anesth Analgesia 2016;123:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall SW. Power for tests of interaction: effect of raising the type I error rate. Epidemiol Perspect Innov 2007;4:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvin S. Statistical Analysis of Epidemiological Data. 2nd ed. New York: Oxford University Press; 1996:213–214. [Google Scholar]
- 26.Meher S, Duley L, Hunter K, Askie L. Antiplatelet therapy before or after 16 weeks' gestation for preventing preeclampsia: an individual participant data meta-analysis. Am J Obstet Gynecol 2017;216:121–128.e122. [DOI] [PubMed] [Google Scholar]
- 27.American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy: report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 28.Cadavid AP. Aspirin: the mechanism of action revisited in the context of pregnancy complications. Front Immunol 2017;8:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed A, Rezai H, Broadway-Stringer S. Evidence-based revised view of the pathophysiology of preeclampsia. Adv Exp Med Biol 2016;956:355–374. [DOI] [PubMed] [Google Scholar]
- 30.George EM, Granger JP. Mechanisms and potential therapies for preeclampsia. Curr Hypertens Rep 2011;13:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng SB, Sharma S. Preeclampsia and health risks later in life: an immunological link. Semin Immunopathol 2016;38:699–708. [DOI] [PubMed] [Google Scholar]
- 32.Esenwa CC, Elkind MSV. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol 2016;12:594–604. [DOI] [PubMed] [Google Scholar]
- 33.Ros HS, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S. Pulmonary embolism and stroke in relation to pregnancy: how can high-risk women be identified? Am J Obstet Gynecol 2002;186:198–203. [DOI] [PubMed] [Google Scholar]
- 34.Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy-related stroke: frequency, trends, risk factors, and outcomes. Obstet Gynecol 2015;125:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammer ES, Cipolla MJ. Cerebrovascular dysfunction in preeclamptic pregnancies. Curr Hypertens Rep 2015;17:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craici I, Wagner S, Garovic VD. Review: preeclampsia and future cardiovascular risk: formal risk factor or failed stress test? Ther Adv Cardiovasc Dis 2008;2:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garovic VD, August P. Preeclampsia and the future risk of hypertension: the pregnant evidence. Curr Hypertens Rep 2013;15:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeh JS, Cheng HM, Hsu PF, et al. Synergistic effect of gestational hypertension and postpartum incident hypertension on cardiovascular health: a nationwide population study. J Am Heart Assoc 2014;3:e001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esteve-Valverde E, Ferrer-Oliveras R, Gil-Aliberas N, Baraldes-Farre A, Llurba E, Alijotas-Reig J. Pravastatin for preventing and treating preeclampsia: a systematic review. Obstet Gynecol Surv 2018;73:40–55. [DOI] [PubMed] [Google Scholar]
- 40.Synhaeve NE, Arntz RM, van Alebeek ME, et al. Women have a poorer very long-term functional outcome after stroke among adults aged 18-50 years: the FUTURE study. J Neurol 2016;263:1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Leading causes of death in females, United States, 2015 (current listing). Available at: cdc.gov/women/lcod/2015/index.htm. Accessed August 29, 2018.
- 42.Gall SL, Tran PL, Martin K, Blizzard L, Srikanth V. Sex differences in long-term outcomes after stroke: functional outcomes, handicap, and quality of life. Stroke 2012;43:1982–1987. [DOI] [PubMed] [Google Scholar]
- 43.Whitson HE, Landerman LR, Newman AB, Fried LP, Pieper CF, Cohen HJ. Chronic medical conditions and the sex-based disparity in disability: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 2010;65:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young B, Hacker MR, Rana S. Physicians' knowledge of future vascular disease in women with preeclampsia. Hypertens Pregnancy 2012;31:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American College of Cardiology/American Heart Association. ASCVD risk calculator. Available at: static.heart.org/riskcalc/app/index.html#!/baseline-risk. Accessed March 21, 2018.
- 46.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 2009;113:1299–1306. [DOI] [PubMed] [Google Scholar]
- 47.Roberts CL, Bell JC, Ford JB, Hadfield RM, Algert CS, Morris JM. The accuracy of reporting of the hypertensive disorders of pregnancy in population health data. Hypertens Pregnancy 2009;27:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barton JR, O'Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: progression and outcome. Am J Obstet Gynecol 2001;184:979–983. [DOI] [PubMed] [Google Scholar]
- 49.Geller SE, Ahmed S, Brown ML, Cox SM, Rosenberg D, Kilpatrick SJ. International Classification of Diseases-9th revision coding for preeclampsia: how accurate is it? Am J Obstet Gynecol 2004;190:1629–1634. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy 2003;22:203–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data from the CTS are housed in an online secure data warehouse maintained by the City of Hope in collaboration with the Supercomputing Center of the University of California, San Diego. Data and statistical code used for the current analysis are available to other investigators on request to the CTS.






