Abstract
Over the last few decades, the improved diagnostic criteria, the wide use of MRI, and the growing availability of effective pharmacologic treatments have led to substantial advances in the management of multiple sclerosis (MS). The importance of early diagnosis and treatment is now well-established, but there is still no consensus on how to define and monitor response to MS treatments. In particular, the clinical relevance of the detection of minimal MRI activity is controversial and recommendations on how to define and monitor treatment response are warranted. An expert panel of the Magnetic Resonance Imaging in MS Study Group analyzed and discussed published studies on treatment response in MS. The evolving concept of no evidence of disease activity and its effect on predicting long-term prognosis was examined, including the option of defining a more realistic target for daily clinical practice: minimal evidence of disease activity. Advantages and disadvantages associated with the use of MRI activity alone and quantitative scoring systems combining on-treatment clinical relapses and MRI active lesions to detect treatment response in the real-world setting were also discussed. While most published studies on this topic involved patients treated with interferon-β, special attention was given to more recent studies providing evidence based on treatment with other and more efficacious oral and injectable drugs. Finally, the panel identified future directions to pursue in this research field.
With the progress of therapeutics, we have learned that the natural history of multiple sclerosis (MS) can be modified by treatment and that beginning treatment early in the disease course results in better medium-term clinical outcomes than delaying treatment. Given the rapidly expanding range of treatments available for relapsing-remitting MS (RRMS), it would be of crucial importance to predict the individual patient's response in order to make optimal therapeutic decisions. However, we are still far from precision medicine in the MS field, since at present no predictive and easily reproducible biomarkers have been found beyond clinical assessment, conventional MRI measures, and neutralizing antibodies to some drugs. Furthermore, we have to consider that (1) there is no shared definition for treatment response in MS; (2) there is still much debate about which markers (clinical relapses, confirmed disability worsening, MRI activity, or a combination of all) should be used to predict treatment response upon implementation of a specific disease-modifying drug; (3) the vast majority of studies on this topic were conducted on interferon-β (IFN-β)–treated patients, thus raising the question of whether we can use the same tools to predict response across different treatments.
Despite these difficulties, the early prediction of treatment response is a central goal in the field of MS research. In this review, recommendations on how MRI should be used to define and monitor treatment response are given and knowledge gaps to be filled in this research field are discussed on the basis of the published evidence and expert opinion.
Methodologic issues
An international workshop was held in Rome, Italy, in September 2015 under the auspices of MAGNIMS (Magnetic Resonance Imaging in MS), an intellectually independent network of European clinical research groups that have an interest in the use of MRI to study patients with MS (magnims.eu). The panel convened to the workshop was composed of experts in the diagnosis and management of MS, and included neurologists, neuroradiologists, statisticians, and physicists from MAGNIMS-affiliated institutions.
The panel met to present and discuss data from published research studies and to consider the recommendations contained in previous articles related to the management of patients with MS under pharmacologic treatment. After the meeting, the panel continued to discuss the latest evidence in this field, in order to set out specific and fully updated recommendations, and the relevant publications on the topic were reviewed (until December 2017). The first draft of the manuscript was written by the principal author and was based on contributions from each panelist. The draft was then circulated to all members, who iteratively modified the document until a final consensus was reached.
Data availability
E-references are available from MAGNIMS (magnims.eu/?protected-download=16119).
Definition of treatment response
The concepts of response and nonresponse, as well as the time frame during which this is assessed, are widely mixed in the literature. Nonresponse to a treatment is defined on the basis of 3 outcomes or their combination: worsening of disability, incidence of relapses, and presence of active MRI lesions (defined as new/enlarged T2 lesions or gadolinium-enhanced lesions).
In many studies based on a short/medium-term follow-up period, the definition of a nonresponse has been based on a 1.0-point increase in the Expanded Disability Status Scale (EDSS) confirmed at 6 months,1 independently of the duration of clinical follow-up (usually ranging from 1 to 6 years). In longer-term studies (15–16 years), nonresponders were defined on the basis of a net increase in the EDSS,2 the milestone of reaching EDSS ≥6.0, and a transition to the secondary progressive (SP) phase.3 Other definitions of nonresponse proposed in the literature encompass the combination of EDSS worsening or relapses,1,4,5 an increased relapse rate as compared to the pretreatment relapse frequency,6,7 the presence of active MRI lesions during treatment,8,9 and switching from one drug to another.10,11
No evidence vs minimal evidence of disease activity
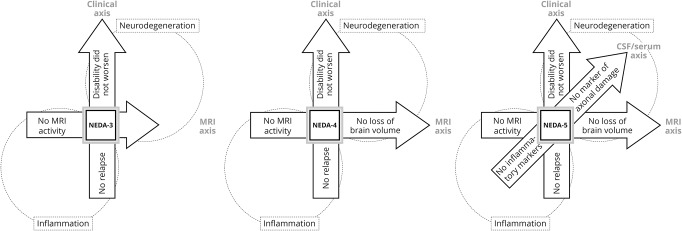
With increasingly effective therapies, no evidence of disease activity (NEDA) has gathered increasing consideration as a treatment goal. NEDA is a composite score based on the zero tolerance concept entailing the absence of relapses, no confirmed EDSS worsening, and no active lesions on annual brain MRI scans.12 This latter definition, also so-called NEDA-3, is mainly driven by focal inflammatory activity rather than neurodegenerative processes. Therefore, recently, it has been proposed to also include brain atrophy (NEDA-4) (figure 1) based on a meta-analysis13 demonstrating that both focal T2 lesions and an annual brain volume loss >0.4% during treatment explained 75% of the variance of disability worsening over 2 on-treatment years, and that their combination is better than either metric alone.14 CSF or serum biomarkers (neurofilament light chain as a specific marker of neuroaxonal integrity seems particularly promising) might also help in assessing the persistence of subtle inflammation and insidious neurodegenerative process.15
Figure 1. Definition of the concept of no evidence of disease activity (NEDA).
Other metrics assessing cognitive status, neurophysiologic features, retinal structure, patient-reported outcomes (including quality of life), as well as spinal cord imaging, could also be incorporated into the NEDA definition in the future to provide a composite endpoint for better assessing treatment efficacy in clinical trials. On the other hand, all the aforementioned assessments are currently not applicable routinely in daily clinical practice. As a consequence, NEDA-3 appears to be the only goal we are able to assess in a real-world setting at present, but we must take into account that the achievement of a NEDA-3 status does not preclude cognitive deterioration, neurodegeneration, or disease progression. Moreover, we must also consider that NEDA-3 is MRI-biased, since MRI lesions are more frequent than clinical attacks.
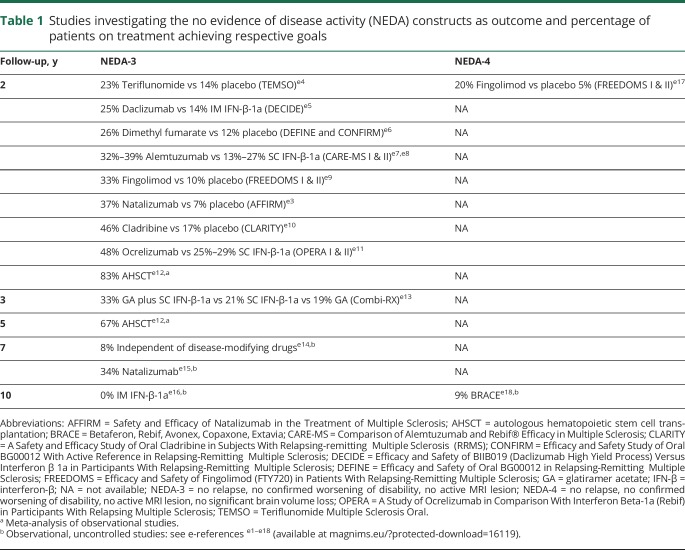
From a more practical point of view, the most important question concerns the persistence of NEDA over time and its accuracy for predicting long-term prognosis.16–18 Preliminary data suggest that NEDA is difficult to sustain over the long term with platform therapies (from 0% to less than 10% over a time period of 7–10 years). Indeed, longer-term disease remission (about 30%–40% over a time period of 5–7 years) has been instead described with monoclonal antibodies or after autologous hematopoietic stem cell transplantation (67% at 5 years), but with a greater burden in terms of surveillance and adverse events (table 1).e1–e18 It must be stressed, however, that comparisons of independent studies conducted on different treatment populations should be interpreted with caution. Since NEDA-3 might be considered a too stringent treatment goal, tolerance of a minimal evidence of disease activity (MEDA) may represent a more realistic goal to strive for, without exposing patients at risk of long-term poor outcomes. However, only a few studies explored the predictive value of early NEDA and MEDA in identifying the IFN-β-treated patients who will attain clinically meaningful disability outcomes in the long-term period. In one study,18 MEDA was defined as experiencing either 1 relapse with <3 new T2 lesions or <2 gadolinium-enhancing lesions during the first year of treatment. A minimal degree of activity did not seem predictive of EDSS worsening over a 7-year follow-up. More recently, Prosperini et al.19 provided real-world data showing that the adopted definition of MEDA affected the long-term risk of reaching the disability milestone of EDSS 6.0 in 1,195 patients treated with IFN-β or glatiramer acetate (GA). A definition of MEDA based upon <2 gadolinium-enhancing lesions and <3 new T2 lesions did not imply an increased risk, whereas the occurrence of even a single relapse during the first treatment year was associated with long-term poor outcome.
Table 1.
Studies investigating the no evidence of disease activity (NEDA) constructs as outcome and percentage of patients on treatment achieving respective goals
Indicators of treatment response
There is confusion in the literature on the properties of baseline factors distinguishing prognostic markers from markers of response to treatment. A prognostic factor is a baseline measure that is associated with a subsequent outcome (such as death, relapse, or disability progression). The definition of a prognostic factor is independent from treatments and prognostic markers assessment can be done with or without a control group. A treatment effect modifier is a baseline factor that is associated with a higher or lower treatment effect. An effect modifier can be defined only in relation to a specific treatment and the assessment of a treatment effect modifier must be done in presence of a control group, by an interaction analysis.
Demographic, clinical, and MRI markers at baseline that can be considered treatment effect modifiers have been assessed in a recent meta-analysis of subgroup analyses of randomized trials performed in patients with RRMS, showing that a higher treatment benefit is associated with baseline characteristics of earlier (younger age and lower EDSS) and more active disease on MRI (higher gadolinium enhancement activity).20 Earlier observational studies6,7,21 and more recent large studies from the MSBase Study Group22,23 explored which demographic and clinical characteristics, considered at treatment start, were associated with prognosis over short-term follow-up periods. Modifiers of treatment response comprised age, disease duration, disease course, previous relapse activity, disability, predominant relapse phenotype, and previous therapy, but the magnitude and direction of these associations are highly variable across different therapies and are affected by the considered outcome (relapse, disability worsening, or regression).23 Although these baseline indicators were often described as predictors, they should be considered merely as prognostic indicators since they were analyzed without a control group of nontreated patients.20
A dynamic scenario is necessary to talk about surrogate markers: variables that can be detected earlier after treatment initiation than the final outcome and that mediate the effect of the treatment on such an outcome. In other words, the treatment effect on the surrogate marker should predict the treatment effect on the final clinical outcome of interest.
According to different studies, the treatment effect on relapses in the first treatment year predicted ∼62% and ∼80% of the treatment effect on the 2-year disability worsening in IFN-β- and natalizumab-treated patients, respectively.24,25 In analogy, the treatment effect on new T2 lesions in the first treatment year predicted ∼60% of the treatment effect on relapses26 and ∼57% of the treatment effect on the 2-year disability worsening.27 A combined measure of 1-year changes in MRI lesions and relapses predicted 100% of the treatment effect on 2-year disability worsening in IFN-β-treated patients.24 The combined treatment effect on relapses and yearly percentage brain volume change over 2 years resulted to predict ∼73% of treatment effect on disability worsening in fingolimod-treated patients.28
Monitoring treatment response clinically
Relapses are the main feature of RRMS and it is well-established that relapse rate in the first year of treatment appears to be correlated with short-term disability.
The pivotal trial of intramuscular IFN-β-1a demonstrated that patients with ≥2 relapses during the first 2 treatment years had a higher risk of being in the worst EDSS quartile at 15 years (odds ratio 4.44; p = 0.01).3 Interestingly, experiencing ≥2 relapses in patients treated with placebo was not associated with this poor outcome. This latter observation raised the hypothesis that those who present persistent inflammatory activity early on IFN-β treatment represent a pathogenetically distinct subset of patients whose fingerprint is an exaggerated molecular response to IFN-β.29 Similarly, another long-term study of patients included in the pivotal trial on subcutaneous IFN-β-1b showed that changes in EDSS (p < 0.001) and relapse rate (p = 0.025) during the study (0–2 years) were associated with a higher risk of reaching an EDSS score of 6.0 or transitioning to SPMS after 16 years.3 Again, in a cohort of patients who began IFN-β therapy between 1995 and 2001 in Barcelona, the presence of relapses or EDSS worsening during the first 2 treatment years was associated with an increased risk of developing SPMS, attaining an EDSS score of 7.5, or exhibiting an increase of ≥5 EDSS steps after 12 years.18 However, the presence of an isolated relapse without changes in EDSS during the first 2 treatment years did not significantly increase the risk of developing marked long-term disability, supporting an earlier postmarketing study that pointed out the low accuracy of clinical relapses in predicting accumulation of disability over a median time period of 5 years.21 Overall, clinical activity measures at 1 or 2 years had moderate to good specificity and low to moderate sensitivity to this outcome.18,30 Other recent studies have also confirmed that clinical activity, defined as EDSS change or relapses during the first years of IFN-β treatment, had a very negative effect on long-term prognosis.17,22
It is clear, therefore, that the presence of clinical and MRI activity in the short term is predictive of negative prognosis and vice versa. There are, however, some concerns when only relapses are adopted to predict treatment response:
Relapse rate is influenced by both a regression to the mean phenomenon (due to inclusion of active patients) and a general shift in trials to milder MS populations (in an era with increasingly effective drugs).
Relapses can be affected by recall bias from patients and observer bias from clinicians.
Excluding pseudoexacerbations is not always possible.
Waiting for a relapse may expose patients to risk of accumulating disability.
Severity and recovery from relapses usually is not considered.
Monitoring treatment response with MRI
Conventional MRI (i.e., T2-weighted and contrast-enhanced T1-weighted sequences) represents a powerful tool to monitor disease activity in MS. Active lesions on MRI are 5–10 times more frequent than clinical relapses, especially in patients with RRMS. Therefore, the persistence of the inflammatory component of the disease, such as gadolinium-enhancing lesions or new/enlarged T2-hyperintense lesions, suggests a partial or even absent biological effect of treatment. However, T1-hypointense lesions (black holes), representing areas of chronic axonal loss more strictly correlated with current disability, were considered hardly ever and thereby there are limited data on the treatment effect on this MRI marker.31
A post hoc analysis of the original trial on intramuscular IFN-β-1a found poor disability outcome associated with the occurrence of ≥2 on-study relapses in both active and placebo groups, whereas accumulation of >2 new/enlarged T2 lesions was associated with poor disability outcome in the active group, but not in the placebo group.32 This latter observation supports the greater sensitivity of MRI measures over clinical features in determining the responsiveness of IFN-β treatment.
Post hoc analyses of clinical trials on IFN-β-1a and -1b clearly showed that the treatment effect on new T2 lesions in the first treatment year accounted for a relevant proportion of treatment effect on relapses (∼70%–80%) and disability worsening (∼60%) over the subsequent year.24,26,27
Postmarketing MRI-based studies have suggested a clinically relevant association between the occurrence of MRI activity after 6–12 months of treatment and poorer outcomes over the medium-term period. This relationship survives even in absence of early clinical activity (i.e., in the first 6–12 months of treatment) and irrespective of the adopted definition of treatment response (e.g., occurrence of relapses or confirmed EDSS worsening).4,30,33 However, one can argue that—in the absence of a comparison group—the detection of MRI activity may reflect a poor disease prognosis rather than a lack of treatment response.
Two studies described a dose–effect relationship between new MRI lesions and the risk of future relapses34 or accumulation of disability.30 Active MRI lesions in functionally eloquent areas, such as brainstem and spinal cord, were related with an increased risk of future EDSS worsening.34 Such infratentorial (especially brainstem) or spinal cord damage is closely associated with clinical disability, independently from brain atrophy and other MRI metrics.35,36
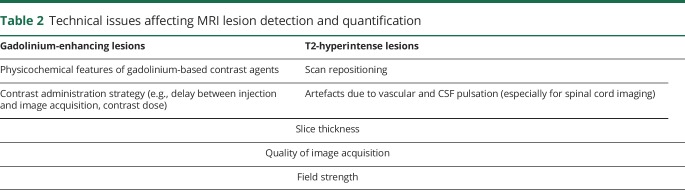
There is controversy about what degree or threshold of MRI activity should be accepted before defining a patient as a treatment nonresponder. Published studies suggest different cutoff of MRI lesions (in some cases even just 1 or 2 new lesions), and differently weighted the risk of treatment failure if the new lesion was gadolinium-enhancing or not.5,30,33,34,37 These discrepancies across studies are mainly due to technical issues in lesion detection and quantification (table 2):
Accurate detection and counting of new/enlarged T2 lesions is closely dependent on technical factors such as scan repositioning, slice thickness, and quality of image acquisition.38
Detection and counting of gadolinium-enhancing lesions is easier and more reliable, but not sufficiently sensitive, since an overt breakdown of the blood–brain barrier (BBB) is typically short-lived (few weeks) and occurs almost exclusively in newly acute lesions, while a low-grade BBB disruption occurs also in chronic active (nonenhancing) lesions.
Detection and counting of gadolinium-enhancing lesions also depends on MRI acquisition protocol, contrast administration strategy (e.g., delay between injection and image acquisition, contrast dose), and physicochemical features of gadolinium-based contrast agents (brain deposition of gadolinium-based contrast agents in patients who had repeated administrations39 should be also taken into account in the future selection of contrast agents for safety reasons).
Active lesion assessment is usually performed visually, resulting in almost perfect reliability for gadolinium-enhancing lesions, but moderate to substantial reliability for new T2 lesions and low reliability for enlarging T2 lesions.40
Residual disease activity can still occur in the first few months of treatment, due to a delay in the treatment response; thus, the timing of the baseline MRI should be based on the pharmacodynamics of the treatment concerned, i.e., after a sufficient time frame allowing the drug to start working, usually 3–6 months. For example, the maximal effect on MRI activity was found to start after 3 months for dimethyl fumarate and 7 months for GA.41,42 However, the kinetics of the onset of action and the time for reaching the full benefit are not known precisely for any drug.
Spinal cord lesions are often neglected. Although many spinal cord lesions are clinically symptomatic, new spinal cord lesions may occur even in the absence of clinical signs or symptoms, and without subclinical brain MRI activity.43 Spinal cord lesions may be more strongly correlated with future disability accrual even while on treatment.34 However, image artefacts due to vascular and CSF pulsation during spinal cord MRI acquisition make spinal cord MRI difficult to standardize. As a consequence, the use of spinal cord imaging for assessing treatment response seems rather limited.38
Table 2.
Technical issues affecting MRI lesion detection and quantification
Scoring treatment response
The first attempt of combining clinical and MRI activity measures to predict treatment response was made by the Canadian MS Working Group (CMSWG) that provided the Treatment Optimization Recommendations (TOR) in 2004.44 The CMSWG presented an analogic model, derived from expert consensus, based on different levels of concern about the occurrence of disability worsening, relapses, and MRI activity during treatment. These levels of concern were classified as low, medium, and high. Although the TOR framework did not provide quantitative rules, when applied to Prevention of Relapses and disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis (PRISMS) data (using relapse and disability worsening only), 89% of patients classified as having a medium or high level of concern after 1 year of treatment went on to develop further breakthrough disease over the next 2–4 years.45 More recently, the CMSWG proposed an updated framework, suggesting that a change in treatment might be considered if there is a high level of concern in any one domain (relapses, progression, or MRI activity), a medium level of concern in any 2 domains, or a low level of concern in all 3 domains.46
Río et al.37 analyzed 222 patients treated with one IFN-β formulation for ≥1 year, combining clinical relapses, disability worsening (as measured by a 1-point EDSS increase confirmed at 6 months), and active MRI (defined as >2 new T2-hyperintense or gadolinium-enhancing lesions). Patients who were positive for at least 2 of the 3 criteria analyzed after the first year of treatment were found to have a higher probability of having a new relapse or disability increase in the subsequent 2 years. These individuals would, therefore, be strong candidates for a treatment switch (although this has not been tested in a randomized trial). Of note, the isolated presence of relapses or MRI activity during year 1 did not predict the risk of new clinical activity or disease progression in the ensuing 2 years. In addition, an increase in disability alone during the first treatment year was a poor predictor of subsequent disability worsening.
After this publication, Sormani and coworkers47 simplified the assessment proposed by Río et al.37 through a scoring system (the so-called Modified Río Score) based on only new T2 lesions and relapses as components. The rationale for using only these 2 markers came from evidence that the effect of IFN-β on the combination of MRI activity and relapses in the first year accounts for almost 100% of treatment effect on disability worsening at 2 years.24 Cutoff values for the number of relapses and the number of new T2 lesions counted over the first treatment year were established by statistical modeling of data provided by the active arms of the PRISMS trial (training set).47 The results of this analysis were validated using the real-world dataset that was used for developing the original Rio Score (validation set). The Modified Río Score stratified patients into 3 risk groups according to the probability of disability worsening: low (24%), medium (33%), and high risk (65%), with excellent specificity (97%) and very low sensitivity (24%) in discriminating treatment response. The Modified Rio Score was further refined to encompass a new clinical visit and a new brain MRI at 6 months (for identifying relapses or the presence of ≥2 new T2 lesions) after the first treatment year, in order to allow a better classification of patients in the medium risk group, who were the most difficult to classify in terms of future treatment response and planning.48
One criticism of the Modified Rio Score is the somewhat higher threshold of MRI activity (>4 new T2 lesions) required to define substantial MRI activity that translates into a low sensitivity to detect treatment response. The latter likely reflects the disease activity as assessed on an old dataset such as the PRISMS cohort (used as the training set), especially if compared with more recent trial cohorts and real-world populations.
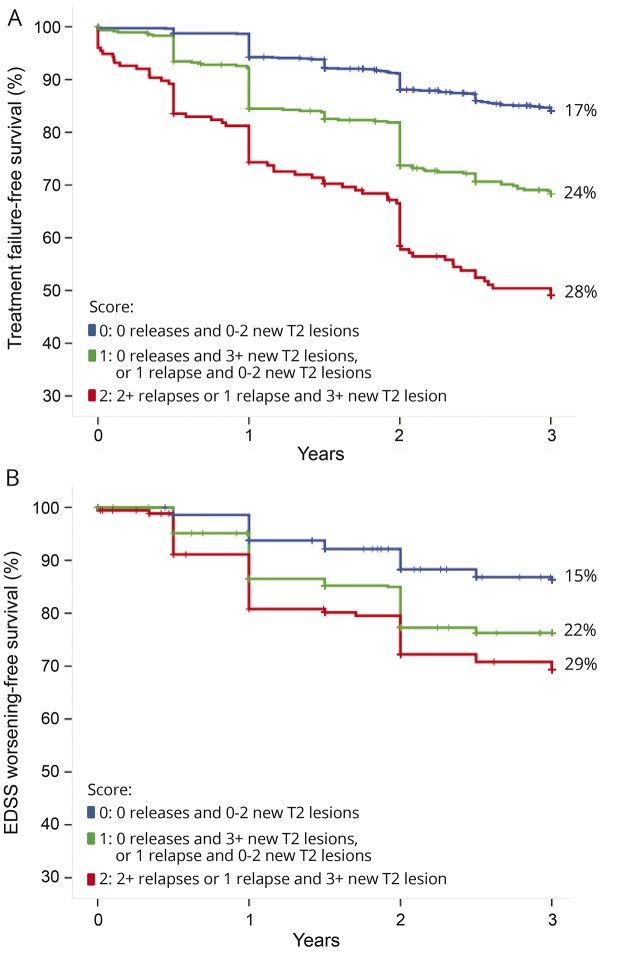
Subsequently, a larger and more recent multicenter dataset was collected within the MAGNIMS Study Group to explore whether minimal MRI activity can be accepted or substantial MRI activity (eventually in combination with clinical relapses) should alert for a suboptimal treatment response.11 The presence of relapses during the first IFN-β year was the main predictor of the risk of treatment failure over the subsequent 3 years. Moreover, the presence of substantial MRI activity, here defined by ≥3 new T2 lesions, increased the ability to predict treatment failure (figure 2). The study concludes that substantial MRI activity during the first year of treatment with IFN-β, particularly if in combination with clinical relapses, indicates significant risk of treatment failure and EDSS worsening in the short term.
Figure 2. Treatment failure–free survival and disability worsening–free survival.
Treatment failure–free survival (defined as switch to a second-line treatment for lack of efficacy) (A) and disability worsening–free survival (defined as an at least 1-point Expanded Disability Status Scale [EDSS] increase) (B) over 3 years, according to the different combinations of new T2 lesions and relapses during the first year of therapy grouped in a 3-level score, in a cohort of 1,280 patients treated with interferon-β (modified from Sormani et al.11).
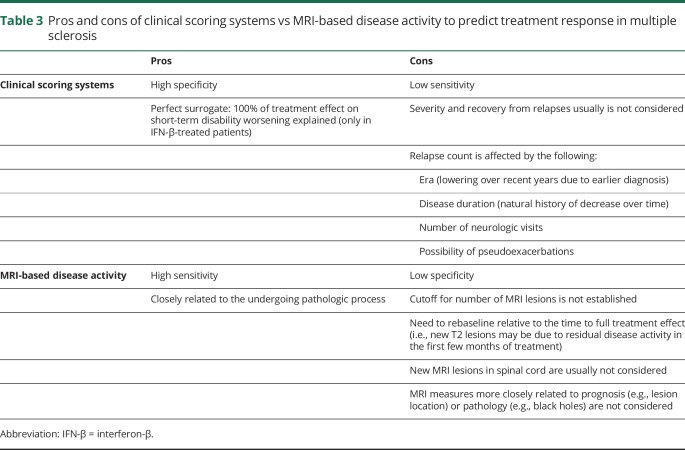
Table 3 summarizes the advantages and disadvantages associated with the use of MRI activity alone and quantitative scoring systems.
Table 3.
Pros and cons of clinical scoring systems vs MRI-based disease activity to predict treatment response in multiple sclerosis
Assessing treatment response to drugs other than IFN-β
One important weakness when evaluating tools for assessing treatment response is that the vast majority of studies are based upon patients on IFN-β treatment, with limited data from cohorts of patients treated with other drugs.
In 2014, Río et al.49 evaluated if their previous scoring system used to assess response to IFN-β could also be applied to GA. Patients were classified as active (clinical and MRI activity), inactive (none), and partially active (clinical or MRI activity) after the first treatment year. Patients classified as active during the first year were at an increased risk of continued clinical activity over the next 2 years. Interestingly, while the prognosis of active patients on GA was similar to that reported in patients treated with IFN-β, the partially active group showed a different clinical evolution. Patients with isolated clinical activity had a significant risk of new activity in the next 2 years. By contrast, patients with isolated MRI activity during the first year of treatment did not show a significant risk of future disease activity. These findings can be explained by the different mechanisms of action of the 2 drugs. While IFN-β rapidly inhibits BBB leakage and gadolinium enhancement within 2 weeks,50 GA shows a slower rate of BBB stabilization and a less dramatic resolution of gadolinium-enhancing activity.51 Consequently, MRI measures used to assess treatment response in patients receiving IFN-β are not necessarily applicable to patients with GA.
Attempts to define treatment response to the newer MS drugs fingolimod and teriflunomide have been made recently. Boster et al.52 investigated whether MRI activity or relapses during the first 12 months of treatment with fingolimod (M0–M12) predicted disease activity over the following 36 months on treatment in the pooled extension populations of the Efficacy and Safety of Fingolimod (FTY720) in Patients With Relapsing-Remitting Multiple Sclerosis (FREEDOMS) and FREEDOMS II trials. They found that either relapses or MRI activity or their combination effectively predicted future relapses and disability worsening.
Sormani et al.53 confirmed that ≥1 relapses during the first year of fingolimod increased the risk of confirmed disability worsening over 4 years on treatment in patients in the FREEDOMS and FREEDOMS II trials. To investigate the added value of MRI, patients who were relapse-free during the first treatment year were analyzed separately. Persistent lesion formation (defined as presence of new T2 lesions in both the first and second 6 months of treatment) represented a better predictor than the crude number of new T2 lesions in identifying patients at higher risk of future disability. Moreover, the combination of 1-year persistent MRI lesion activity and substantial brain atrophy (defined here as a percentage brain volume loss >0.8%) was associated with a higher risk of disability worsening compared with no persistent lesion formation and percentage brain volume loss ≤0.8%.
The risk of disability worsening with teriflunomide was investigated in the Teriflunomide Multiple Sclerosis Oral (TEMSO) core study and extension population54 using the score previously developed by the MAGNIMS Study Group, based on the occurrence of relapses (0 to ≥2) and new/enlarging T2 lesions (<3 or ≥3).11 After the first year of teriflunomide treatment, patients were classified into risk score categories as follows: 0 = low, 1 = intermediate, either 2 or 3 = high. The risk of confirmed disability worsening over the 7-year follow-up period was significantly higher for patients in categories 2 or 3 than in those scoring 0 (hazard ratio [HR] 1.96, p = 0.004). At 18-month follow-up, patients in the intermediate-risk group (26% of the whole population) were reclassified as responders (0 relapses or <2 new T2 lesions) or nonresponders (≥1 relapses or ≥2 new T2 lesions). Patients reclassified as nonresponders had a higher risk of disability worsening than those reclassified as responders (HR 1.92, p < 0.001).
Suggestions for changing treatment when using injectable therapies and, to a certain degree, oral licensed drugs, are probably different from those of more effective drugs, such as natalizumab and alemtuzumab. While treatment failure was often associated with early disease activity on IFN-β or GA,8 carryover disease activity was described in a subgroup of natalizumab-treated patients who experienced MRI activity in the first treatment year without subsequent relapses or EDSS worsening,55 especially in case of higher baseline MRI activity.56 On the other hand, any new lesions beyond 18 months of natalizumab therapy should be considered as a suspected adverse event of progressive multifocal leukoencephalopathy.38 Disease activity occurring after immune cell-depletive agents, such as alemtuzumab or cladribine, can be contemplated as an indication to retreat the patient.57
Recommendations and future directions
Future clinical trials should adopt NEDA as composite endpoint to evaluate treatment efficacy, since it is more sensitive than traditional clinical measures (relapse rates and disability worsening) and requires fewer patients especially for head-to-head study design.
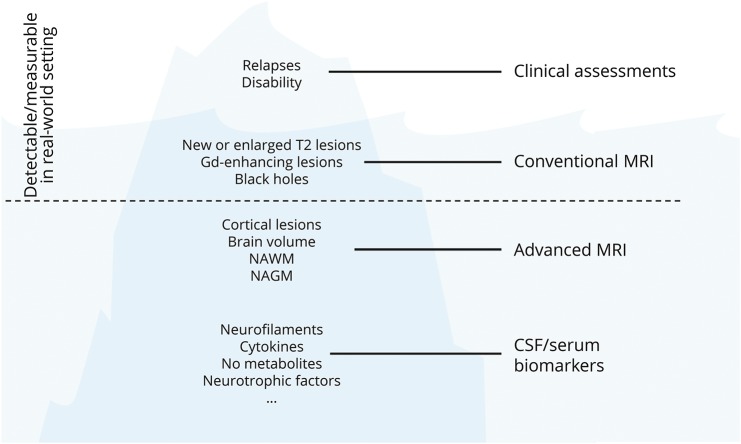
Many other phenomena could be incorporated into the NEDA endpoint, with the ultimate goal of suppressing all aspects of disease activity and progression (figure 3).
In the real-world setting, NEDA currently seems to be an unrealistic treatment goal over the long term, thereby identifying a need to define a MEDA that can be tolerated before treatment switching.
The residual disease activity carrying over during the first few months of treatment should be always taken into account when using MRI to monitor effects of treatment. The residual disease activity could affect the assessment of NEDA, which should therefore account for the time frame allowing the drug to reach efficacy (usually 3–6 months).
Future investigation is warranted to better elucidate if drugs with different mechanisms of action share (or not) the same predictors of treatment response.
Although methods for precise quantitative analyses of MRI lesion already exist and have been used in clinical trials for many years, strong efforts are being made to develop automated quantitative lesion segmentation by dedicated software with the aim of increasing accuracy in lesion detection while decreasing time of analyses.58–60 Application of automated MRI subtraction can improve interpretation of serial imaging, but this approach cannot be incorporated into daily clinical practice until validation studies are performed and technical improvements are achieved.
Figure 3. The iceberg of multiple sclerosis phenomena.
Gd = gadolinium; NAGM = normal-appearing grey matter; NAWM = normal-appearing white matter; NO = nitric oxide.
The MAGNIMS Study Group discussed how to define and monitor response to MS treatments, and concluded that there is no shared consensus on definition of treatment response. While NEDA may represent a future endpoint in the experimental setting, its attainability in the real world is controversial. Clinical and MRI activity in isolation may be not sufficient to determine treatment response, whereas the combination of these measures using composite scores appears preferable. Although this article is based on the most recent literature data, periodic updates are needed in the light of the near introduction of newer drugs active against MS and technological innovations in MRI lesion detection and counting.
Acknowledgment
T.A.Y., O.C., and F.B. are supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Glossary
- BBB
blood–brain barrier
- CMSWG
Canadian MS Working Group
- EDSS
Expanded Disability Status Scale
- FREEDOMS
Efficacy and Safety of Fingolimod (FTY720) in Patients With Relapsing-Remitting Multiple Sclerosis
- GA
glatiramer acetate
- HR
hazard ratio
- IFN-β
interferon-β
- MEDA
minimal evidence of disease activity
- MS
multiple sclerosis
- NEDA
no evidence of disease activity
- PRISMS
Prevention of Relapses and disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis
- RRMS
relapsing-remitting multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
- TOR
Treatment Optimization Recommendations
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
C. Gasperini: fees as invited speaker or travel expenses for attending meeting from Biogen, Merck-Serono, Teva, Sanofi, Novartis, and Genzyme. L. Prosperini: consulting fees from Biogen, Novartis, and Roche; speaking honoraria from Almirall, Biogen, Genzyme, Merck Serono, Novartis, and Teva; travel grants from Biogen, Genzyme, Novartis, and Teva; research grants from the Italian MS Society (Associazione Italiana Sclerosi Multipla) and Genzyme. M. Tintore: support for scientific meetings and honorariums for advisory work from Almirall, Bayer, Biogen, Genzyme, Merck Serono, Novartis, Sanofi-Aventis, Roche, and Teva; co-editor of MSJ-ETC. M. Sormani: personal compensation for consulting services and for speaking activities from Genzyme, Merck Serono, Teva, Synthon, Roche, Novartis, and Biogen. M. Filippi: Editor-in-Chief of the Journal of Neurology; compensation for consulting services and/or speaking activities from Biogen Idec, Merck-Serono, Novartis, and Teva Pharmaceutical Industries; research support from Biogen Idec, Teva Pharmaceutical Industries, Novartis, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, Cure PSP, Alzheimer's Drug Discovery Foundation (ADDF), the Jacques and Gloria Gossweiler Foundation (Switzerland), and ARiSLA (Fondazione Italiana di Ricerca per la SLA). J. Rio: speaking honoraria and personal compensation for participating in advisory boards from Almirall, Bayer-Schering Healthcare, Biogen-Idec, Genzyme, Merck-Serono, Novartis, Teva, and Sanofi-Aventis. J. Palace: funding for highly specialized services to run a national congenital myasthenia service and a neuromyelitis service; support for scientific meetings and honorariums for advisory work from Merck Serono, Biogen Idec, Novartis, Teva, Abide, Chugai Pharma, Alexion, MedDay, Argenx, Bayer Schering, and Medimmune, and unrestricted grants from Merck Serono, Novartis, Biogen Idec, Chugai, Alexion, and Bayer Schering; MS Society and Guthy-Jackson Foundation research grants. M. Rocca: speaking honoraria from Biogen Idec, Novartis, Teva Neurosciences, Genzyme, Merck Serono, and Roche; research support from the Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla. O. Ciccarelli: support by the National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre (BRC); consulting fees from Roche, Teva, Sanofi-Genzyme, Novartis, and Biogen. F. Barkhof: support by the NIHR UCLH BRC; consultancy fees from Bayer, Roche, Merck, Sanofi-Genzyme, Teva, IXICO, Biogen, GeNeuro, Apitope, and Novartis, either as central reader or by serving on steering committees, advisory boards, and data safety monitoring committees. J. Sastre-Garriga: compensation for participating on advisory boards, speaking honoraria, and travel expenses for scientific meetings, consulting services, or research support from Celgene, Novartis, Biogen, Teva, Merck, Almirall, and Genzyme. H. Vrenken: research support from Merck Serono, Novartis, Pfizer, and Teva; speaker honoraria from Novartis; all funds were paid directly to his institution. J. Frederiksen: funding for scientific advisory boards and for travel related to these activities as well as honoraria from Biogen Idec, Merck Serono, Sanofi-Aventis, Teva, Novartis, Genzyme, and Almirall; speaking honoraria from Santhera, Biogen Idec, Merck Serono, Teva, and Novartis; advisor on preclinical development for Takeda. T. Yousry: research support from Biogen, GlaxoSmithKline, Novartis, and Schering AG; honoraria from Biogen, Bayer Schering, Hikma, and Novartis. C. Enzinger: funding for travel and speaking honoraria from Biogen, Bayer Schering, Merck Serono, Novartis, Shire, Genzyme, Teva Pharmaceutical Industries Ltd., and Sanofi-Aventis. A. Rovira: scientific advisory boards for Novartis, Sanofi-Genzyme, Icometrix, SyntheticMRI, and OLEA Medical; speaking honoraria from Bayer, Sanofi-Genzyme, Bracco, Merck-Serono, Teva Pharmaceutical Industries Ltd., Novartis, Roche, and Biogen Idec; agreements with Siemens AG and Icometrix. L. Kappos' institute (University Hospital Basel, Switzerland): steering committee, advisory board, and consultancy fees from Actelion, Addex, Bayer, Biogen, Biotica, Genzyme, Eli-Lilly, Merck, Mitsubishi, Novartis, Ono Pharma, Pfizer, Receptos, Sanofi, Santhera, Siemens, Teva, UCB, and XenoPort; speaker fees from Bayer, Biogen, Merck, Novartis, Sanofi, and Teva; support of educational activities from Bayer, Biogen, CSL Behring, Genzyme, Merck, Novartis, Sanofi, and Teva; royalties from Neurostatus; grants from Bayer Healthcare, Biogen, Merck, Novartis, Roche, and Roche Research Foundations. C. Pozzilli: scientific advisory boards for Actelion, Biogen, Genzyme, Hoffmann-La Roche Ltd., Merck-Serono, Novartis, Sanofi, and Teva; consulting and/or speaking fees, research support, and travel grants from Allergan, Almirall, Biogen, Genzyme, Hoffmann-La Roche Ltd., Merck-Serono, Novartis, Sanofi, and Teva. X. Montalban: personal fees for consultancy and speaking from Actelion, Bayer, Biogen, Celgene, Hoffmann-La Roche, Merck, Novartis, Oryzon Genomics, Sanofi Genzyme, and Teva. N. De Stefano: honoraria from Biogen, Genzyme, Merck, Novartis, Schering, and Teva for consulting services, speaking, and travel support; advisory boards for Biogen, Merck, and Novartis. Go to Neurology.org/N for full disclosures.
References
- 1.Río J, Nos C, Tintoré M, et al. Defining the response to interferon-beta in relapsing-remitting multiple sclerosis patients. Ann Neurol 2006;59:344–352. [DOI] [PubMed] [Google Scholar]
- 2.Bermel RA, You X, Foulds P, et al. Predictors of long-term outcome in multiple sclerosis patients treated with interferon β. Ann Neurol 2013;73:95–103. [DOI] [PubMed] [Google Scholar]
- 3.Goodin DS, Traboulsee A, Knappertz V, et al. Relationship between early clinical characteristics and long term disability outcomes: 16 year cohort study (follow-up) of the pivotal interferon β-1b trial in multiple sclerosis. J Neurol Neurosurg Psychiatry 2012;83:282–287. [DOI] [PubMed] [Google Scholar]
- 4.Tomassini V, Paolillo A, Russo P, et al. Predictors of long-term clinical response to interferon beta therapy in relapsing multiple sclerosis. J Neurol 2006;253:287–293. [DOI] [PubMed] [Google Scholar]
- 5.Durelli L, Barbero P, Bergui M, et al. MRI activity and neutralising antibody as predictors of response to interferon beta treatment in multiple sclerosis. J Neurol Neurosurg Psychiatry 2008;79:646–651. [DOI] [PubMed] [Google Scholar]
- 6.Waubant E, Vukusic S, Gignoux L, et al. Clinical characteristics of responders to interferon therapy for relapsing MS. Neurology 2003;61:184–189. [DOI] [PubMed] [Google Scholar]
- 7.Portaccio E, Zipoli V, Siracusa G, Sorbi S, Amato MP. Response to interferon-beta therapy in relapsing-remitting multiple sclerosis: a comparison of different clinical criteria. Mult Scler 2006;12:281–286. [DOI] [PubMed] [Google Scholar]
- 8.Romeo M, Martinelli-Boneschi F, Rodegher M, et al. Clinical and MRI predictors of response to interferon-beta and glatiramer acetate in relapsing-remitting multiple sclerosis patients. Eur J Neurol 2013;20:1060–1067. [DOI] [PubMed] [Google Scholar]
- 9.Hartung HP, Kappos L, Goodin DS, et al. Predictors of disease activity in 857 patients with MS treated with interferon beta-1b. J Neurol 2015;262:2466–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadavid D, Kim S, Peng B, et al. Clinical consequences of MRI activity in treated multiple sclerosis. Mult Scler 2011;17:1113–1121. [DOI] [PubMed] [Google Scholar]
- 11.Sormani MP, Gasperini C, Romeo M, et al. Assessing response to interferon-β in a multicenter dataset of patients with MS. Neurology 2016;87:134–140. [DOI] [PubMed] [Google Scholar]
- 12.Giovannoni G, Tomic D, Bright JR, Havrdová E. “No evident disease activity”: the use of combined assessments in the management of patients with multiple sclerosis. Mult Scler 2017;23:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kappos L, De Stefano N, Freedman MS, et al. Inclusion of brain volume loss in a revised measure of “no evidence of disease activity” (NEDA-4) in relapsing-remitting multiple sclerosis. Mult Scler 2016;22:1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol 2014;75:43–49. [DOI] [PubMed] [Google Scholar]
- 15.Disanto G, Barro C, Benkert P, et al. ; Swiss Multiple Sclerosis Cohort Study Group. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017;81:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cree BA, Gourraud PA, Oksenberg JR, et al. ; University of California, San Francisco MS-EPIC Team. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol 2016;80:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015;72:152–158. [DOI] [PubMed] [Google Scholar]
- 18.Río J, Rovira À, Tintoré M, et al. Disability progression markers over 6-12 years in interferon-β-treated multiple sclerosis patients. Mult Scler 2018;24:322–330. [DOI] [PubMed] [Google Scholar]
- 19.Prosperini L, Mancinelli CR, Sgarlata E, et al. Minimal or no evidence of disease activity: which target to prevent long-term disability in multiple sclerosis? Mult Scler 2017;23(suppl 3):386 (P769). [Google Scholar]
- 20.Signori A, Schiavetti I, Gallo F, Sormani MP. Subgroups of multiple sclerosis patients with larger treatment benefits: a meta-analysis of randomized trials. Eur J Neurol 2015;22:960–966. [DOI] [PubMed] [Google Scholar]
- 21.O'Rourke K, Walsh C, Antonelli G, et al. Predicting beta-interferon failure in relapsing-remitting multiple sclerosis. Mult Scler 2007;13:336–342. [DOI] [PubMed] [Google Scholar]
- 22.Jokubaitis VG, Spelman T, Kalincik T, et al. Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Ann Neurol 2016;80:89–100. [DOI] [PubMed] [Google Scholar]
- 23.Kalincik T, Manouchehrinia A, Sobisek L, et al. ; MSBase Study Group. Towards personalized therapy for multiple sclerosis: prediction of individual treatment response. Brain 2017;140:2426–2443. [DOI] [PubMed] [Google Scholar]
- 24.Sormani MP, Li DK, Bruzzi P, et al. Combined MRI lesions and relapses as a surrogate for disability in multiple sclerosis. Neurology 2011;77:1684–1690. [DOI] [PubMed] [Google Scholar]
- 25.Wang YC, Sandrock A, Richert JR, Meyerson L, Miao X. Short-term relapse quantitation as a fully surrogate endpoint for long-term sustained progression of disability in RRMS patients treated with natalizumab. Neurol Res Int 2011;2011:195831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sormani MP, Stubinski B, Cornelisse P, et al. Magnetic resonance active lesions as individual-level surrogate for relapses in multiple sclerosis. Mult Scler 2011;17:541–549. [DOI] [PubMed] [Google Scholar]
- 27.Sormani MP, Bruzzi P, Beckmann K, et al. MRI metrics as surrogate endpoints for EDSS progression in SPMS patients treated with IFN beta-1b. Neurology 2003;60:1462–1466. [DOI] [PubMed] [Google Scholar]
- 28.Sormani MP, De Stefano N, Francis G, et al. Fingolimod effect on brain volume loss independently contributes to its effect on disability. Mult Scler 2015;21:916–924. [DOI] [PubMed] [Google Scholar]
- 29.Rudick RA, Rani MR, Xu Y, et al. Excessive biologic response to IFNβ is associated with poor treatment response in patients with multiple sclerosis. PLoS One 2011;6:e19262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prosperini L, Gallo V, Petsas N, Borriello G, Pozzilli C. One-year MRI scan predicts clinical response to interferon beta in multiple sclerosis. Eur J Neurol 2009;16:1202–1209. [DOI] [PubMed] [Google Scholar]
- 31.Sahraian MA, Radue EW, Haller S, Kappos L. Black holes in multiple sclerosis: definition, evolution, and clinical correlations. Acta Neurol Scand 2010;122:1–8. [DOI] [PubMed] [Google Scholar]
- 32.Rudick RA, Lee JC, Simon J, Ransohoff RM, Fisher E. Defining interferon beta response status in multiple sclerosis patients. Ann Neurol 2004;56:548–555. [DOI] [PubMed] [Google Scholar]
- 33.Prosperini L, Mancinelli CR, De Giglio L, et al. Interferon beta failure predicted by EMA criteria or isolated MRI activity in multiple sclerosis. Mult Scler 2014;20:566–576. [DOI] [PubMed] [Google Scholar]
- 34.Galassi S, Prosperini L, Logoteta A, et al. A lesion topography-based approach to predict the outcomes of patients with multiple sclerosis treated with interferon beta. Mult Scler Relat Disord 2016;8:99–106. [DOI] [PubMed] [Google Scholar]
- 35.Tintore M, Rovira A, Arrambide G, et al. Brainstem lesions in clinically isolated syndromes. Neurology 2010;75:1933–1938. [DOI] [PubMed] [Google Scholar]
- 36.Kearney H, Altmann DR, Samson RS, et al. Cervical cord lesion load is associated with disability independently from atrophy in MS. Neurology 2015;84:367–373. [DOI] [PubMed] [Google Scholar]
- 37.Río J, Castilló J, Rovira A, et al. Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler 2009;15:848–853. [DOI] [PubMed] [Google Scholar]
- 38.Wattjes MP, Rovira À, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis: establishing disease prognosis and monitoring patients. Nat Rev Neurol 2015;11:597–606. [DOI] [PubMed] [Google Scholar]
- 39.Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 2017;16:564–570. [DOI] [PubMed] [Google Scholar]
- 40.Altay EE, Fisher E, Jones SE, et al. Reliability of classifying multiple sclerosis disease activity using magnetic resonance imaging in a multiple sclerosis clinic. JAMA Neurol 2013;70:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging–measured disease activity and burden in patients with relapsing multiple sclerosis. Ann Neurol 2001;49:290–297. [PubMed] [Google Scholar]
- 42.Kappos L, Gold R, Miller DH, et al. Effect of BG-12 on contrast-enhanced lesions in patients with relapsing-remitting multiple sclerosis: subgroup analyses from the phase 2b study. Mult Scler 2012;18:314–321. [DOI] [PubMed] [Google Scholar]
- 43.Lycklama G, Thompson A, FIlippi M, et al. Spinal-cord MRI in multiple sclerosis. Lancet Neurol 2003;2:555–562. [DOI] [PubMed] [Google Scholar]
- 44.Freedman MS, Patry DG, Grand'Maison F, et al. Treatment optimization in multiple sclerosis. Can J Neurol Sci 2004;31:157–168. [DOI] [PubMed] [Google Scholar]
- 45.Freedman MS, Forrestal FG. Canadian Treatment Optimization Recommendations (TOR) as a predictor of disease breakthrough in patients with multiple sclerosis treated with interferon beta-1a: analysis of the PRISMS study. Mult Scler 2008;14:1234–1241. [DOI] [PubMed] [Google Scholar]
- 46.Freedman MS, Selchen D, Arnold DL, et al. Treatment optimization in MS: Canadian MS Working Group updated recommendations. Can J Neurol Sci 2013;40:307–323. [DOI] [PubMed] [Google Scholar]
- 47.Sormani MP, Rio J, Tintorè M, et al. Scoring treatment response in patients with relapsing multiple sclerosis. Mult Scler 2013;19:605–612. [DOI] [PubMed] [Google Scholar]
- 48.Sormani M, Signori A, Stromillo M, De Stefano N. Refining response to treatment as defined by the modified Rio score. Mult Scler 2013;19:1246–1247. [DOI] [PubMed] [Google Scholar]
- 49.Río J, Rovira A, Tintoré M, et al. Evaluating the response to glatiramer acetate in relapsing-remitting multiple sclerosis (RRMS) patients. Mult Scler 2014;20:1602–1608. [DOI] [PubMed] [Google Scholar]
- 50.Calabresi PA, Stone LA, Bash CN, Frank JA, McFarland HF. Interferon beta results in immediate reduction of contrast-enhanced MRI lesions in multiple sclerosis patients followed by weekly MRI. Neurology 1997;48:1446–1448. [DOI] [PubMed] [Google Scholar]
- 51.Sormani MP, Bruzzi P, Comi G, Filippi M. The distribution of the magnetic resonance imaging response to glatiramer acetate in multiple sclerosis. Mult Scler 2005;11:447–449. [DOI] [PubMed] [Google Scholar]
- 52.Boster A, Hawker K, Ritter S, Tomic D, Sprenger T. Disease activity in the first year predicts longer-term clinical outcomes in the pooled population of the phase III FREEDOMS and FREEDOMS II studies (P7.239). Neurology 2015;84(14 suppl):P7.239. [Google Scholar]
- 53.Sormani MP, Kappos L, Piani-Meier D. Persistent MRI lesion activity and brain volume loss as predictors of long term disability progression under treatment in relapsing-remitting multiple sclerosis. Eur J Neurol 2016;23(suppl):205–206. [Google Scholar]
- 54.Sormani MP, Truffinet P, Thangavelu K, Rudi P, Simonson C, De Stefano N. Predicting long-term disability outcomes in patients with MS treated with teriflunomide in TEMSO. Neurol Neuroimmunol Neuroinflamm 2017;4:e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bates D, Bartholomé E. Treatment effect of natalizumab on relapse outcomes in multiple sclerosis patients despite ongoing MRI activity. J Neurol Neurosurg Psychiatry 2012;83:55–60. [DOI] [PubMed] [Google Scholar]
- 56.Prosperini L, Giannì C, Barletta V, et al. Predictors of freedom from disease activity in natalizumab treated-patients with multiple sclerosis. J Neurol Sci 2012;323:104–112. [DOI] [PubMed] [Google Scholar]
- 57.Berger T, Elovaara I, Fredrikson S, et al. Alemtuzumab use in clinical practice: recommendations from European multiple sclerosis experts. CNS Drugs 2017;31:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Battaglini M, Rossi F, Grove RA, et al. Automated identification of brain new lesions in multiple sclerosis using subtraction images. J Magn Reson Imaging 2014;39:1543–1549. [DOI] [PubMed] [Google Scholar]
- 59.Valverde S, Oliver A, Roura E, et al. Quantifying brain tissue volume in multiple sclerosis with automated lesion segmentation and filling. Neuroimage Clin 2015;9:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Sitter A, Steenwijk MD, Ruet A, et al. ; MAGNIMS study group and for neuGRID. Performance of five research-domain automated WM lesion segmentation methods in a multi-center MS study. Neuroimage 2017;163:106–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
E-references are available from MAGNIMS (magnims.eu/?protected-download=16119).