Abstract
Purpose—
Understanding the mechanism of radioresistance could help develop strategies to improve therapeutic response of patients with PDAC. The SMAD4 gene is frequently mutated in pancreatic cancer. In this study, we investigated the role of SMAD4 deficiency in pancreatic cancer cells’ response to radiotherapy.
Experimental design—
We downregulated SMAD4 expression with SMAD4 siRNA or SMAD4 shRNA and overexpressed SMAD4 in SMAD4 mutant pancreatic cancer cells followed by clonogenic survival assay to evaluate their effects on cell radioresistance. To study the mechanism of radioresistance, the effects of SMAD4 loss on reactive oxygen species (ROS) and autophagy were determined by Flow Cytometry and immunoblot analysis, respectively. Furthermore, we measured radioresistance by clonogenic survival assay after treatment with autophagy inhibitor (Chloroquine) and ROS inhibitor (N-acetyl-L-cysteine) in SMAD4-depleted pancreatic cancer cells. Finally, the effects of SMAD4 on radioresistance were also confirmed in an orthotopic tumor model derived from SMAD4-depleted Panc-1 cells.
Results—
SMAD4-depleted pancreatic cancer cells were more resistant to radiotherapy based on clonogenic survival assay. Overexpression of wild type SMAD4 in SMAD4-mutant cells rescued their radiosensitivity. Radioresistance mediated by SMAD4 depletion was associated with persistently higher levels of ROS and radiation-induced autophagy. Finally, SMAD4 depletion induced in vivo radioresistance in Panc-1-derived orthotopic tumor model (P = 0.038). More interestingly, we observed that the protein level of SMAD4 is inversely correlated with autophagy in orthotopic tumor tissue samples.
Conclusion—
Our results demonstrate that defective SMAD4 is responsible for radioresistance in pancreatic cancer through induction of ROS and increased level of radiation-induced autophagy.
Keywords: pancreatic cancer, SMAD4, radiotherapy, autophagy, reactive oxygen species
Translational Relevance
Adjuvant radiotherapy is often given following surgery for pancreatic ductal adenocarcinoma (PDAC). However there is still controversy about whether the patients benefit from radiotherapy. In the United States, chemoradiotherapy is accepted as appropriate adjuvant therapy of PDAC, but standard adjuvant treatment in Europe is chemotherapy alone. There might be a certain group of PDAC patients who could get some measurable benefit from radiotherapy, however that group is not well defined and further evaluation of biomarkers might help to more precisely determine that group. Gene mutations could affect therapy responses of cancer cells, and SMAD4 is mutated in 55% PDAC patients. This study documents that SMAD4 depletion increases radioresistance of pancreatic cancer cells both in vitro and in vivo. SMAD4 depletion induces high levels of reactive oxygen species (ROS) and autophagy. Pre-treatment with N-acetyl-L-cysteine (NAC), a ROS inhibitor, or Chloroquine (CQ), an autophagy inhibitor, could re-sensitize SMAD4-depleted cells to IR. Future studies are needed to investigate the relationship of SMAD4 status and survival advantages of chemoradiotherapy in patients with PDAC, which would be helpful to guide the administration of targeted therapies in the adjuvant setting based on SMAD4 status.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignant disease of the exocrine pancreas, and is the fourth most common cause of cancer deaths worldwide, leading to estimated 227,000 deaths annually(1, 2). Despite advances in conventional therapies (surgical, chemotherapy and radiotherapy), little improvement has been observed in the survival rate over the past 30 years(3). The median survival of patients with PDAC is less than 6 months, and the 5-year survival rate is less than 5%(1–3). Since early-stage pancreatic cancer is usually clinically silent, most patients have already developed locally advanced or metastatic disease at diagnosis, and only 10–15% of the patients are eligible for surgical resection(4, 5). Most pancreatic cancer patients are treated with chemotherapy in the United States, either alone or in combination with radiotherapy(6–8), while chemotherapy is frequently used alone in patients in Europe, based on the European organization for Research and Treatment of Cancer (EORTC) trail(9). However, the US study was criticized for poor patient accrual, early termination, small patient number and suboptimal radiotherapy dose. At the same time, there are some defects in EORTC trial design, including the mixing up of pancreas and peri-ampullary cancers, underpowered analysis for survival benefit, and use of antiquated radiotherapy and chemotherapy techniques. A growing body of evidence showed no survival benefit for adjuvant chemoradiotherapy but revealed a potential benefit for adjuvant chemotherapy(10–13). However, the true benefit of the addition of radiotherapy is still unknown(14). The underlying reason for the striking difference in guidelines of PDAC treatment between these different regions is still unclear. Because many gene mutations affect cell growth and drug responses of cancer cells, we suspect that the difference in the mutational status of some key genes in the pancreatic cancer patients may contribute to resistance to radiotherapy.
Mutations in multiple genes such as SMAD4, KRAS, TP53 and CDKN2A/INK4A(15, 16) have been demonstrated to be important in PDAC progression. Among these, the SMAD4 status is considered to be an important molecular feature which distinguishes two major classes of PDAC. The tumor suppressor gene SMAD4 encoding a common intracellular mediator of the TGF-β superfamily is mutated or deleted in 55% pancreatic cancers(16, 17). This gene is also inactivated at varying frequency in breast, colorectal and gastric cancer(18, 19). Loss of SMAD4 promotes pancreatic tumor progression and increases metastasis(20, 21). SMAD4 is reported as a negative prognostic factor for overall survival(17, 22–24). Growing evidence showed that the loss of SMAD4 induces resistance to chemotherapy in colorectal, breast, head and neck cancers(25–27). However, the role of SMAD4 in radioresistance of pancreatic cancer and the underlying molecular mechanism have not been fully elucidated.
In this study, we showed that SMAD4 depletion renders pancreatic cancer cells resistant to ionizing radiation (IR) both in vitro and in vivo. Mechanistically, SMAD4 depletion induces high levels of autophagy and ROS, which appear to contribute to such radioresistance.
Materials and Methods
Cell lines and culture
The human pancreatic cancer cell lines Panc-1 and MIA PaCa-2 were purchased from the American Type Culture Collection (ATCC, Rockefeller, MD, USA). Cells were maintained in DMEM medium (GIBCO, Grand Island, NY) supplemented with 10% or 20% fetal bovine serum and 100 U/ml penicillin (GIBCO, Carlsbad, CA, USA). Panc-1 cells transfected with shRNA (Panc-1-shControl and Panc-1-shSMAD4) were maintained in DMEM medium (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 1 μg/ml puromycin (Sigma, St. Louis, MO, USA). All cell lines were cultured in a 37°C incubator with 95% air and 5% CO2. Each cell line was authenticated and regularly tested for mycoplasma contamination.
Reagents and antibodies
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was applied to transfect PcDNA3-Flag-SMAD4 plasmid (Addgene plasmid #80888) into cells by following manufacturers’ instruction. N-acetyl-L-cysteine (NAC) and chloroquine (CQ) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and were reconstituted in dimethyl sulfoxide (DMSO) and phosphate-buffered saline (PBS) at 100mM, respectively. Antibodies against SMAD4, Rad51, BRCA-1, LC3, FLAG and γ-H2AX were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). A monoclonal anti-β-actin antibody was obtained from Sigma (St Louis, MO, USA).
Measurement of ROS
Cells were seeded for 24 h at a density of 3 × 105 cells/ml prior to IR treatment. The level of intracellular ROS was measured using the fluorescent probe 5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) following manufacturer’s instruction (Life Technologies, Carlsbad, CA, USA). In brief, cells were incubated with 10 μM CM-H2DCFDA for 30 min at 37°C in the dark and then washed in serum-free minimum essential medium without phenol red. The cells were then analyzed in a FACSCanto apparatus (BD Biosciences, Heidelberg, Germany).
Small interfering RNA (siRNA) transfection
Cells were grown to confluence in 6-cm tissue culture plates, and transfected with one of the four SMAD4-specific siRNA oligos at 50 nM final concentration (si-SMAD4-a: 5’-AGAUGAAUUGGAUUCUUUAdTdT-3’; si-SMAD4-b: 5’-GUGUGCAGUUGGAAUGUAAdTdT-3’; si-SMAD4-c: 5’-GUACUUCAUACCAUGCCGAdTdT-3’; si-SMAD4-d: 5’-CAUCCUAGUAAAUGUGUUAdTdT-3’) using INTERFERin™ (Illkirch, France) as the transfection reagent according to the manufacturer’s instruction. A scrambled siRNA (siScr: 5’-ACGCGUAACGCGGGAAUUUdTdT-3’) was applied as a negative control. Cells were harvested 72 h later for gene expression analysis by western blot.
Retroviral shRNA production
Retroviral vectors carrying a human SMAD4-specific shRNA (target sequence 1: 5’-GGTGTGCAGTTGGAATGTA-3’ for clone #35 and #46. Target sequence 2: 5’-GATTAACACTGCAGAGTAA-3’ for supplementary figures) or a scrambled non-targeting control (sequence: 5’-AAAATGCAGTTGGAATGTA-3) were purchased from Addgene (Cambridge, MA, USA). The shSMAD4 and shControl recombinant viruses were generated by transient transfection with packaging plasmids pMLg/pRRE, pRSV.rev, and pHCMV-G into 293T cells(28). Virus-containing supernatant was collected 72 h later, and applied to transfect Panc-1 cells, and then selected three individual clones as Panc-1-shSMAD4 #35 and #46 (for target sequence 1) and #2 (for target sequence 2).
In vitro and in vivo IR
For in vitro studies, cells were irradiated at various doses using an XRAD 320 X-ray irradiator (Precision X-Ray, Inc., CT, USA) at a dose rate of 75 cGy/min. For in vivo IR, mice were anesthetized with ketamine/xylazine (100mg/kg+10mg/kg) and placed in well-ventilated custom jigs (Precision X-Ray), allowing for local delivery of radiation using an XRad 320 X-ray irradiator (Precision X-Ray, Inc., CT, USA) at a dose rate of 289.8 cGy/min.
Clonogenic assay to measure cell survival
Cell survival was determined using a standard clonogenic assay. Cells with or without SMAD4 knockdown were trypsinized to generate a single-cell suspension and seeded into 6-well plates in triplicate. After incubation for 16 h, 800 cells were treated with IR (0–6 Gy). After 7–10 incubation, the colonies were fixed with 4% paraformaldehyde and then stained with 0.5% crystal violet. After counting the number of colonies (containing more than 50 cells), the surviving fraction was calculated.
Western blot analysis
Cells were rinsed twice with PBS and lysed for 2 h with Radio Immunoprecipitation Assay (RIPA) Lysis buffer (Thermo Scientific, Waltham, MA, USA) supplemented with a protease inhibitor and a protease inhibitor cocktail. Cell debris was removed by centrifugation at 12,000 rpm for 10 min at 4°C. Protein concentration in the supernatant was determined with the BCA Protein Assay Kit (Pierce, USA). The protein samples were separated by electrophoresis using a 4–20% Mini-PROTEAN Tetra Cell polyacrylamide gel (Bio-Rad, Hercules, CA, USA) and then transferred to polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, USA). The membrane was blocked with 5% skim milk, and incubated with the indicated primary antibodies, followed by incubation with horseradish peroxidase-labeled secondary antibodies. Antibody-bound proteins were detected using the Enhanced Chemiluminescence (ECL) reagent (GenDEPOT, Katy, TX, USA) according to the manufacturer’s instructions. The protein levels were quantified using the ImageJ software (NIH, Bethesda, MD, USA) and then normalized by the expression levels in control cells for each group.
Confocal microscopy analysis
Cells were seeded onto 4-well LabTek II chamber slides (NUNC, Rochester, NY, USA) for 24 h, and then treated with IR at 2, 4 or 6 Gy. After incubation for 2 h, cells were fixed with 4% paraformaldehyde for 10 min, washed 3 times with PBS and blocked with 3% BSA and 0.1% Triton-X100 in PBS for 30 min at room temperature. After removing the blocking buffer, cells were incubated with the primary antibodies (rabbit γ-H2AX antibody, LC3 antibody and mouse SMAD4 antibody) overnight at 4°C. After being washed three times with PBS, cells were incubated with Alexa Fluor 488-conjugated anti-rabbit IgG and Alexa Fluor 594-conjugated anti-mouse IgG secondary antibodies (Invitrogen, Grand Island, NY, USA) for 2 h in the dark. The cells were then washed and mounted with mounting medium containing DAPI (VECTOR Laboratories, Burlingame, CA, USA). Finally, a Nikon A1 confocal microscope was applied to examine and quantitate γ-H2AX, LC3 and SMAD4 signals. At least 300 nuclei were evaluated for each data point.
In vivo studies
All mouse studies were performed in compliance with the guidelines of the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals following protocols approved by the Institutional Animal Care and Use Committee (IACUC). Six-week-old athymic nude mice were purchased from Charles River Laboratories (Boston, MA, USA). Mice (8 per group) were injected with 2 × 106 Panc-1-shControl or Panc-1-shSMAD4 cells suspended in 100 μl medium with 5% Matrigel directly into the pancreas for orthotopic tumor development. Three weeks after inoculation, the mice received 4 Gy radiation for 3 times with 2-day intervals between irradiations. The mice were sacrificed at 3 weeks after the last treatment, and the tumors were removed and weighed.
Immunohistochemical (IHC) staining of tissue samples
IHC staining was performed as previously described(29). Briefly, tumor sections were deparaffinized, rehydrated, and treated with 10 mM citrate buffer (pH 6.0) at 95°C to retrieve antigens. After quenching endogenous peroxidase activity with H2O2 and blocking with 10% horse serum, the sections were incubated sequentially with the primary antibodies mouse anti-SMAD4 (1:100; Cell Signaling Technology Inc. CA, USA), rabbit anti-LC3 (1:100; Cell Signaling Technology Inc. CA, USA), rabbit anti-γ-H2AX (1:100; Cell Signaling Technology Inc. CA, USA), biotinylated secondary antibodies, and the Avidin-Biotin Complexes (ABC) reagent (Gene Tech. San Francisco, CA, USA). The immunostaining was visualized with 3.3-diaminobenzidine (Gene Tech. San Francisco, CA, USA). The sections were then counterstained with hematoxylin. Negative controls were performed in each case by replacing the primary antibody with PBS.
Statistical analysis
All experiments were performed in triplicate. The data were expressed as means ± SD. Statistical analyses were performed using Student’s t-test and ANOVA. Differences across groups selected to be compared were detected via repeated measures ANOVA. Values of P <0.05 were considered statistical significant, and all the statistical test were two sides. All statistical analyses were conducted in R program (R core team, version 3.4.2).
Result
SMAD4 knockdown in pancreatic cancer cells results in IR resistance.
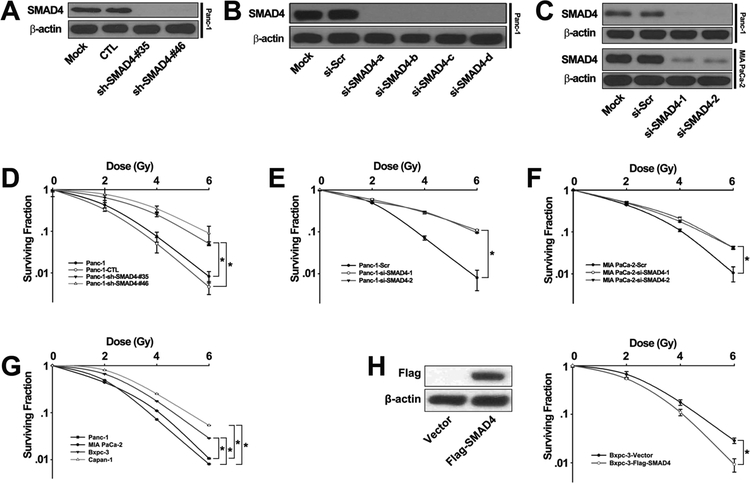
In order to examine whether SMAD4 was involved in response to IR, we knocked down SMAD4 gene expression in Panc-1 cells with retroviral shRNA constructs (sh-SMAD4 #35 and sh-SMAD4 #46) or siRNA oligos (si-SMAD4-1 and si-SMAD4-2) targeting the SMAD4 gene. To exclude the off-target effect of shRNA, we also established cell lines stably expressing another shRNA construct which targeted different sites in SMAD4. Western blot analysis showed that SMAD4 was depleted by siRNA or shRNA (Fig. 1A-B, Fig. S1). The same siRNA oligos were effective in suppressing SMAD4 expression in MIA PaCa-2 cells (Fig. 1C). The cancer cells were treated with various doses of IR, and then seeded on cell culture plates for colony formation. Interestingly, Panc-1 cells infected with the SMAD4 gene-specific shRNA retrovirus were more resistant to IR than both the untreated cells and those treated with retrovirus carrying a control scramble shRNA (Fig. 1D). Similar results were obtained when Pan-1 cells treated with siRNA oligos (Fig. 1E). Consistent with Panc-1 cells, MIA-PaCa-2 cells treated with SMAD4 siRNA oligos were more resistant to IR than those treated with the control siRNA oligos (Fig. 1F). We also found that SMAD4 WT pancreatic cancer cell lines (Panc-1, MIA PaCa-2) were more sensitive to IR than SMAD4 mutated cell lines (Bxpc-3, Capan-1) (Fig. 1G). Furthermore, we found that the Bxpc-3 cells transfected with Flag-SMAD4 plasmid were more sensitive to IR than control cells with empty vector (Fig. 1H). These results indicate that suppression of SMAD4 expression increases radioresistance in pancreatic cancer cells.
Figure 1. SMAD4 knockdown resulted in irradiation resistance in PDAC cells.
(A-C) Panc-1 cells were transfected with a control shRNA retroviral vector or two independent shRNA retroviral vector against SMAD4 (A), Panc-1 cells were transfected with scrambled siRNA or four SMAD4-specific siRNA for 72h (B), Panc-1, MIA PaCa-2 cells were transfected with scrambled siRNA or pool of SMAD4-specific siRNA for 72h (C), and total cell lysates were harvested followed by immunoblotting with the indicated antibodies. (D-F) Panc-1 cells were transfected with a control shRNA retroviral vector or two independent shRNA retroviral vector targeting SMAD4 (D) or pool of SMAD4-specific siRNA (E), MIA PaCa-2 cells were transfected with scrambled siRNA or SMAD4-specific siRNA pool (F), and then treated with indicated doses of IR. Colony formation assays were conducted and more than 50 cells were counted. (G) Colony formation assays were conducted in two SMAD4 mutated cell lines (Bxpc-3, Capan-1) and two SMAD4 WT cell lines (Panc-1, MIA PaCa-2). The differences between SMAD4 WT and mutated cells selected to be compared were detected using repeated measures ANOVA. * , P < 0.05. (H) Bxpc-3 cells were transfected with Flag-SMAD4 plasmid or empty vector, followed by colony formation assays. Shown are the averages of triplicate samples. Standard errors are shown by error bars. The differences in the two groups selected to be compared were detected using repeated measures ANOVA. * , P < 0.05. (si-SMAD4-1=si-SMAD4-a+si-SMAD4-b; si-SMAD4-2=si-SMAD4-c+si-SMAD4-d)
SMAD4 depletion results in DNA damage and genomic instability.
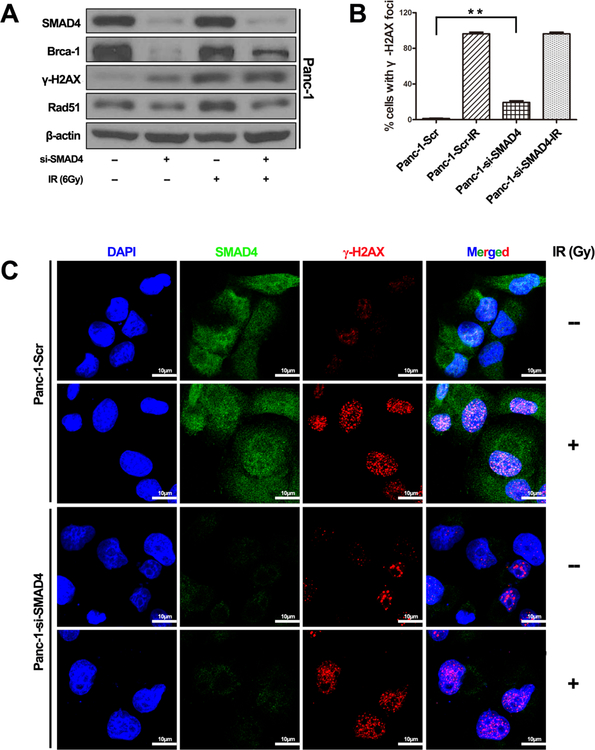
Radiation kills cancer cells by inducing DNA damage which triggers DNA damage response (DDR), including genomic instability. To investigate whether SMAD4 loss affects genomic stability, we examined several DDR endpoints. Knockdown of SMAD4 expression activated phosphorylation of the H2AX histone (γ-H2AX), a molecular marker for DNA double strand breaks (DSB)(30), in Panc-1 cells without IR (Fig. 2A). After IR treatment, the γ-H2AX level increased in both control and SMAD4 depleted cells (Fig. 2A, Fig. S2). Interestingly, the levels of Brca1 and Rad51, two key proteins involved in DNA DSB repair, were reduced in cells with suppressed SMAD4 expression (Fig. 2A, Fig. S2). Immunostaining revealed a similar pattern of γ-H2AX levels in Panc-1 cells (Fig. 2B and C). There was a larger number of γ-H2AX foci in Panc-1 cells treated with si-SMAD4 before IR treatment, and massive staining of γ-H2AX foci were readily visible within 2 h of IR in the nuclei of Panc-1 cells treated with either the control siRNA or SMAD4 siRNA (Fig. 2B, 2C). These results indicate that the SMAD4 protein is required for maintaining a proper DNA damage response machinery in cancer cells.
Figure 2. SMAD4 depletion impaired DNA Double-Strand Break Signaling in Panc-1 cells in response to ionizing radiation.
(A) Panc-1 cells were transfected with scrambled siRNA, or four independent SMAD4-specific siRNAs for 72 h, and then total cell lysates were harvested followed by immunoblotting with the indicated antibodies. (B, C) Panc-1 cells were transfected with scrambled siRNA or pool of SMAD4-specific siRNAs and then exposed to IR (6 Gy). Laser confocal microscopy images of pancreatic cancer cell lines labeled with fluorescent antibodies to γ-H2AX (red channel), SMAD4 (green channel) and DAPI (blue channel) at 2 h post irradiation or mock treatment. Focal g-H2AX staining signals were quantified in cells. (C) The typical staining of γ-H2AX in Panc-1 cells before and after the treatment with irradiation. The average data point was calculated from three independent experiments. At least 1000 cells were counted for each cell line. The differences in those two groups were detected using t-test. **, P <0.01.
Pancreatic cancer cells with suppressed SMAD4 expression exhibits increased level of autophagy.
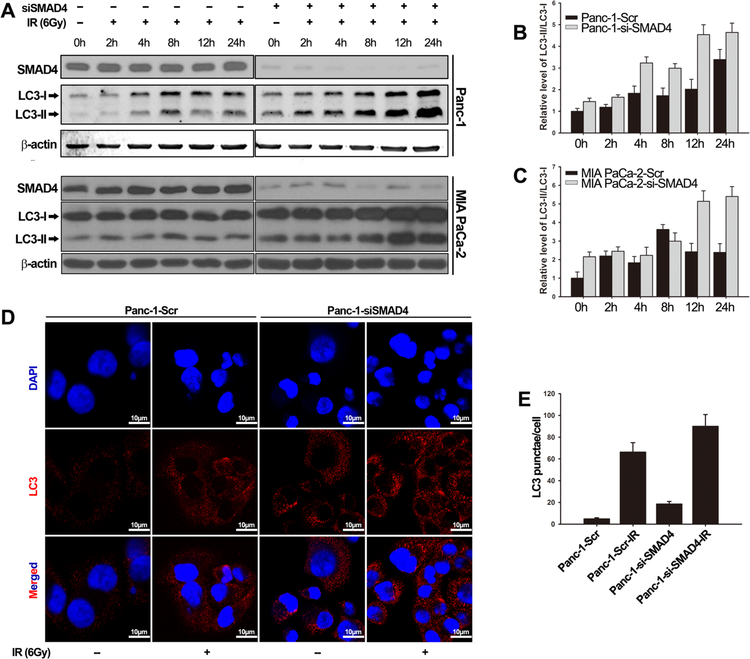
It has been well documented that autophagy occurs in response to IR, which rescues cells from IR-induced cellular damage, thereby contributing to radioresistance(31–33). Inhibition of autophagy has been shown to sensitize tumor cells to the cytotoxic effects of both chemotherapy and radiotherapy(34, 35). In order to determine whether autophagy played a role in SMAD4-dependent resistance to IR, we knocked down SMAD4 expression in Panc-1 and MIA Paca-2 cells, and measured cellular levels of the autophagy marker protein LC3-II, a lipidated LC3 protein that is associated with autophagosomes(36). Western blot analysis revealed that knockdown of SMAD4 promoted the conversion of LC3-I to LC3-II in Panc-1 (1.45-fold increase) and MIA PaCa-2 (2.15-fold increase) cells (Fig. 3A-C). Upon IR (6 Gy) treatment, LC3-II levels increased steadily in a time-dependant manner in both control cells and SMAD4-depleted cells. The peak reached at the 24-hour time point in both cell lines. However, LC3-II increase was more profound in cells with suppressed SMAD4 expression compared with that in control cells. We also observed similar results in the Panc-1 cells stable expressing SMAD4 shRNA (Fig. S2A). During authophagy, autophagic flux could be easily visualized by fluorescence microscopy using LC3-II antibody, which is tightly bound to the membrane of autophagosome. Immunofluorescent imaging analysis (Fig. 3D-E, Fig. S2B) showed that, in SMAD4-depleted cells, LC3 signals shifted from a diffuse cytoplasmic pattern to a punctate membrane pattern. In addition, SMAD4-depleted cells displayed a higher level of membrane LC3 signal, indicating autophagosome formation (Fig. 3D-E, Fig. S2B). Collectively, our results suggest that radioresistance mediated by SMAD4 depletion is accompanied by an increase in autophagic flux in pancreatic cancer cells.
Figure 3. SMAD4 depletion induced high level of autophagy in response to ionizing radiation.
(A-C) Panc-1 and MIA PaCa-2 were transfected with scrambled siRNA or SMAD4-specific siRNA for 48 h and then were treated with or without IR (6 Gy). Cell lysates were harvested at the indicated time points after IR. (A) Immunoblot of LC3 isoforms were measured using antibody against LC3. Quantification of the LC3-II/LC3-I ratio in Panc-1 cells (B) and MIA PaCa-2 cells (C). The protein level of the β-actin was used as the internal standard. Shown are the averages of triplicate experiments. (D) Representative confocal images of endogenous LC3 staining in Panc-1 transfected with scramble or SMAD4-specific siRNA for 48 h, and treated with or without IR (6 Gy). Cells were stained with antibody to LC3 (red) and DAPI (blue). Scale bars, 10 μm. (E) Quantification of LC3 puncta in Panc-1 cell lines.
SMAD4 depletion causes persistent ROS increase in pancreatic cancer cells after irradiation exposure.
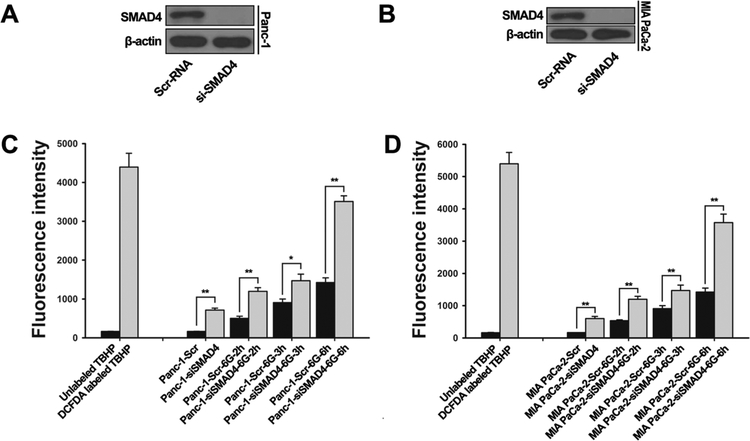
It has been previously reported that autophagy occurs in response to stress, such as ROS(37, 38) and nutrient starvation(39). IR induce intracellular ROS production, and ROS accumulation is necessary for the initiation of autophagy(40–43). We next examined ROS levels in SMAD4-depleted pancreatic cancer cells based on CM-H2DCFDA fluorescence staining (Fig. 4, Fig. S3). ROS levels were increased within 2 to 6 hours after IR treatment in Panc-1 and MIA PaCa-2 cell lines (Fig. 4C-D). The basal levels of ROS were elevated in both cell lines with SMAD4 depletion, and radiation-induced ROS levels were higher in SMAD4-depleted cells than those in control cells within 2 to 6 hours after IR treatment (Fig. 4C-D, Fig. S3B). The result indicates that induction of autophagy was accompanied by accumulation of ROS in SMAD4-depleted pancreatic cancer cells.
Figure 4. SMAD4 depletion increased reactive oxygen species generation in PDAC cells.
(A, B) Panc-1 and MIA PaCa-2 cells were transfected with scrambled siRNA or SMAD4-specific siRNA for 48 h and then treated with IR (6 Gy). SMAD4 depletion was confirmed by Western blot. (C, D) Cells were then incubated with ROS indicator (CM-H2DCFDA), and fluorescence intensity was assessed by flow cytometry at indicated time points. Labeled tert-butyl hydrogen peroxide (TBHP, 50μM) serve as a positive control. The differences in those two groups were detected using t-test. * , P < 0.05, **, P < 0.01.
Small molecule inhibitors of ROS and autophagy reverse SMAD4-depletion induced IR resistance.
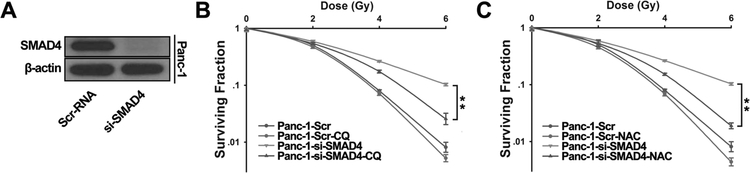
Since SMAD4 depletion caused higher level of autophagy and ROS production in pancreatic cancer cells, we set up experiments to test whether autophagy and ROS play a critical role on IR resistance induced by SMAD4 depletion in PDAC. We transfected Panc-1 cells with a control scramble siRNA or SMAD4-specific siRNA (Fig. 5A), and also treated cells with chloroquine (CQ), an autophagy inhibitor. These cells were subjected to increasing doses of IR treatment, and then seeded for colony formation. While only marginal difference was observed in the control Panc-1 cells with or without CQ treatment, cells transfected with SMAD4 siRNA were significantly more resistant to IR at 4 Gy and 6 Gy doses compared with control cells, and CQ treatment partially reduced such resistance (Fig, 5B). In a separate experiment, Panc-1 cells transfected with a control or SMAD4-specific siRNA were treated with N-acetyl-L-cysteine (NAC), a ROS inhibitor, before IR. Similar to the result of CQ co-treatment, pre-treatment with NAC re-sensitized cells to IR in SMAD4-depleted cells, but not in control cells (Fig. 5C). Similar phenomena were observed in the Panc-1 cells expressing SMAD4 shRNA after NAC and CQ treatment (Fig. S4). Furthermore, we found that SMAD4-induced autophagy is partially rescued by ROS scavenger (Fig. S5A) and NAC or CQ did not affect Rad 51 and Brca-1 protein levels (Fig. S5B). Taken together, our data show that the autophagy and ROS inhibitors sensitized pancreatic cancer cells to IR, indicating that SMAD4 loss induced IR resistance is at least partially due to increased level of ROS and autophagy.
Figure 5. SMAD4-induced IR-resistance is partially rescued by pretreatment of autophagy inhibitor or ROS scavenger.
(A) Panc-1 cells were transfected with scrambled siRNA or SMAD4-specific siRNA for 72h. SMAD4 depletion was confirmed by Western blot. (B, C) Cells were pretreated with 10μM chloroquine (CQ) (B) or 3mM N-acetyl-L-cysteine (NAC) (C) for 1 h, and then treated with indicated doses of IR. Colony formation assays were conducted and more than 50 cells counted. Shown are the averages of triplicate samples. Standard errors are shown by error bars. The differences between the two groups selected to be compared were detected using repeated measures ANOVA. *, P < 0.05.
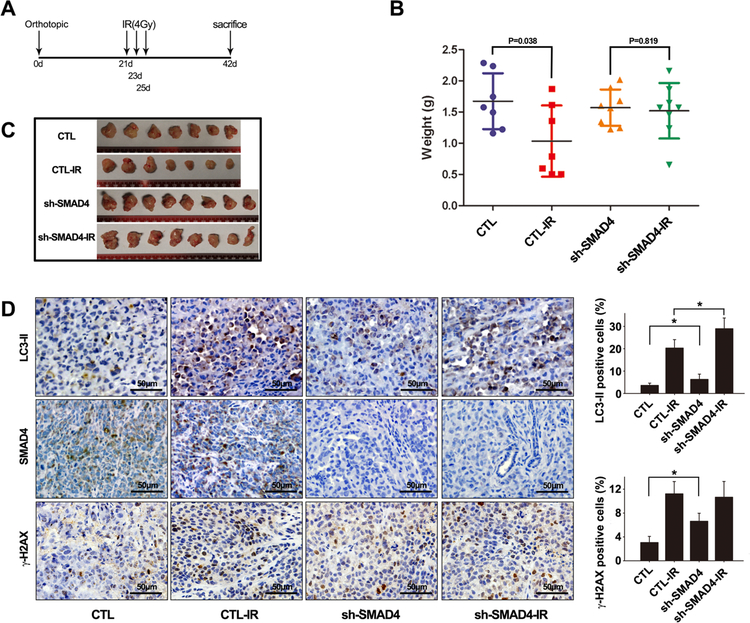
Pancreatic tumors with SMAD4 depletion are resistant to radiotherapy in vivo.
To validate whether the above cell-based findings were applicable in vivo, we generated orthotopic pancreatic cancer murine models by intra-pancreatic inoculation of Panc-1 cells, and treated tumor-bearing mice with IR (Fig. 6A). While growth of the control Panc-1 tumors was significantly inhibited by IR treatment (P = 0.038), SMAD4 depletion largely abolished such radio-sensitivity of Panc-1 tumors (P = 0.819) (Fig. 6B-C). Consistent with the cell-based results, SMAD4 depletion promoted basal LC3II and γ-H2AX expression in tumor tissues(Fig. 6D). We also found that γ-H2AX expression level was higher in tumor sections derived from SMAD4 shRNA-expressing cells than those from control shRNA cells. Similar to the cell-based study (Fig. 2), LC3-II and γ-H2AX expression levels were further increased after IR (Fig. 6D). These results suggest that SMAD4 is essential for pancreatic cancer radiosensitivity.
Figure 6. SMAD4 depletion induced radio-resistance in an orthotopic pancreatic cancer mouse model.
(A) Schemes for the establishment and treatment of the pancreatic cancer orthotopic model. (B, C) Nude mice were injected with 1×106 Panc-1 cells infected with control retroviral vector, or shSMAD4 vector with 5% Matrigel into the pancreas. After 21 days, tumors were irradiated thrice with 4-Gy dose of IR every other day. The mice were sacrificed at day 42, and the tumors were isolated, weighed, and compared between the groups using ANOVA. (D) Immunohistochemistry (IHC) was performed on serial sections using antibodies against SMAD4, LC3 and g-H2AX in tumor tissues.
Discussion
Chemotherapy alone or chemotherapy combined with radiotherapy is used both after surgical resection (adjuvant therapy) and prior to the tumor resection (neoadjuvant therapy) of PDAC in North America(6–8). However, many patients with PDAC are either intrinsically resistant or develop acquired resistance to radiotherapy(44). It is crucial to understand the molecular mechanism of such radioresistance, so as, to develop effective targeted therapies for pancreatic cancer. In this study, we identified a critical role of SMAD4 in mediating pancreatic cancer cells’ response to radiotherapy. Our in vitro and in vivo studies demonstrated that SMAD4 depletion led to radioresistance through induction of autophagy and ROS.
It was reported that 55% of PDAC showed SMAD4 inactivation(16, 17). Therefore, the SMAD4 status is considered to be a molecular biomarker for PDAC. However, how SMAD4 mutations contribute to the efficacy of radiotherapy of PDAC remains elusive. Homozygous deletion of SMAD4 gene or its inactivating mutation was observed in several types of cancer including pancreatic cancer(15) and colorectal cancer(45, 46). Multiple clinical studies showed that the PDAC patients with intact SMAD4 more commonly had local dominant pattern of tumor progression, while the patients with SMAD4 loss commonly had distant disease progression(17, 22–24). Recent research reported that the combination of Runx3 expression levels and SMAD4 status coordinately modulate the balance between dissemination and cell division in PDAC(47). However, very few studies have reported the correlation between SMAD4 and radioresistance in pancreatic cancer. Here we evaluated the effect of SMAD4 loss on the radioresistance of pancreatic cancer cells and in the animal model. We discovered that SMAD4 mutant cell lines were more resistant to IR than SMAD4 WT cell lines. SMAD4 depletion significantly increased IR resistance, and SMAD4 restoration significantly increased IR sensitivity of pancreatic cancer cells in vitro. Consistently, depletion of SMAD4 decreases the IR radiosensitivity of pancreatic tumor in vivo. Mechanistically, we found the SMAD4 depletion-induced IR resistance occurred via elevated levels of ROS and autophagy in pancreatic cancer cells. Pre-treatment of autophagy inhibitor CQ or ROS scavenger NAC significantly increased radio-sensitivity of SMAD4-depleted pancreatic cancer cells.
Ionizing radiation generates large amount of intracellular free radicals and ROS(37, 38), and excessive production of ROS could induce autophagy(40, 41). Moderate autophagy, in turn, assists in the clearance of excessive ROS to protect cells from oxidative damage, which may reflect the balance of either cell survival or death(41, 43). There has been compelling evidence that clinically anticancer therapeutics, including DNA-damaging agents and IR, induce autophagy in cell culture and animal models(48, 49). Autophagy plays a cytoprotective role during anticancer therapy, and its inhibition can sensitize cancer cells to these therapies(31–33, 50). Activation of DNA damage response (DDR) may evoke autophagy as an early adaptive response, which can be considered as the DDR mechanisms of DNA damage tolerance. It is therefore expected that dysregulated autophagy may protect pancreatic cancer cells from IR-induced cellular damage, thereby enhancing radioresistance.
Indeed, we observed elevated level of autophagy in SMAD4-depleted pancreatic cancer cells after IR, and correlates with reduced radiosensitivity. Consistent with above reports, our results suggest that autophagy may function as a cytoprotective mechanism during radiotherapy in pancreatic cancer. Given that the process of autophagy may be used by cells to escape or delay apoptotic death in response to IR-induced DNA damage, molecules regulating autophagy and autophagic cell death may serve as viable targets for eliminating resistance to pancreatic cancer treatment. Resistance to IR in pancreatic cancer has contributed to treatment challenges(9–13). Thus, understanding the molecular mechanisms underlying radioresistance is an important pursuit. In this study, we discovered SMAD4 loss-induced radioresistance, and elucidated the underlying mechanism, which may explain the phenomenon of which have been reported in previously clinical studies(23, 24). Our findings suggested that SMAD4 expression level correlated with radio-sensitivity of pancreatic cancer cells via regulating ROS and autophagy pathway. Thus, assessment of SMAD4 status may represent a vital screening test before radiotherapy. However, further clinical investigations are warranted to evaluate the relationship of SMAD4 status and survival advantages of chemoradiotherapy in patients with pancreatic cancer, which would be helpful to guide the administration of targeted therapies in the adjuvant setting based on SMAD4 status.
In conclusion, we have shown that SMAD4 depletion induced radioresistance in pancreatic cancer cells both in vitro and in vivo through elevated levels of ROS and autophagy under both basal and after IR treatment. However, the special mechanism by which SMAD4 participates into or regulates autophagy need to be confirmed on patient tumor tissues. Our investigation of the role of SMAD4 in regulating autophagy and radiosensitivity revealed a novel function for this frequently mutated gene in PDAC, which may have significant implication in deciding on the proper treatment plan for PDAC patients.
Supplementary Material
Acknowledgments
Financial Support:
The authors acknowledge financial support from the following sources: National Institute of Health grants (1R01CA193880, U54CA143837 and U54CA210181), Department of Defense grants (W81XWH-12–1-0414), National Natural Science Foundation of China (No. 31771497, 81472578), and the Ernest Cockrell Jr. Presidential Distinguished Chair.
Footnotes
Conflict of interest statement:
There are no financial disclosures or conflicts of interest to report for any of the authors.
References
- 1.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- 2.Makohon-Moore A, Iacobuzio-Donahue CA. Pancreatic cancer biology and genetics from an evolutionary perspective. Nature reviews Cancer. 2016;16:553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 4.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP. Adjuvant treatment of pancreatic cancer. European journal of cancer. 2011;47 Suppl 3:S378–80. [DOI] [PubMed] [Google Scholar]
- 6.Tempero MA, Arnoletti JP, Behrman SW, Ben-Josef E, Benson AB, Casper ES, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012;10:703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loehrer PJ Sr., Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kooby DA, Gillespie TW, Liu Y, Byrd-Sellers J, Landry J, Bian J, et al. Impact of adjuvant radiotherapy on survival after pancreatic cancer resection: an appraisal of data from the national cancer data base. Ann Surg Oncol. 2013;20:3634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–82; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–85. [DOI] [PubMed] [Google Scholar]
- 11.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. [DOI] [PubMed] [Google Scholar]
- 12.Adler G, Seufferlein T, Bischoff SC, Brambs HJ, Feuerbach S, Grabenbauer G, et al. [Carcinoma of the pancreas: summary of guidelines 2007, issued jointly by 15 German specialist medical societies]. Dtsch Med Wochenschr. 2007;132:1696–700. [DOI] [PubMed] [Google Scholar]
- 13.Barhoumi M, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouche O, et al. [Locally advanced unresectable pancreatic cancer: Induction chemoradiotherapy followed by maintenance gemcitabine versus gemcitabine alone: Definitive results of the 2000–2001 FFCD/SFRO phase III trial]. Cancer Radiother. 2011;15:182–91. [DOI] [PubMed] [Google Scholar]
- 14.Werner J, Combs SE, Springfeld C, Hartwig W, Hackert T, Buchler MW. Advanced-stage pancreatic cancer: therapy options. Nat Rev Clin Oncol. 2013;10:323–33. [DOI] [PubMed] [Google Scholar]
- 15.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–3. [DOI] [PubMed] [Google Scholar]
- 17.Blackford A, Serrano OK, Wolfgang CL, Parmigiani G, Jones S, Zhang X, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 2009;15:4674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolic A, Kojic S, Knezevic S, Krivokapic Z, Ristanovic M, Radojkovic D. Structural and functional analysis of SMAD4 gene promoter in malignant pancreatic and colorectal tissues: detection of two novel polymorphic nucleotide repeats. Cancer epidemiology. 2011;35:265–71. [DOI] [PubMed] [Google Scholar]
- 19.Wang LH, Kim SH, Lee JH, Choi YL, Kim YC, Park TS, et al. Inactivation of SMAD4 tumor suppressor gene during gastric carcinoma progression. Clin Cancer Res. 2007;13:102–10. [DOI] [PubMed] [Google Scholar]
- 20.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes & development. 2006;20:3130–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer cell. 2007;11:229–43. [DOI] [PubMed] [Google Scholar]
- 22.Tascilar M, Skinner HG, Rosty C, Sohn T, Wilentz RE, Offerhaus GJ, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115–21. [PubMed] [Google Scholar]
- 23.Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crane CH, Varadhachary GR, Yordy JS, Staerkel GA, Javle MM, Safran H, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3037–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bornstein S, White R, Malkoski S, Oka M, Han G, Cleaver T, et al. Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. The Journal of clinical investigation. 2009;119:3408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhola NE, Balko JM, Dugger TC, Kuba MG, Sanchez V, Sanders M, et al. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. The Journal of clinical investigation. 2013;123:1348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papageorgis P, Cheng K, Ozturk S, Gong Y, Lambert AW, Abdolmaleky HM, et al. Smad4 inactivation promotes malignancy and drug resistance of colon cancer. Cancer research. 2011;71:998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang Y, Ling J, Suzuki R, Roife D, Chopin-Laly X, Truty MJ, et al. SMAD4 regulates cell motility through transcription of N-cadherin in human pancreatic ductal epithelium. PloS one. 2014;9:e107948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, Xu L, Guo C, Ke A, Hu G, Xu X, et al. Identification of RegIV as a novel GLI1 target gene in human pancreatic cancer. PloS one. 2011;6:e18434. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Valdiglesias V, Giunta S, Fenech M, Neri M, Bonassi S. gammaH2AX as a marker of DNA double strand breaks and genomic instability in human population studies. Mutation research. 2013;753:24–40. [DOI] [PubMed] [Google Scholar]
- 31.Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer research. 2008;68:1485–94. [DOI] [PubMed] [Google Scholar]
- 32.Rieber M, Rieber MS. Sensitization to radiation-induced DNA damage accelerates loss of bcl-2 and increases apoptosis and autophagy. Cancer Biol Ther. 2008;7:1561–6. [DOI] [PubMed] [Google Scholar]
- 33.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. The Journal of clinical investigation. 2007;117:326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenzelmann Broz D, Spano Mello S, Bieging KT, Jiang D, Dusek RL, Brady CA, et al. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes & development. 2013;27:1016–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang P, Zhang J, Zhang L, Zhu Z, Fan J, Chen L, et al. MicroRNA 23b regulates autophagy associated with radioresistance of pancreatic cancer cells. Gastroenterology. 2013;145:1133–43e12. [DOI] [PubMed] [Google Scholar]
- 36.Tanida I Autophagosome formation and molecular mechanism of autophagy. Antioxidants & redox signaling. 2011;14:2201–14. [DOI] [PubMed] [Google Scholar]
- 37.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell death and differentiation. 2008;15:171–82. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Chen Y, Gibson SB. Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell Signal. 2013;25:50–65. [DOI] [PubMed] [Google Scholar]
- 40.Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–7. [DOI] [PubMed] [Google Scholar]
- 41.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–8. [DOI] [PubMed] [Google Scholar]
- 42.Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–77. [DOI] [PubMed] [Google Scholar]
- 43.Filomeni G, Desideri E, Cardaci S, Rotilio G, Ciriolo MR. Under the ROS…thiol network is the principal suspect for autophagy commitment. Autophagy. 2010;6:999–1005. [DOI] [PubMed] [Google Scholar]
- 44.Yoneyama H, Takizawa-Hashimoto A, Takeuchi O, Watanabe Y, Atsuda K, Asanuma F, et al. Acquired resistance to gemcitabine and cross-resistance in human pancreatic cancer clones. Anticancer Drugs. 2015;26:90–100. [DOI] [PubMed] [Google Scholar]
- 45.Yu J, Wu WK, Li X, He J, Li XX, Ng SS, et al. Novel recurrently mutated genes and a prognostic mutation signature in colorectal cancer. Gut. 2015;64:636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleming NI, Jorissen RN, Mouradov D, Christie M, Sakthianandeswaren A, Palmieri M, et al. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Cancer research. 2013;73:725–35. [DOI] [PubMed] [Google Scholar]
- 47.Whittle MC, Izeradjene K, Rani PG, Feng L, Carlson MA, DelGiorno KE, et al. RUNX3 Controls a Metastatic Switch in Pancreatic Ductal Adenocarcinoma. Cell. 2015;161:1345–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell death and differentiation. 2004;11:448–57. [DOI] [PubMed] [Google Scholar]
- 49.Chen JH, Zhang P, Chen WD, Li DD, Wu XQ, Deng R, et al. ATM-mediated PTEN phosphorylation promotes PTEN nuclear translocation and autophagy in response to DNA-damaging agents in cancer cells. Autophagy. 2015;11:239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gewirtz DA The four faces of autophagy: implications for cancer therapy. Cancer research. 2014;74:647–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.