Abstract
Objective.
To determine the role of brown adipose tissue (BAT) in cancer activity.
Materials and Methods.
The study group comprised 142 patients (121 f, 21 m; mean age: 49±16 yrs) who underwent F18-FDG PET/CT (PET/CT) for staging or surveillance of cancer and who were BAT-positive on PET/CT. BAT volume by PET/CT, abdominal (visceral and subcutaneous) fat and paraspinous muscle cross sectional areas (CSA) were assessed. Groups with and without active cancer on PET/CT were compared using a 2-sided paired t-test. Linear regression analyses between BAT and body composition parameters were performed.
Results.
There were 62 patients (54 f, 8 m) who had active cancer on PET/CT and 80 patients (67 f, 13 m) without active cancer. Groups were similar in age and BMI (p≥0.4), abdominal fat and muscle CSA, fasting glucose, and outside temperature at time of scan (p≥0.2). Patients who had active cancer on PET/CT had higher BAT volume compared to patients without active cancer (p=0.009). In patients without active cancer, BAT was positively associated with BMI and abdominal fat depots (r= 0.46 to r=0.59, p<0.0001) while there were no such associations in patients with active cancer (p≥0.1). No associations between BAT and age or muscle CSA were found (p≥0.1).
Conclusion.
BAT activity is greater in patients with active cancer compared to age, sex and BMI-matched BAT positive patients without active cancer, suggesting a possible role of BAT in cancer activity.
Keywords: FDG-PET/CT, brown adipose tissue (BAT), body composition, cancer activity
Introduction
Recent studies have shown that adipose tissue plays an important role in the development and progression of cancer (1–3). Once considered an inert fat depot, adipose tissue has been recognized as an endocrine and metabolic organ (4). Humans exhibit two types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT), which usually perform opposite physiological functions. WAT represents the largest component of adipose tissue and stores extra energy in the form of triglycerides, breaks down triglycerides and supplies fatty acids to other organs when needed. (5). WAT also secretes adipokines, hormones, growth factors and mesenchymal progenitor cells that can stimulate systemic cell growth and tumor progression (6, 7). WAT is found in subcutaneous and visceral compartments and excess accumulation of WAT, especially visceral adipose tissue (VAT), is associated with increased metabolic risk (8, 9).
BAT, on the other hand, consumes energy by generating heat through the expression of uncoupling protein 1 (UCP1), a process called non-shivering thermogenesis [1]. BAT also plays a role in glucose and lipid metabolism by consuming fatty acids and glucose and by regulating energy homeostasis. BAT is activated by cold exposure, higher in women and in young and lean subjects (10). BAT is characterized by high mitochondrial content and high vascularity (11).
Whereas BAT has been mainly investigated in the context of obesity and metabolic disease (12, 13), new evidence suggests a role in cancer activity and associated metabolic disturbances (14–17). A study in mice has shown accelerated tumor growth following tumor implantation into BAT (17). In addition, significantly increased BAT in the adult mammary fat pad was found in a mouse model of Breast Cancer gene 1 (BRCA1) breast cancer compared to mammary glands from wild-type mice (18). Furthermore, a recent study in humans has indicated a potential role of BAT in breast cancer progression (14). BAT can be quantified non-invasively using 18 F-fluorodeoxyglucose Positron Emission Tomography/Computerized Tomography (PET/CT) (19, 20) and PET/CT is routinely used for staging and surveillance of malignant neoplasms.
The purpose of our study was to determine the role of BAT in cancer activity using PET/CT. We hypothesized that patients with active cancer have more BAT compared to patients with successfully treated cancer.
Materials and Methods
Our study was IRB approved and complied with HIPAA guidelines with exemption status for individual informed consent.
Patients
A retrospective search was performed of all18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) examinations obtained at our institution from January 2006 to December 2015 (n=21,262). We included consecutive patients older than 18 years with a history of malignant neoplasm prior to PET/CT who were BAT positive on PET/CT. We excluded patients who had a history of surgery or radiation therapy to the neck and supraclavicular area which could confound assessment of BAT. Patients with hyperthyroidism were excluded given the increased metabolism of BAT in hyperthyroidism (21). Because BAT FDG uptake might decrease in response to anxiolytic agents or sympathetic blockade (22, 23), patients on beta blockers and benzodiazepines were excluded. Fasting glucose, assessed prior to injection of radiotracer, was recorded. Medical records were reviewed for the presence of cancer cachexia, defined as weight loss greater than 5% over the past 6 months, or weight loss greater than 2% in individuals with BMI <20 kg/m2 or with sarcopenia (24). Outdoor temperatures in Boston for the dates of scans were obtained from the National Weather Service. Clinical characteristics of 45 subjects have been reported previously (25), however, no data on the remaining subjects have been reported and no data on BAT in relation to cancer activity has been published in any of the subjects.
18F-FDG-PET/CT
Whole-body PET/CT (Siemens Biograph 16 or 64, Siemens, Erlangen, Germany or GE Discovery, GE Healthcare, Milwaukee, WI, USA) was performed per standard clinical protocol. Patients fasted 6 hours before the exam in a room in which the ambient temperature was set at 75°F. Blood glucose levels were measured upon arrival and 18F-FDG was injected only if blood glucose was ≤ 200 mg/ dl. 18F-FDG was produced using an on-site 230 MeV isochronous cyclotron. The dose injected was based on patient’s BMI (BMI<30, 15 mCi; 30.1 ≤ BMI ≤44, 20 mCi; BMI >44, 25 mCi). After injection, the patient relaxed in a semi-reclined chair and PET/CT was performed 60 minutes following the injection of FDG. Attenuation correction CT obtained in mid-expiration phase without intravenous contrast (slice thickness 5 mm; table feed per rotation, 18 mm; time per table rotation, 0.5 s; tube voltage, 120 kVp; tube current, 11 mAs; field of view, 48 cm) and PET images were acquired. 3D PET images were obtained from the skull base to the mid-thigh, with 6–8 bed positions lasting 3–7 minutes each. Images were reconstructed to a slice thickness of 2.4 mm. Subsequently, diagnostic contrast-enhanced CT was performed (slice thickness 5 mm; table feed, 15 mm/sec; tube voltage, 120 kVp; tube current– time product, 200 mAs). Images were reconstructed with 2-mm section thickness at 2-mm intervals. The CT scanners used in this study were tested on an annual basis according to American Association of Physicists in Medicine (AAPM) and American College of Radiology (ACR) guidelines (AAPM report #74 and #96 and ACR CT QC manual) and standard clinical quality assurance measures were performed to assess for reproducibility of scans over time.
Image analysis
PET/CTs were reviewed for abnormal radiotracer uptake indicating active primary malignancy or metastatic disease using standard clinical methods. Verification of detected lesions was performed using additional imaging studies performed at time of PET/CT and/or histology. In addition, medical records and follow-up imaging studies were reviewed to confirm the presence/absence of active malignancy at time of PET/CT.
Semiquantitative and qualitative evaluation of BAT was performed on fused FDG-PET and CT images. BAT activity was assessed by measuring FDG uptake along the neck, supraclavicular, mediastinal and paravertebral regions corresponding to adipose tissue attenuation on CT by creating a region of interest to determine standardized uptake values (SUV). SUV were calculated using the following formula: SUV (bw) = Ctis/Dinj/bw, where SUV (bw) is SUV normalized for body weight, Ctis is tissue concentration expressed as megabecquerels per milliliter, Dinj is injected dose expressed in megabecquerels, and bw is body weight expressed as kilograms. The SUVs were used to quantify the volume of BAT (cm3) as the sum of voxel volumes within suspected BAT regions where SUV ≥ 1.5 and HU are between −190 and −10 (19). Analyses were performed using PET-CT Viewer shareware (26) (Supplementary Figure 1).
The non-contrast attenuation-correction CT images were used to assess abdominal adipose tissue and paraspinous muscle cross-sectional areas (CSA) (cm2). Adipose tissue and muscle measurements were performed in the abdomen at the mid-portion of the 4th lumbar vertebra. Measurements performed at the L4 level have been shown to correlate with whole body adiposity (27). Automated thresholding methods were applied using a threshold set for −50 to −250 HU to identify abdominal adipose tissue (28), and −29 to 150 HU to identify muscle tissue (29) (Osirix software version 3.2.1; www.osirix-viewer.com/index.html). Total abdominal (TAT), abdominal subcutaneous (SAT) and visceral (VAT) adipose tissue and paraspinous muscle areas were outlined and mean cross sectional areas (CSA) (cm2) were assessed (Supplemental Figure 2). Intra-reader variability coefficients of variation (CV) for these measurements are 0.6% to 3.8% and interclass correlation coefficients (ICC) are 0.98 to 1.0 (95% confidence interval (CI) 0.83 to 1.0). For inter-reader variability CVs are 3.1% to 3.3% and ICCs are 0.98 to 1.0 (95% CI 0.85 to 1.0).
Statistical Analysis
Statistical analysis was performed using JMP (SAS Institute, Carry, NC) software. Variables were tested for normality of distribution using the Shapiro-Wilk test. Variables that were not normally distributed were log transformed. Groups with and without active cancer on PET/CT were compared using a 2-sided paired t-test. ANCOVA was used to control for cancer type between the groups. Linear regression analyses between BAT and measures of body composition were performed. Standard least squares regression modeling was performed to control for age, BMI, and cancer type. Separate correlation analyses within the groups with and without active cancer were also performed. P < 0.05 was used to denote significance.
Results
Clinical characteristics and body composition, including BAT, are shown in Table 1. We identified 142 patients, 121 women and 21 men with a mean age: 49±16 years (range 20 to 88 years) with a history of cancer who were BAT positive on PET/CT.
Table 1.
Clinical characteristics and body compositions in patients with and without active cancer on PET/CT. Data are presented as mean±SD for continuous variables and n for categorical variables.
| Variable | No active cancer (n=80) |
Active cancer (n=62) |
p-value |
|---|---|---|---|
| Age (years)* | 48±14 | 51±17 | 0.4 |
| Women / Men | 67/13 | 54/8 | 0.6 |
| Outside temperature at time of PET/CT (°C) | 9.3±7.3 | 7.8±7.9 | 0.2 |
| Fasting glucose (mg/dL) | 102.3±12.0 | 103.6±12.4 | 0.6 |
| BMI (kg/m2)* | 25±5 | 25±4 | 0.7 |
| Total abdominal adipose tissue CSA (cm2)* | 278±148 | 272±117 | 0.9 |
| Visceral adipose tissue CSA (cm2)* | 75±64 | 72±46 | 0.9 |
| Subcutaneous adipose tissue CSA (cm2)* | 203±108 | 200±83 | 0.8 |
| Paraspinous muscle CSA (cm2)* | 77±18 | 73±17 | 0.3 |
| Brown adipose tissue volume (cm3)* | 12±16 | 24±45 | 0.009 |
CSA: cross sectional area
Comparison performed on log-transformed data
There were 62 patients (54 women, 8 men) who had active cancer on PET/CT and 80 patients (67 women, 13 men) without active cancer. None of the patients in the active and non-active cancer groups had cancer cachexia. There was no significant difference in the time interval between the last form of treatment and PET/CT between the groups (active vs non-active cancer: 11.5±19.5 months vs 19.6±20.7 months, p=0.2). Outside temperature at time of PET/CT was similar between the groups with and without active cancer. Groups were similar in age, sex, fasting glucose, and BMI, abdominal and muscle CSA. Patients who had active cancer on PET/CT had higher BAT volume compared to patients without active cancer (Figures 1 and 2).
Fig.1.
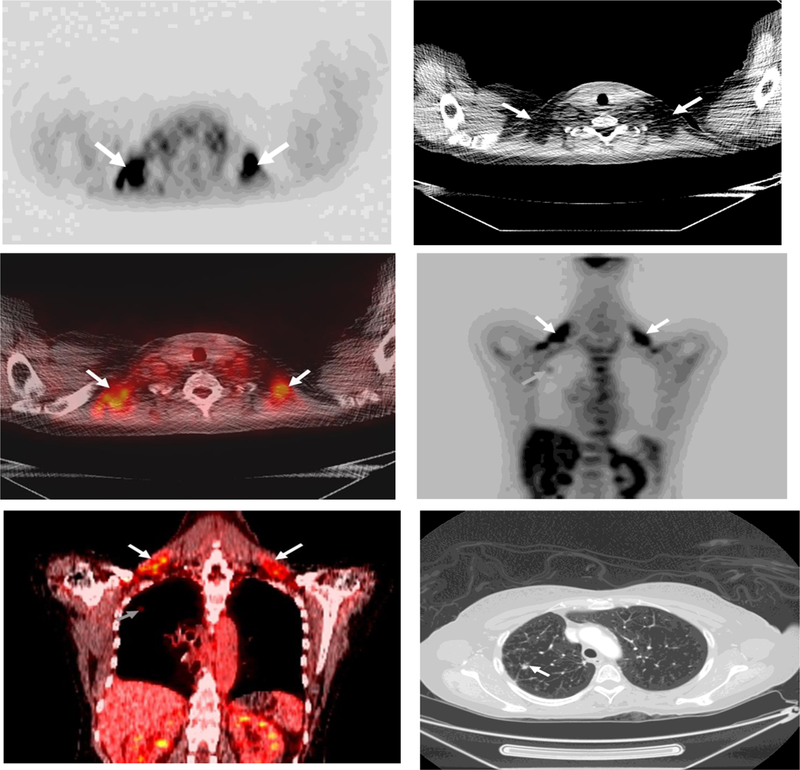
56 year-old woman with non small cell lung cancer (BMI: 25 kg/m2).
Axial PET image (A) demonstrates increased FDG uptake in the supraclavicular areas, which corresponds to fat attenuation on the CT (B) and fused PET/CT (C) (white arrows). Coronal PET (D) and fused PET/CT images (E) demonstrate the extent of brown adipose tissue (BAT) which was 28 mL (white arrows). Note increased FDG uptake of active lung cancer (gray arrows). Axial CT (F) demonstrates the non small cell lung cancer (arrow).
Fig.2.
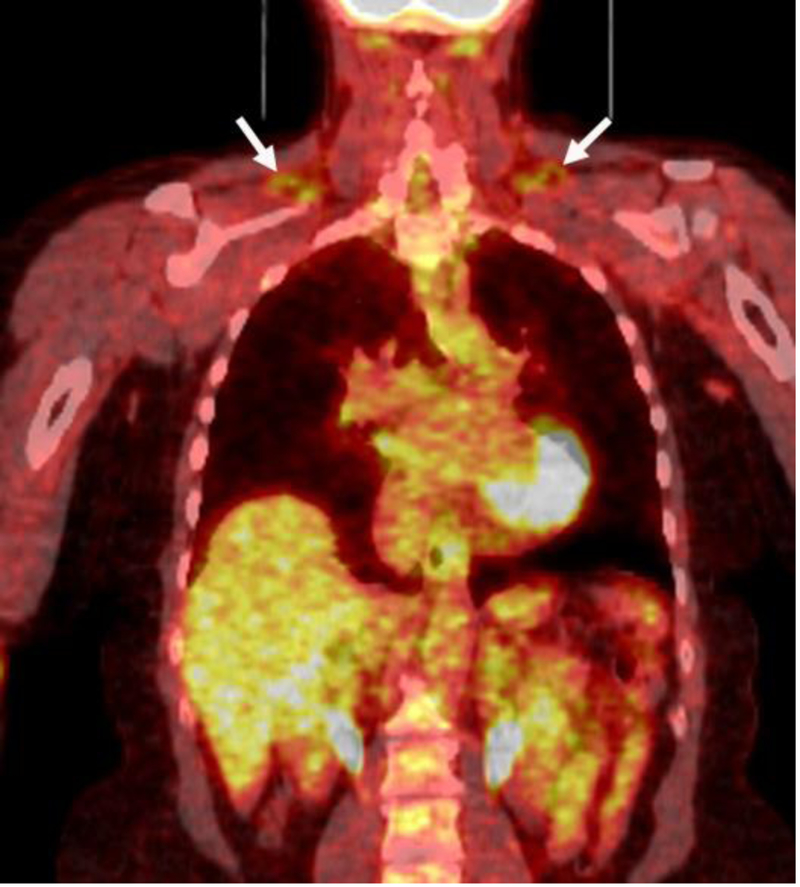
53 year-old woman with successfully treated breast cancer and no active disease on PET/CT (BMI: 25 kg/m2). Coronal fused PET/CT demonstrates a small amount of BAT in the supraclavicular areas (BAT volume: 6 mL).
The primary cancer types are listed in Table 2. After controlling for cancer type, the difference in BAT volume between the groups remained significant (p=0.02).
Table 2.
Overview of cancer types.
| Cancer type | Total n=142 |
No active cancer n=80 |
Active cancer n=62 |
|---|---|---|---|
| Lymphoma | 38 | 27 | 11 |
| Lung cancer | 23 | 4 | 19 |
| Gastrointestinal cancer | 21 | 14 | 7 |
| Breast cancer | 18 | 7 | 11 |
| Melanoma | 12 | 8 | 4 |
| Thyroid cancer | 10 | 7 | 3 |
| Genitourinary cancer | 11 | 8 | 3 |
| Sarcoma/carcinoma of
unknown origin |
9 | 5 | 4 |
Correlation analyses between BAT volume and body composition are shown in Table 3. Within the entire group and within patients without active cancer, BAT was positively associated with abdominal fat (p≤0.0001), independent of age, BMI, and cancer type, while there were no such associations in patients with active cancer (p≥0.1). Similarly, BMI was positively associated with BAT in the entire group and patients without active cancer, independent of age, and cancer type, but not in patients with active cancer (p=0.5). No associations between BAT and age or muscle CSA were found in any group (p≥0.1) (Table 3).
Table 3.
Associations between brown adipose tissue volume and body composition
| log Brown adipose tissue volume | ||||||
|---|---|---|---|---|---|---|
| Variable | Combined | No active cancer | Active cancer | |||
| r | p | r | p | r | p | |
| Age | 0.05 | 0.5 | 0.10 | 0.4 | 0.03 | 0.8 |
| log BMI | 0.32 | 0.0001* | 0.46 | <0.0001* | 0.09 | 0.5 |
| log TAT CSA | 0.43 | <0.0001** | 0.59 | <0.0001** | 0.19 | 0.1 |
| log VAT CSA | 0.36 | <0.0001** | 0.53 | <0.0001** | 0.15 | 0.3 |
| log SAT CSA | 0.40 | <0.0001** | 0.55 | <0.0001** | 0.19 | 0.1 |
| log Muscle CSA | 0.13 | 0.1 | 0.12 | 0.3 | 0.21 | 0.1 |
TAT: total abdominal adipose tissue; VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue, muscle: paraspinous muscle; CSA: cross-sectional area;
Significant after controlling for age and cancer type
Significant after controlling for age, BMI, and cancer type
Discussion
Our study showed that BAT activity is greater in patients with active cancer compared to age, sex, and BMI-matched BAT positive patients without active cancer. Furthermore, BAT was positively associated with abdominal fat depots in patients who did not have active cancer on PET/CT, while no such associations were present in patients with active cancer. Our findings suggest a possible role of BAT in cancer progression and associated metabolic disturbances.
There has been increasing interest in the role of adipose tissue in the development and progression of cancer. While obesity, defined as excess accumulation of WAT, is linked to an increased risk of cancer and reduced survival in cancer patients (30–32), less is known about the role of BAT in cancer activity. While BAT was considered exclusive to rodents and neonates, with the advent of PET/CT, metabolically active BAT has been identified in humans (10, 33, 34).
BAT dissipates nutrient energy as heat through non-shivering thermogenesis by uncoupling protein 1 (UCP1), a protein located in the inner mitochondrial membrane of brown adipocytes (11). Two types of BAT have been identified: the classical brown adipocytes which originate from stem cells of muscle lineage (35) and beige or brite cells which are derived from white adipose cell lineage. The formation of beige cells within WAT is referred to as ‘browning” (36, 37). The myokine irisin, secreted by skeletal muscle, has been shown to induce the expression of UCP-1 and the transformation of white adipocytes into beige/brite cells (38). We therefore matched our groups for fat and muscle areas.
In adults, BAT is primarily found in the neck, supraclavicular, and paravertebral regions and consists of a mix of classical brown and beige cells (39, 40).
Given its high metabolic activity, studies in humans have focused mainly on increasing BAT mass or activity as a new therapeutic approach to obesity and associated metabolic diseases (12, 13). However, recent studies in animals and humans suggest that BAT is involved in cancer activity and its metabolic disturbances. Jones et al. (18) identified UCP-1 positive cells in the adult mammary fat pad in a mouse model of BRCA1 breast cancer compared to mammary glands from wild-type mice. UCP-1 mRNA levels in the Brca1 mutant mice were 50-fold elevated compared to wild-type mice. Furthermore, regions of increased vascularity, as indicated by increased protein expression of CD31, a marker for angiogenesis, were found. They hypothesized that BAT plays a role in breast cancer development by favoring tumor growth through characteristics, such as increased vascularity (18). Lim et al. (17) implanted different cancer types in BAT and found accelerated tumor growth, increased neovascularization, blood perfusion and decreased hypoxia. Cao et al. (14) examined 96 women who had undergone PET/CT for staging of breast cancer and 96 age-and weight-matched women who underwent PET/CT for other malignancies, predominately, colon cancer. Prevalence of positive BAT was significantly higher in patients with breast cancer (16.7%) compared to patients with other malignancies (5.2%) (14). Huang et al. examined PET/CTs of 1740 patients with a history of cancer and of 569 patients without cancer history. Patients with cancer history had higher activity of BAT, which was positively associated with cancer stage (15).
In our study BAT volume was significantly higher in patient with active cancer compared to patient with successfully treated cancer. Multiple factors can affect the activity of BAT, such as age, sex, BMI, blood sugar, cold exposure, or β-adrenergic stimulation (10, 41). We therefore examined only patients who underwent PET/CT using a standardized clinical protocol and who were BAT positive on PET/CT. In addition, to account for the effect of cancer and associated therapy on BAT, we only included patients who had a history of cancer. Furthermore, our groups showed no significant differences in age, sex, fasting glucose, medication use, and BMI, abdominal white fat depots and muscle mass, and comparisons were adjusted for cancer type. Using these strict criteria, our observed higher volume of BAT in patients with active cancer remained significant, suggesting a role of BAT in cancer activity.
Recent studies in animals have implicated BAT in the development of cancer cachexia (16). Cancer cachexia is a complex syndrome that involves profound metabolic imbalances between energy intake and energy expenditure and is a negative prognostic factor for overall survival (42). Enhanced thermogenesis and energy expenditure in BAT is suggested as a reason for the hypermetabolic state of patients with cancer cachexia. Mice with cachexia-inducing colorectal tumors showed increased BAT activity despite thermoneutrality. In addition, inflammatory signaling was observed in BAT as an energetically “wasteful” response in the setting of cachexia (43). Furthermore, browning of WAT was observed in the initial stages of cancer cachexia in mice before significant loss of muscle and fat mass (44).
We observed positive associations between BAT and abdominal fat depots in patients without active cancer, independent of age, BMI, and cancer type, while no such associations were found in patients with active cancer. These findings suggest that BAT plays a different role in modulating body composition depending on cancer activity. There were no associations between BAT and muscle mass. Of note, none of the patients in our cohort had cancer cachexia.
Our study had several limitations including its retrospective nature, and the heterogeneous patient population, with different cancer types. However, we only included BAT positive patients who had a history of cancer and were imaged using a standardized PET/CT protocol. Because we only included BAT positive patients, there was a significantly higher proportion of women, due to the known higher prevalence of BAT in women. This might limit generalizability of our results. In addition, anxiety could have played a role in increased FDG uptake in BAT. A limitation was the use of different imaging equipment over time. However, as both groups were imaged over the same time period, we do not think that those changes would introduce systemic bias. We also performed standard clinical quality assurance measures to assess for reproducibility of scans over time. Moreover, PET/CT is an imperfect standard of reference given its heterogeneity of response and sensitivity to experimental or environmental factors (45). Strengths of our study include the large number of BAT positive patients with detailed assessment of BAT and body composition and the matched groups of patients with and without active cancer.
In conclusion, our preliminary investigation showed that BAT activity is greater in patients with active cancer compared to age, sex and BMI-matched BAT positive patients without active cancer. Moreover, BAT is positively associated with abdominal fat in patients without active cancer, while no such associations are present in patients with active cancer. Our findings suggest a possible role of BAT in cancer activity and associated metabolic disturbances. Prospective longitudinal studies are necessary to assess the effects of BAT on cancer activity and progression.
Supplementary Material
Acknowledgments
Funding: NIH grants K24 DK-109940 and P30DK040561
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was waived for this retrospective study.
References
- 1.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 2011;71(7):2455–65. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Power surge: supporting cells “fuel” cancer cell mitochondria. Cell Metab 2012;15(1):4–5. [DOI] [PubMed] [Google Scholar]
- 3.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta 2013;1831(10):1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89(6):2548–56. [DOI] [PubMed] [Google Scholar]
- 5.Cinti S The adipose organ at a glance. Dis Model Mech 2012;5(5):588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klopp AH, Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res 2011;18(3):771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res 2009;69(12):5259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 2001;50(4):425–35. [DOI] [PubMed] [Google Scholar]
- 9.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21(6):697–738. [DOI] [PubMed] [Google Scholar]
- 10.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360(15):1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84(1):277–359. [DOI] [PubMed] [Google Scholar]
- 12.Whittle AJ, Lopez M, Vidal-Puig A. Using brown adipose tissue to treat obesity - the central issue. Trends Mol Med 2011;17(8):405–11. [DOI] [PubMed] [Google Scholar]
- 13.Wu C, Cheng W, Sun Y, Dang Y, Gong F, Zhu H, et al. Activating brown adipose tissue for weight loss and lowering of blood glucose levels: a microPET study using obese and diabetic model mice. PLoS One 2014;9(12):e113742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Q, Hersl J, La H, Smith M, Jenkins J, Goloubeva O, et al. A pilot study of FDG PET/CT detects a link between brown adipose tissue and breast cancer. BMC Cancer 2014;14:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YC, Chen TB, Hsu CC, Li SH, Wang PW, Lee BF, et al. The relationship between brown adipose tissue activity and neoplastic status: an (18)F-FDG PET/CT study in the tropics. Lipids Health Dis 2011;10:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kir S, Spiegelman BM. Cachexia and Brown Fat: A Burning Issue in Cancer. Trends Cancer 2016;2(9):461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim S, Hosaka K, Nakamura M, Cao Y. Co-option of pre-existing vascular beds in adipose tissue controls tumor growth rates and angiogenesis. Oncotarget 2016;7(25):38282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones LP, Buelto D, Tago E, Owusu-Boaitey KE. Abnormal Mammary Adipose Tissue Environment of Brca1 Mutant Mice Show a Persistent Deposition of Highly Vascularized Multilocular Adipocytes. J Cancer Sci Ther 2011(Suppl 2). [DOI] [PMC free article] [PubMed]
- 19.Chen KY, Cypess AM, Laughlin MR, Haft CR, Hu HH, Bredella MA, et al. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): Recommendations for Standardized FDG-PET/CT Experiments in Humans. Cell Metab 2016;24(2):210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampath SC, Sampath SC, Bredella MA, Cypess AM, Torriani M. Imaging of Brown Adipose Tissue: State of the Art. Radiology 2016;280(1):4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahesmaa M, Orava J, Schalin-Jantti C, Soinio M, Hannukainen JC, Noponen T, et al. Hyperthyroidism increases brown fat metabolism in humans. J Clin Endocrinol Metab 2014;99(1):E28–35. [DOI] [PubMed] [Google Scholar]
- 22.Gelfand MJ, O’Hara S M, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol 2005;35(10):984–90. [DOI] [PubMed] [Google Scholar]
- 23.Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med 2007;32(5):351–7. [DOI] [PubMed] [Google Scholar]
- 24.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12(5):489–95. [DOI] [PubMed] [Google Scholar]
- 25.Bredella MA, Gill CM, Rosen CJ, Klibanski A, Torriani M. Positive effects of brown adipose tissue on femoral bone structure. Bone 2014;58:55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbaras L, Tal I, Palmer MR, Parker JA, Kolodny GM. Shareware program for nuclear medicine and PET/CT PACS display and processing. AJR Am J Roentgenol 2007;188(6):W565–8. [DOI] [PubMed] [Google Scholar]
- 27.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 2004;97(6):2333–8. [DOI] [PubMed] [Google Scholar]
- 28.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr 1982;36(1):172–7. [DOI] [PubMed] [Google Scholar]
- 29.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1998;85(1):115–22. [DOI] [PubMed] [Google Scholar]
- 30.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348(17):1625–38. [DOI] [PubMed] [Google Scholar]
- 31.Chen J Multiple signal pathways in obesity-associated cancer. Obes Rev 2011;12(12):1063–70. [DOI] [PubMed] [Google Scholar]
- 32.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371(9612):569–78. [DOI] [PubMed] [Google Scholar]
- 33.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360(15):1500–8. [DOI] [PubMed] [Google Scholar]
- 34.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360(15):1518–25. [DOI] [PubMed] [Google Scholar]
- 35.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A 2007;104(11):4401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010;298(6):E1244–53. [DOI] [PubMed] [Google Scholar]
- 37.Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab 2012;302(1):E19–31. [DOI] [PubMed] [Google Scholar]
- 38.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012;481(7382):463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 2013;19(5):635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150(2):366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96(1):192–9. [DOI] [PubMed] [Google Scholar]
- 42.Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14(11):754–62. [DOI] [PubMed] [Google Scholar]
- 43.Tsoli M, Moore M, Burg D, Painter A, Taylor R, Lockie SH, et al. Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer Res 2012;72(17):4372–82. [DOI] [PubMed] [Google Scholar]
- 44.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 2014;20(3):433–47. [DOI] [PubMed] [Google Scholar]
- 45.Cypess AM, Haft CR, Laughlin MR, Hu HH. Brown fat in humans: consensus points and experimental guidelines. Cell Metab 2014;20(3):408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


