Abstract
Introduction:
Accurate diagnosis of an inhibitor, a neutralizing antibody to infused factor VIII (FVIII), is essential for appropriate management of haemophilia A (HA). Low-titre inhibitors may be difficult to diagnose due to high rates of false-positive inhibitor results in that range. Transient low-titre inhibitors and false-positive inhibitors may be due to the presence of a lupus anticoagulant (LA) or other non-specific antibodies. Fluorescence immunoassay (FLI) to detect antibodies to FVIII is a sensitive method to identify inhibitors in HA. Evaluations of antibody profiles by various groups have demonstrated that haemophilic inhibitors detected by Nijmegen-Bethesda (NBA) and chromogenic Bethesda (CBA) assays correlate with positivity for anti-FVIII immunoglobulin (Ig) G1 and G4.
Aim:
This study sought to determine whether FLI could distinguish false-positive FVIII inhibitor results related to LAs from clinically relevant FVIII inhibitors in HA patients.
Methods:
Samples from haemophilic and non-haemophilic subjects were tested for LA, specific FVIII inhibitors by NBA and CBA, and anti-FVIII immunoglobulin profiles by FLI.
Results:
No samples from LA-positive non-haemophilic subjects were positive by FLI for anti-FVIII IgG4. Conversely, 91% of NBA-positive samples from haemophilia subjects were positive for anti-FVIII IgG4. Two of 11 haemophilia subjects had samples negative for anti-FVIII IgG4 and CBA, which likely represented LA rather than FVIII inhibitor presence.
Conclusions:
Assessment of anti-FVIII profiles along with the CBA may be useful to distinguish a clinically relevant low-titre FVIII inhibitor from a transient LA in HA patients.
Keywords: factor VIII inhibitor, fluorescence immunoassay, lupus anticoagulant
1 |. INTRODUCTION
A serious complication of haemophilia is the development of inhibitors—neutralizing antibodies against infused exogenous factor VIII (FVIII) in haemophilia A (HA) or factor IX (FIX) in haemophilia B.1,2 Inhibitors are quantified through clot-based assays, such as the Bethesda, Nijmegen-Bethesda (NBA) or chromogenic Bethesda (CBA) assays. These measure functional inhibition of FVIII but not FVIII-specific immunoreactivity.3 Peak inhibitor titres below 5 Bethesda Units (BU) are low-responding inhibitors; while those with peak titres of 5 BU or greater are high-responding inhibitors4 and require use of alternative therapies, such as bypassing agents, to control or prevent bleeding.2,5,6 Immune tolerance induction (ITI) is utilized in certain circumstances to eradicate the inhibitor and return patients to their pre-inhibitor baseline state, but is costly, time-consuming and not uniformly successful.5,6 Assays to accurately determine the presence of a specific FVIII inhibitor are therefore essential for clinical management of patients.
In haemophilia patients, the antibody response against FVIII is polyclonal involving multiple IgG subclasses. Previous studies have demonstrated that IgG1 and IgG4 are the most common anti-FVIII antibody subclasses present in NBA-positive samples.3,7,8 Anti-FVIII IgG4 is found almost exclusively in patients with functional FVIII inhibitors, whereas anti-FVIII IgG1 is also common in patients without functional inhibitors and has been suggested to be predictive of inhibitor development.3,5,8 Hofbauer et al8 have detected high-affinity anti-FVIII IgG1 and anti-FVIII IgG4 antibodies in haemophilia patients over 500 days before the first detection of a FVIII inhibitor by traditional methods. As the relative abundance of IgG4 is the lowest of all of IgG subclasses in human serum,9 the exclusivity of anti-FVIII IgG4 to an evolving inhibitor or inhibitor-positive sample makes it a compelling marker for FVIII inhibitors.
Unlike specific haemophilic inhibitors, nonspecific inhibitors of coagulation do not directly neutralize FVIII or FIX activity but instead interfere with assays measuring coagulation factor levels, independent of FVIII or FIX function.10,11 The archetypical nonspecific factor inhibitor is the lupus anticoagulant (LA), which was reported in 21% of HA patients.12 A LA, through its phospholipid binding, can interfere with measurement of FVIII-specific inhibitors in clot-based assays,10,11,13 resulting in false-positive FVIII inhibitor titres.13,14 Conversely, FVIII inhibitors are reported to interfere with LA assays, producing false-positive results for those tests.15,16 Currently no single laboratory test, such as Dilute Russell’s Viper Venom Time (DRVVT), APTT-LA, Staclot-LA or Dilute Protime, definitively identifies all LAs.12,14,15
As patients with HA may develop a LA in response to common stimuli, including infection, a subset of HA patients may carry both types of antibodies; however, as baseline coagulation assays such as the APTT are prolonged in haemophilia patients, a LA may go unnoticed unless specific studies are performed. Distinguishing a LA from a specific factor neutralizing inhibitor has important implications for research, surveillance and clinical management of patients with haemophilia.3,13 In clinical trials and surveillance programs, false-positive specific factor inhibitor results may contribute to erroneous prevalence and incidence calculations, and mischaracterization of patients.13 In the clinical setting, interference by LAs makes it difficult to identify specific factor inhibitors, which is critically important to determine appropriate therapy.
Although assays to identify a specific FVIII inhibitor, without interference from non-specific inhibitors of coagulation, have been proposed,15,17 they require validation. In this observational study, we tested samples from haemophilia and non-haemophilic subjects to determine whether the NBA, CBA or fluorescence immunoassay (FLI) could discriminate between a LA and a specific FVIII inhibitor.
2 |. MATERIAL S AND METHODS
2.1 |. Subjects
This descriptive study was conducted at the Indiana Hemophilia & Thrombosis Center (IHTC) in collaboration with the US Centers for Disease Control and Prevention (CDC). The investigational protocol was approved by Institutional Review Boards at the CDC and IHTC. Over a 4-year period, blood samples were collected with informed consent from subjects with HA and a diagnosis of a specific FVIII inhibitor; subjects with a suspected or confirmed LA with an underlying diagnosis of HA; and subjects with a LA, but without an underlying diagnosis of haemophilia. The IHTC subjects were followed for 1-year following closure of the study to determine further changes in inhibitor status.
Additional de-identified blood samples obtained through a research agreement with Mid America Clinical Laboratories (MACL) were notable for positive markers for a LA: a positive DRVVT, activated partial thromboplastin-Lupus Anticoagulant (APTT LA), Staclot-LA or Dilute Protime. An additional subset of HA samples was obtained from subjects previously enrolled in the US Hemophilia Inhibitor Research Study (HIRS) conducted by the CDC.18
During the observational study period, 78 samples were collected, 5 of which could not be fully analysed due to laboratory processing concerns. The remaining 73 samples from 53 patients were analysed, as described below. The inhibitor status of 1 IHTC subject changed during the 1-year follow-up period: 2 samples from this subject (collected during routine clinical care) were reviewed and included in this analysis following guidance from the IRB. Previously published data from 116 samples collected from 97 HA patients (HIRS) and 56 samples from 56 paid donors without haemophilia were used as reference groups for comparison.3,13
2.2 |. FVIII inhibitor testing
The NBA and CBA were performed at CDC as previously described13 with positive NBA and CBA results defined as ≥0.5 Nijmegen-Bethesda units (NBU) and chromogenic Bethesda Units (CBU), respectively. The coefficients of variation (CV) for the NBA and CBA were 9.8% and 5.9% for a negative control and 10.2% and 6.9% for a 1 NBU positive control, respectively.
2.3 |. Anti-FVIII antibody testing
FLI was performed at the CDC as previously described.3 The threshold for positivity was set at 2 standard deviations above mean fluorescence intensity (MFI) of results obtained for 56 healthy donors. Results were expressed as MFI. Due to a change in IgG4 detection reagent during this study, the threshold for positivity was 8.3 MFI for samples tested prior to November 10, 2015 and 11.4 MFI thereafter. FLI CV for 3 negative controls were 16.2%, 6.0% and 12.8%. FLI CV for 3 positive controls were 4.1%, 3.8% and 6.1%.
2.4 |. LA testing
Dilute Russell’s Viper Venom Time was performed using LA1 screening and LA2 confirmation reagents (Siemens, Washington, DC, USA) at MACL and with DVVtest and DVVconfirm reagents (American Diagnostica, Stamford, CT, USA) at CDC. A hexagonal phase assay was performed using Staclot-LA (Diagnostica Stago, Parsippany, NJ, USA) and APTT-LA was performed per STAGO PTT-LA (Diagnostica Stago) at MACL. Dilute Protime (dPT) was performed by a MACL modified aPTT assay using citrated plasma (50 μl) and a 1:33 dilution of Innovin (50 μl, Siemens) in Owrens Veronal Buffer (Siemens). After incubation for 3 minutes at 37°C, CaCl2 (50 μl, Siemens) was added and clot time was recorded and compared to the normal range for the dPT assay. Quality control for the dPT assay was performed using a normal and weak lupus positive control.
3 |. RESULTS
Table 1 shows the demographics of the haemophilia study group. Table 2 shows comparative laboratory data from the haemophilia study group, the non-haemophilia study group with positive LA tests and previously published data from a haemophilia reference group of 97 subjects collected during HIRS.13 NBA-negative subjects in the haemophilia reference group had no history of an inhibitor. Eight of 11 subjects in the haemophilia study group had a prior history of ITI therapy or received ITI during the study.
TABLE 1.
Demographics of the haemophilia study group
| Subject | Age at 1st sample draw (y) | Race and ethnicity | Historical peak titre (BU) | Factor VIII mutation | Inhibitor risk |
|---|---|---|---|---|---|
| H1 | 25.6 | C/NH | 2.1 | Intron 22 inversion, c.6429 + ?_6430-?inv | Reported |
| H2 | 65.1 | C/NH | 8.5 | Large deletion, exon 10 (c.1444-?_1537 + ?del) | Reported |
| H3 | 5.8 | C/NH | 9 | Missense c.5188A>G; p.Met1730Val | Unreported |
| H4 | 18.2 | C/NH | 18 | c.5111_5112insAA; p.Asn1704Lysfs*28 | Unreported |
| H5 | 1.5 | C/NH | 9.2 | Not available | NA |
| H6 | 4.8 | C/NH | 52 | Intron 22 inversion, c.6429 + ?_6430-?inv | Reported |
| H7 | 36 | C/NH | 40 | c.1585delC; p.Leu453Phefs*10 | Reported |
| H8 | 2.2 | C/NH | NA | c.4926delA; Glu1642Glufs*21 | Unreported |
| H9 | 3.5 | C/NH | 200 | Intron 22 inversion, c.6429 + ?_6430-?inv | Reported |
| H10 | 2.5 | C/NH | 4 | Intron 22 inversion, c.6429 + ?_6430-?inv | Reported |
| H11 | 1.5 | AA/NH | None | Missense c.901C>T;p.Arg301Cys | Unreported |
AA, African American; C, Caucasian; NH, non-Hispanic; NA, not applicable.
All subjects had severe haemophilia A. The inhibitor risk is based on a literature search of that specific mutation being known to increase the risk of an inhibitor. Reported indicates that there are literature data to indicate that an inhibitor is associated with that mutation. Unreported indicates that that mutation was not known to be associated with an increased inhibitor risk in the literature or other databases.
TABLE 2.
Results of factor VIII (FVIII) inhibitor tests by Nijmegen-Bethesda assay (NBA) and chromogenic Bethesda assay (CBA), anti-FVIII antibodies by fluorescence immunoassay and lupus anticoagulant (LA) tests
| Group | NBA result | Samples, N | Anti-FVIII IgG1 positive | Anti-FVIII IgG4 positive | CBA positive | LA positive |
|---|---|---|---|---|---|---|
| Haemophilia reference group | NBA negative | 50 | 14 (28%) | 2 (4%) | 0 | – |
| NBA positive | 66 | 60 (91%) | 60 (91%) | 56 (85%) | 2/24 | |
| Haemophilia study group | NBA negative | 9 | 8 (89%) | 8 (89%) | 2 (22%) | 3/5 |
| NBA positive | 23 | 22 (96%) | 19 (83%) | 16 (70%) | 9/16 | |
| Non-haemophilia study group | NBA negative | 29 | 3 (10%) | 0 | 2 (7%) | 29/29 |
| NBA positive | 12 | 1 (8%) | 0 | 0 | 12/12 |
Samples were reviewed from: (i) The haemophilia reference group (a summary of previously published data for a group of 97 haemophilia A patients tested as part of the Hemophilia Inhibitor Research Study and used for reference); (ii) The haemophilia study group (the study group of 11 haemophilia patients listed in Table 4); and (iii) The non-haemophilia study group (the study group of 41 non-haemophilia patients with positive LA tests).
In the haemophilia reference group, anti-FVIII IgG4 was positive in 91% of 66 NBA-positive samples and 4% of 50 NBA-negative samples. CBA was positive in 85% of NBA-positive samples and in none of the NBA-negative samples.3,13 In the haemophilia study group, anti-FVIII IgG4 was positive in 83% of 23 NBA-positive samples and 89% of 9 NBA-negative samples; CBA was positive in 70% of NBA-positive samples and 22% of NBA-negative samples.
Among 41 samples from 41 subjects in the non-haemophilia study group (subjects without haemophilia but with a positive LA test), none had a positive anti-FVIII IgG4 result; however, 4 samples (10%) had a positive anti-FVIII IgG1 result. Twelve samples (29%) had a positive NBA titre, and 2 samples (5%) had a positive CBA result. One CBA-positive patient had titres of 0.5 CBU and 0.2 NBU; while the other had titres 0.8 CBU and 0.3 NBU. Table 3 compares anti-FVIII IgG and IgM results for these subjects to the non-heamophilia reference group (previously published data for a paid group of non-haemophilia subjects).3 There was no correlation between individual LA test positivity and either NBA, CBA or FLI result (Table S1).
TABLE 3.
Summary of positive samples in different FLI assays from the non-haemophilia study group (non-haemophilia subjects with a known LA) and in the non-haemophilia reference group (a summary of previously published data from paid donors without haemophilia)
| Group | Anti-FVIII IgG1 positive | Anti-FVIII IgG2 positive | Anti-FVIII IgG3 positive | Anti-FVIII IgG4 positive | FLI-IgGM positive |
|---|---|---|---|---|---|
| Non-haemophilia study group, N = 41 | 4 (10%) | 12 (29%) | 2 (5%) | 0 | 8 (20%) |
| Non-haemophilia reference group, N = 56 | 3 (5.4%) | 3 (5.4%) | 1 (1.8%) | 1 (1.8%) | 4 (7.1%) |
Test results from the 11 subjects in the haemophilia study group are listed in Table 4. LA tests were run based on clinical need (12/21 samples were positive for a LA). Nine subjects (H1-H9) displayed clinical symptoms of a haemophilic inhibitor. Eight of these 9 subjects (H1-H7 and H9) showed positivity for anti-FVIII IgG4 in all samples tested. The inhibitor status of subject H9 changed during the 1-year post-study follow-up period at which time ITI therapy was initiated. Two additional samples (H9-D and H9-E) were reported during this period. None of the other subjects had a change in inhibitor status during this period. One subject (H8) was IgG4 negative in his initial sample; he initiated ITI therapy and at 1-month follow-up his IgG4 was positive. Upon successful tolerization, his IgG4 results once again became negative. Three samples from the remaining 2 subjects (H10 and H11) were positive by NBA. These 2 subjects did not exhibit IgG4 antibodies, were negative by CBA, had positive LA tests and lacked clinical symptoms of an inhibitor through 3 and 2 years of follow-up, respectively.
Table 4.
Laboratory characteristics of samples from haemophilia study group subjects
| Sample ID | Sample interval (months) | NBA result | CBA result | FLI subclass positivity | LA positivity | ITI history/status | Current therapy |
|---|---|---|---|---|---|---|---|
| H1-A | 0 | 0.5 | 0.0 | 1,4 | D | None | BPA |
| H1-B | 26 | 1.5 | 1.5 | 1,4 | — | None | BPA |
| H2-A | 0 | 1.8 | 1.4 | 1,2,4 | — | None | BPA |
| H2-B | 23 | 1.6 | 2.0 | 1,2,4 | D | None | BPA |
| H3-A | 0 | 1.6 | 0.8 | 1,2,4 | A | Previously tolerized | PP |
| H4-A | 0 | 0.3 | 0.1 | 1,4 | A, D, S | Previously tolerized | MP |
| H4-B | 7 | 0.5 | 0.7 | 1,4 | NEG | Ongoing | ITI |
| H4-C | 12 | 0.2 | 0.6 | 1,4 | NEG | Ongoing | ITI |
| H4-D | 29 | 0.1 | 0.1 | 1,2,4 | D | Ongoing | ITI |
| H4-E | 31 | 0.4 | 0.3 | 1,4 | D | Ongoing | ITI |
| H4-F | 36 | 0.9 | 3.4 | 1,4 | D | Ongoing | ITI |
| H5-A | 0 | 1.4 | 0.3 | 1,2,3,4 | D | Ongoing | ITI |
| H6-A | 0 | 3.5 | >2.0 | 1,2,3,4 | NEG | Ongoing | ITI |
| H6-B | 1 | — | — | 1,4 | — | Ongoing | ITI |
| H6-C | 2 | 0.3 | 0.4 | 1,4 | — | Ongoing | ITI |
| H6-D | 5 | 0.5 | 0.3 | 1,4 | NEG | Ongoing | ITI |
| H6-E | 9 | 1.2 | 1.6 | 1,2,3,4 | NEG | Ongoing | ITI |
| H6-F | 10 | 1.9 | 0.8 | 1,4 | NEG | Ongoing | ITI |
| H6-G | 11 | 2.1 | 0.9 | 1,4 | — | Ongoing | ITI |
| H6-H | 15 | 0.8 | 1.1 | 1,4 | — | Ongoing | ITI |
| H7-A | 0 | 4.5 | >2.0 | 1,2,4 | — | Failed | BPA |
| H8-A | 0 | 1.4 | 0.8 | 1,3,M | NEG | None | PP |
| H8-B | 1 | 0.4 | 0.5 | 1,3,4,M | — | Ongoing | ITI |
| H8-C | 2 | 0.1 | 0.4 | 1,2,4,M | — | Ongoing | ITI |
| H8-D | 5 | 0.2 | <0.1 | 1 | — | Tolerized | PP |
| H9-A | 0 | 0.7 | 1.2 | 1,4 | A | Previously tolerized | MP |
| H9-B | 1 | 0.5 | 0.4 | 1,4 | — | Previously tolerized | MP |
| H9-C | 3 | 0.2 | 0.4 | 4 | NEG | Previously tolerized | MP |
| H9-D | 17 | 1.4 | 1.6 | 1,4 | A,S | Previously tolerized | MP |
| H9-E | 27 | 3.3 | 3.4 | 1,4 | — | Ongoing | ITI |
| H10-A | 0 | 0.5 | 0.0 | 1 | D | Previously tolerized | PP |
| H11-A | 0 | 0.8 | 0.0 | 1,2,M | D, S | None | PP |
| H11-B | 1 | 0.5 | 0.3 | None | NEG | None | PP |
A, APTT-LA; BPA, bypassing agent therapy; D, DRVVT; ITI, immune tolerance induction therapy; MP, modified prophylaxis; NEG, negative result; PP, primary prophylaxis; S, STACLOT.
All samples were tested in the FLI assay; 32/33 samples were tested in both the NBA and CBA; 21/33 samples were tested in at least 1 LA assay. Samples were tested for a LA based on the determination of the attending physician, and so not all samples were tested for a LA. Normal range: NBA<0.5 NBU and CBA<0.5 CBU.
Twenty-three of 32 tested samples in the haemophilia study group were NBA-positive, 16 of which (70%) were also positive by CBA (Table 2). Of 7 CBA-negative, NBA-positive samples, 4 (H1-A, H5-A, H10-A, and H11-A) were positive in a LA assay; 2 (H6-D and H11-B) were negative for LA; and one (H9-B) was not tested. Two samples (H4-C and H8-B) were positive in the CBA and negative in the NBA; both were positive in anti-FVIII IgG1 and anti-FVIII IgG4 assays. Seven samples were negative by both NBA and CBA, 6 of which had detectable anti-FVIII IgG4 (discussed below).
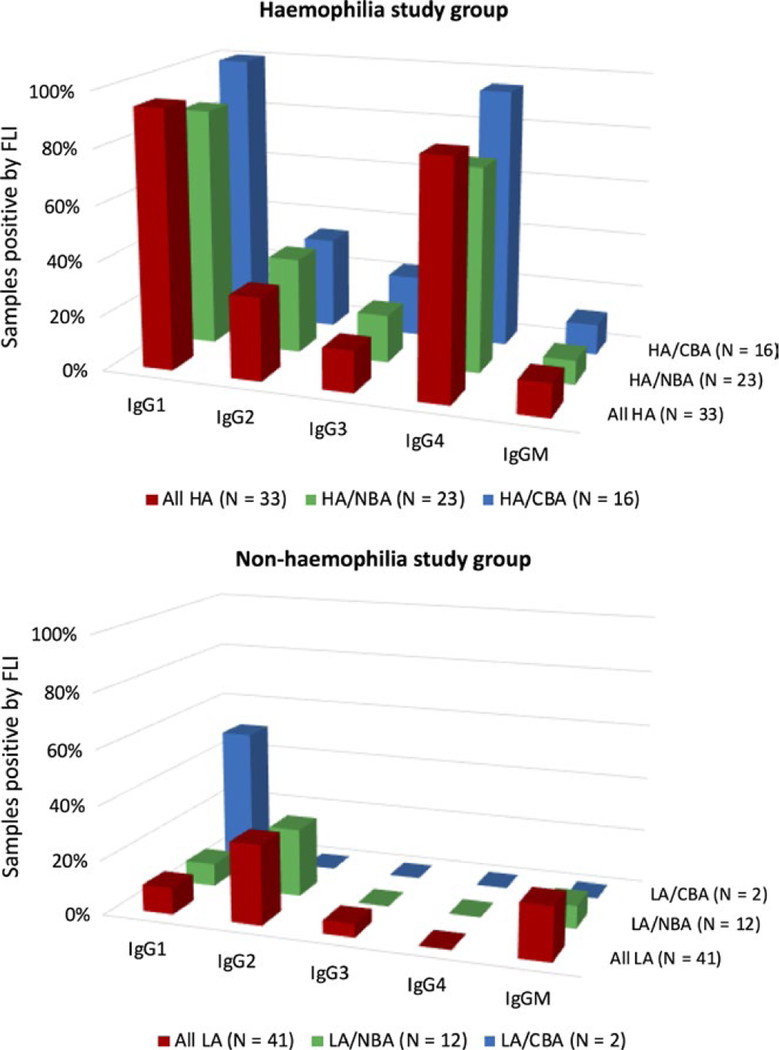
In the haemophilia study group, NBA and CBA results correlated best with positivity for anti-FVIII IgG1 and anti-FVIII IgG4, whereas anti-FVIII IgG2, IgG3 and IgM showed much lower frequencies (Figure 1). Anti-FVIII IgG4 was negative in all samples from the non-haemophilia study group, including those with a positive NBA or CBA titre; anti-FVIII IgG1 was present in 1 of 12 samples with positive NBA and 1 of 2 samples with positive CBA.
FIGURE 1.
Percentage of samples with positive FLI assay results, stratified by: (i) all samples; (ii) samples with a positive NBA titre; and (iii) samples with a positive CBA titre. The “All HA” group is the haemophilia study group; and the “All LA” is the non-haemophilia study group. The “HA/NBA” and “LA/NBA” columns reflect only samples with positive NBA titres; and the “HA/CBA” and “LA/CBA” columns reflect only samples with positive CBA titres
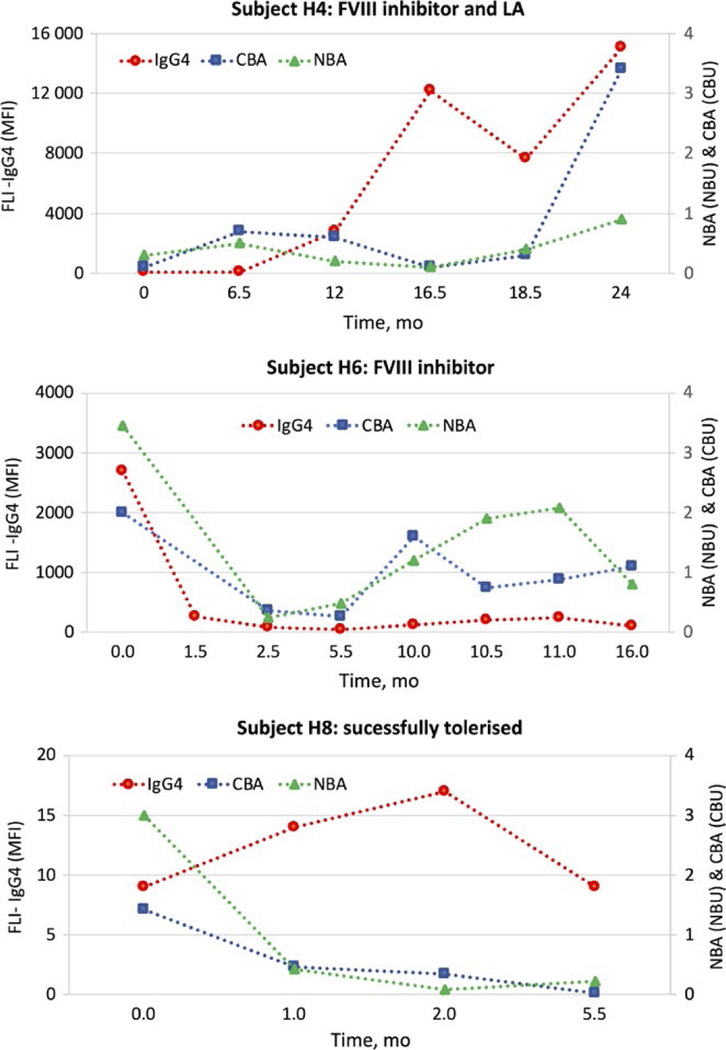
Figure 2 shows longitudinal analyses of anti-FVIII IgG4, NBA and CBA for 3 subjects: subject H4 appeared to have both a clinically confirmed inhibitor and a LA; subject H6 had a history of a persistent inhibitor but no LA; and subject H8 initiated ITI while on the study and was successfully tolerized, as indicated by negative NBA, CBA and anti-FVIII IgG4 results on analysis of his final sample. For these 3 subjects, the NBA and CBA titres were similar, while the levels of anti-FVIII IgG4 varied greatly among individuals (Figure 2). These differences in antibody levels (despite similar functional inhibition) may represent unique characteristics of the antibodies such as affinity and epitope specificity.
FIGURE 2.
Longitudinal analyses of 3 subjects receiving ITI. Results for anti-FVIII IgG4 (MFI), NBA (NBU) and CBA (CBU) are plotted over time using time, t = 0 for the subject’s initial study sample. All anti-FVIII IgG4 results were positive for subjects H4 and H6. NBA and CBA titres varied, giving positive and negative readings at different timepoints—but neither subject was successfully tolerized during the study or 1-year follow-up period. Subject H8 initially had a negative anti-FVIII IgG4, which became positive during the course ITI of ITI therapy. At the final timepoint, subject H8 was considered tolerized, which was reflected in his negative anti-FVIII IgG4, NBA and CBA results and he has remained tolerized during the 1-y post-study follow-up period
4 |. DISCUSSION
To determine appropriate management for patients with inhibitors, clinicians rely on composite assessment of clinical history, bleeding manifestations and inhibitor titre as measured by functional assays; however, LAs are known to cause false-positive results in the NBA.12,13 This problem is exacerbated by the lack of a definitive diagnostic test for LAs and by documented inconsistencies in laboratory testing for FVIII inhibitors.19,20 The CBA is less influenced by a LA than the NBA or BA; and anti-FVIII IgG1 and anti-FVIII IgG4 subtypes have been shown to correlate better with detection of a neutralizing haemophilic inhibitor by CBA than by NBA.5,13
The current study aimed to improve understanding of the effect of a LA on different FVIII inhibitor assays. Our hypothesis was that the immunoreactive profile generated by anti-FVIII FLI could be used to distinguish a haemophilia patient with a LA from a haemophilia patient with a clinically relevant FVIII inhibitor.
The discriminatory value of the anti-FVIII IgG4 assay for distinguishing LAs from FVIII inhibitors is supported by our data which show that none of the 41 samples from the non-haemophilia study group (with positive LA tests)—including those with either a positive NBA or CBA titre—were positive in the anti-FVIII IgG4 assay. These results are similar to those observed in healthy subjects by Whelan et al7 using an ELISA and by Boylan et al using the FLI. The negative results in the FLI strongly support our hypothesis that this assay is unaffected by the presence of Las, whereas the commercial anti-FVIII ELISA, which is reported to measure IgG but is not specific for IgG4, has been reported to give positive results in some LA patients.21 Our results show IgG subclasses other than IgG4 may be present in LA patients and could influence results of this ELISA test. Our data suggest that a specific anti-FVIII IgG4 assay is able to discern a LA from a low-titre FVIII inhibitor with a high discriminatory value, while anti-FVIII IgG1, IgG2, IgG3 and IgGM are less useful. The observation that 12 (29%) LA-positive samples from the non-haemophilia study group were positive in the NBA and 2 (5%) in the CBA in the absence of anti-FVIII IgG4 confirm that these functional assays may be subject to interference.
Data from the haemophilia reference group show a low incidence of anti-FVIII IgG4 antibodies in subjects with a negative NBA (similar to the non-haemophilia reference group) and a high incidence in NBA-positive samples.3 Our haemophilia study group also showed a high correlation between a positive NBA titre and a positive result in the IgG4 assay (19/23, 79%), with stronger correlation being observed between a positive CBA titre and a positive anti-FVIII IgG4 result (17/18, 94%). The single discordant result was from a newly diagnosed inhibitor subject (H8) who, although initially testing negative for anti-FVIII IgG4, became positive within 4 weeks of his initial sample. These correlations support previous findings noted by Miller et al13.
We found that 29/30 samples (97%) from 9 subjects (H1-H9) who exhibited clinical symptoms of a haemophilic inhibitor either during the study or the 1-year follow-up period were positive for anti-FVIII IgG1 and that 28/30 samples (93%) were positive for anti-FVIII IgG4 (Table 4). The two samples negative for anti-FVIII IgG4 were the initial and final samples from a single patient (H8), who developed anti-FVIII IgG4 (and was successfully tolerized) in the interim. It has been previously reported that anti-FVIII IgG4 antibodies are typically observed only in samples with high-titre inhibitors, whereas low-titre inhibitors are predominantly anti-FVIII IgG1-positive.22,23 Our study using the FLI found the vast majority of low-titre inhibitor samples to be both anti-FVIII IgG1 and IgG4 positive.3 Furthermore, our data support the view that anti-FVIII IgG2, IgG3 and IgM are less useful in confirming the presence of a clinically relevant haemophilic inhibitor. As anti-FVIII IgG1 may be present in inhibitor-negative samples, this leaves anti-FVIII IgG4 as the single best indicator of clinically relevant inhibitor.
Samples from subjects H9, H10 and H11 show the value of performing additional testing in questionable cases. Subject H9 had been previously tolerized and was following a modified prophylaxis regimen but his IgG4 levels were consistently elevated. Two years after his initial sample, he showed clinical and laboratory evidence of hemophilic inhibitor through Bethesda, NBA and CBA and reinitiated ITI therapy. The consistently elevated IgG4 levels may have indicated the recurrence of an inhibitor, justifying the need for continued testing. Subjects H10 and H11 were classified as inhibitor positive based on NBA, but this was not confirmed by CBA or FLI. Both subjects were positive in one or more LA assays. The lack of clinical symptoms of a haemophilic inhibitor and the negative anti-FVIII IgG4 results supported the hypothesis that these 2 subjects were instead presenting with a LA.
Longitudinal analyses of subjects undergoing ITI therapy with clinically confirmed, low-titre inhibitors, suggest that the presence of a persistent positive anti-FVIII IgG4 result may have a greater diagnostic value than either the NBA or CBA. Negative back-to-back inhibitor titres were observed for subject H4 (CBA and NBA) and subject H6 (CBA only) during ITI therapy, suggesting successful ITI; however, these assay results were not supported by clinical observation and the subjects’ anti-FVIII IgG4 remained elevated. Within 8 months their NBA and CBA titres once again became positive, consistent with the underlying anti-FVIII IgG4 results and clinical data (Figure 2). Conversely, 1 subject, H8, was successfully tolerized during the study, a result noted in not just his final negative NBA and CBA titres, but also in his final anti-FVIII IgG4 result being negative. Although the significance of these results is limited by the small size of the study, in these cases, the presence or absence of anti-FVIII IgG4 correlated with the necessary continuance of ITI therapy or with successful tolerization, respectively.
There are limitations inherent to this study: most noticeably, the study evaluated a relatively limited number of subjects and samples, and may not be representative of the broader inhibitor population; secondly, recommended repeat LA assay results were not consistently available to confirm presence of a LA, which could potentially impact some of the observed trends; and thirdly, the FLI assay cannot distinguish a low affinity from a high affinity antibody, and so might record a positive result in the absence of a clinically relevant inhibitor.
This study demonstrates the clinical utility of immunologic testing for distinguishing specific anti-FVIII antibodies from LA. A high correlation exists between the previously recommended CBA and the anti-FVIII IgG4 FLI assay, which has a more rapid turn-around time and is less expensive to perform. While cost, delays and uncertainty associated with additional assays may be high, the cost of repeat patient visits, testing and unnecessary therapy is far greater. Based on our observations, we recommend that all samples with low-titre haemophilic inhibitors or with potential LAs are screened in immunologic assays. Recognizing that our data set is relatively small, we further recommend that a prospective study be initiated to further investigate the role of FLI in distinguishing haemophilic inhibitors from interfering LAs.
Supplementary Material
ACKNOWLEDG EMENTS
We thank Meadow Heiman and Angie Summers of IHTC for contributing to the development of the IRB protocol and data collection; Anne Rice, Jennifer Driggers and Dorothy Ellingsen of CDC’s Hemostasis Laboratory, who performed all NBA, CBA, FLI and a subset of DRVVT analyses; Dr. Terrence Cudahy, Director, and the staff of MACL who performed local laboratory testing and retained LA plasma samples for analysis through the research protocol. We also wish to thank both Sonia Nasr, PhD and Ian Mitchell, PhD of Gloval Consulting, Broomfield, CO, for their expert assistance in writing and reviewing this manuscript.
Footnotes
DISCLOSURES
The authors stated that they had no interests which might be perceived as posing a conflict or bias.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Lawrence JS, Johnson JB. The presence of a circulating anticoagulant in a male member of a hemophiliac family. Trans Am Clin Climatol Assoc 1941;57:223–231. [PMC free article] [PubMed] [Google Scholar]
- 2.Kempton CL, White GC 2nd. How we treat a hemophilia A patient with a factor VIII inhibitor. Blood 2009;113:11–17. [DOI] [PubMed] [Google Scholar]
- 3.Boylan B, Rice AS, Dunn AL, et al. Characterization of the anti-factor VIII immunoglobulin profile in patients with hemophilia A by use of a fluorescence-based immunoassay. J Thromb Haemost 2015;13:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White GC 2nd, Rosendaal F, Aledort LM, et al. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost 2001;85:560. [PubMed] [Google Scholar]
- 5.DiMichele DM. Inhibitors in hemophilia: a primer. Treatment of Hemophilia Monographs 2008; No.7:1–9. [Google Scholar]
- 6.Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood 2014;124:3365–3372. [DOI] [PubMed] [Google Scholar]
- 7.Whelan SF, Hofbauer CJ, Horling FM, et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood 2013;121:1039–1048. [DOI] [PubMed] [Google Scholar]
- 8.Hofbauer CJ, Whelan SF, Hirschler M, et al. Affinity of FVIII-specific antibodies reveals major differences between neutralizing and nonneutralizing antibodies in humans. Blood 2015;125: 1180–1188. [DOI] [PubMed] [Google Scholar]
- 9.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014;5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen H, Mackie IJ, Anagnostopoulos N, Savage GF, Machin SJ. Lupus anticoagulant, anticardiolipin antibodies, and human immunodeficiency virus in haemophilia. J Clin Pathol 1989;42:629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro AD, Thiagarajan P. Lupus anticoagulants. In: Spaet T, ed. Progress in Thrombosis and Haemostasis New York, NY: Grune & Stratton; 1982:263–285. [PubMed] [Google Scholar]
- 12.Blanco AN, Cardozo MA, Candela M, Santarelli MT, Perez Bianco R, Lazzari MA. Anti-factor VIII inhibitors and lupus anticoagulants in haemophilia A patients. Thromb Haemost 1997;77:656–659. [PubMed] [Google Scholar]
- 13.Miller CH, Rice AS, Boylan B, et al. Comparison of clot-based, chromogenic and fluorescence assays for measurement of factor VIII inhibitors in the US Hemophilia Inhibitor Research Study. J Thromb Haemost 2013;11:1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verbruggen B. Diagnosis and quantification of factor VIII inhibitors. Haemophilia 2010;16:20–24. [DOI] [PubMed] [Google Scholar]
- 15.Tripodi A Testing for lupus anticoagulants: all that a clinician should know. Lupus 2009;18:291–298. [DOI] [PubMed] [Google Scholar]
- 16.Brandt J, Barna L, Triplett D. Laboratory identification of lupus anticoagulants: results of the Second International Workshop for Identification of Lupus Anticoagulants. On behalf of the Subcommittee on Lupus Anticoagulants/Antiphospholipid Antibodies of the ISTH. Thromb Haemost 1995;74:1597–1603. [PubMed] [Google Scholar]
- 17.Blanco AN, Alcira Peirano A, Grosso SH, Gennari LC, Perez Bianco R, Lazzari MA. A chromogenic substrate method for detecting and titrating anti-factor VIII antibodies in the presence of lupus anticoagulant. Haematologica 2002;87:271–278. [PubMed] [Google Scholar]
- 18.Soucie J, Miller C, Kelly F, et al. A study of prospective surveillance for inhibitors among persons with haemophilia in the United States. Haemophilia 2014;20:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peerschke EI, Castellone DD, Ledford-Kraemer M, Van Cott EM, Meijer P, Committee NPT. Laboratory assessment of factor VIII inhibitor titer: the North American Specialized Coagulation Laboratory Association experience. Am J Clin Pathol 2009;131:552–558. [DOI] [PubMed] [Google Scholar]
- 20.Miller CH, Rice AS, Boylan B, et al. Characteristics of hemophilia patients with factor VIII inhibitors detected by prospective screening. Am J Hematol 2015;90:871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahud M, Zhukov O, Mo K, Popov J, Dlott J. False-positive results in ELISA-based anti FVIII antibody assay may occur with lupus anticoagulant and phospholipid antibodies. Haemophilia 2012;18:777–781. [DOI] [PubMed] [Google Scholar]
- 22.Minno GD, Santagostino E, Pratt K, Konigs C. New predictive approaches for ITI treatment. Haemophilia 2014;20(Suppl. 6):27–43. [DOI] [PubMed] [Google Scholar]
- 23.van Helden PM, van den Berg HM, Gouw SC, et al. IgG subclasses of anti-FVIII antibodies during immune tolerance induction in patients with hemophilia A. Br J Haematol 2008;142:644–652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.