Abstract
What happens to early acquired but later abandoned motor skills? To investigate effects of disuse on early‐developing motor skills, we examined crawling in two groups of habitual crawlers (34 6–12‐month‐old infants and five adults with Uner Tan Syndrome) and two groups of rusty crawlers (27 11–12‐year‐old children and 13 college‐aged adults). Habitual crawlers showed striking similarities in gait patterns, limbs supporting the body, and crawling speed, despite dramatic differences in crawling practice, posture, and body size. Habitual crawlers trotted predominantly, whereas rusty crawlers showed a variety of gait patterns. Within sequences, habitual crawlers and children showed more switches in gait patterns than young adults. Children crawled faster and kept fewer limbs on the grounds than the other groups. Old crawling patterns were retained despite disuse, but new ones were also added. Surprisingly, results indicate that nothing was lost with disuse, but some features of crawling were gained or altered.
Keywords: crawling, disuse, experience, locomotion, motor development, quadrupedal gait
1. INTRODUCTION
As the old saying goes, you never forget how to ride a bike. But is it true? What is the fate of disused motor skills? People acquire new motor skills across the lifespan. Infants learn to reach, sit, crawl, and walk. Children, adolescents, and adults learn to ride a bike, swim, drive a car, play the piano, and perform the kicks, throws, pirouettes, and cartwheels required for sports and other athletic activities. Some early acquired skills remain in use for a lifetime, but others fall by the wayside. Infants abandon crawling in favor of walking, adolescents give up soccer for other leisure activities, and adults forgo their chosen athletics to focus on their careers and families.
Among the roll‐call of abandoned skills, certain early motor skills hold a unique status. They are foundational in infancy but abandoned in childhood, although they remain possible in child‐ hood and adulthood. Coordinating breathing while suckling ensures infants’ survival; crawling and cruising provide infants’ first—and for a time only—means of locomotion. Adults could presumably coordinate interleaved breathing and swallowing, crawl, and cruise, but typically they don’t. Previous work, however, has not examined how the radical change in habitual practice affects motor performance.
Perhaps one reason why researchers have ignored the fate of disused infant motor skills is that habitual practice is confounded with chronological age and body size. It is relatively easy, of course, to find children and adults who can demonstrate a long‐ abandoned infant skill such as crawling, but older participants do not retain baby‐sized bodies and proportions. Likewise, finding children or adults who habitually perform an infant motor skill is extremely difficult. As it turns out, the availability of such a sample of habitual adult crawlers (Shapiro, Cole, et al., 2014) presented us with the opportunity to investigate the fate of motor skills—crawling in particular—that normally are abandoned with development.
1. 1. Locomotion on four limbs
Crawling presents an ideal test case for studying the fate of abandoned infant motor skills. From a practical standpoint, crawling is relatively easy to elicit at various points in the lifespan. Babies, older children, and adults will crawl on demand from one end of a walkway to the other, and older participants will also crawl on a motorized treadmill. Habitual use of infantile skills in adults is rare, and there‐ fore not normally feasible to study. However, we had the fortuitous opportunity to observe habitual crawling in five siblings with Uner Tan Syndrome (UTS) whose primary form of locomotion is crawling (Humphrey, Skoyles, & Keynes, 2005; Tan, 2006a). The siblings live in a remote region of Turkey and the film company (Passionate Productions, www.passionateproductions.co.uk) that documented their unusual form of locomotion agreed to share their videos with us.
Crawling is also ideal for conceptual reasons. Researchers can ex‐ amine both “use” and “disuse.” Most infants crawl for several weeks or months before they walk, and accordingly, the developmental trajectory of crawling proficiency and coordination is well documented (Adolph, Vereijken, & Denny, 1998; Ames, 1937; Freedland & Bertenthal, 1994; Patrick, Noah, & Yang, 2009, 2012 ).
Crawling can be assessed by standard measures of locomotor skill, such as speed and consistency. Across skills, faster, more consistent movements are considered more proficient. However, because crawling involves locomotion on four limbs, it has unique features compared with other forms of locomotion. By changing the sequence and relative timing of limb movements, crawling humans (and other animals that use quadrupedal gaits) can locomote with a wide variety of coordination patterns.
Four‐legged gaits are classified formally in terms of the sequence and relative timing of limb movements (Hildebrand, 1980). Speed is not directly included in formal gait classifications. Thus, formal gait classifications do not map neatly onto the behaviors most lay‐ people imagine when they think of walking, trotting, and galloping. According to the formal classification system, in a perfect trot, the front limb moves at the same time as the hind limb on the opposite side of the body; these limb pairs move in alternation, 50% out of phase with each other. That’s why riding a trotting horse feels so bumpy; the horse bounces from one diagonal limb pair to the next (Schmitt, Cartmill, Griffin, Hanna, & Lemelin, 2006). For the sake of simplicity, in the current paper, we do not consider the exact sequence of footfalls. That is, we consider a gait to be “trot‐like” any time participants display diagonal limb pairings, without specifying which limb in the diagonal pair lands first (i.e., we make no distinction between “lateral” and “diagonal” limb sequences). In a “pace‐like” gait, limbs on the same side of the body move together; imagine a camel swaying from side to side with each step. A variety of quadrupeds also display “single‐foot” gaits—so‐called because each limb moves by itself, with no limb pairings. The equestrian’s notion of horses cantering and galloping are both examples of “galloping” gaits, where footfalls on the left and right side of the body are un‐ evenly spaced in time. When both front legs move roughly together followed by both back legs moving roughly together—like a rabbit fleeing a sudden noise—this is a “bounding” gait.
1. 2. Crawling in infants, children, and adults
Previous studies of crawling cannot address the question of disuse due to differences in procedures, measures, and populations, and no study compared infants, children, and adults. However, previous work suggests important differences and similarities among age groups. Infant crawling is typically trot‐like on hands and knees or hands and feet (Adolph et al., 1998; Freedland & Bertenthal, 1994; Patrick, Noah, & Yang, 2009). Diagonal limb pairings are present from the first weeks of crawling, indicating that prolonged practice is not required to learn the pattern. Adult crawling on hands and knees is more varied than in infants, and includes both trot‐ and pace‐like gaits (Patrick et al., 2009). On hands and feet, adults exhibit more pace‐like gaits, but also exhibit trot‐like and single‐foot gaits (MacLellan, Ivanenko, Cappellini, Labini, & Lacquaniti, 2012; Patrick et al., 2009; Sparrow, 1989; Sparrow & Newell, 1994), and one adult in one study produced two strides of galloping (Patrick et al., 2009). We previously reported that the siblings with Uner Tan Syndrome favor trot‐like gaits but do not use them exclusively (Shapiro, Cole, et al., 2014). Only Hildebrand has described crawling in school‐aged children, a group that shares adults’ lack of recent practice crawling, but is younger, lighter, and possibly more spry. He reported awkward, inconsistent gait patterns in 6‐ to 11‐year‐old children crawling on hands and feet, describing their gait as “conspicuously unnatural and labored” (Hildebrand, 1967, p. 126).
Although Sparrow and Newell (1994) required adults to practice crawling for several weeks prior to testing, they did not compare practiced and unpracticed adult crawlers. Regardless, we cannot know whether several weeks of intermittent practice is equivalent to a lifetime of habitual crawling. Moreover, previous work did not compare the prevalence of gait types, consistency of gait patterns, speed, and limb support (number of limbs touching the floor at one time) among infants, children, and adults—and no previous researchers had the opportunity to compare these age groups with adult habitual crawlers.
1.3. Current study
The primary aim of the current study was to investigate the role of habitual practice on crawling skill: What is gained, lost, or altered with habitual practice and after a decade or two of disuse? We compared crawling in four groups of participants with different amounts of habitual practice (and consequently different ages and bodies) moving on all fours: infants for whom crawling was their primary means of locomotion; 11‐ to 12‐year‐old children who had not crawled habitually in years; young adults who had not crawled habitually in decades; and a unique group of five adult siblings diagnosed with Uner Tan Syndrome (UTS), who had used crawling as their primary form of locomotion for their entire lives. Both the children and young adults lacked recent practice with crawling, but the children were younger, smaller, and possibly more agile than the adults—potentially altering how they coordinate movement on all fours. As habitual crawlers, infants are the usual reference point for studies of human crawling. But most infants crawl habitually only for a few weeks or months. The UTS group gave us the unique opportunity to describe crawling after years, not weeks, of practice.
Children and young adults were instructed to crawl on both hands and knees and hands and feet. Habitual crawlers were observed in their typical posture—infants on hands and knees and the UTS group on hands and feet. Thus, we could not observe both postures in all four groups, but we could observe habitual crawling in both postures— on hands and knees in the infants and on hands and feet in the UTS adults. All participants were tested crawling over ground rather than on a motorized treadmill with a predetermined speed, so that we could observe their spontaneous gait coordination. We asked children and young adults to demonstrate crawling on the spot so that we could examine crawling with no recent practice.
We tested group differences in four critical aspects of crawling. Gait types represent particular patterns of inter‐limb coordination, and switches between gait types reflect the overall consistency of gait coordination across a sequence. Based on previous work, we expected the infants and adults with UTS to display more trot‐like gaits and children and young adults to display both trotting and pacing. We expected the two groups with habitual practice (infants and adults with UTS) to display more intraindividual consistency in gait types compared with the two groups without recent practice (children and young adults).
Speed is a classic measure of locomotor skill. Larger bodies normally lead to higher speed of locomotion—think of a short‐legged person walking beside a long‐legged person. However, with speed normalized to body size, and for cadence (steps/second), which is unrelated to body size, we expected the habitual crawlers to move faster than the “rusty” child and young adult crawlers. Limb support refers to the number of limbs on the ground at one time. It is related to speed, but is not dependent on speed—think of adults running, where both feet are off the ground at one time, and athletes “speed walking,” where one foot is always on the ground but they move at tremendous speeds. Similar to speed, we expected the habitual crawlers to exhibit strides with lower values of limb support com‐ pared with the groups without recent practice.
2. METHOD
2. 1. Participants and procedure
We observed crawling in 83 participants across a range of ages, body sizes, and levels of crawling experience (Figure 1): 34 infants (26 observed once, eight observed longitudinally), 27 school‐age children, and 18 adults (five habitual crawlers, 13 with no recent crawling experience). Data from the infant and UTS groups were culled from three previous studies (Adolph et al., 1998; Shapiro, Cole, et al., 2014; Soska, Robinson, & Adolph, 2015); data from child and young adult groups were collected for the present study. Given that the various datasets used different recording procedures, we only re‐ port measures that are not influenced by differences in the procedures. Videos and illustrative excerpts are shared in the Databrary library (databrary.org).
FIGURE 1.
Four groups of crawlers: (a–b) habitual infant crawlers, (c–d) rusty children, (e–f) rusty young adults, and (g–h) adults with Uner Tan Syndrome. Infants are shown crawling on hands and knees on the (a) gridded foam and (b) carpeted walkways. Both still frames show infants supported on four limbs. Children are shown crawling on (c) hands and knees and (d) hands and feet. Both still frames show children supported on only one limb—a hand in (c) and a foot in (d). Photos are blurry because children were moving so fast. Young adults are shown crawling on (e) hands and knees and (f) hands and feet. The young adult on hands and knees is demonstrating a trot‐like gait, and the crawler on hands and feet is displaying a pace‐like gait. Habitual adult crawlers are shown crawling on hands and feet in (g) and (h)
2.1.1. Infant crawlers
All infants crawled as their habitual method of locomotion and none could walk—verified by the research team at each session. Families were recruited from maternity wards in the New York City area or from published birth announcements in Bloomington, IN. Most families were white and middle income. Twenty‐six 6‐ to 12‐month‐ olds were observed once (15 boys, 11 girls, M age = 9.81 months). Parents reported their infants’ first day of crawling retrospectively in a structured interview (N = 18) or infants were tracked prospectively (N = 8) until they crawled to criterion (at least 3 m on hands and knees without stopping for more than 0.5 s). Crawling experience ranged from 0.06 to 5.5 months (M = 1.9). An additional eight infants were observed twice, at their first and tenth weeks of crawling (five boys, three girls, M age at first visit = 8.96 months). All were tracked prospectively. Families in both groups received small gifts as souvenirs of participation.
Infants in both the cross‐sectional and longitudinal groups were encouraged to crawl across an elevated walkway (Figure 1a,b) covered either with gridded high‐density foam (walkway dimensions: 100 cm wide × 500 cm long × 64 cm high) or plush carpet (walk‐ way dimensions: 83.6 cm wide × 244.5 cm long × 75.3 cm high). Caregivers stood at the far end of the walkway and encouraged their infants to crawl to the end of the walkway using praise, toys, and snacks as incentives. An experimenter followed alongside infants to ensure their safety. Infants wore only diapers and an undershirt so their limbs were clearly visible. Trials were video recorded (30 fps) from a panning view perpendicular to the walkway. We collected 2–11 sequences per infant per session (M = 6.0 trials).
At the end of the session, we measured infants’ body dimensions. Recumbent height ranged from 65.0 cm to 78.7 cm (M = 72.9), and weight ranged from 7.3 kg to 11.8 kg (M = 9.2), and there were no differences in body size between boys and girls.
2.1.2. Children
Twenty‐seven 11‐ and 12‐year‐olds (14 boys, 13 girls) from the New York City area participated as part of a school science field trip (Figure 1c,d). Most were white or Asian and middle or lower income. None had recently practiced crawling. Children crawled two times on hands and knees and two times on hands and feet, counterbalanced for order, across a gridded foam mat (100 cm wide × 596 cm long × 5 cm high) wearing gym shorts and t‐shirts. Children were encouraged to crawl from one end of the mat to the other on either hands and knees or hands and feet with no other instructions about how to crawl. Trials were videotaped (30 fps) with a rolling camera that followed alongside children as they crawled.
At the end of the session, we measured children’s body dimensions. Standing height ranged from 142.5 to 177.5 cm (M = 154.7), and weight ranged from 29.3 to 80.1 kg (M = 46.3). We found no differences in body size between boys and girls.
2.1.3. Young adults
Thirteen college‐aged adults (five men, eight women) from the New York City area volunteered or participated for course credit. Participants were predominantly white and middle class. None had recently practiced crawling. As shown in Figure 1e,f, they crawled across a gridded foam mat (same dimensions as the mat used with children) in gym shorts and t‐shirts for six trials each (three on hands and knees, three on hands and feet). The extra trial was added to ensure a sufficient number of strides in this longer‐legged group. The young adults were asked to crawl to the far end of the gridded foam on either hands and knees or hands and feet, with no other instructions about how to crawl. Trials were videotaped (30 fps) from a series of stationary cameras perpendicular to the walkway.
After the session, we measured adults’ body dimensions. Standing height ranged from 151.5 to 180.2 (M = 169.0), and weight ranged from 48.5 to 85.5 (M = 62.5). Men tended to be larger than women.
2.1.4. Habitual adult crawlers
The habitual adult crawlers (approximately 19–35 years of age) were five siblings (one man, four women) from a family in Turkey; all five crawled as their primary means of locomotion and had been crawling since infancy (Figure 1g,h). The five siblings were diagnosed with Uner Tan Syndrome (Humphrey et al., 2005; Shapiro, Cole, et al., 2014; Tan, 2006b), a disorder characterized by impaired cognitive abilities, an underdeveloped cerebellum, and habitual quadrupedal gait. Similar cerebellar abnormalities cause difficulties with balance, but do not necessarily preclude walking upright in patients who receive therapeutic interventions (see Humphrey et al., 2005). We obtained raw footage of the siblings from the archives of Passionate Productions, who had originally recorded the siblings as part of a television documentary, “The Family That Walks On All Fours” (www.passionateproductions.co.uk/film_family_walks.html). The siblings were videotaped with handheld cameras at 25 fps during their daily routines and also crawled on demand for the documentary. They crawled over a variety of surfaces including flat ground and shallow slopes, sand, mud, linoleum, concrete, dirt and shallow water. They were fully clothed; the man wore loose‐fitting pants and occasion‐ ally shirts, and the women wore ankle‐length traditional skirts, billowy blouses, and headscarves. All five sometimes wore shoes on their hands and/or feet. The siblings contributed 1–20 sequences each (M = 11.8).
2.2. Data coding and processing
Using the video coding software Datavyu (www.datavyu.org), we identified all sequences of crawling in which each step moved the shoulders or hips forward relative to the ground without pauses greater than half a second. Sequences with fewer than five consecutive steps visible on camera were excluded from analyses because at least five steps are required for standard measures of periodic quadrupedal gait (one of each of three limbs and two on the limb that moves first and last, termed a “stride”). Note, however, that five consecutive steps do not constitute a standard stride if more than one limb takes multiple steps over the stride interval. For the included sequences, we coded the stance (stationary) periods of each limb. The onset of stance was the first frame of non‐sliding contact between limb and ground and the offset was the first frame where the limb no longer touched the ground or began sliding forward. To ensure accurate timing measures, a second coder scored the sequences, and disagreements were resolved through discussion. Inter‐rater reliability between the two coders was high. Coders agreed within two frames (67 ms) on 95.3% of stance onsets and 91.2% of stance offsets, and the duration of stance periods identified by the two coders was highly correlated, r = 0.97, p < 0.001. All dependent measures were calculated from these coded stance times.
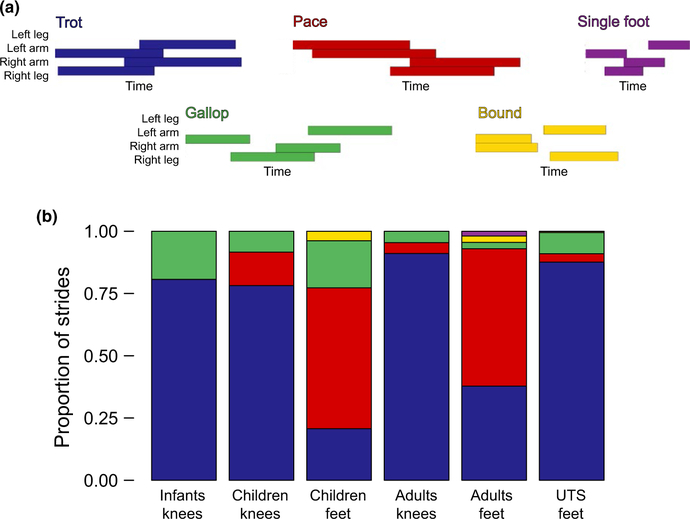
To describe inter‐limb coordination, we calculated gait type (trot‐like, pace‐like, gallop, bound, single‐foot) using a simplified version of standard definitions of quadrupedal gait in animals, Figure 2a (see Cartmill, Lemelin, & Schmitt, 2002; Hildebrand, 1966, 1967, 1977 ). When the arms alternate and the legs alternate (roughly 50% phasing), the pattern is classified as a symmetrical gait: a trot, pace, or single‐foot. We classified gaits as trot‐like when diagonal limbs moved roughly together (i.e., the lag between the left arm and the left leg is 50% ± 25% of the cycle). We classified gaits as pace‐like when limbs on the same side of the body moved together (i.e., the lag between the left arm and the left leg is 0% ± 25% of the cycle). Symmetrical gaits that retained consistent, periodic limb movements but had no limb pairings (i.e., each limb moved independently but in order) were classified as single‐foot gaits. When either the arms or the legs do not alternate, it is an asymmetrical gait: a gallop or bound. We classified gaits as bounds when the arms moved together, the legs moved together, or both. Asymmetrical gaits with no homologous limb pairings were classified as gallops. By definition, calculations of gait type require periodic gait. We therefore identified the subset of crawling steps that formed periodic gait sequences. We excluded stumbles, falls, and steps in which one limb repeated before the other three limbs moved forward. Using a moving window, we identified strides of periodic gait, rather than relying on a predefined reference limb throughout the crawling sequence. This approach allowed us to calculate gait types from any usable portion of a crawling sequence.
FIGURE 2.
Gait types for sequences of periodic gait. (a) Timelines depicting strides in each gait type: trot‐like (diagonal limbs move together), pace‐like (same‐side limbs move together), gallop (arms and legs move independently and asymmetrically), bound (front then back limbs move together), and single‐foot (arms and legs move independently but symmetrically). (b) Average proportion of strides of each gait type for each group crawling on hands and knees or hands and feet
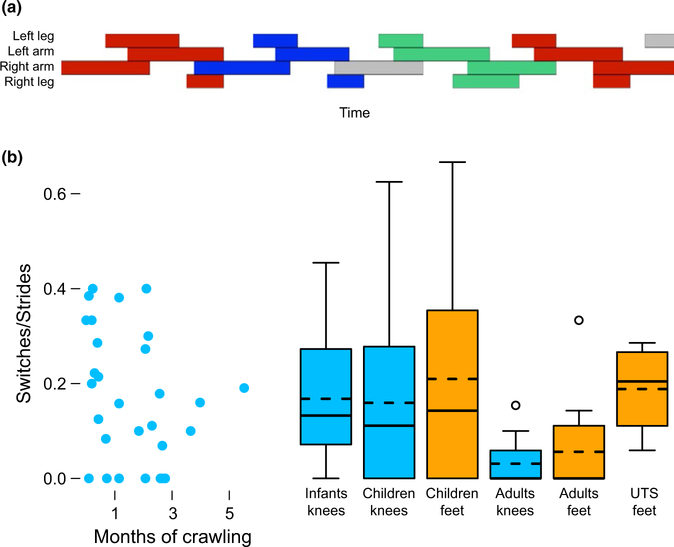
Participants did not always maintain a single gait throughout a crawling sequence; instead, they often switched gaits from one stride to the next, as shown in Figure 3a. We therefore characterized the consistency of gait type by calculating the number of switches within a sequence divided by the total number of strides taken. We chose to normalize switches to strides taken because participants with longer sequences—whether due to longer walk‐ ways or shorter stride lengths—by definition had more opportunities to switch gait types. Note gait types are categorical definitions based on changes in continuous measures. Thus, maintaining the same gait type does not imply perfect within‐gait type consistency in kinematics; conversely, transitioning to a new gait type may reflect either a gradual change sufficient to constitute a different coordination pattern or an abrupt, discontinuous change.
FIGURE 3.
Consistency of gait types. (a) Timeline depicting a single crawling sequence where the participant (a child) switched from a pace‐like gait to a trot‐like gait to a gallop and then back to a pace. (b) Average number of within‐ sequence switches normalized by total number of strides for each participant. Left side: scatterplot showing average number of switches/strides across months of crawling experience in the infants. Each symbol depicts data from one session. Right side: box plots showing average switches/strides for each group crawling on hands and knees or hands and feet. Solid lines denote medians, dashed lines denote means, and circles denote outliers beyond 1.5 times the interquartile range. Note, for young adults, the medians are zero
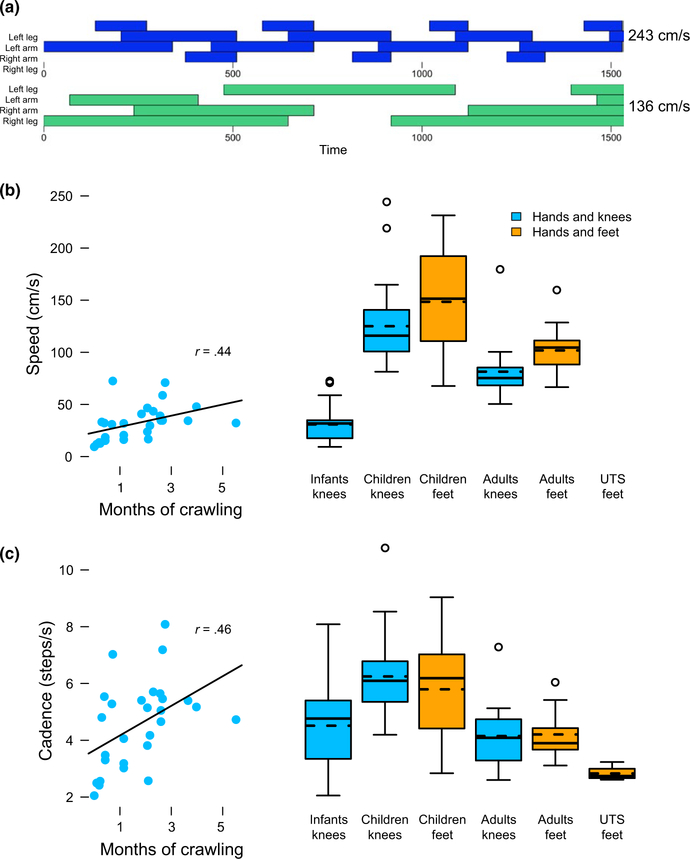
Cursory examination of the continuous crawling sequences revealed that sometimes participants’ gait was not periodic. Novice infants sometimes used flailing, variable combinations of limbs to crawl; more experienced infants and older participants occasionally added or skipped a step. Thus, for all remaining measures, we did not restrict our analysis to the subset of steps reflecting periodic gait. We calculated crawling speed and cadence—both classic measures of locomotor skill—based on the coded times. Crawling speed (cm/second) was the total distance traveled in each laboratory trial di‐ vided by the total time in motion. In common parlance, some gaits imply a certain level of speed—the word “gallop” conjures the image of a sprinting horse, whereas the word “trot” does not. However, a gallop is not by definition faster than a trot; it is possible to gal‐ lop slowly or to trot quickly, as shown in Figure 4a. Because speed is confounded with body size (larger participants take larger steps), we also calculated normalized speed by dividing the distance traveled by participants’ standing height; the resulting measure can be thought of as body lengths/second. Because the siblings with Uner Tan Syndrome were filmed in their everyday environment, we could not obtain accurate distances of their crawling steps and thus could not calculate speed. However, cadence (steps/second) was available for all groups, and was highly correlated with speed in past work (Grieve & Gear, 1966) and in the current study (group r’s from 0.74 to 0.95, ps < 0.001). Cadence is thus included as an indirect measure of crawling speed to allow comparisons across all groups.
FIGURE 4.
Speed and cadence. (a) Timelines depicting a trot (top) and a gallop (bottom) of similar speeds. (b) Average crawling speed (cm/second). Left side: scatterplot showing average speed across months of crawling experience in the infants. Each symbol depicts mean data from one session. Right side: box plots showing average crawling speed for each group crawling on hands and knees or hands and feet. Solid lines denote medians, dashed lines denote means, and circles denote outliers beyond 1.5 times the interquartile range. (c) Average crawling cadence (steps/second). Left side: scatterplot showing average cadence across months of crawling experience in the infants. Each symbol depicts data from one session. Right side: box plots showing average cadence for each group crawling on hands and knees or hands and feet. Solid lines denote medians, dashed lines denote means, and circles denote outliers beyond 1.5 times the interquartile range
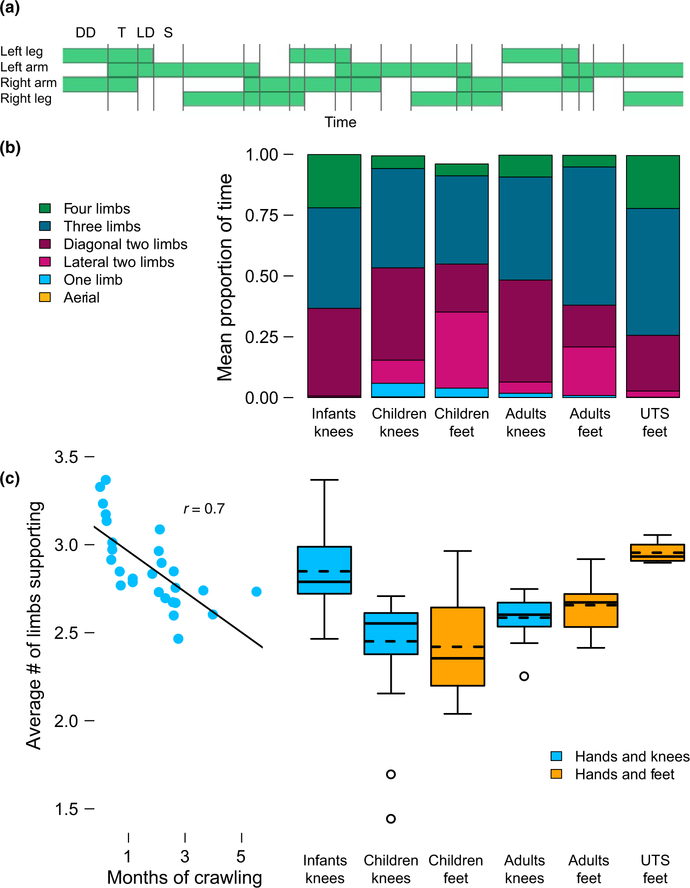
During a single bipedal walking step, the body is supported first by two limbs, then by one; when running, a “flight phase” with no limbs supporting the body is added. In the same way, a quadrupedal crawler experiences multiple combinations of limbs supporting the body during a single stride (Figure 5a). We characterized these patterns of limb support in two ways. First, we calculated the pro‐ portion of time participants were supported by four, three, two, one, or no limbs. Given that flight phases were rare—only eight in our combined dataset of 11,107 crawling steps—the authors confirmed each aerial phase by watching the videos to ensure at least one video frame with all four limbs off the ground. In addition, we calculated the average number of limbs supporting the body throughout the trial. This summary measure takes into account which types of sup‐ port participants experienced (e.g., aerial phases, or phases of sup‐ ported by one, two, three, or four limbs) and how long each type of support lasted during the trial (Shapiro, Young, & VandeBerg, 2014).
FIGURE 5.
Support and flight. (a) Timeline depicting a single crawling sequence (from a child) containing periods of support on one, two, and three limbs (denoted by gray vertical lines). Note, zero‐ and four‐limb support are not depicted. DD = diagonal double limb support, T = triple limb support, LD = lateral double limb support, S = single limb support. (b) Average proportion of time spent in each combination of limbs for each group crawling on hands and knees or hands and feet. Aerial periods were so brief they are not visible in the graph. Average number of limbs supporting the body. Left side: scatter plot showing average number of limbs used for support across months of crawling experience in the infants. Each symbol depicts data from one session. Right side: box plots showing average number of limbs used for support for each group crawling on hands and knees or hands and feet. Solid lines denote medians, dashed lines denote means, and circles denote outliers beyond 1.5 times the interquartile range
2.3. Data analyses
To analyze effects of crawling experience in the infants, we used Generalized Estimating Equations (GEE), which allow for a mix of cross‐sectional and longitudinal data, and treat crawling experience as a continuous variable. For group comparisons of crawling on hands and knees (infants, children, and young adults) and crawling on hands and feet (children, young adults, adults with UTS), we used one‐way ANOVAs; for the longitudinal infants, we included data only from their tenth week of crawling in the ANOVAs so that they contributed only one data point. We used 2 (children and young adults) × 2 (handsknees and hands‐feet) mixed measures ANOVAs to assess differences between postures in the two groups who crawled in both postures.
3. RESULTS
3.1. Periodic gait types
As expected based on past work, trot‐like gaits were predominant, especially during crawling on hands and knees and in the UTS group on hands and feet (Figure 2b); every participant who crawled on hands and knees trotted at least once. But we also observed all five of the gait types we considered (trot, pace, single foot, gallop, bound). In addition to trotting and pacing, we witnessed galloping in every group, including beautifully fluid sequences of galloping in the male with UTS, single‐foot gaits in the young adults, and, to our astonishment, one child who literally bounded across the room like a rabbit and an adult with UTS who bounded for a few strides. We also observed gaits which—although used consistently over several “strides”—did not fit into any existing quadrupedal gait type definition.
3.1.1. Hands and knees crawling
As a group, infants predominantly used trot‐like gaits (M = 79.0% of strides), but also produced strides of galloping (M = 17.4%) and infrequent pace‐like gaits (M = 0.1% of strides). More experienced infants were more likely to trot, as confirmed by a main effect of experience on the proportion of trot‐like strides, χ2 = 7.00, p = 0.008. But a lack of recent experience did not cause the pattern to be lost from the repertoire: Children and young adults showed a similar preference for trotting when crawling on hands and knees (Ms = 77.9% and 93.1% of strides, respectively). Both children and young adults also paced (Ms = 13.5% and 4.4% of strides) and galloped (Ms = 8.6% and 2.5% of strides) while on hands and knees. Young adults were slightly more likely to trot, resulting in a marginal main effect of group on the proportion of hands‐knees strides that were trot‐like, F(2,73) = 2.81, p = 0.07.
3.1.2. Hands and feet crawling
As expected, both children and young adults were less likely to trot on hands and feet compared to crawling on hands and knees, F(1,38) = 91.90, p < 0.001. Children used trot‐like gaits on only M = 20% of strides; instead, they paced (M = 52.9%), galloped (M = 17.2%), or bounded (M = 2.5%). Young adults trotted on only M = 36.6% of strides, instead using pace‐like gaits (M = 56.1%), gal‐ lops (M = 1.9%), and single‐foot gaits (M = 1.1%). In contrast, the UTS group predominantly used trot‐like gaits—M = 83.5% of strides— despite crawling on hands and feet. The UTS group paced on only M = 3% of strides. They also galloped (M = 8.8%), and more rarely bounded (M = 0.7%). The ANOVA confirmed a main effect of group on the proportion of trot‐like strides, F(2,73) = 7.50, p = 0.002; the UTS group were more likely to trot than either children, p = 0.001, or young adults, p = 0.04.
3.1.3. Unclassifiable steps
Many sequences of steps could not be categorized into any known quadrupedal gait type. In some cases, these unclassifiable steps were a result of a brief deviation in an otherwise visible pattern: Participants occasionally deviated from periodic gait by repeating a footfall sooner than expected (e.g., moving the right hand twice within the same stride) or by skipping a footfall altogether (e.g., taking an entire stride without the right hand touching down). In other cases, however, participants continually used coordination patterns that simply do not match any known gait. For example, one child consistently took two steps with the arms for every one step with the feet; although this pattern was cyclical and used consistently during the sequence, such a gait cannot be described by existing periodic gait definitions.
Counting both steps excluded by the moving window and gaits considered unclassifiable, 4.3% of infants’ steps, 4.7% and 1.8% of children and adults’ steps, and 2.9% of steps in the UTS group did not fit into existing definitions of periodic gait. These low group averages conceal substantial inter‐individual variability; although every group contained participants with no unclassifiable steps, the maximum portion of steps excluded was 39% for one infant, 100% for one of the children while crawling on hands and feet, 33% for one young adult, and 11% for one of the adults with UTS. Thus, although trotting was by far the most common gait observed across ages and postures, both it—and existing periodic gait types more broadly—do not accurately describe all patterns of inter‐limb coordination for all crawlers; see also Lee, Cole, Golenia, and Adolph (2018).
3.2. Consistency of coordination within sequences
Even when displaying recognizable gait types, participants did not always produce the same gait consistently within sequences. As depicted in Figure 3a, participants sometimes switched gait types mid‐sequence, occasionally exhibiting multiple switches in a single sequence. In some cases, participants switched back and forth be‐ tween two gaits; in other cases, they switched between three different gaits over the course of a single crawling sequence. The number of mid‐sequence switches ranged from 0–6 in the infants, 0–4 in the children, and 0–2 in the young adults. In the adults with UTS—for whom very long sequences were available—the maximum number of switches observed was 28. Because longer sequences permit more opportunity to switch gait types, below we report all consistency measures relative to the total number of strides.
3.2.1. Hands and knees crawling
Although we expected that more habitual practice would lead to greater consistency, we found no benefit of additional months of crawling experience on how often infants switched gait types within a sequence, χ2 = 2.12, p = 0.15. Likewise, we found no penalty for disuse: Young adults were more consistent than both infants and children, Figure 3b. Only four young adults switched gait types at any point during a sequence, and on average they did so every 11.13 strides. In contrast, 26 of the 34 infants switched gait types (switching every M = 6.38 strides) and 15 of the 27 children switched gaits (switching every M = 4.76 strides). ANOVAs on switches/strides (which includes participants who never switched) and strides/switch (which describes the rate of switching only in those who switched gaits at some point) confirmed the greater consistency in young adults, Fs > 4.42, ps < 0.02. The young adults were more consistent than either infants or children on both measures, ps < 0.04.
3.2.2. Hands and feet crawling
Crawling posture did not affect consistency in the children and young adults, F(1,38) = 0.058, p = 0.81. As on hands and knees, young adults showed the greatest consistency while crawling on hands and feet, Figure 3b. Only four of the 13 young adults switched gait types, com‐ pared to 18 of the 27 children and all participants in the UTS group. The ANOVA confirmed a main effect of age group on the number of switches/stride, F(2,73) = 3.69, p = 0.04. Young adults were more consistent than children, p = 0.03; all other group comparisons were non‐significant. However, unlike hands and knees crawling, there was no main effect of age group on how many strides participants averaged before switching, F(2,43) = 2.00, p = 0.16. Young adults who switched at least once changed gait types every 2.5 strides on average, children who switched changed gaits every 2.4 strides, and the adults with UTS changed gaits every 6.0 strides. Put another way, fewer young adults switched gait types—resulting in a lower aver‐ age number of switches/total strides in this group—but those who switched at least once did so at similar rates across groups.
3.3. Crawling speed & cadence
Figure 4 shows crawling speed and cadence for each group. As in previous work, experienced infants crawled faster than novices. But to our surprise, the groups without recent crawling experience (children and young adults) crawled faster than the habitual crawlers (infants and adults with UTS), even when controlling for differences in body size. Children and young adults also crawled faster on hands and feet than on hands and knees.
3.3.1. Hands and knees crawling
As expected, more experienced infants were faster crawlers than less experienced infants, as revealed by main effects of experience on crawling speed, χ2 = 6.24, p = 0.01 and cadence, χ2 = 20.36, p < 0.001 (scatterplots in Figure 4b). However, the older, rusty crawlers were not hampered by lack of recent experience (box plots in Figure 4b). Young adults crawled faster than infants, and children crawled faster than both groups. Children’s advantage held for normalized speed, indicating that body size was not responsible for the group effect. Children crawled faster (M = 0.58 body lengths/second) than infants (M = 0.29 body lengths/second) and young adults (M = 0.40 body lengths/second). Similarly, children’s cadence was higher than infants and young adults, Figure 4c. The ANOVAs con‐ firmed main effects of group on speed, F(2,70) = 81.03, p < 0.001, normalized speed, F(2,58) = 24.96, p < 0.001, and cadence, F(2,70) = 15.51, p < 0.001, and post hocs confirmed the group differences, ps < 0.001.
3.3.2. Hands and feet crawling
Compared to hands and knees, both children and young adults crawled faster on hands and feet (compare orange and blue bars in Figure 4b), F(1,38) = 17.39, p < 0.001. As on hands‐and‐knees, children crawled faster than adults, F(1,38) = 13.56, p = 0.001. Children averaged normalized speeds of 0.66 body lengths/second, whereas young adults averaged 0.46 body lengths/second (speed data were unavailable for the UTS group). Children were also faster crawlers on hands and feet as measured by cadence: The ANOVA confirmed a main effect of group, F(2,41) = 11.23, p < 0.001. Children’s cadence was higher than young adults (M = 5.79 compared to M = 4.20), p = 0.005, and higher than the UTS group (M = 2.83), p = 0.001, Figure 4c.
3.4. Support and flight
Children not only displayed unexpected gaits and impressive speeds, at times they literally flew down the walkway. We observed seven aerial phases in the children and one in a rusty adult, five on hands and feet and three on hands and knees. However, aerial phases were also extremely brief—only 42.25 ms, on average. In fact, the duration of all types of support could vary widely within a sequence. For example, in the sequence depicted in Figure 5a, the child spent 646 ms in triple support, 1,598 ms in double support, 272 ms in single support, and no time with either zero or four limbs supporting the body. Thus, it is important to take into account how long each type of support lasted. Figure 5b shows the average proportion of each stride spent supported by one, two, three, or four limbs. From the data depicted in Figure 5b, we calculated the average number of limbs that supported the body over the course of the stride, shown in Figure 5c.
3.4.1. Hands and knees crawling
With crawling experience, infants used fewer limbs of support on average, χ2 = 7.68, p = 0.006 (scatterplot in Figure 5c). However, as shown in Figure 5b, infants spent more time supported by all four limbs as compared to the children or young adults, resulting in a higher average limb support—M = 2.80 limbs of support over the cycle (boxplots in Figure 5c). In contrast, the children and young adults spent less time supported by four limbs and more time sup‐ ported by two limbs, yielding an average limb support of 2.45 in the children and 2.58 in the adults. The ANOVA confirmed a main effect of age group, F(2,70) = 17.51, p < 0.001. Infants had higher support values than the children and adults, ps < 0.02.
3.4.2. Hands and feet crawling
Like infants, habitual adult crawlers in the UTS group also spent a greater amount of time supported by all four limbs, resulting in a mean limb support of 2.96. As shown in Figure 5b, the children and young adults spent far less time supported by four limbs, and more time supported by one or two limbs, resulting in a decrease in the average number of limbs supporting the body. Children averaged 2.40 limbs of support over the cycle, and adults averaged 2.66 limbs of support (boxplots in Figure 5c). The ANOVA confirmed a main effect of age group on the average number of limbs sup‐ porting the body, F(2,42) = 11.09, p < 0.001. Children averaged fewer limbs of support than either young adults or the UTS group, ps < 0.02; young adults were not significantly different from the UTS group, p = 0.10.
3.4.3. Types of support
All of the combinations of support seen in the habitual crawlers— the infants and the adults with UTS—were also seen in participants without recent crawling experience (Figure 5b). However, crawlers without recent experience used several patterns of support that were largely absent in the habitual crawlers. All but one of the aerial phases we observed occurred in the children. Children spent M = 6% of the time crawling on hands and knees with only one limb on the ground, yet both infants and adults with UTS spent less than M = 1% of their time with only one limb on the ground. Likewise, habitual crawlers largely avoided lateral two‐limb sup‐ port (body supported by two limbs on the same side of the body) regardless of posture: On average, infants spent only M = 0.1% of their time in lateral two‐limb support, and the adults with UTS spent only 2.7% of the time in lateral two‐limb support. In contrast, children and young adults respectively averaged 31.4% and 21.7% lateral two‐limb support while crawling on hands and feet, and even showed lateral two‐limb support while crawling on hands and knees.
4. DISCUSSION
The primary aim of the current study was to investigate the role of habitual practice on crawling skill: What is gained, lost, or altered with habitual practice and after decades of disuse? To put the answer simply: Nothing was lost. All of the ways of moving favored by habitual crawlers—the gaits, the combinations of limbs used for support, the speeds, and so on—were still observed in groups who had not habitually crawled in years. But just because children and adults can crawl the way they did as infants does not mean they will. Old patterns were retained, but new ones were also added. Children and young adults displayed gaits not seen in habitual crawlers; adults showed greater stride‐to‐stride consistency than infants, children, and UTS adults; and children used speeds and combinations of limb support not seen in habitual crawlers or young adults.
4.1. No penalty for disuse
Speed is a common measure of locomotor skill (Adolph et al., 1998; Bril & Breniere, 1989; Hallemans, De Clercq, & Aerts, 2006; Ivanenko et al., 2004; Sutherland, Olshen, Cooper, & Woo, 1980), and speed indeed increased with crawling experience in our infant sample. Similarly, novice infant walkers spend more time with two limbs on the ground for support than experienced walkers, and with experience infants increase the amount of time spent on one limb (Hallemans et al., 2006; Sutherland et al., 1980). But with no recent practice, the children we observed were incredibly fast, flying down the walkway on all fours—completely contrary to Hildebrand’s (1967) “conspicuously unnatural and labored” characterization of children crawling. Children averaged the fewest number of limbs supporting the body, and even showed true flight phases.
Previous work considered the presence of multiple gait types as evidence of flexibility (Patrick et al., 2009); yet it was the children, not either group of habitual crawlers, who showed the greatest range of crawling gaits. In addition to the trotting and pacing reported in previous work, we also observed sustained galloping and even bounding gaits—neither of which were previously reported. The unusual gaits may have emerged because we included school‐age children in addition to young adults, or because we tested participants crawling overground rather than on a treadmill. Although Patrick et al. (2009) observed two strides of galloping in one adult, the researchers were forced to stop the treadmill due to safety concerns.
Typically, speed is correlated with changes in support and flight. However, the speed‐support relation is not mandatory. It is possible, for example, to increase speed without adding a flight phase as when bipedal speed walkers move quickly while always keeping one foot on the ground. Similarly, primates that move on all fours on tree limbs use single‐foot “ambling” gaits at fast speeds to ensure that at least one limb is always safely in contact with the support sur‐ face. Likewise, extremely heavy animals such as elephants use am‐ bling gaits to avoid whole‐body flight phases when moving quickly (Hutchinson, Famini, Lair, & Kram, 2003; Schmitt et al., 2006). Thus, although the increased speed displayed by the children may have affected other dependent measures, it cannot fully explain their high performance.
In short, in all the markers of locomotor skill tested in the cur‐ rent study, rusty child and young adult crawlers performed as well as—or better than—habitual crawlers. However, school‐age children showed a clear advantage: For most measures, children out‐per‐ formed the young adults. Like the children, adults showed multiple gait types. But crawling speed and the number of limbs supporting the body were at similar levels in young adults and infants. Young adults only surpassed the other groups in the stride‐to‐stride consistency of gait types; they were more consistent than any other group. Yet even in the young adults, nothing was lost. Although adults were less impressive than the school‐age children, they too showed no penalty for disuse. All the ways of moving displayed by habitual crawlers were present after decades of disuse. The gait favored by habitual crawlers—trotting—was observed at least once in all participants, even rusty crawlers. The combinations of limb support seen in habitual crawlers—two‐, three‐, and four‐limb support—were present in all groups. Speed and consistency were similar. We simply found no evidence that what was “known” in infancy was lost or un‐ available to rusty crawlers.
Disuse did not harm crawling ability. But it likely did not help crawling ability either; lack of practice surely did not make children and young adults such proficient crawlers. Rather, during those years or decades of disuse, participants continued to develop. The older participants were bigger, stronger, and likely better coordinated after decades of moving their body in a host of ways. For example, Patrick et al. (2009) theorized that adults may pace in addition to the trotting seen in infants because of their longer legs and better balance control. Children’s performance supports the idea that a “better” body played a role. Children and young adults both lacked recent crawling experience, but the children were lighter, smaller, and likely more agile than the young adults—and were likewise faster, required fewer limbs of support, and exhibited a greater variety of gait types. Moreover, other body characteristics (e.g., body proportions and spinal curvature) may differ between children and adults (Shefi, Soudack, Konen, & Been, 2013; Tardieu, Hasegawa, & Haeusler, 2017). Indeed, Dunbar and Badamb (1998) called the juvenile period “the golden age” of posture and locomotion due to well‐developed strength and coordination combined with a relatively small body size. Nonetheless, it is possible that the children were simply more adventurous than the adults. Children, after all, still play. In the lab‐ oratory, that playfulness may have led children to be more creative and carefree; outside of the laboratory, it may have led to past experiences with other unusual motor activities that facilitate crawling. Further work is needed to elucidate the role of body proportions in crawling proficiency in habitual crawlers and people without recent crawling experience.
4.2. Benefits of habitual practice
As expected, infants showed gains in crawling skill on most measures with experience. Over weeks of crawling, infants displayed more trot‐like strides, faster speeds, and fewer limbs on the floor for support. However, despite decades of additional practice, adults with UTS performed no better than infants on any measure. At first blush, this may appear puzzling: Shouldn’t years of habitual practice outshine weeks or months of practice? However, motor skill acquisition typically shows asymptotic performance, where skill increases dramatically at first, then tapers off. By 5–7 years of age, for example, children’s gait is as mature as adult gait for many parameters (Breniere & Bril, 1998; Bril & Ledebt, 1998; Sutherland et al., 1980).
Moreover, children and young adults outperformed both habitual groups. So is there really no benefit of habitual practice after a period of initial skill acquisition? The logical answer would be no. Children and adults were flashy, but impractical and inefficient. Flight phases are clearly risky and high speeds require great energy expenditure. Children and adults likewise used potentially unstable gaits and types of limb support. The child who bounded down the walk‐ way provides an extreme example, but even the relatively common pacing (and corresponding lateral two‐limb support) is potentially unstable. Lateral two‐limb support requires weight to be supported entirely on the left or right side of the body. Diagonal two‐limb sup‐ port, in contrast, spreads weight evenly across the body; crawling gaits that entail diagonal, rather than lateral, two‐limb support may therefore be more stable (Cartmill et al., 2002). Differences in stability may explain why our habitual crawlers avoided lateral two‐limb support and gaits such as the pace.
In fact, it is particularly interesting that the UTS group displayed so little pacing, despite crawling on hands and feet. Longer‐legged animals frequently pace, presumably to avoid having the back legs “bump into” the front legs as they would during a trotting gait (Cartmill et al., 2002; Hildebrand, 1968; Shapiro & Raichlen, 2005; Young, 2012). Crawling on hands and feet extends the length of the back legs, and accordingly, our rusty child and young adult crawlers performed more pacing while on hands and feet. But despite crawling in the same posture, the UTS group did not use pacing as the solution to the problem of long limbs. Instead, they stuck with the likely more stable trot‐like patterns, just as they displayed more moderate speeds.
Possibly the laboratory testing situation for children and young adults favored short bursts of impractical behavior because there was little penalty for error or inefficiency. The foam mats used in the lab‐ oratory testing might have been more forgiving of errors than other environments, and the short distances might have allowed for more flamboyant behavior. If we had asked children to crawl for extended periods of time, perhaps looping around a track, they might eventually have settled into the same patterns as the habitual crawlers. However, the testing conditions in the laboratory cannot fully explain habitual crawlers’ reluctance to use “flashy” gaits or speeds. Infants were tested in the same laboratory setting and encouraged to crawl quickly; the adults in the UTS group were filmed in the real world, but were explicitly asked by the filmmakers to demonstrate their skill as crawlers. Indeed, the filmmakers commented that the male in the UTS group eagerly “showed off” his skills, and produced gorgeous sequences of galloping. Thus, both groups had the opportunity to be‐ have as the children and young adults did, but they either could not or would not. During everyday life, habitual crawlers accumulate longer times and longer stretches of crawling per day in a more variable con‐ text, where energy efficiency and stability pay off; even when these constraints were removed, habitual crawlers did not switch to the less practical ways of crawling seen in the children. It could be that the habitual crawlers were incapable of the feats of coordination seen in the children. Or, it might simply be that habitual crawlers saw no rea‐ son to abandon perfectly functional, habitual ways of crawling. Either way, habitual crawlers were, in a word, habitual.
The fact that habitual crawlers stick to more functional, safe gaits even when given the opportunity to demonstrate “flashier” forms of crawling highlights a related point. Skill measured in the straight‐ line paradigm (fast crawling with multiple possible gaits) may not indicate skill in more real‐world challenges such as adapting gait to changing terrain. The straight‐line skill paradigm, after all, bears little resemblance to real‐world locomotion (Adolph et al., 2012; Lee et al., 2018). Locomotor experience is crucial for infants to accurately perceive the limits of their abilities (Adolph, 1997, 2000 ; Kretch & Adolph, 2013), and even adults judging their abilities for otherwise familiar actions show increased accuracy following brief practice in the laboratory (Cole, Chan, Vereijken, & Adolph, 2013; Franchak, van der Zalm, & Adolph, 2010). The straight‐path task used here does not emphasize these kinds of flexible, adaptive judgments about locomotion; it is designed only to index coordination and skill under optimal conditions. In a more functional task that requires coping with a variable, changing environment by either adapting movements or deciding which movements are possible, the UTS group may have shown an advantage from their decades of practice, and children and young adults may have shown a deficit.
4.3. Consistency as a marker of skill
In many locomotor tasks—indeed, in most motor tasks involving a degree of repetition—more consistent performance is considered more skillful performance (Adolph, Cole, & Vereijken, 2015). The UTS group was not more consistent than any other group, perhaps due to their extensive experience or their underlying pathology. Regardless, the infants did not show gains in consistency with experience, as might be expected. Likewise, all groups showed deviations from periodic gait. Our laboratory set‐up involved crawling over flat, even ground in a straight line—arguably the best scenario for observing consistent, repeating gaits. Nevertheless, we saw no evidence that habitual crawlers optimized for consistency, and all groups— even the young adults, who showed the greatest stride‐to‐stride consistency—produced steps that deviated from periodic gait.
Although it may seem counter‐intuitive, inconsistent performance in experts is not necessarily surprising. Variability is not inherently good or bad; its meaning depends on the context and functional consequences of inconsistency (for reviews, see Adolph et al., 2015; Adolph & Robinson, 2015; Fetters, 2010; Vereijken, 2010). In situations where variability has a penalty, it typically de‐ creases as skill increases. A wobbly, lurching toddler will likely fall; a newly‐reaching infant’s flailing hand is likely to miss its target. Yet in other situations, moving adaptively requires variability. Moving over rough, uneven terrain requires step‐to‐step variability as the walker or crawler adjusts each step to the changing conditions. Likewise, atypically developing infants show higher motor variability than their typically developing peers in some instances (Looper, Wu, Barroso, Ulrich, & Ulrich, 2006), but lower variability—leading to rigid, stereo‐ typed behavior—in others (Hadders‐Algra, 2002). Variability adheres to the “Goldilocks principle”: Behavior must be consistent enough to achieve success, but variable enough to allow for future flexibility and learning (Fetters, 2010).
Thus, consistency does not automatically go hand‐in‐hand with greater skill. Rather, consistency will be selected when the setting does not require adaptations of behavior, and when inconsistency incurs a penalty. When accuracy, efficiency, or safety are crucial and are threatened by inconsistent performance, skilled actors avoid inconsistency. But without such constraints, some degree of variability and inconsistency will persist. For example, even experienced belly‐crawling infants are wildly inconsistent, moving with different combinations of arms, legs, and torso from day to day, trial to trial, and stride to stride (Adolph et al., 1998). After infants begin crawling on hands and knees—lifting the torso up off the ground— balance becomes more crucial, and variability is sharply reduced.
It would appear that the constraints on our crawlers were relatively mild; the biomechanical and energetic constraints were not strong enough to completely reduce variability. The constraints may have been sufficient to cause the habitual crawlers to focus on a more limited number of gaits, but were not sufficient to make them use the same gait on stride after stride. Likewise, all groups occasionally deviated from periodic gait—even the young adults, who showed the greatest stride‐to‐stride consistency. In our testing scenario, inconsistency from one stride to the next or brief deviations from periodic gait had no clear benefit, but they likewise had no clear penalty. Without any particular pressure to remove it, a certain amount of inconsistency persisted—as it does in many behaviors.
5. CONCLUSION
So, what is the fate of disused motor skills? In the case of crawling, and presumably for some of the other motor skills we may abandon in the course of development (e.g., riding a bicycle or playing piano), it is possible that disuse comes with no penalty—at least on some critical metrics (keeping balance on the bicycle, coordinating finger movements with key location, etc.). However, for other motor skills, habitual practice may be necessary to maintain the basic level of performance (e.g., turning a cartwheel or doing a headstand). And for still other skills, performance may deteriorate even with years of habitual practice (e.g., gymnastics or ballet dancing). Some aspects of some skills are not lost with disuse; some aspects of some skills are lost even with habitual practice. In all cases, changes in body size, agility, information‐processing speed, and other factors likely play a role.
ACKNOWLEDGMENTS
This research was supported by National Institute of Health and Human Development Grant R37‐HD33486 to Karen E. Adolph. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD. We would like to thank Passionate Productions for providing video footage. We also thank Gladys Chan, Shoshana Leftin, Omran Majumder, and Dejana Mladenovic for coding assistance, and the members of the NYU Infant Action Lab for assistance with data collection.
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: R37-HD33486
REFERENCES
- Adolph KE (1997). Learning in the development of infant locomotion. Monographs of the Society for Research in Child Development, 62(3), 1–140. 10.2307/1166199. [DOI] [PubMed] [Google Scholar]
- Adolph KE (2000). Specificity of learning: Why infants fall over a veritable cliff. Psychological Science, 11, 290–295. 10.1111/1467-9280.00258 [DOI] [PubMed] [Google Scholar]
- Adolph KE, Cole WG, Komati M, Garciaguirre JS, Badaly D, Lingeman JM, … Sotsky RB (2012). How do you learn to walk? Thousands of steps and dozens of falls per day. Psychological Science, 23, 1387–1394. 10.1177/0956797612446346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Cole WG, & Vereijken B (2015). Intra‐individual variability in the development of motor skills in childhood. In Diehl M, Hooker K, & Sliwinski M (Eds.), Handbook of intra‐individual variability across the lifespan (pp. 59–83). New York: Routledge/Taylor & Francis Group. [Google Scholar]
- Adolph KE, & Robinson SR (2015). Motor development. In Liben L, & Muller U (Eds.), Handbook of child psychology and developmental science (7th edn., Vol. 2 Cognitive Processes, pp. 114–157). New York: Wiley. [Google Scholar]
- Adolph KE, Vereijken B, & Denny MA (1998). Learning to crawl. Child Development, 69, 1299–1312. 10.1111/j.1467-8624.1998.tb06213.x. [DOI] [PubMed] [Google Scholar]
- Ames LB (1937). The sequential patterning of prone progression in the human infant. Genetic Psychology Monographs, 19, 409–460. [Google Scholar]
- Breniere Y, & Bril B (1998). Development of postural control of gravity forces in children during the first 5 years of walking. Experimental Brain Research, 121, 255–262. 10.1007/s002210050458 [DOI] [PubMed] [Google Scholar]
- Bril B, & Breniere Y (1989). Steady‐state velocity and temporal structure of gait during the first six months of autonomous walking. Human Movement Science, 8, 99–122. 10.1016/0167-9457(89)90012-2 [DOI] [Google Scholar]
- Bril B, & Ledebt A (1998). Head coordination as a means to assist sensory integration in learning to walk. Neuroscience and Biobehavioral Reviews, 22, 555–563. 10.1016/S0149-7634(97)00044-4 [DOI] [PubMed] [Google Scholar]
- Cartmill M, Lemelin P, & Schmitt D (2002). Support polygons and symmetrical gait in mammals. Zoological Journal of the Linnean Society, 136, 401–420. 10.1046/j.1096-3642.2002.00038.x. [DOI] [Google Scholar]
- Cole WG, Chan GLY, Vereijken B, & Adolph KE (2013). Perceiving affordances for different motor skills. Experimental Brain Research, 225, 309–319. 10.1007/s00221-012-3328-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar DC, & Badamb GL (1998). Development of posture and locomotion in free‐ranging primates. Neuroscience and Biobehavioral Reviews, 22, 541–546. 10.1016/S0149-7634(97)00042-0 [DOI] [PubMed] [Google Scholar]
- Fetters L (2010). Perspective on variability in the development of human action. Physical Therapy, 90, 1860–1867. 10.2522/ptj.2010090. [DOI] [PubMed] [Google Scholar]
- Franchak JM, van der Zalm D, & Adolph KE (2010). Learning by doing: Action performance facilitates affordance perception. Vision Research, 50, 2758–2765. 10.1016/j.visres.2010.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland RL, & Bertenthal BI (1994). Developmental changes in interlimb coordination: Transition to hands‐and‐ knees crawling. Psychological Science, 5, 26–32. 10.1111/j.1467-9280.1994.tb00609.x. [DOI] [Google Scholar]
- Grieve DW, & Gear RJ (1966). The relationships between length of stride, step frequency, time of swing and speed of walking for children and adults. Ergonomics, 5, 379–399. 10.1080/00140136608964399 [DOI] [PubMed] [Google Scholar]
- Hadders‐Algra M (2002). Variability in infant motor behavior: A hallmark of the healthy nervous system. Infant Behavior and Development, 25, 433–451. 10.1016/S0163-6383(02)00144-3 [DOI] [Google Scholar]
- Hallemans A, De Clercq D, & Aerts P (2006). Changes in 3D joint dynamics during the first 5 months after the onset of independent walking: A longitudinal follow‐up study. Gait and Posture, 24, 270–279. 10.1016/j.gaitpost.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Hildebrand M (1966). Analysis of the symmetrical gaits of tetrapods. Folia Biotheoretica, 6, 9–22. [Google Scholar]
- Hildebrand M (1967). Symmetrical gaits of primates. American Journal of Physical Anthropology, 26, 119–130. 10.1002/ajpa.1330260203 [DOI] [Google Scholar]
- Hildebrand M (1968). Symmetrical gaits of dogs in relation to body build. Journal of Morphology, 124, 353–360. 10.1002/jmor.1051240308 [DOI] [PubMed] [Google Scholar]
- Hildebrand M (1977). Analysis of asymmetrical gaits. Journal of Mammalogy, 58, 131–156. 10.2307/1379571 [DOI] [Google Scholar]
- Hildebrand M (1980). The adaptive significance of tetrapod gait se‐ lection. American Zoologist, 20, 255–267. 10.1093/icb/20.1.255 [DOI] [Google Scholar]
- Humphrey N, Skoyles JR, & Keynes R (2005). Human hand‐walkers: Five siblings who never stood up [online] LSE Research Online: Retrieved from https://eprints.lse.ac.uk/archive/00000463
- Hutchinson J, Famini D, Lair R, & Kram R (2003). Are fast‐moving elephants really running? Nature, 422, 493–494. 10.1038/422493a [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Dominici N, Cappellini G, Dan B, Cheron G, & Lacquaniti F (2004). Development of pendulum mechanism and kinematic coordination from the first unsupported steps in toddlers. The Journal of Experimental Biology, 207, 3797–3810. 10.1242/jeb.01214. [DOI] [PubMed] [Google Scholar]
- Kretch KS, & Adolph KE (2013). Cliff or step? Posture‐specific learning at the edge of a drop‐off. Child Development, 84, 226–240. 10.1111/j.1467-8624.2012.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Cole WG, Golenia L, & Adolph KE (2018). The cost of simplifying complex developmental phenomena: A new perspective on learning to walk. Developmental Science, 21, e12615 10.1111/desc.12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looper J, Wu J, Barroso RA, Ulrich D, & Ulrich BD (2006). Changes in step variability of new walkers with typical development and with down syndrome. Journal of Motor Behavior, 38, 367–372. 10.3200/JMBR.38.5.367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan MJ, Ivanenko YP, Cappellini G, Labini FS, & Lacquaniti F (2012). Features of hand‐foot crawling behavior in human adults. Journal of Neurophysiology, 107, 114–125. 10.1152/jn.00693.2011 [DOI] [PubMed] [Google Scholar]
- Patrick SK, Noah JA, & Yang JF (2009). Interlimb coordination in human crawling reveals similarities in development and neural control with quadrupeds. Journal of Neurophysiology, 101, 603–613. 10.1152/jn.91125.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SK, Noah JA, & Yang JF (2012). Developmental constraints of quadrupedal coordination across crawling styles in human infants. Journal of Neurophysiology, 107, 3050–3061. 10.1152/jn.00029.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt D, Cartmill M, Griffin TM, Hanna JB, & Lemelin P (2006). Adaptive value of ambling gaits in primates and other mammals. Journal of Experimental Biology, 209, 2042–2049. 10.1242/jeb.02235 [DOI] [PubMed] [Google Scholar]
- Shapiro LJ, Cole WG, Young JW, Raichlen DA, Robinson SR, & Adolph KE (2014). Human quadrupeds, primate quadrupedal‐ ism, and Uner Tan Syndrome. PLoS ONE, 9, e101758 10.1371/journal.pone.0101758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LJ, & Raichlen DA (2005). Lateral sequence walking in infant Papio cynocephalus: Implications for the evolution of diagonal sequence walking in primates. American Journal of Physical Anthropology, 126, 205–213. 10.1002/ajpa.20049 [DOI] [PubMed] [Google Scholar]
- Shapiro LJ, Young JW, & VandeBerg JL (2014). Body size and the small branch niche: Using marsupial ontogeny to model primate loco‐ motor evolution. Journal of Human Evolution, 68, (14–31). 10.1016/j.jhevol.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Shefi S, Soudack M, Konen E, & Been E (2013). Development of the lumbar lordotic curvature in children from age 2 to 20 years. Spine, 38, E602–E608. 10.1097/BRS.0b013e31828b666b [DOI] [PubMed] [Google Scholar]
- Soska KC, Robinson SR, & Adolph KE (2015). A new twist on old ideas: How sitting reorients crawlers. Developmental Science, 18, 206–218. 10.1111/desc.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow WA (1989). Creeping patterns of adults and infants. American Journal of Physical Anthropology, 78, 387–401. 10.1002/ajpa.1330780307 [DOI] [PubMed] [Google Scholar]
- Sparrow WA, & Newell KM (1994). The coordination and control of human creeping with increases in speed. Behavioural Brain Research, 63, 151–158. 10.1016/0166-4328(94)90086-8 [DOI] [PubMed] [Google Scholar]
- Sutherland DH, Olshen R, Cooper L, & Woo S (1980). The development of mature gait. Journal of Bone and Joint Surgery, 62, 336–353. 10.2106/00004623-198062030-00004 [DOI] [PubMed] [Google Scholar]
- Tan U (2006a). Evidence for “Uner Tan syndrome” as a human model for reverse evolution. International Journal of Neuroscience, 116, 1539–1547. 10.1080/10623320600934325 [DOI] [PubMed] [Google Scholar]
- Tan U (2006b). A new syndrome with quadrupedal gait, primitive speech, and severe mental retardation as a live model for human evolution. International Journal of Neuroscience, 116, 361–369. 10.1080/00207450500455330 [DOI] [PubMed] [Google Scholar]
- Tardieu C, Hasegawa K, & Haeusler M (2017). How did the pelvis and vertebral column become a functional unit during the transition from occasional to permanent bipedalism? The Anatomical Record, 300, 912–931. 10.1002/ar.23577 [DOI] [PubMed] [Google Scholar]
- Vereijken B (2010). The complexity of childhood development: Variability in perspective. Physical Therapy, 90, 1850–1859. 10.2522/ptj.20100019 [DOI] [PubMed] [Google Scholar]
- Young JW (2012). Gait selection and the ontogeny of quadrupedal walking in squirrel monkeys (Saimiri boliviensis). American Journal of Physical Anthropology, 147, 580–592. 10.1002/ajpa.22016 [DOI] [PubMed] [Google Scholar]