Abstract
Fatiguing exercise promotes oxidation of intracellular thiols, notably glutathione. Interventions that oppose or reverse thiol oxidation can inhibit fatigue. The reduced cysteine donor l-2-oxothiazolidine-4-carboxylate (OTC) supports glutathione synthesis and is approved for use in humans but has not been evaluated for effects on skeletal muscle. We tested the hypotheses that OTC would 1) increase reduced glutathione (GSH) levels and decrease oxidized glutathione, and 2) inhibit functional indexes of fatigue. Diaphragm fiber bundles from adult male ICR mice were incubated for 1 or 2 h at 37°C with buffer (control, C) or OTC (10 mM). N-acetylcysteine (NAC; 10 mM) was used as a positive control. We measured GSH metabolites and fatigue characteristics. We found that muscle GSH content was increased after 1-h incubation with OTC or NAC but was not altered after 2-h incubation. One-hour treatment with OTC or NAC slowed the decline in force with repetitive stimulation [mean (SD) fatigue index at 300 s: OTC = 34 ± 6% vs. C = 50 ± 8%, P < 0.05; NAC = 55 ± 4% vs. C = 65 ± 8%, P < 0.05] as did the 2-h OTC treatment (OTC = 38 ± 9% vs. C = 51 ± 9%, P < 0.05). These results demonstrate that OTC modulates the muscle GSH pool and opposes fatigue under the current experimental conditions.
Keywords: exercise, diaphragm, respiratory muscle, oxidative stress
fatigue of limb and respiratory skeletal muscle contributes importantly to the morbidity and mortality of chronic heart failure (40), chronic obstructive pulmonary disease (21), cancer (59), rheumatoid arthritis (15), and other chronic diseases (30). Fatigue inhibits the tasks of daily living and decreases the quality of life for millions of individuals. At present, there is no pharmacological therapy for fatigue.
Rational drug discovery must accommodate the cellular mechanism of muscle fatigue, which involves complex changes in membrane excitability, calcium regulation, metabolism, and myofibrillar protein function (2, 34). Accumulating evidence suggests that many of these changes can be linked to thiol oxidation, a fundamental component of the fatigue process (17, 58). Oxidation of thiol groups can promote fatigue directly if the affected groups are in myofibrillar or membrane-linked proteins or indirectly if kinases and/or phosphatases are affected by oxidation. Accordingly, compounds that oppose thiol oxidation have been tested extensively for their capacities to inhibit or reverse fatigue (12, 13, 25, 33, 35, 36, 42, 50, 55, 57, 60, 61).
N-acetylcysteine (NAC), a reduced cysteine donor, is the only thiol-targeted intervention that consistently reduces fatigue in animals and humans (20, 48). The efficacy of NAC primarily stems from its capacity to increase reduced glutathione (GSH) concentration in tissues (37, 54). GSH is a major intracellular antioxidant, and its synthesis is limited by cysteine availability (37). NAC is hydrolyzed in the intra- and extracellular space to yield cysteine (53, 54), thereby supporting GSH synthesis. NAC is safe for human use but has minor side effects (32, 33, 50) that are likely dose dependent and can limit its practical applications in humans.
The thiazolidine compound l-2-oxothiazolidine-4-carboxylate (OTC; commercial name Procysteine) raises both cysteine and GSH levels after being metabolized in the intracellular space (3, 4, 8, 38, 39, 63, 64). OTC is approved for human use (11, 47, 62) and may be more effective than NAC in raising tissue cysteine and GSH content (7, 8, 63). Thus OTC represents a theoretical alternative to NAC for clinical treatment of fatigue. However, OTC effects on glutathione homeostasis are not well defined for skeletal muscle, and OTC effects on muscle fatigue have not been tested.
We developed the current study with two hypotheses in mind. First, we postulated that OTC would raise muscle GSH levels and decrease the total amount of oxidized glutathione, mimicking the effects of NAC. Second, we hypothesized that in vitro treatment with OTC, like NAC, would slow the decline in force seen during fatiguing muscle contractions. Confirmation of these hypotheses would set the stage for future translational experiments.
METHODS
Animals.
Experiments were performed using 64 male ICR mice (6–8 wk old; Harlan, Indianapolis, IN) to compare drug-treated vs. contemporary control muscles from littermate animals. Mice were maintained in a 12:12-h dark-light cycle and received water and food ad libitum. Each animal was deeply anesthetized by inhalation of isoflurane (Aerrane; Baxter Healthcare, Deerfield, IL) and killed by cervical dislocation. All procedures conformed to the guiding principles for use and care of laboratory animals of the American Physiological Society and were approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
Glutathione analyses.
The diaphragm muscle was quickly excised after cervical dislocation, and fiber bundles were dissected from the lateral costal region. Diaphragm fiber bundles were either processed immediately for measurement of GSH, glutathione disulfide (GSSG), and cysteine-glutathione disulfide (CySSG) or preincubated under conditions used in contractile studies. In the latter case, fiber bundles were tied to plastic frames at a length that equaled the average optimal length (Lo) as determined during contractile studies (∼10 mm). Control bundles were placed in Krebs-Ringer solution (in mM: 137 NaCl, 5 KCl, 1 MgSO4, 1 NaH2PO4, 24 NaHCO3, and 2 CaCl2) bubbled continuously using 95%O2-5%CO2 to maintain a pH of ∼7.4 at 37°C. Experimental bundles were incubated in Krebs-Ringer solution containing OTC (10 mM; Sigma-Aldrich, St. Louis, MO) or NAC (10 mM; Sigma-Aldrich). The NAC concentration was based on that of Khawli et al. (26); the OTC concentration was matched to NAC.
OTC solution was prepared by sonicating the compound in Krebs-Ringer solution (Sonic Dismembrator model 100; Fisher Scientific, Pittsburgh, PA) until dissolved (30–40 s). NAC solution was prepared by adding the chemical to Krebs-Ringer solution and stirring until dissolved. All solutions were prepared on the day of the experiment. Incubation times were 1 or 2 h based on the reported efficacies of OTC (16) and NAC (26).
After incubation, fiber bundles were removed, trimmed of bone and connective tissue, snap frozen in liquid nitrogen, weighed, and placed in microcentrifuge tubes containing 0.5 ml of ice-cold 5% (wt/vol) perchloric acid with 0.2 M boric acid and 10 μM γ-glutamylglutamate (24). The sample then was centrifuged at 16,000 g for 20 min at 4°C. An aliquot (300 μl) of supernatant was removed and stored at −80°C for later determination of GSH, GSSG, and CySSG concentrations by high-performance liquid chromatography at the Emory Clinical Biomarkers Laboratory (Emory University, Atlanta, GA) (24). Total oxidized glutathione (RSSG) was calculated as 2(GSSG) + CySSG, assuming GSSG is formed by oxidation of two GSH molecules and CySSG is formed by oxidation of one cysteine (CySH) and one GSH (23). Total glutathione content was calculated as GSH + 2(GSSG) + CySSG. Values were normalized for muscle wet weight.
Fatigue protocol.
After the animal was killed, the diaphragm muscle was quickly excised and placed in Krebs-Ringer solution bubbled continuously with 95%O2-5%CO2. A fiber bundle with its associated rib and central tendon was dissected from the left hemidiaphragm. The rib was tied to a glass rod, and the central tendon was attached to a force transducer (BG Series 100g; Kulite, Leonia, NJ) using silk suture (4-0). The force transducer was mounted on a micrometer that was used to adjust muscle length. The muscle was positioned between platinum plate electrodes located within a water-jacketed organ bath and was stimulated using supramaximal voltage. Fiber bundle length was adjusted to elicit the highest twitch force (Lo). The temperature of the organ bath was then increased to 37°C, and the solution bathing the muscle was changed to Krebs-Ringer solution (control), OTC (10 mM), or NAC (10 mM). All solutions were prepared on the day of the experiment and contained d-tubocurarine (0.025 mM).
Muscle contraction was evoked using direct electrical stimuli (Grass S48; Quincy, MA) using pulse and train durations of 0.3 and 250 ms, respectively. In a subset of animals, maximal tetanic contractions were stimulated (300 Hz) during drug equilibration; changes in maximal tetanic force (Po) were used to evaluate viability of the preparation over time. After drug equilibration (60 or 120 min), we conducted a fatigue protocol consisting of tetanic contractions every 2 s for 600 s (stimulus frequency 50 Hz, train duration 500 ms, pulse duration 0.3 ms). The output of the force transducer was monitored using a digital oscilloscope (546601B; Hewlett Packard, Palo Alto, CA), and tracings of interest were recorded on a flatbed recorder (BD-11E; Kipp and Zonen, Delft, The Netherlands).
After the fatigue protocol, muscle length was measured using an electronic caliper (CD-6” CS; Mitutoyo America, Aurora, IL). The fiber bundle was then removed from the organ bath, trimmed of bone and connective tissues, blotted dry, and weighed. Muscle weight and Lo were used to estimate cross-sectional area according to Close (10). Specific forces were expressed (N/cm2). Unstimulated force was determined by measuring baseline force immediately before a stimulus train during the fatigue protocol. Stimulated force was equal to the difference between baseline and peak tetanic force elicited by electrical stimulation during the fatigue protocol. Changes in stimulated and unstimulated forces measured during the fatigue protocol were normalized for the stimulated force in the first contraction of the protocol (F0). The decline in force after 300 s (fatigue index, FI) was calculated as [(F0 − F300)/F0] × 100.
Statistical analyses.
Biochemical measurements of thiol-related compounds passed tests for normality and equal variance; differences were tested using one-way ANOVA. Differences in fatigue characteristics were tested using two-way repeated-measures ANOVA (within factor, time; between factor, group). Post hoc comparisons were performed using the Fisher least significant difference test. Statistical analyses were conducted using commercial software (SigmaStat; SPSS, Chicago, IL). Statistical significance was accepted when P < 0.05. Data are means ± SD.
RESULTS
Viability of muscle preparations.
Po was monitored as an index of diaphragm fiber bundle viability in vitro. Among eight experimental groups in our two protocols, Po declined at mean rates of 0.0–7.5%/h. These values compare favorably to reported rates of 5–10%/h for this preparation (49). Average Po values among groups ranged from 21.0 to 26.3 N/cm2. Mean Po did not differ between antioxidant-treated fiber bundles and their corresponding control groups in any comparison (P > 0.91).
Glutathione regulation.
Basal levels of glutathione metabolites were measured in muscle samples frozen quickly after death. Mean values (nmol/g wet wt) were as follows: GSH, 463 ± 85; GSSG, 130 ± 43; CySSG, 15 ± 7; and RSSG, 275 ± 85. In vitro incubation times for muscle fiber bundles corresponded to the efficacies of NAC and OTC at 1 and 2 h, respectively (16, 26). Relative to basal values, incubation in drug-free buffer did not alter glutathione metabolites (Figs. 1 and 2).
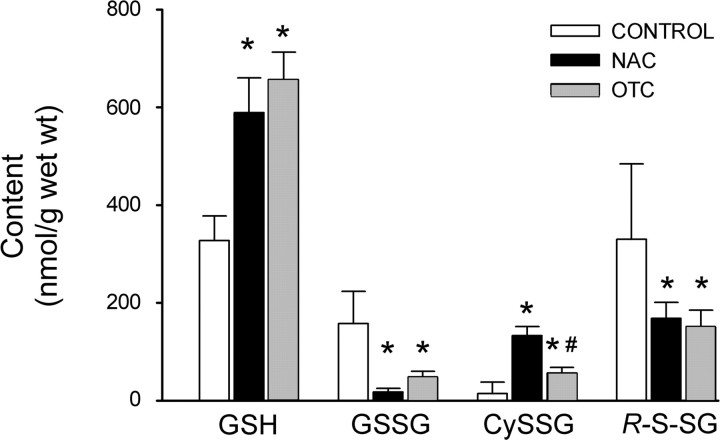
Fig. 1.
Effects of 1-h l-2-oxothiazolidine-4-carboxylate (OTC) or N-acetylcysteine (NAC) incubation on the intramuscular glutathione pool. Bars depict mean glutathione (GSH), glutathione disulfide (GSSG), cysteine-glutathione disulfide (CySSG), and total oxidized glutathione (RSSG) in diaphragm muscles incubated for 1 h in buffer (control), NAC (10 mM), or otc (10 mM). RSSG was calculated as 2(GSSG) + CySSG. Values are means ± SD; n = 3 per group. *P < 0.05 vs. control. #P < 0.05 vs. NAC.
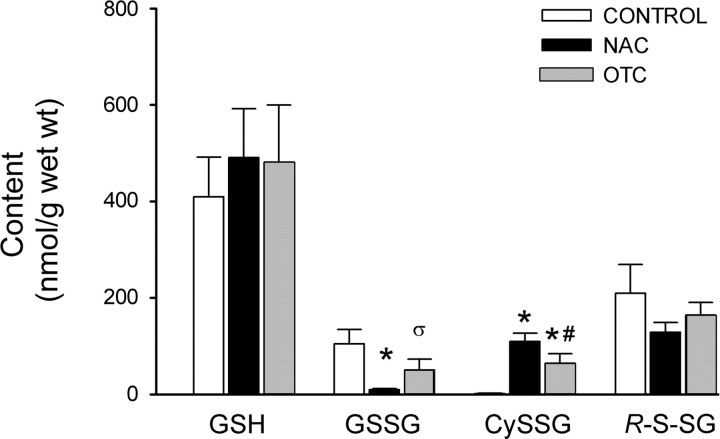
Fig. 2.
Effects of 2-h OTC or NAC incubation on glutathione. Bars depict mean GSH, GSSG, CySSG, and RSSG in diaphragm muscles incubated for 2 h in buffer (control), NAC, or OTC. Values are means ± SD; n = 3/group. *P < 0.05; σP < 0.07 vs. control. #P < 0.05 vs. NAC. In RSSG analyses, the power of 1-way ANOVA was <0.80, so negative results should be interpreted with caution.
Over a 1-h time period, incubation in OTC caused tissue GSH levels to approximately double (Fig. 1). This increase was accompanied by decrements in the oxidized glutathione conjugates GSSG and RSSG, which fell by 71 and 55%, respectively. Despite an overall shift from oxidized to reduced glutathione, OTC increased tissue CySSG levels. This reflects the mechanism of OTC action as a reduced cysteine donor; parallel increases in intracellular cysteine and GSH favor CySSG production. CySSG levels were greater after NAC (8-fold increase) than OTC (3-fold increase; P < 0.05). Otherwise, NAC effects on glutathione metabolites were indistinguishable from those of OTC.
The effects of OTC on glutathione metabolism were less evident after 2 h. As shown in Fig. 2, only the elevation in CySSG persisted. GSH, GSSG, and RSSG shifts were smaller, relative to control, and none was statistically resolvable. After 2 h, NAC effects were also blunted. Changes in GSSG and CySSG persisted, but previous shifts in GSH and RSSG were undetectable.
Neither OTC nor NAC altered total glutathione content. Tissue levels (nmol/g wet wt) ranged from 620 ± 35 to 810 ± 35 in our experimental groups. There were no systematic differences among groups or between treatment times.
Fatigue characteristics.
The fatigue protocol induced stereotypical changes in force that are presented in Fig. 3. Tetanic forces developed during electrical stimulation (stimulated force) underwent a biphasic decline, falling rapidly over the first 120–180 s and more slowly thereafter. This was accompanied by a transient increase in the residual forces sustained by fiber bundles during the periods between tetanic stimuli (unstimulated force). This unstimulated force peaked at 180–360 s and then declined monotonically.
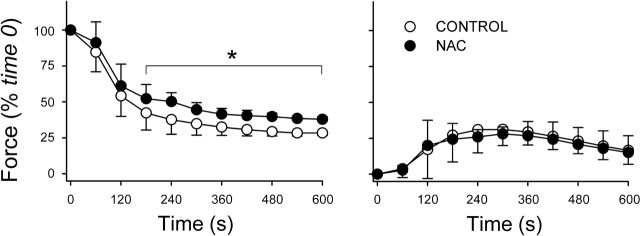
Fig. 3.
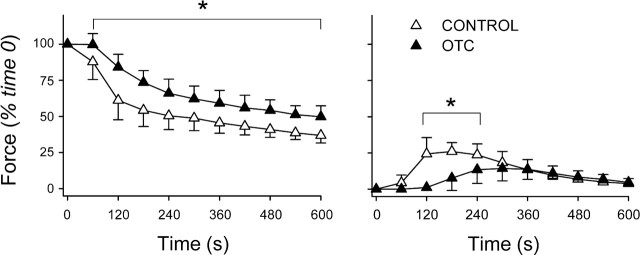
One-hour incubation with NAC inhibits fatigue. Fatigue was induced using repetitive 50-Hz stimulations; NAC-treated fiber bundles were compared with buffer-only controls using a time- and stimulus-matched protocol. Left: changes in stimulated force over time. Specific forces at time 0 did not differ between NAC (11.8 ± 1.6 N/cm2; n = 5) and control (12.8 ± 1.4 N/cm2; n = 6). Right: changes in unstimulated force normalized for stimulated muscle force at time 0. Values are means ± SD. *P < 0.05 vs. control.
Consistent with the oxidized-to-reduced glutathione shift (above), 1 h of preincubation with NAC blunted the decline of stimulated force (Fig. 3) and decreased the fatigue index at 300 s from 65 ± 7% (control) to 55 ± 5% (P < 0.05). NAC did not affect unstimulated force (Fig. 3). After 2 h, smaller changes in glutathione suggested that NAC might be less effective against fatigue. This was true. NAC did not alter stimulated force (Fig. 4) or FI (control, 57 ± 8%; NAC, 55 ± 2%; P > 0.75) and had small, variable effects on unstimulated force (Fig. 4).
Fig. 4.
NAC does not inhibit fatigue after 2-h incubation. Left: changes in stimulated force over time. Specific forces at time 0 averaged 10.3 N/cm2 in both NAC and control groups (n = 4/group). Right: changes in unstimulated force normalized for stimulated force at time 0. Values are means ± SD. *P < 0.05 vs. control.
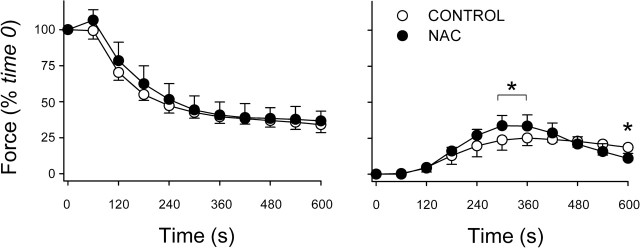
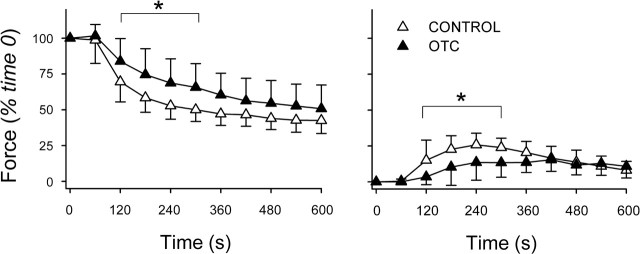
As with NAC, preincubation in OTC for 1 h diminished fatigue. OTC slowed the decline in stimulated force during early stages of the protocol (Fig. 5) and lessened FI at 300 s from 50 ± 8% (control) to 34 ± 16% (P < 0.05). Unlike NAC, OTC blunted the rise of unstimulated force (Fig. 5), evidence of more effective relaxation between tetanic contractions. Glutathione data at 2 h predicted that, like NAC, OTC would be less protective against fatigue. This was not so. OTC preserved stimulated force more effectively than at 1 h, increasing force at every time point in the fatigue protocol (Fig. 6). OTC lessened FI from 51 ± 9% (control) to 38 ± 9% (P < 0.05) and continued to blunt fatigue-induced increases in unstimulated force (Fig. 6).
Fig. 5.
Fatigue is diminished by 1-h incubation with OTC. Left: changes in stimulated force over time. Specific forces at time 0 did not differ between the OTC (11.3 ± 1.5 N/cm2; n = 7) and control (12.3 ± 2.2 N/cm2; n = 7). Right: changes in unstimulated force normalized for stimulated force at time 0. Values are means ± SD. *P < 0.05 vs. control.
Fig. 6.
OTC effects on fatigue are retained after 2-h incubation. Left: changes in stimulated force over time. At time 0, OTC-treated fiber bundles developed less specific force (12.1 ± 1.8 N/cm2; n = 5) than control bundles (14.2 ± 1.7 N/cm2; n = 5). Right: changes in unstimulated force normalized for stimulated force at time 0. Values are means ± SD. *P < 0.05 vs. control.
DISCUSSION
The main hypotheses of our study were that OTC would promote glutathione reduction and delay diaphragm muscle fatigue. Our data following 1-h incubation support both hypotheses, providing the first information about OTC effects on skeletal muscle function. OTC opposed several aspects of fatigue more effectively than NAC. OTC had greater effects on unstimulated force, and unlike NAC, OTC diminished FI after prolonged (2 h) incubation. The actions of OTC were not tightly linked to changes in the glutathione redox state.
OTC effects on glutathione biochemistry.
Glutathione is a major component of the antioxidant defenses in eukaryotic cells (37, 54). Under normal physiological conditions, GSH synthesis is limited by cysteine availability (37). OTC is transported into cells and metabolized to cysteine by the enzyme 5-oxoprolinase (64), thereby supporting GSH synthesis. Radioactive tracer studies indicate skeletal muscle is capable of OTC uptake and metabolism (38). In contrast to OTC, NAC is deacetylated to form cysteine in the extracellular space as well as the intracellular compartment (54). By increasing the availability of reduced cysteine, OTC and NAC increase GSH levels in a variety of cell types and tissues (3, 4, 6, 8, 39, 53, 63, 64). However, the relative efficacy of these two compounds depends on the experimental model and tissue. For instance, in liver (63) and blood vessels (8), OTC is more effective than NAC in raising GSH content, whereas in cultured neurons, NAC is more effective than OTC (14).
In our in vitro diaphragm preparation, OTC effects on glutathione metabolism closely resembled those of NAC. One-hour preincubation caused the anticipated changes, increasing GSH and CySSG levels while lowering GSSG and RSSG. After 2 h, OTC and NAC effects were still detectable in measurements of GSSG (depressed) and CySSG (elevated) but not in GSH and RSSG. These data suggest thiol donor effects on skeletal muscle are time dependent and illustrate the potential complexity of glutathione regulation in this cell type. For example, decrements in drug effects on GSH and RSSG after 2 h may reflect the cumulative, pro-oxidant effect of a high Po2 environment in vitro. Also, OTC may have increased cysteine but not GSH due to a negative feedback mechanism controlling intracellular GSH levels (51).
Thiol chemistry in muscle fatigue.
Thiol oxidation is a robust characteristic of fatiguing exercise. Human studies indicate that blood levels of GSSG increase (19, 33, 56), whereas GSH and total glutathione in the blood and muscle decrease (19, 36), during exercise. These conditions favor oxidation of vicinal thiols on key regulatory proteins, e.g., Na+-K+-ATPase (9), sarcoplasmic reticulum (SR) Ca2+ release channels (1), SR Ca2+-ATPase (65), troponin (18), and myosin (28), which promotes sarcolemmal depolarization (29), altered calcium handling (5, 65), and myofibrillar dysfunction (5). Conversely, interventions that limit thiol oxidation tend to oppose fatigue. NAC delays fatigue in animals (12, 26, 41, 57, 60) and humans (25, 27, 35, 36, 50, 61). Modulation of GSH has similar effects; fatigue is slower with GSH supplementation (31, 45) and faster after GSH depletion (43, 55). The thiol-specific reducing agent dithiothreitol slows fatigue of single fibers (42) and speeds recovery from fatigue in diaphragm muscle (13).
We reasoned that NAC and OTC treatment would increase the intramyocyte GSH pool, promoting thiol reduction and conferring protection against fatigue. Data from our experiments generally support this model. The exception is OTC treatment for 2 h. Fatigue was delayed without significant changes in GSH or RSSG. It is possible that OTC effects on GSH and RSSG could not be resolved statistically using our current sample size, a type II error (power calculations suggested a total of ∼250 mice to resolve the effects of OTC on GSH), Alternatively, OTC may have acted via GSH-related antioxidant systems or thiol-linked enzymes, e.g., glutaredoxin and thioredoxins (23). Thus our findings with 2-h incubation suggest that the fatigue-sparing properties of cysteine donors are not linked to changes in GSH per se. We speculate that protection against fatigue may be linked to the redox state of cysteine. A slower rate of increase in intracellular cysteine with OTC compared with NAC would lessen cysteine auto-oxidation (3), increasing the proportion of (reduced) cysteine-to-total cysteine (reduced + oxidized), thereby delaying fatigue. Lower CySSG in OTC- compared with NAC-treated muscles (Figs. 1 and 2) is consistent with this postulate.
Thiol donors as therapeutic agents.
The best-defined compound in this category is NAC. NAC treatment in vitro decreases the rate of muscle fatigue by ∼16–40% (12, 26, 41), results that were reproduced in the current study. Because NAC is approved for human use (46) and has no serious side effects (32), the positive results obtained in vitro were used to justify preclinical research in healthy humans. NAC was subsequently shown to delay fatigue during electrically stimulated muscle contractions (50), breathing against an inspiratory load (61), handgrip exercise (33), and whole body cycling exercise (25, 35, 36). These findings suggest NAC is safe and effective against muscle fatigue in humans. The stage is now set for clinical trials in an appropriate patient population (e.g., Ref. 27).
We do not suppose that NAC is the only, or even the best, compound in this category. Other thiol donors may be more effective against fatigue. Our current data identify OTC as a potential alternative. Like NAC, OTC inhibits fatigue in vitro and is approved for human use (7, 11, 44, 47, 62), and no serious side effects have been reported. Preclinical studies can be pursued immediately in healthy subjects. Indeed, OTC could be tested head to head against NAC as a “gold standard,” and if warranted, clinical trials could follow. The availability of OTC broadens opportunities for translational research and increases the likelihood that a treatment can eventually be developed for clinically significant fatigue.
Methodological considerations.
The stability of isolated muscle preparations is compromised in a time-, temperature-, and stimulus-dependent manner (52), a potentially confounding factor during in vitro experimentation. Previous studies have demonstrated the relative stability of mouse diaphragm fiber bundles, in which maximal force declines at 5–10%/h (49). Our current studies, in which force fell by < 7%/h, reinforce the usefulness of this preparation.
A more fundamental limitation of in vitro experimentation is that elements of the physiological fatigue process (blood flow, endocrine mediators, and neuromuscular activation, among others) are eliminated (2). Thus our present findings reflect OTC effects on muscle fibers and provide no information on systemic actions of the drug. Moreover, the concentration of OTC that we used (10 mM) is ∼10 times the peak plasma concentration achieved when the drug is given in therapeutic doses (22). In vitro experiments require higher concentration than in vivo to elicit physiologically relevant effects. For substances administered to the whole tissues in vitro, the surface area available for diffusion is smaller and diffusion distances are greater compared with drug delivery in vivo. This being said, in vitro studies (12, 26) using bath concentrations 10–20 times the plasma value achieved with therapeutic dosages have proven useful predictors of NAC effects on muscle fatigue in living animals and humans (33, 35, 36, 50, 57, 61). The current data justify future studies of OTC in more physiological systems, intact animals, and eventually humans.
Conclusions.
This study identifies OTC as a novel inhibitor of skeletal muscle fatigue that increases GSH content and decreases total oxidized glutathione in a time-dependent manner. Protection against fatigue is not a simple function of glutathione redox state, suggesting a more complex mechanism of action via thioredoxin, glutaredoxin, or other thiol-based pathways. More importantly, OTC is approved for use in humans. The present findings identify OTC as an attractive investigational tool for use in humans and a potential treatment for clinical fatigue.
GRANT
This study was supported by National Space Biomedical Research Institute Grant NASA MA00209 (to M. B. Reid) and American Heart Association Postdoctoral Fellowship 0725334B (to L. F. Ferreira).
Acknowledgments
We thank the late Brian J. Hardin for technical support and discussions on the mechanisms of muscle fatigue.
REFERENCES
- 1.Aghdasi B, Zhang JZ, Wu Y, Reid MB, Hamilton SL. Multiple classes of sulfhydryls modulate the skeletal muscle Ca2+ release channel. J Biol Chem : 3739–3748, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev : 287–332, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Anderson ME, Meister A. Intracellular delivery of cysteine. Methods Enzymol : 313–325, 1987. [DOI] [PubMed] [Google Scholar]
- 4.Anderson ME, Meister A. Marked increase of cysteine levels in many regions of the brain after administration of 2-oxothiazolidine-4-carboxylate. FASEB J : 1632–1636, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol : 565–575, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banks MF, Stipanuk MH. The utilization of N-acetylcysteine and 2-oxothiazolidine-4-carboxylate by rat hepatocytes is limited by their rate of uptake and conversion to cysteine. J Nutr : 378–387, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest : 164–172, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Boesgaard S, Poulsen HE, Aldershvile J, Loft S, Anderson ME, Meister A. Acute effects of nitroglycerin depend on both plasma and intracellular sulfhydryl compound levels in vivo. Effect of agents with different sulfhydryl-modulating properties. Circulation : 547–553, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Boldyrev A, Kurella E. Mechanism of oxidative damage of dog kidney Na/K-ATPase. Biochem Biophys Res Commun : 483–487, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev : 129–197, 1972. [DOI] [PubMed] [Google Scholar]
- 11.Cudkowicz ME, Sexton PM, Ellis T, Hayden DL, Gwilt PR, Whalen J, Brown RH Jr. The pharmacokinetics and pharmaco-dynamics of Procysteine in amyotrophic lateral sclerosis. Neurology : 1492–1494, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Diaz PT, Brownstein E, Clanton TL. Effects of N-acetylcysteine on in vitro diaphragm function are temperature dependent. J Appl Physiol : 2434–2439, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Diaz PT, Costanza MJ, Wright VP, Julian MW, Diaz JA, Clanton TL. Dithiothreitol improves recovery from in vitro diaphragm fatigue. Med Sci Sports Exerc : 421–426, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Dringen R, Hamprecht B. N-acetylcysteine, but not methionine or 2-oxothiazolidine-4-carboxylate, serves as cysteine donor for the synthesis of glutathione in cultured neurons derived from embryonal rat brain. Neurosci Lett : 79–82, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Ekdahl C, Broman G. Muscle strength, endurance, and aerobic capacity in rheumatoid arthritis: a comparative study with healthy subjects. Ann Rheum Dis : 35–40, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ensunsa JL, Hirschberger LL, Stipanuk MH. Catabolism of cysteine, cystine, cysteinesulfinate, and OTC by isolated perfused rat hindquarter. Am J Physiol Endocrinol Metab : E782–E789, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira LF, Reid MB. Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol : 853–860, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs F, Liou YM, Grabarek Z. The reactivity of sulfhydryl groups of bovine cardiac troponin C. J Biol Chem : 20344–20349, 1989. [PubMed] [Google Scholar]
- 19.Gohil K, Viguie C, Stanley WC, Brooks GA, Packer L. Blood glutathione oxidation during human exercise. J Appl Physiol : 115–119, 1988. [DOI] [PubMed] [Google Scholar]
- 20.Goldfarb AH. Nutritional antioxidants as therapeutic and preventive modalities in exercise-induced muscle damage. Can J Appl Physiol : 249–266, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med : 961–966, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Gwilt PR, Radick LE, Li XY, Whalen JJ, Leaf CD. Pharmacokinetics of 2-oxothiazolidine-4-carboxylate, a cysteine prodrug, and cysteine. J Clin Pharmacol : 945–950, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. New York: Oxford University Press, 2007.
- 24.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol : 93–112, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Kelly MK, Wicker RJ, Barstow TJ, Harms CA. Effects of N-acetylcysteine on respiratory muscle fatigue during heavy exercise. Respir Physiol Neurobiol : 67–72, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Khawli FA, Reid MB. N-acetylcysteine depresses contractile function and inhibits fatigue of diaphragm in vitro. J Appl Physiol : 317–324, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Koechlin C, Couillard A, Simar D, Cristol JP, Bellet H, Hayot M, Prefaut C. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am J Respir Crit Care Med : 1022–1027, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Konczol F, Lorinczy D, Belagyi J. Effect of oxygen free radicals on myosin in muscle fibres. FEBS Lett : 341–344, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol Cell Physiol : C1–C24, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med : 10–48, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Leeuwenburgh C, Ji LL. Glutathione and glutathione ethyl ester supplementation of mice alter glutathione homeostasis during exercise. J Nutr : 2420–2426, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Mant TG, Tempowski JH, Volans GN, Talbot JC. Adverse reactions to acetylcysteine and effects of overdose. Br Med J : 217–219, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matuszczak Y, Farid M, Jones J, Lansdowne S, Smith MA, Taylor AA, Reid MB. Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve : 633–638, 2005. [DOI] [PubMed] [Google Scholar]
- 34.McKenna MJ, Hargreaves M. Resolving fatigue mechanisms determining exercise performance: integrative physiology at its finest! J Appl Physiol : 286–287, 2008. [DOI] [PubMed] [Google Scholar]
- 35.McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol : 279–288, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol : 1477–1485, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Meister A, Anderson ME. Glutathione. Annu Rev Biochem : 711–760, 1983. [DOI] [PubMed] [Google Scholar]
- 38.Meister A, Anderson ME, Hwang O. Intracellular cysteine and glutathione delivery systems. J Am Coll Nutr : 137–151, 1986. [DOI] [PubMed] [Google Scholar]
- 39.Mesina JE, Page RH, Hetzel FW, Chopp M. Administration of l-2-oxothiazolidine-4-carboxylate increases glutathione levels in rat brain. Brain Res : 181–183, 1989. [DOI] [PubMed] [Google Scholar]
- 40.Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, Kubler W, Haass M. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation : 2153–2158, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Mishima T, Yamada T, Matsunaga S, Wada M. N-acetylcysteine fails to modulate the in vitro function of sarcoplasmic reticulum of diaphragm in the final phase of fatigue. Acta Physiol Scand : 195–202, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Moopanar TR, Allen DG. The activity-induced reduction of myofibrillar Ca2+ sensitivity in mouse skeletal muscle is reversed by dithiothreitol. J Physiol : 191–200, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales CF, Anzueto A, Andrade F, Levine SM, Maxwell LC, Lawrence RA, Jenkinson SG. Diethylmaleate produces diaphragmatic impairment after resistive breathing. J Appl Physiol : 2406–2411, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Morris PE, Papadakos P, Russell JA, Wunderink R, Schuster DP, Truwit JD, Vincent JL, Bernard GR. A double-blind placebo-controlled study to evaluate the safety and efficacy of l-2-oxothiazolidine-4-carboxylic acid in the treatment of patients with acute respiratory distress syndrome. Crit Care Med : 782–788, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Novelli GP, Falsini S, Bracciotti G. Exogenous glutathione increases endurance to muscle effort in mice. Pharmacol Res : 149–155, 1991. [DOI] [PubMed] [Google Scholar]
- 46.Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol : 77–82, 1988. [DOI] [PubMed] [Google Scholar]
- 47.Porta P, Aebi S, Summer K, Lauterburg BH. l-2-Oxothiazolidine-4-carboxylic acid, a cysteine prodrug: pharmacokinetics and effects on thiols in plasma and lymphocytes in human. J Pharmacol Exp Ther : 331–334, 1991. [PubMed] [Google Scholar]
- 48.Powers SK, Deruisseau KC, Quindry J, Hamilton KL. Dietary antioxidants and exercise. J Sports Sci : 81–94, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med : 479–484, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Reid MB, Stokic DS, Koch SM, Khawli FA, Leis AA. N-acetylcysteine inhibits muscle fatigue in humans. J Clin Invest : 2468–2474, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richman PG, Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem : 1422–1426, 1975. [PubMed] [Google Scholar]
- 52.Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol Cell Physiol : C265–C270, 1985. [DOI] [PubMed] [Google Scholar]
- 53.Sen CK. Glutathione homeostasis in response to exercise training and nutritional supplements. Mol Cell Biochem : 31–42, 1999. [PubMed] [Google Scholar]
- 54.Sen CK. Nutritional biochemistry of cellular glutathione. J Nutr Biochem : 660–672, 1997. [Google Scholar]
- 55.Sen CK, Atalay M, Hanninen O. Exercise-induced oxidative stress: glutathione supplementation and deficiency. J Appl Physiol : 2177–2187, 1994. [DOI] [PubMed] [Google Scholar]
- 56.Sen CK, Rankinen T, Vaisanen S, Rauramaa R. Oxidative stress after human exercise: effect of N-acetylcysteine supplementation. J Appl Physiol : 2570–2577, 1994. [DOI] [PubMed] [Google Scholar]
- 57.Shindoh C, DiMarco A, Thomas A, Manubay P, Supinski G. Effect of N-acetylcysteine on diaphragm fatigue. J Appl Physiol : 2107–2113, 1990. [DOI] [PubMed] [Google Scholar]
- 58.Smith MA, Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol : 229–241, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Stone P, Hardy J, Broadley K, Tookman AJ, Kurowska A, A'Hern R. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer : 1479–1486, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Supinski GS, Stofan D, Ciufo R, DiMarco A. N-acetylcysteine administration alters the response to inspiratory loading in oxygen-supplemented rats. J Appl Physiol : 1119–1125, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Travaline JM, Sudarshan S, Roy BG, Cordova F, Leyenson V, Criner GJ. Effect of N-acetylcysteine on human diaphragm strength and fatigability. Am J Respir Crit Care Med : 1567–1571, 1997. [DOI] [PubMed] [Google Scholar]
- 62.Vita JA, Frei B, Holbrook M, Gokce N, Leaf C, Keaney JF Jr. l-2-Oxothiazolidine-4-carboxylic acid reverses endothelial dysfunction in patients with coronary artery disease. J Clin Invest : 1408–1414, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williamson JM, Boettcher B, Meister A. Intracellular cysteine delivery system that protects against toxicity by promoting glutathione synthesis. Proc Natl Acad Sci USA : 6246–6249, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williamson JM, Meister A. Stimulation of hepatic glutathione formation by administration of l-2-oxothiazolidine-4-carboxylate, a 5-oxo-l-prolinase substrate. Proc Natl Acad Sci USA : 936–939, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu KY, Zweier JL, Becker LC. Hydroxyl radical inhibits sarcoplasmic reticulum Ca2+-ATPase function by direct attack on the ATP binding site. Circ Res : 76–81, 1997. [DOI] [PubMed] [Google Scholar]