Abstract
Double-chamber plethysmography is a well established noninvasive method of assessing airflow obstruction in small lab animals. It allows measurement of the specific airway resistance (sRaw), which unlike enhanced pause (Penh), is a meaningful airway mechanics parameter. Since sRaw is measured in spontaneously breathing mice, a limitation of the method is the inability to exclude nasal resistance changes. We recently showed that mice are not truly obligate nasal breathers and that after nasal occlusion, nasally breathing mice can transition to an oral mode of breathing. We now show that it is experimentally possible to algebraically separate the average nasal and pulmonary (including laryngeal) components of total airway resistance change by a series of measurements made across groups of mice breathing nasally or orally, assuming that oral resistance remains constant. Using this approach, we show that nasal resistance change comprises one-half or more of the total resistance change during methacholine challenge. Inhibition of mucin secretion from airway goblet cells attenuates pulmonary but not nasal airway hyperresponsiveness (AHR), and nasal AHR in a murine model of rhinitis may be related to edema.
Keywords: mucus, MARCKS, asthma, airway mechanics
an ideal method to determine airflow obstruction in mice would be reproducible, noninvasive, performed under physiological conditions, and provide physiologically meaningful output. The Bates uncertainty principle elegantly discusses why this does not seem possible (7). At one extreme lies simple unrestrained plethysmography with its often-criticized output, enhanced pause (Penh), which is difficult to relate to any physiological meaning (11), whereas at the other end lies the forced oscillation technique, which requires invasive measurements in anesthetized and mechanically ventilated mice (6). A useful middle is restrained plethsmography in spontaneously breathing mice, based on either Pennock's (14) or Agrawal's method (5). Both methods provide a framework for measurement of sRaw in nasally breathing animals, where sRaw equals the product of thoracic gas volume (TGV) and airway resistance (Raw). A limitation of these methods is their inability to separately assess the pulmonary airway resistance that is considerably smaller than the total resistance. We took advantage of the fact that mice transition to an oral mode of breathing after nasal occlusion (4) to address this limitation. Assuming the oral resistance to be relatively constant during methacholine challenge and lung volume to be similar before and after nasal occlusion, the change in sRaw in orally breathing mice (ΔsRawnose closed) is proportional to the change in pulmonary airway resistance (ΔRawpulmonary). Similarly, in nasally breathing mice, ΔsRawnose open is proportional to the change in the total airway resistance (ΔRawpulm + ΔRawnasal). The values of ΔRaw in each case can be approximated by division by the normal thoracic gas volume at functional residual capacity (TGVFRC) (0.5 ml). The use of a constant value for thoracic gas volume during induced constriction to estimate the absolute values for airway resistance will introduce a significant error that will increase as airway narrowing increases in severity. Since TGVFRC increases during methacholine challenge in spontaneously breathing mice (10), the derived ΔRaw, as used by us, is equivalent to actual ΔRaw normalized to baseline functional residual capacity. Given two successive measurements of sRaw (sRaw1=Raw1 × TGV1; sRaw2=Raw2 × TGV2), ΔRaw as calculated by us equals [Raw2 × (TGV2/TGV1)] − Raw1, rather than Raw2-Raw1. This compensates for the effect of increase in TGVFRC that offsets airway narrowing by other mechanisms. For example, if a spontaneously breathing mouse with baseline Raw of 0.5 ml·s·cmH2O−1 developed airway obstruction compensated by hyperinflation such that the TGVFRC doubled from 0.5 to 1 ml, Raw remained nearly constant (similar to that reported by Lai-Fook et al., Ref. 10), and sRaw doubled, the directly measured ΔRaw would be near zero, whereas ΔRaw as derived by us would be 0.5 ml·s·cmH2O−1. Thus, for detection of airway obstruction, the use of sRaw-derived Raw is a valid and useful measure in spontaneously breathing mice. Also, because the same error in estimating ΔRaw would occur when mice breathe with their nose patent and with their nose closed, this error would not affect the partition of Raw into nasal and pulmonary Raw. It follows that when averaged across groups, the difference between ΔRawnose open and ΔRawnose closed will be equal to ΔRawnose, thus allowing calculation of pulmonary resistance and nasal resistance changes in spontaneously breathing mice. We tested the utility of this approach in mice models of asthma and rhinitis and found it to be useful in understanding the mechanisms of airway obstruction in nasal and pulmonary airways.
METHODS
This study was conducted in conformity with the American Physiological Society's Guiding Principles in the Care and Use of Animals. Experimental protocols and study design were approved by the institutional review boards at the Institute of Genomic and Integrative Biology, New Delhi, India. BIO-110006, a MARCKS protein related peptide that inhibits mucin secretion, was provided by BioMarck Pharmaceuticals (1).
Induction of AHR, experimental allergic rhinitis, experimental allergic asthma.
The study was conducted using male, inbred BALB/c mice (16–20 gm body wt) obtained from V. P. Chest Institute (New Delhi, India) mice breeding program that were between 8 and 12 wk in age. Mucous metaplasia and AHR was induced by weekly intraperitoneal injection of 20 μg ovalbumin (Ova) in 2.25 mg alum for 4 wk followed by airway challenge with 3% Ova aerosol (Ova/Ova) within 30 days of the last sensitization. Reference mice were sensitized but, instead of challenge with aerosolized Ova, aerosolized saline was administered (Ova/Sal). In selected mice, allergic rhinitis was induced by daily intranasal administration of 20 μl of 3% Ova solution in normal saline (NS), to sensitized mice, for 7 days.
Quantification of airflow obstruction.
sRaw was measured as the time lag between flows recorded from the head and thoracoabdominal chambers in consciously breathing, restrained mice, using the two-chambered body plethysmograph system devised by Buxco (PLY-3351, Biosystem XA software). Briefly, this equipment consists of two plastic cylinders used as the thoracoabdominal and head compartments that can be attached to one another with an orifice, such that the head and neck of a mouse can pass through but not its shoulders. Mice were acclimatized to being restrained in the plethysmograph during the sensitization and challenge phase. To measure sRaw, mice were placed in the thoracoabdominal chamber, maneuvered to pass their head through the orifice, and gently wedged between the orifice and a posterior piston. Before attaching the head chamber, a suitably cut latex sheet was passed snugly over the neck and interposed between the two chambers to ensure that the chambers would be airtight. Phase lag between the airflow signals recorded from the two chambers was approximated using the time lag at zero flow, and sRaw was calculated. Other parameters such as respiratory rate and respiratory volumes were also recorded.
Experimental protocol.
After the protocol for development of AHR was completed, mice were subjected to methacholine (MCh) challenge to cause airway smooth muscle contraction, mucin hypersecretion, and airflow obstruction. Prior to MCh challenge, each mouse was administered either 5 or 10 mM BIO-11006 (BioMarck Pharmaceuticals) or a control random NH2-terminal sequence (RNS) peptide as aerosols over 30 min to inhibit mucin secretion. After completion of aerosol administration, the nostrils of mice were either occluded with adhesive tape or left open, followed by either a dose-response or a single-challenge experiment performed as follows. To obtain a dose-response curve, stepwise increasing concentrations of MCh (0–240 mM solution in NS) were administered during 1.5-min aerosolizations using an Aerogen micropump nebulizer (Buxco) connected to the plethysmograph and were followed by continuous measurement of specific airway conductance (sGaw) for 3 min. In single-challenge experiments, a single 3-min MCh aerosol challenge (60 mM solution in NS) was administered to mice pretreated with BIO-11006 or RNS, followed by continuous measurement of sGaw. Stable sGaw values associated with artifact-free flow traces at the end of 3-min windows were recorded for analysis. These were first converted to Raw by dividing the reciprocal (sRaw) by 0.5 ml, the estimated thoracic gas volume for such mice. The nasal vs. pulmonary components of airway obstruction were approximated subsequently by calculating the average MCh-induced ΔRaw in groups of mice with noses either open or closed (ΔRawnose open, ΔRawnose closed, respectively). The following assumptions were used in this determination: 1) ΔRawnose open = ΔRawnose + ΔRawpulmonary; 2) ΔRawnose closed = ΔRawpulmonary; 3) ΔRawnose open − ΔRawnose closed = ΔRawnose (from combining eqs. 1 and 2).
With these assumptions, the total change in Raw can be partitioned into pulmonary (eq. 2) and nasal (eq. 3) components. All values were calculated as means ± SD.
Histological analysis of mucin secretion.
In selected animals, to validate the in vivo efficacy of BIO-11006 in inhibition of mucin secretion, intracellular mucin remaining in the airway cells was assessed by periodic acid Schiff (PAS) staining. Serial sections of the main axial bronchus along with minor daughter branches that we refer to as penetrating bronchi were used for quantitative morphometry. Quantitative morphometry was performed on comparable stained sections from the proximal airways using the freely downloadable ImageJ software version 1.38 (National Institutes of Health, http://rsb.info.hih.gov/ij/). Briefly, stained sections were converted to 10× digitized images. With the use of the ImageJ software, images representing the region of interest were split into constituent colors, and threshold was adjusted manually such that only pixels representing a positive stain were seen. The threshold was defined using a control slide from each batch of slides stained together and kept constant for all additional measurements. Automated particle analysis was used to calculate the pixel area. Two sections per lung specimen and specimens from at least three different animals were used per group. All values were expressed as percentage of the mucin content of airways from mice with mucous metaplasia that had not been subjected to MCh challenge.
Statistical analysis.
Two-tailed Student's t-test were used to ascertain significance of difference of means. The threshold of significance was set at 0.05.
RESULTS
Mice are preferential but not obligate nasal breathers. After nasal occlusion, most mice switch to an oral mode of breathing with no apparent discomfort for experimental durations ranging from 15 to 30 min. For ethical considerations, mice showing discomfort by struggling were allowed to breathe naturally. This was seen on only four occasions out of more than forty recordings.
Nasal occlusion causes slower and deeper breathing.
To determine whether there was substantial alteration in the breathing pattern that could influence measurement of sRaw, we compared the visual flow traces and objective respiratory parameters between nasally or orally breathing mice (Table 1). Nasal occlusion was associated with a statistically significant decline in average respiratory rate from 332 ± 40 to 287 ± 37 breaths/min (mean ± SD, P < 0.001) and a compensatory increase in tidal volume from 0.16 ± 0.03 to 0.20 ± 0.04 ml (mean ± SD, P < 0.01). Respiratory flows and fractional respiratory times were not changed significantly. As can be expected from the numbers above, minute ventilation was slightly higher after nasal occlusion but did not reach statistical significance. This breathing pattern alteration most likely reflects a compensatory response to increased dead space and/or resistance while breathing orally and was not felt to be likely to significantly impact the assumptions underlying the method. No evidence of fatigue was noted over the experimental period.
Table 1.
Respiratory parameters during normal nasal breathing and during oral breathing post nasal occlusion
| Nasal Breathing | Oral Breathing | P Value | |
|---|---|---|---|
| Respiratory rate, breaths/min | 332±40 | 287±37 | P < 0.05 |
| Tidal volume, ml | 0.16±0.03 | 0.195±0.04 | P < 0.05 |
| Minute ventilation, ml | 51.7±7.5 | 55±11.9 | NS |
| Peak inspiratory flow, ml/s | 2.66±0.38 | 2.61±0.45 | NS |
| Peak expiratory flow, ml/s | 2.51±0.40 | 2..80±0.49 | NS |
| Estimated Raw, cmH2O·ml·s−1 | 1.8±0.38 | 2.6±0.26 | P < 0.05 |
All values are mean ± SD, n = 18 each. Nasal occlusion causes slower and deeper breathing in mice. P values of <0.05 after adjusting for multiple comparisons were considered significant.
Oral mode of breathing is associated with increased baseline airflow resistance.
To determine whether oral breathing is associated with higher resistance, the baseline Raw values in nasally and orally breathing mice were compared. Nasal occlusion was associated with a significant increase in average Raw (calculated as sRaw/0.5 ml) from 1.8 ± 0.38 to 2.6 ± 0.26 cmH2O·s·ml−1 (mean ± SD, P < 0.01, n = 18 each). Orally breathing mice had more stable breathing patterns with less variation, confirmed by the lower dispersion around the mean (SD/mean, nasal vs. oral; 21 vs. 10%). To ensure that the oral resistance was not a major source of error, five Ova/Sal mice were lightly sedated prior to nasal occlusion and suitably cut pipette tips were placed into the mouth like oral airways. While the baseline Raw was slightly lower than in conscious mice without oral airways (2.2 vs. 2.6 cmH2O·s·ml−1, P < 0.05), there was no such trend in the ΔRaw after 60 mM MCh (0.3 vs. 0.25 cmH2O·s·ml−1).
Increase in nasal resistance is similar in magnitude to the increase in pulmonary resistance.
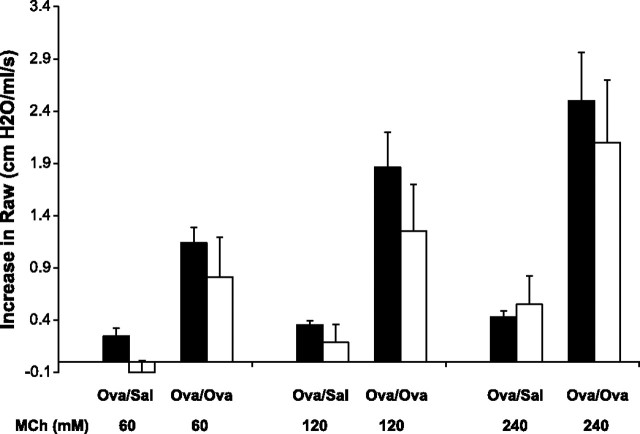
To determine the nasal vs. pulmonary effects of MCh in spontaneously breathing mice, the partitioned components of the total MCh response between control and AHR mice at varying doses of MCh were compared (Fig. 1). The increase in nasal resistance was similar in magnitude and dose dependence to the increase in pulmonary resistance. Mice with AHR had significantly greater increases in both pulmonary and nasal resistances. It was not possible to separate nasal and pulmonary components of the MCh response for resistance changes lower than ∼1 cmH2O·s·ml−1. In our experimental setup, such changes were seen at MCh concentrations lower than 60 mM in mice with experimental AHR and 120 mM in control mice.
Fig. 1.
Increase in nasal resistance is similar in magnitude to the increase in pulmonary resistance. Methacholine (MCh)-induced increase in pulmonary and nasal airway resistance (mean ± SD, shaded and open bars, respectively), is shown for control mice (Ova/Sal, n = 10) and sensitized and challenged mice (Ova/Ova, n = 10).
Treatment with the MARCKS related peptide BIO-11006 inhibits mucin secretion, attenuates the increase in pulmonary resistance, but does not affect nasal resistance.
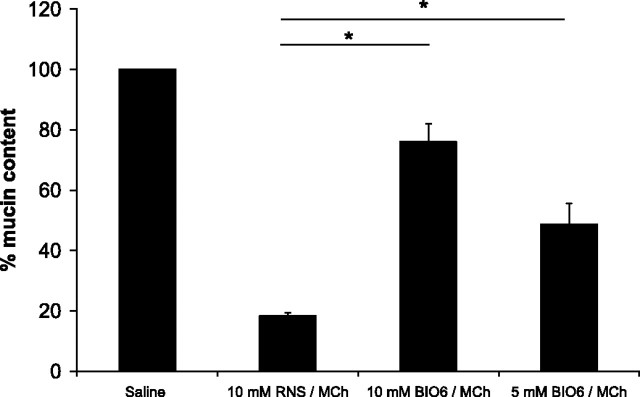
Mice treated with either 5 or 10 mM aerosol of BIO-11006 had a significantly greater retention of mucin content in the airway mucous cells (implying a lesser release into their pulmonary airways) (Fig. 2) and significantly lower increase in pulmonary resistance following MCh treatment (Fig. 3) compared with mice treated with the control peptide RNS. While the 10-mM dose of BIO-11006 appeared to be possibly more efficacious than 5 mM in inhibiting mucin secretion histologically, the difference was not statistically significant and no such trend was observed in physiological measurements. Since the nasal airway contains mucous glands, it could not be histologically established whether BIO-11006 inhibits nasal mucin secretion. However, BIO-11006 treatment was not associated with attenuation of the increase in nasal resistance (Fig. 3).
Fig. 2.
Treatment with BIO-11006 aerosol inhibits MCh-induced mucin secretion from airway mucous cells. Average mucin content of airway sections from methacholine-challenged Ova/Ova mice pretreated with BIO-11006 (Bio6) or RNS aerosol as listed (n = 6, 2 airway sections from 3 mice), expressed as a percentage of average mucin content in saline challenged Ova/Ova mice (n = 10, 2 airway sections from 5 mice). *Statistically significant difference (P < 0.05).
Fig. 3.
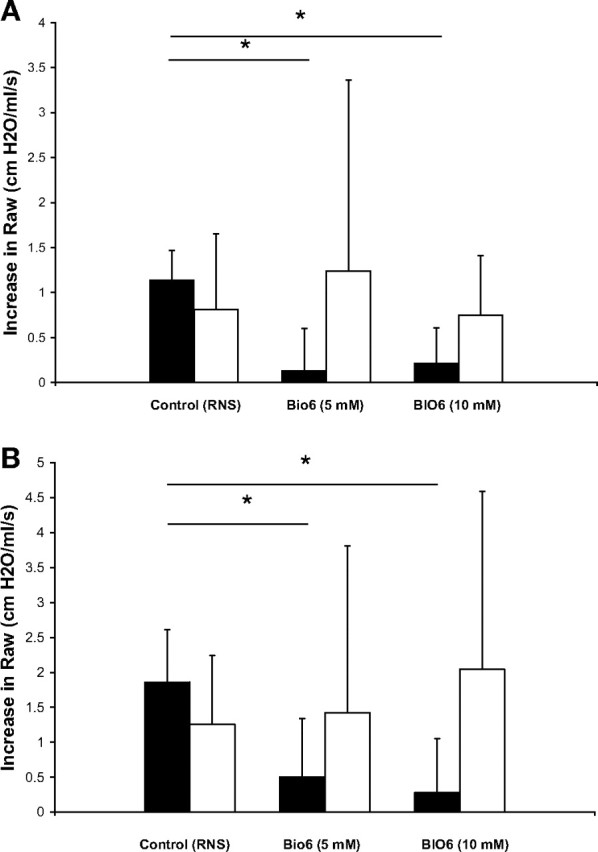
Treatment with BIO-11006 attenuates pulmonary but not nasal airflow obstruction during MCh challenge. MCh-induced increase in pulmonary and nasal airway resistance (mean ± SD, shaded and open bars, respectively) is shown for mice pretreated with 10 mM RNS (left, n = 10); 5 mM BIO-11006 (BIO6, middle, n = 8); and 10 mM BIO-11006 (BIO6, right, n = 8). A: the response to 60 mM MCh; B: the response to 120 mM MCh.
Nasal AHR may be related to edema.
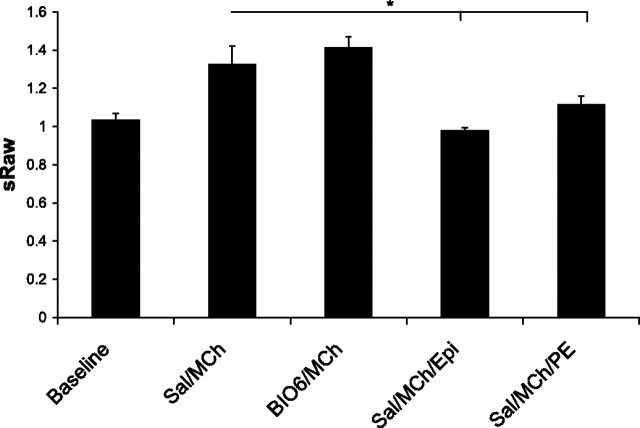
To determine the possible role of nasal edema in airflow obstruction in the nasal airways, mice with experimentally induced allergic rhinitis were treated with topical vasoconstrictors prior to MCh challenge. These mice, with experimentally induced allergic rhinitis, have predominantly nasal inflammation compared with those with experimentally induced asthma that have both nasal and pulmonary inflammation (13). The mice with allergic rhinitis did not demonstrate pulmonary AHR during oral breathing. During a single challenge with 60 mM MCh, the pulmonary resistance increase was only 0.4 ± 0.3 cmH2O·s·ml−1, comparable to naive control mice (0.3 ± 0.4 cmH2O·s·ml−1). Thus only sRaw measurements during nasal breathing were further obtained in these mice (Fig. 4). BIO-11006 did not have a significant effect on the total increase in sRaw after a single 60 mM MCh challenge, but intranasal administration of 20 μl 0.01% epinephrine or phenylephrine significantly attenuated the MCh-induced airflow obstruction.
Fig. 4.
Topical vasoconstrictors but not BIO-11006 inhibit nasal AHR. Changes in specific airway resistance (sRaw) due to MCh challenge (60 mM) in mice with experimental rhinitis treated with saline (n = 4) or BIO-11006 (n = 5) followed by MCh; and 10 μl of 0.01% wt/vol solution of epinephrine (epi, n = 3) or phenylephrine (pe, n = 5) are shown.
DISCUSSION
We describe a novel adaptation of restrained plethysmography that allows noninvasive distinction of pulmonary and nasal airflow resistance changes during spontaneous breathing. However, there are many limitations to this approach. First, whereas changes in pulmonary airflow resistance are measured directly, changes in nasal airflow resistance can only be derived based on the difference between averaged measurements from 1) a group of mice breathing orally (i.e., a measurement of pulmonary resistance) and 2) a group of mice breathing nasally (i.e., a measurement of nasal and pulmonary resistance). It follows that the standard errors associated with algebraically determined change in nasal resistance would be larger and thus associated with poorer resolution for small changes. This implies that for adequately powered statistical comparisons of nasal resistance changes between groups, the sample size must be suitably high, and experimental conditions must be optimized. Second, in our experimental definition, the transition point between nasal and pulmonary airways is at a level above the larynx where the nasal airways join the oral cavity. Changes in laryngeal resistance would be included in the pulmonary component. Since this is also true for plethysmography in humans, this is not considered to be a major problem but would recommend caution during comparisons with studies where animals were intubated or tracheostomized. Third, the oral resistance that we assume to be constant is significantly greater than the nasal resistance, presumably due to the close apposition between the palate and epiglottis in rodents, and can be a potential source of error, although our own investigations suggest that it is relatively constant within the limits of our experimental protocol. Fourth, it is assumed that pulmonary airway resistance change and the thoracic gas volume would be equal for animals breathing nasally or orally. While it seems reasonable to assume that thoracic gas volume would be similar because nasal occlusion is unlikely to cause significant changes in FRC because of the unchanged static mechanics of the lung and chest wall, the small increase in tidal volume noted by us (0.035 ml) may introduce a small error. The use of sRaw-derived Raw values, which are normalized to baseline FRC, would be expected to limit this error. The slower and deeper breathing associated with nasal occlusion may, however, significantly influence the MCh-induced pulmonary airway obstruction (15). Also, MCh effect may be different depending on the route of inhalation. However, such effects are unlikely to be large because in a small subset of six mice challenged with MCh before or after nasal occlusion, no difference between the post-MCh sRaw was observed. Lastly, nasal occlusion is not possible if nasal airflow is directly measured at the nostrils, as in Agrawal's loop method (3). Such a setup was recently described in mice (9) and appears to be easier to perform with less experimental variability, although it is not yet commercially available.
With the use of this approach, it was found that an increase in nasal resistance is an important component of MCh-induced airflow obstruction in control mice as well as those with experimental asthma. It was also determined that the nasal and pulmonary components of the MCh response are approximately equal in a mouse model of asthma. The baseline total resistance, as well as the magnitude of increase in pulmonary resistance during MCh challenge, as estimated, is within the range of reported invasive measurements in spontaneously breathing or tracheostomized animals, respectively (6).
This approach was also used to successfully examine the effects of aerosolized BIO-11006, an inhibitor of mucin secretion, on MCh-induced airflow obstruction in nasal and pulmonary airways (Table 2). From the first two columns, it can be seen that the effects of MCh challenge in mice are not restricted to the nasal or pulmonary airways. Yet BIO-11006 therapy only attenuates the pulmonary component of the MCh response. Thus the noninvasive mouse model of asthma reflects potentially two distinct processes, i.e., pulmonary and nasal AHR. The inhibition of pulmonary AHR by BIO-11006 was similar to that previously reported by us for intranasal MANS peptide; an older prototype of the MARCKS related peptides that inhibit mucin secretion (4). It was recently reported that airway closure is the major mechanism of AHR in allergically inflamed mice lungs (12, 16), the distribution of which corresponds well to the expected patterns of mucous obstruction (2). Our experimental design is optimized to maximize the mucin secretion that peaks near 5 to 7 days after allergen challenge, by which time inflammation and airway smooth muscle contractility have started diminishing (8). In this context, it is not surprising that inhibiting mucin hypersecretion causes ∼80% reductions in MCh-related pulmonary airflow obstruction. Interestingly, pretreatment of mice with BIO-11006 did not attenuate nasal AHR. This suggested that mechanisms other than mucin secretion may be important in nasal AHR and/or that BIO-11006 was ineffective in inhibiting nasal mucin secretion. Unlike pulmonary airways of mice, which secrete mucus exclusively by exocytosis of mucin granules from mucous cells followed by hydration, nasal airways contain glands that secrete mucus. While we were unable to histologically measure glandular secretion in the nasal airways, we did find that application of a topical vasoconstrictor was sufficient to normalize nasal AHR. Thus edema is likely to be an important component of MCh-induced nasal airflow obstruction.
Table 2.
Percentage of total airflow obstruction attributable to nasal and pulmonary airways at each MCh dose in sensitized and challenged mice (Ova/Ova) and the BIO-11006-induced improvement
| MCh | Nasal Resistance Increase as % of Total Resistance Increase | Pulmonary Resistance Increase as % of Total Resistance Increase | BIO-11006-Induced %Improvement in Nasal Resistance Increase | BIO-11006-Induced %Improvement in Pulmonary Resistance Increase |
|---|---|---|---|---|
| 60 mM | 42 | 58 | −7 | 85 |
| 120 mM | 40 | 60 | −16 | 79 |
| 240 mM | 46 | 54 | 13 | 78 |
Ratios were derived from group means for each category, and, therefore, variance could not be calculated and statistical tests of difference were not performed.
In summary, partitioning of nasal and pulmonary components of airflow obstruction during spontaneous breathing is feasible and useful in addressing questions related to the airway response mechanisms.
GRANTS
The study was funded in part by BioMarck Pharmaceuticals, USA, and by Council of Scientific and Industrial Research, India, via task force project NWP-0033.
DISCLOSURE
A. Agrawal was provided $55,000 grant support by BioMarck Pharmaceuticals between 2005 and 2007.
I. Parikh and E. C. Murphy are employees of Biomarck Pharmaceuticals.
Acknowledgments
We thank Dr. Balaram Ghosh and Dr. Arjun Ram for invaluable advice and support.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agrawal A, Singh VP, Singh SK, Murphy EC, Parikh I. Inhibition of mucin hypersecretion by BIO-11006, a MARCKS related peptide, improves airway hyperresponsiveness to methacholine in a murine model of mucous hypersecretion. Am J Respir Crit Care Med : A944. 4–15–2008. [Google Scholar]
- 2.Agrawal A. Mucous obstruction and airway hyperresponsiveness in mice. Am J Respir Crit Care Med : 1171–1172, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal A, Ram A. Commentary on “restrained whole body plethysmography for measure of strain-specific and allergen-induced airway responsiveness in conscious mice.” J Appl Physiol : 2411–2413, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal A, Rengarajan S, Adler KB, Ram A, Ghosh B, Fahim M, Dickey BF. Inhibition of mucin secretion with MARCKS-related peptide improves airway obstruction in a mouse model of asthma. J Appl Physiol : 399–405, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal KP. Specific airways conductance in guinea pigs: normal values and histamine induced fall. Respir Physiol : 23–30, 1981. [DOI] [PubMed] [Google Scholar]
- 6.Bates JH. Point:Counterpoint: Lung impedance measurements are/are not more useful than simpler measurements of lung function in animal models of pulmonary disease. J Appl Physiol : 1900–1901, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol : 1297–1306, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, Stripp BR, Dickey BF. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol : 382–394, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flandre TD, Leroy PL, Desmecht DJ. Effect of somatic growth, strain, and sex on double-chamber plethysmographic respiratory function values in healthy mice. J Appl Physiol : 1129–1136, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Lai-Fook SJ, Houtz PK, Lai YL. End-expiratory and tidal volumes measured in conscious mice using single projection x-ray images. J Appl Physiol : 521–533, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Lundblad LK, Irvin CG, Hantos Z, Sly P, Mitzner W, Bates JH. Penh is not a measure of airway resistance! Eur Respir J : 805, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, Bates JH. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med : 768–774, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakaya M, Dohi M, Okunishi K, Nakagome K, Tanaka R, Imamura M, Baba S, Takeuchi N, Yamamoto K, Kaga K. Noninvasive system for evaluating allergen-induced nasal hypersensitivity in murine allergic rhinitis. Lab Invest : 917–926, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Pennock BE, Cox CP, Rogers RM, Cain WA, Wells JH. A noninvasive technique for measurement of changes in specific airway resistance. J Appl Physiol : 399–406, 1979. [DOI] [PubMed] [Google Scholar]
- 15.Thorpe CW, Salome CM, Berend N, King GG. Modeling airway resistance dynamics after tidal and deep inspirations. J Appl Physiol : 1643–1653, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol : 2019–2027, 2004. [DOI] [PubMed] [Google Scholar]