Abstract
The beneficial effects of caloric restriction in increasing longevity and forestalling age-related diseases are well known. Dietary restriction of methionine also renders similar benefits. We recently showed in a renal epithelial cell culture system that reduction of culture medium methionine by 80% resulted in altered tight junctional (TJ) claudin composition and also improved epithelial barrier function (51). In the current study, we examined the effect of dietary restriction of methionine on TJ barrier function in rat gastrointestinal tissue to see whether this phenomenon also holds true in a tissue model and for a different epithelial cell type. After 28 days on methionine-restricted (MR) diet, rats showed small but significant reductions in the plasma and (intracellular) colonocyte levels of methionine. Colon mucosal sheets from rats on the MR diet showed increased transepithelial electrical resistance with concomitant decrease in paracellular diffusion of 14C-d-mannitol, suggesting improved barrier function relative to rats on control diet. This improved barrier function could not be explained by changes in colon crypt length or frequency. Neither was the colonocyte mitotic index nor the apoptotic frequency altered significantly. However, TJ composition/structure was being altered by the MR diet. RT-PCR and Western blot analysis showed an increase in the abundance of claudin-3 and an apparent change in the posttranslational modification of occludin, data reinforcing a paracellular barrier alteration. Overall, our data suggest that reduction in dietary intake of methionine results in improved epithelial barrier function by inducing altered TJ protein composition.
Keywords: claudin-3, occludin, mannitol flux, paracellular
epithelial cell layers, such as skin and the mucosal lining of the gastrointestinal (GI) tract, are designed to restrict the movement of unwanted substances from the external environment to the bloodstream. At the same time, the epithelial cell sheet, which lines the GI tract, should allow the absorption of desired compounds such as nutrients and electrolytes. This movement of compounds across epithelial layers is mediated both through the cells (transcellular pathway) and through the interspace between cells (paracellular pathway). Epithelial cell layers of different tissues of an organism and even the same tissue of different organisms differ in the unique claudin composition of that tissue's epithelial tight junction (TJ) and thereby in their “leakiness” or paracellular permeability to various compounds based on compound size, charge, and other characteristics (32, 48).
The TJ, a gasket-like proteinaceous seal that spans the apicolateral border of the plasma membrane and links to the actomyosin cytoskeleton within epithelial cells, plays a major role in preventing free solute diffusion along the paracellular space (32, 33, 48). For example, antigens found in the bronchial/alveolar airways or the duodenal lumen cannot freely cross into the stromal compartment and engender an inflammatory cascade. Similarly, infectious microbes and viruses on the luminal surface of, e.g., the colon or the cornea, are prevented from gaining access to interstitial fluid and vasculature by the barrier function of TJs.
It was first shown over 30 years ago that TJ alteration and compromised barrier function occur in neoplasia (27). These changes have since been shown in ovarian cancer (43), breast cancer (22), glioblastoma (25), liver cancer (63), and colon cancer (52). Our group has previously shown that tumor-promoting agents, via activation of protein kinase C, are extraordinarily adept at inducing junctional leak (7, 10, 36, 37). It has also been demonstrated that increased epithelial barrier permeability preceded polyp-like foci formation in a differentiated kidney epithelial cell culture model (35, 36).
Decreased barrier function, as measured by decreased electrical resistance or by increased nonelectrolyte leak, is thought to be intrinsic to inflammatory bowel diseases (16, 20, 26). An improved mucosal barrier could impose better control/restriction on unregulated antigen presentation to the gastrointestinal stroma, i.e., reduced basal inflammation. Recent studies show that targeted dysfunction of TJs leads to immune activation and contributes to the development of colitis (53).
Studies spanning the past 7 decades demonstrated that caloric restriction (CR) consistently slowed the rate of aging and extended average and maximal life span (11, 28, 58). Methionine restriction (MR) has also been demonstrated by many investigators to extend life span in various organisms, with rodents being the most extensively investigated (30, 39, 45, 64). These nutritional interventions do not simply extend life span but also forestall the onset of age-related diseases including hypercholesterolemia (54), cancer (9), etc.
In addition to the involvement of methionine in life span extension, it has been known for many years that tumor cells have a much greater dependence on methionine than do normal cells (19). Substituting homocysteine for methionine in culture medium can halt the growth of many cancer cells while allowing for continued growth of nontransformed cells (4, 24). The level of interest has been increasing in using MR as an adjunct to conventional cancer chemotherapy, either by restricting dietary methionine intake or using orally administered methioninase as a means of metabolically eliminating ingested methionine from the upper GI lumen (6, 21).
It has been very well established that methionine-deficient diets are causing widespread changes in DNA-methylation patterns, perhaps mediated by S-adenosylmethionine. This would have the capacity to result in changes in gene expression (41). Methionine restriction also decreases mitochondrial oxidative stress (5, 46) in addition to inducing apoptosis in—and affecting the motility of—androgen-independent prostate cancer cells (13, 14).
The very few agents (e.g., dexamethasone, EGF, etc.) that can cause a tightening of TJs are also known to positively affect health and/or augment longevity (50, 62). Therefore, it is conceivable that MR and CR may achieve their beneficial ends and the physiological and immunological benefits that come along with them, at least in part, through enhanced barrier function. Using a differentiated epithelial cell culture model, we observed that MR alters TJ structure and composition, decreases TJ permeability, and thereby enhances epithelial barrier function (51). This is one of extremely few modalities known to be able to induce improved barrier function, in contrast to a growing list of pathogenic agents capable of causing TJ leak (33).
In this present study we sought to extend our tissue culture studies by looking at the effects of restriction of dietary methionine intake on epithelial barrier function at the tissue level in an animal model. Here we report for the first time that rats fed on an MR diet for a brief period (4 wk) showed improved TJ barrier function due apparently to an altered TJ protein expression pattern.
MATERIALS AND METHODS
Animals and dietary regimens.
The animal protocols followed in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the Lankenau Institute for Medical Research. In this study, male Sprague-Dawley rats of 350–400 g size from Harlan Labs were utilized. As in earlier MR studies by others (39, 44, 45, 64), rats at 6 wk of age were purchased and once in our colony, maintained on regular rat diet for 2 wk. Animals were observed twice weekly for any signs of morbidity, and weights were recorded twice each week as well. At the end of each experiment, the rats were killed (for harvesting of the large intestine and its mucosal epithelial tissue) with a guillotine to avoid known effects of anesthesia on junctional properties (49).
Rat feed was purchased from Harlan Labs (Teklad) in pelletized form. The complete amino acid complement diet (AA diet) was the AIN-76 diet with protein (casein) being replaced by an essential amino acid mixture. The MR diet contained 0.17% l-methionine (wt/wt) and (elevated) 3.39% l-glutamic acid (rather than the normal 0.86% methionine and 2.70% glutamic acid in the AA diet) (44). Other sulfur-containing amino acids, cysteine and cystine, were eliminated totally from this diet. Instead of the 2× complement of vitamins used in the longevity studies of Richie Jr. et al. (44) and others, we used diets with 1× vitamins. Both groups received food and water ad libitum. The reduction in methionine was compensated by an increase in glutamate content for the overall nitrogen content of the diet (45).
Blood chemistry and analysis of amino acid contents.
Analysis of blood plasma/serum samples collected from rats after euthanasia was performed at the Mainline Clinical Labs of the Lankenau Hospital (Wynnewood, PA). For the analysis of intracellular amino acid contents, cell scrapings from rat colons were sonicated on ice for 60 s, cooled on ice for 30 s, and sonicated again on ice for 60 s. Suspensions were then flash-frozen using a methanol-dry ice bath and shipped overnight on dry ice along with plasma samples to Amino Acids.com (St. Paul, MN) where free, intracellular, and plasma amino acid pools were determined using Hitachi Model L-8900 dedicated HPLC amino acid analyzers.
Transepithelial physiology.
After the 4-wk period, rats were euthanized by guillotine and (trunk) blood samples were collected immediately in heparinized sample collection tubes. Determination of transepithelial electrical resistance (Rt) and transepithelial mannitol flux (Jm) was done as described previously (36). In brief, the colon was removed from euthanized animals, sliced longitudinally, and cleaned of any fecal content by ice-cold, pH 7.35, bicarbonate-buffered saline [Krebs-Ringer bicarbonate (KRB)]. It was then placed on its mucosal surface and the serosal membrane and muscularis propria were surgically removed. Remaining tissue was mounted in an Ussing chamber, with tissue bathed in 37°C KRB stirred by gas-lift (95% O2, 5% CO2) oxygenation. This chamber permits the separation and sampling of luminal vs. antiluminal fluid compartments, with the epithelial tissue itself serving as the barrier between the fluid compartments, mimicking in vivo barrier function, and allowing precise study of transport and barrier function by the epithelium. Transepithelial electrical resistance (Rt) and transepithelial voltage were measured every 2 min using Ag/AgCl electrodes in series with 1 M NaCl agar bridges and a standard current/voltage clamp delivering 1-s current pulses of 40 μA (McGrath Research and Technology, Phoenix, AZ). After 30–45 min of incubation, the mucosal tissue was physiologically stabilized and the maximal Rt was measured. The greater the Rt value, the lower the permeability (to electrolytes). Short circuit current and potential difference measurements were also performed. 14C-d-mannitol (Perkin-Elmer, Boston, MA) (along with nonradiolabeled mannitol) was then added to the mucosal hemichamber (final activity 0.1 μCi/ml, final concentration 0.1 mM). Aliquots from the serosal fluid hemichamber were taken every 15 min for 60 min for liquid scintillation counting. From linear regression of a graph of cpm/min, and using the measured specific activity of the 14C-probe in the mucosal hemichamber, we calculated the transepithelial probe flux rate (pmol·min−1·cm−2 tissue). This value reflects the permeability of the TJs to that particular probe. The 14C-d-mannitol (180 molecular weight) probe is not metabolizable by any GI tissue studied here.
Colon crypt morphology and cell kinetics.
For histologic analysis, colon tissue samples from rats on AA and MR diets were fixed in 4% neutral buffered formalin for 24 h, transferred to 70% ethanol, and stored until paraffin embedding. Sections (6 μm) were prepared, stained with hematoxylin and eosin (H&E) , and photographed on an upright microscope by standard methods (42). Ki-67 immunohistochemistry following antigen retrieval was done as mentioned elsewhere (3) using the mouse monoclonal 1° antibody from Abcam (Cambridge, MA) and the Vectastain ABC kit from Vector Labs (Burlingame, CA).
Quantitative analysis of claudin-3 and occludin mRNA levels by real-time RT-PCR.
RNA was isolated with TRIzol (Invitrogen) from rat tissues according to the manufacturer's protocol. One microgram of total RNA was used to generate cDNA using Taqman Reverse Transcription Reagents (PE Applied Biosystems, Foster City, CA). For PCR amplification (17), oligonucleotide primers for rat claudin-3 (CLDN3; rCLDN3-F: CATCGCAGCTACTTGCCAGT and rCLDN3-R: TTTTTTTTTTTTTTTT-GCAAAAACGA) and for rat occludin (OCLN; rOCLN-F: AAGAGTCGACAGTCCAATGG and rOCLN-R: TGAAGTCATCCACGGACAAG) were designed and used at a concentration of 450 nM (for CLDN3) or 300 nm (for OCLN). The SYBR Green I assay and the GeneAmp 7300 Sequence Detection System (PE Applied Biosystems) were used for the quantification of the PCR products. The comparative CT method (where CT is cycle number at threshold; PE Applied Biosystems) was used to determine the relative CLDN3 and OCLN expression level of each sample to the CT value of control tissues. GAPDH values were used for normalization.
Analyses of TJ proteins.
Methionine-restricted (MR) and control (AA) rats were killed in parallel as described above. After being washed with ice-cold KRB, the mucosal epithelium was carefully scraped away with a stainless steel spatula and placed in a 4°C nondetergent buffer with inhibitors of calcium-dependent and calcium-independent proteases as previously described (36). After initial sonication, using successive centrifugations and detergent extractions (first Triton X-100 then SDS), PAGE and Western immunoblot analyses were performed. Primaries antibodies for detection of various claudins and occludin were from Zymed (San Francisco, CA).
Statistical analysis.
Data are reported throughout as means ± SE. Experimental and control groups were compared throughout by unpaired Student's t-tests, with statistical significance being ascribed when P < 0.05.
RESULTS
Effect of dietary methionine restriction on growth, blood chemistry, and the levels of sulfur-containing amino acids.
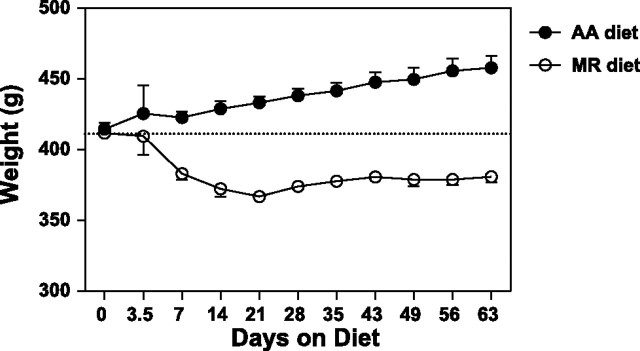
We followed the well-established formulations of a full-complement amino acid versus a slightly modified methionine-restricted diet (AA and MR diets, respectively, as described in materials and methods) used in previous longevity studies (39, 45). As mentioned, these diets involve replacing protein (casein) in the animal feed with the nitrogen equivalent of free amino acids. One group of rats was given the AA diet ad libitum, whereas the other received the MR diet ad libitum. On the basis of the observed appearance and activity level, both groups of rats appeared healthy throughout the experimental period. While the rats on the AA diet gained weight, the rats on the MR diet lost weight for the first 2 wk before beginning to recover body weight (Fig. 1).
Fig. 1.
Body weights of animals on complete amino acid complement (AA) and methionine-restricted (MR) diets. Data are reported as means ± SE (n = 6 animals) and span 60 days of diet. Animals were 7–8 wk of age at the start of the diets.
Measured at 28 days on diet, there was no significant difference in the serum albumin or total protein content between rats on AA diet vs. MR diet (Table 1). Also there was no significant change in any of the plasma electrolytes analyzed. Blood creatinine level, a measure of renal function, did not show any significant difference either. Vitamin B12 level, an indicator of a significant deviation from normal food intake, remained similar in both groups on AA and MR diets. There was a significant yet unexplained decrease in the blood urea nitrogen (BUN) and fasting glucose levels between animals on AA diet and MR diet.
Table 1.
Serological components of animals on AA and MR diets
| Parameter (Plasma) | AA Diet | MR Diet | P |
|---|---|---|---|
| Albumin | 1.3 ± 0.05 | 1.2 ± 0.04 | NS |
| BUN | 14.4 ± 1.0 | 10.4 ± 0.5 | 0.037 |
| Calcium | 10.9 ± 0.1 | 10.7 ± 0.2 | NS |
| Creatinine | 0.88 ± 0.06 | 0.76 ± 0.04 | NS |
| Glucose | 109.4 ± 3.4 | 91.6 ± 1.9 | 0.018 |
| Magnesium | 1.96 ± 0.04 | 1.94 ± 0.05 | NS |
| Potassium | 6.4 ± 0.3 | 7.3 ± 0.6 | NS |
| Sodium | 140.6 ± 1.9 | 157.6 ± 9.0 | NS |
| Total protein | 6.8 ± 0.1 | 6.5 ± 0.1 | NS |
| Vitamin B12 (serum) | 1,378 ± 18 | 1,415 ± 59 | NS |
Values are means ± SE; n = 5. AA, amino acid; MR, methionine restriction; BUN, blood urea nitrogen; NS, not significant.
MR diet decreases colonocyte methionine level.
There was a significant decrease in the plasma levels of methionine (17.4%) and its metabolite taurine (29.4%) in plasma of rats on the MR diet compared with rats on the AA diet (Fig. 2A). There was no significant change in the plasma cystine levels. The decrease in colonocyte intracellular methionine level from rats on the MR diet compared with the AA diet (12.1%) was very modest but statistically significant (Fig. 2B).
Fig. 2.
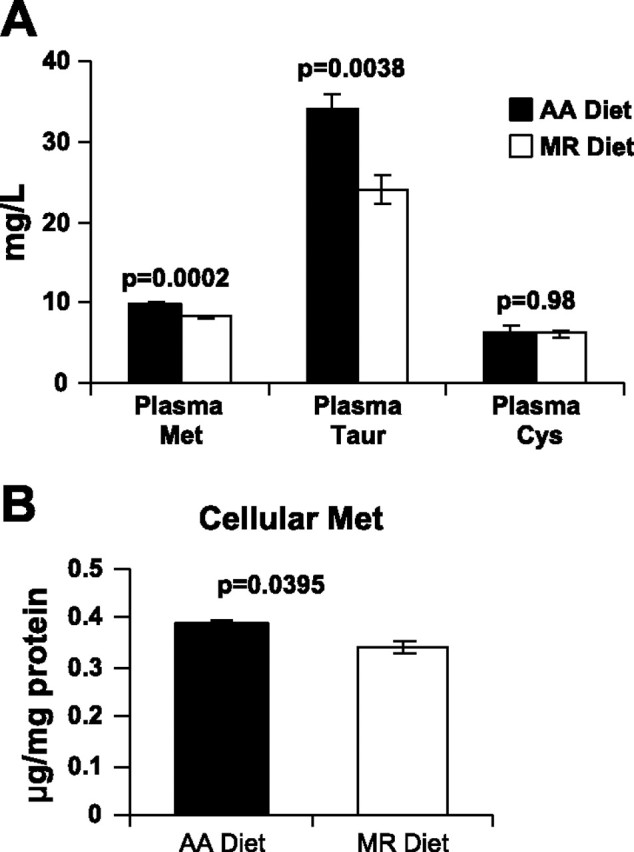
Dietary methionine restriction decreases plasma and colonocyte methionine levels. A: plasma levels of methionine (Met), taurine (Taur), and cystine (Cys) from animals on AA and MR diets (n = 3 animals). B: intracellular methionine levels of colonocytes from rats on AA and MR diets (n = 3 animals). Values shown represent means ± SE.
Methionine-restricted diet affects tight junction barrier function.
Measurement of electrical properties of rat distal colon tissue sheets revealed a significant increase (∼24%; P < 0.01) in the transepithelial electrical resistance of distal colon tissue sheets (Fig. 3A) from rats fed on MR diet for a period of 4 wk over colon tissue from rats on the AA diet. Concomitant with this observation, there was a significant (∼45%; P < 0.03) reduction in the transepithelial flux of 14C-d-mannitol across the distal colon tissue (Fig. 3B) from rats on the MR diet. Both imply a decrease in paracellular leak and an improvement in the TJ barrier function. No significant changes in colon short circuit current were observed (data not shown).
Fig. 3.
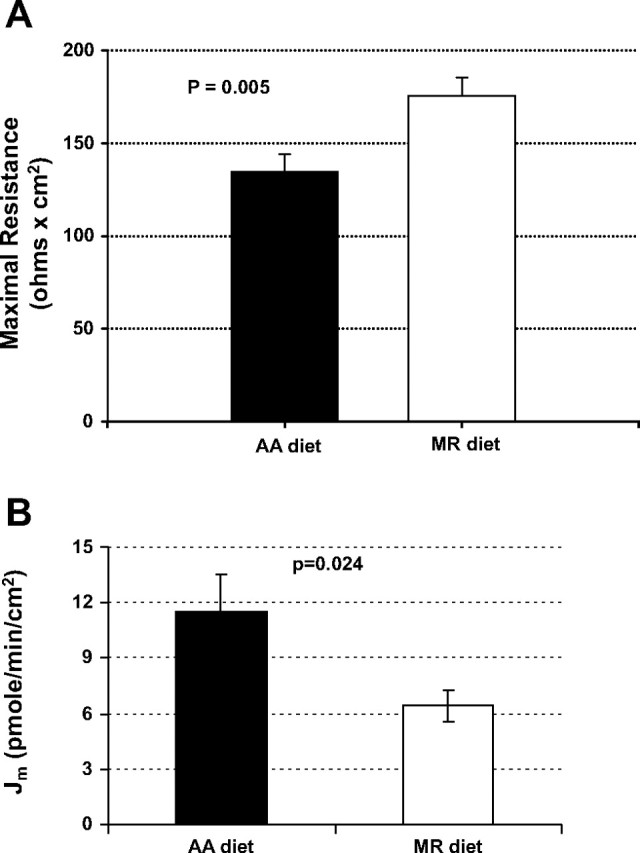
Methionine restriction diet affects tight junction (TJ) barrier function. A: transepithelial resistance (Rt) (Ω × cm2) across distal colon tissues from rats on AA and MR diets (n = 17 and 18 tissues, respectively) (maximal resistance recorded during incubation period). B: 14C-mannitol flux (Jm) (pmol·min−1·cm−2) across distal colon tissues from rats on AA and MR diets (n = 10 tissues). Values shown represent means ± SE.
Time course of methionine restriction-induced change in colon barrier function.
In these studies, rats were maintained on control (AA) or MR diets as described above, out to 63 days. At each reported time point, animals were euthanized and colon mucosal electrophysiology and mannitol permeability studies were performed, also as described. As shown in Fig. 4, significant change in transepithelial electrical resistance or transepithelial mannitol flux was observed as early as day 14. This significant difference persisted on out to (at least) 63 days, the longest time point tested.
Fig. 4.
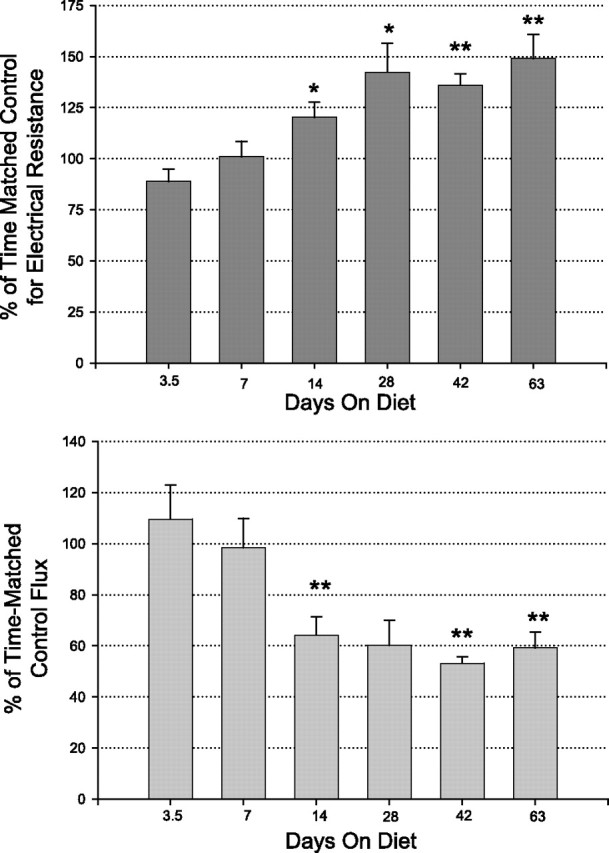
Time course of methionine-restriction-induced changes in colon epithelial barrier function. Top: relative change of transepithelial electrical resistance compared with time-matched controls. Bars indicate means ± SE (n = 6 to 12 separate time-matched tissues). Bottom: transepithelial mannitol flux on the same tissues. *P < 0.05; **P < 0.001 (Student's t-test) for comparison of tissues from MR diet vs. AA diet animals.
Improved barrier function is not due to altered colon architecture or colonocyte cell kinetics.
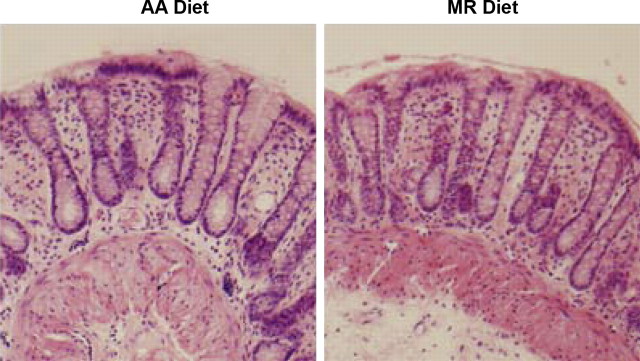
There are several factors, in addition to changes in TJ permeability, that may be responsible for our observation of an improved barrier function in the rats on the MR diet. The histological architecture and cell proliferation of colon tissues from rats fed with the AA diet or the MR diet were evaluated by paraffin sectioning and H&E staining. The histology of colon sections showed no apparent differences between the groups (Fig. 5). Architecture of the crypts (length and frequency of crypts) and general thickness of the colonic mucosa were similar in both groups (see Table 2). Cell proliferation was evaluated by semiquantitative Ki-67 labeling and mitotic indexes. The Ki-67 labeled cells were in the appropriate compartment within the epithelium, most densely just above the base of the crypts in both groups. This indicator of cell proliferation showed no differences between the two groups (data not shown). There was also no difference in the rate of apoptosis between the two groups or the observed mitotic frequency (Table 2). In summary, methionine restriction did not appear to alter the histological phenotype or cell cycle kinetics of the rat colon mucosa.
Fig. 5.
Improved barrier function is not due to altered colon architecture. Hematoxylin and eosin (H&E) staining of colon tissue sections from rats on AA (left) and MR (right) diets shows no differences in crypt frequency, length, or architecture. The quantitative data of Table 2 are derived from H&E-stained sections such as these.
Table 2.
Morphological and cell kinetic parameters of colon mucosa from control and MR distal colon mucosa
| Control Diet | MR Diet | n | P | |
|---|---|---|---|---|
| Crypt length | 0.27 ± 0.01 | 0.26 ± 0.03 | 3 | 0.70 (NS) |
| Mitotic frequency | 1.17 ± 0.27 | 0.83 ± 0.20 | 12 | 0.33 (NS) |
| Apoptotic frequency | 0.50 ± 0.19 | 0.42 ± 0.15 | 12 | 0.73 (NS) |
Values are means ± SE. Crypt length is measured in millimeters in hematoxylin and eosin-stained sections of formalin-fixed distal colon tissue from rats kept on the two different diets for 28 days. Mitotic and apoptotic frequency was determined from numbers of detected mitotic cells and apoptotic bodies in the same sections. P values are from Student's t-tests on tissue from the two different diets.
MR diet improves barrier function by altering tight junction protein composition.
We observed approximately an 80% increase in claudin-3 mRNA in distal colon tissue of MR diet rats by RT-PCR; there was no significant change in occludin expression (Fig. 6A). Methionine restriction did thus affect transcription of certain TJ genes. We then looked at the abundance of certain TJ proteins. Western blot analysis revealed an increase in the abundance of claudin-3 in colon samples from rats on the MR diet compared with the AA group (Fig. 6, B and C). There was no significant change in the protein abundance of two other claudins tested, claudins 1 and 7. Although the abundance of occludin was not altered, there was an apparent change in posttranslational modification of occludin protein (Fig. 6B, top) in colonocytes from the MR diet group, with the appearance in MR colonocytes of an additional occludin band 3–5 kDa above the original occludin band.
Fig. 6.
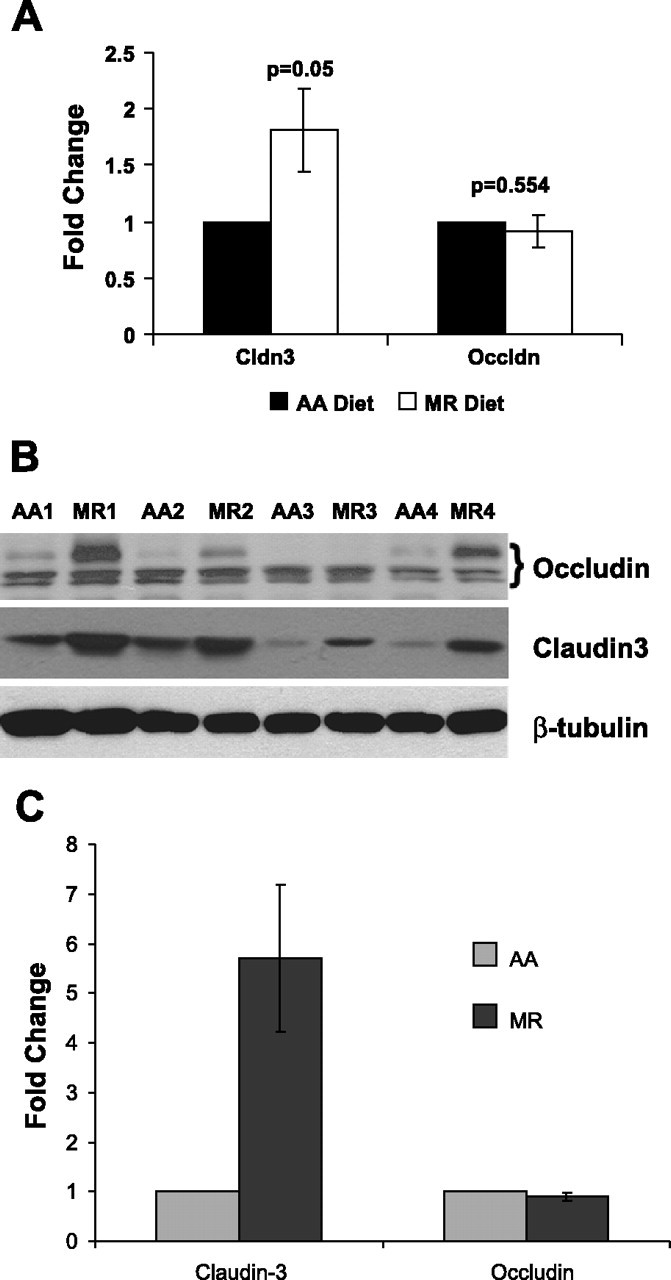
Methionine restriction alters mRNA expression and protein abundance of certain tight junction components. A: RT-PCR of CLDN3 and OCLN in the distal colon of animals on AA and MR diet (means ± SE, n = 3 tissues); GAPDH values were used for normalization. B: Western blot analysis showing the steady-state level of tight junction proteins (top, occludin; middle, claudin-3) in the distal colon of animals on AA and MR diets [numbers indicate the appropriate matched animal pairs (AA1, MR1, AA2, etc.); the various occludin species are marked by a bracket, with a new species appearing due presumably to posttranslational modification]. Sample loading was 20 μg total protein per lane, but inequalities were monitored by β-tubulin levels (bottom) (n = 4 tissues). C: quantitation and normalization of claudin-3 and occludin levels were based on the β-tubulin levels in each lane (means ± SE, n = 4 tissues).
DISCUSSION
Methionine restricted (MR) diet seems to be beneficial for the overall health of an organism. Several studies using rodent models report improved life expectancy (39, 45, 55). MR diet also seems to have certain tumor-suppressing activities as demonstrated by cell culture and clinical studies of prostate cancer (12, 14), GI cancer (23, 59), etc. In addition, restricted intake of methionine abated mitochondrial reactive oxygen species in rats (5, 18) and resulted in improved lipogenic/lipolytic balance (40) and possibly hypercholesterolemia (54). We extended our previous renal epithelial cell culture studies on restricted methionine to a more medically relevant, animal tissue model, to see whether MR also enhances epithelial barrier properties in gastrointestinal tissue.
In the present study, we only restricted the intake of methionine to 20% of the regular diet and did not completely eliminate it from the diet. Animals were on control (AA) or MR diet ad libitum for 4 wk (enough time for 4–7 rounds of epithelial refoliation of the GI tract). Our careful observations of rats indicated that the MR diet did not produce any unhealthy effects on the animals. With the exception of glucose and BUN, plasma electrolytes and nonelectrolytes remained unchanged after switching to the MR diet. The body weights of animals on the MR diet initially declined by 10% over the first 2 wk then began to recover, ending only 7% below initial weights at our longest time point (63 days). At this point, average weights were 18% less than time-matched controls, whose weight increased steadily over this time. Arguably these changes, including initial weight loss, are not considered to be unhealthy changes. The overall physical appearance and the activity level of the rats on low methionine diet seemed to be similar to that of rats on the full complement amino acids diet as observed on a biweekly basis.
The main result of our current study is that restricted dietary intake of methionine improved colon epithelial barrier function in rats, as we observed earlier for renal epithelial cells in vitro (51). We observed a significant increase in the transepithelial electrical resistance accompanied by decreased transepithelial paracellular flux of a radioactive tracer (14C-mannitol) across rat colon mucosa. No significant difference in the short circuit current across the colon tissue sheets from animals on AA vs. MR diets was observed (data not shown). This suggested that there was no difference in sodium reabsorption or chloride secretion from the colonocytes and that the difference seen in transepithelial resistance was in fact due to a difference in the TJ permeability, rather than differential transcellular permeability.
We did not see any gross changes in colon crypt architecture nor in colon cell kinetics in rats on the MR diet. However, we did observe a moderate change in the plasma and colonocyte (intracellular) methionine levels. Methionine is a major precursor of S-adenosylmethionine, which in turn acts as the main donor of methyl groups in the methylation reactions of several macromolecules including DNA. DNA methylation is one of the important epigenetic mechanisms known to alter gene expression. For these reasons, future studies are needed to focus on determining whether methionine-related changes in DNA methylation are responsible for the tight junctional alterations we observe in the MR diet (31, 56). Understanding the mechanism(s) behind the effects of restricted methionine diet on epithelial barrier function might help us to design new strategies to combat environmental and pathological conditions that target and affect epithelial barrier function in various tissues.
The epithelial and endothelial cell layers (and the TJs that bind the neighboring cells) exist to prevent free interchange of nutrients, water, toxins, and antigens between the luminal and antiluminal compartments along the paracellular route. Antigens or microbes found for example in bronchial/alveolar airways or the duodenal lumen cannot, therefore, freely cross into the stromal compartment and engender an inflammatory cascade. Microbial infectious agents on the skin, the surface of the colon, or the cornea are thereby prevented from gaining access to interstitial fluid and vasculature. Yet disease agents as diverse as the enterotoxins of bacteria, or proteases in feces of dust mites, can specifically and amazingly target individual TJ proteins as a means of gaining access to a more favorable environment on the other side of the barrier (and in the process triggering significant morbidity) (1, 2, 57). The disease ramifications of paracellular leak across any epithelial/endothelial barrier are myriad (34, 47).
In a previous study (51) with a kidney epithelial cell line (LLC-PK1), we observed that low methionine-containing medium resulted in an increase in the abundance of claudin-5, one of the renal TJ proteins. Therefore, we also looked at the gene expression (by RT-PCR) and the corresponding abundance of various colonocyte TJ proteins (by Western blot analysis) in the current study. Here, in colon, the abundance of claudin-3 was significantly increased in the MR diet while a posttranslational change in (but not the overall abundance of) occludin was also observed. Our group earlier observed posttranslational changes in occludin to accompany induced TJ permeability changes (8). In both the cell culture and the animal models the end result of the MR diet, viz. improvement in barrier function, is the same, while the particular TJ proteins involved are different. This difference in the change in the profile of TJ proteins involved in the kidney epithelial cells and the rat distal colon tissue likely reflects the specificity of the TJ protein composition of different epithelial tissues with similar biological function.
It is important to state that the changes we report in claudin-3 and occludin are not necessarily the molecular mechanism for the enhanced barrier function. They are merely structural changes we observed that may underlie the functional changes we recorded. In addition, it should be noted that MR is likely inducing many other changes to the epithelium besides simply tight junctional barrier changes. Cytoskeletal, cell signaling, and cell adhesion changes are also likely and need to be investigated. Beyond this, one needs to consider that imposition of MR on the entire organism will cause changes well beyond the GI tract. For example, the effect that we observe of MR on BUN could result from MR-induced changes in intermediary metabolism (liver or muscle) and/or changes in barrier function in the kidney.
A recent study (14) reported that methionine deprivation reduced the amount of Ras-GTP and Rho-GTP, two of the small GTPases that are involved in directing cell movement and regulating cytoskeleton arrangement. This then increased the amount of two actin-binding proteins, profilin and cofilin (and phosphorylation of cofilin-Ser3), in DU145 prostate cancer cells but reduced the amount of profilin in PC3 cells (15, 29). These specific effects of MR on cytoskeletal dynamics were shown to modulate the directionality and motility of prostate cancer cells (14). It is interesting to note the parallels between the differential effects MR has on proteins involved in cell motility and TJ barrier function, both being dependent on the cytoskeletal actin dynamics.
In summary, for the first time, we report here that restricted dietary intake of a single essential amino acid, namely, methionine, improved colon epithelial barrier function in rats as we observed a significant increase in the transepithelial resistance accompanied by decreased flux of a radioactive tracer (14C-mannitol) across the rat colon. We hypothesize for future studies that the improved barrier function was accomplished by the altered composition of TJ proteins that could have resulted from the lowered colonocyte DNA methylation, albeit with only a moderate change in the plasma and colonocyte methionine levels. Future studies should test some of the medical applications of the MR diet and its ability to enhance epithelial TJs and barrier function in various diseases whose etiology likely involves TJ compromise. For example, compromise of the gastrointestinal barrier in patients with Crohn's disease, one of the inflammatory bowel diseases, as evaluated by radiolabeled tracer leakage, electrical resistance, and/or electrical impedance, was later traced to alterations of the claudin TJ proteins mentioned above (60, 61). Another scenario where improvement of barrier function can be of significant value is in treating critical care patients with multiple organ failures (e.g., acute respiratory distress syndrome) and on prolonged enteric feeding. Although methionine restriction per se may be inappropriate or nonfeasible in certain medical conditions, its effects on tight junctions may provide a basis for other, more clinically practical therapies.
GRANTS
The current study was supported in part by a generous grant from the Sharpe-Strumia Research Foundation of the Bryn Mawr Hospital (Bryn Mawr, PA) and by the Broad Medical Research Program of The Broad Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Gwendolyn Gilliard for help with the histology work and Yvette Mercer, and Loretta Rossino, Editorial Office, Lankenau Institute for Medical Research, for assistance with the manuscript preparation and submission.
REFERENCES
- 1. Balkovetz DF , Katz J. Bacterial invasion by a paracellular route: divide and conquer. Microbes Infect : 613–619, 2003. [DOI] [PubMed] [Google Scholar]
- 2. Berkes J , Viswanathan V , Savkovic S , Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut : 439–451, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bromley M , Rew D , Becciolini A , Balzi M , Chadwick C , Hewitt D , Li Y , Potten C. A comparison of proliferation markers (BrdUrd, Ki-67, PCNA) determined at each cell position in the crypts of normal human colonic mucosa. Eur J Histochem : 89–100, 1996. [PubMed] [Google Scholar]
- 4. Cao WX , Ou JM , Fei XF , Zhu ZG , Yin HR , Yan M , Lin YZ. Methionine-dependence and combination chemotherapy on human gastric cancer cells in vitro. World J Gastroenterol : 230–232, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caro P , Gomez J , Lopez-Torres M , Sanchez I , Naudi A , Jove M , Pamplona R , Barja G. Forty percent and eighty percent methionine restriction decrease mitochondrial ROS generation and oxidative stress in rat liver. Biogerontology : 183–196, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Cellarier E , Durando X , Vasson MP , Farges MC , Demiden A , Maurizis JC , Madelmont JC , Chollet P. Methionine dependency and cancer treatment. Cancer Treat Rev : 489–499, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Clarke H , Marano CW , Soler AP , Mullin JM. Modification of tight junction function by protein kinase C isoforms. Adv Drug Deliv Rev : 283–301, 2000. [DOI] [PubMed] [Google Scholar]
- 8. Clarke H , Soler AP , Mullin JM. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J Cell Sci : 3187–3196, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Colman RJ , Anderson RM , Johnson SC , Kastman EK , Kosmatka KJ , Beasley TM , Allison DB , Cruzen C , Simmons HA , Kemnitz JW , Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science : 201–204, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Souza T , Indig FE , Morin PJ. Phosphorylation of claudin-4 by PKCepsilon regulates tight junction barrier function in ovarian cancer cells. Exp Cell Res : 3364–3375, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dirks AJ , Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev : 1–7, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Epner D , Morrow S , Wilcox M , Houghton J. Nutrient intake and nutritional indexes in adults with metastatic cancer on a phase I clinical trial of dietary methionine restriction. Nutr Cancer : 158–166, 2002. [DOI] [PubMed] [Google Scholar]
- 13. Fu YM , Zhang H , Ding M , Li YQ , Fu X , Yu ZX , Meadows GG. Selective amino acid restriction targets mitochondria to induce apoptosis of androgen-independent prostate cancer cells. J Cell Physiol : 522–534, 2006. [DOI] [PubMed] [Google Scholar]
- 14. Fu YM , Yu ZX , Lin H , Fu X , Meadows GG. Selective amino acid restriction differentially affects the motility and directionality of DU145 and PC3 prostate cancer cells. J Cell Physiol : 184–193, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall A. Rho GTPases and the actin cytoskeleton. Science : 509–514, 1998. [DOI] [PubMed] [Google Scholar]
- 16. Hawker PC , McKay JS , Turnberg LA. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology : 508–511, 1980. [PubMed] [Google Scholar]
- 17. Hewitt K , Agarwal R , Morin P. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer : 186, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hipkiss AR. On methionine restriction, suppression of mitochondrial dysfunction and aging. Rejuvenation Res : 685–688, 2008. [DOI] [PubMed] [Google Scholar]
- 19. Hoffman RM. Altered methionine metabolism and transmethylation in cancer. Anticancer Res : 1–30, 1985. [PubMed] [Google Scholar]
- 20. Hollander D. Crohn's disease–a permeability disorder of the tight junction? Gut : 1621–1624, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kokkinakis DM. Methionine-stress: a pleiotropic approach in enhancing the efficacy of chemotherapy. Cancer Lett : 195–207, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Kominsky SL , Argani P , Korz D , Evron E , Raman V , Garrett E , Rein A , Sauter G , Kallioniemi OP , Sukumar S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene : 2021–2033, 2003. [DOI] [PubMed] [Google Scholar]
- 23. Komninou D , Leutzinger Y , Reddy BS , Richie JP. methionine restriction inhibits colon carcinogenesis. Nutr Cancer : 202–208, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Kreis W , Baker A , Ryan V , Bertasso A. Effect of nutritional and enzymatic methionine deprivation upon human normal and malignant cells in tissue culture. Cancer Res : 634–641, 1980. [PubMed] [Google Scholar]
- 25. Liebner S , Fischmann A , Rascher G , Duffner F , Grote EH , Kalbacher H , Wolburg H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol (Berl) : 323–331, 2000. [DOI] [PubMed] [Google Scholar]
- 26. Marin ML , Greenstein AJ , Geller SA , Gordon RE , Aufses AH. A freeze fracture study of Crohn's disease of the terminal ileum: changes in epithelial tight junction organization. Am J Gastroenterol : 537–547, 1983. [PubMed] [Google Scholar]
- 27. Martinez-Palomo A. Ultrastructural modifications of intercellular junctions between tumor cells. In Vitro : 15–20, 1970. [DOI] [PubMed] [Google Scholar]
- 28. Masoro EJ. Caloric restriction and aging: controversial issues. J Gerontol A Biol Sci Med Sci : 14–19, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Meili R , Firtel RA. Two poles and a compass. Cell : 153–156, 2003. [DOI] [PubMed] [Google Scholar]
- 30. Miller RA , Buehner G , Chang Y , Harper JM , Sigler R , Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell : 119–125, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miranda TB , Jones PA. DNA methylation: the nuts and bolts of repression. J Cell Physiol : 384–390, 2007. [DOI] [PubMed] [Google Scholar]
- 32. Mullin JM. Epithelial barriers, compartmentation, and cancer. Sci STKE : pe2, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Mullin JM , Agostino N , Rendon-Huerta E , Thornton JJ. Keynote review: epithelial and endothelial barriers in human disease. Drug Discov Today : 395–408, 2005. [DOI] [PubMed] [Google Scholar]
- 34. Mullin JM , Leatherman JM , Valenzano MC , Huerta ER , Verrechio J , Smith DM , Snetselaar K , Liu M , Francis MK , Sell C. Ras mutation impairs epithelial barrier function to a wide range of nonelectrolytes. Mol Biol Cell : 5538–5550, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mullin JM , Marano CW , Laughlin KV , Nuciglio M , Stevenson BR , Peralta Soler A. Different size limitations for increased transepithelial paracellular solute flux across phorbol ester and tumor necrosis factor-treated epithelial cell sheets. J Cell Physiol : 226–233, 1997. [DOI] [PubMed] [Google Scholar]
- 36. Mullin JM , Peralta Soler A , Laughlin KV , Kampherstein JA , Russo LM , Saladik DT , George K , Shurina RD , O'Brien TG. Chronic exposure of LLC-PK1 epithelia to the phorbol ester TPA produces polyp-like foci with leaky tight junctions and altered protein kinase C-alpha expression and localization. Exp Cell Res : 12–22, 1996. [DOI] [PubMed] [Google Scholar]
- 37. Mullin JM , Snock KV , Shurina RD , Noe J , George K , Misner L , Imaizumi S , O'Brien TG. Effects of acute vs. chronic phorbol ester exposure on transepithelial permeability and epithelial morphology. J Cell Physiol : 35–47, 1992. [DOI] [PubMed] [Google Scholar]
- 38. Novikova SI , He F , Bai J , Cutrufello NJ , Lidow MS , Undieh AS. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS ONE : e1919, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Orentreich N , Matias JR , DeFelice A , Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr : 269–274, 1993. [DOI] [PubMed] [Google Scholar]
- 40. Perrone CE , Mattocks DAL , Hristopoulos G , Plummer JD , Krajcik RA , Orentreich N. Methionine restriction effects on 11 -HSD1 activity and lipogenic/lipolytic balance in F344 rat adipose tissue. J Lipid Res : 12–23, 2008. [DOI] [PubMed] [Google Scholar]
- 41. Pogribny IP , Karpf AR , James SR , Melnyk S , Han T , Tryndyak VP. Epigenetic alterations in the brains of Fisher 344 rats induced by long-term administration of folate/methyl-deficient diet. Brain Res : 25–34, 2008. [DOI] [PubMed] [Google Scholar]
- 42. Ramalingam A , Duhadaway JB , Sutanto-Ward E , Wang Y , Dinchuk J , Huang M , Donover PS , Boulden J , McNally LM , Soler AP , Muller AJ , Duncan MK , Prendergast GC. Bin3 deletion causes cataracts and increased susceptibility to lymphoma during aging. Cancer Res : 1683–1690, 2008. [DOI] [PubMed] [Google Scholar]
- 43. Rangel LB , Sherman-Baust CA , Wernyj RP , Schwartz DR , Cho KR , Morin PJ. Characterization of novel human ovarian cancer-specific transcripts (HOSTs) identified by serial analysis of gene expression. Oncogene : 7225–7232, 2003. [DOI] [PubMed] [Google Scholar]
- 44. Richie JP , Komninou D , Leutzinger Y , Kleinman W , Orentreich N , Malloy V , Zimmerman JA. Tissue glutathione and cysteine levels in methionine-restricted rats. Nutrition : 800–805, 2004. [DOI] [PubMed] [Google Scholar]
- 45. Richie JP , Leutzinger Y , Parthasarathy S , Malloy V , Orentreich N , Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J : 1302, 1994. [DOI] [PubMed] [Google Scholar]
- 46. Sanz A , Caro P , Ayala V , Portero-Otin M , Pamplona R , Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J : 1064–1073, 2006. [DOI] [PubMed] [Google Scholar]
- 47. Sawada N , Murata M , Kikuchi K , Osanai M , Tobioka H , Kojima T , Chiba H. Tight junctions and human diseases. Med Electron Microsc : 147–156, 2003. [DOI] [PubMed] [Google Scholar]
- 48. Schneeberger EE , Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol : C1213–C1228, 2004. [DOI] [PubMed] [Google Scholar]
- 49. Simons RM , Laughlin KV , Kampherstein JA , Desai DC , Shurina RD , Mullin JM. Pentobarbital affects transepithelial electrophysiological parameters regulated by protein kinase C in rat distal colon. Dig Dis Sci : 632–640, 1998. [DOI] [PubMed] [Google Scholar]
- 50. Singh AB , Harris RC. Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J Biol Chem : 3543–3552, 2004. [DOI] [PubMed] [Google Scholar]
- 51. Skrovanek S , Valenzano MC , Mullin JM. Restriction of sulfur-containing amino acids alters claudin composition and improves tight junction barrier function. Am J Physiol Regul Integr Comp Physiol : R1046–R1055, 2007. [DOI] [PubMed] [Google Scholar]
- 52. Soler AP , Miller RD , Laughlin KV , Carp NZ , Klurfeld DM , Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis : 1425–1431, 1999. [DOI] [PubMed] [Google Scholar]
- 53. Su L , Shen L , Clayburgh D , Nalle S , Sullivan E , Meddings J , Abraham C , Turner J. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology : 551–563, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sugiyama K , Yamakawa A , Kumazawa A , Saeki S. Methionine content of dietary proteins affects the molecular species composition of plasma phosphatidylcholine in rats fed a cholesterol-free diet. J Nutr : 600–607, 1997. [DOI] [PubMed] [Google Scholar]
- 55. Sun L , Sadighi Akha AA , Miller RA , Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci : 711–722, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tost J. DNA methylation: an introduction to the biology and the disease-associated changes of a promising biomarker. Methods Mol Biol : 3–20, 2009. [DOI] [PubMed] [Google Scholar]
- 57. Wan H , Winton HL , Soeller C , Taylor GW , Gruenert DC , Thompson PJ , Cannell MB , Stewart GA , Garrod DR , Robinson C. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy : 279–294, 2001. [DOI] [PubMed] [Google Scholar]
- 58. Wolf G. Calorie restriction increases life span: a molecular mechanism. Nutr Rev : 89–92, 2006. [DOI] [PubMed] [Google Scholar]
- 59. Xin L , Cao WX , Fei XF , Wang Y , Liu WT , Liu BY , Zhu ZG. Applying proteomic methodologies to analyze the effect of methionine restriction on proliferation of human gastric cancer SGC7901 cells. Clin Chim Acta : 206–212, 2007. [DOI] [PubMed] [Google Scholar]
- 60. Zeissig S , Bojarski C , Buergel N , Mankertz J , Zeitz M , Fromm M , Schulzke JD. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor alpha antibody treatment. Gut : 1295–1302, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zeissig S , Burgel N , Gunzel D , Richter J , Mankertz J , Wahnschaffe U , Kroesen AJ , Zeitz M , Fromm M , Schulzke JD. Changes in expression and distribution of claudin-2, -5 and -8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut : 61–72, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zettl KS , Sjaastad MD , Riskin PM , Parry G , Machen TE , Firestone GL. Glucocorticoid-induced formation of tight junctions in mouse mammary epithelial cells in vitro. Proc Natl Acad Sci USA : 9069–9073, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhong Y , Enomoto K , Tobioka H , Konishi Y , Satoh M , Mori M. Sequential decrease in tight junctions as revealed by 7H6 tight junction-associated protein during rat hepatocarcinogenesis. Jpn J Cancer Res : 351–356, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zimmerman JA , Malloy V , Krajcik R , Orentreich N. Nutritional control of aging. Exp Gerontol : 47–52, 2003. [DOI] [PubMed] [Google Scholar]