Abstract
Aging and cardiovascular disease are associated with the loss of nitric oxide (NO) signaling and a decline in the ability to increase coronary blood flow reserve (CFR). Thrombospondin-1 (Thbs-1), through binding of CD47, has been shown to limit NO-dependent vasodilation in peripheral vascular beds via formation of superoxide (O2−). The present study tests the hypothesis that, similar to the peripheral vasculature, blocking CD47 will improve NO-mediated vasoreactivity in coronary arterioles from aged individuals, resulting in improved CFR. Isolated coronary arterioles from young (4 mo) or old (24 mo) female Fischer 344 rats were challenged with the NO donor, DEA-NONO-ate (1 × 10−7 to 1 × 10−4 M), and vessel relaxation and O2− production was measured before and after Thbs-1, αCD47, and/or Tempol and catalase exposure. In vivo CFR was determined in anesthetized rats (1–3% isoflurane-balance O2) via injected microspheres following control IgG or αCD47 treatment (45 min). Isolated coronary arterioles from young and old rats relax similarly to exogenous NO, but addition of 2.2 nM Thbs-1 inhibited NO-mediated vasodilation by 24% in old rats, whereas young vessels were unaffected. Thbs-1 increased O2− production in coronary arterioles from rats of both ages, but this was exaggerated in old rats. The addition of CD47 blocking antibody completely restored NO-dependent vasodilation in isolated arterioles from aged rats and attenuated O2− production. Furthermore, αCD47 treatment increased CFR from 9.6 ± 9.3 (IgG) to 84.0 ± 23% in the left ventricle in intact, aged animals. These findings suggest that the influence of Thbs-1 and CD47 on coronary perfusion increases with aging and may be therapeutically targeted to reverse coronary microvascular dysfunction.
Keywords: aging, thrombospondin-1, CD47, nitric oxide, coronary blood flow
NEW & NOTEWORTHY
Advanced age increases the potency of thrombospondin-1 action on coronary arterioles, resulting in increased superoxide production and suppressed vasodilation. CD47 blocking antibodies attenuate the action of thrombospondin-1 in coronary arterioles and improve coronary flow reserve in a rodent model of aging.
changes in vascular phenotype, such as decreases in nitric oxide (NO) signaling and increases in oxidative stress, have been linked with aging (12, 21, 24, 25, 42). NO is the primary vasodilator employed by the coronary vasculature and, in particular, the arterioles whose vasoactivity is largely responsible for increasing myocardial perfusion to meet demand (15, 29). In addition, loss of NO signaling leads to a substrate switch of the proatherosclerotic molecule, H2O2 (6, 32, 33). Several studies have shown that a reduction in NO bioavailability contributes to the development of endothelial dysfunction (5, 39). Not surprisingly, loss of NO signaling and increases in oxidative stress are established markers of cardiovascular disease (9, 31, 33, 43, 45). Notably, endothelial dysfunction and loss of NO-mediated dilation have been shown to be effective predictors of future adverse cardiovascular events (2, 27).
The matricellular protein thrombospondin-1 (Thbs-1) has been shown to reduce NO-dependent vasodilation, with impact demonstrated on systemic blood pressure and in multiple vascular settings, including the aorta, kidney, and lung (3, 4, 10, 36). This effect occurs through inhibition of NO signaling at multiple levels, including blocking NO production through endothelial NO synthase (eNOS) and inhibiting the activity of soluble guanylyl cyclase (sGC), the major downstream effector of NO in vasodilation (3, 30, 46). Thbs-1 has additionally been shown to induce production of superoxide through NADPH oxidase and uncoupling of endothelial NO synthase (4, 10). Superoxide is known to rapidly react with NO to form peroxynitrite, reducing the availability of NO for vascular signaling and thereby pushing the balance of vasoactivity toward vasoconstriction (8). Binding to the cell surface receptor CD47 on both endothelial cells and vascular smooth muscle cells appears to facilitate the vascular actions of Thbs-1 (3, 10). In fact, the interaction of Thbs-1 and CD47 has been implicated in the progression of pulmonary arterial hypertension and renal ischemia-reperfusion injury (4, 36).
Because of the association with cardiovascular risk, it is important to understand the mechanisms by which NO signaling in the vasculature is impaired in aging, especially in women. This is because >50% of women that present with symptoms of coronary ischemia do not have large-vessel obstruction, but instead have coronary microvascular dysfunction (34). Our laboratory has previously demonstrated that NO-mediated dilation is decreased with advancing age in coronary arterioles of female rats (24). Thbs-1 is known to potently reduce NO signaling, but has not been studied in the context of coronary microvascular reactivity or advancing age in women. Understanding this process is likely to identify therapeutic targets for addressing coronary dysfunction in aging and the rationale for the use of female rodents in the present study. Additionally, peptides that mimic the CD47 binding domain of Thbs-1 have shown efficacy as anti-angiogenic therapies (17). Humanized antibodies against CD47 have shown promise for cancer treatment in a preclinical setting and have progressed on to clinical trials (28). We hypothesize that Thbs-1 interaction with CD47 contributes to the loss of NO signaling with aging, and that use of antibodies designed to block the activation of CD47 will be effective for the treatment of age-related coronary microvascular dysfunction.
METHODS
Animals.
All animal surgeries were performed in accordance with protocols approved by the University of Louisville Institutional Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, 2011). All animals used were female Fischer 344 rats. Young rats were purchased from Harlan Laboratories and were used at 4 mo of age. Old rats were provided by the National Institute on Aging and were used at 24 mo of age. All animals were anesthetized with 5% isoflurane before being euthanized by removal of the heart.
Isolated vessel experiments.
Coronary arterioles (100–150 μm diameter) branching from the left anterior descending artery (LAD) were isolated and cannulated between pipettes in a microvascular perfusion chamber (isolated vessel systems). Vessels were maintained at 37°C in a 3-ml physiological saline solution (PSS) bath containing Ca2+ Ringer solution, MOPS, NaH2PO4, glucose, pyruvic acid, EDTA, and bovine serum albumin. PSS was changed every 20 min to refresh substrates required for vasoactivity. Pressure across the vessel was stabilized at 45 mmHg. The luminal vessel diameter was measured with a video caliper system (Colorado Video Systems). Pressure and diameter measurements were recorded throughout the experiment with LabScribe3 software (iWorx). Vasodilation experiments were begun once vessels reached and maintained a spontaneous tone of 20% or greater, spontaneous tone = 100 × (maximum diameter − resting diameter)/maximum diameter. Vessels that failed to reach a spontaneous tone of 20% or greater were not used for experiments. Maximum dilation was measured after 30-min incubation in Ca2+-free PSS, followed by addition of sodium nitroprusside.
NO-dependent dilation was induced by addition of the NO donor diethylamine NONOate (DEA-NONO-ate, Cayman Chemical) to the vessel perfusion bath in 2-min increments to cover a concentration range from 1 × 10−7 to 1 × 10−4 M in half-log increments. Vessel diameter at the end of each 2-min incubation was recorded. In dilation experiments involving Thbs-1, vessels were incubated with 2.2 nM Thbs-1 (recombinant human, Athens Research and Technology) for 1 h before as well as during the concentration response of DEA-NONO-ate. Vessels were incubated with the SOD mimetic 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (Tempol, 0.1 mM, Sigma) and the enzyme polyethylene glycol-catalase (catalase, 500 U/ml, Sigma), along side Thbs-1 for experiments involving ROS scavengers. CD47 blockade experiments were carried out with a CD47 COOH-terminal domain functional blocking antibody (αCD47, Abcam, ab33852). Vessels were incubated with 2 μg/ml αCD47 for 20 min before the addition of Thbs-1, followed by coincubation for 1 h before beginning the DEA-NONO-ate concentration response.
The fluorescent dye dihydroethidium (DHE; Molecular Probes, Life Technologies) was used to measure generation of superoxide. DHE was dissolved in 1 ml of PSS without albumin to a concentration of 5 μM, which was then perfused through the vessel over a period of 5 min followed by a 10-min incubation period. After incubation, vessels were flushed with 1 ml of PSS without albumin for 5 min, and the vessel bath was replaced with fresh PSS without albumin. Two minutes after these washes, baseline images were obtained with a 10× objective using bright-field (auto exposure), 4,6-diamidino-2-phenylindole (200-ms exposure), and tetramethylrhodamine isothiocyanate (40-ms exposure) filters. Vessels were then incubated with Thbs-1 at 2.2 nM for 20 min, and imaging was repeated. In separate experiments, baseline images were obtained, followed by 10-min incubation with 2 μg/ml αCD47 and subsequent 20-min coincubation with Thbs-1 and αCD47 before imaging was repeated. A third set of experiments was performed in which baseline images were obtained, followed by 20 min coincubation with Thbs-1 and Tempol (0.1 mM) and catalase (500 U/ml) before imaging was repeated. The extent of DHE oxidation by superoxide under each condition was determined within a 1-mm2 region of interest placed over the vessel wall. DAPI signal (unoxidized DHE) was subtracted from tetramethylrhodamine isothiocyanate signal (oxidized DHE) to normalize for dye loading (23). Fluorescence change from baseline was calculated and reported as arbitrary units for each treatment, Δfluorescence = fluorescencetreatment − fluorescencebaseline.
ELISA.
Measurement of circulating levels of Thbs-1 was performed in triplicate by ELISA assay (Cloud Gene) on rat plasma, according to manufacturer specifications. Blood was collected from the left ventricle (LV) of young and old rats before death in K2-EDTA-coated tubes (Becton Dickinson), spun down for 15 min at 1,000 g, and the plasma supernatant was retrieved and stored at −20°C.
Western blot.
Protein levels of Thbs-1 and CD47 in the heart were detected by Western blotting. Heart tissue was homogenized on ice in lysis buffer containing 1 mM DTT, 0.1% protease inhibitor (Sigma, P-8340), and 0.81 mM sodium-orthovanadate. Lysates were centrifuged, and supernatant was collected. Protein concentrations were determined by a Bradford Protein Quantification assay (Bio-Rad). Lysis buffer was used to bring all samples to a protein concentration of 1 μg/μl. Fifteen micrograms of each sample were prepared using 2 × Laemmli buffer (Bio-Rad) containing 5% β-mercaptoethanol. Electrophoresis was performed using precast 4–20% gradient gels (Bio-Rad), followed by overnight transfer to polyvinylidene difluoride membranes. Membranes were blocked in 5% milk before overnight incubation with primary antibodies against Thbs-1 (mouse anti-Thbs-1, 1:200, Thermo Scientific), CD47 (goat anti-CD47, 1:1,000, Santa Cruz), and β-actin (mouse anti-β-actin, 1:2,000, Santa Cruz). Incubation with the fluorescently labeled secondary antibodies goat anti-mouse 488 and rabbit anti-goat 594 (Jackson ImmunoResearch) were carried out for 1 h at 1:1,000 dilutions. Imaging was carried out on a Typhoon FLA 7000 biomolecular imager (GE). ImageJ 1.47v software (National Institutes of Health) was used to perform densitometry, with β-actin serving as a loading control for each sample.
Quantitative PCR.
Thbs-1 and CD47 transcript levels were detected in LV tissue and coronary arterioles. RNA was isolated from LV tissue using RNA-bee (Amsbio) and phase-lock gel tubes (5 Prime). A RNeasy Mini Kit (Qiagen) was used according to the manufacturer's protocols to isolate RNA from coronary arterioles. Following RNA isolation, cDNA was synthesized using the Affinity Script quantitative PCR (qPCR) cDNA synthesis kit (Agilent), according to manufacturer instructions. Quantitative real-time PCR was performed on the generated complimentary DNA to measure Thbs-1 and CD47 transcript levels, with readings being normalized to the reference gene dynactin. All reactions were run on a Rotor Gene 6000 real-time PCR machine (Corbett Life Science). The following rat primers (Integrated DNA Technologies) were used: Thbs-1 forward CGGTTTGATCAGAGTGGTGA, Thbs-1 reverse CGGCACTCGTATTTCATGTC, CD47 forward CTGGGCTATGTCCTTGCTGT, CD47 reverse CTGGTTGGAAGCGACAAACT, dynactin forward CGAGAAGCTCAAGGATGAGG, and dynactin reverse GAAGGTCACTTTGCCCATGT. Fold changes were determined by the 2−ΔΔCT method, in which ΔCT = CT(target gene) − CT(reference gene) and ΔΔCT = ΔCT(target sample) − ΔCT(reference sample).
Immunofluorescence and confocal imaging.
CD47 expression and localization within isolated coronary arterioles was analyzed by immunofluorescent labeling and confocal imaging. Coronary arterioles of 100- to 150-μm diameter were isolated from the LAD, fixed with 2% paraformaldehyde (Electron Microscopy Sciences), and permeabilized with 0.5% Triton X-100 (Sigma Aldrich). Blocking was carried in PBS containing 0.1% Triton X-100, 10% normal rabbit serum (Jackson ImmunoResearch), and 0.5% bovine serum albumin (Sigma Aldrich). Vessels were incubated with goat anti-CD47 primary antibody (Santa Cruz) diluted 1:50 in blocking solution. Negative control vessels received goat IgG (Life Technologies) in place of primary antibody. The fluorescently tagged secondary antibody rabbit anti-goat AF594 (1:200, Life Technologies) was applied, followed by nuclear staining with RedDot2 (1:200, Biotium). Vessels were placed in Fluoromount-G (Southern Biotech) and imaged using an Olympus BX61WI confocal microscope with a ×60 objective (numerical aperture 0.9) using a 1-μm step size and total imaging depth of ∼120 μm. CD47 was detected using the AF594 excitation/emission filter set, and RedDot2 was detected using the AF647 excitation/emission filter set. AMIRA v6.0.1 software (FEI) was used to generate three-dimensional reconstructions from confocal images.
Coronary blood flow reserve measurements.
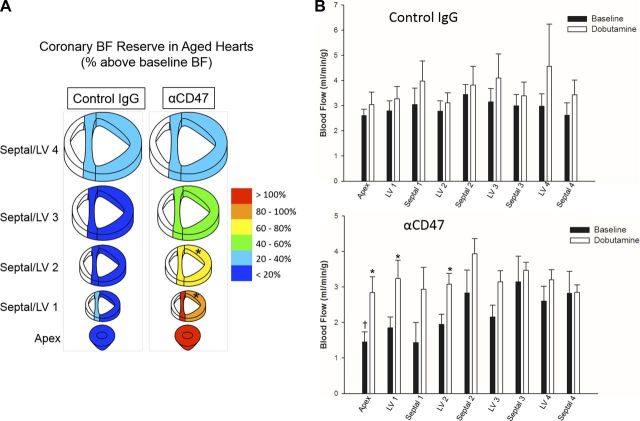
Twenty-four-month-old female rats received 100 μg of either control IgG (Abcam, ab18437) or CD47 functional blocking antibody (αCD47, Abcam, ab33852) in 200 μl of sterile saline intravenously via tail vein injection. Antibody dose was selected to achieve ∼0.4 μg antibody/g body wt (35). One hour following tail vein injection, coronary blood flow (BF) measurements were obtained by injection of colored microspheres (BioPal), as previously described (26). Briefly, 1.2 × 106 microspheres were injected into the LV transapically while carotid blood was concurrently sampled at a constant withdrawal rate. Distinct isotopes were injected for a baseline sampling, and this was followed by a second sampling taken at the end of a 5-min intravenous dobutamine injection (10 μg·kg−1·min−1). Following microsphere delivery during dobutamine stress, hearts were explanted and divided into nine sections consisting of the apex, and four regions each of the LV and septum (see Fig. 5A). This sectioning allowed for analysis of regional BF, including portions of the LV that are supplied by the LAD. Heart sections and blood samples underwent neutron activation at BioPal for sensitive detection of labeled microspheres. Coronary BF (ml·min−1·g−1) was calculated by the following: BF = {[blood sample withdraw rate (ml/min) × dpm in tissue]/dpm in blood sample}/tissue weight (g), where dpm is disintegrations/min. Blood samples were withdrawn at 500 μl/min for 2 min. Coronary blood flow reserve (CFR) was calculated as percent increase in BF over baseline: 100 × (dobutamine BF − baseline BF)/baseline BF.
Fig. 5.
CD47 blockade improves coronary blood flow reserve (CFR) in advanced age. A: CFR was calculated from measurements of blood flow at baseline and with dobutamine stimulation. Hearts from control IgG-treated (n = 4) and αCD47-treated (n = 5) 24-mo-old rats were sectioned to allow for analysis of regional perfusion changes. Color for each section corresponds to percent increase in blood flow with dobutamine stimulation over baseline. *Significant difference between αCD47 and control IgG sections. B: absolute blood flow (ml·min−1·g−1) under baseline and dobutamine conditions is shown for heart sections from control IgG- and αCD47-treated old rats. Values are means ± SE. *Significant difference between dobutamine stimulated and baseline blood flow. †Difference between control IgG and αCD47 treated rats.
Statistics.
Vessel relaxation and superoxide experiments were analyzed using fixed effects, mixed-model ANOVA. Bonferroni post hoc analysis was used to make subsequent pairwise comparisons. Experiments directly evaluating age-related differences without multiple treatments were analyzed by a Student's t-test. In all cases, the threshold for significance was set to P ≤ 0.05. All statistical analysis was performed using either SPSS (IBM) or SigmaPlot v11.2 software packages (Systat Software).
RESULTS
Thbs-1 reduces NO-dependent dilation in coronary arterioles of aged rats.
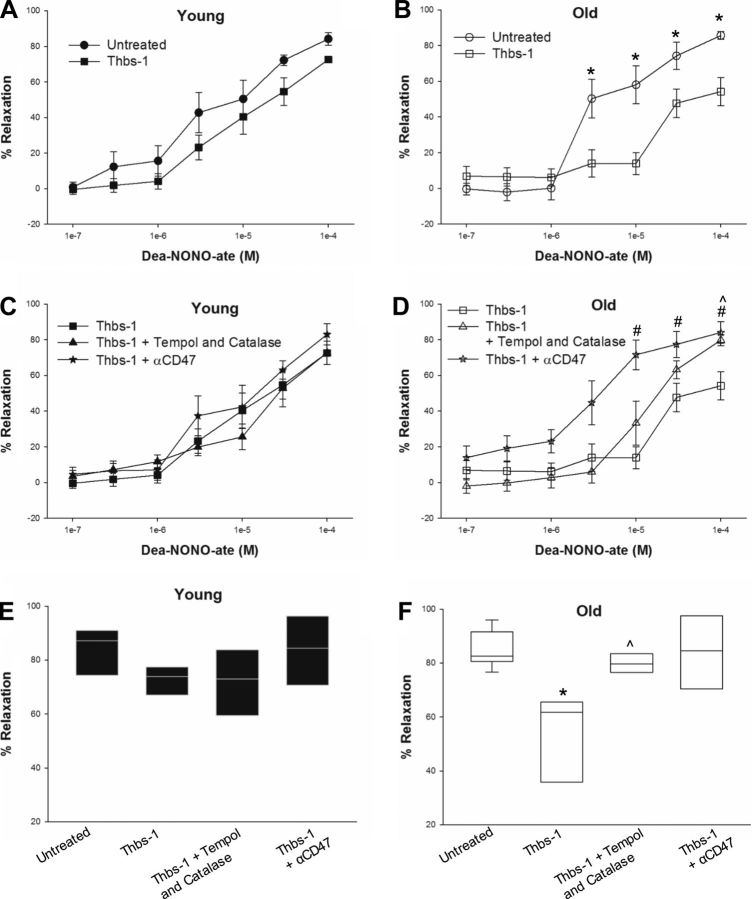
Under basal conditions (untreated at an intravascular pressure of 45 mmHg), coronary arterioles from young (4 mo) and old (24 mo) female rats showed similar levels of maximal diameter and spontaneous tone (Table 1) and relaxation to DEA-NONO-ate (Fig. 1, A and B). The addition of exogenous Thbs-1, at physiologically relevant levels (10), into the vessel bath did not inhibit NO-dependent dilation in arterioles from young rats (Fig. 1A). In contrast, the presence of exogenous Thbs-1 led to significant impairment of NO-dependent dilation in arterioles from old rats at 3 × 10−6 M (percent relaxation, untreated 47.32 ± 11.85% and Thbs-1 16.34 ± 10.33%), 1 × 10−5 M (54.22 ± 11.20 and 16.28 ± 8.16%), 3 × 10−5 M (72.25 ± 8.34 and 59.06 ± 3.58%), and 1 × 10−4 M (84.96 ± 2.39 and 65.48 ± 2.80%, respectively) DEA-NONO-ate (Fig. 1B).
Table 1.
Age group characteristics
| Young Female | Old Female | |
|---|---|---|
| Age, mo | 4 | 24 |
| Total n | 28 | 35 |
| Body weight, g | 194.9 ± 2.5 | 291.4 ± 6.8* |
| Heart weight, mg | 510.7 ± 10.2 | 727.9 ± 16.8* |
| Maximum vessel diameter, μm | 137.0 ± 5.0 | 143.2 ± 4.6 |
| Baseline | ||
| n | 6 | 9 |
| Spontaneous tone, % | 53.9 ± 4.7 | 44.5 ± 4.4 |
| Intitial steady-state diameter, μm | 60.7 ± 9.3 | 84.5 ± 7.1 |
| Thbs-1 | ||
| n | 8 | 8 |
| Spontaneous tone, % | 33.6 ± 1.7 | 31.7 ± 3.0 |
| Intitial steady-state diameter, μm | 90.1 ± 5.9 | 100.25 ± 9.5 |
| Thbs-1 + Tempol and catalase | ||
| n | 6 | 6 |
| Spontaneous tone, % | 40.8 ± 5.2 | 34.6 ± 3.0 |
| Intitial steady-state diameter, μm | 77.2 ± 10.6 | 89.4 ± 9.2 |
| Thbs-1 + αCD47 | ||
| n | 6 | 6 |
| Spontaneous tone, % | 34.0 ± 4.4 | 37.9 ± 4.3 |
| Intitial steady-state diameter, μm | 103.0 ± 9.9 | 90.7 ± 6.3 |
Values are means ± SE; n, no. of rats. Animal and isolated coronary arteriole characteristics are presented for young and old age female groups. Old female rats exhibited increased body weight and heart weight compared with young females. Initial steady-state diameter values were measured for each treatment before addition of DEA-NONO-ate, but no age-related or treatment-dependent differences were noted. Spontaneous tone was calculated by the formula, %spontaneous tone = 100 × (maximum diameter − initial diameter)/maximum diameter.
Significant age-related differences.
Fig. 1.
Thbs-1 reduces NO-dependent vasodilation in coronary arterioles through the cell surface receptor CD47. Line and scatter plots show percent relaxation of isolated coronary arterioles at concentrations of DEA-NONO-ate ranging from 1 × 10−7 to 1 × 10−4 M. A: addition of 2.2 nM Thbs-1 did not significantly reduce vasodilation in arterioles from young (4 mo) rats. B: arterioles from old (24 mo) rats showed reduced vasodilation in the presence of Thbs-1 at 3 × 10−6, 1 × 10−5, 3 × 10−5, and 1 × 10−4 M DEA-NONO-ate. C: providing the antioxidants Tempol and catalase or a CD47 blocking antibody (αCD47) along with Thbs-1 did not significantly alter vasodilation in arterioles from young rats. D: treatment with αCD47 prevented Thbs-1-associated decreases in vasodilation at 1 × 10−5, 3 × 10−5, and 1 × 10−4 M DEA-NONO-ate in arterioles from old rats. Addition of Tempol and catalase improved vasodilation compared with Thbs-1 alone at 1 × 10−4 M DEA-NONO-ate. E and F: percent relaxation for all treatment groups at 1 × 10−4 M DEA-NONO-ate is represented by box plots showing the median, 1st quartile, and 3rd quartile (E, young; F, old). Error bars show the 95% confidence interval for groups with n ≥ 9. Significant differences are denoted by *between untreated and Thbs-1 groups, #between Thbs-1 and Thbs-1 + α-CD47 groups, and ^between Thbs-1 and Thbs-1 + Tempol and catalase groups. For all groups, n = 6–9.
CD47 blockade improves NO-dependent dilation in aging.
In a separate set of experiments, coronary arterioles from young and old female rats were cotreated with Thbs-1 and αCD47. Arterioles from young rats did not show differences in vasodilation with αCD47 treatment compared with Thbs-1 alone or no treatment (Fig. 1, C and E). However, αCD47 treatment improved NO-dependent vasodilation in the presence of Thbs-1 in coronary arterioles from old rats, with significant improvements at 1 × 10−5 M (percent relaxation, Thbs-1 16.28 ± 8.16% and Thbs-1 + αCD47 71.55 ± 8.33%), 3 × 10−5 M (59.06 ± 3.58 and 77.34 ± 7.24%), and 1 × 10−4 M (65.48 ± 2.80 and 84.02 ± 6.01%, respectively) DEA-NONO-ate (Fig. 1, D and 1F). Additionally, arterioles from old rats treated with αCD47 showed significantly increased dilation to low concentrations of NO compared with untreated arterioles (3 × 10−7 M, untreated 4.56 ± 9.16% and Thbs-1 + αCD47 19.10 ± 7.16%; 1 × 10−6 M, 4.06 ± 5.98 and 23.13 ± 6.57%, respectively) (compare Fig. 1, B and D). Notably, cotreatment of old arterioles with αCD47 and Thbs-1 improved vasodilation across all concentrations of DEA-NONO-ate to levels equal or greater than those seen in young arterioles under basal conditions (compare Fig. 1, A and D).
ROS scavengers minimally restore NO-dependent vasodilation in aging.
Since Thbs-1 has been shown to increase superoxide levels, the SOD mimetic Tempol and the enzyme catalase were employed to convert superoxide to oxygen and water (4, 10). Arterioles from young rats cotreated with Thbs-1, Tempol, and catalase showed no difference in NO-dependent relaxation compared with Thbs-1 only and untreated vessels (Fig. 1, C and E). Cotreatment of coronary arterioles from old rats with Thbs-1, Tempol, and catalase improved NO-dependent vasodilation at only the 1 × 10−4 M concentration of DEA-NONO-ate compared with Thbs-1 treatment alone (Fig. 1, D and F; percent relaxation, Thbs-1 65.48 ± 2.80% and Thbs-1 + Tempol and catalase 79.71 ± 3.03%).
CD47 blockade prevents Thbs-1-induced production of superoxide.
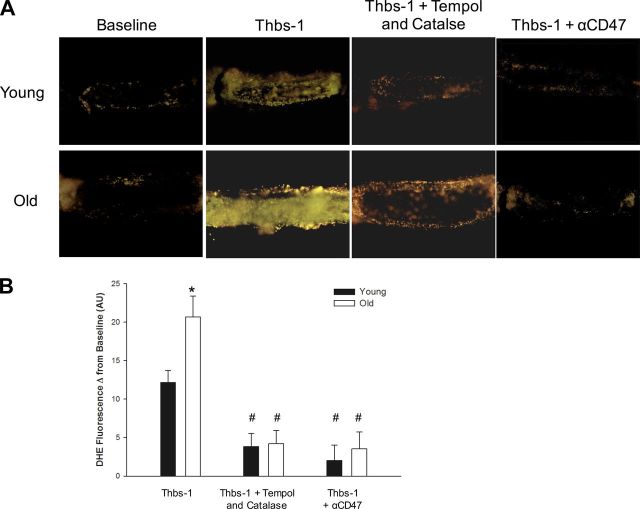
Superoxide levels, as measured by DHE fluorescence intensity, were similar for arterioles from both young and old rats, with increases observed after addition of Thbs-1 (Fig. 2). Notably, Thbs-1 induced significantly greater superoxide production in arterioles from old rats compared with young [Fig. 2B; fluorescence intensity change, young 12.14 ± 1.60 and old 20.68 ± 2.72 arbitrary units (AU)]. As expected, incubation with a combination of Thbs-1, Tempol, and catalase prevented increases in superoxide levels, resulting in a significant difference compared with Thbs-1 alone for both young (Thbs-1 12.14 ± 1.60 and Thbs-1 + Tempol and catalase 3.85 ± 1.70 AU) and old (20.68 ± 2.72 and 4.21 ± 1.72 AU, respectively) rats (Fig. 2). Suppression of superoxide production was also achieved when αCD47 was provided along with Thbs-1 in both young (Thbs-1 12.14 ± 1.60 and Thbs-1 + αCD47 2.02 ± 1.99 AU) and old (20.68 ± 2.72 and 3.54 ± 2.18 AU, respectively) rats (Fig. 2). Age-associated differences in superoxide production seen with Thbs-1 treatment were attenuated by addition of Tempol and catalase or αCD47 (Fig. 2B).
Fig. 2.
Blockade of CD47 binding attenuates Thbs-1-induced increases in superoxide in coronary arterioles. A: representative DHE fluorescence images of coronary arterioles isolated from young and old rats provide a measurement of superoxide levels. Images were obtained at baseline and after treatment with Thbs-1, Thbs-1 + Tempol and catalase, or Thbs-1 + αCD47. B: DHE fluorescence intensities are quantified as change from baseline for each treatment. When treated with Thbs-1, arterioles from old rats show significantly increased superoxide production compared with young. For both young and old, Tempol and catalase or αCD47 treatment reduced superoxide production compared with Thbs-1 treatment alone. Values are means ± SE; for all groups, n = 6–8. Significant differences are denoted by *between age groups within a treatment, and #between the indicated treatment and Thbs-1 alone within an age group.
Thbs-1 and CD47 expression in aging.
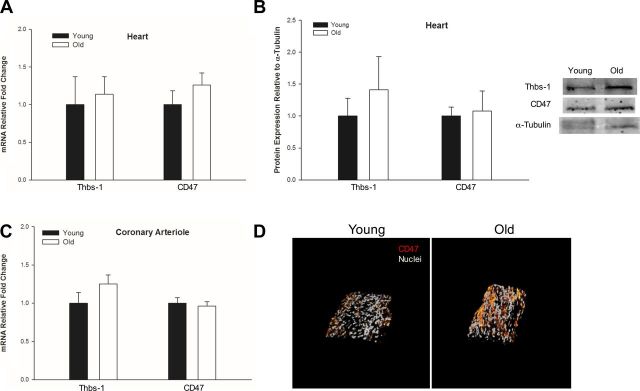
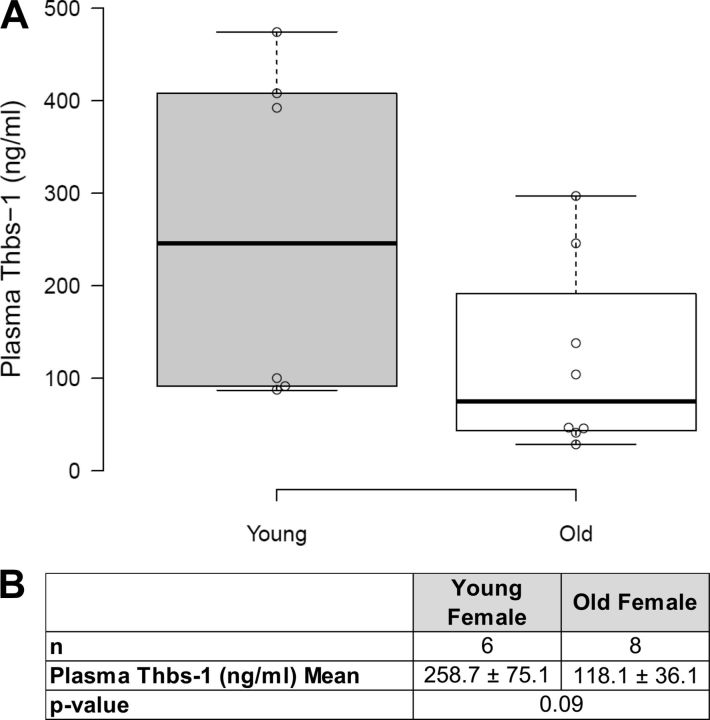
Transcript levels of Thbs-1 and CD47 measured by qPCR showed no age-related expression differences in either heart tissue or coronary arterioles (Fig. 3, A and C). Western blot performed on heart tissue homogenate showed equal protein levels of Thbs-1 and CD47, regardless of age (Fig. 3B). Immunofluorescent imaging of CD47 on isolated coronary arterioles revealed increased surface expression on old coronary arterioles compared with young, but these were not statistically significant (Fig. 3D). To further characterize the coronary microvascular environment, circulating Thbs-1 levels were measured in plasma by ELISA with no age-related differences being found (Fig. 4).
Fig. 3.
Thbs-1 and CD47 expression were not found to be altered in advanced age. qPCR for Thbs-1 and CD47 was performed on heart tissue (A) or on coronary arterioles (C) isolated from young and old female rats (n = 5–6, all groups). B: age-specific protein levels of Thbs-1 and CD47 in heart tissue were analyzed by Western blot (n = 3, all groups), and a representative blot is shown to the right. A–C: expression levels were normalized to young for each target. Values are means ± SE. D: representative confocal immunofluorescence images taken at ×60 show nuclei (gray) and CD47 (red) on coronary arterioles from young and old rats (young n = 6, old n = 7). Although arterioles from old hearts exhibit increased CD47 staining, age-related differences in expression were not detected.
Fig. 4.
Plasma Thbs-1 concentrations do not vary with age. A: plasma Thbs-1 levels were measured in young (4 mo) and old (24 mo) female rats by ELISA. Box plots show the median, 25th, and 75th percentiles. Whiskers indicate 1.5 times the interquartile range from the 25th and 75th percentiles. Data points are plotted as open circles. Plasma Thbs-1 levels of young female rats appeared to fall into two distinct groups: high and low concentration. Four of eight measurements from old rats show plasma Thbs-1 concentrations that are intermediate to those observed in young females. B: mean Thbs-1 plasma concentrations and sample sizes are provided for young and old rats. The difference in plasma Thbs-1 values did not reach statistical significance. Values are means ± SE.
Intravenous administration of αCD47 improves CFR in aged rats.
Microsphere blood tracers were used to measure BF under baseline (resting) and dobutamine-stimulated (working) conditions in different regions of the heart. Twenty-four-month-old rats receiving intravenous administration of αCD47 showed increased CFR (%increase in BF with dobutamine over baseline) in heart sections supplied by the LAD compared with rats receiving control IgG. Significant improvements in CFR were seen in the LV 1 (%increase, IgG 9.7 ± 9.3 and αCD47 84.0 ± 23.2%) and LV 2 (IgG 7.3 ± 9.9 and αCD47 67.5 ± 15.7%, respectively) heart sections (Fig. 5A). Absolute BF (ml·min−1·g−1) did not significantly differ between baseline and dobutamine conditions in any sections of the heart for control IgG-treated rats. In contrast, significant differences between baseline and dobutamine BF for αCD47-treated rats were seen in the apex (BF, baseline 1.45 ± 0.28 and dobutamine 2.84 ± 0.45 ml·min−1·g−1), LV 1 (baseline 1.85 ± 0.30 and dobutamine 3.24 ± 0.52 ml·min−1·g−1), and LV 2 (baseline 1.94 ± 0.29 and dobutamine 3.08 ± 0.31 ml·min−1·g−1) heart sections. Additionally, a significant difference was approached in the LV 3 (P = 0.06) heart section of αCD47-treated rats. Notably, absolute BF measurements did not significantly differ between control IgG and αCD47-treated rats, except in the apex section at baseline (IgG 2.60 ± 0.26 and αCD47 1.45 ± 0.28 ml·min−1·g−1) (Fig. 5B).
DISCUSSION
The mechanisms by which aging contributes to vascular dysfunction and loss of NO signaling are incompletely understood. Our findings show that there is an age-dependent dysfunction in coronary arterioles related to Thbs1-CD47 activity, leading to decreased vasodilation to NO and increased superoxide production within the coronary microvessel wall. Exogenous Thbs-1 significantly decreased NO-dependent vasodilation in coronary arterioles from rats of advanced age (Fig. 1, B and F), while coronary arterioles from young rats treated with Thbs-1 did not show altered NO responsiveness (Fig. 1, A and E). These data suggest that aging increases the response of the coronary microvessel wall to Thbs-1, resulting in suppression of NO-dependent vasodilation (Fig. 6). This raises the possibility that Thbs-1 is a contributor to the underlying coronary vascular pathology associated with aging.
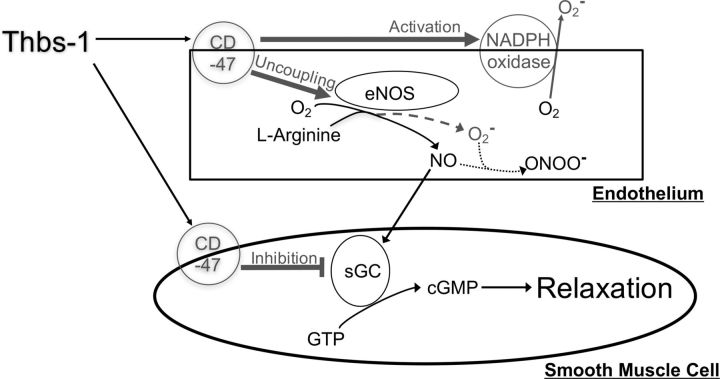
Fig. 6.
Pathways downstream of CD47 are implicated in the exacerbated response to Thbs-1 observed in coronary arterioles from aged rats. Coronary arterioles from old rats show increased superoxide production and decreased NO-dependent vasodilation in response to Thbs-1 compared with coronary arterioles from young rats. These effects are independent of Thbs-1 and CD47 concentration. Thbs-1 was added exogenously at equal levels to both age groups, and CD47 density on coronary arterioles was not found to be altered in aging. As such, more potent activation of CD47 and greater effect on downstream effectors are implicated in the exacerbated response to Thbs-1 seen in arterioles from aged rats. Downstream effects of CD47 include activation of NADPH oxidase, uncoupling of endothelial NO synthase (eNOS), and inhibition of soluble guanylyl cyclase (sGC).
Given the growing body of literature showing that Thbs-1 suppresses NO signaling in general (3, 30, 46), it is somewhat surprising that coronary arterioles from young rats did not also show impaired NO-dependent vasodilation. A possibility is that healthy coronary arterioles are protected from the action of Thbs-1 through mechanisms such as antioxidant defense, and that this protection is lost in aging. In fact, impaired antioxidant defenses are known contributors to the development of vascular dysfunction in aging (1, 9, 11, 12, 14). To investigate this possibility, the effect of aging on superoxide production downstream of Thbs-1 was evaluated. Our results show that advanced age predisposes coronary arterioles to higher levels of superoxide production when challenged with Thbs-1 (Fig. 2). We originally hypothesized that the increased presence of superoxide was reducing NO bioavailability and driving age-dependent inhibition of vasodilation by Thbs-1. However, treating vessels with the antioxidants Tempol and catalase to reduce superoxide levels only minimally rescued vasodilation in arterioles from aged rats (Fig. 1, D and F). These results argue that superoxide generation is not the primary mechanism by which Thbs-1 suppresses NO-dependent vasodilation in aging coronary arterioles. Thbs-1 has previously been shown to directly inhibit sGC, and this represents a non-superoxide-driven pathway by which Thbs-1 could be exerting an age-dependent effect on vasodilation (30). Interestingly, decreased expression and activity of sGC have previously been noted in advanced age (22, 37, 41).
Both suppression of NO-dependent vasodilation and production of superoxide in the presence of Thbs-1 were attenuated by treatment with a CD47 blocking antibody in coronary arterioles of old rats (Figs. 2 and 1, D and F). This adds to other reports that highlight CD47 as the necessary cell surface receptor for Thbs-1 regulation of NO signaling (3, 10, 18, 30). The cell surface receptor CD36 has also been described as a binding partner for Thbs-1, mediating many of the same cellular responses as CD47, particularly in the context of angiogenesis and platelet aggregation (13, 19, 20). However, inhibition of NO signaling by physiological concentrations of Thbs-1 is attenuated in CD47-null, but not CD36-null, models (18). This agrees with our results, which show that, in coronary arterioles, the effect of Thbs-1 on superoxide production and vasodilation can be counteracted by blocking CD47. As such, our work supports the targeting of CD47 for treating age-related vascular dysfunction.
The positive effect of αCD47 treatment on vasodilation seen in isolated vessel experiments was also observed in vivo. Aged rats treated with αCD47 showed improved CFR in sections of the heart supplied by the LAD and responsible for generating much of the contractile force of the LV (Fig. 5). Decreased CFR, associated with aging and cardiovascular disease, limits the ability of the heart to respond to increased demand and is associated with cardiovascular disease (16, 38). Blocking of CD47 appears to be a promising therapeutic strategy for increasing CFR and addressing age-related coronary microvascular dysfunction. Humanized CD47 blocking antibodies are currently in phase I clinical trials for cancer treatment (28). CD47 is frequently overexpressed by tumor cells, where it acts to suppress immune clearance through interaction with signal regulatory protein-α on myeloid cells (44). These trials will evaluate the safety and efficacy of CD47 blocking therapeutics, potentially bringing CD47 blockade as a therapy for vascular disease one step closer to reality.
The mechanism by which age contributes to increased coronary microvascular dysfunction downstream of Thbs-1 is not yet understood. In this study, Thbs-1 was provided at 2.2 nM to reflect reported circulating concentrations. Plasma levels of Thbs-1 for young and old female rats were both within error of this reported value, with concentrations found to be 1.72 and 0.95 nM, respectively (Fig. 4). Plasma, heart, and coronary arteriole levels of Thbs-1 also did not differ statistically based on age in our female rats (Figs. 3, A–C, and 4). Our study is the first to report Thbs-1 expression in young or old female rats. The lack of age-related changes in Thbs-1 expression contrasts with two previous reports that found Thbs-1 levels to increase with age in myocardial tissue and skin biopsies (7, 35). Both of these studies used male mice, whereas we have employed a female rat model, suggesting this difference may be related to sex or species. Decreased antioxidant defense is known to occur in aging and may play a role in increasing superoxide levels in coronary arterioles of old rats after treatment with Thbs-1 (9, 43). Increased superoxide levels alone do not account for the full age-dependent effect of Thbs-1, as scavenging of reactive oxygen species did not completely reverse Thbs1 effects on vasodilation (Fig. 1D). Increased vascular density of CD47 in aging would contribute to increased Thbs-1 binding events and signal transduction, but qPCR and immunofluorescence of coronary arterioles did not show significant variation in CD47 receptor expression with age in females (Fig. 3, C and D). Straface et al. (40) demonstrated that, in patients with metabolic syndrome, there was a significant decrease in CD47 in red blood cells from men compared with women, but no sex difference was found in cells from age-matched healthy donors. To date, there have been very few sex-specific comparisons of the Thbs-1/CD47 signaling pathway and expression as age progresses, which implies that this is an understudied research area. Our results suggest that, even without increases in concentration in either Thbs-1 or CDd47 in our female rats, Thbs-1 induces increased vascular impairment in aging via enhanced CD47 activity.
We have shown that Thbs-1, at circulating levels typical of young rats, leads to suppression of NO-dependent vasodilation and heightened superoxide production in advanced age. Regardless of the mechanism, age-dependent response to Thbs-1 may be a direct contributor to the development of a negative vascular phenotype in aging (Fig. 6), with consequences for cardiovascular health. Blockade of CD47 attenuates the effects of Thbs-1 and, therefore, represents a therapeutic target for age-related cardiovascular dysfunction that deserves further attention.
GRANTS
This study was supported by American Heart Association Grant 12BGIA9310043 (A. J. LeBlanc), the Gheens Foundation (A. J. LeBlanc), and National Institute of General Medical Sciences Grant P30 GM-103507.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.N., G.M., K.C., J.A., S.H., and A.J.L. performed experiments; C.N., G.M., K.C., J.A., S.H., and A.J.L. analyzed data; C.N., G.M., K.C., J.A., S.H., J.B.H., and A.J.L. interpreted results of experiments; C.N., G.M., K.C., J.A., S.H., and A.J.L. prepared figures; C.N. and A.J.L. drafted manuscript; C.N., J.B.H., and A.J.L. edited and revised manuscript; C.N., G.M., K.C., J.A., S.H., J.B.H., and A.J.L. approved final version of manuscript; A.J.L. conception and design of research.
REFERENCES
- 1.Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol Heart Circ Physiol : H1015–H1022, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation : 163–169, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res : 471–481, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, Champion HC, Zuckerbraun BS, Calzada MJ, Isenberg JS. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res : 682–693, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boger RH, Lentz SR, Bode-Boger SM, Knapp HR, Haynes WG. Elevation of asymmetrical dimethylarginine may mediate endothelial dysfunction during experimental hyperhomocyst(e)inaemia in humans. Clin Sci (Lond) : 161–167, 2001. [PubMed] [Google Scholar]
- 6.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res : 26–36, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Cai H, Yuan Z, Fei Q, Zhao J. Investigation of thrombospondin-1 and transforming growth factor-beta expression in the heart of aging mice. Exp Ther Med : 433–436, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirkov YY, Horowitz JD. Impaired tissue responsiveness to organic nitrates and nitric oxide: a new therapeutic frontier? Pharmacol Ther : 287–305, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res : e42–e54, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Csanyi G, Yao M, Rodriguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, Pagano PJ. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol : 2966–2973, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell : 783–797, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res : 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol : 707–717, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res : 1659–1666, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Durand MJ, Gutterman DD. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation : 239–247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galderisi M, Rigo F, Gherardi S, Cortigiani L, Santoro C, Sicari R, Picano E. The impact of aging and atherosclerotic risk factors on transthoracic coronary flow reserve in subjects with normal coronary angiography. Cardiovasc Ultrasound : 20, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henkin J, Volpert OV. Therapies using anti-angiogenic peptide mimetics of thrombospondin-1. Expert Opin Ther Targets : 1369–1386, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem : 26069–26080, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood : 613–623, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med : 41–48, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Kang LS, Chen B, Reyes RA, Leblanc AJ, Teng B, Mustafa SJ, Muller-Delp JM. Aging and estrogen alter endothelial reactivity to reactive oxygen species in coronary arterioles. Am J Physiol Heart Circ Physiol : H2105–H2115, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kloss S, Bouloumie A, Mulsch A. Aging and chronic hypertension decrease expression of rat aortic soluble guanylyl cyclase. Hypertension : 43–47, 2000. [PubMed] [Google Scholar]
- 23.LeBlanc AJ, Moseley AM, Chen BT, Frazer D, Castranova V, Nurkiewicz TR. Nanoparticle inhalation impairs coronary microvascular reactivity via a local reactive oxygen species-dependent mechanism. Cardiovasc Toxicol : 27–36, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBlanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, Muller-Delp JM. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am J Physiol Regul Integr Comp Physiol : R1713–R1723, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leblanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol : H2280–H2288, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leblanc AJ, Touroo JS, Hoying JB, Williams SK. Adipose stromal vascular fraction cell construct sustains coronary microvascular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol : H973–H982, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lind L, Berglund L, Larsson A, Sundstrom J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation : 1545–1551, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, Willingham S, Howard M, Prohaska S, Volkmer J, Chao M, Weissman IL, Majeti R. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One : e0137345, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcus ML, Chilian WM, Kanatsuka H, Dellsperger KC, Eastham CL, Lamping KG. Understanding the coronary circulation through studies at the microvascular level. Circulation : 1–7, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Miller TW, Isenberg JS, Roberts DD. Thrombospondin-1 is an inhibitor of pharmacological activation of soluble guanylate cyclase. Br J Pharmacol : 1542–1547, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res : e31–e40, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol Heart Circ Physiol : H459–H465, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol : H93–H100, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J : 735–741, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Rogers NM, Roberts DD, Isenberg JS. Age-associated induction of cell membrane CD47 limits basal and temperature-induced changes in cutaneous blood flow. Ann Surg : 184–191, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers NM, Yao M, Novelli EM, Thomson AW, Roberts DD, Isenberg JS. Activated CD47 regulates multiple vascular and stress responses: implications for acute kidney injury and its management. Am J Physiol Renal Physiol : F1117–F1125, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruetten H, Zabel U, Linz W, Schmidt HH. Downregulation of soluble guanylyl cyclase in young and aging spontaneously hypertensive rats. Circ Res : 534–541, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Schindler TH. Myocardial blood flow: putting it into clinical perspective. J Nucl Cardiol. In press. [DOI] [PubMed]
- 39.Schulze F, Lenzen H, Hanefeld C, Bartling A, Osterziel KJ, Goudeva L, Schmidt-Lucke C, Kusus M, Maas R, Schwedhelm E, Strodter D, Simon BC, Mugge A, Daniel WG, Tillmanns H, Maisch B, Streichert T, Boger RH. Asymmetric dimethylarginine is an independent risk factor for coronary heart disease: results from the multicenter Coronary Artery Risk Determination investigating the Influence of ADMA Concentration (CARDIAC) study. Am Heart J : 493 e491–e498, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Straface E, Gambardella L, Mattatelli A, Canali E, Boccalini F, Agati L, Malorni W. The red blood cell as a gender-associated biomarker in metabolic syndrome: a pilot study. Int J Cell Biol : 204157, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sverdlov AL, Chan WP, Procter NE, Chirkov YY, Ngo DT, Horowitz JD. Reciprocal regulation of NO signaling and TXNIP expression in humans: impact of aging and ramipril therapy. Int J Cardiol : 4624–4630, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Sverdlov AL, Ngo DT, Chan WP, Chirkov YY, Horowitz JD. Aging of the nitric oxide system: are we as old as our NO? J Am Heart Assoc : e000973, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol : H363–H372, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua MS, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PO, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NN, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A : 6662–6667, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Z, Li J, Kong J, Wu S. Impairment of vascular endothelial function following reperfusion therapy in patients with acute myocardial infarction. J Int Med Res : 1074–1078, 2013. [DOI] [PubMed] [Google Scholar]
- 46.Yao M, Roberts DD, Isenberg JS. Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharm Res : 13–22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]