Abstract
Sedentary aging leads to left ventricular (LV) and vascular stiffening due in part to advanced glycation end-products (AGEs) cross-linking of extracellular matrix proteins. Vigorous lifelong exercise ameliorates age-related cardiovascular (CV) stiffening and enhances exercise LV function, although this effect is limited when exercise is initiated later in life. We hypothesized that exercise training might be more effective at improving the impact of age-related CV stiffening during exercise when combined with an AGE cross-link breaker (Alagebrium). Sixty-two seniors (≥60 yr) were randomized into four groups: sedentary + placebo, sedentary + Alagebrium, exercise + placebo, and exercise + Alagebrium for 1 yr. Moderate-intensity aerobic exercise was performed 3-4 sessions/wk; controls underwent similar frequency of yoga/balance training. Twenty-four similarly-aged, lifelong exercisers (4–5 sessions/wk) served as a comparator for the effect of lifelong exercise on exercising LV function. Oxygen uptake (Douglas bags), stroke index (SI; acetylene rebreathing), and effective arterial elastance (Ea) were collected at rest and submaximal and maximal exercise. Maximum O2 uptake (23 ± 5 to 25 ± 6 ml·kg−1·min−1) increased, while SI (35 ± 11 to 39 ± 12 ml/m2) and Ea (4.0 ± 1.1 to 3.7 ± 1.2 mmHg·ml−1·m−2) were improved across all conditions with exercise, but remained unchanged in controls (exercise × time, P ≤ 0.018). SI or Ea were not affected by Alagebrium (medication × time, P ≥ 0.468) or its combination with exercise (interaction P ≥ 0.252). After 1 yr of exercise plus Alagebrium, exercise SI and Ea remained substantially below that of lifelong exercisers (15–24 and 9–22%, respectively, P ≤ 0.415). In conclusion, Alagebrium plus exercise had no synergistic effect on exercise LV function and failed to achieve levels associated with lifelong exercise, despite a similar exercise frequency.
Keywords: Alagebrium, exercise, physical training, left ventricular, stroke volume
NEW & NOTEWORTHY
Exercise for 1 yr improved stroke volume and effective arterial elastance during exercise in previously sedentary seniors; however, these adaptations were not enhanced by the advanced glycation end-product cross-link breaker Alagebrium. Exercise plus Alagebrium for 1 yr failed to restore exercising left ventricular function to levels associated with lifelong exercise, despite a similar exercise frequency.
progressive stiffening of the heart and central vasculature appears to be an inevitable consequence of sedentary human aging (1, 15, 41). The accumulation and cross-linking of advanced glycation end-products (AGEs) on long-lived extracellular matrix proteins contribute to cardiovascular (CV) stiffening with aging (3, 38). This stiffening of the CV system is believed to contribute to the pathophysiology of several CV conditions (systolic hypertension, atrial fibrillation, heart failure with preserved ejection fraction) reported in the elderly (23).
The benefits of vigorous lifelong exercise on CV stiffening and left ventricular (LV) function during exercise has been previously reported (1, 7, 10, 41). A year of progressive and vigorous exercise in previously sedentary seniors improved measures of exercise LV function, including stroke index (SI) and effective arterial elastance (Ea); however, it failed to reverse LV stiffening (17) or improve an intrinsic structural index of central arterial stiffness (42) potentially due to the accumulation of AGEs that could not be reversed by exercise alone. Thus a concurrent pharmacological therapy that either reduces AGE formation and/or breaks AGE cross-links may be necessary to elicit the full expression of exercise training benefits on LV stiffness and function when AGEs are in substantial quantities.
Alagebrium (a thiazolium derivative) is an effective agent in breaking AGE's cross-links and reverses CV stiffness in aged animals (39, 46, 51). When combined with exercise, Alagebrium was more effective at reducing LV and vascular stiffness and improving LV systolic and diastolic function compared with either treatment alone in aged rodents (46). Our laboratory has recently demonstrated that 1-yr treatment of Alagebrium, combined with moderate-intensity exercise, reduced LV stiffness equivalent to ∼10–15 yr of sedentary aging in healthy seniors, while Alagebrium or exercise alone failed to improve LV stiffness (16). Whether a combination therapy of Alagebrium and exercise acts synergistically to improve measures of LV function during exercise compared with either treatment alone in sedentary seniors has not been examined previously.
Thus the primary purpose of this present study was to examine whether Alagebrium, combined with moderate-intensity exercise for 1 yr, improves exercise LV function in previously sedentary seniors. A secondary purpose was to examine the effect of 1 yr of exercise plus Alagebrium vs. lifelong exercise on LV function during exercise when a comparable exercise “dose” as indicated by weekly exercise frequency is performed.
We hypothesized that, given the unique effects of Alagebrium and exercise on CV stiffness and function, the combination therapy of Alagebrium and moderate-intensity exercise would improve exercise stroke volume (SV) and Ea to a greater degree than either treatment alone and would achieve levels equivalent to that observed with lifelong exercise at a similar exercise dose.
METHODS
Participant population and study design.
Details of the design of this present study have been reported previously (16): a prospective, randomized, double-blind, placebo-controlled (Alagebrium only), parallel-group study for 1 yr evaluating the efficacy of the combination of Alagebrium (200 mg daily) or placebo and aerobic exercise training or contact control in sedentary but otherwise healthy seniors (http://www.clinicaltrials.gov identifier: NCT01014572). Sixty-two seniors (68 ± 6 yr, 40 women) were recruited from three primary sources: the Dallas Heart Study (52), the Cooper Center Longitudinal Study (12), and a random sample of employees of Texas Health Resources, the third largest employer in the Dallas-Fort Worth metroplex and a diverse health care company (15). Participants were randomly assigned to four groups, 1) sedentary + placebo (CONT); 2) sedentary + Alagebrium (CONT + ALG); 3) moderate-intensity exercise + placebo (EX); and 4) moderate-intensity exercise + Alagebrium (EX + ALG), by an independent statistician. Participants who were randomized into the sedentary groups underwent yoga or balance training as contact control for the year period.
To investigate the effect of a comparable lifelong dose of training on exercise LV performance, 24 (69 ± 6 yr, 5 women) committed (4-5 exercise sessions/wk) lifelong exercisers served as an exercise dose control. The goal of this control population was to determine whether the combination of Alagebrium and exercise training could fully reverse the effect of sedentary aging equivalent to individuals with a lifetime of exercise training, which would be a powerful test of the AGE's hypothesis of sedentary aging. Additional information regarding the recruitment of these participants can be found elsewhere (10). All participants were rigorously screened for comorbidities, including obesity, lung disease, diabetes mellitus, hypertension (24-h blood pressure > 140/90 mmHg), and coronary artery disease or structural heart disease by an exercise stress test and echocardiogram, respectively. The experimental procedures were explained to all participants, with written, informed consent obtained as approved by the institutional review boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas.
Measurement of resting and exercise variables.
Measurements of exercise function were collected at baseline, 6 mo (data not presented), and 1 yr. At each testing session, O2 uptake (V̇o2), cardiac output (Q̇c), and blood pressure were determined at the following treadmill conditions: 1) quiet standing rest; 2) low-intensity [∼30–45% of maximal V̇o2 (V̇o2 max)] steady-state submaximal exercise; 3) moderate-intensity (∼60–75% of V̇o2 max) steady-state submaximal exercise; and 4) maximal exercise. One participant was tested on an upright cycle because of orthopedic concerns. Gas fractions were analyzed by mass spectrometry and ventilatory volumes by a Tissot spirometer, as previously reported (1). V̇o2 max was defined as the highest V̇o2 measured from at least a 30-s Douglas bag. Q̇c was measured with the acetylene rebreathing method, which has been previously validated in this laboratory (21). Systemic arteriovenous oxygen difference (a-vO2diff) was calculated from the Fick equation (a-vO2diff = V̇o2/Q̇c). Heart rate was measured via a 12-lead ECG, while SV was calculated as Q̇c/heart rate. SV was scaled relative to body surface area (SI) and fat-free mass (SV-FFM) to reduce the confounding effect of body size and composition (9).
Upright resting and exercise blood pressures were measured on the left arm by ECG gated electrosphygmomanometry (Tango; SunTech Medical). Resting blood pressure was collected with the subjects left arm hanging relaxed, while it was allowed to swing freely during exercise. All subjects were carefully monitored to ensure that they did not hang onto and/or tightly grip the treadmill handrail during the measurement of exercise blood pressure.
Systemic vascular resistance (SVR) was calculated by the following formula: mean arterial pressure (MAP)/Q̇c × 80, where 80 is a conversion factor to dyn·s−1·cm−5. Ea, an index of total arterial load on the LV, was estimated as ESP/SV, in which ESP is end-systolic blood pressure, by multiplying brachial systolic blood pressure by 0.9 (11). Ea was scaled relative to body surface area, as previously reported in our laboratory and by others (10, 34). Systemic arterial compliance (SAC) was calculated as SI/pulse pressure.
Measurement of body composition.
Body density and composition were determined by underwater weighing with correction for residual lung volume (53). Each participant performed at least three adequate measurements defined as a definite plateau in underwater weight, and the mean value was calculated.
Measurement of total blood volume.
Total blood volume (TBV) was measured using a carbon monoxide rebreathing method modified from that described by Burge and Skinner (8) and has been described in detail elsewhere (19). Typical error of measurement, expressed as a coefficient of variation (%) for test-retest reproducibility for hemoglobin mass, which is used as a marker of carbon monoxide distribution, is <3% for repeated measures in our laboratory (19). To reduce the confounding effect of body size and composition on TBV (9), absolute values were scaled relative to total body mass and FFM.
Exercise training program.
Details of the exercise intervention have been previously reported (16). The training program was individually prescribed using heart rate monitoring and training zones derived from baseline exercise testing. Initially, participants walked or jogged 3 sessions weekly for 25 min/session at the “base pace,” which targets heart rates equivalent to ∼70-80% of maximal heart rate. From the 3rd mo, “base pace” was gradually prolonged to 35 min/session. At the 4th mo, a 30 min/session of higher intensity maximal steady state (MSS; ventilatory threshold), was added monthly, and the frequency of MSS was increased to 2 sessions/mo from the 5th mo. From the 6th to 12th mo, participants exercised at least 4 sessions/wk (3 × base pace for 35-40 min and 1 × MSS for 40 min). Training sessions for the exercise intervention were supervised for the first 2 wk and reviewed monthly thereafter. The exercise control groups underwent yoga or balance training 3-4 sessions/wk and were reviewed monthly to control for the increased contact associated with monitoring the exercise training sessions.
Training impulse.
Exercise training dose was assessed by training impulse (TRIMP), as described by Banister et al. (5). The calculation of TRIMP has been described in a previous paper from our laboratory (32).
Sample size estimation.
As previously reported (16), sample size estimation (α = 0.05; β < 0.20) was based on changes in LV stiffness with a 4-wk treatment of Alagebrium in aged canines (4). Alagebrium improved LV stiffness 24 units (57.1 ± 6.8 to 33.1 ± 4.6 mmHg·m−2·ml−1) with a SD of 13. Assuming this effect was equivalently potent in humans in an unpaired comparison, 13 subjects per group would be required to detect this effect. With an expected drop-out of ∼15% (2 subjects) in each group for 1 yr, we planned to enroll 15 subjects per group.
Statistical analysis.
Measurements of exercise function, body composition, and TBV were collected and analyzed by investigators blinded to the group assignment (Alagebrium or placebo). After all measurements and data analyses were completed, drug codes were broken and made available for statistical analysis. A one-factor ANOVA was used to examine differences in baseline characteristics. Mixed-model repeated-measures ANOVAs were used to determine the effect of treatment and time on V̇o2 max and measures of LV function. The effect of Alagebrium (medication × time) or exercise (exercise × time) over time were first assessed, followed by an interaction effect of Alagebrium and exercise over time (medication × exercise × time). When an interaction effect of Alagebrium or exercise over time achieved P < 0.10, further analysis between experimental conditions was performed. Comparison of 1 yr vs. lifelong exercise on exercise variables were determined by unpaired t-tests, with nonparametric data analyzed via Mann-Whitney rank-sum test. All statistical analysis was performed using SigmaStat (Systat Software) and SAS version 9 (SAS Institute). P < 0.05 was considered statistically significant. Data are presented as means ± SD in Table 1 and mean ± SE in Figs. 2–4.
Table 1.
Body size and composition measures, total blood volume, and training volume
| CONT | CONT + ALG | EX | EX + ALG | Lifelong† | |
|---|---|---|---|---|---|
| Age, yr | 70 (66–78) | 65 (64–69) | 66 (63–67) | 66 (64–70) | 69 (5–71) |
| Sex, female, n (%) | 10 (67) | 9 (64) | 11 (73) | 9 (57) | 5 (21) |
| Weight, kg | |||||
| Baseline | 67 ± 13 | 74 ± 10 | 72 ± 12 | 74 ± 11 | 73 ± 11 |
| 1-yr | 67 ± 13 | 74 ± 10 | 71 ± 12 | 70 ± 9 | |
| BSA, m2 | |||||
| Baseline | 1.76 ± 0.21 | 1.86 ± 0.16 | 1.82 ± 0.18 | 1.87 ± 0.19 | 1.88 ± 0.18 |
| 1-yr | 1.75 ± 0.20 | 1.85 ± 0.17 | 1.82 ± 0.19 | 1.82 ± 0.16 | |
| FFM, kg | |||||
| Baseline | 44 ± 9 | 48 ± 10 | 46 ± 10 | 49 ± 10 | 52 ± 9 |
| 1-yr | 44 ± 10 | 48 ± 10 | 47 ± 11 | 48 ± 11 | |
| TBV, ml/kg | |||||
| Baseline | 66 ± 9 | 64 ± 4 | 65 ± 11 | 65 ± 10 | 73 ± 8 |
| 1-yr | 68 ± 10 | 64 ± 6 | 69 ± 15* | 72 ± 12* | |
| TBV, ml/kg FFM | |||||
| Baseline | 100 ± 12 | 100 ± 8 | 102 ± 11 | 99 ± 7 | 104 ± 9 |
| 1-yr | 103 ± 11 | 101 ± 7 | 106 ± 9 | 106 ± 8* | |
| TRIMP, 1-yr | 9,066 ± 3,285 | 9,477 ± 2,754 |
Values are means ± SD, except for age, which is median (25–75% range); n, no. of subjects.
BSA, body surface area; FFM, fat-free mass; TBV, total blood volume; TRIMP, training impulse.
P < 0.05, 1-yr vs. baseline.
Descriptive characteristics of lifelong committed exercisers are presented elsewhere (8).
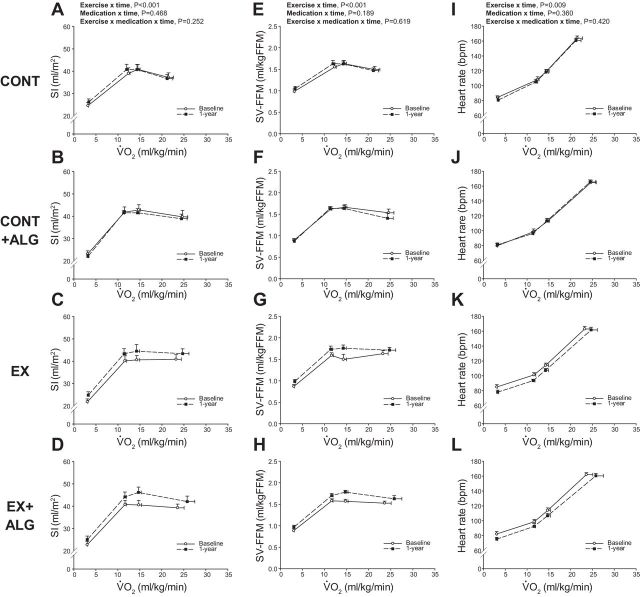
Fig. 2.
SI (A–D), SV-FFM (E–H), and heart rate (I–L) as a function of scaled V̇o2 at baseline and 1 yr. CONT, sedentary (A, E, and I); CONT + ALG, sedentary plus Alagebrium (B, F, and J); EX, exercise training only (C, G, and K); EX + ALG, exercise training plus Alagebrium (D, H, and L). bpm, Beats/min Values are means ± SE.
Fig. 4.
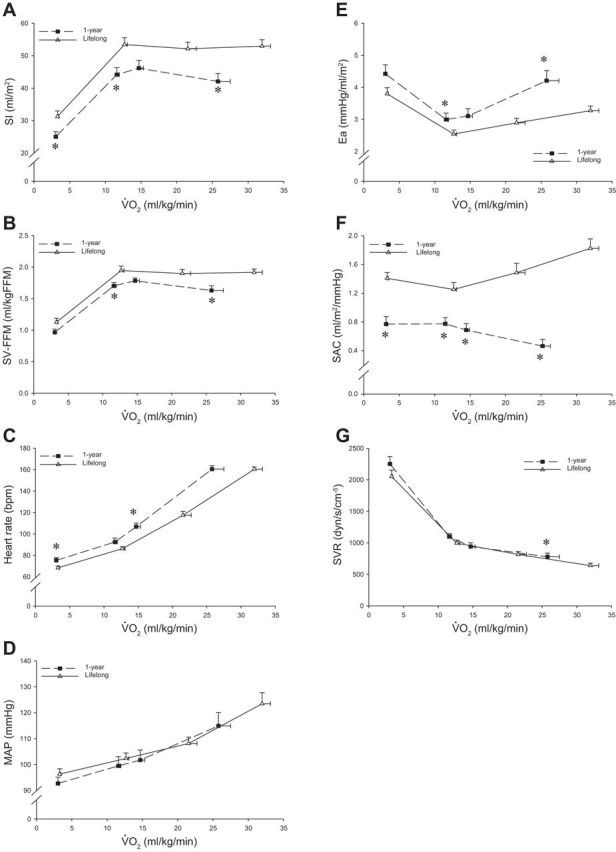
SI (A), SV-FFM (B), heart rate (C), MAP (D), Ea (E), SAC (F), and SVR (G) as a function of scaled V̇o2 after 1 yr of EX + ALG (n = 14) and lifelong exercise (n = 24). bpm, Beats/min. Values are means ± SE. *P < 0.05, committed lifelong exercise vs. after 1 yr of EX + ALG.
RESULTS
Participants characteristics.
Age, cardiac morphology, LV pressure-volume curves, and V̇o2 max at baseline have been previously reported (16). Body size and composition measures, TBV, and V̇o2 max were not different among groups at baseline (Table 1) (P ≥ 0.355).
LV function during exercise.
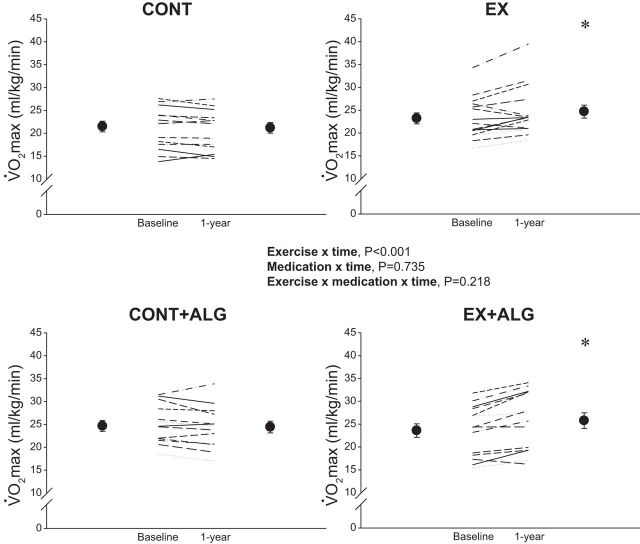
Fifty-eight participants completed the 1-yr intervention. Exercise and medication compliance rates have been previously detailed (16); for Alagebrium adherence was >90% (pill counts), and all participants completed >85% of exercise sessions. Measures of body size or composition were not different among groups at 1 yr (P ≥0.072). TBV and V̇o2 max scaled to total body mass increased with exercise (P ≤ 0.005), but not Alagebrium or contact control (Table 1 and Fig. 1). TRIMP over the 1-yr training period was not different between EX (n = 14) and EX + ALG (n = 12) groups (9,066 ± 3,285 vs. 9,477 ± 2,754 for EX and EX + ALG, respectively, P = 0.735).
Fig. 1.
Scaled V̇o2 max at baseline and 1 yr. Circles and error bars represent group means ± SE. CONT, sedentary; CONT + ALG, sedentary plus Alagebrium; EX, exercise training only; EX + ALG, exercise training plus Alagebrium. *P < 0.05 vs. baseline.
Figure 2 depicts the relationship between V̇o2 expressed relative to total body mass, and SI, SV-FFM, and heart rate at baseline and 1 yr. SI and SV-FFM increased (upward shift) with exercise training (P < 0.001), but was unaffected by Alagebrium (P ≥ 0.189). Exercise training resulted in a reduction in submaximal exercise heart rate (rightward shift) (P = 0.010) (Fig. 2, I–L).
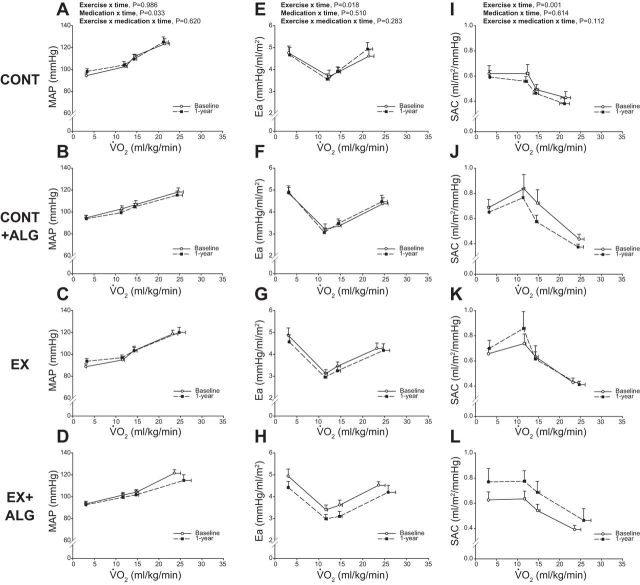
Figure 3 plots the relationship between V̇o2 expressed relative to total body mass and MAP, Ea, and SAC at baseline and 1 yr. MAP was lower (downward shift) at 1 yr with Alagebrium (P = 0.033), but exercise training had little impact (P = 0.986) (Fig. 3, A–D). Conversely, Ea was improved after 1 yr of exercise training (downward shift) (P = 0.018) (Fig. 3, E–H), but not with Alagebrium (P = 0.510). Similarly, SAC during exercise was only improved (upward shift) with exercise training (P < 0.001) (Fig. 3, K and L).
Fig. 3.
MAP (A–D), Ea (E–H), and SAC (I–L) as a function of scaled V̇o2 at baseline and 1 yr. CONT, sedentary (A, E, and I); CONT + ALG, sedentary plus Alagebrium (B, F, and J); EX, exercise training only (C, G, and K); EX + ALG, exercise training plus Alagebrium (D, H, and L). Values are means ± SE.
SVR and a-vO2diff were not significant improved after 1 yr of exercise or Alagebrium (P ≥ 0.311).
One year vs. lifelong training.
Age, body size, and composition measurements, LV pressure-volume curves, cardiac morphology, and V̇o2 max of committed lifelong exercisers have been reported previously (7, 10).
Figure 4 plots the relationship between V̇o2 expressed relative to total body mass and SI, SV-FFM, heart rate, MAP, Ea, SAC, and SVR after 1 yr of moderate-intensity exercise training plus Alagebrium compared with a committed level of lifelong exercise. Exercise SI and SV-FFM remained smaller, while submaximal exercise heart rate remained higher at a given V̇o2 after 1 yr compared with lifelong exercise (Fig. 4, A–C). Ea, SAC, and SVR were lower at maximal exercise with lifelong exercise (P ≤ 0.047) (Fig. 4, E–G), while exercise MAP was not different between groups (P ≥ 0.210) (Fig. 4D).
DISCUSSION
The major findings from this study are as follows: 1) contrary to our hypothesis, a combination of Alagebrium and exercise did not act synergistically on exercise SV and Ea, nor did it elicit greater adaptations in SAC, SVR, TBV, or V̇o2 max compared with either treatment alone; and 2) 1 yr of Alagebrium combined with exercise did not restore exercise LV function to the levels achieved with lifelong exercise in previously sedentary seniors.
The effects of Alagebrium on LV function and exercise performance.
In humans, LV and central vasculature stiffness increase with advancing age (1, 15, 41). Collagen accumulation and AGE cross-linking of extracellular matrix proteins is proposed to contribute substantially to these age-related changes (3, 38). Alagebrium reduces CV stiffness and improves LV systolic and diastolic function in aged animal models (4, 26, 39, 46, 51).
Moreover, in aged rodents and humans, a combination of Alagebrium and exercise elicited greater improvements in LV and vascular stiffness compared with either Alagebrium or exercise alone (16, 46). Thus we hypothesized that, when Alagebrium and exercise are used concurrently, more prominent and physiological meaningful improvements in SV and Ea would be observed during exercise when preload and ventricular-arterial coupling are stressed. Contrary to our hypothesis, exercise combined with Alagebrium failed to positively impact exercise SV or Ea beyond that obtained by exercise training alone.
The effect of 1 yr of aerobic exercise training on exercise SV and Ea in previously sedentary seniors: SV.
An important observation of this study is that 1 yr of moderate-intensity exercise training increased exercise SV in previously sedentary seniors. Previous training studies in seniors have consistently reported an increased SV during submaximal exercise (27, 31, 37, 43, 48); however, there has been an absence of concordant findings related to maximal SV (6, 13, 14, 16, 17, 27, 31, 37, 43, 44, 48). Nevertheless, a recent meta-analysis of 16 studies in predominantly untrained late middle-aged adults and seniors reported that an increased maximal Q̇c and thus maximal SV primary mediates the improvement in V̇o2 max with 2–12 mo of exercise training (29).
The importance of LV diastolic function for the training-related change in exercise SV in previously sedentary seniors.
Unfortunately, measures of exercise LV volumes were not collected in this present study; thus we can only speculate on the mechanisms for the larger SV with 1 yr of training. The importance of LV end-diastolic volume to exercise training-related increases in SV in seniors has been demonstrated in cross-sectional and longitudinal training studies (13, 20, 35, 36, 48). A larger and more complaint LV, coupled with hypervolemia, augments the facilitation of the Starling mechanism during exercise in trained young and older individuals (7, 24, 25, 47). Two previous reports in seniors from our laboratory (16, 17) have reported that 1 yr of exercise training increased LV mass without a change in cardiac compliance. In contrast to previous examinations involving a shorter exercise training intervention (12 wk) in similarly aged men and women (18, 33, 45), we found that exercise training for 1 yr resulted in an expansion of TBV. Thus we speculate that these key adaptations would have resulted in a larger LV end-diastolic volume during exercise in our older adults, if we had been able to measure it. This contention is supported by a larger LV end-diastolic volume at a similar filling pressure during saline infusion after 1 yr of training in the current exercise training cohort, suggestive of improved LV diastolic filling during conditions of increased preload such as exercise (16).
Ea.
Ea represents the net arterial load imposed on the LV (afterload) and reflects the complex interaction of peripheral vascular resistance, systemic arterial compliance, and heart rate (22, 49). The age-related increase in resting Ea predominantly results from central arterial stiffening (34). Our group has previously shown that exercise Ea is enhanced with lifelong exercise (10) and after 1 yr of vigorous exercise in previously sedentary seniors (17), suggesting that improved ventricular-arterial coupling is an important training-related adaptation in seniors.
The importance of structural and functional vascular adaptations for the training-related change in exercise Ea in previously sedentary seniors.
Increased systemic arterial compliance and/or a reduction in peripheral vascular resistance are potential adaptations for an improved exercise Ea with exercise training. Relatively short-term (3 mo) exercise training results in more “youthful” levels of central vascular stiffness in healthy middle-aged adults (50), while peripheral vascular resistance during exercise is reduced (27, 37) after 3–12 mo of training in previously sedentary older adults. These findings suggest that both structural and functional vascular adaptations may be important for an improved Ea with exercise training in seniors.
Shibata and Levine (42) showed that 1 yr of vigorous and progressive exercise training improved resting SV, Ea, and SAC in sedentary seniors; however, these changes in Ea and SAC were eliminated when SV was restored to pretraining levels via lower body negative pressure. These findings suggest that an improved Ea after training in older adults represents a functional adaptation secondary to an increase in SV. This contention is strengthened by the finding that biological aortic age, an index that specifically reflects the intrinsic structural characteristics of vascular stiffness, was unaffected at rest or during preload reduction after training in the same cohort.
Effects of 1 yr of exercise plus Alagebrium vs. lifelong exercise on exercise LV function.
Our laboratory has previously reported the superior exercise LV function in lifelong committed exercisers and Masters athletes (10). Committed lifelong exercisers provide a unique physiological comparison due to the following: 1) the current exercise trained cohort performed a similar weekly exercise frequency as committed lifelong exercisers; 2) by performing this frequency of exercise, current and committed lifelong exercisers would likely achieve the current recommendations for weekly physical activity (≥150 min/wk); and 3) our laboratory's previous examination showed that 4-5 sessions/wk was the minimum lifelong exercise frequency that resulted in a significant improvements in LV function during exercise (10).
One year of moderate-intensity exercise training combined with Alagebrium failed to achieve the levels of SV and Ea associated with lifelong exercise of a similar weekly training frequency (Fig. 4, A and E). Instead, the values achieved at 1 yr for V̇o2 max, LV mass, and TBV are more closely associated with a casual lifelong exercise frequency (2–3 sessions/wk) (see Tables 1 and 3 in Ref. 10 for details).
There are several possibilities why 1 yr of exercise plus Alagebrium failed to improve exercise LV function to the levels associated with a committed level of lifelong exercise. First, while notable central and peripheral adaptations have been reported in seniors with short and relatively long-term (3–12 mo) exercise training (6, 13, 17, 30, 31, 35, 40), the intrinsic structural adaptations associated with improved LV and vascular compliance (1, 7, 41, 42) in highly trained seniors likely require several years of vigorous training stimuli (17, 42). Moreover, several decades of sustained training may be required for pericardial remodeling, which facilitates LV diastolic filling (1, 7).
Second, the intensity or overall dose of exercise may have been too low to provoke the ventricular and arterial remodeling associated with lifelong exercise. High-intensity exercise training results in rapid and substantial gains in V̇o2 max and partially reverses the CV effects of aging in healthy seniors (27, 28, 37). Previous works from our laboratory (17) and others (27) that have incorporated or exclusively used repeated bouts of high-intensity exercise have demonstrated a substantial increase in V̇o2 max and exercise SV in seniors. It is possible that, when routinely performed over a sufficient duration, the hemodynamic conditions of high-intensity and/or long-duration exercise provide the stimulus for more profound adaptations in LV and central vascular structure and function. For example, a recent study in sedentary young adults who performed a training program that is typical for competitive endurance athletes by incorporating periods of long-duration and high-intensity interval training showed that, while exercise intensity and volume may differentially influence the magnitude and pattern of LV and right ventricular remodeling, a greater exercise dose generally leads to more substantial adaptations (2).
Lastly, exercise may have been started too late in life to favorably influence cardiac and central vascular stiffening. Fujimoto et al. (15) showed that LV stiffness increases from the transition from early to late middle age and thus may represent the “sweet spot” when exercise may elicit the most beneficial effects on CV structure and function, including LV and central arterial stiffness. A randomized, 2-yr training program in sedentary, early to late middle-aged adults (ClinicalTrials.gov identifier: NCT02039154) is nearing completion and may provide more conclusive evidence for this contention.
Study limitations.
We only examined moderate-intensity exercise training in this present study, and, therefore, our findings may differ if higher exercise intensities or a greater exercise dose is performed. A myocardial biopsy was not performed to measure AGE's content; therefore, we cannot be certain that Alagebrium had the desired effect in this present cohort. The comparison of the present exercise cohort vs. committed lifelong exercise was based on a similar weekly exercise frequency; however, this approach fails to strictly control for other components of an exercise training program, including intensity, duration, or mode of exercise, all of which may have an important impact on LV function during exercise. Finally, our senior subjects were nonobese and normotensive and were screened for occult CV disease; therefore, it is unclear whether these current results are applicable to the larger population.
Conclusion.
In summary, moderate-intensity exercise for 1 yr improved SV and Ea during exercise in previously sedentary seniors, but these adaptations were not enhanced by the AGE cross-link breaker Alagebrium. Despite a comparable weekly exercise frequency, exercise SV and Ea after 1 yr of exercise in combination with Alagebrium did not achieve the levels associated with lifelong exercise. This latter finding suggests that several years of training stimuli, especially started early in life before AGE cross-links or other metabolic pathways are firmly established, may be required to obtain the exercise LV function associated with lifelong exercise in previously sedentary seniors.
GRANTS
This study was supported by National Institute on Aging Grant AG-17479.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.C.C.-R., N.F., K.M.S., J.L.H., S.S., M.D.P., K.N.B., and B.D.L. performed experiments; G.C.C.-R. and B.D.L. analyzed data; G.C.C.-R., N.F., K.M.S., J.L.H., S.S., M.D.P., K.N.B., and B.D.L. interpreted results of experiments; G.C.C.-R. prepared figures; G.C.C.-R., N.F., K.M.S., J.L.H., and S.S. drafted manuscript; G.C.C.-R. and B.D.L. edited and revised manuscript; J.L.H. and B.D.L. conception and design of research; B.D.L. approved final version of manuscript.
REFERENCES
- 1.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation : 1799–1805, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Arbab-Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams-Huet B, Haykowsky MJ, Levine BD. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation : 2152–2161, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens : 3–12, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P, Cerami A, Brines M, Regan TJ. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci U S A : 2809–2813, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banister EW, Morton RH, Fitz-Clarke J. Dose/response effects of exercise modeled from training: physical and biochemical measures. Ann Physiol Anthropol : 345–356, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation : 1085–1094, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Bhella P, Hastings J, Fujimoto N, Shibata S, Carrick-Ranson G, Adams-Huet B, Palmer M, Levine BD. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol : 1257–1266, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burge CM, Skinner SL. Determination of hemoglobin mass and blood volume with CO: evaluation and application of a method. J Appl Physiol : 623–631, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Carrick-Ranson G, Hastings JL, Bhella PS, Shibata S, Fujimoto N, Palmer D, Boyd K, Levine BD. The effect of age-related differences in body size and composition on cardiovascular determinants of V̇o2 max. J Gerontol A Biol Sci Med Sci : 608–616, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrick-Ranson GC, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E, Levine BD. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol : 736–745, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol : 2028–2034, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Das S, Barlow CE, Grundy S, Lakoski SG. Fitness, fatness, and systolic blood pressure: data from the Cooper Center Longitudinal Study. Am Heart J : 166–170, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Ehsani AA, Ogawa T, Miller TR, Spina RJ, Jilka SM. Exercise training improves left ventricular systolic function in older men. Circulation : 96–103, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Ehsani AA, Spina RJ, Peterson LR, Rinder MR, Glover KL, Villareal DT, Binder EF, Holloszy JO. Attenuation of cardiovascular adaptations to exercise in frail octogenarians. J Appl Physiol : 1781–1788, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto N, Hastings JL, Bhella PS, Shibata S, Gandhi NK, Carrick-Ranson G, Palmer D, Levine BD. Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. J Physiol : 1871–1880, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto N, Hastings JL, Carrick-Ranson G, Shafer KM, Shibata S, Bhella PS, Abdullah SM, Barkley KW, Adams-Huet B, Boyd KN, Livingston SA, Palmer D, Levine BD. Cardiovascular effects of 1 year of Alagebrium and endurance exercise training in healthy older individuals. Circ Heart Fail : 1155–1164, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation : 1797–1805, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gass G, Gass E, Wicks J, Browning J, Bennett G, Morris N. Rate and amplitude of adaptation to two intensities of exercise in men aged 65–75 yr. Med Sci Sports Exerc : 1811–1818, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Gore CJ, Rodriguez FA, Truijens MJ, Townsend NE, Stray-Gundersen J, Levine BD. Increased serum erythropoietin but not red cell production after 4 wk of intermittent hypobaric hypoxia (4,000–5,500 m). J Appl Physiol : 1386–1393, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Hagberg J, Goldberg A, Lakatta L, O'Connor F, Becker L, Lakatta E, Fleg J. Expanded blood volumes contribute to the increased cardiovascular performance of endurance-trained older men. J Appl Physiol : 484–489, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol : 867–874, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation : 513–521, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. II. The aging heart in health: links to heart disease. Circulation : 346–354, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Levine BD. Regulation of central blood volume and cardiac filling in endurance athletes: the Frank-Starling mechanism as a determinant of orthostatic tolerance. Med Sci Sports Exerc : 727–732, 1993. [PubMed] [Google Scholar]
- 25.Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation : 1016–1023, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Masurekar MR, Vatner DE, Jyothirmayi GN, Regan TJ, Vatner SF, Meggs LG, Malhotra A. Glycation end-product cross-link breaker reduces collagen and improves cardiac function in aging diabetic heart. Am J Physiol Heart Circ Physiol : H2587–H2591, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Makrides L, Heigenhauser GJ, Jones NL. High-intensity endurance training in 20- to 30- and 60- to 70-yr-old healthy men. J Appl Physiol : 1792–1798, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Molmen HE, Wisloff U, Aamot IL, Stoylen A, Ingul CB. Aerobic interval training compensates age related decline in cardiac function. Scand Cardiovasc J : 163–171, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Montero D, Diaz-Canestro C. Endurance training and maximal oxygen consumption with ageing: Role of maximal cardiac output and oxygen extraction. Eur J Prev Cardiol : 733–743, 2016. [DOI] [PubMed] [Google Scholar]
- 30.Murias JM, Kowalchuk JM, Paterson DH. Mechanisms for increases in V̇o2 max with endurance training in older and young women. Med Sci Sports Exerc : 1891–1898, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Murias JM, Kowalchuk JM, Paterson DH. Time course and mechanisms of adaptations in cardiorespiratory fitness with endurance training in older and young men. J Appl Physiol : 621–627, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Okazaki K, Iwasaki K, Prasad A, Palmer MD, Martini ER, Fu Q, Arbab-Zadeh A, Zhang R, Levine BD. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol : 1041–1049, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Okazaki K, Kamijo Y, Takeno Y, Okumoto T, Masuki S, Nose H. Effects of exercise training on thermoregulatory responses and blood volume in older men. J Appl Physiol (1985) : 1630–1637, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation : 2254–2262, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Schulman SP, Fleg JL, Goldberg AP, Busby-Whitehead J, Hagberg JM, O'Connor FC, Gerstenblith G, Becker LC, Katzel LI, Lakatta LE, Lakatta EG. Continuum of cardiovascular performance across a broad range of fitness levels in healthy older men. Circulation : 359–367, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Seals D, Hagberg J, Spina R, Rogers M, Schechtman K, Ehsani A. Enhanced left ventricular performance in endurance trained older men. Circulation : 198–205, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Endurance training in older men and women. I. Cardiovascular responses to exercise. J Appl Physiol : 1024–1029, 1984. [DOI] [PubMed] [Google Scholar]
- 38.Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation–a mini-review. Gerontology : 227–237, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro BP, Owan TE, Mohammed SF, Meyer DM, Mills LD, Schalkwijk CG, Redfield MM. Advanced glycation end products accumulate in vascular smooth muscle and modify vascular but not ventricular properties in elderly hypertensive canines. Circulation : 1002–1010, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata S, Hastings JL, Prasad A, Fu Q, Okazaki K, Palmer MD, Zhang R, Levine BD. “Dynamic” Starling mechanism: effects of ageing and physical fitness on ventricular-arterial coupling. J Physiol : 1951–1962, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibata S, Levine BD. Biological aortic age derived from the arterial pressure waveform. J Appl Physiol : 981–987, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata S, Levine BD. Effect of exercise training on biologic vascular age in healthy seniors. Am J Physiol Heart Circ Physiol : H1340–H1346, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spina R, Ogawa T, Kohrt W, Holloszy J, Ehsani A. Differences in cardiovascular adaptations to endurance exercise training between older men and women. J Appl Physiol : 849–855, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Spina RJ, Ogawa T, Miller TR, Kohrt WM, Ehsani AA. Effect of exercise training on left ventricular performance in older women free of cardiopulmonary disease. Am J Cardiol : 99–104, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Stachenfeld NS, Mack GW, DiPietro L, Morocco TS, Jozsi AC, Nadel ER. Regulation of blood volume during training in post-menopausal women. Med Sci Sports Exerc : 92–98, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Steppan J, Tran H, Benjo AM, Pellakuru L, Barodka V, Ryoo S, Nyhan SM, Lussman C, Gupta G, White AR, Daher JP, Shoukas AA, Levine BD, Berkowitz DE. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol : 565–572, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stickland MK, Welsh RC, Petersen SR, Tyberg JV, Anderson WD, Jones RL, Taylor DA, Bouffard M, Haykowsky MJ. Does fitness level modulate the cardiovascular hemodynamic response to exercise? J Appl Physiol : 1895–1901, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation : 1648–1655, 1994. [DOI] [PubMed] [Google Scholar]
- 49.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol Heart Circ Physiol : H773–H780, 1983. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation : 1270–1275, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Vaitkevicius PV, Lane M, Spurgeon H, Ingram DK, Roth GS, Egan JJ, Vasan S, Wagle DR, Ulrich P, Brines M, Wuerth JP, Cerami A, Lakatta EG. A cross-link breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proc Natl Acad Sci U S A : 1171–1175, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH; Dallas Heart Study Investigators. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol : 1473–1480, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Wilmore JH, Behnke AR. An anthropometric estimation of body density and lean body weight in young men. J Appl Physiol : 25–31, 1969. [DOI] [PubMed] [Google Scholar]