Abstract
The neuropeptides orexins are important in regulating the neurobiological systems that respond to stressful stimuli. Furthermore, orexins are known to play a role many of the phenotypes associated with stress-related mental illness such as changes in cognition, sleep-wake states, and appetite. Interestingly, orexins are altered in stress-related psychiatric disorders such as Major Depressive Disorder and Anxiety Disorders. Thus, orexins may be a potential target for treatment of these disorders. In this review, we will focus on what is known about the role of orexins in acute and repeated stress, in stress-induced phenotypes relevant to psychiatric illness in preclinical models, and in stress-related psychiatric illness in humans. We will also briefly discuss how orexins may contribute to sex differences in the stress response and subsequent phenotypes relevant to mental health, as many stress-related psychiatric disorders are twice as prevalent in women.
Keywords: Orexins, Hypocretins, Stress, HPA axis, Habituation, Cognition, Sleep, Appetite
1. Introduction
The neuropeptides orexins have well-described roles in arousal, appetite, and cognition, all of which are affected in stress-related mental illness (Chemelli et al., 1999; Dube et al., 1999; Lambe et al., 2005; Mahler et al., 2014; Sakurai et al., 1998; Sweet et al., 1999; Vanitallie, 2002). Moreover, orexins are altered in anxious and depressed patients (Brundin et al., 2007; Johnson et al., 2010; Strawn et al., 2010). Thus, orexins may contribute to the stress response and subsequent phenotypes relevant to mental health. In this review, we will focus on what is known about the role of orexins in acute and repeated stress, in stress-induced phenotypes relevant to psychiatric illness in preclinical models, and in stress-related psychiatric illness in humans.
2. Background on orexins
2.1. Orexins
Orexins (also called hypocretins) are excitatory neuropeptides generated from the prepro-orexin precursor that is exclusively localized in cells of the lateral and posterior hypothalamic region (de Lecea et al., 1998; Sakurai et al., 1998). Prepro-orexin is cleaved into two highly structurally related and highly conserved peptides, orexin A (composed of 33 amino acids) and orexin B (composed of 28 amino acids) (Sakurai et al., 1998). There are approximately 10,000–20,000 orexinergic neurons in the human brain (Nishino et al., 2000) and roughly 1100 orexinergic neurons in the rat brain (de Lecea et al., 1998). Orexin neurons can be round, elliptical, or fusiform, and either bipolar or unipolar (Cheng et al., 2003). In addition, orexin neurons are large, with a diameter of 21 μm on average (Cheng et al., 2003). Orexin neurons are intrinsically in a depolarized state due to constitutively active non-selective cation currents (Horvath and Gao, 2005). Orexin activity, including peptide levels and neural function, varies across the circadian cycle, supporting the involvement of orexins in regulating arousal (Taheri et al., 2000). Almost all orexin neurons (94%) also synthesize the peptide dynorphin, an opioid receptor agonist (Chou et al., 2001), while about 50% of orexin neurons are thought to also contain the excitatory neurotransmitter glutamate (Henny et al., 2010; Rosin et al., 2003; Torrealba et al., 2003). Some studies have suggested that there is functional dichotomy for orexin neurons; specifically, that orexin neurons in the perifornical and dorsomedial hypothalamic areas are involved in stress and arousal, while those in the lateral hypothalamus are more involved in addiction (Harris et al., 2005; Harris and Aston-Jones, 2006). However, this anatomical specificity has not been investigated in the majority of the current literature.
2.2. Orexin receptors
Orexins bind to two G-protein coupled orexin receptors (GPCRs), the orexin 1 and orexin 2 receptors (OX1R and OX2R, respectively) (de Lecea et al., 1998; Sakurai et al., 1998). OX1R binds Orexin A with a much higher affinity than Orexin B (roughly 10–1000 times higher), whereas OX2R binds both peptides with similar affinity (Gotter et al., 2012b). Both receptors can couple to Gq, Gs, and Gi, (Holmqvist et al., 2005; Magga et al., 2006; Tang et al., 2008), and the specific G protein associated with each receptor varies among different neuronal populations (Bernard et al., 2002; van den Pol et al., 1998; Yang et al., 2003). Activation of these G proteins leads to a variety of downstream signaling cascades including phospholipase C (PLC), adenylate cyclase (AC), and extracellular signal-regulated kinase (ERK) (Selbach et al., 2010; Uramura et al., 2001). While many brain regions express both orexin receptors, some regions exhibit differential expression (Marcus et al., 2001). For example, the cingulate cortex and locus coeruleus selectively express OX1R, while the paraventricular nuclei of the hypothalamus (PVN) and shell neurons of nucleus accumbens preferentially express OX2R (Marcus et al., 2001; Trivedi et al., 1998).
2.3. Projection sites
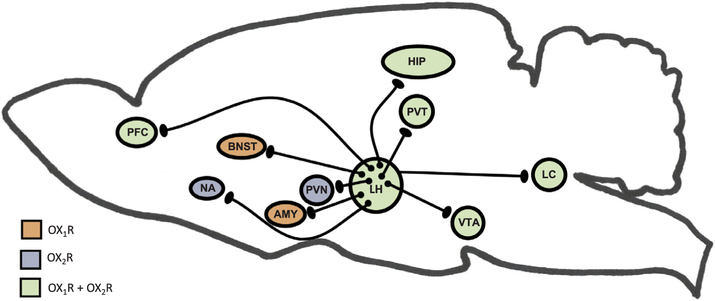
Orexin neurons widely project to all regions of the brain including the hypothalamus (de Lecea et al., 1998; Peyron et al., 1998), thalamus, cortex, brain stem, and spinal cord (van den Pol, 1999). Orexin input to brain regions important for arousal, such as the locus coeruleus, help to regulate the response to a stressor (Hagan et al., 1999). Moreover, orexin containing nerve terminals project to stress-sensitive centers such as the amygdala and bed nucleus of the stria terminalis (Date et al.,1999). Orexin neurons also project to the paraventricular nucleus of the hypothalamus (PVN) (Winsky-Sommerer et al., 2004), where neurons expressing Corticotropin Releasing Hormone (CRH) initiate the Hypothalamic Pituitary Adrenal (HPA) Axis. Further, orexin neurons densely project to the paraventricular nucleus of the thalamus (PVT) (Kirouac et al., 2005), which plays a role in regulating neuroendocrine and behavioral adaptations to severe or chronic stress (Hsu et al.,2014). Specifically, orexins may influence gene expression of the CRH type 1 receptor (CRH1R) in the paraventricular nucleus of the thalamus (Heydendael et al., 2012, 2011), and this brain region may then regulate the HPA axis via multisynaptic pathways through the BNST to the PVN. The orexin neuropeptides orexin A and orexin B interact with noradrenergic, cholinergic, serotonergic, histaminergic, and dopaminergic systems, in addition to the HPA axis (Sutcliffe and de Lecea, 2000). Thus, orexins have the potential to regulate the stress response through actions at multiple projection sites. For an illustration of orexin projections to stress-relevant brain regions, please see Fig. 1.
Fig. 1. Orexin projections to stress-relevant brain regions.
This illustration depicts orexin neurons in the lateral hypothalamus (LH) and several stress-relevant projection sites. The expression of orexin receptors within these brain regions is also shown. Orexins are known directly innervate the prefrontal cortex (PFC) and Hippocampus (HIP), and orexin activation during stress underlie cognitive impairments. Orexin receptors in the Nucleus Accumbens (NA) and Ventral Tegmental Area (VTA) mediate stress-induced reward seeking. Orexins regulate activity in the hypothalamic pituitary adrenal (HPA) axis through projections to the paraventricular nucleus of the hypothalamus (PVN), where neurons expressing Corticotropin Releasing Hormone (CRH) are located. Further, orexin neurons densely project to the paraventricular nucleus of the thalamus (PVT), which plays a role in regulating neuroendocrine and behavioral adaptations to repeated or chronic stress. Orexins mediate behaviors relevant to affect and mood in humans (depression, anxiety, fear) through actions in the Bed Nucleus of the Stria Terminalis (BNST) and the Amygdala (AMY). Orexins also have inputs to brain regions important for arousal, such as the locus coeruleus (LC), which in turn can regulate activity in the limbic, thalamic and hypothalamic structure that are directly regulated by orexins.
3. Orexins and the stress response
Extending beyond their role in mediating general arousal and wakefulness, orexins are important in the response to stressful stimuli which requires the animal to shift from a basal to a reactive state (Berridge and España, 2005). Accordingly, orexins are important in regulating the neurobiological systems that respond to stressful stimuli. Most of the literature has identified a role for orexins in regulating the acute stress response, while less is known about the role of these neuropeptides in adaptations to repeated stress. Below, we will discuss what is currently known about the involvement of orexins in regulating responses to both acute and repeated stress.
3.1. Acute stress
Administration of orexins affects behaviors impacted by stress and induces both HPA and sympathetic activity (discussed further in Sections 3.1.1 and 3.1.2) For example, central administration of orexins induces grooming, face washing, and burrowing (Ida et al., 2000). These behavioral effects are blocked by an OX1R antagonist (Duxon et al., 2001). Moreover, stimulation of orexins (by administration of clozapine-N-oxide (CNO), which binds to Gs-coupled Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) expressed in orexin neurons) increases struggling behavior during restraint stress, and this is prevented by an OX2R antagonist (Grafe et al., 2017b). Interestingly, orexin-deficient mice have a diminished behavioral response to stress, as measured by decreased activity in a resident-intruder paradigm (Kayaba et al., 2003), further highlighting the importance of this neuropeptide in producing stress-related behaviors.
The interaction between orexins and stress is reciprocal. Consequently, in addition to orexin increasing stress-related behaviors, acute stress activates orexins neurons. For example, acute psychological stressors such as forced swim stress (Chang et al., 2007) and restraint stress (Grafe et al., 2017b) produce increases in both the percentage of orexin neurons dual labeled with cFos (a measure of neural activation) and orexin A levels in the cerebrospinal fluid. Restraint stress was also found to increase orexin neuron activity as measured by increased calcium signals via fiber optometry (González et al., 2016). Acute physical stressors such as intraperitoneal injections and exercise have also been shown to increase orexin neural activation (Messina et al., 2016; Panhelainen and Korpi, 2012). Remarkably, acute stress can have long lasting effects on orexin gene expression, as increased prepro-or-exin can be observed 2 weeks following an acute 2-minute foot shock stress (Chen et al., 2013).
3.1.1. Hypothalamic-pituitary-adrenal axis
In general, orexins have been shown to promote the HPA axis response to acute stress at all levels of the HPA axis. (Berridge et al., 2010; Johnson et al., 2012; Winsky-Sommerer et al., 2005, 2004). For example, central infusion of orexins activates CRH neurons. Additionally, optogenetic stimulation of orexin neurons increases cFos expression in the PVN (Bonnavion et al., 2015). Moreover, CRH neurons in the PVN abundantly express the OX2R (Trivedi et al., 1998). Central administration of orexins also increases downstream HPA hormones ACTH and corticosterone, and this can be reversed with a CRH antagonist (Al-Barazanji et al., 2001; Jászberényi et al., 2000; Kuru et al., 2000; Spinazzi et al., 2006). Orexins promote release of ACTH through its actions on both OX1R and OX2R in the pituitary (Date et al., 2000). Interestingly, narcolepsy patients with low levels of orexins have blunted ACTH levels, which suggests that orexins are important in promoting this branch of the HPA response (Kok et al., 2002). Orexins stimulate glucocorticoid secretion via OX1R in the adrenal gland, though studies in both rat and human adrenal glands have indicated that both orexin receptors are present (López et al., 1999; Mazzocchi et al., 2001; Ziolkowska et al., 2005). While both orexin 1 and 2 receptors have been implicated in acute HPA activity, the role of each receptor appears dependent on the paradigm of stress used. For example, while the orexin 1 receptor modulates ACTH release by restraint stress (Samson et al., 2007), it does not modulate ACTH release induced by cage exchange stress (where the rodent is placed in a dirty cage previously occupied by another mouse) (Bonaventure et al., 2015). The role each receptor plays during each type of acute stress is likely dependent upon the circuitry activated, as the relative expression of each orexin receptor differs between brain regions.
While orexins are known to promote HPA activity, it has also been shown that HPA activation induces orexin activity. Specifically, electron microscopy has demonstrated that CRH boutons contact orexin neurons (Horvath et al., 1999; Winsky-Sommerer et al., 2004), and that orexin neurons express both CRH2R and CRH-BP mRNA and protein (Slater et al., 2016). Moreover, electrophysiological data demonstrate that CRH administration increases orexin neuron activity (Winsky-Sommerer et al., 2004). These studies also found that orexin neurons were less sensitive to foot shock or restraint stress in CRH1R knockout mice. These data, along with previous receptor localization data, suggest that both CRH1R and CRH2R play a role in activating orexins during stress.
3.1.2. Sympathetic nervous system
Orexins are known to acutely promote sympathetic nervous system activity. For example, central orexin administration increases blood pressure, heart rate, and renal sympathetic nerve activity (Shirasaka et al., 1999; Smith et al., 2002). However, orexins have been shown to be involved in promoting the sympathetic response to only certain types of stressors. For example, contextual fear conditioning-induced increases in heart rate and blood pressure are reduced with the dual orexin receptor antagonist almorexant, but restraint- and cold exposure-induced pressor and tachycardic responses are not (Furlong et al., 2009). Moreover, sympathetic outflow during defense responses are clearly regulated by orexins, as orexin knockout mice display attenuated blood pressure and heart rate in response to emotional stress in the resident-intruder test compared with controls (Kayaba et al., 2003). Recent experiments using specific receptor antagonists (rather than dual receptor antagonists) have indicated that both the OX1R and OX2R promote cardiovascular and locomotive responses to stress (Beig et al.,2015). Orexin neurons exert much of their effects on the sympathetic response through actions on the medulla and nucleus of the solitary tract (NTS) in the brainstem (de Oliveira et al., 2003; Zheng et al., 2005).
3.2. Repeated stress
While the role of orexins in acute stress appears to be straightforward (orexins promote the acute behavioral and neuroendocrine response to stress, and acute stress activates orexin neurons), the role of the orexin system in regulating responses to repeated stress is not as clearly defined. Whether orexin function is increased or decreased with repeated stress may depend on the type, intensity, and duration of the stressor, and whether habituation and other adaptations take place. With repeated exposure to moderately intense stressors, individuals typically habituate to that stress as indicated by decreasing responsivity in behavioral, HPA and autonomic measures (Grissom et al., 2008; Grissom and Bhatnagar, 2009). Failure to habituate to a stressor is a hallmark of stress-related illnesses such as post-traumatic stress disorder (PTSD) and panic disorder (Johnson et al., 2012). Interestingly, patients with panic anxiety symptoms have higher levels of orexin in their cerebrospinal fluid (CSF) (Johnson et al., 2010).
In studies using repeated restraint stress, both hypoactivity and hyperactivity of the orexin system have been reported. For example, both orexin neuronal activation (as measured by dual orexin and cFos immunohistochemistry) and orexin A levels in the cerebrospinal fluid decreased after HPA habituation to repeated restraint in male rats (Grafe et al., 2017b). Thus, in this case, habituation to a repeated stressor is associated with decreased orexin function. Another study found that orexins were upregulated in the basolateral amygdala of male mice after 14 days of repeated restraint stress (Kim et al., 2015). As this paradigm did not examine HPA habituation to repeated restraint, and used 2 h of restraint per day (compared with 30-min per day in the previously mentioned restraint study), it is possible that these rodents did not habituate, which would explain their increased orexin activity. In addition, the Kim et al. study reported upregulation of orexin mRNA transcripts specifically in the amygdala, whereas the Grafe et al. study examined orexin neuronal activation in the lateral hypothalamus and overall orexin levels in the cerebrospinal fluid. Thus, these different types of assessments may yield quite different results. Ultimately, it will be important to examine orexin function in a variety of locations during repeated stress (e.g. both in orexinergic neurons in the lateral hypothalamus and in specific projection regions) in order to truly understand the role these neuropeptides play.
Both decreased and increased orexin function have been observed in rodents exposed to repeated social defeat. Specifically, decreased prepro-orexin mRNA and orexin A and B peptides were observed in the hypothalamus after social defeat paradigms in both mice and rats (Lutter et al., 2008; Nocjar et al., 2012). Though HPA habituation was not assessed in these paradigms, it has been suggested that when a stressor is chronic, predictable, and inescapable, this leads to cessation of coping behaviors, orexin system hypoactivity, and may lead to depressive-like behavior (Mahler et al., 2014). However, previous literature has noted that there are naturally occurring differences in response to social defeat, where some rodents display a passive coping strategy while others display an active coping strategy (Wood et al., 2010). The passive coping strategy, assessed by a short latency to defeat, is typically associated with delayed habituation to repeated defeat and depressive-like behavior, whereas the active coping strategy is not (Finnell et al., 2017; Wood et al., 2010). In a recent study, we found that active coping behaviors in social defeat are associated with lower orexin function in male rats. Moreover, we found that inhibiting orexins (by administering CNO, which binds to Gi-coupled DREADDs expressed in orexin neurons) promotes resilience to repeated social defeat in previously passive coping rodents (Grafe et al., 2018). Consistent with our data, another social defeat study found that lower orexin function may be indicative of resilience, rather than vulnerability (Chung et al., 2014). Thus, it appears that orexins may contribute to individual differences in response to social defeat stress, whereupon lower orexins exhibited by actively coping animals is associated with resilience to social defeat. However, in situations where animals are exposed to inescapable stress (and exhibit reduced coping), lower orexins are associated with vulnerability to stress.
A study using 8 weeks of unpredictable chronic mild stress found that orexin neurons were activated (using dual immunohistochemical staining for orexin and cFos) (Nollet et al., 2011). Typically, unpredictable chronic mild stress prevents habituation through variation in the type of stressor, which might explain the increase in orexin activation after repeated stress in this case. Indeed, in a study of male and female repeated restraint, female rats did not habituate as fully as males, and thus, had higher levels of orexin neuronal activation (as measured by dual orexin and cFos immunohistochemical labeling) (Grafe et al., 2017a). In this simplistic view of the orexin system in repeated stress, if habituation does not take place, orexin function is increased, whereas if habituation does take place, orexin function is decreased.
There are several gaps in our knowledge that need to be addressed to fully understand the role that orexins play in repeated stress. First, we must examine orexin function in a variety of locations, rather than just in orexinergic neurons, during repeated stress in order to gain a better understanding of how these neuropeptides modulate repeated stress. While many brain regions receiving orexin input may be important in regulating the response to repeated stress, a study using repeated swim stress found that OX1R signaling in the paraventricular thalamus was critical for adaptation to repeated stress (Heydendael et al., 2011). There are likely many more brain regions in which orexins act to mediate habituation to stress, including the amygdala, PVN, and LC (Grafe et al., 2017b). Next, we must examine orexin function in individuals rather than just treatment groups to determine if these neuropeptides mediate differential responses to stress (as was observed in social defeat stress). Lastly, it will be important to determine whether differences in orexin function are present before stress occurs, or as a result of it. Pre-existing differences in orexin expression may impact future responses to stress. Currently, our reliable measures of orexins (mRNA, protein in brain and csf) require terminal procedures. Further, plasma levels of orexins do not differ between narcoleptic patients and normal controls, and thus, are likely not a reliable indication of central orexin activity (Dalai et al., 2001). Consequently, we cannot directly determine whether differences in orexin expression pre-exists exposure to stress. With these caveats in mind, the main conclusion we can draw is that the orexin system responds to repeated stress differentially, depending on the nature of the stressor and how one adapts to it. Ultimately, it appears that orexin function decreases with habituation, and is lower in rats resilient to the consequences of repeated stress. For a summary of orexin measures after various repeated stress paradigms, please see Table 1.
Table 1. Summary of orexin measures after repeated stress.
Summary of the literature reporting orexin measures after various repeated stress paradigms (including repeated restraint, social defeat, and unpredictable chronic mild stress). Details concerning the type, duration, and intensity of each stressor are reported, as well as the type and location of the orexin measure, whether it was increased or decreased, and citation for the study.
| Stress paradigm | Orexin measure | orexin levels after stress | Citation |
|---|---|---|---|
| Repeated restraint (30min/day × 5 days) |
Levels in CSF Orexin Neuron Activity (Orx/cFos) |
Decreased Decreased |
Grafe et al., 2017a |
| Repeated restraint (2 h/day × 14 days) |
Orexin mRNA in Amygdala | Increased | Kim et al., 2015 |
| Repeated social defeat (Defeat 15 min, sensory contact up to 30 min × 7 days) |
Orexin mRNA in LH | Decreased in Resilient Rats compared to Vulnerable Rats | Grafe et al., 2018 |
| Repeated social defeat (Defeat 5min/day, sensory contact for rest of 24 h × 10 days) |
Orexin mRNA in LH | Decreased in Resilient Rats compared to Vulnerable Rats | Chung et al., 2014 |
| Repeated social defeat (Defeat 30 min/day × 5 days) |
Orexin A & B peptide in mPFC, VTA, and LH | Decreased | Nocjar et al., 2012 |
| Repeated social defeat (Defeat 10 min/day, sensory contact for rest of 24 h × 10 days) |
Orexin mRNA in LH Orexin protein in LH |
Decreased Decreased |
Lutter et al., 2008 |
| Unpredictable chronic mild stress (alterations in bedding, cage tilt, cage shift (placed in empty cage of another male), restraint stress; randomly ordered × 8 weeks) |
Orexin Neuron Activity (Orx/cFos) | Increased | Nollet et al., 2011 |
3.3. Summary
The neuropeptides orexins are important regulators of the stress response. The role orexins play in acute stress is relatively straightforward, with numerous studies providing evidence of orexins increasing HPA and sympathetic activity, as well as stress-related behaviors. Moreover, acute stress produces reliable increases in orexin actions, thus, this relationship between orexins and acute stress is reciprocal. While orexins modulate the response to repeated stress, less data is available on this topic. Currently, it appears that the involvement of the orexin system in repeated stress depends on the type, intensity, and duration of the stressor, and whether one adapts to this repeated stress. More research should be done on the particular brain regions that orexins modulate during repeated stress. Additionally, it would be ideal to measure orexin function in the same individuals before, during, and after stress in order to determine exactly how this system changes in response to repeated stress.
4. Orexins and phenotypes relevant to mental health
Orexin neurons receive a variety of signals related to environmental, physiological and emotional stimuli, and project broadly to the entire central nervous system. Orexin neurons are implicated in a wide variety of neuroendocrine and behavioral responses that are disrupted in stress-related psychiatric disease including sleep, cognition, appetite, addiction, emotion and pain (Allard et al., 2004; Bingham et al., 2001; Chemelli et al., 1999; Harris et al., 2005; Lambe et al., 2005; Sakurai, 2014; Sakurai et al., 1998; Suzuki et al., 2005a). Below, we will discuss preclinical studies that shed light on how orexins contribute to each phenotype associated with stress-related mental illness.
4.1. Sleep
The role of orexins in narcolepsy was discovered in humans, dogs, and mice in quick succession, highlighting the importance of these peptides in arousal, which can be defined as an increase in sensory alertness, motor activity, and emotional reactivity (Chemelli et al., 1999; Lin et al., 1999; Nishino et al., 2000; Pfaff et al., 2002; Reilly, 1999). Notably, orexin knockout mice displayed reduced arousal (periods of behavioral arrest during the dark period as measured by video photography), which was further characterized as narcolepsy-like behavior (up close videos revealed collapse of head and neck, with simultaneous buckling of the limb, and EEG confirmed this was REM sleep) (Chemelli et al., 1999). Ox2R knockout mice also show decreased arousal (behaviorally abnormal attacks of non REM sleep, and disrupted wake), but their phenotype is milder than orexin null mice (Willie et al., 2003). In contrast, OX1R knockout mice do not display any overt behavioral abnormalities (Sakurai, 2007). Interestingly, targeted restoration of OX1R in the locus coeruleus or Ox2R in the dorsal raphe in orexin receptor knockout mice prevent pathological fragmentation of wakefulness and cataplexy, respectively (Hasegawa et al., 2014). Moreover, lesioning the amygdala reduces cataplexy in orexin knockout mice, which may explain why these episodes are often triggered by strong emotions (Burgess et al., 2013). Thus, orexins promote arousal through both the OX1R and OX2R in brain regions such as the dorsal raphe and locus coeruleus, with afferent input from the amygdala.
Preclinical data supports the hypothesis that orexin hyperactivity plays a role in insomnia (Prober et al., 2006). (1) Central administration of exogenous orexin promotes arousal and wakefulness (Nagahara et al., 2015). (2) Optogenetic activation of orexin neurons increases the likelihood of sleep-wake transitions (Adamantidis et al., 2007). (3), Endogenous orexin levels increase during wakefulness and decrease during sleep (Hagan et al., 1999). (4) Orexin receptors antagonists are an effective treatment for insomnia, further supporting the idea that orexin hyperactivity contributes to insomnia (Gotter et al., 2012a; Nakamura and Nagamine, 2017). In contrast, loss of orexins (as in narcolepsy) results in hypersomnia, including disrupted REM sleep, excessive daytime sleepiness, and cataplexy (Nakamura et al., 2011). Indeed, decreased levels or orexins in the cerebrospinal fluid levels can be used as diagnostic criteria for narcolepsy (Mignot et al., 2002).
Changes in arousability are a key phenotype of stress-related psychiatric disorders, often predating a formal diagnosis of these disorders (Benca, 1996). For example, increased arousability is observed in PTSD, as measured by an exaggerated startle response, irritability, hypervigilance, and sleep disturbances (Jorge, 2015). Studies in the rat have found altered orexin signaling underlies stress-induced abrupt awakening, which can be observed even 21 days after the stress has ended, when rats are re-exposed to the original footshock context (Yu et al.,2016). The abrupt awakening was present in female rats that had undergone footshock stress and those that had witnessed cage mates undergoing footshock stress, demonstrating that observing a stressful event is sufficient to cause these orexin-mediated disruptions in arousal. A novel orexin 1 receptor antagonist has recently been discovered that will reduce panic-like responses in rodents with no apparent sedative effects, and consequently, may be a good candidate for treatment of anxiety disorders (Bonaventure et al., 2017). Collectively, it is clear that the orexins contribute to changes in arousability in response to stress. This may lead to dysregulation of sleep, which is a key phenotype in psychiatric illness.
4.2. Cognition
Given that orexins promote arousal, and changes in arousal can modulate cognitive function (Maran et al., 2017; Packard and Goodman, 2012), these neuropeptides likely play an important role in cognition. In support of this, narcoleptic patients show specific cognitive impairments (Blackwell et al., 2017). Consistently, much of the preclinical literature suggests that orexins enhance cognitive function. Accordingly, when orexin antagonists are used, different types of cognitive function, such as attention, spatial memory, and social memory, are impaired. For example, studies in rodents demonstrate that orexin administration into the prefrontal cortex increases attentional performance in a self-paced task of visuospatial sustained and divided attention, while systemic administration of orexin receptor 1 antagonist SB334867 decreases performance in a sustained attention task (Boschen et al., 2009; Lambe et al., 2005). Moreover, transnasal delivery of orexin A in monkeys ameliorates sleep deprivation-induced cognitive deficits in a delayed match-to-sample memory task assessing short term memory (Deadwyler et al., 2007). Previous studies have demonstrated via both lesion experiments and neural activity measurements that the medial prefrontal cortex (mPFC) is required for attention-related tasks and short-term memory tasks (Fuster and Alexander, 1971; Miner et al., 1997; Pardo et al., 1991; Sakurai and Sugimoto, 1985). Moreover, the literature indicates that mRNA for both orexin 1 and 2 receptors are expressed in the medial prefrontal cortex (Marcus et al., 2001). Thus, orexin actions in the mPFC likely promote attention.
Inactivation of orexin 1 receptors in the hippocampus impairs acquisition, consolidation, and retrieval in the Morris Water Maze, a spatial memory task (Akbari et al., 2006). However, systemic administration of the dual orexin antagonist almorexant failed to impact spatial memory in the Morris Water Maze (Dietrich and Jenck, 2010). As the latter study used systemic administration, it is possible that the antagonist was not used at a high enough dose to prevent orexin action in the hippocampus of these rats. Additionally, the sleep-promoting properties of almorexant may have indirectly elicited a beneficial effect on cognitive function, but this has not been extensively tested. In sum, there is some evidence that orexins promote spatial memory through actions in the hippocampus.
Orexin-A also appears to enhance social memory, as Orexin/ataxin-3-transgenic mice, in which orexin neurons degenerate by 3 months of age, display deficits in long-term social memory, which was measured using an automated “two-enclosure homecage” social test (where mice had to choose between approaching a previously investigated mouse or a novel mouse after a 60 min delay) (Yang et al., 2013). Nasal administration of exogenous orexin-A restored social memory and enhanced synaptic plasticity in the hippocampus in these ataxin mice. This experiment, studying how orexins contribute to social memory, along with the previously discussed experiments, focusing on the role of orexins in attention and spatial memory, support the idea that orexin activation enhances several different types of cognitive function.
However, when orexin activity is increased during stress, it is possible that cognition might be negatively impacted. For example, we recently found that stimulating orexins (by administering CNO, which binds to Gs-coupled DREADDs expressed in orexin neurons) during social defeat stress leads to subsequent impairments in the novel object recognition test (unpublished). This test assays spatial and recognition memory and depends on hippocampal and entorhinal cortex structures, which express both OX1R and OX2R (Marcus et al., 2001; Stackman et al., 2016; Wilson et al., 2013; Yang et al., 2013). Interestingly, stimulating orexins did not impair novel object recognition in non-stressed rats, thus, the specific interaction between increased orexins and stress caused cognitive impairment. It may be that the combination of CNO-induced increases in orexin activity and stress-induced increases in orexin activity leads to excessive orexin-induced arousal, and as a result, cognition is impaired. The relationship between orexin activity and cognitive function may be an inverted U curve, consistent with the understanding of GPCR regulation (Black et al., 2016). Specifically, when orexin levels are too low, as in narcolepsy, cognition is impaired; however, when orexin levels are too high, as in conditions of stress, cognition may also be impaired, possibly due to receptor desensitization or downregulation (Black et al., 2016). An appropriate amount of orexins is required to maintain optimal cognitive ability. One possible mechanism by which stress might interact with orexins to impair cognitive function is through its actions in the prefrontal cortex. Particularly, several laboratories have shown that repeated restraint causes atrophy in the apical dendrites in layer II/III pyramidal cells in the PFC, which are directly innervated by orexin (Lambe et al., 2005). This pathway only explains the role of orexins in cognitive tasks that are PFC-dependent (and likely not the hippocampally-mediated novel object recognition task). Future research should investigate other brain regions involved in orexin-mediated changes in cognition after stress. In sum, orexins play an important role in stress-induced cognitive impairment, which is an important phenotype in stress-related psychiatric disorders.
4.3. Appetite
Orexins first emerged because of their significant appetite inducing effects (Sakurai et al., 1998). Specifically, many preclinical studies demonstrate that orexins promote food intake (Dube et al., 1999; Sweet et al., 1999). Conversely, orexin antagonists reduce food consumption (Haynes et al., 2000). Moreover, the orexin system appears to be regulated by nutritional states. For instance, overnight food deprivation, a physiological stressor, increases expression of both prepro-orexin (Cai et al., 1999) and its receptors (Karteris et al., 2005). While some argue that orexin-induced hyperphagia is a consequence of enhanced arousal, one study demonstrated that this was not the case (Kotz et al., 2002). Specifically, orexin A stimulated feeding the same amount whether in the presence or absence of a running wheel. Therefore, orexin-induced feeding is not always coincident with increased activity suggesting that the hyperphagic response is not merely an artifact of arousal (Rodgers et al., 2002).
As central orexins increase food intake, and orexin receptor antagonists decrease food intake, one might expect orexin deficient mice to be lean. However, transgenic mice with gradual loss of orexin neurons show feeding abnormalities and dysregulation in energy homeostasis, leading to obesity despite the reduced food intake (Chieffi et al., 2017). Reduced physical activity appears the main cause of weight gain in these models resulting in energy imbalance. In parallel, narcoleptic patients show an increased incidence of obesity (Dahmen et al., 2001). Oppositely, transgenic overexpression of orexin confers resistance to obesity, possibly due to increased locomotor activity, sympathetic tone, and higher metabolic rate (Funato et al., 2009). In sum, stable orexin deficiency or overexpression (as opposed to transient changes in orexin levels) appear to affect body weight via changes in energy expenditure.
Several studies have examined how orexin neurons regulate appetitive behavior during and after stress. For example, orexins were found to play a role in stress-induced binge eating, as their levels are significantly elevated in response to a high fat diet in previously restricted mice (Pankevich et al., 2010). Moreover, orexins are thought to interact with reward systems to selectively regulate intake of highly palatable food, and thus might be a good target for control of compulsive eating (Piccoli et al., 2012). After sleep deprivation, which is fundamentally stressful, rats show an increase in orexin mRNA expression and an associated increase in appetite (Martins et al., 2010). Interestingly, in this same study, sleep recovery reduced levels of orexin mRNA and animals became hypophagic. Stress that occurs prenatally has also been found to impair orexin function in response to starvation (Boersma et al., 2016). Thus, a dysfunction in this neuropeptide system after stress may lead to an anorexic phenotype. In addition to regulating appetite during stress, orexins may affect overall body weight through changes in physical activity. Indeed, it has been suggested that orexin dysfunction may lead to hyperactivity observed in patients with anorexia nervosa (Baranowska et al., 2008). Consistently, we have found that stimulation of orexins prior to each of 5 days of repeated restraint stress causes a decrease in body weight, coincident with an increase in struggle behavior during restraint (Grafe et al., 2017b). Again, the type, duration, and intensity of the stressor may dictate how orexins regulate subsequent changes in appetite and physical activity. Nonetheless, the majority of work suggests that orexins mediate increases in appetite and feeding. This remains an important area of research because feeding and metabolism are important phenotypes in stress-related psychiatric illness.
4.4. Addiction
While orexins are known to be involved in natural rewards such as food intake (Sharf et al., 2010) and sexual behaviors (Di Sebastiano et al., 2011), these neuropeptides have also been implicated in drugseeking behavior (Borgland et al., 2006; Harris et al., 2005). There is anatomical evidence of orexin fibers projecting to the ventral tegmental area and both orexin 1 and 2 receptor mRNA are present in dopaminergic neurons (Korotkova et al., 2003). In addition, orexins have been shown to promote excitation in dopaminergic neurons (Korotkova et al., 2003). However, others report that few orexinergic projections end in synaptic contacts with dopaminergic neurons, suggesting that orexin might be released extrasynaptically (Balcita-Pedicino and Sesack, 2007). Regardless, activation of orexins increases preference for cues indicating drug reward and reinstates extinguished drug seeking behavior (Harris et al., 2005). The OX1R (rather than the OX2R) appears to be more important in regulating drug seeking behavior and self-administration (Prince et al., 2015; Zarepour et al., 2014). Orexins also facilitate reward by attenuating the antireward effects of its cotransmitter dynorphin in the ventral tegmental area (Muschamp et al.,2014). In short, orexins may be important in promoting drug reward seeking behaviors.
Accompanying this role of orexins in mediating drug reward, orexins also play an important role in stress-induced reinstatement of drug seeking behavior. Evidence for this includes that an orexin antagonist prevents foot shock-induced reinstatement (Boutrel et al., 2005). Additionally, several studies highlight the importance of the CRH system interacting with orexins to mediate drug seeking. For example, recent studies have found that the CRH1R and OX1R oligomerize, associating with the cocaine target ol receptor, and modulating dopamine release in the VTA. This provides a mechanism by which orexins are involved in stress-induced cocaine seeking (Navarro et al.,2015). In addition to cocaine seeking, the CRH1R has been shown to mediate orexin activation in morphine reward (Lasheras et al., 2015). Ultimately, orexin and CRH may modulate drug seeking by modulating NMDA receptor function in the VTA (Borgland et al., 2010). Overall, the literature supports a dynamic interaction between orexins and the stress/reward pathways, highlighting how these neuropeptides may contribute to addictions that are highly comorbid with stress-related psychiatric illness.
4.5. Emotions
4.5.1. Anxiety-like behavior
Much of the preclinical literature indicates that orexins promote anxiety-like behaviors. For example, central injections of orexins decrease time spent in the light in the light-dark test and decrease time spent in the open arms in the elevated plus maze (Avolio et al., 2011; Li et al., 2010; Suzuki et al., 2005b). In contrast, OX1R receptor antagonist SB-334867 has been shown to reduce anxiety-like behaviors in mice exposed to cat odors (as measured by a decrease in hiding and increase in approach toward the cat odor) (Vanderhaven et al., 2015). SB-334867 pretreatment was also shown to reduce sodium lactate-induced anxiety-like behavior in mice. Specifically, SB-334867 pretreatment increased social interaction behavior and time spent in the center in an open field test compared to vehicle pretreated groups (Johnson et al., 2010). Thus, the OX1R appears to regulate anxiety-like behaviors in several different behavioral paradigms. Interestingly, the spontaneously hypertensive rat, which is known for its anxiety-like behaviors (as measured by decreased social interaction in the social interaction test, decreased time spent in the center in the open field test, and decreased time spent in the open arms on the elevated plus maze compared to other strains (Ramos et al., 1997)), has increased expression of orexins neurons compared to other rat strains (Clifford et al., 2015). This provides further support for the idea that orexins promote anxiety-like behaviors.
Orexin action during stress induces anxiety-like behavior. For instance, when orexins are stimulated during social defeat stress (by administering CNO, which binds to Gs-coupled DREADDs expressed in orexin neurons), subsequent social interaction time decreases (unpublished). However, stimulation of orexin neurons in the absence of stress was not sufficient to change subsequent social behavior. Thus, perhaps CRH or another stress related peptide must synergize to create this effect. In a separate study, inhibition of orexin action during social defeat (by administering CNO, which binds to Gi-coupled DREADDs expressed in orexin neurons) increased the amount of social interaction time in passively coping rats, with total interaction time at a comparable level to that of actively coping rats (Grafe et al., 2018). Thus, inhibiting orexins throughout social stress decreased the socially relevant anxiety-like behavior. The brain regions in which orexins act during stress to regulate anxiety-like behaviors are not fully elucidated. However, orexins have dense projections to the PVT and BNST (Peyron et al., 1998), and previous studies have demonstrated that orexins act in these brain regions to induce anxiety-like behavior (Heydendael et al., 2013, 2011; Li et al., 2010; Lungwitz et al., 2012). Collectively, the preclinical data indicate that increased orexin signaling contributes to increased measures of anxiety-like behaviors, which is a key phenotype in many stress-related psychiatric disorders.
4.5.2. Depressive-like behavior
Many preclinical studies indicate that depressive-like behavior in rodents is associated with reduced orexin system function. For example, Wistar-Kyoto rats, which demonstrate hormonal and behavioral traits analogous to those observed in depressed patients (Will et al., 2003), express a lower number of orexin neurons (Allard et al., 2004). Moreover, orexin immunoreactivity in the lateral hypothalamus of adult male Nile grass rats (a diurnal equatorial rodent species) was attenuated in a model of seasonal affective disorder using dim light conditions, consisting of 12-hr of light and 12-h dark, but only 50 lx light for the dim lighft condition, rather than a bright light condition of 1000 lx for 4 weeks total (Deats et al., 2014). In socially defeated rats that displayed increased immobility in the forced swim test, orexin system function was also reduced (Lutter et al., 2008). A more recent study found that this social defeat-induced immobility in the forced swim test could be reversed with a central orexin agonist (Chung et al., 2014).
However, other preclinical studies examining depressive-like behaviors demonstrate increased orexin system function. For example, Flinders Sensitive Line rats that are used as a genetic model of depression display increased orexin neurons compared with controls (Mikrouli et al., 2011). Moreover, chronically restrained C57BL/6 mice that show multiple measures of depressive-like behavior (namely, decreased social interaction in the U-field test, decreased sucrose consumption in the sucrose preference test, and increased immobility in both the tail suspension test and forced swim test) had increased orexin mRNA in the amygdala; knocking down orexin expression reversed these behaviors (Kim et al., 2015). Repeated corticosterone injections have also been shown to increase orexin system function and depressive-like behavior. Specifically, corticosterone injections concurrently increase the number of orexin neurons in the hypothalamus and increase immobility in the forced swim test and tail suspension test (Jalewa et al., 2014). Also supportive of the idea that high orexins underlie depressive-behavior, OX1R knockout mice show decreased immobility time in the forced swim test (Abbas et al., 2015). Moreover, pharmacological blockade of the orexin system during unpredictable chronic mild stress reduces subsequent immobility in the tail suspension test (Nollet et al., 2012).
It is likely that orexins are acting in multiple brain regions to regulate depressive-like behaviors. For example, one recent study found that orexin 1 receptors in the amygdala regulate immobility in the forced swim test, a measure of depressive-like behavior (Arendt et al., 2013). Other experiments indicate that orexin interaction with the dorsal raphe may be important in regulating depressive-like behaviors (Brown et al., 2001; Muraki et al., 2004). There are likely many more brain regions of dense orexin innervation that contribute to these depressive behaviors after stress. However, whether these regions are receiving more or less orexin signaling may depend on the duration, intensity, and type of stressor. Moreover, the orexin receptors in these various brain regions may have divergent roles in depressive-like behavior, as OX1R and OX2R knockouts have antidepressant and pro-depressant-like effects (measured by the forced swim test and tail suspension test), respectively (Scott et al., 2011). Overall, preclinical data support a role for orexins in depressive-like behavior, but the exact mechanism by which orexins exert their effects remains unknown. Nonetheless, these neuropeptides are altered in multiple measures of depressive-like behavior after different stress paradigms, which highlights their potential importance in stress-related psychiatric disease.
4.5.3. Fear
Preclinical data indicate that orexins contribute to freezing behavior in fear conditioning paradigms. For example, a study in mice has found that prepro-orexin mRNA is increased after repeated foot shock stress, and the amount of prepro-orexin mRNA is positively correlated with the amount of freezing in response to the shock chamber. Moreover, a dual orexin receptor antagonist decreased freezing behavior in the shock chamber (Chen et al., 2014). These data, together with an additional study showing that orexin neurons are activated upon re-exposure to a footshock chamber (Furlong et al., 2009), indicate that orexins contribute to contextual fear conditioning, and this may be mediated by both orexin receptors. A dual orexin receptor antagonist also reduced fear-potentiated startle, which suggests that both orexin receptors may be involved in cued fear conditioning (Steiner et al., 2012). Delineation of which orexin receptors might be important for particular types of fear conditioning paradigms came first with the help of knockout mice. Specifically, mice lacking the OX1R show impaired freezing responses and reduced neuronal activation in the lateral amygdala in response to both cued and contextual fear conditioning (Soya et al., 2013). Restoring OX1R selectively in the locus coeruleus ameliorated these behavioral and neuronal responses. In contrast, mice lacking OX2R show reduced freezing in a contextual but not cued fear paradigms (Soya et al., 2013). However, a more recent study using specific orexin antagonists has found that the OX1R, and not the OX2R is important in regulating contextual fear (Wang et al., 2017). While much of contextual and cued fear is attributed to the hippocampus and amygdala (Johnson et al., 2012), which express both orexin receptors (Marcus et al., 2001), the PVT (which receives dense orexinergic projections) has also been found to be important regulating the fear response; lesioning the PVT decreases freezing in response to cued fear conditioning (Li et al., 2014). However, another study using a dual orexin receptor antagonist in the PVT had no effect on cued or conditioned fear, thus orexins in the PVT do not appear to contribute to the expression of learned fear (Dong et al., 2015). Collectively, these preclinical data indicate that orexins contribute to the fear response through multiple brain regions, and the type of orexin receptor that mediates these behaviors is dependent upon the type of fear conditioning paradigm used (and the brain regions engaged). While the formation of fear memories after stress exposure is typically an adaptive process that helps one avoid similar situations and improve coping strategies, dysregulation of the fear response occurs in anxiety disorders and phobias (Flores et al., 2015). Orexins appear to regulate fear; thus, they may contribute to some of these stress-related illnesses.
4.6. Pain
Orexin fibers project to many areas of the central nervous system, including regions involved in modulating pain (Peyron et al., 1998). Orexins are thought to be anti-nociceptive, as orexin knockout mice show increased sensitivity to pain and less stress-induced analgesia (Watanabe et al., 2005). Moreover, chronic pain is more common and more disabling in narcolepsy patients (Li et al., 2016). Conversely, peripheral and central administration of orexin A and B show antinociceptive effects in thermal, mechanical, and chemical assays (Bingham et al., 2001; Mobarakeh et al., 2005). It was recently discovered that orexins induce long term depression in the spinal cord dorsal horn, which might explain the mechanism by which these neuropeptides exert their analgesic effects (Park and Weon, 2017).
There has been increasing evidence supporting the link between orexin function and headaches. Studies initially identified a polymorphism in the hypocretin receptor 2 gene that is associated with headache (Rainero et al., 2004). Additional experiments have found reduced orexin levels in cerebrospinal fluid in cluster headache (Bartsch et al., 2004). A recent study indicated that retinal ganglion cells project, in part, to orexin neurons in the lateral hypothalamus, which then project to nuclei in the brainstem and spinal cord, strengthening the link between bright light and migraines (Noseda et al., 2017). Intranasal administration of orexin A is a potential treatment for headaches, and is currently used for narcolepsy with cataplexy (Weinhold et al., 2014).
Though chronic stress generally exacerbates pain, acute stress may suppress pain (Ahmad and Zakaria, 2015). While there is not much research on the role of orexins in chronic stress-induced hyperalgesia, recent work indicates that orexins contribute to some aspects of stress-induced analgesia. For example, OX1R induces endocannabinoid signaling in the periaqueductal gray, which can increase hot plate latency in mice; this was prevented with treatment of OX1R antagonist SB334867 (Lee et al., 2016). Thus, orexin activation may reduce pain in response to acute stress. More research is needed to better understand the role of orexins in chronic stress-induced pain. Regardless, it is clear that orexins modulate pain, including headaches, which are comorbid in stress-related psychiatric disorders (Antonaci et al., 2011; Felice et al., 2015). Thus, these neuropeptides may be an important target for this key phenotype in stress-related illness.
4.7. Summary
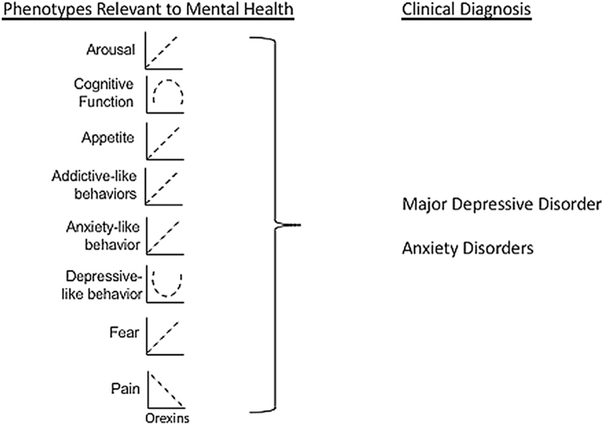
Preclinical data support the role for orexins in stress-induced changes in sleep, cognition, appetite, addiction, emotion and pain. Specifically, orexins mediate stress-induced changes in arousal, impairing both sleep and cognition. Additionally, orexins are important in stress-induced increases in both natural reward and drug seeking behaviors. Orexins have also been shown to play a role in stress-induced anxiety-like behaviors, depressive-like behaviors, and fear responses. Lastly, orexins are important in modulating pain, including headaches. As disruptions in all of these behaviors are important phenotypes in stress-related psychiatric illness, orexins may be a potential target for treatment of these disorders. For a summary of orexin action in phenotypes relevant to mental health, please see Fig. 2.
Fig. 2. Orexin action in phenotypes relevant to mental health.
This illustration depicts orexins actions in phenotypes relevant to mental health, as discussed in the preclinical portion of this review. Activity of orexin cells or orexin signaling is depicted on the x axis is labeled (increasing from left to right) and the expression of each behavioral phenotype is depicted on the y axis (increasing from bottom to top). Most behavioral phenotypes are more pronounced with higher orexin action (eg higher arousal, appetite, addictive-like behaviors, anxiety-like behaviors, and fear), whereas some behavioral phenotypes have a curvilinear relationship with orexin action (cognitive function, depressive-like behavior), and one behavioral phenotype shows a negative relationship with orexin action (pain). These relationships are all based on evidence from current literature, but it is possible that further experiments could change the way these relationships are portrayed. For example, perhaps some of these relationships are not linear, and if tested when orexins were at much lower or higher levels, would demonstrate curvilinear relationships. These differing relationships between orexin action and phenotypes relevant to mental health may be reflective of differential orexin receptor expression/function and/or brain circuitry involved. All of these phenotypes can be observed in humans and would then contribute to a clinical diagnosis of stress-related mental illnesses such as Major Depressive Disorder and Anxiety Disorders.
5. Orexins and stress-related psychiatric illness
Altered orexin system function has been observed in many different stress-related psychiatric disorders. Below, we discuss what is known about the role of orexins in specific stress-related illnesses such as Major Depressive Disorder and Anxiety Disorders.
5.1. Major depressive disorder
The link between the orexinergic system and depression remains equivocal, as clinical models report conflicting results. Specifically, different studies measuring orexin A in both the cerebrospinal fluid and plasma indicate that either hypoactivity (Brundin et al., 2007) or hyperactivity (Salomon et al., 2003; von der Goltz et al., 2011) of the orexinergic system is associated with Major Depressive Disorder. Orexin mRNA levels in peripheral blood cells have been negatively correlated with scores on the Hamilton rating scale for depression, suggesting that orexin expression may be associated with severity of depression (Rotter et al., 2011). Interestingly, reduced diurnal variation of orexin levels in the cerebrospinal fluid has been reported in MDD (Salomon et al., 2003), which might explain disruptions in sleep-wake patterns in these patients. Remarkably, these levels could be restored with antidepressant treatment. In psychologically normal, epileptic patients, microdialysis probes implanted in the amygdala revealed that higher orexin levels were observed during stronger emotions but lower orexin levels were present during flat affect, demonstrating that orexin activity is important in regulating emotions and not just arousal related symptoms in mood disorders (Blouin et al., 2013). The OX1R gene has also recently been implicated in Major Depressive Disorder, as a polymorphism in this receptor is more common in MDD patients compared with controls (Rainero et al., 2011). While it is hypothesized that this polymorphism alters orexin receptor function, its exact function remains unknown. Thus, it is clear that orexin function is altered in Major Depressive Disorder, but it is unclear if these neuropeptides are important in the development of this illness.
5.2. Anxiety disorders
On the whole, the clinical literature suggests that orexins are anxiogenic. For example, orexin levels in the cerebrospinal fluid are increased in patients with Panic Disorder (Johnson et al., 2010), which is an anxiety disorder characterized by unexpected attacks of heightened arousal, somatic symptoms, and fear (Hasan and Mooney, 1986). Remarkably, silencing orexins with RNA interference or an orexin 1 receptor antagonist blocked panic responses in an animal model of panic disorder (Johnson et al., 2010). Thus, hyperactivity of the orexin system appears to contribute to panic disorder, and orexin 1 receptor antagonists may constitute a novel treatment for panic disorder. A link between the orexin 2 receptor and panic disorder has also been identified; namely, the Iso allele of the Val380Iso polymorphism in the orexin 2 receptor is more frequent in panic patients than in controls (Annerbrink et al., 2011). The functional significance of this polymorphism is unknown, but has been suggested to hinder the dimerization of the receptor (as either a homodimer or a heterodimer with the orexin 1 receptor) (Rainero et al., 2008).
In a clinical study using the anti-panic drug sertraline (a selective serotonin reuptake inhibitor which specifically blocks the SERT transporter), orexin levels in the cerebrospinal fluid were reduced, suggesting that orexins are anxiogenic (Salomon et al., 2003). Further supporting this notion, the drug buproprion, which has lower efficacy in treating panic disorder (but is better for treating depression), does not decrease orexin levels in the cerebrospinal fluid (Salomon et al., 2003). Thus, reducing orexin function could be an effective treatment of panic disorder. However, it should be noted that this study is correlational in nature, and thus, does not provide concrete evidence that orexins are anxiogenic.
In contradiction to this prior evidence, many narcoleptic patients with impaired orexin system function display panic anxiety. However, this does not preclude orexins from playing an important role in panic anxiety. As with most phenotypes, panic anxiety is likely regulated by a variety of neurotransmitters, including norepinephrine, which may compensate if other arousal peptides like orexins are low (as they are in narcolepsy). In sum, orexins appear to play a convincing role in panic anxiety, and may be a good target for treatment.
5.3. Summary
Inconsistencies in reports of hypoactivity versus hyperactivity of the orexin system in stress-related psychiatric disorders may result from the heterogeneity of these disorders as well as limitations in measuring orexin levels and baseline state of orexin neuron activity or orexin concentrations. Both genetic and environmental factors contribute to the heterogeneity of these disorders (Gregory et al., 2008; Koenen et al., 2008; Pigoni et al., 2017; Poulton et al., 2008). As previously mentioned, various polymorphisms have been noted in orexin system function in these disorders. These polymorphisms have all recently been found, and there may be many more to discover. Moreover, these polymorphisms will likely not be uniform in all patients for a given mental illness. In addition, environmental exposure to different types, intensities, and durations of stressors may affect orexin system function, as we saw in preclinical models. Each person will encounter unique stressors that may increase the risk of developing these psychiatric illnesses. Lastly, whether measures of orexin in plasma or cerebrospinal fluid are physiologically meaningful and can act as proxy for central orexin system activity remains to be established. Orexin levels in plasma are close to the resolving limit of radioimmunoassay, thus comparing plasma levels of orexin in control versus depressed patients is likely not a reliable measure (Chen et al., 2015). Going forward, it would be ideal to determine more reliable measures of orexin function in clinical studies so that we may discern the contribution of this system in psychiatric illness. For a summary of orexin measures in stress-related mental illness, please see Table 2.
Table 2. Summary of orexin measures in stress-related mental illness.
Summary of the literature reporting orexin measures in clinically diagnosed stress-related mental illnesses such as Major Depressive Disorder, Panic Disorder, and Schizophrenia. Details of the type of orexins measured, whether increased or decreased activity was associated with each mental illness, and citations are noted. Csf: cerebrospinal fluid.
| Clinical diagnosis | Orexin measures | Orexin activity | Citations |
|---|---|---|---|
| Major depressive disorder | Orexin concentrations in csf, orexin mRNA in blood cells | Hypoactivity in Depression | Brundin et al., 2007; Rotter et al., 2011 |
| Orexin concentrations in csf, plasma orexin concentrations | Hypoactivity in Depression | Salomon et al., 2003; von der Goltz et al., 2011 | |
| Orexin 1 Receptor Polymorphism | More common in MDD | von der Goltz et al., 2011 | |
| Panic disorder | Orexin concentrations in csf | Hyperactivity in Panic Disorder | Johnson et al., 2010 |
| Orexin 2 Receptor Polymorphism | More common in Panic Disorder | Annerbrink et al., 2011 |
6. Sex differences in orexins
Women are twice as likely as men to suffer from stress-related psychiatric disorders, such as post-traumatic stress disorder and depression, however, the biological basis of these sex differences is unknown (Keane et al., 2006; Kessler et al., 1994; Nestler et al., 2002; Seeman, 1997; Sheikh et al., 2002). As previously mentioned, orexins regulate the stress response and are altered in anxious and depressed patients (Brundin et al., 2007; Johnson et al., 2010; Strawn et al., 2010). Thus, it is possible that orexins may contribute to these sex differences in stress-related psychiatric disorders.
Most studies examining orexin function, whether preclinical or clinical, have been performed in males. While sex differences in orexin precursor prepro-orexin mRNA have been previously reported (Johren et al., 2002), neither the mechanisms underlying this sex difference nor the functional consequences of this disparity were understood. Our lab conducted a detailed examination of sex differences in the HPA response to repeated stress and in subsequent cognitive flexibility using the Attentional Set Shifting Test (AST) (Grafe et al., 2017a). Using inhibitory DREADDs specifically targeted to orexin neurons, we determined the role of orexins in mediating the effects of stress. We determined that elevated orexins in female rats are responsible for heightened HPA responses to repeated stress and stress-induced cognitive deficits in the side reversal task of AST (Grafe et al., 2017a). This suggests a novel role for orexins in mediating sex differences in functions that are altered in stress-related psychiatric disorders. Our data also suggest that glucocorticoid receptors act directly on the orexin promoter to increase prepro-orexin expression in females, providing a novel regulatory mechanism for the control of orexin system activity. Another study reports that estrogens increase expression of both orexins and their receptors, but the exact mechanism by which this occurs is unknown (Silveyra et al., 2010).
Other recent preclinical studies have also noted sex differences in orexin function. Specifically, female rats have recently been reported to have a higher sensitivity to metabolic cues than males through orexin neuron activation (Fukushima et al., 2015). Moreover, at a particular pharmacological dose, blocking orexin 1 receptor disrupts addictive-like behaviors for sucrose and cocaine in males but not females (Cason and Aston-Jones, 2014; Zhou et al., 2012). These studies provide support for the idea that orexin function is increased in female rodents. Specifically, fasting caused more orexin neurons to be active in females than males (Fukushima et al., 2015) and a higher dose of the orexin antagonist may have been required to reduce orexin activity in female rats compared with male rats to inhibit addictive behavior (Cason and Aston-Jones, 2014; Zhou et al., 2012). On the other hand, if high levels of orexin mediate these addictive behaviors in females, perhaps one might expect that even a low dose of orexin antagonist would produce an observable effect. Regardless, food and drug seeking behavior engages the orexin system differentially in males and females. Further support for increased orexin function in females comes from a recent study in which OX2R expression in the PVN was found to be higher in female rats compared with male rats (Loewen et al., 2017). Increased OX2R expression might suggest increased orexin action at the apex of the HPA response; this might explain, in part, how orexins exacerbate the response to stress in female rats. Lastly, a model of chronic unpredictable mild stress in both sexes found that there was a significant positive correlation between CRH mRNA and prepro-orexin mRNA and a significant increase in OX1R in female but not male rats (Lu et al., 2017). Together, these data strengthen support for sexually dimorphic orexin system changes, namely, that female rodents demonstrate higher orexin system activity compared with male rodents.
Sex differences in orexin action have recently been reported in the clinical literature as well. For example, orexin immunoreactivity in the anterior cingulate cortex and dorsolateral prefrontal cortex was reported to be increased in female patients with MDD compared with male patients with MDD (Lu et al., 2017). This may indicate that orexins are important in the etiology of depression, as it is more common in females. Moreover, another study examining control and Alzheimer’s patients found that females had higher orexin levels in the cerebrospinal fluid than males, regardless of the diagnosis (Schmidt et al., 2013). Interestingly, narcolepsy, due to hypofunction of orexins, is more common in men, whereas insomnia, possibly due to overactive orexin action, is more common in women (Mallampalli and Carter, 2014; Nakamura et al., 2011; Prober et al., 2006). Orexin antagonists are currently used to treat insomnia, thus, sex differences in orexin activity could signify a future need to develop sex-specific guidelines in dosing (as exists with zolpidem, a GABA receptor agonist (Farkas et al., 2013; Mallampalli and Carter, 2014)). As orexins regulate the stress response, cognitive function, sleep, food intake, autonomic responses, and emotional memory (Sakurai, 2014), these treatments may ameliorate many phenotypes of stress-related illness in a sex-specific way.
7. Conclusions
The orexin system is critical for our response to stress, and modulates many behaviors relevant to mental health. In general, orexins promote arousal, which is a key component underlying the response to stressful stimuli. Thus, orexins are important in inducing the response to stress. Moreover, conditions of stress reciprocally promote high orexin activity, which then leads to ongoing increased wakefulness, disrupted cognition, changes in appetite, addictive behaviors, dysregulated emotions, and heightened sensitivity to pain, which are all key phenotypes in stress-related psychiatric disorders. Importantly, there is increased evidence of individual differences in the role of orexins in responses to repeated stress. Current evidence suggests that if an individual habituates to repeated stress, such as to repeated restraint and to some extent repeated social defeat, then low orexin expression is associated with resilience. However, exposure to intense stressors such as repeated footshock or swim, stimuli to which habituation does not occur because it is not adaptive, lower orexin expression seems to be associated with vulnerability. Thus, the physiological significance of low orexin expression in an individual depends on the environmental demands, both intrinsic and extrinsic, imposed on that individual. A fuller understanding of the role of orexins in such individual differences to repeated stress requires repeated assessment of expression of orexins and their receptors over time. Furthermore, there may be orexin expressing cells outside of the lateral hypothalamus that are extremely low expressing basally, but which may increase their expression in case of physiological demand. There are likely orexin-expressing cells in the periphery that have yet to be fully characterized but would explain the robust expression of orexin receptors in some peripheral tissues.
Orexin neurons excite many neural systems, and dysfunction of the orexin system may contribute to stress-related psychiatric disorders. Indeed, dysfunction of the orexin system has been reported in stress-related illnesses such as Major Depressive Disorder and Anxiety Disorders. Given the current use of orexin receptor antagonists for treatment of insomnia (Winrow and Renger, 2014), and the wide ranging effects of orexins, it is important to delineate specific circuits, behaviors, and phenotypes affected by each orexin receptor in different contexts of stress. Moreover, recent work has found that orexins are novel regulators of sex differences in behavioral and neuroendocrine adaptations to repeated stress and in the cognitive consequences of repeated stress. Thus, orexins could be important in the etiology of those stress-related psychiatric disorders that present differently in men and women.
Acknowledgments
Funding
The authors were supported by grants from NIMH to SB (R01MH109975) and to LG (F32MH109269).
References
- Abbas MG, Shoji H, Soya S, Hondo M, Miyakawa T, Sakurai T, 2015. Comprehensive behavioral analysis of male Ox1r −/−mice showed implication of orexin receptor-1 in mood, anxiety, and social behavior. Front. Behav. Neurosci 9, 324 10.3389/fnbeh.2015.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L, 2007. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424. 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad AH, Zakaria R, 2015. Pain in times of stress. Malays. J. Med. Sci 22, 52–61. [PMC free article] [PubMed] [Google Scholar]
- Akbari E, Naghdi N, Motamedi F, 2006. Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav. Brain Res 173, 47–52. http://dx.doi.Org/10.1016/j.bbr.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Al-Barazanji KA, Wilson S, Baker J, Jessop DS, Harbuz MS, 2001. Central orexin-A activates hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats. J. Neuroendocrinol 13, 421–424. [DOI] [PubMed] [Google Scholar]
- Allard JS, Tizabi Y, Shaffery JP, Ovid Trouth C, Manaye K, 2004. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides 38, 311–315. http://dx.doi.Org/10.1016/j.npep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Annerbrink K, Westberg L, Olsson M, Andersch S, Sjodin I, Holm G, Allgulander c., Eriksson E, 2011. Panic disorder is associated with the Val308Iso polymorphism in the hypocretin receptor gene. Psychiatr. Genet 21, 85–89. 10.1097/YPG.0b013e328341a3db. [DOI] [PubMed] [Google Scholar]
- Antonaci F, Nappi G, Galli F, Manzoni GC, Calabresi P, Costa A, 2011. Migraine and psychiatric comorbidity: a review of clinical findings. J. Headache Pain 12, 115–125. 10.1007/s10194-010-0282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt DH, Ronan PJ, Oliver KD, Callahan LB, Summers TR, Summers CH, 2013. Depressive behavior and activation of the orexin/hypocretin system. Behav. Neurosci 127, 86–94. 10.1037/a0031442. [DOI] [PubMed] [Google Scholar]
- Avolio E, Alò R, Carelli A, Canonaco M, 2011. Amygdalar orexinergic-GABAergic interactions regulate anxiety behaviors of the Syrian golden hamster. Behav. Brain Res 218, 288–295. http://dx.doi.Org/10.1016/j.bbr.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Sesack SR, 2007. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and γ-aminobutyric acid neurons. J. Comp. Neurol 503, 668–684. 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- Baranowska B, Baranowska-Bik A, Bik W, Martynska L, 2008. The role of leptin and orexins in the dysfunction of hypothalamo-pituitary-gonadal regulation and in the mechanism of hyperactivity in patients with anorexia nervosa. Neuro Endocrinol. Lett 29, 37–40. [PubMed] [Google Scholar]
- Bartsch T, Levy MJ, Knight YE, Goadsby PJ, 2004. Differential modulation of nociceptive dural input to [hypocretin] orexin A and B receptor activation in the posterior hypothalamic area. Pain 109, 367–378. http://dx.doi.Org/10.1016/j.pain.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Beig MI, Dampney BW, Carrive P, 2015. Both Ox1r and Ox2r orexin receptors contribute to the cardiovascular and locomotor components of the novelty stress response in the rat. Neuropharmacology 89, 146–156. http://dx.doi.Org/10.1016/j.neuropharm.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Benca RM, 1996. Sleep in psychiatric disorders. Neurol. Clin 14, 739–764. [DOI] [PubMed] [Google Scholar]
- Bernard R, Lydic R, Baghdoyan HA, 2002. Hypocretin-1 activates G proteins in arousal-related brainstem nuclei of rat. Neuroreport 13, 447–450. [DOI] [PubMed] [Google Scholar]
- Berridge CW, España RA, 2005. Hypocretins: waking, arousal, or action? Neuron 46, 696–698. 10.1016/j.neuron.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Berridge CW, España RA, Vittoz NM, 2010. Hypocretin/orexin in arousal and stress. Brain Res. 1314, 91–102. 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham S, Davey PT, Babbs AJ, Irving EA, Sammons MJ, Wyles M, Jeffrey P, Cutler L, Riba I, Johns A, Porter RA, Upton N, Hunter AJ, Parsons AA, 2001. Orexin-A, an hypothalamic peptide with analgesic properties. Pain 92, 81–90. [DOI] [PubMed] [Google Scholar]
- Black JB, Premont RT, Daaka Y, 2016. Feedback regulation of G protein-coupled receptor signaling by GRKs and arrestins. Semin. Cell Dev. Biol 50, 95–104. 10.1016/j.semcdb.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell JE, Alammar HA, Weighall AR, Kellar I, Nash HM, 2017. A systematic review of cognitive function and psychosocial well-being in school-age children with narcolepsy. Sleep Med. Rev 34, 82–93. 10.1016/j.smrv.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Blouin AM, Fried I, Wilson CL, Staba RJ, Behnke EJ, Lam HA, Maidment NT, Karlsson K.ñ., Lapierre JL, Siegel JM, 2013. Human hypocretin and melaninconcentrating hormone levels are linked to emotion and social interaction. Nat. Commun 4, 1547 10.1038/ncomms2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma GJ, Liang N-C, Lee RS, Albertz JD, Kastelein A, Moody LA, Aryal S, Moran TH, Tamashiro KL, 2016. Failure to upregulate Agrp and Orexin in response to activity based anorexia in weight loss vulnerable rats characterized by passive stress coping and prenatal stress experience. Psychoneuroendocrinology 67, 171–181. 10.1016/j.psyneuen.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Dugovic C, Shireman B, Preville C, Yun S, Lord B, Nepomuceno D, Wennerholm M, Lovenberg T, Carruthers N, Fitz SD, Shekhar A, Johnson PL, 2017. Evaluation of JNJ-54717793 a novel brain penetrant selective orexin 1 receptor antagonist in two rat models of panic attack provocation. Front. Pharmacol 8, 357 10.3389/fphar.2017.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Yun S, Johnson PL, Shekhar A, Fitz SD, Shireman BT, Lebold TP, Nepomuceno D, Lord B, Wennerholm M, Shelton J, Carruthers N, Lovenberg T, Dugovic C, 2015. A Selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J. Pharmacol. Exp. Ther 352, 590–601. 10.1124/jpet.114.220392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnavion P, Jackson AC, Carter ME, de Lecea L, 2015. Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat. Commun 6, 6266 10.1038/ncomms7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A, 2006. Orexin A in the VTA Is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49, 589–601. 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Ungless MA, Bonci A, 2010. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: emerging players in addiction. Brain Res. 1314, 139–144. 10.1016/j.brainres.2009.10.068. [DOI] [PubMed] [Google Scholar]
- Boschen KE, Fadel JR, Burk JA, 2009. Systemic and intrabasalis administration of the orexin-1 receptor antagonist, SB-334867, disrupts attentional performance in rats. Psychopharmacology 206, 205–213. 10.1007/s00213-009-1596-2. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L, 2005. Role for hypocretin in mediating stress-induced reinstatement of cocaineseeking behavior. Proc. Natl. Acad. Sci 102, 19168–19173. 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Sergeeva O, Eriksson KS, Haas HL, 2001. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology 40, 457–459. [DOI] [PubMed] [Google Scholar]
- Brundin L, Björkqvist M, Petersén A, Träskman-Bendz L, 2007. Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur. Neuropsychopharmacol 17, 573–579. 10.1016/j.euroneuro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Burgess CR, Oishi Y, Mochizuki T, Peever JH, Scammell TE, 2013. Amygdala lesions reduce cataplexy in orexin knock-out mice. J. Neurosci 33, 9734–9742. 10.1523/JNEUROSCI.5632-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, Tadayyon M, Clapham JC, Wilding J, Williams G, 1999. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes 48, 2132–2137. [DOI] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G, 2014. Role of orexin/hypocretin in conditioned sucroseseeking in female rats. Neuropharmacology 86, 97–102. 10.1016/j.neuropharm.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Saito T, Ohiwa N, Tateoka M, Deocaris CC, Fujikawa T, Soya H, 2007. Inhibitory effects of an orexin-2 receptor antagonist on orexin A- and stress-induced ACTH responses in conscious rats. Neurosci. Res 57, 462–466. 10.1016/j.neures.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M, 1999. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451. [DOI] [PubMed] [Google Scholar]
- Chen Q, de Lecea L, Hu Z, Gao D, 2015. The hypocretin/orexin system: an increasingly important role in neuropsychiatry. Med. Res. Rev 35, 152–197. 10.1002/med.21326. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang H, Lin Z, Li S, Li Y, Bergen HT, Vrontakis ME, Kirouac GJ, 2014. Orexins (hypocretins) contribute to fear and avoidance in rats exposed to a single episode of footshocks. Brain Struct. Funct 219, 2103–2118. 10.1007/s00429-013-0626-3. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang H, Lin Z, Li S, Li Y, Bergen HT, Vrontakis ME, Kirouac GJ, 2013. Orexins (hypocretins) contribute to fear and avoidance in rats exposed to a single episode of footshocks. Brain Struct. Funct 10.1007/s00429-013-0626-3. [DOI] [PubMed] [Google Scholar]
- Cheng S-B, Kuchiiwa S, Gao H-Z, Kuchiiwa T, Nakagawa S, 2003. Morphological study of orexin neurons in the hypothalamus of the Long-Evans rat, with special reference to co-expression of orexin and NADPH-diaphorase or nitric oxide synthase activities. Neurosci. Res 46, 53–62. [DOI] [PubMed] [Google Scholar]
- Chiefifi S, Carotenuto M, Monda V, Valenzano A, Villano I, Precenzano F, Tafuri D, Salerno M, Filippi N, Nuccio F, Ruberto M, De Luca V, Cipolloni L, Cibelli G, Mollica MP, Iacono D, Nigro E, Monda M, Messina G, Messina A, 2017. Orexin system: the key for a healthy life. Front. Physiol 8, 357 10.3389/fphys.2017.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE, 2001. Orexin (hypocretin) neurons contain dynorphin. J. Neurosci 21, RC168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H-S, Kim J-G, Kim J-W, Kim H-W, Yoon B-J, 2014. Orexin administration to mice that underwent chronic stress produces bimodal effects on emotion-related behaviors. Regul. Pept 194–195, 16–22. 10.1016/j.regpep.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Clifford L, Dampney BW, Carrive P, 2015. Spontaneously hypertensive rats have more orexin neurons in their medial hypothalamus than normotensive rats. Exp. Physiol 100, 388–398. 10.1113/expphysiol.2014.084137. [DOI] [PubMed] [Google Scholar]
- Dahmen N, Bierbrauer J, Kasten M, 2001. Increased prevalence of obesity in narcoleptic patients and relatives. Eur. Arch. Psychiatry Clin. Neurosci 251, 85–89. [DOI] [PubMed] [Google Scholar]
- Dalal MA, Schuld A, Haack M, Uhr M, Geisler P, Eisensehr I, Noachtar S, Pollmächer T, 2001. Normal plasma levels of orexin A (hypocretin-1) in narcoleptic patients. Neurology 56, 1749–1751. [DOI] [PubMed] [Google Scholar]
- Date Y, Mondal MS, Matsukura S, Ueta Y, Yamashita H, Kaiya H, Kangawa K, Nakazato M, 2000. Distribution of orexin/hypocretin in the rat median eminence and pituitary. Brain Res. Mol. Brain Res 76, 1–6. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M, 1999. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc. Natl. Acad. Sci. U. S. A 96, 748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino L, Siegel JM, Hampson RE, 2007. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J. Neurosci 27, 14239–14247. 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG, 1998. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A 95, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira CVR, Rosas-Arellano MP, Solano-Flores LP, Ciriello J, 2003. Cardiovascular effects of hypocretin-1 in nucleus of the solitary tract. Am. J. Physiol. - Hear. Circ. Physiol 284, H1369–H1377. 10.1152/ajpheart.00877.2002. [DOI] [PubMed] [Google Scholar]
- Deats SP, Adidharma W, Lonstein JS, Yan L, 2014. Attenuated orexinergic signaling underlies depression-like responses induced by daytime light deficiency. Neuroscience 272, 252–260. 10.1016/j.neuroscience.2014.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sebastiano AR, Wilson-Pérez HE, Lehman MN, Coolen LM, 2011. Lesions of orexin neurons block conditioned place preference for sexual behavior in male rats. Horm. Behav 59, 1–8. 10.1016/j.yhbeh.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Dietrich H, Jenck F, 2010. Intact learning and memory in rats following treatment with the dual orexin receptor antagonist almorexant. Psychopharmacology 212, 145–154. 10.1007/s00213-010-1933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Li Y, Kirouac GJ, 2015. Blocking of orexin receptors in the paraventricular nucleus of the thalamus has no effect on the expression of conditioned fear in rats. Front. Behav. Neurosci 9, 161 10.3389/fnbeh.2015.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube MG, Kalra SP, Kalra PS, 1999. Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res. 842, 473–477. [DOI] [PubMed] [Google Scholar]
- Duxon MS, Stretton J, Starr K, Jones DN, Holland V, Riley G, Jerman J, Brough S, Smart D, Johns A, Chan W, Porter RA, Upton N, 2001. Evidence that orexin-A-evoked grooming in the rat is mediated by orexin-1 (0X1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology 153, 203–209. [DOI] [PubMed] [Google Scholar]
- Farkas RH, Unger EF, Temple R, 2013. Zolpidem and driving impairment-identifying persons at risk. N. Engl. J. Med 369, 689–691. 10.1056/NEJMp1307972. [DOI] [PubMed] [Google Scholar]
- Felice VD, Moloney RD, Cryan JF, Dinan TG, O’Mahony SM, 2015. Visceral pain and psychiatric disorders. Mod. Trends Pharmacopsychiatry 2, 103–119. 10.1159/000435936. [DOI] [PubMed] [Google Scholar]
- Finnell JE, Lombard CM, Melson MN, Singh NP, Nagarkatti M, Nagarkatti P, Fadel JR, Wood CS, Wood SK, 2017. The protective effects of resveratrol on social stress-induced cytokine release and depressive-like behavior. Brain. Behav. Immun 59, 147–157. 10.1016/j.bbi.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores Á, Saravia R, Maldonado R, Berrendero F, 2015. Orexins and fear: implications for the treatment of anxiety disorders. Trends Neurosci. 38, 550–559. 10.1016/j.tins.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Fukushima A, Hagiwara H, Fujioka H, Kimura F, Akema T, Funabashi T, 2015. Sex differences in feeding behavior in rats: the relationship with neuronal activation in the hypothalamus. Front. Neurosci 9, 88 10.3389/fnins.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, Yanagisawa M, 2009. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 9, 64–76. 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong TM, Vianna DML, Liu L, Carrive P, 2009. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur. J. Neurosci 30, 1603–1614. 10.1111/j.1460-9568.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE, 1971. Neuron activity related to short-term memory. Science 173, 652–654. [DOI] [PubMed] [Google Scholar]
- González JA, Iordanidou P, Strom M, Adamantidis A, Burdakov D, 2016. Awake dynamics and brain-wide direct inputs of hypothalamic MCH and orexin networks. Nat. Commun 7, 11395 10.1038/ncomms11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter AL, Roecker AJ, Hargreaves R, Coleman PJ, Winrow CJ, Renger JJ, 2012a. Orexin receptors as therapeutic drug targets. Prog. Brain Res 198, 163–188. 10.1016/B978-0-444-59489-1.00010-0. [DOI] [PubMed] [Google Scholar]
- Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ, 2012b. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol. Rev 64, 389–420. 10.1124/pr.111.005546. [DOI] [PubMed] [Google Scholar]
- Grafe LA, Cornfeld A, Luz S, Valentino R, Bhatnagar S, 2017a. Orexins mediate sex differences in the stress response and in cognitive flexibility. Biol. Psychiatry 81, 683–692. 10.1016/j.biopsych.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Eacret D, Dobkin J, Bhatnagar S, 2018. Reduced orexin system function contributes to resilience to repeated social stress. eneuro 5, ENEURO.0273–17.2018. 10.1523/ENEURO.0273-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Eacret D, Luz S, Gotter AL, Renger JJ, Winrow CJ, Bhatnagar S, 2017b. Orexin 2 receptor regulation of the hypothalamic-pituitary-adrenal (HPA) response to acute and repeated stress. Neuroscience 348, 313–323. 10.1016/j.neuroscience.2017.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, Lau JYF, Eley TC, 2008. Finding gene-environment interactions for phobias. Eur. Arch. Psychiatry Clin. Neurosci 258, 76–81. 10.1007/S00406-007-0786-3. [DOI] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S, 2009. Habituation to repeated stress: get used to it. Neurobiol. Learn. Mem 92, 215–224. 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, Kerr W, Bhatnagar S, 2008. Struggling behavior during restraint is regulated by stress experience. Behav. Brain Res 191, 219–226. 10.1016/j.bbr.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N, 1999. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Natl. Acad. Sci. U. S. A 96, 10911–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G, 2006. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 29, 571–577. 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G, 2005. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437, 556–559. 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hasan MK, Mooney RP, 1986. Panic disorder: a review. Compr. Ther 12, 3–7. [PubMed] [Google Scholar]
- Hasegawa E, Yanagisawa M, Sakurai T, Mieda M, 2014. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J. Clin. Invest 124, 604–616. 10.1172/JCI71017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR, 2000. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul. Pept 96, 45–51. [DOI] [PubMed] [Google Scholar]
- Henny P, Brischoux F, Mainville L, Stroh T, Jones BE, 2010. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience 169, 1150–1157. 10.1016/j.neuroscience.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydendael W, Sengupta A, Beck S, Bhatnagar S, 2013. Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior. Physiol. Behav 10.1016/j.physbeh.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydendael W, Sengupta A, Bhatnagar S, 2012. Putative genes mediating the effects of orexins in the posterior paraventricular thalamus on neuroendocrine and behavioral adaptations to repeated stress. Brain Res. Bull 89, 203–210. 10.1016/j.brainresbull.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydendael W, Sharma K, Iyer V, Luz S, Piel D, Beck S, Bhatnagar S, 2011. Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology 152, 4738–4752. 10.1210/en.2011-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist T, Johansson L, Östman M, Ammoun S, Åkerman KEO, Kukkonen JP, 2005. OX1 orexin receptors couple to adenylyl cyclase regulation via multiple mechanisms. J. Biol. Chem 280, 6570–6579. 10.1074/jbc.M407397200. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Gao X-B, 2005. Input organization and plasticity of hypocretin neurons: possible clues to obesity’s association with insomnia. Cell Metab. 1, 279–286. 10.1016/j.cmet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van Den Pol AN, 1999. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J. Comp. Neurol 415, 145–159. [PubMed] [Google Scholar]
- Hsu DT, Kirouac GJ, Zubieta J-K, Bhatnagar S, 2014. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front. Behav. Neurosci 8, 73 10.3389/fnbeh.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N, 2000. Possible involvement of orexin in the stress reaction in rats. Biochem. Biophys. Res. Commun 270, 318–323. 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- Jalewa J, Wong-Lin K, McGinnity TM, Prasad G, Hölscher C, 2014. Increased number of orexin/hypocretin neurons with high and prolonged external stress-induced depression. Behav. Brain Res 272, 196–204. 10.1016/j.bbr.2014.05.030. [DOI] [PubMed] [Google Scholar]
- Jászberényi M, Bujdosó E, Pataki I, Telegdy G, 2000. Effects of orexins on the hypothalamic-pituitary-adrenal system. J. Neuroendocrinol 12, 1174–1178. [DOI] [PubMed] [Google Scholar]
- Jöhnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A, 2012. Orexin, stress, and anxiety/panic states. Prog. Brain Res 198, 133–161. 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A, 2010. A key role for orexin in panic anxiety. Nat. Med 16, 111–115. 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johren O, Neidert SJ, Rummer M, Dominiak P, 2002. Sexually dimorphic expression of prepro-orexin mRNA in the rat hypothalamus. Peptides 23,1177–1180. 10.1016/S0196-9781(02)00052-9. [DOI] [PubMed] [Google Scholar]
- Jorge RE, 2015. Posttraumatic stress disorder. Contin. Lifelong Learn. Neurol 21, 789–805. 10.1212/01.CON.0000466667.20403.b1. [DOI] [PubMed] [Google Scholar]
- Karteris E, Machado RJ, Chen J, Zervou S, Hillhouse EW, Randeva HS, 2005. Food deprivation differentially modulates orexin receptor expression and signaling in rat hypothalamus and adrenal cortex. AJP Endocrinol. Metab 288, E1089–E1100. 10.1152/ajpendo.00351.2004. [DOI] [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T, 2003. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 285, R581–R593. 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- Keane TM, Marshall AD, Taft CT, 2006. Posttraumatic stress disorder: etiology, epidemiology, and treatment outcome. Annu. Rev. Clin. Psychol 2,161–197. 10.1146/annurev.clinpsy.2.022305.095305. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG, 1994. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J. Affect. Disord 30, 15–26. [DOI] [PubMed] [Google Scholar]
- Kim T-K, Kim J-E, Park J-Y, Lee J-E, Choi J, Kim H, Lee E-H, Kim S-W, Lee J-K, Kang H-S, Han P-L, 2015. Antidepressant effects of exercise are produced via suppression of hypocretin/orexin and melanin-concentrating hormone in the basolateral amygdala. Neurobiol. Dis 79, 59–69. 10.1016/j.nbd.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S, 2005. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 1059, 179–188. 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Nugent NR, Amstadter AB, 2008. Gene-environment interaction in posttraumatic stress disorder. Eur. Arch. Psychiatry Clin. Neurosci 258, 82–96. 10.1007/s00406-007-0787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok SW, Roelfsema F, Overeem S, Lammers GJ, Strijers RL, Frolich M, Meinders AE, Pijl H, 2002. Dynamics of the pituitary-adrenal ensemble in hypocretin-deficient narcoleptic humans: blunted basal adrenocorticotropin release and evidence for normal time-keeping by the master pacemaker. J. Clin. Endocrinol. Metab 87, 5085–5091. 10.1210/jc.2002-020638. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE, 2003. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J. Neurosci 23, 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz CM, Teske JA, Levine JA, Wang C, 2002. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul. Pept 104, 27–32. [DOI] [PubMed] [Google Scholar]
- Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, Yamashita H, 2000. Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport 11, 1977–1980. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK, 2005. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J. Neurosci 25, 5225–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasheras MC, Laorden ML, Milanés MV, Núñez C, 2015. Corticotropin-releasing factor 1 receptor mediates the activity of the reward system evoked by morphine-induced conditioned place preference. Neuropharmacology 95, 168–180. 10.1016/j.neuropharm.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Chang L-Y, Ho Y-C, Teng S-F, Hwang L-L, Mackie K, Chiou L-C, 2016. Stress induces analgesia via orexin 1 receptor-initiated endocannabinoid/CBl signaling in the mouse periaqueductal gray. Neuropharmacology 105, 577–586. 10.1016/j.neuropharm.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-B, Jones JR, de Lecea L, 2016. Hypocretins, neural systems, physiology, and psychiatric disorders. Curr. Psychiatry Rep 18, 7 10.1007/s11920-015-0639-0. [DOI] [PubMed] [Google Scholar]
- Li Y, Dong X, Li S, Kirouac GJ, 2014. Lesions of the posterior paraventricular nucleus of the thalamus attenuate fear expression. Front. Behav. Neurosci 8, 94 10.3389/fnbeh.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ, 2010. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology 212, 251–265. 10.1007/s00213-010-1948-y. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E, 1999. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98, 365–376. [DOI] [PubMed] [Google Scholar]
- Loewen SP, Paterson AR, Loh SY, Rogers MF, Hindmarch CCT, Murphy D, Ferguson AV, 2017. Sex-specific differences in cardiovascular and metabolic hormones with integrated signalling in the paraventricular nucleus of the hypothalamus. Exp. Physiol 10.1113/EP086436. [DOI] [PubMed] [Google Scholar]
- López M, Señaris R, Gallego R, Garcia-Caballero T, Lago F, Seoane L, Casanueva F, Dieguez C, 1999. Orexin receptors are expressed in the adrenal medulla of the rat. Endocrinology 140, 5991–5994. 10.1210/endo.140.12.7287. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhao J, Balesar R, Fronczek R, Zhu Q-B, Wu X-Y, Hu S-H, Bao A-M, Swaab DF, 2017. Sexually dimorphic changes of hypocretin (orexin) in depression. EBioMedicine 18, 311–319. 10.1016/j.ebiom.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungwitz EA, Molosh A, Johnson PL, Harvey BP, Dirks RC, Dietrich A, Minick P, Shekhar A, Truitt WA, 2012. Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol. Behav 107, 726–732. 10.1016/j.physbeh.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ, 2008. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J. Neurosci 28, 3071–3075. 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magga J, Bart G, Oker-Blom C, Kukkonen JP, Åkerman KEO, Näsman J, 2006. Agonist potency differentiates G protein activation and Ca2+ signalling by the orexin receptor type 1. Biochem. Pharmacol 71, 827–836. 10.1016/j.bcp.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G, 2014. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat. Neurosci 17, 1298–1303. 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallampalli MP, Carter CL, 2014. Exploring sex and gender differences in sleep health: a Society for Women’s Health Research Report. J. Womens. Health (Larchmt) 23, 553–562. 10.1089/jwh.2014.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maran T, Sachse P, Martini M, Weber B, Pinggera J, Zuggal S, Furtner M, 2017. Lost in time and space: states of high arousal disrupt implicit acquisition of spatial and sequential context information. Front. Behav. Neurosci 11, 206 10.3389/fnbeh.2017.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK, 2001. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol 435, 6–25. [DOI] [PubMed] [Google Scholar]
- Martins PJF, Marques MS, Tufik S, D’Almeida V, 2010. Orexin activation precedes increased NPY expression, hyperphagia, and metabolic changes in response to sleep deprivation. AJP Endocrinol. Metab 298, E726–E734. 10.1152/ajpendo.00660.2009. [DOI] [PubMed] [Google Scholar]
- Mazzocchi G, Malendowicz LK, Gottardo L, Aragona F, Nussdorfer GG, 2001. Orexin A stimulates cortisol secretion from human adrenocortical cells through activation of the adenylate cyclase-dependent signaling cascade. J. Clin. Endocrinol. Metab 86, 778–782. 10.1210/jcem.86.2.7233. [DOI] [PubMed] [Google Scholar]
- Messina G, Di Bernardo G, Viggiano A, De Luca V, Monda V, Messina A, Chieffi S, Galderisi U, Monda M, 2016. Exercise increases the level of plasma orexin A in humans. J. Basic Clin. Physiol. Pharmacol 27, 611–616. 10.1515/jbcpp-2015-0133. [DOI] [PubMed] [Google Scholar]
- Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, Vankova J, Black J, Harsh J, Bassetti C, Schrader H, Nishino S, 2002. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch. Neurol 59, 1553–1562. [DOI] [PubMed] [Google Scholar]
- Mikrouli E, Wörtwein G, Soylu R, Mathé AA, Petersén Å, 2011. Increased numbers of orexin/hypocretin neurons in a genetic rat depression model. Neuropeptides 45, 401–406. 10.1016/j.npep.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Miner LA, Ostrander M, Sarter M, 1997. Effects of ibotenic acid-induced loss of neurons in the medial prefrontal cortex of rats on behavioral vigilance: evidence for executive dysfunction. J. Psychopharmacol 11, 169–178. 10.1177/026988119701100210. [DOI] [PubMed] [Google Scholar]
- Mobarakeh JI, Takahashi K, Sakurada S, Nishino S, Watanabe H, Kato M, Yanai K, 2005. Enhanced antinociception by intracerebroventricularly and intrathecally-administered orexin A and B (hypocretin-1 and −2) in mice. Peptides 26, 767–777. 10.1016/j.peptides.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Muraki Y, Yamanaka A, Tsujino N, Kilduff TS, Goto K, Sakurai T, 2004. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J. Neurosci 24, 7159–7166. 10.1523/JNEUROSCI.1027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA, 2014. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc. Natl. Acad. Sci. III, E1648–E1655. 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara T, Saitoh T, Kutsumura N, Irukayama-Tomobe Y, Ogawa Y, Kuroda D, Gouda H, Kumagai H, Fujii H, Yanagisawa M, Nagase H, 2015. Design and synthesis of non-peptide, selective orexin receptor 2 agonists. J. Med. Chem 58, 7931–7937. 10.1021/acs.jmedchem.5b00988. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kanbayashi T, Sugiura T, Inoue Y, 2011. Relationship between clinical characteristics of narcolepsy and CSF orexin-A levels. J. Sleep Res 20, 45–49. 10.1111/j.l365-2869.2010.00870.x. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nagamine T, 2017. Neuroendocrine, autonomic, and metabolic responses to an orexin antagonist, suvorexant, in psychiatric patients with insomnia. Innov. Clin. Neurosci 14, 30–37. [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Quiroz C, Moreno-Delgado D, Sierakowiak A, McDowell K, Moreno E, Rea W, Cai N-S, Aguinaga D, Howell LA, Hausch F, Cortés A, Mallol J, Casadó V, Lluís C, Canela EI, Ferré S, McCormick PJ, 2015. Orexin-corticotropin-releasing factor receptor heteromers in the ventral tegmental area as targets for cocaine. J. Neurosci 35, 6639–6653. 10.1523/JNEUROSCI.4364-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM, 2002. Neurobiology of depression. Neuron 34, 13–25. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E, 2000. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 355, 39–40. 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Zhang J, Feng P, Panksepp J, 2012. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience 218, 138–153. 10.1016/j.neuroscience.2012.05.033. [DOI] [PubMed] [Google Scholar]
- Nollet M, Gaillard P, Minier F, Tanti A, Belzung C, Leman S, 2011. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology 61, 336–346. 10.1016/j.neuropharm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Nollet M, Gaillard P, Tanti A, Girault V, Belzung C, Leman S, 2012. Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology 37, 2210–2221. 10.1038/npp.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R, Lee AJ, Nir R-R, Bernstein CA, Kainz VM, Bertisch SM, Buettner C, Borsook D, Burstein R, 2017. Neural mechanism for hypothalamic-mediated autonomic responses to light during migraine. Proc. Natl. Acad. Sci 114, E5683–E5692. 10.1073/pnas.1708361114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Goodman J, 2012. Emotional arousal and multiple memory systems in the mammalian brain. Front. Behav. Neurosci 6, 14 10.3389/fnbeh.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panhelainen AE, Korpi ER, 2012. Evidence for a role of inhibition of orexinergic neurons in the anxiolytic and sedative effects of diazepam: a c-Fos study. Pharmacol. Biochem. Behav 101, 115–124. 10.1016/j.pbb.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Teegarden SL, Hedin AD, Jensen CL, Bale TL, 2010. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J. Neurosci 30, 16399–16407. 10.1523/JNEUROSCI.1955-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME, 1991. Localization of a human system for sustained attention by positron emission tomography. Nature 349, 61–64. 10.1038/349061aO. [DOI] [PubMed] [Google Scholar]
- Park KB, Weon H, 2017. Orexin receptors mediate long-term depression of excitatory synaptic transmission in the spinal cord dorsal horn. Neurosci. Lett 660, 12–16. 10.1016/j.neulet.2017.08.068. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS, 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D, Frohlich J, Morgan M, 2002. Hormonal and genetic influences on arousal-sexual and otherwise. Trends Neurosci. 25, 45–50. [DOI] [PubMed] [Google Scholar]
- Piccoli L, Micioni Di Bonaventura MV, Cifani C, Costantini VJA, Massagrande M, Montanari D, Martinelli P, Antolini M, Ciccocioppo R, Massi M, Merlo-Pich E, Di Fabio R, Corsi M, 2012. Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharmacology 37, 1999–2011. 10.1038/npp.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigoni A, Delvecchio G, Altamura AC, Soares JC, Fagnani C, Brambilla P, 2017. The role of genes and environment on brain alterations in Major Depressive Disorder: a review of twin studies. J. Affect. Disord 10.1016/j.jad.2017.10.036. [DOI] [PubMed] [Google Scholar]
- Poulton R, Andrews G, Millichamp J, 2008. Gene-environment interaction and the anxiety disorders. Eur. Arch. Psychiatry Clin. Neurosci 258, 65–68. 10.1007/S00406-007-0784-5. [DOI] [PubMed] [Google Scholar]
- Prince CD, Rau AR, Yorgason JT, Espana RA, 2015. Hypocretin/orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem. Neurosci 6, 138–146. 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober DA, Rihel J, Onah AA, Sung R-J, Schier AF, 2006. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J. Neurosci 26, 13400–13410. 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainero I, Gallone S, Valfrè W, Ferrero M, Angilella G, Rivoiro C, Rubino E, De Martino P, Savi L, Ferrone M, Pinessi L, 2004. A polymorphism of the hypocretin receptor 2 gene is associated with cluster headache. Neurology 63, 1286–1288. [DOI] [PubMed] [Google Scholar]
- Rainero I, Martino P De, Pinessi, L., 2008. Hypocretins and primary headaches: neurobiology and clinical implications. Expert Rev. Neurother 8, 409–416. 10.1586/14737175.8.3.409. [DOI] [PubMed] [Google Scholar]
- Rainero I, Ostacoli L, Rubino E, Gallone S, Picci LR, Fenoglio P, Negro E, Rosso C, De Martino P, De Marchi M, Furlan PM, Pinessi L, 2011. Association between major mood disorders and the hypocretin receptor 1 gene. J. Affect. Disord 130, 487–491. 10.1016/j.jad.2010.10.033. [DOI] [PubMed] [Google Scholar]
- Ramos A, Berton O, Mormede P, Chaouloff F, 1997. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav. Brain Res 85, 57–69. [DOI] [PubMed] [Google Scholar]
- Reilly CE, 1999. I. Mutation in the hypocretin (orexin) receptor 2 gene causes canine narcolepsy. J. Neurol 246 985–986. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Ishii Y, Halford JCG, Blundell JE, 2002. Orexins and appetite regulation. Neuropeptides 36, 303–325. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG, 2003. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J. Comp. Neurol 465, 593–603. 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- Rotter A, Asemann R, Decker A, Kornhuber J, Biermann T, 2011. Orexin expression and promoter-methylation in peripheral blood of patients suffering from major depressive disorder. J. Affect. Disord 131, 186–192. 10.1016/j.jad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Sakurai T, 2014. The role of orexin in motivated behaviours. Nat. Rev. Neurosci 15, 719–731. 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- Sakurai T, 2007. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat. Rev. Neurosci. 8, 171–181. 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, 1998. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Sugimoto S, 1985. Effects of lesions of prefrontal cortex and dorsomedial thalamus on delayed go/no-go alternation in rats. Behav. Brain Res 17, 213–219. [DOI] [PubMed] [Google Scholar]
- Salomon RM, Ripley B, Kennedy JS, Johnson B, Schmidt D, Zeitzer JM, Nishino S, Mignot E, 2003. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol. Psychiatry 54, 96–104. [DOI] [PubMed] [Google Scholar]
- Samson WK, Bagley SL, Ferguson AV, White MM, 2007. Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. Am. J. Physiol. Regul. Integr. Comp. Physiol 292, R382–R387. 10.1152/ajpregu.00496.2006. [DOI] [PubMed] [Google Scholar]
- Schmidt FM, Kratzsch J, Gertz H-J, Tittmann M, Jahn I, Pietsch U-C, Kaisers UX, Thiery J, Hegerl U, Schönknecht P, 2013. Cerebrospinal fluid melaninconcentrating hormone (MCH) and hypocretin-1 (HCRT-1, Orexin-A) in Alzheimer’s disease. PLoS One 8, e63136 10.1371/journal.pone.0063136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, Nestler EJ, Elmquist JK, Lutter M, 2011. Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behav. Brain Res 222, 289–294. 10.1016/j.bbr.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman MV, 1997. Psychopathology in women and men: focus on female hormones. Am. J. Psychiatry 154, 1641–1647. [DOI] [PubMed] [Google Scholar]
- Selbach O, Bohla C, Barbara A, Doreulee N, Eriksson KS, Sergeeva OA, Haas HL, 2010. Orexins/hypocretins control bistability of hippocampal long-term synaptic plasticity through co-activation of multiple kinases. Acta Physiol. 198, 277–285. 10.1111/j.1748-1716.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ, 2010. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol. Psychiatry 67, 753–760. 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JI, Leskin GA, Klein DF, 2002. Gender differences in panic disorder: findings from the National Comorbidity Survey. Am. J. Psychiatry 159, 55–58. [DOI] [PubMed] [Google Scholar]
- Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H, 1999. Sympathetic and cardiovascular actions of orexins in conscious rats. Am. J. Physiol. 277, R1780–R1785. [DOI] [PubMed] [Google Scholar]
- Silveyra P, Cataldi NI, Lux-Lantos VA, Libertun C, 2010. Role of orexins in the hypothalamic-pituitary-ovarian relationships. Acta Physiol. (Oxf) 198, 355–360. 10.1111/j.1748-1716.2009.02049.x. [DOI] [PubMed] [Google Scholar]
- Slater PG, Noches V, Gysling K, 2016. Corticotropin-releasing factor type-2 receptor and corticotropin-releasing factor-binding protein coexist in rat ventral tegmental area nerve terminals originated in the lateral hypothalamic area. Eur. J. Neurosci 43, 220–229. http://dx.doi.org/10.l11l/ejn.13113. [DOI] [PubMed] [Google Scholar]
- Smith PM, Connolly BC, Ferguson AV, 2002. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res. 950, 261–267. [DOI] [PubMed] [Google Scholar]
- Soya S, Shoji H, Hasegawa E, Hondo M, Miyakawa T, Yanagisawa M, Mieda M, Sakurai T, 2013. Orexin receptor-1 in the locus coeruleus plays an important role in cue-dependent fear memory consolidation. J. Neurosci 33, 14549–14557. 10.1523/JNEUROSCI.1130-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG, 2006. Orexins in the regulation of the hypothalamic-pituitary-adrenal axis. Pharmacol. Rev 58, 46–57. 10.1124/pr.58.1.4. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Cohen SJ, Lora JC, Rios LM, 2016. Temporary inactivation reveals that the CA1 region of the mouse dorsal hippocampus plays an equivalent role in the retrieval of long-term object memory and spatial memory. Neurobiol. Learn. Mem 133, 118–128. 10.1016/j.nlm.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MA, Lecourt H, Jenck F, 2012. The brain orexin system and almorexant in fear-conditioned startle reactions in the rat. Psychopharmacology 223, 465–475. 10.1007/s00213-012-2736-7. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Pyne-Geithman GJ, Ekhator NN, Horn PS, Uhde TW, Shutter L.a., Baker DG, Geracioti TD, 2010. Low cerebrospinal fluid and plasma orexin-A (hypocretin-1) concentrations in combat-related posttraumatic stress disorder. Psychoneuroendocrinology 35, 1001–1007. 10.1016/j.psyneuen.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L, 2000. The hypocretins: excitatory neuromodulatory peptides for multiple homeostatic systems, including sleep and feeding. J. Neurosci. Res 62, 161–168. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T, 2005. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res. 1044, 116–121. 10.1016/j.brainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Sweet DC, Levine AS, Billington CJ, Kotz CM, 1999. Feeding response to central orexins. Brain Res. 821, 535–538. [DOI] [PubMed] [Google Scholar]
- Taheri S, Sunter D, Dakin C, Moyes S, Seal L, Gardiner J, Rossi M, Ghatei M, Bloom S, 2000. Diurnal variation in orexin A immunoreactivity and prepro-orexin mRNA in the rat central nervous system. Neurosci. Lett 279, 109–112. [DOI] [PubMed] [Google Scholar]
- Tang J, Chen J, Ramanjaneya M, Punn A, Conner AC, Randeva HS, 2008. The signalling profile of recombinant human orexin-2 receptor. Cell. Signal 20, 1651–1661. 10.1016/j.cellsig.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Yanagisawa M, Saper CB, 2003. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 119, 1033–1044. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM, 1998. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 438, 71–75. [DOI] [PubMed] [Google Scholar]
- Uramura K, Funahashi H, Muroya S, Shioda S, Takigawa M, Yada T, 2001. Orexin-a activates phospholipase C- and protein kinase C-mediated Ca2+ signaling in dopamine neurons of the ventral tegmental area. Neuroreport 12, 1885–1889. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, 1999. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J. Neurosci 19, 3171–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB, 1998. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J. Neurosci 18, 7962–7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaven MW, Cornish JL, Staples LG, 2015. The orexin-1 receptor antagonist SB-334867 decreases anxiety-like behavior and c-Fos expression in the hypothalamus of rats exposed to cat odor. Behav. Brain Res 278, 563–568. 10.1016/j.bbr.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Vanitallie TB, 2002. Stress: a risk factor for serious illness. Metabolism. 51, 40–45. [DOI] [PubMed] [Google Scholar]
- von der Goltz C, Koopmann A, Dinter C, Richter A, Grosshans M, Fink T, Wiedemann K, Kiefer F, 2011. Involvement of orexin in the regulation of stress, depression and reward in alcohol dependence. Horm. Behav 60, 644–650. 10.1016/j.yhbeh.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Wang H, Li S, Kirouac GJ, 2017. Role of the orexin (hypocretin) system in contextual fear conditioning in rats. Behav. Brain Res 316, 47–53. 10.1016/j.bbr.2016.08.052. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kuwaki T, Yanagisawa M, Fukuda Y, Shimoyama M, 2005. Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport 16, 5–8. [DOI] [PubMed] [Google Scholar]
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Goder R, Baier PC, 2014. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav. Brain Res 262, 8–13. 10.1016/j.bbr.2013.12.045. [DOI] [PubMed] [Google Scholar]
- Will CC, Aird F, Redei EE, 2003. Selectively bred Wistar-Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol. Psychiatry 8, 925–932. 10.1038/sj.mp.4001345. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, Leonard CS, Richardson JA, Hammer RE, Yanagisawa M, 2003. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron 38, 715–730. [DOI] [PubMed] [Google Scholar]
- Wilson DIG, Langston RF, Schlesiger MI, Wagner M, Watanabe S, Ainge JA, 2013. Lateral entorhinal cortex is critical for novel object-context recognition. Hippocampus 23, 352–366. 10.1002/hipo.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winrow CJ, Renger JJ, 2014. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br. J. Pharmacol 171, 283–293. 10.1111/bph.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, de Lecea L, 2005. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol. Neurobiol 32, 285–294. 10.1385/MN:32:3:285. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L, 2004. Interaction between the corticotropinreleasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J. Neurosci 24, 11439–11448. 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S, 2010. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology 151, 1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Samson WK, Ferguson AV, 2003. Excitatory effects of orexin-A on nucleus tractus solitarius neurons are mediated by phospholipase C and protein kinase C. J. Neurosci 23, 6215–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zou B, Xiong X, Pascual C, Xie J, Malik A, Xie J, Sakurai T, Xie XS, 2013. Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. J. Neurosci 33, 5275–5284. 10.1523/JNEUROSCI.3200-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Cui S-Y, Zhang X-Q, Cui X-Y, Li S-J, Sheng Z-F, Cao Q, Huang Y-L, Xu Y-P, Lin Z-G, Yang G, Song J-Z, Ding H, Zhang Y-H, 2016. Mechanisms underlying footshock and psychological stress-induced abrupt awakening from posttraumatic “nightmares”. Int. J. Neuropsychopharmacol 19, pyv113 10.1093/ijnp/pyv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarepour L, Fatahi Z, Sarihi A, Haghparast A, 2014. Blockade of orexin-1 receptors in the ventral tegmental area could attenuate the lateral hypothalamic stimulation-induced potentiation of rewarding properties of morphine. Neuropeptides 48,179–185. 10.1016/j.npep.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud H-R, 2005. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J. Comp. Neurol 485, 127–142. 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE, 2012. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J. Pharmacol. Exp. Ther 340, 801–809. 10.1124/jpet.111.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowska A, Spinazzi R, Albertin G, Nowak M, Malendowicz LK, Tortorella C, Nussdorfer GG, 2005. Orexins stimulate glucocorticoid secretion from cultured rat and human adrenocortical cells, exclusively acting via the 0X1 receptor. J. Steroid Biochem. Mol. Biol 96, 423–429. 10.1016/j.jsbmb.2005.05.003. [DOI] [PubMed] [Google Scholar]