Abstract
Background:
Therapeutic drug monitoring is useful in the treatment of tuberculosis to assure adequate exposure, minimize antibiotic resistance, and reduce toxicity. Salivary therapeutic drug monitoring could reduce the risks, burden, and costs of blood-based therapeutic drug monitoring. This systematic review compared human pharmacokinetics of antituberculosis drugs in saliva and blood to determine if salivary therapeutic drug monitoring could be a promising alternative.
Methods:
On December 2, 2016, PubMed and the Institute for Scientific Information Web of Knowledge were searched for pharmacokinetic studies reporting human salivary and blood concentrations of antituberculosis drugs. Data on study population, study design, analytical method, salivary Cmax, salivary area under the time–concentration curve, plasma/serum Cmax, plasma/serum area under the time–concentration curve, and saliva–plasma or saliva–serum ratio were extracted. All included articles were assessed for risk of bias.
Results:
In total, 42 studies were included in this systematic review. For the majority of antituberculosis drugs, including the first-line drugs ethambutol and pyrazinamide, no pharmacokinetic studies in saliva were found. For amikacin, pharmacokinetic studies without saliva–plasma or saliva–serum ratios were found.
Conclusions:
For gatifloxacin and linezolid, salivary therapeutic drug monitoring is likely possible due to a narrow range of saliva– plasma and saliva–serum ratios. For isoniazid, rifampicin, moxifloxacin, ofloxacin, and clarithromycin, salivary therapeutic drug monitoring might be possible; however, a large variability in saliva– plasma and saliva–serum ratios was observed. Unfortunately, salivary therapeutic drug monitoring is probably not possible for doripenem and amoxicillin/clavulanate, as a result of very low salivary drug concentrations.
Keywords: tuberculosis, therapeutic drug monitoring, saliva, oral fluid
INTRODUCTION
Tuberculosis (TB) is an infectious disease that is still a huge problem worldwide, although it is curable with antibiotics. In 2015, approximately 10.4 million people worldwide had TB for the first time, including 480,000 patients with multi–drug-resistant TB (MDR-TB).1 MDR-TB is caused by strains of Mycobacterium tuberculosis resistant to at least the first-line drugs isoniazid and rifampicin. Drug-susceptible TB is treated with a standard combination of isoniazid, rifampicin, ethambutol, and pyrazinamide during 2 months followed by 4 months of only isoniazid and rifampicin.2 The treatment of MDR-TB consists of a combination of at least 5 antibiotics that are likely to be effective.3
Therapeutic drug monitoring (TDM) can be used to assure adequate exposure, minimize antibiotic resistance, and reduce side effects.4 TDM is, however, not a part of the standard TB treatment according to the World Health Organization (WHO) guidelines. Subtherapeutic drug concentrations cause decreased cure rates and can induce antibiotic resistance.5,6 On the other hand, too high concentrations of some anti-TB drugs can lead to serious toxicity.4,7 In addition, pharmacokinetics of anti-TB drugs show large inter-individual variability.8 Thus, applying TDM in TB therapy could be helpful to achieve therapeutic drug concentrations in an early stage of treatment.
Although blood samples have been routinely used for TDM, venipuncture is an invasive procedure with increased risks of infection, local hematoma, and pain at the puncture site.9,10 In addition, pain-related fear plays a major role for patients.9 In addition, venipuncture is rather expensive because it requires qualified staff and appropriate materials.9,10 Blood sampling is undesirable for some patient groups because of limited blood supply (eg, neonates), less accessible veins (eg, elderly), or religious objections.9 Because of these disadvantages, alternatives to regular blood sampling (eg, saliva) are being studied.
Oral fluid is a mixture of saliva secreted by all glands present in the oral cavity.11 The terms saliva and oral fluid are used interchangeably in the literature.
Saliva sampling is less complicated compared with taking blood samples and reduces costs.10,12 An economic study about saliva collection in children showed 58% savings with the saliva sampling procedure alone compared with blood sampling, caused by a shorter sampling time and less expensive materials.13 If parents were collecting saliva samples instead of medical staff, the savings could increase up to 90%.13 Collecting saliva samples is also experienced as more comfortable by patients.9,12,14 For certain patient groups, such as children, elderly, and people with disabilities, saliva sampling is a preferred method.10,12,14 Stimulated saliva samples can be taken by chewing on absorbent cotton rolls, paraffin, or after applying citric acid under the tongue. For nonstimulated saliva samples, the passive drooling technique is regularly used.
Dried blood spot (DBS) sampling is another less invasive method. However, DBS sampling can be painful, is more complicated, and has higher failure rates than saliva sampling.15 The drug concentrations in DBS are influenced by the hematocrit value and spot volume.16 In addition, free (unbound) drug concentrations are not determinable in DBS,16 whereas salivary concentrations generally represent the free (unbound) drug concentrations.14,17
Distribution of drugs from blood to saliva generally occurs by passive diffusion. Protein binding, negative log of acid dissociation constant (pKa), molecular mass, lipid solubility, and chemical stability in saliva are physicochemical properties of drugs that influence the salivary drug concentration. Salivary pH value, salivary flow rate, and some diseases of the oral cavity are physiological properties that determine drug penetration into saliva.12,18 Actively stimulating saliva flow will increase the excretion of bicarbonate and therefore can influence the drug distribution and concentration in saliva.11,14 Generally, concentrations in saliva reflect the free (unbound) drug concentrations in plasma at a certain ratio.14,17 The saliva–plasma ratio can be determined not only by calculating the mean saliva–plasma ratio of all chosen time points but also by using the area under the time– concentration curve (AUC) values of the time–concentration curves in saliva and plasma. For some anti-TB drugs, saliva– plasma or saliva–serum ratios are studied, but a clear overview of the comparison of salivary to blood-based TDM for anti-TB drugs is not available.
The aim of this systematic review was to investigate whether TDM of anti-TB drugs using saliva samples is feasible, and if so, for which of these drugs which bioanalytical assays for saliva-based TDM should be established and validated.
MATERIALS AND METHODS
A protocol of this systematic review was registered at PROSPERO with registration number CRD42017051749 and available through www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42017051749. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used for this review.19
For this review, the first-line and second-line anti-TB drugs were selected from the WHO guidelines.2,3 Ertapenem, faropenem, doripenem, ofloxacin, and clarithromycin were added to this list.
PubMed and Institute for Scientific Information (ISI) Web of Knowledge searches were performed on the December 2, 2016. The keywords used for this systematic search were (isoniazid OR rifampicin OR pyrazinamide OR ethambutol OR levofloxacin OR moxifloxacin OR gatifloxacin OR amikacin OR capreomycin OR kanamycin OR streptomycin OR ethionamide OR prothionamide OR cycloserine OR terizidone OR linezolid OR clofazimine OR bedaquiline OR delamanid OR paraaminosalicylic acid OR imipenem/cilastatin OR imipenem OR cilastatin OR meropenem OR amoxicillin/clavulanate OR amoxicillin OR clavulanate OR thiacetazone OR ertapenem OR faropenem OR doripenem OR ofloxacin OR clarithromycin) AND saliva AND (pharmacokinetics OR saliva–plasma ratio OR saliva–serum ratio OR TDM OR penetration OR distribution OR drug concentration). No limitation of publication date was used. A second reviewer checked the reproducibility of the search using the stated keywords.
After duplicate articles were removed, titles and abstracts were screened for eligibility, and the selected manuscripts were read by 2 independent reviewers. Exclusion factors were as follows: no human study, no anti-TB drug concentration was measured in saliva or plasma/serum, and if the manuscript was a review article. Primary references of the excluded reviews were checked and included if the study was relevant and obtainable.
Data extraction of the included articles was performed by 1 person. A reviewer independently checked the data extraction afterward. Data on study population, study design, saliva sampling method, analytical method, peak concentration (Cmax) in saliva, AUC in saliva, Cmax in plasma or serum, AUC in plasma or serum, and saliva–plasma or saliva–serum ratio were extracted from the included articles. Authors of included articles were contacted if numerical Cmax values were missing, although a time–concentration curve was stated.
If the article contained a time–concentration curve of the drug, but no numerical Cmax value was available, the Cmax was estimated using the graph. If AUC values of both saliva and plasma or serum were given, the ratio was manually calculated by dividing the salivary AUC by the plasma or serum AUC. The saliva–plasma or saliva–serum ratio was calculated (1/plasma–saliva ratio or 1/serum–saliva ratio, respectively) if the article only mentioned the plasma–saliva or serum–saliva ratio. All calculated ratios and estimated Cmax values were marked in the table.
As no validated tool for risk of bias assessment of pharmacokinetic studies is available, we used the Risk Of Bias In Nonrandomized Studies—of Interventions (ROBINS-I) tool.20 This tool was validated for nonrandomized intervention studies. Changes were made in the confounding section to make the tool more suitable for pharmacokinetic studies. The assessment was checked by a second reviewer.
RESULTS
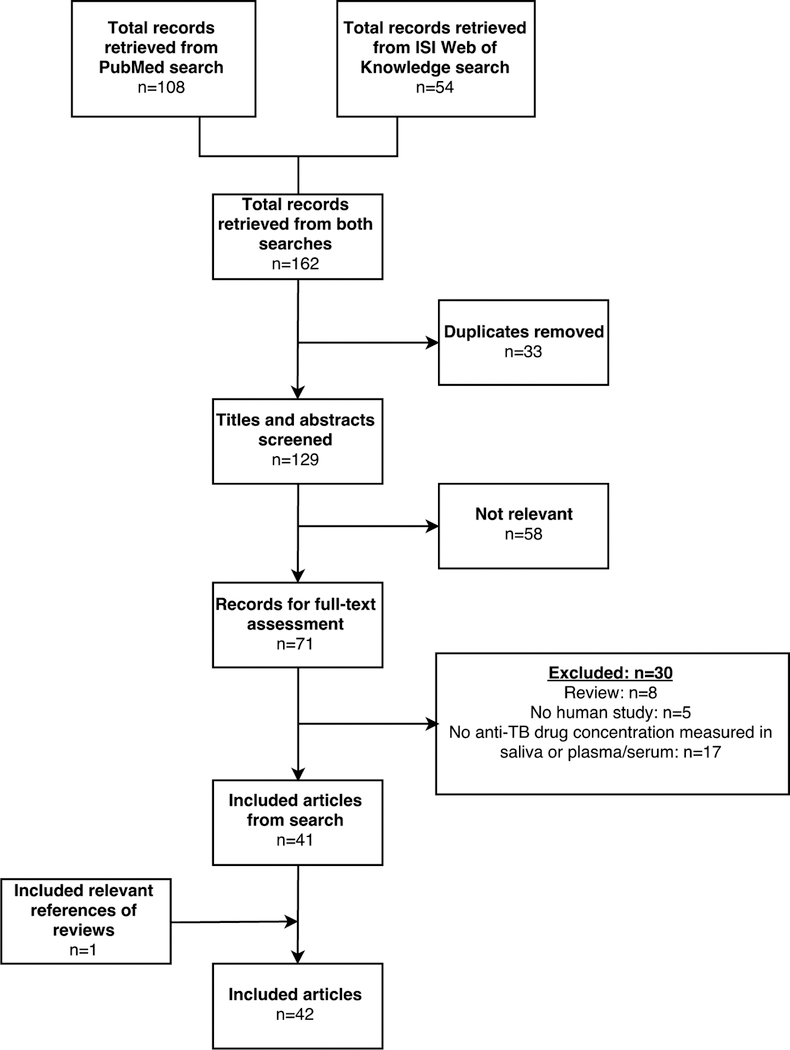
A total of 162 records were found in the PubMed (n = 108) and ISI Web of Knowledge (n = 54) search (Figure 1). After duplicates were removed, a number of 129 articles remained, of which 58 were classified as not relevant based on title and abstract. After full-text assessment, 30 records were excluded. One article, Ichihara21 was included after searching the references of the excluded review articles. Overall, 42 articles were included in this systematic review.
FIGURE 1.
Results of searches and study selection. Using the search terms, 162 records were found, 71 of which were assessed as relevant. After full-text assessment, 30 articles were excluded. A total of 42 articles were included in this systematic review.
No articles concerning salivary pharmacokinetics of first-line anti-TB drugs ethambutol, pyrazinamide and second-line anti-TB drugs levofloxacin, capreomycin, kanamycin, streptomycin, ethionamide, prothionamide, cycloserine, terizidone, clofazimine, bedaquiline, delamanid, paraaminosalicylic acid, imipenem/cilastatin, meropenem, thiacetazone, ertapenem, or faropenem were found in the systematic search.
Study populations of the included articles were composed of healthy volunteers, patients with TB, children, neonates, or patients with numerous diseases and ranged from studies as few as 2 to as many as 80 participants. For each anti-TB drug, variable dosage regimens were administered, and multiple saliva sampling methods as well as several analytical methods were used (Table 1).
TABLE 1.
Data of Included Pharmacokinetic Studies Comparing Salivary and Blood Anti-TB Drug Peak Concentrations, Values of AUC, and the Saliva-Plasma or Saliva-Serum Ratio in Humans
| Drug | Study | Study Population | Studv Design | Dose | Saliva Sampling Method |
|---|---|---|---|---|---|
| Isoniazid | Brown et al24 | HV; N = 5 | Open-label cross-over | 300 mg, single dose | S; unflavored chewing gum |
| Gummurthy et al31 | PTB and 1TB patients; N = 30 | Open-label | 300 mg, single dose | S; unflavored chewing gum | |
| Hutchings et al79 | Patients with various diseases; N = 22 | Open-label | 200 mg, single dose | S; chewing teflon tape | |
| Suryawati et al40 | HV; N = 8 | Open-label | 10 mg/kg, single dose | ND | |
| Rifampicin | Gummurthy et al31 | PTB and 1TB patients; N = 30 | Open-label | 10 mg/kg, single dose | S; unflavored chewing gum |
| Orisakwe et al32 | HV; N = 5 | Open-label cross-over | 600 mg, single dose | S; chewing gum | |
| Ezejiofor et al30 | HV; N = 5 | Open-label cross-over | 600 mg, single dose | S; unflavored chewing gum | |
| Darouiche et al29 | HV; N = 5 | Open-label | 600 mg, for 4 d | ND | |
| McCracken et al80 | Children (6–58 mo old) with impetigo or cellulitis; N = 38 | Open-label | 10 mg/kg, single dose | Capillary pipettes | |
| Murthy et al28 | PTB patients; N = 20 | Open-label | 450/600 mg, single dose | Wide, capped bottle | |
| Orisakwe et al33 | Male HV; N = 6 | Open-label | 600 mg, single dose | ND | |
| Moxifloxacin | Burkhardt et al38 | Male, Caucasian HV; N = 12 | Double-blind; randomized cross-over | 400 mg, for 7 d | S; Salivette |
| Müller et al37 | Male HV; N = 13 | Randomized; open-label cross-over | 400 mg, single dose p.o and i.v. (during 60 min) | S; Salivette | |
| Stass et al36 | Male, Caucasian HV; N = 39 | Double-blind; randomized cross-over and group comparison | 50–800 mg, single dose | S; chew on cotton roll | |
| Burkhardt et al35 | Male patients with SCI and decubitus ulcer; N = 4 | Open-label | 400 mg, single dose | S; Salivette | |
| Kumar et al31 | HV; N = 24 | Open-label | 400 mg, single dose | S; unflavored chewing gum | |
| Ofloxacin | Kozjek et al44 | Male HV; N = 6 | Randomized parallel group | 400 mg, single dose | NS |
| Koizumi et al41 | Patients with chronic respiratory tract infections; N = 18 | Open-label | 300 mg, single dose | Sterile glass dishes | |
| Warlich et al45 | HV; N = 6 | Open-label | 200 mg b.i.d., for 3 d | S; chewing parafilm | |
| Leigh et at46 | HV; N = 11 | Open-label | 200 mg b.i.d., for 3.5 d | NS | |
| Immanuel et al | Male HV; N = 7 | Open-label | 600/800 mg, single dose | S; unflavored chewing gum | |
| Miya et al81 | PTB or NSCLC patients; N = 12 | Open-label | 200 mg ti.d., for at least 7 d | ND | |
| Ohkubo et al27 | Male HV; N = 4 | Open-label | 100/200 mg, single dose | S; chewing parafilm | |
| Fujita et al25 | Patients with infections or antibiotic prophylaxis and 11V; N = 80 | Open-label | 100 mg alt. d.−200 mg ti.d., (depending on renal function), for 5 d | ND | |
| Edlund et al48 | Gastric surgery patients; N = 20 | Open-label | 400 mg, single dose | Sterile glass tubes | |
| Ichihara et al21 | Male HV; N = 19 | Open-label | 100/300/600 mg, single dose | ND | |
| Tsubakihara et al49 | Patients with renal failure; N = 12 (6 HD, 6 non-HD) | Open-label | 100 mg, single dose | ND | |
| Gatifloxacin | Nakashima et al53 | Male, Asian HV;N = 30 | Open-label | 100/200/400/600 mg, single dose 300 mg b.i.d., for 6.5 d | NS |
| Mignot et al*54 | Male, Caucasian HV; N = 36 | Double-blind, randomized, placebo controlled | 400/600 mg, single dose and for 10 d | NS | |
| Amikacin | Masumi et al39 | Neonates (2-and 12-day old); N = 2 | Open-label | 3.0–6.0 mg/kg i.v. | ND |
| Biasini et al23 | Children with CF and pneumonia; N = ND | Open-label | 10 mg/kg i.v. injection | ND | |
| Linezolid | Bolhuis et al51 | MDR-TB patients (5 African, 1 Caucasian, 1 Asian); N = 7 | Open-label | 300 mg b.i.d. at steady state | S; Salivette |
| Hara et al82 | HV; N = 4 | Open-label | 600 mg, single dose | S; Salivette | |
| Amoxicillin/clavulanate | Goddard et al26 | Male HV; N = 8 | Double-blind, randomized, placebo-controlled cross-over | 750 mg (amoxicillin), for 5 d | ND |
| Ortiz et al62 | HV; N = 26 | Open-label, randomized, cross-over | 500 mg (amoxicillin), single dose | ND | |
| Ginsburg et al61 | Children (4 54-month old) with AOM; N = 24 | Open-label, cross-over | 15 and 25 mgkg (amoxicillin), single dose | Capillary pipettes | |
| Baglie et al22 | HV; N = 20 | Open-label; randomized cross-over | 875 mg (amoxicillin), single dose | NS, Sterile glass tubes | |
| Wüst et al60 | HV; N = 10 | Open-label | 750 mg (amoxicillin); single dose | ND | |
| Doripenem | Burian et al59 | Male HV; N = 6 | Open-label | 500 mg i.v. in 1 h, single dose | ND |
| Claritliromycin | Fassbender et al83 | HV; N = 10 | Randomized, crossover | 500 mg b.i.d., for 3 d | S; chewing on cotton roll |
| Kees et al50 | Male HV; N = 12 | Open-label, randomized, crossover | 500 mg q.d./250 mg b.i.d., for 5 d | NS; dental tampon | |
| Burkhardt et al38 | Male, Caucasian HV; N = 12 | Double-blind, randomized, cross-over | 500 mg b.i.d., for 7 d | S; Salivette | |
| Bolhuis et al51 | MDR-TB patients (5 African, 1 Caucasian, 1 Asian); N = 7 | Open-label | 250 mg at steady state | S; Salivette | |
| Goddard et al26 | Male HV; N = 8 | Double-blind, randomized, placebo-controlled, crossover | 500 mg, for 5 d | ND | |
| Edlund et al52 | HV; N = 10 | Double-blind, randomized | 500 mg b.i.d., for 10 d | NS; Glass tubes | |
| Wüst et al60 | HV; N = 10 | Open-label | 500 mg, single dose | ND | |
| Morihana et al84 | Male HV; N = 3 | Open-label | 300 mg, single dose | NS | |
| Drug | Analytical Method | Saliva Cmax (mcg/mL) and AUC (mcg · h · mL–1) | Plasma or Serum Cmax (mcg/ML) AUC AUC(mcg · h · mL2–1) | Saliva-Plasma or Saliva-Serum Ratio | Characteristics of Ratio |
| Isoniazid | UV (saliva), Ehrlich reagent and UV (plasma) | Cmax: 1.70 ± 0.10 | Plasma Cmax: 4.50 ± 20 | 0.14 | Conc |
| AUC0–24 h: 8.96 ± 0.37 | Plasma AUC0–24 h: 65.50 ± 6.82 | 0.14‡ | AUC0–24 h | ||
| AUC0-inf 10.06 ± 0.43 | Plasma AUC0-inf: 65.90 ± 6.67 | 0.15‡ | AUC0–inf | ||
| UV | Cmax: Slow acetylators: 7.6 (5.4–13.2) | Serum Cmax: Slow acetylators: 7.8 (4.8–15.0) | Slow acetylators: 0.95‡ Rapid acetylators: 0.94‡ | AUC | |
| Rapid acetylators: 6.0 (4.8–7.4) AUC: Slow acetvlators: 37 (20–58); | Rapid acetylators: 5.9 (4.6–8.7) Serum AUC: Slow acetvlators: 39 (21–62) | ||||
| Rapid acetylators: 17(12–22) | Rapid acetylators: 18(11–27) | ||||
| HPLC-UVC | Cmax: Slowacetylators: 2.5†; Rapidacetylators: 2.3† | PlasmaCmax: Slowacetylators: 2.0†;Rapidacetylators: 1.7† | — | — | |
| AUC:ND | Plasma AUC:ND | ||||
| UV | Cmax:ND | Serum Cmax:ND | 0.80 ± 0.05;Elimination: 0.81 ± 0.05;Absorption:1.09 ± 0.29 | AUC0–inf Conc | |
| AUC0–inf: 31.88 ± 9.57 | Serum AUC0–inf: 38.66 ± 10.53 | ||||
| Rifampicin | Plate diffusion assay with Staphylococcus aureus UV | Cmax: 0.9 | Serum Cmax: 8.5 | 0.07–0.13 | Conc |
| AUC: ND | Serum AUC: ND | ||||
| UV | Cmax: 12.8 ± 0.33 | Plasma Cmax: 17.8 ± 1.04 | 0.67‡ | AUC0–24 h | |
| AUC0–24 h: 63.6 ± 1.4 | Plasma AUC0–24 h: 95.5 ± 2.2 | 0.66‡ | AUC0–inf | ||
| AUC0–inf: 68.1 ± 1.8 | Plasma AUC0–inf: 103.6 ± 3.6 | ||||
| UV | Cmax: 9.00 ± 0.70 | PlasmaCmax: 16 ± 2.12 | 0.15 | Conc | |
| AUC0–24 h: 68.85 ± 5.48 | Plasma AUC0–24 h: 485.60 ± 60.57 | 0.14‡ | AUC0–24 h | ||
| AUC0–inf:72.1868.18 | PlasmaAUC0–inf:505.60±77.13 | 0.14‡ | AUC0–inf | ||
| HPLC-UV | Cmax: ND | SerumCmax: ND | — | — | |
| Highest measured conc at 2 h: 0.42 ± 0.12 | Highest measured serum conc at 5 h: 10.65 ± 4.55 | ||||
| AUC: ND | Serum AUC: ND | ||||
| Agar disk diffusion micro-method with Sarcina lutea | Cmax: ND | Serum Cmax: ND | — | — | |
| Median conc at t = 2 h | Highest measured serum conc at 1 h: | ||||
| Suspension: 1.7 (0.54–7.2) | Suspension: 10.7 ± 0.81 | ||||
| Suspension in apple sauce: 1.6 (0.48–4.0) | Suspension in applesauce: 8.9 ± 1.29 | ||||
| Powder in applesauce: 2.4 (0.85–3.8) | Powder in applesauce: 11.5 ± 2.3 | ||||
| AUC: ND | Serum AUC: Suspension: 56 Suspension in applesauce: 38 Powder in applesauce: 57 | ||||
| RP-HPLC-EC | Cmax: 450 mg: 0.84 ± 0.21, 600 mg: 1.23 ± 0.17 | Serum Cmax: ND; Highest measured serum conc at t = 3 h: 450 mg: 7.99 ± 1.98, 600 mg: 12.18 ± 1.92 | 600 mg: 0.1, 450 mg: 0.11–0.31 | Conc | |
| AUC: 450 mg: 10.59 ± 4.36, 600 mg: 15.13 ± 2.81 | Serum AUC: ND | ||||
| UV | Cmax: 11.6 ± 4.9 | Plasma Cmax: 17.8 ± 5.1 | 0.53‡ | AUC0–24h | |
| AUC0–24 h: 49.68 ± 9 | Plasma AUC0–24 h: 94.15 ± 18 | 0.52‡ | AUC0–inf | ||
| AUC0–inf: 50.01 ± 11 | Plasma AUC0–inf: 96.76 ± 12 | ||||
| Moxifloxacin | HPLC-Fluor | Cmax: day 1: 3.6†, day 7: 4.8† | Serum Cmax: day 1: 3.10 ± 0.60, day 7: 3.98 ± 1.10 | t >2 h: 0.8 | Conc |
| AUC: ND | Serum AUC0–12 h: dav 1: 28.2 ± 4.1, day 7: 39.5 ± 6.6 | ||||
| Serum AUC0–inf: day 1: 35.6 ± 6.5 | |||||
| HPLC-Fluor | Cmax | Plasma Cmax | 0.83 ± 0.20 | AUC0–12 h | |
| p.o.: 3.6 ± 1.0 | p.o.: 3.2 ± 0.6 | p.o.: 0.88‡ | AUC0–12 h | ||
| i.v.: 5.1 ± 1.4 | i.v.: 3.7 ± 0.7 | i.v.: 0.93‡ | |||
| AUC0–12 h | Plasma AUC0–12 h | ||||
| p.o.: 17.6 ± 2.7 | p.o.: 19.8 ± 1.5 | ||||
| i.v.: 21.4 ± 5.0 | i.v.: 22.9 ±11.1 | ||||
| HPLC-Fluor | Cmax | Plasma Cmax | 50 mg: 0.72‡ | AUC0–inf | |
| 50 mg: 0.31 ± 1.55 | 50 mg: 0.29 ± 1.25 | 100 mg: 0.97‡ | |||
| 100 mg: 0.84 ± 1.74 | 100 mg: 0.59 ± 1.21 | 200 mg: 0.91‡ | |||
| 200 mg: 1.62 ± 1.44 | 200 mg: 1.16 ± 1.35 | ||||
| 400 mg: 2.50 ± 1.31 | |||||
| 600 mg: 3.19 ± 1.19 | |||||
| 800 mg: 4.73 ± 1.16 | |||||
| AUC0–inf | Plasma AUC0–inf | ||||
| 50 mg: 2.81 ± 1.40 | 50 mg: 3.88 ± 1.13 | ||||
| 100 mg: 8.27 ± 1.54 | 100 mg: 8.51 ± 1.21 | ||||
| 200 mg: 14.0 ± 1.29 | 200 mg: 15.4 ± 1.20 | ||||
| 400 mg: 26.9 ± 1.18 | |||||
| 600 mg: 39.9 ± 1.11 | |||||
| 800 mg: 59.9 ± 1.24 | |||||
| HPLC-Fluor | Cmax: 1.4 ± 0.4 | Serum Cmax: 4.4 ± 2.7 | 0.45 | Conc | |
| AUC0–8 h: 4.7 ± 3.0 | Serum AUC0–8 h: 15.0 ± 9.7 | 0.31‡ | AUC0–8 h | ||
| RP-HPLC-Fluor | Cmax: ND | Plasma Cmax: ND | 0.54 | Conc | |
| AUC: ND | Plasma AUC: ND | ||||
| Ofloxacin | RP-HPLC-Fluor | Cmax: 1.71 ± 0.44 | Plasma Cmax: 3.66 ± 0.72 | 0.43 ± 0.02 | Conc |
| AUC: 6.41 ± 1.08 | Plasma AUC: 18.22 ± 2.52 | 0.36 ± 0.07 | AUC | ||
| 0.455 | Corr | ||||
| RP-HPLC-Fluor | Cmax: 4.53 ± 0.75 | Serum Cmax: 4.25 ± 0.41 | T = 0–4 h: <1 | Conc | |
| AUC: 63.0 ±8.9 | Serum AUC: 51.5 ± 5.7 | T = 4–8 h: increases from <1 to >1 | AUC | ||
| T = 8–16 h: >1 T = 16 h: 1.14 ± 0.11 1.22‡ 0.61 ± 0.03 | |||||
| RP-HPLC-Fluor | Cmax: 2.07 ± 0.38 | Serum Cmax: 2.96 ± 0.30 | 0.61 ± 0.03 | Conc | |
| AUC0–12 h: 10.8 ± 0.8 | Serum AUC0–12 h: 17.8 ± 0.5 | 0.606 | AUC0–12 h | ||
| Micro-biological assay with Bacillus subtilis | Cmax | Serum Cmax | 0.78 | Corr | |
| 1st dose: 1.9 ± 0.7 | 1st dose: 2.7 ± 0.7 | 1st dose: 0.64‡ | AUC0–8 h | ||
| 7th dose: 2.6 ± 0.7 | 7th dose: 3.4 ± 0.5 | 7th dose: 0.74‡ | AUC0–inf | ||
| AUC0–8 h | Serum AUC0–8 h | 1st dose: 0.64‡ | |||
| 1st dose: 8.9 ± 3.1 | 1st dose: 13.9 ± 3 | 7th dose: 0.73‡ | |||
| 7th dose: 12.9 ±4.5 | 7th dose: 17.5 ± 3.6 | ||||
| AUC0-inf | Serum AUC0–inf | ||||
| 1st dose: 14.8 ± 5.0 | 1st dose: 23.0 ± 5.3 | ||||
| 7th dose: 20.7 ±8.5 | 7th dose: 28.2 ± 7.4 | ||||
| RP-HPLC-Fluor | Cmax | Plasma Cmax | 600 mg: 0.40–0.57 | Conc | |
| 600 mg: 4.1 | 600 mg: 8.0 (7.4–8.6) | 800 mg: 0.40–0.56 | AUC0–24 h | ||
| 800 mg: 4.2 | 800 mg: 9.8 (8.2–11.4) | 600 mg: 0.49‡ | |||
| AUC0–24 h | Plasma AUC0–24 h | 800 mg: 0.47‡ | |||
| 600 mg: 29.7 | 600 mg: 60.8 (54.2–67.4) | ||||
| 800 mg: 40.2 | 800 mg: 85.3 (69.4–101.2) | ||||
| Plasma AUC0-inf | |||||
| 600 mg: 67.9 (60.9–74.9) | |||||
| 800 mg: 93.1 (79.7–106.5) | |||||
| HPLC-Fluor | Cmax: ND | Serum Cmax:ND | — | — | |
| Conc at day 3,t=2 h: 3.36 ± 2.23 | Serum conc at day 3,t=2 h: 3.15 ± 1.52 | ||||
| AUC: ND | Serum AUC: ND | ||||
| HPLC-UVC | Cmax | Serum Cmax: ND | 0. 508 | Corr | |
| 100 mg: 0.5133–0.7333 | 100 mg: 0.7682–1.1785 | 100 mg: 0.42–0.71 | AUC0–6h | ||
| 200 mg: 0.9442–2.0530 | 200 mg: 1.8792–3.0890 | 200 mg: 0.40–0.63 | |||
| AUC0–6 h | Serum AUC0–6 h | ||||
| 100 mg: 1.7368–2.4653 | 100 mg:2.8755–4.6179 | ||||
| 200 mg: 3.8850–6.5199 | 200 mg: 7.0148–10.0860 | ||||
| Paper disk method with Bacillus subtilis and Escherichia coli | Cmax: ND | Serum Cmax: ND | 0.9969 | Corr | |
| AUC: ND | Serum AUC: ND | ||||
| Agar-well diffusion method with Escherichia coli | No Cmax detectedin 40% of samples of day2 Conc: 0.1–0.7 | Serum Cmax: 3.6 ± 1.7 | — | — | |
| AUC: ND | Serum AUC0–inf : 47.3 ± 28.3 | ||||
| RP-HPLC-UV (serum), paper disk-plate method with Bacillus subtilis or Escherichia coli (serum and saliva) | Cmax: ND | Serum Cmax of single dose | 0.655 | Corr | |
| Highest measured conc of single doses | 100 mg: 0.95 ± 0.17 | ||||
| 100 mg: 0.77 ± 0.17 at 2 h | 300 mg: 2.65 ± 0.41 | ||||
| 300 mg: 2.51 ± 0.24 at 2 h | 300 mg fasting: 3.86 ± 0.85 | ||||
| 300 mg fasting: 3.02 ± 1.20 at 1 h | 600 mg: 6.64 ± 0.76 | ||||
| 600 mg: 4.44 ± 0.79 at 3 h | |||||
| AUC: ND | Serum AU0–24 h of angle doses | ||||
| 100 mg: 6.02 ± 1.05 | |||||
| 300 mg: 21.70 ± 2.63 | |||||
| 300 mg fasting: 29.38 ± 4.74 | |||||
| 600 mg: 68.40 ± 7.61 | |||||
| Paper disk method with Bacillus subtilis and Escherichia coli | Cmax | Serum Cmax | Non-HD: 0.75 | Corr | |
| Non-HD: 1.32 | Non-HD: 1.68 | HD: 1.07 | |||
| HD: ND | HD: ND | Non-HD: 0.61‡ | AUC | ||
| AUC | Serum AUC | ||||
| Non-HD: 64.29 | Non-HD: 105.23 | ||||
| HD: ND | HD: ND | ||||
| Gatifloxacin | RP-HPLC-Fluor | Cmax | Serum Cmax | 0.81 | Corr |
| 200 mg: 1.55 ± 0.51 | 100 mg: 0.873 ±0.187 | ||||
| 400 mg: 3.05 ± 0.74 | 200 mg: 1.71 ± 0.35 | ||||
| 400 mg: 3.35 ± 0.55 | |||||
| 600 mg: 5.41 ± 1.13 | |||||
| Serum 300 mg b.i.d.: | |||||
| Day 1: 2.77 ± 0.54 | |||||
| Day 4: 3.45 ± 0.63 | |||||
| Day 7: 3.36 ± .46 | |||||
| AUC: ND | Serum AUC0–inf | ||||
| 100 mg: 7.00 ± 1.36 | |||||
| 200 mg: 14.5 ± 2.6 | |||||
| 400 mg: 32.4 ± 4.1 | |||||
| 600 mg: 53.5 ± 2.6 | |||||
| HPLC-Fluor | Cmax | Plasma Cmax | About 1 | Conc | |
| 400 mg: day 1: 3.2† | 400 mg: day 1: 3.682 ± 0.75, day 15:4.226 ± 1.283 | ||||
| 600 mg:day 1:7.0† | 600 mg: day1: 5.266 ± 1.237, Day 15: 5.811 ± 1.043 | ||||
| AUC: ND | Plasma AUC0–inf | ||||
| 400 mg: day 1: 30.871 ± 4.390 | |||||
| 600 mg: day 1: 51.728 ± 7.625 | |||||
| Plasma AUC0–24 h: | |||||
| 400 mg: day 15: 34.409 ± 5.740 | |||||
| 600 mg: day 15: 61.763 ± 10.198 | |||||
| Amikacin | Paper disk method with Bacillus subtilis | Cmax: ND | Serum Cmax: ND | — | — |
| AUC: ND | Serum AUC: ND | ||||
| ND | Cmax: ND | Serum Cmax: ND | — | — | |
| AUC: ND | Serum AUC: ND | ||||
| Linezolid | HPLC-MS/MS | Cmax: 10.1 (8.2–10.7) | Serum Cmax: 10.9 (6.8–11.5) | 0.97 | Conc serum-saliva |
| AUC0–12 h: 62.1 (50.5–89.2) | Serum AUC0–12 h: 63.9 (47.8–83.8) | 1.03‡ | Conc saliva-serum | ||
| 0.97‡ | AUC0–12 h | ||||
| 1.05 | Corrserum-saliva | ||||
| 0.95‡ | Corr saliva-serum | ||||
| HPLC-UV | Cmax: ND | Plasma Cmax: ND | — | — | |
| Highest measured mean conc at t = 3h:7.1–17.0 | Highest measured mean plasma conc at t=3 h: 10.4–14.1 | ||||
| AUC: ND | Plasma AUC: ND | ||||
| Amoxicillin/clavulanate | Bioassay with Sarcina lutea | Not detected | Plasma Cmax: 14.56 (11.03–18.1) | — | — |
| AUC: ND | Plasma AUC0–4h: 24.4 (21.1–27.6) | ||||
| Plasma AUC0–inf: 25.9 (21.8–30.1) | |||||
| RP-HPLC-UV | Not detected | Plasma Cmax | — | — | |
| H. Pylori−: 2:51.9 (29.0–74.8) | |||||
| H. Pylori+: 41.7 (23.3–60.0) | |||||
| AUC: ND | Plasma AUC0–2h | ||||
| H. Pylori−: 1587.7 (1208.2–1967.2), H. Pylori+: 1203.3 (989.3–1417.3) | |||||
| Plasma AUC0–inf | |||||
| H. Pylori−: 1755.1 (1394.0–2116.2), H. Pylori+: 1358.4 (1135.4–1581.4) | |||||
| Micro-method with Sarcina lutea | Cmax= ND Highest measured conc at t= 2 h |
Serum Cmax: ND Highest measured serum conc at t=1 h |
— | — | |
| 15 mg/kg: 0.3 (0–0.36); Detected in 50% of samples | 15 mg/kg: Fasting: 5.4 ± 0.76; Fed: 3.2 ± 0.48 | ||||
| 25 mg/kg: 0.17 (0–0.4); Detected in70% of samples | 25 mg/kg: Fasting: 8.9 ± 1.4; Fed: 7.9 ± 1.7 | ||||
| AUC: ND | Serum AUC | ||||
| 15 mg/kg: fasting: 16; 15 mg/kg, fed:14 | |||||
| 25 mg/kg: fasting: 24; 25 mg/kg, fed: 24 | |||||
| RP-LC-ESI-MS (plasma), RPHPLC-UV (saliva) | Cmax | Plasma Cmax | Amoxil: 0.47‡ | AUC0–8 h | |
| Amoxil: 6.37 ± 3.63 | Amoxil: 14.37 ± 6.01 | Amoxicillin EMS: 0.34‡ | |||
| Amoxicillin EMS: 6.23 ± 4.89 | Amoxicillin EMS: 16.94 ± 6.39 | Amoxil: 0.55‡ | |||
| AUC0–8 h | Plasma AUC0–8 h | Amoxicillin EMS: 0.34‡ | AUC0–inf | ||
| Amoxil: 22.83 ± 13.92 | Amoxil: 48.28 ± 20.00 | ||||
| Amoxicillin EMS: 18.78 ± 14.62 | Amoxicillin EMS: 55.10 ± 14.25 | ||||
| AUC0–inf | Plasma AUC0–inf | ||||
| Amoxil: 26.29 ± 14.27 | Amoxil: 47.62 ± 18.42 | ||||
| Amoxicillin EMS: 18.50 ± 15.06 | Amoxicillin EMS: 54.14 ± 12.38 | ||||
| Agar diffusion method with Bacillus subtilis | Cmax: ND | Serum Cmax: ND | — | — | |
| Conc at est Tmax(2 h): 0.03 ± 0.01 | Serum conc at est Tmax(2 h): 7.16 ± 2.53 | ||||
| AUC: ND | Serum AUC: ND | ||||
| Doripenem | UHPLC-MS/MS | Cmax: 0.5 ± 0.2 | Plasma Cmax: 15.3 ± 6.00 | 0.04 ± 0.03 | AUC0–inf |
| AUC0–8 h: 0.9 ± 0.5 | Plasma AUC0–8 h: 26.0 ± 9.90 | 0.03‡ | AUC0–8 h | ||
| AUC0–inf: 1.0 ± 0.5 | Plasma AUC0–inf: 26.3 ± 10.1 | ||||
| Clarithromycin | RP-HPLC-coulometric detection | Cmax at steady state | Serum Cmax | — | — |
| Day 3: 1.9† | Day 1: 2.1 ± 0.7 | ||||
| Highest measured conc | Day 3:2.3 ± 1.0 | ||||
| Day 1 at 4 h: 1.06 ± 0.7 | |||||
| Day 3 at 4 h: 1.87 ± 1.3 | |||||
| AUC: ND | Serum AUC0–inf | ||||
| Day 1: 15.3 ± 4.8 | |||||
| Day 3: 27.9 ± 12.4 | |||||
| HPLC-EC | Cmax: 500 mg q.d. | Serum Cmax: 500 mg q.d.: | 0.25–0.40 | Conc | |
| Day 1: 0.89 ± 0.32, day 5: 1.06 ± 0.38 | Day 1: 2.10 ± 0.49, day 5: 2.33 ± 0.58 | ||||
| 250 mg b.i.d. | 250 mg b.i.d. | ||||
| Day 1: 0.31 ± 0.15, day 5: 0.29 ± 0.07 | Day 1: 0.94 ± 0.33, day 5: 1.23 ± 0.37 | ||||
| AUC: ND | Serum AUC0–12 h | ||||
| 250 mg b.i.d., day 1: 5.21 ± 1.31 | |||||
| Serum AUC0–inf | |||||
| 500 mg q.d., day 1: 15.63 ± 4.46 | |||||
| 250 mg b.i.d., day 1: 5.80 ± 1.31 | |||||
| Serum AUCss | |||||
| 500 mg q.d., day 5: 18.32 ± 4.77 | |||||
| 250 mg b.i.d., day 5: 7.85 ± 2.00 | |||||
| HPLC-EC | Cmax | Serum Cmax | Around 0.5 | Conc | |
| Day 1: 0.9† | Day 1: 1.76 ± 0.51 | ||||
| Day 7: 1.6† | Day 7: 2.41 ± 0.81 | ||||
| AUC: ND | Serum AUC0–12 h | ||||
| Day 1: 10.6 ± 2.51 | |||||
| Day 7: 18.0 ± 5.0 | |||||
| AUC0–inf | |||||
| Day 1: 12.6 ± 3.34 | |||||
| HPLC-MS/MS | Cmax: 2.8 (2.0–3.4) | Serum Cmax: 1.7 (1.3–2.7) | 3.07 | Conc serum-saliva | |
| AUC0–12 h: 10.7 (9.4–12.1) | Serum AUC0–12 h: 8.2 (6.2–12.2) | 0.33‡ | Conc saliva-serum | ||
| 1.30‡ | AUC0–12 h | ||||
| 2.67 | Corr serum-saliva | ||||
| 0.37‡ | Corr saliva-serum | ||||
| Bioassay with Sarcina lutea | Cmax: 3.87 (3.03–4.72) | Plasma Cmax : 5.39 (4.54–6.23) | 0.75‡ | AUC0–4h | |
| AUC0–4 h: 9.48 (7.56–11.41) | Plasma AUC0–4 h: 12.7 (11.5–13.9) | ||||
| Plasma AUC0–inf: 29.5 (20.2–38.8) | |||||
| Agar plate diffusion method with Bacillus subtilis | Cmax | Plasma Cmax | Day 1: 0.73‡ | AUC0–10 h | |
| Day 1: 2.38 (0.78–4.58) | Day 1: 2.98 (1.74–4.94) | Day 10: 0.99‡ | |||
| Day 10: 4.29 (2.67–7.39) | Day 10: 3.87 (2.23–7.41) | ||||
| AUC0–10 h | Plasma AUC0–10 h | ||||
| Day 1: 13.3 (5.2–28.4) | Day 1: 18.1 (9.8–27.8) | ||||
| Day 10: 27.4 (20.2–35.9) | Day 10: 27.8 (18.8–42.8) | ||||
| Agar diffusion method with Micrococcus luteus | Cmax: ND | Serum Cmax: ND | — | — | |
| Conc at estimated Tmax (2h): 2.72 ± 0.87 | Serum conc at estimated Tmax (2 h): 4.04 ± 1.14 | ||||
| AUC: ND | Serum AUC | ||||
| Paper disk method with Micrococcus luteus | Cmax: 1.93457 | Serum Cmax: 1.486240 | 0.95‡ | AUC | |
| AUC: 17.7031 | Serum AUC: 18.584 | ||||
The legend of the graph in the article referred to the upper curve as a result of a 4<X)-mg dose. We assumed this was a mistake; therefore, the Cmax values of -MX) and 600 mg arc exchanged. Authois of the article were contacted but did not respond.
Estimated value.
Calculated value.
alt. d., every other day; AOM, acme otitis media; AUC. area under the time-concentration curve; b.id.. twice a day; Cmax. peak concentration; conc, concentration; com. slope of correlation of saliva and plasma or scrum; EC. clcctro-chcmical; fluor, fluorescence; HD. hemodialysis; HPLC. high-performance liquid chromatography; HV. healthy volunteers; ITB. intestinal TB, i.v.. intrav enous; ND. not defined; NS. non-stimulatcd; NSCLC. non-small cdl lung cancer, p.o.. per oral; PI B. pulmonary TB; q.d., once a d3y; RP. reversed phase; S. stimulated; SCI. spinal cord injury; SP. spectrophotometry; t.i.d.. three times a day; Tmax, time of peak concentration; UV, ultraviolet-visible spectrophotometry.
All included articles were assessed for risk of bias. Baglie et al,22 Biasini et al,23 Brown et al,24 Fujita et al,25 Goddard et al,26 and Ohkubo et al27 were considered at a serious risk of bias (Table 2). This means that the studies have some serious problems with bias for a nonrandomized study.20 Baglie et al22 and Brown et al24 both used different analytical methods for saliva and plasma. This could have introduced bias in the measurement of outcomes. Fujita et al25 and Biasini et al23 were judged at a serious risk of bias because important information, for instance, the sampling or analytical procedure, was scarcely described. Fujita et al25 did not mention any validation of the analytical method, whereas Biasini et al23 provided too little information about the analytical procedures to estimate the risk of bias. Goddard et al26 did not use paired sampling for all time points. Ohkubo et al27 sampled saliva after tooth brushing. This could have contaminated the samples with blood. All other studies were estimated at a moderate risk of bias, meaning the study provides evidence for a nonrandomized study but is not comparable with a well-performed randomized trial.20
TABLE 2.
Results of Risk of Bias Assessment of Included Articles Using Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) Tool
| Study | Confounding | Selection of Participants |
Classification of Interventions |
Deviations From Interventions |
Missing Data |
Measurement of Outcomes |
Selection of Reported Result |
Overall |
|---|---|---|---|---|---|---|---|---|
| Baglie et al | + | + | + | + | + | − | +/− | − |
| Biasini et al | − | + | + | + | − | ? | +/− | − |
| Bolhuis et al | + | + | + | + | + | + | +/− | +/− |
| Brown et al | + | + | + | + | + | − | +/− | − |
| Burian et al | + | + | + | + | + | + | +/− | +/− |
| Burkhardt et al, 2006 |
+ | + | + | + | + | + | +/− | +/− |
| Burkhardt et al, 2002 |
+ | + | + | + | + | + | +/− | +/− |
| Darouiche et al | + | + | + | + | + | + | +/− | +/− |
| Edlund et al, 2000 | + | + | + | + | + | + | +/− | +/− |
| Edlund et al, 1998 | + | + | + | + | + | + | +/− | +/− |
| Ezejiofor et al | + | + | + | + | + | + | +/− | +/− |
| Fassbender et al | + | + | + | + | + | + | +/− | +/− |
| Fujita et al | − | + | + | + | + | + | +/− | − |
| Ginsburg et al | + | + | + | + | + | + | +/− | +/− |
| Goddard et al | − | + | + | + | + | + | +/− | − |
| Gurumurthy et al | + | + | + | + | + | + | +/− | +/− |
| Hara et al | + | + | + | + | + | + | +/− | +/− |
| Hutchings et al | + | + | + | + | + | + | +/− | +/− |
| Ichihara et al | + | + | + | + | +/− | + | +/− | +/− |
| Immanuel et al | + | + | + | + | + | + | +/− | +/− |
| Kees et al | + | + | + | + | + | + | +/− | +/− |
| Koizumi et al | + | + | + | + | + | + | +/− | +/− |
| Kozjek et al | + | + | + | + | + | + | +/− | +/− |
| Kumar et al | + | + | + | + | + | + | +/− | +/− |
| Leigh et al | + | + | + | + | + | + | +/− | +/− |
| Masumi et al | + | + | + | + | + | + | +/− | +/− |
| McCracken et al | + | + | + | + | + | + | +/− | +/− |
| Mignot et al | + | + | + | + | + | + | +/− | +/− |
| Miya et al | + | + | + | + | + | + | +/− | +/− |
| Morihana et al | + | + | + | + | + | + | +/− | +/− |
| Müller et al | + | + | + | + | + | + | +/− | +/− |
| Murthy et al | + | + | + | + | + | + | +/− | +/− |
| Nakashima et al | + | + | + | + | + | + | +/− | +/− |
| Ohkubo et al | − | + | + | + | + | + | +/− | − |
| Orisakwe et al, 2004 |
+ | + | + | + | + | + | +/− | +/− |
| Orisakwe et al, 1996 |
+ | + | + | + | + | + | +/− | +/− |
| Ortiz et al | + | + | + | + | + | + | +/− | +/− |
| Stass et al | + | + | + | + | + | + | +/− | +/− |
| Suryawati et al | + | + | + | + | + | + | +/− | +/− |
| Tsubakihara et al | + | + | + | + | + | + | +/− | +/− |
| Warlich et al | + | + | + | + | + | + | +/− | +/− |
| Wüst et al | + | + | + | + | + | + | +/− | +/− |
Low risk of bias (+), moderate risk of bias (+/−), serious risk of bias (−). and no information (?).
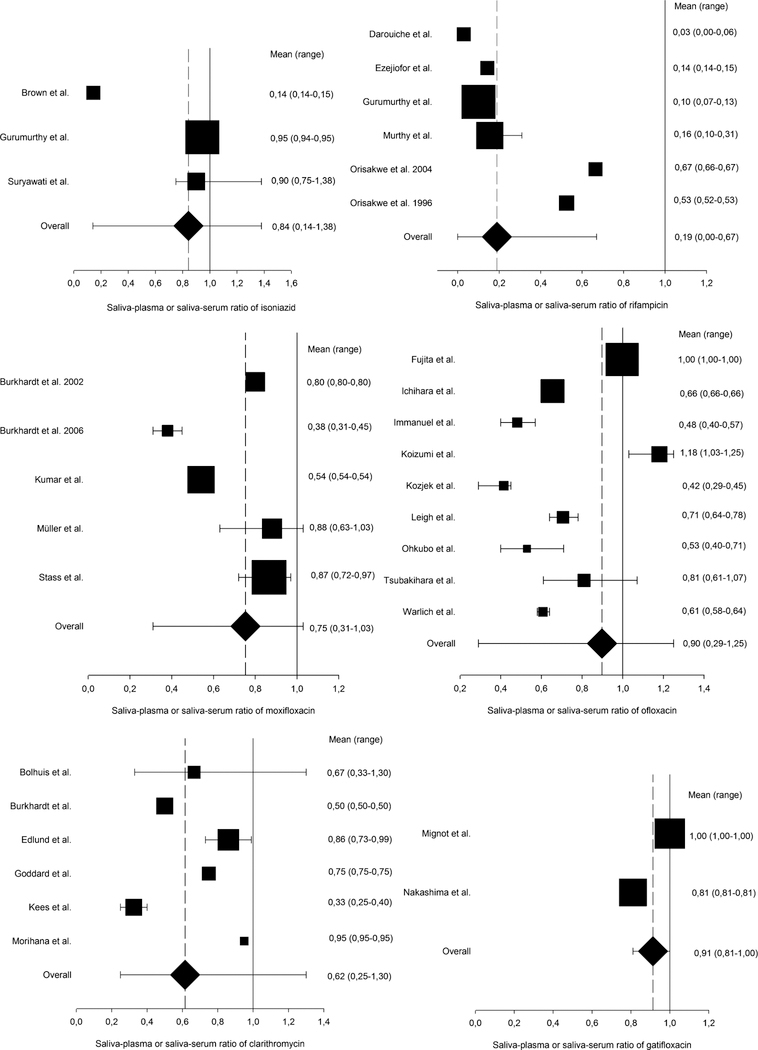
In general, a large variability in saliva–plasma and saliva–serum was observed for isoniazid, rifampicin, moxifloxacin, ofloxacin, and clarithromycin (Figures 2 and 3). The saliva–plasma and saliva–serum ratios of rifampicin were clustered in 2 groups: Murthy and Kumar,28 Darouiche et al,29 Ezejiofor et al,30 and Gurumurthy et al,31 with ratios of 0.1–0.2, in contrast to Orisakwe et al,32 and Orisakwe and Ofoefule33 with ratios around 0.6. A similar clustering effect was seen with moxifloxacin. Kumar et al34 and Burkhardt et al35 reported saliva–plasma and saliva–serum ratios of 0.4– 0.6, whereas Stass et al,36 Müller et al,37 and Burkhardt et al38 found ratios of 0.8–0.9. Isoniazid, ofloxacin, and clarithromycin showed an overall large diversity of reported saliva–plasma and saliva–serum ratios. For gatifloxacin, linezolid, and doripenem, relatively small ranges of saliva– plasma and saliva–serum ratios were found.
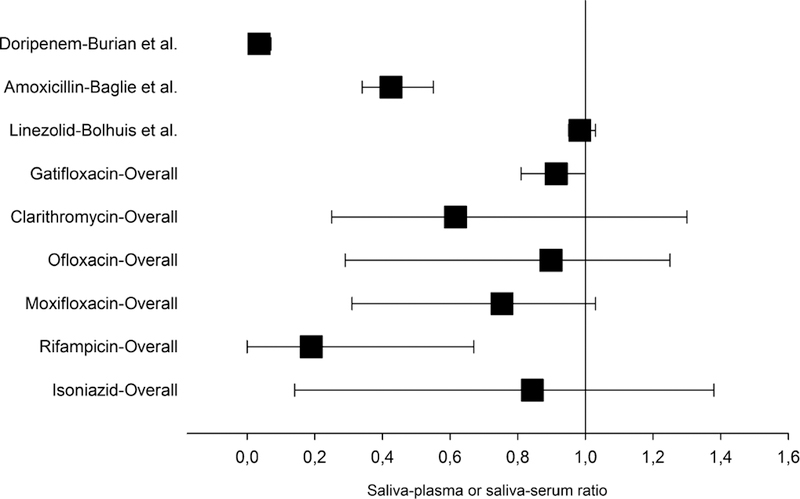
FIGURE 2.
Saliva–plasma or saliva–serum ratio of anti-TB drugs. The weighted mean () and range of saliva–plasma or saliva–serum ratio are displayed per drug. Mean (range) of doripenem: 0.04 (0.01–0.07); amoxicillin: 0.43 (0.34–0.55); linezolid: 0.98 (0.95–1.03); gatifloxacin: 0.91 (0.81–1.00); clarithromycin: 0.62 (0.25–1.30); ofloxacin: 0.90 (0.29–1.25); moxifloxacin: 0.75 (0.31–1.03); rifampicin: 0.19 (0.00–0.67); and isoniazid: 0.84 (0.14–1.38). For doripenem, amoxicillin, and linezolid, only 1 study with a saliva–plasma or saliva– serum ratio was included. For the other drugs, the numbers of included studies were as follows: gatifloxacin (n = 2), clarithromycin (n = 6), ofloxacin (n = 9), moxifloxacin (n = 5), rifampicin (n = 6), and isoniazid (n = 3).
FIGURE 3.
Saliva–plasma or saliva–serum ratios of anti-TB drugs. Top left: isoniazid; top right: rifampicin; middle left: moxifloxacin; middle right: ofloxacin; bottom left: clarithromycin; and bottom right: gatifloxacin. As per drug, the saliva–plasma or saliva–serum ratios of the included articles are displayed as weighted mean () with range. In addition, the overall mean (♦) and range were determined for each drug. All numerical values of mean and range are presented to the right of the graphs.
All included studies of amoxicillin/clavulanate administered only amoxicillin instead of the combination with clavulanate that is used in TB treatment. The small range of saliva– plasma ratios for amoxicillin is distorted. In fact, all studies, except Baglie et al,22 reported a very low or even no detectable salivary concentration of amoxicillin, indicating a saliva–plasma or saliva–serum ratio of close to 0. By contrast, Baglie et al22 reported amoxicillin quantifiable salivary Cmax and AUC values as well asa saliva–plasma ratio of 0.34–0.55. The 2 included studies of amikacin, Masumi et al39 and Biasini et al23 did not report any saliva–plasma or saliva–serum ratios.
Several studies reported a time-dependent saliva–plasma or saliva–serum ratio. Suryawati and Santoso40 reported a rifampicin saliva–serum ratio of 1.09 ± 0.29 during the absorption phase and 0.81 ± 0.05 during the elimination phase. For moxifloxacin, Burkhardt et al38 and Müller et al37 observed a saliva–plasma or saliva–serum ratio higher than 1 during the first 2 hours after administration. Thereafter, the ratio declined to below 1. A time-dependent saliva–serum ratio was also found for ofloxacin by Koizumi et al.41 During the first 4 hours after administration, the saliva–serum ratio was below 1, and during the following 4 hours, the ratio increased to above 1 and remained above 1 during 8–16 hours after administration. After 16 hours, a mean saliva–serum ratio of 1.14 was measured.
DISCUSSION
In this systematic review, we aimed to investigate whether TDM of anti-TB drugs using saliva samples is feasible. We found this to be likely possible for linezolid and gatifloxacin, whereas possible for isoniazid, rifampicin, ofloxacin, moxifloxacin, and clarithromycin. For other anti-TB drugs, either too few data were available, or the drugs seemed unlikely to be feasible for salivary TDM.
The review was strengthened by the inclusion of all WHO-approved anti-TB drugs as well as ertapenem, faropenem, and doripenem because interest in using these other carbapenems as part of anti-TB treatment has increased.42 Ofloxacin and clarithromycin were still included, despite the WHO recommendation to not use these drugs.3 In specific situations, ofloxacin and clarithromycin might be useful to treat difficult cases.43 The information gained from this systematic review could also be applied to other infectious diseases.
Isoniazid,24,31,40 moxifloxacin,34–38 ofloxacin,21,25,27,41,44–49 and clarithromycin26,38,50–52 showed varying saliva–plasma and saliva–serum ratios. The same issue applied to rifampicin, although rifampicin showed some low saliva–plasma and saliva–serum ratios that could complicate the detection of the drug in saliva for low-dosage regimes. A wide range of saliva–plasma and saliva–serum ratios is especially caused by highly varying mean ratios across studies, not by wide ranges of study-specific ratios. A wide range of saliva–plasma and saliva–serum ratios could be caused by differences in study population, dose, saliva sampling method, and analytical method between the studies. The influences of these factors on the saliva–plasma and saliva–serum ratio are hard to determine because of the great variation of these factors among the included studies. Salivary TDM of these 5 anti-TB drugs may be possible; however, 1 workable saliva–plasma or saliva–serum ratio is required (Table 3). For instance, if the saliva–plasma ratio of isoniazid of 0.14 as found by Brown et al24 is applied to predict AUC values in blood using salivary AUC, the calculated AUC in blood will be almost 7 times higher than if the ratio of Gurumurthy et al31 (0.95) or of Suryawati and Santoso40 (0.90) is used. These substantial differences could have an effect on dosing recommendations based on such TDM results. However, the quality of Brown et al24 was unclear, as said study was classified as at a serious risk of bias.
TABLE 3.
Summary of Salivary TDM Potentials of all Anti-TB Drugs
| Group | Anti-TB Drug | Conclusion | Comments |
|---|---|---|---|
| First-line drugs | Isoniazid | Maybe possible | Wide range of saliva-plasma and saliva-serum ratios. |
| Rifampicin | Maybe possible | Wide range of saliva-plasma and saliva-serum ratios. Some low ratios reported. |
|
| Ethambutol | No data | Studies needed. | |
| Pvrazi namtde | No data | Studies needed. | |
| Group A: fluoroquinolones | Levofloxacin | No data | Studies needed. |
| Moxifloxacin | Maybe possible | Wide range of saliva-plasma and saliva-serum ratios. |
|
| Gatifloxacin | Likely possible | Promising saliva-plasma and saliva- serum ratios. Additional study in patients with TB needed. |
|
| Group B: second-line injectable agents |
Amikacin | No data | Studies needed. Included studies did measure salivary concentrations, but no Cmax, AUC, or saliva- plasma or saliva-serum ratio was reported. |
| Capreomycin | No data | Studies needed. | |
| Kanamycin | No data | Studies needed. | |
| Streptomycin | No data | Studies needed. | |
| Group C: other core second-line agents |
Ethionamide | No data | Studies needed. |
| Prothionamide | No data | Studies needed. | |
| Cycloserine | No data | Studies needed. | |
| Terizidone | No data | Studies needed. | |
| Linezolid | Likely possible | Promising saliva-serum ratios. More studies with other dosage regimes needed. |
|
| Clofazimine | No data | Studies needed. | |
| Group Dl: add-on agents | Pyrazinamide | See first-line drugs | See first-line drugs. |
| Ethambutol | |||
| High-dose isoniazid | |||
| Group D2: add-on agents | Bedaquiline | No data | Studies needed. |
| Delamanid | No data | Studies needed. | |
| Group D3: add-on agents | p-aminosalicylic acid | No data | Studies needed. |
| Imipenem/cilastatin | No data | Studies needed. | |
| Meropenem | No data | Studies needed. | |
| Amoxicillin/clavulanate | Probably not possible | Low or undetectable drug concentrations in saliva, probably due to low lipophilicity. |
|
| Thioacetazone | No data | Studies needed. | |
| Ofloxacin | Maybe possible | Wide range of saliva-plasma and saliva-serum ratios. |
|
| Clarithromycin | Maybe possible | Wide range of saliva-plasma and saliva-serum ratios. |
|
| Ertapenem | No data | Studies needed. | |
| Doripenem | Probably not possible | Low saliva-plasma ratio, probably due to low lipophilicity. More studies with other dosage regimes needed. |
|
| Faropenem | No data | Studies needed. |
The conclusion of this systematic review is displayed as per anti-TB drug using “No data.” “Probably not possible.” “Maybe possible.” and “Likely possible.” Besides, comments are added to clarify these conclusions.
For gatifloxacin and linezolid, salivary TDM is likely possible because of the narrow range of saliva–serum and saliva–plasma ratios.51,53,54 An additional study of gatifloxacin, preferably in patients with TB, should be performed to confirm the reported findings because pharmacokinetic parameters could significantly differ in patients with TB using several anti-TB drugs compared with healthy volunteers. However, in 2006, the US Food and Drug Administration (FDA) officially warned that gatifloxacin is associated with an elevated risk of dysglycemia.55,56 So, gatifloxacin might be replaced in TB treatment by other fluoroquinolones, such as moxifloxacin or levofloxacin, in the future. Additional studies of linezolid using other dosages are necessary to rule out any dose dependency of the saliva–serum ratio and to complete the salivary pharmacokinetic profile of linezolid.
For doripenem and amoxicillin/clavulanate, salivary TDM is probably not possible because of very low salivary drug concentrations (Table 3). Both doripenem and amoxicillin are hydrophilic drugs and this complicates passage through membranes.57,58 This problem could also apply to the other carbapenems. More studies comparing doripenem concentrations in blood and saliva are needed to confirm the results of Burian et al59 and to rule out any dose dependency. Nearly all studies regarding amoxicillin/clavulanate reported undetectable amoxicillin concentrations in saliva.26,60–62 Only Baglie et al22 reported a substantial salivary concentration of amoxicillin and a saliva–plasma ratio. A possible reason is that this study administered the highest dose of all included studies. Besides, the variant results of Baglie et al22 could also be explained by the serious risk of bias.
More information is needed about the salivary pharmacokinetics of amikacin because no saliva–plasma or saliva– serum ratios or salivary AUC values are reported in the analyzed articles.23,39
For many anti-TB drugs, salivary pharmacokinetic information is lacking, even for the first-line drugs pyrazinamide and ethambutol (Table 3). As the incidence of drug-susceptible TB is significantly greater than the incidence of MDR-TB, the first-line drugs have to be prioritized in future studies of salivary TDM. Especially, for pyrazinamide, more information about the pharmacokinetic parameters in saliva versus blood is important, as it is part of the MDR-TB regimen.3 Besides, pyrazinamide is one of the few anti-TB drugs for which low serum concentrations are associated with poor treatment outcomes.63,64 The priority of second-line drugs should be ranked according to the grouping system of WHO as shown in Table 3. Anti-TB drugs in group A are considered the most beneficial in MDR-TB treatment and will be often used, whereas groups D2 and D3 contain add-on anti-TB drugs that will be less frequently prescribed.
Obviously, more pharmacokinetic studies comparing anti-TB drug concentrations in saliva and plasma or serum are needed before salivary TDM could be implemented in the treatment of TB. To overcome the observed variability in saliva–plasma and saliva–serum ratios, large study populations and comparable study designs, study populations, dosage regimes, saliva sampling methods (stimulated versus nonstimulated), and analytical methods should be used in future studies.
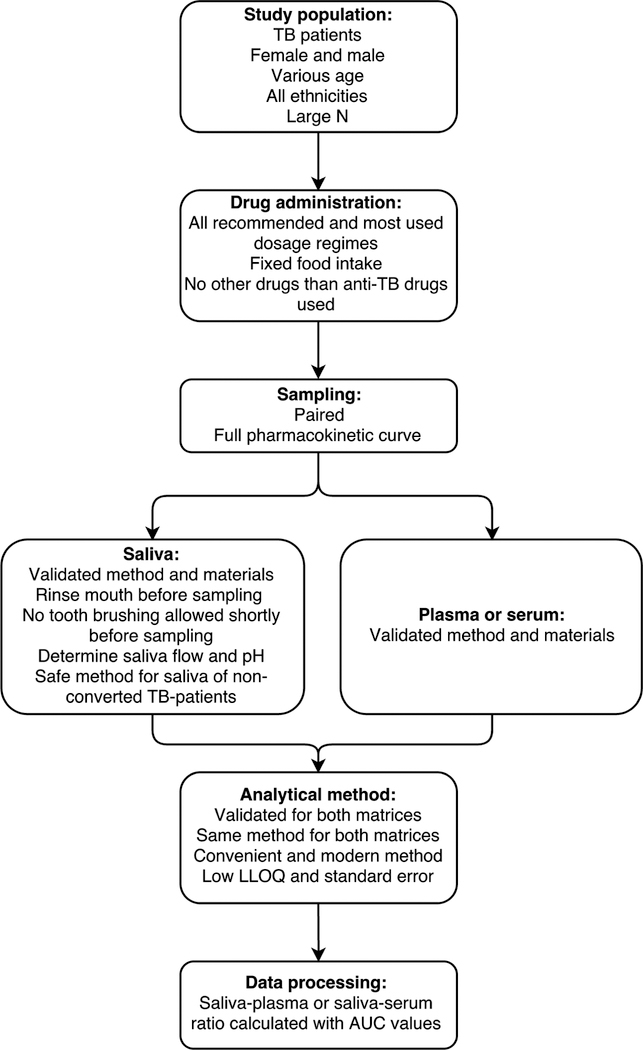
An ideal design for this kind of study is proposed in Figure 4 to assist and advice all future researchers. Most important factors are inclusion of patients with TB, paired sampling, validation, salivary flow, salivary pH, and saliva–plasma or saliva–serum ratios calculated using AUC values.
FIGURE 4.
Ideal study design for pharmacokinetic studies comparing anti-TB drug concentrations in saliva and plasma or serum. LLOQ, lower limit of quantification; N, number.
A limitation of this systematic review is that many studies included healthy volunteers instead of patients with TB. It is hard to extrapolate the findings of these studies to the clinic because the effect of TB on the salivary pharmacokinetics is unknown. Furthermore, almost none of the included studies reported the saliva flow and pH, although both can influence the salivary drug concentration.12,18 The salivary flow and pH values were not included in this review because of a lack of information. In future studies of salivary pharmacokinetics, salivary flow and pH should be measured to provide a complete profile. Besides, risk of bias assessment of the included articles was problematic because no tool is validated for pharmacokinetic studies. The ROBINS-I tool was not used in its validated structure as a result of changes in the confounding section. A validated and appropriate tool for the risk of bias assessment of pharmacokinetic studies is needed to assess the quality of these studies. Overall, our review found predictable saliva–plasma or saliva–serum ratios of less than 1. However, 3 studies of isoniazid and moxifloxacin reported saliva–plasma or saliva–serum ratios with values of above 1 during the absorption phase.37,38,41 A high ratio during the absorption phase could be explained by drug adhesion to the oral mucosa.38 Normally, this effect is averted by rinsing the mouth with water before sampling, but this precaution was not reported in the 2 moxifloxacin studies.37,38 An active transport system across the salivary epithelium can also cause a high concentration in saliva.37 However, this seems unlikely because not all studies of isoniazid and moxifloxacin reported this high saliva–plasma or saliva– serum ratios.
In the future, many TB endemic settings may benefit from TDM with saliva samples, particularly if the saliva sample collection is standardized and sample analysis is optimized. For instance, salivary TDM would allow patients the option to sample themselves at any location and afterward bring their saliva samples to a local health post. Importantly, for the first-line drugs isoniazid and rifampicin, several analytical methods using ultraviolet-visible (UV-VIS) spectrophotometry have been used in several studies.65–67 In addition, for ethambutol,68 moxifloxacin,69 levofloxacin,70 ofloxacin,71 paraaminosalicylic acid,72 amoxicillin/clavulanate,73 and imipenem/cilastatin,74 UV-VIS spectrophotometry methods were described in literature. Remarkably, 1 analytical method that determines isoniazid, rifampicin, and pyrazinamide simultaneously with a UV-VIS spectrophotometer was published.75 After validation in both blood and saliva, these UV-VIS methods could easily be implemented in referral laboratories of more resource-limited settings because of their relative simplicity and lower costs. Of caution, however, before implementing salivary TDM, the chemical stability of anti-TB drugs in saliva should be thoroughly studied to determine the necessity for rapid sample analysis. Isoniazid, for instance, is known to be unstable in both saliva and blood.76,77 Furthermore, the eventuality of M. tuberculosis being culturable from the saliva of nonconverted patients with TB is an extra factor that must be taken into account. The sampling method should be thoroughly designed and tested in advance to create a safe technique for the investigators working with the saliva samples and all other people involved. A recent study showed that membrane filtration (pore size 0.22 mcg) is suitable for decontamination of saliva samples containing M. tuberculosis.78 However, before membrane filtration can be implemented in salivary TDM, recovery testing should rule out any adhesion of the drug to membranes.
CONCLUSION
In this systematic review, we summarized the current knowledge about the salivary and blood concentrations of anti-TB drugs and their saliva–plasma or saliva–serum ratio in humans and determined for which anti-TB drugs salivary TDM should be further investigated either in basic pharmacokinetic studies or in larger validation cohorts.
Unfortunately, for most anti-TB drugs, salivary pharmacokinetic information is entirely lacking. For these drugs, such as pyrazinamide, pharmacokinetic studies comparing drug concentrations in saliva and blood are needed. For amikacin, pharmacokinetic studies using saliva samples were found but without saliva–plasma or saliva–serum ratios. Salivary TDM is likely possible for gatifloxacin and linezolid because of their promising, narrow-ranged saliva–plasma and saliva–serum ratios. It may be possible for isoniazid, rifampicin, moxifloxacin, ofloxacin, and clarithromycin, but because of the wide range of saliva–plasma and saliva–serum ratios, further well-designed pharmacokinetic studies in patients with TB would be recommended. TDM with salivary samples is probably not feasible for doripenem and amoxicillin/clavulanate because of very low salivary concentrations. Overall, it seems worthwhile to further explore saliva as potential matrix for TDM of anti-TB drugs, especially for children.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2016 Geneva, Switzerland: WHO Press; 2016. [Google Scholar]
- 2.World Health Organization. Treatment of Tuberculosis Guidelines 4th ed. Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- 3.World Health Organization. Treatment Guidelines of Drug-Resistant Tuberculosis Geneva, Switzerland: WHO Press; 2016. [Google Scholar]
- 4.Zuur MA, Bolhuis MS, Anthony R, et al. Current status and opportunities for therapeutic drug monitoring in the treatment of tuberculosis. Expert Opin Drug Metab Toxicol 2016;12:509–521. [DOI] [PubMed] [Google Scholar]
- 5.Weiner M, Benator D, Burman W, et al. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis 2005;40: 1481–1491. [DOI] [PubMed] [Google Scholar]
- 6.Weiner M, Burman W, Vernon A, et al. Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med 2003;167:1341–1347. [DOI] [PubMed] [Google Scholar]
- 7.Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 2014;74:839–854. [DOI] [PubMed] [Google Scholar]
- 8.Heysell SK, Moore JL, Peloquin CA, et al. Outcomes and use of therapeutic drug monitoring in multidrug-resistant tuberculosis patients treated in Virginia, 2009–2014. Tuberc Respir Dis (Seoul) 2015;78:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiang TK, Ensom MH. A qualitative review on the pharmacokinetics of antibiotics in saliva: implications on clinical pharmacokinetic monitoring in humans. Clin Pharmacokinet 2016;55:313–358. [DOI] [PubMed] [Google Scholar]
- 10.Mullangi R, Agrawal S, Srinivas NR. Measurement of xenobiotics in saliva: is saliva an attractive alternative matrix? Case studies and analytical perspectives. Biomed Chromatogr 2009;23:3–25. [DOI] [PubMed] [Google Scholar]
- 11.Aps JK, Martens LC. Review: the physiology of saliva and transfer of drugs into saliva. Forensic Sci Int 2005;150:119–131. [DOI] [PubMed] [Google Scholar]
- 12.Raju KS, Taneja I, Singh SP, et al. Utility of noninvasive biomatrices in pharmacokinetic studies. Biomed Chromatogr 2013;27:1354–1366. [DOI] [PubMed] [Google Scholar]
- 13.Gorodischer R, Burtin P, Hwang P, et al. Saliva versus blood sampling for therapeutic drug monitoring in children: patient and parental preferences and an economic analysis. Ther Drug Monit 1994;16:437–443. [DOI] [PubMed] [Google Scholar]
- 14.Danhof M, Breimer DD. Therapeutic drug monitoring in saliva. Clin Pharmacokinet 1978;3:39–57. [DOI] [PubMed] [Google Scholar]
- 15.Spielberg F, Critchlow C, Vittinghoff E, et al. Home collection for frequent HIV testing: acceptability of oral fluids, dried blood spots and telephone results. HIV Early Detection Study Group. AIDS 2000;14: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 16.Vu DH, Koster RA, Alffenaar JW, et al. Determination of moxifloxacin in dried blood spots using LC-MS/MS and the impact of the hematocrit and blood volume. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879:1063–1070. [DOI] [PubMed] [Google Scholar]
- 17.Patsalos PN, Berry DJ. Therapeutic drug monitoring of antiepileptic drugs by use of saliva. Ther Drug Monit 2013;35:4–29. [DOI] [PubMed] [Google Scholar]
- 18.Jusko WJ, Milsap RL. Pharmacokinetic principles of drug distribution in saliva. Ann N Y Acad Sci 1993;694:36–47. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichihara N Phase I study on DL-8280. Chemotherapy 1984;32: 118–149. [Google Scholar]
- 22.Baglie S, Del Ruenis AP, Motta RH, et al. Plasma and salivary amoxicillin concentrations and effect against oral microorganisms. Int J Clin Pharmacol Ther 2007;45:556–562. [DOI] [PubMed] [Google Scholar]
- 23.Biasini GC, Pistocchi E, Miano A. Antibiotic treatment of lung disease in cystic fibrosis. Pediatr Med Chir 1983;5:157–160. [PubMed] [Google Scholar]
- 24.Brown SA, Ezejiofor NA, Barikpoar E, et al. Isoniazid pharmacokinetics in the presence of ofloxacin and norfloxacin antibiotics. Am J Ther 2014. doi: 10.1097/MJT.0000000000000032. [DOI] [PubMed]
- 25.Fujita K, Matsuoka N, Takenaka I, et al. Pharmacokinetics of ofloxacin—measurement of drug concentration in saliva of patients with impaired renal function. Drugs 1995;49:312–313. [DOI] [PubMed] [Google Scholar]
- 26.Goddard AF, Jessa MJ, Barrett DA, et al. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology 1996;111:358–367. [DOI] [PubMed] [Google Scholar]
- 27.Ohkubo T, Suno M, Kudo M, et al. Column-switching high-performance liquid chromatography of ofloxacin in human saliva and correlation of ofloxacin level in saliva and serum. Ther Drug Monit 1996;18:598–603. [DOI] [PubMed] [Google Scholar]
- 28.Murthy MGK, Kumar TP. Comparative levels of rifampicin in serum and saliva in tuberculosis patients by HPLC method. JEMDS 2016;5: 1827–1831. [Google Scholar]
- 29.Darouiche R, Perkins B, Musher D, et al. Levels of rifampin and ciprofloxacin in nasal secretions: correlation with MIC90 and eradication of nasopharyngeal carriage of bacteria. J Infect Dis 1990;162:1124–1127. [DOI] [PubMed] [Google Scholar]
- 30.Ezejiofor NA, Brown S, Barikpoar E, et al. Effect of ofloxacin and norfloxacin on rifampicin pharmacokinetics in man. Am J Ther 2015; 22:29–36. [DOI] [PubMed] [Google Scholar]
- 31.Gurumurthy P, Rahman F, Narayana AS, et al. Salivary levels of isoniazid and rifampicin in tuberculosis patients. Tubercle 1990;71:29–33. [DOI] [PubMed] [Google Scholar]
- 32.Orisakwe OE, Akunyili DN, Agbasi PU, et al. Some plasma and saliva pharmacokinetics parameters of rifampicin in the presence of pefloxacin. Am J Ther 2004;11:283–287. [DOI] [PubMed] [Google Scholar]
- 33.Orisakwe OE, Ofoefule SI. Plasma and saliva concentrations of rifampicin in man after oral administration. Tokai J Exp Clin Med 1996;21: 45–49. [PubMed] [Google Scholar]
- 34.Kumar AK, Sudha V, Srinivasan R, et al. Simple and rapid liquid chromatography method for determination of moxifloxacin in saliva. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879:3663–3667. [DOI] [PubMed] [Google Scholar]
- 35.Burkhardt O, Derendorf H, Jager D, et al. Moxifloxacin distribution in the interstitial space of infected decubitus ulcer tissue of patients with spinal cord injury measured by in vivo microdialysis. Scand J Infect Dis 2006;38:904–908. [DOI] [PubMed] [Google Scholar]
- 36.Stass H, Dalhoff A, Kubitza D, et al. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother 1998;42:2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller M, Stass H, Brunner M, et al. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob Agents Chemother 1999;43:2345–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burkhardt O, Borner K, Stass H, et al. Singleand multiple-dose pharmacokinetics of oral moxifloxacin and clarithromycin, and concentrations in serum, saliva and faeces. Scand J Infect Dis 2002;34: 898–903. [DOI] [PubMed] [Google Scholar]
- 39.Masumi R, Hirama Y, Narita A, et al. Studies on the intravenous administration of amikacin to neonates. Jpn J Antibiot 1987;40:1146–1156. [PubMed] [Google Scholar]
- 40.Suryawati S, Santoso B. Determination of isoniazid half-life from salivary samples. Int J Clin Pharmacol Ther Toxicol 1986;24:18–22. [PubMed] [Google Scholar]
- 41.Koizumi F, Ohnishi A, Takemura H, et al. Effective monitoring of concentrations of ofloxacin in saliva of patients with chronic respiratory tract infections. Antimicrob Agents Chemother 1994;38:1140–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sotgiu G, D’Ambrosio L, Centis R, et al. Carbapenems to treat multidrug and extensively drug-resistant tuberculosis: a systematic review. Int J Mol Sci 2016;17:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van der Paardt AL, Akkerman OW, Gualano G, et al. Safety and tolerability of clarithromycin in the treatment of multidrug-resistant tuberculosis. Eur Respir J 2017;49. doi: 10.1183/13993003.01612-2016. [DOI] [PubMed] [Google Scholar]
- 44.Kozjek F, Suturkova LJ, Antolic G, et al. Kinetics of 4-fluoroquinolones permeation into saliva. Biopharm Drug Dispos 1999;20:183–191. [DOI] [PubMed] [Google Scholar]
- 45.Warlich R, Korting HC, Schafer-Korting M, et al. Multiple-dose pharmacokinetics of ofloxacin in serum, saliva, and skin blister fluid of healthy volunteers. Antimicrob Agents Chemother 1990;34:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leigh DA, Walsh B, Harris K, et al. Pharmacokinetics of ofloxacin and the effect on the faecal flora of healthy volunteers. J Antimicrob Chemother 1988;22:115–125. [DOI] [PubMed] [Google Scholar]
- 47.Immanuel C, Hemanthkumar AK, Gurumurthy P, et al. Dose related pharmacokinetics of ofloxacin in healthy volunteers. Int J Tuberc Lung Dis 2002;6:1017–1022. [PubMed] [Google Scholar]
- 48.Edlund C, Kager L, Malmborg AS, et al. Effect of ofloxacin on oral and gastrointestinal microflora in patients undergoing gastric surgery. Eur J Clin Microbiol Infect Dis 1988;7:135–143. [DOI] [PubMed] [Google Scholar]
- 49.Tsubakihara Y, Hayashi T, Shoji T, et al. Pharmacokinetic study of ofloxacin using saliva concentration in chronic renal failure. Nihon Jinzo Gakkai Shi 1994;36:246–249. [PubMed] [Google Scholar]
- 50.Kees F, Wellenhofer M, Grobecker H. Serum and cellular pharmacokinetics of clarithromycin 500 mg q.d. and 250 mg b.i.d. in volunteers. Infection 1995;23:168–172. [DOI] [PubMed] [Google Scholar]
- 51.Bolhuis MS, van Altena R, van Hateren K, et al. Clinical validation of the analysis of linezolid and clarithromycin in oral fluid of patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 2013; 57:3676–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edlund C, Alvan G, Barkholt L, et al. Pharmacokinetics and comparative effects of telithromycin (HMR 3647) and clarithromycin on the oropharyngeal and intestinal microflora. J Antimicrob Chemother 2000;46: 741–749. [DOI] [PubMed] [Google Scholar]
- 53.Nakashima M, Uematsu T, Kosuge K, et al. Single- and multiple-dose pharmacokinetics of AM-1155, a new 6-fluoro-8-methoxy quinolone, in humans. Antimicrob Agents Chemother 1995;39:2635–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mignot A, Guillaume M, Brault M, et al. Multiple-dose pharmacokinetics and excretion balance of gatifloxacin, a new fluoroquinolone antibiotic, following oral administration to healthy Caucasian volunteers. Chemotherapy 2002;48:116–121. [DOI] [PubMed] [Google Scholar]
- 55.U.S. Food and Drug Administration. FDA alert “Gatifloxacin (marketed as Tequin)” 2015. Available at: https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm107821.htm Accessed March 23, 2017.
- 56.Park-Wyllie LY, Juurlink DN, Kopp A, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med 2006;354: 1352–1361. [DOI] [PubMed] [Google Scholar]
- 57.National Center for Biotechnology Information. Amoxicillin compound summary 2017.
- 58.National Center for Biotechnology Information. Doripenem compound summary 2017.
- 59.Burian B, Zeitlinger M, Donath O, et al. Penetration of doripenem into skeletal muscle and subcutaneous adipose tissue in healthy volunteers. Antimicrob Agents Chemother 2012;56:532–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wüst J, Hardegger U. Penetration of clarithromycin into human saliva. Chemotherapy 1993;39:293–296. [DOI] [PubMed] [Google Scholar]
- 61.Ginsburg CM, McCracken GH Jr, Thomas ML, et al. Comparative pharmacokinetics of amoxicillin and ampicillin in infants and children. Pediatrics 1979;64:627–631. [PubMed] [Google Scholar]
- 62.Ortiz RA, Calafatti SA, Corazzi A, et al. Amoxicillin and ampicillin are not transferred to gastric juice irrespective of Helicobacter pylori status or acid blockade by omeprazole. Aliment Pharmacol Ther 2002;16:1163–1170. [DOI] [PubMed] [Google Scholar]
- 63.Ramachandran G, Kumar AK, Kannan T, et al. Low serum concentrations of rifampicin and pyrazinamide associated with poor treatment outcomes in children with tuberculosis related to HIV status. Pediatr Infect Dis J 2016;35:530–534. [DOI] [PubMed] [Google Scholar]
- 64.Pasipanodya JG, McIlleron H, Burger A, et al. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 2013; 208:1464–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sunahara S, Nakagawa H. Metabolic study and controlled clinical trials of rifampin. Chest 1972;61:526–532. [DOI] [PubMed] [Google Scholar]
- 66.Rao KV, Kailasam S, Menon NK, et al. Inactivation of isoniazid by condensation in a syrup preparation. Indian J Med Res 1971;59:1343–1353. [PubMed] [Google Scholar]
- 67.Bjornesjo KB, Jarnulf B. Determination of isonicotinic acid hydrazide in blood serum. Scand J Clin Lab Invest 1967;20:39–40. [PubMed] [Google Scholar]
- 68.Ismail-Mohamed AM, Mohamed FA, Atia NN, et al. Ethambutol-Cobalt (II) ions complexation spectral characteristics and applications for quantitative analysis. Pak J Pharm Sci 2015;28:603–609. [PubMed] [Google Scholar]
- 69.Motwani SK, Chopra S, Ahmad FJ, et al. Validated spectrophotometric methods for the estimation of moxifloxacin in bulk and pharmaceutical formulations. Spectrochim Acta A Mol Biomol Spectrosc 2007;68:250–256. [DOI] [PubMed] [Google Scholar]
- 70.Shirkhedkar AA, Surana SJ. Quantitative determination of levofloxacin hemihydrate in bulk and tablets by UV-spectrophotometry and first order derivative methods. Pak J Pharm Sci 2009;22:301–302. [PubMed] [Google Scholar]
- 71.Hopkala H, Kowalczuk D. Application of derivative UV spectrophotometry for the determination of ciprofloxacin, norfloxacin and ofloxacin in tablets. Acta Pol Pharm 2000;57:3–13. [PubMed] [Google Scholar]
- 72.Vetuschi C, Ragno G, Mazzeo P. Determination of p-aminosalicylic acid and m-aminophenol by derivative UV-spectrophotometry. J Pharm Biomed Anal 1988;6:383–391. [DOI] [PubMed] [Google Scholar]
- 73.Gujral RS, Haque SM. Simultaneous determination of potassium clavulanate and amoxicillin trihydrate in bulk, pharmaceutical formulations and in human urine samples by UV spectrophotometry. Int J Biomed Sci 2010;6:335–343. [PMC free article] [PubMed] [Google Scholar]
- 74.Forsyth RJ, Ip DP. Determination of imipenem and cilastatin sodium in Primaxin by first order derivative ultraviolet spectrophotometry. J Pharm Biomed Anal 1994;12:1243–1248. [DOI] [PubMed] [Google Scholar]
- 75.Asadpour-Zeynali K, Saeb E. Simultaneous spectrophotometric determination of rifampicin, isoniazid and pyrazinamide in a single step. Iran J Pharm Res 2016;15:713–723. [PMC free article] [PubMed] [Google Scholar]
- 76.Hutchings A, Spragg BP, Routledge PA. Stability of isoniazid and acetylisoniazid in saliva. Ther Drug Monit 1988;10:234–236. [DOI] [PubMed] [Google Scholar]
- 77.Tron C, Lemaitre F, Pollock D, et al. Stability study of isoniazid in human plasma: practical aspects for laboratories. Ther Drug Monit 2015;37:831–833. [DOI] [PubMed] [Google Scholar]
- 78.van den Elsen SHJ, van der Laan T, Akkerman OW, et al. Membrane filtration is suitable to reliably eliminate Mycobacterium tuberculosis from saliva for therapeutic drug monitoring. J Clin Microbiol 2017; 55:3292–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hutchings AD, Monie RD, Spragg BP, et al. Saliva and plasma concentrations of isoniazid and acetylisoniazid in man. Br J Clin Pharmacol 1988;25:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCracken GH Jr, Ginsburg CM, Zweighaft TC, et al. Pharmacokinetics of rifampin in infants and children: relevance to prophylaxis against Haemophilus influenzae type b disease. Pediatrics 1980;66:17–21. [PubMed] [Google Scholar]
- 81.Miya T, Hamakubo S, Goya T, et al. Ofloxacin concentrations in serum, saliva and pleural effusion of patients with pulmonary tuberculosis and lung cancer. Jpn J Antibiot 1995;48:960–964. [PubMed] [Google Scholar]
- 82.Hara S, Uchiyama M, Yoshinari M, et al. A simple high-performance liquid chromatography for the determination of linezolid in human plasma and saliva. Biomed Chromatogr 2015;29:1428–1431. [DOI] [PubMed] [Google Scholar]
- 83.Fassbender M, Lode H, Schiller C, et al. Comparative pharmacokinetics of macrolide antibiotics and concentrations achieved in polymorphonuclear leukocytes and saliva. Clin Microbiol Infect 1996;1: 235–243. [DOI] [PubMed] [Google Scholar]
- 84.Morihana T, Kaneko A, Tomita F, et al. Penetration of clarithromycin to saliva and its effect on normal salivary bacterial flora. Jpn J Antibiot 1989;42:973–982. [PubMed] [Google Scholar]