Abstract
Background
Unicompartmental knee arthroplasty restores function and improves pain in appropriately selected patients. Scant evidence exists regarding the treatment of periprosthetic joint infection (PJI) after unicompartmental knee arthroplasty (UKA).
Questions/purposes
(1) What was the overall survivorship free from reinfection? (2) What is the survivorship free of all-cause revision? (3) What are the Knee Society scores (KSS) and complications after surgical treatment of UKA PJI?
Methods
This retrospective study with data drawn from a longitudinally maintained institutional registry identified 15 UKA PJIs between 1992 and 2014. The median age at PJI diagnosis was 58 years (range, 41-82 years), nine of 15 were men, and the median body mass index was 29 kg/m2 (range, 23-36 kg/m2). Ten patients (10 of 15) satisfied major Musculoskeletal Infection Society diagnostic criteria. There were five patients (five of 15) with early postoperative infections, five (five of 15) with acute hematogenous infections, and five (five of 15) with chronic PJIs. Two-stage exchange was performed in four patients with PJIs (four of 15), and débridement, antibiotics, and implant retention (DAIR) was performed in 11 patients (11 of 15) with PJIs. We performed Kaplan-Meier survivorship analysis for reinfection and revision procedures. Thirteen patients had a minimum of 2 years’ followup and were included in the clinical analysis. Median followup was 4 years (range, 2-6 years). We calculated KSS.
Results
Infection-free survivorship was 71% at 5 years (95% confidence interval [CI], 46%–96%). Treatment success was higher for patients undergoing two-stage exchange (100% at 5 years; 95% CI, 100%–100%) versus DAIR (61% at 5 years; 95% CI, 31%–92%). Four of 11 patients undergoing DAIR had developed a reinfection at final followup. Survivorship free of any revision was 49% at 5 years (95% CI, 19%–79%). One patient from the two-stage exchange cohort underwent femoral component revision for aseptic loosening 5 years after PJI treatment, and two patients from the DAIR group were converted to TKA for disease progression at a mean of 4 years. In patients with a minimum of 2 years’ followup, median KSS improved from 73 (range, 50-93) before index UKA to 94 (range, 55-100; p = 0.016).
Conclusions
Treatment of UKA PJI with DAIR was associated with a lower infection-free survivorship at 5 years compared with two-stage exchange with conversion to TKA. Among those patients who were infection-free, a number needed reoperations for disease progression (in the DAIR group) or component loosening (in both groups). UKA PJI results in substantial morbidity, and patients with these infections should be followed closely for aseptic causes of failure in addition to infection recurrence.
Level of Evidence
Level IV, therapeutic study.
Introduction
Between 1998 and 2005, the number of unicompartmental knee arthroplasties (UKAs) performed in the United States increased almost eightfold [15]. In the appropriately selected patient, UKA may provide pain relief and restoration of function; however, the mid- to long-term implant survivorship reported in registries is inferior to that of TKA [2, 13, 14]. Reported causes of failure include adjacent compartment arthritis, component loosening, periprosthetic fracture, and infection [5-7].
Periprosthetic joint infections (PJIs) after UKA are rare, and there are limited data evaluating treatment results. Labruyère et al. [10] found that one-stage conversion to TKA in nine chronic PJIs was successful at a mean 60 months and five of these patients had failed prior débridement, antibiotics, and implant retention (DAIR). Singer et al. [18] evaluated 64 infected knee replacements (six were UKAs) and found that after one-stage revision, the six UKAs were infection-free at 3 years. To the authors’ knowledge, there have been no studies evaluating the results of two-stage exchange arthroplasty in UKA PJI. Because of the paucity of studies focusing on UKA PJI [10, 18] and the gaps in knowledge despite those studies, we do not know whether treatment of PJI after UKA should mirror that of TKA, where two-stage exchange is typically recommended for established infection, or whether less aggressive approaches such as DAIR might be used. In addition, the presence of native cartilage raises additional questions about the ideal treatment of UKA PJI. Bacterial destruction of native cartilage and subsequent progressive arthritis is a potential source of unique failure after attempts at DAIR in UKA as compared with TKA.
Specifically, we asked the following questions: (1) What was the overall survivorship free from reinfection? (2) What was the survivorship free of all-cause revision? (3) What were the Knee Society scores (KSS) and complications after surgical treatment of UKA PJI?
Patients and Methods
We identified all patients treated surgically for PJI after UKA between 1992 and 2014 from our longitudinally maintained institutional total joint registry in this retrospective study. Institutional review board approval was attained before study initiation. Between January 1992 and December 2014, we performed 1440 UKAs, which represented 5.75% of our knee arthroplasty practice. We performed UKA when a patient presented with isolated unicompartmental arthritis. Of those who were treated with this approach, nine patients (1%) had died before 2 years’ followup, and 142 (10%) had less than 2 years’ followup, whereas 1262 patients (1288 knees [88%]) were available for followup at a minimum of 2 years (median, 5.1 years; range, 2-16.5 years). Of this group, we identified 11 patients with PJI diagnosed at a median of 0.04 year (range, 0-2 years) after the index procedure. In addition, we identified four patients with PJI diagnosed at a median of 2 years, who were referred for definitive management and had the opportunity for 2-year followup. This left 15 patients with the opportunity for 2-year followup who were included in the study.
We included patients with UKAs who met the diagnostic criteria for PJI outlined by the Musculoskeletal Infection Society (MSIS) [12] who were treated at our institution during the study period. Fifteen patients (15 knees) met the inclusion criteria.
All PJI treatment was performed at a single institution by surgeons with experience in complex knee arthroplasty. Two-stage exchange was performed by removing all components, completing femoral and tibial cuts for subsequent TKA, and placing a high-dose, nonarticulating antibiotic spacer. In general, patients with PJI for longer periods of time (generally close to 4 weeks or longer) or with more severe host and extremity status were treated with two-stage exchange, whereas patients with PJI for shorter periods of time (generally < 4 weeks) were treated with DAIR.
All patients were assessed by an orthopaedic infectious disease specialist pre- and postoperatively to help tailor organism-specific parenteral antibiotics. The duration of antibiotic therapy after spacer insertion was 6 weeks for all patients. For patients undergoing a two-stage exchange protocol, reimplantation was considered once the patient had completed the course of parenteral antibiotics and there were no ongoing concerns for PJI. Specifically, patients had normalization of their erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). The median time from antibiotic cessation to reimplantation was 3 weeks (range, 2–5 weeks). The median ESR was 25 mm/hr (range, 19–35 mm/hr) and the median CRP level was 6 mg/L (range, 1–14 mg/L) immediately before reimplantation. Seven (seven of 15) patients were placed on long-term antibiotic suppression after their treatment for infection.
Patient followup included clinical visits at 3 months, 1 year, 2 years, 5 years, and every 5 years thereafter. Clinical outcomes were calculated using the KSS for patients with at least 2 years’ followup [7].
All 15 patients were included in the survivorship analysis. One patient died, and one patient was lost to followup before 2 years. Although all 15 patients were included in survivorship analysis and perioperative complication reporting, 13 had at least 2 years of clinical followup and were included in clinical analysis. We reported survivorship free of reoperation for infection when initial treatment was DAIR, survivorship free of reoperation for infection when initial treatment was two-stage exchange, survivorship free or reoperation for infection for both treatment groups, and survivorship free of revision for any reason. We report KSS for patients before index UKA and at latest followup after all infection-related operations but before any aseptic revisions. Median followup of these 13 patients was 4 years (range, 2–6 years).
Statistical Analysis
The data were summarized using medians and ranges for all variables. The differences between preoperative and postoperative KSS were compared using Wilcoxon signed-rank test. We used a Kaplan-Meier [8] survivorship analysis to calculate survivorship free of reinfection, revision, or reoperation with 95% confidence intervals (CIs) reported. We analyzed clinical outcomes with JMP software, Version 10.0 (SAS, Cary, NC, USA); a significance value was set at α < 0.05.
Median age at the time of PJI diagnosis was 58 years (range, 41–82 years), nine patients were men (nine of 15), and median body mass index was 29 kg/m2 (range, 23–36 kg/m2).
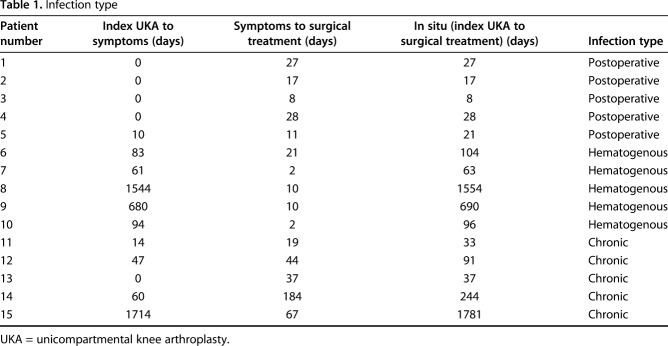
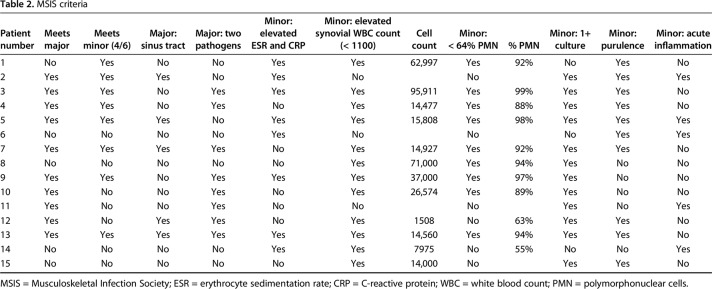
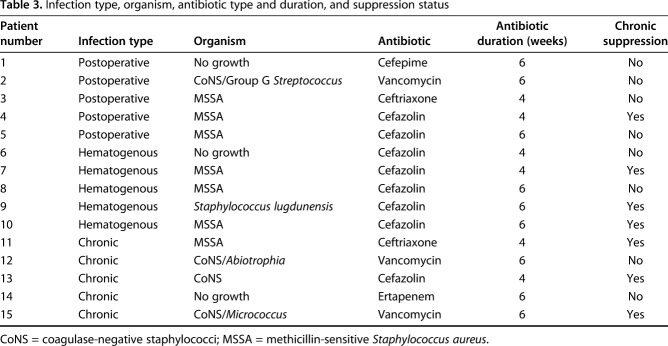
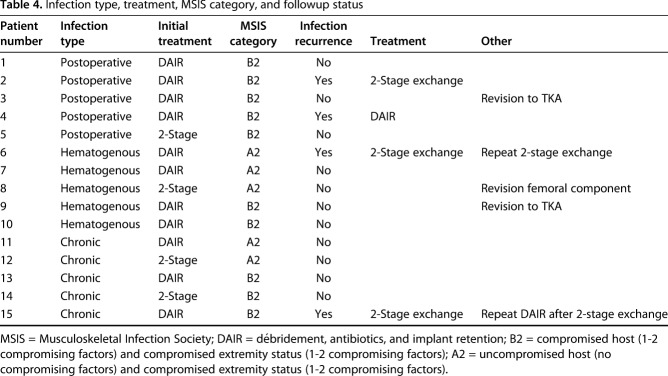
Patient history, physical examination, serology markers, synovial fluid analysis, and microbiologic studies were used to diagnose and classify patients according to the MSIS and McPherson staging systems, respectively [11, 12]. There were five (five of 15) early postoperative infections (< 4 weeks postoperatively), five (five of 15) acute hematogenous infections (< 4 weeks of symptom duration), and five (five of 15) chronic infections (> 4 weeks of symptom duration; Table 1). All acute hematogenous infections were late (> 4 weeks postoperatively). Ten (10 of 15) patients met major MSIS diagnostic criteria, one (one of 15) met four of six minor, and four (four of 15) patients met three of six minor MSIS diagnostic criteria (Table 2). The most common organisms were methicillin-sensitive Staphylococcus aureus in seven patients (seven of 15) and coagulase-negative Staphylococcus sp in five patients (five of 15; Table 3). Five (five of 15) were classified at type A hosts and 10 (10 of 15) were type B hosts. All patients’ local extremity grades were classified as compromised (type 2; Table 4).
Table 1.
Infection type
Table 2.
MSIS criteria
Table 3.
Infection type, organism, antibiotic type and duration, and suppression status
Table 4.
Infection type, treatment, MSIS category, and followup status
Eleven (11 of 15) patients were initially treated with DAIR. Of these 11 knees, eight (eight of 15) had polyethylene exchange. The remaining four (four of 15) patients were initially treated with two-stage exchange arthroplasty (Table 4).
Results
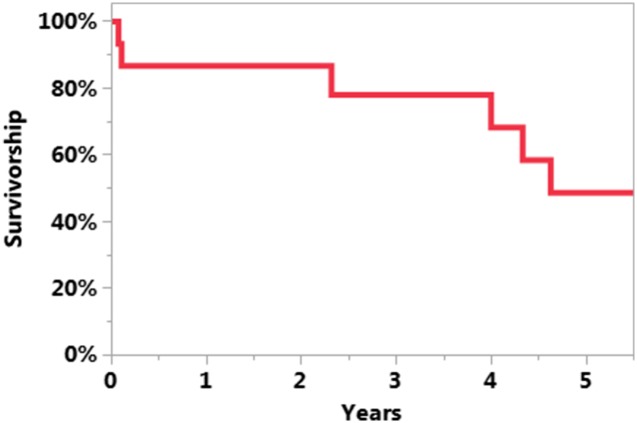
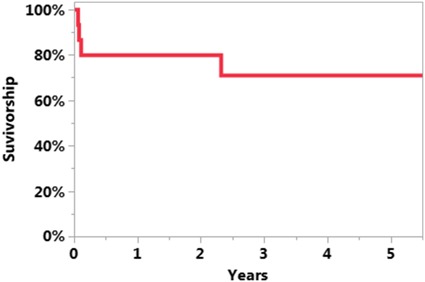
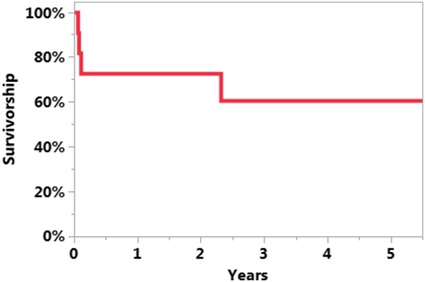
The survivorship free of reinfection after UKA PJI treatment was 71% at 5 years (95% CI, 46%–96%; Fig. 1). The survivorship free of reinfection when DAIR was the initial treatment was 61% at 5 years (95% CI, 31%–92%; Fig. 2). The survivorship free of reinfection after UKA PJI when initial treatment was two-stage exchange was 100% at 5 years (95% CI, 100%–100%; Fig. 3). Four of 11 patients undergoing DAIR developed a reinfection at final followup. One patient had repeat DAIR, and the infection was controlled. Three patients who did not respond to DAIR underwent two-stage exchange arthroplasty. At the time of reimplantation, two received posterior-stabilized TKAs and one received a constrained TKA with stems on both the femur and the tibia and augments on the femur. Two of three of these two-stage exchange arthroplasties became reinfected; one was treated with repeat two-stage exchange and the other had repeat DAIR. In the posterior-stabilized TKA that underwent repeat two-stage exchange at the time of initial reimplantation, a constrained TKA with stems and sleeves on both the femur and tibia were used during the subsequent reimplantation (Fig. 4).
Fig. 1.
The Kaplan-Meier curve representing survivorship free of reinfection after UKA PJI treatment was 71% at 5 years (95% CI, 46%–96%).
Fig. 2.
The Kaplan-Meier curve representing survivorship free of reinfection after UKA PJI when initial treatment was DAIR was 61% at 5 years (95% CI, 31%–92%).
Fig. 3.
The Kaplan-Meier curve representing survivorship free of reinfection after UKA PJI when initial treatment was two-stage exchange was 100% at 5 years (95% CI, 100%–100%).
Fig. 4.
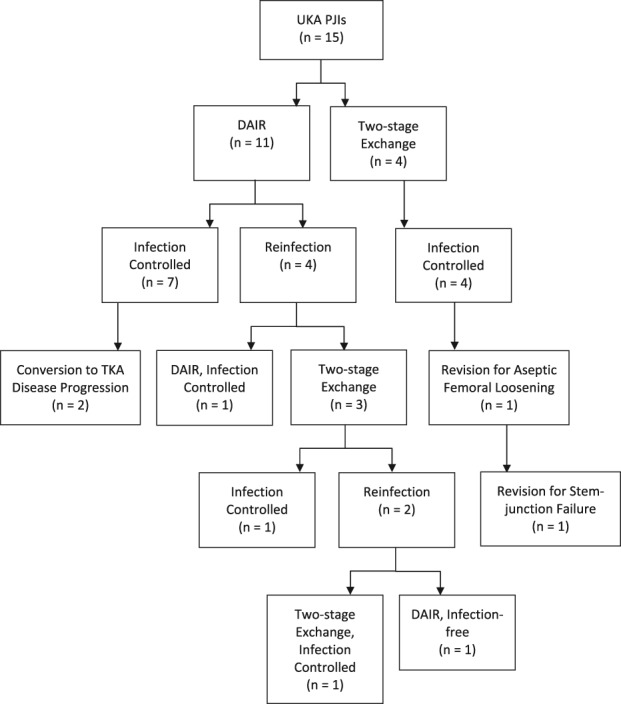
Presented is a tree diagram showing the different treatment outcomes.
The survivorship free of revision for any reason after initial infection treatment was 49% at 5 years (95% CI, 19%–79%; Fig. 5). One patient developed aseptic femoral loosening at 5 years after a two-stage exchange for infection. This knee was revised to a constrained TKA with stems and augments on both the femur and tibia and a trabecular metal cone on the femoral side. Five years after this revision, there was stem-junction failure causing metallosis. This patient then underwent revision to a constrained TKA with stems on the femur and tibia, a cone on the femur, and patellar bone grafting. There were no other reoperations in the two-stage exchange cohort.
Fig. 5.
The Kaplan-Meier curve representing survivorship free of any revision was 49% at 5 years (95% CI, 19%–79%).
Two knees in the DAIR group underwent conversion to TKA for arthritis progression at a mean of 4 years. Both patients were converted to posterior-stabilized TKAs. Neither received augments or bone grafting.
Median KSS improved from 73 (range, 50-93) before index UKA to 94 (range, 55-100; p = 0.016) after definitive PJI treatment and before any aseptic revision. Excluding reinfection and revision, one of the 13 patients had a complication, which was a deep vein thrombosis.
Discussion
UKAs have grown in popularity in the United States [15]. There is a paucity of data reporting the surgical management of PJI after UKA; specifically, no studies have evaluated two-stage exchange for PJI in UKA [10, 18]. This study reports the results of surgical treatment of UKA PJI with either DAIR or two-stage exchange. Reoperations for reinfection and all-cause revisions were high with survivorship free from reinfection and revision of 71% and 49% at 5 years, respectively. A two-stage exchange protocol resulted in higher infection eradication for PJI after UKA compared with DAIR.
There are several limitations to this study. This is a retrospective study that lacks a control group. Patients may have had recurrence of infection that went undetected in our study because they were treated at an outside hospital. In general, patients who had a longer duration of PJI or had more severe host and extremity status received two-stage exchange and those who had a shorter duration of PJI received DAIR, but this was not completely standardized, which limits the validity of the study. In addition, the results are from a single institution, making the results most applicable to academic surgeons with greater experience with reoperation and revision of UKAs. The small study cohort and limited followup reduce the ability to confidently identify patient and microbial variables that affect treatment success and failures. Because of the small sample, we felt the need to remove any analysis of risk factors; specifically, there was sparse data bias. One or two more or fewer patients in any group would change the result, and the CIs were too wide to be useful. We evaluated reinfection using Kaplan-Meier survivorship curves; whereas we felt these were an appropriate way to estimate reinfection, the wide CIs do to some extent limit the estimate. In addition, the study was over 20 years during which time the diagnosis and treatment of PJI have changed substantially. The use and indication for UKAs had changed over time [15]. Because of these changes, there may have been patients with UKA PJI that went undiagnosed causing us to underreport UKA PJI at our institution.
In this series, we report a survivorship free of PJI of 61% in patients treated initially with DAIR. These results are similar to those obtained with DAIR for infected TKA. Previous authors have reported a failure risk between 26% and 48% after TKA [3, 4, 9], which is comparable to our failure risk of 39%. This study is unique in that it reports data on DAIR for PJI in UKA. There are few studies presenting results on DAIR for PJI in UKA [10]. The authors recommend that DAIR only be considered in patients with early postoperative (< 4 weeks) or acute hematogenous infections (< 4 weeks of symptoms). In addition, if the decision is made to go ahead with DAIR, the surgeon should perform aggressive débridement, exchange of modular implants, and synovectomy in the same fashion as with TKA infection to improve procedure success.
We found that DAIR was associated with inferior infection-free survivorship compared with two-stage exchange. Labruyère et al. [10] also reported on inferior results with DAIR with five patients failing DAIR for PJI in UKA. To the authors’ knowledge, this is the first series to report on two-stage exchange for PJI in UKA. In our series, at the time of resection, the UKA components and cartilage are removed by making bone cuts. The importance of removing all cartilage at the time of the resection cannot be overemphasized. The results obtained with a two-stage exchange in this patient group were outstanding if performed as initial treatment but were less successful if the patient did not respond to DAIR. This has also been reported for infected TKA by Sherrell et al. [16], who reported a failure risk of 34% for two-stage exchange after DAIR.
We regard the clinical outcomes after treatment of PJI in UKA in our series to be acceptable, although not ideal. Patients had clinical improvement after DAIR or two-stage exchange. At final followup, KSS improved and the results are similar to what has been reported in other studies on conversion of UKA to TKA [1, 17, 19].
In conclusion, treatment for UKA PJI was associated with a high risk of reoperation as a result of reinfection, implant loosening, and disease progression at 5 years. Treatment of UKA PJI with DAIR was associated with a lower survivorship free of reinfection at 5 years compared with two-stage exchange with conversion to TKA. Among those patients who were infection-free, a number underwent subsequent reoperations for either progression of disease (in the DAIR group) or component loosening (in both groups). UKA PJI results in substantial morbidity and patients should be followed closely for aseptic causes of failure in addition to infection recurrence.
Footnotes
One of the authors (MWP) received personal fees from DePuy Synthes (Warsaw, IN, USA) outside the submitted work. One of the authors (RJS) received a grant and personal fees from Zimmer Biomet (Warsaw, IN, USA) outside the submitted work. One of the authors (MPA) received personal fees from Stryker Orthopaedics (Kalamazoo, MI, USA) outside the submitted work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23:159–163. [DOI] [PubMed] [Google Scholar]
- 2.Berger RA, Nedeff DD, Barden RM, Sheinkop MM, Jacobs JJ, Rosenberg AG, Galante JO. Unicompartmental knee arthroplasty. Clinical experience at 6- to 10-year followup. Clin Orthop Relat Res. 1999;367:50–60. [PubMed] [Google Scholar]
- 3.Buller LT, Sabry FY, Easton RW, Klika AK, Barsoum WK. The preoperative prediction of success following irrigation and débridement with polyethylene exchange for hip and knee prosthetic joint infections. J Arthroplasty. 2012;27:857–864.e1-4. [DOI] [PubMed] [Google Scholar]
- 4.de Vries L, van der Weegen W, Neve WC, Das H, Ridwan BU, Steens J. The effectiveness of débridement, antibiotics and irrigation for periprosthetic joint infections after primary hip and knee arthroplasty. A 15 years retrospective study in two community hospitals in the Netherlands. J Bone Jt Infect. 2016;1:20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foran JR, Brown NM, Della Valle CJ, Berger RA, Galante JO. Long-term survivorship and failure modes of unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2013;471:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furnes O, Espehaug B, Lie SA, Vollset SE, Engesaeter LB, Havelin LI. Failure mechanisms after unicompartmental and tricompartmental primary knee replacement with cement. J Bone Joint Surg Am. 2007;89:519–525. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton WG, Collier MB, Tarabee E, McAuley JP, Engh CA, Jr, Engh GA. Incidence and reasons for reoperation after minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2006;21:98–107. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481 [Google Scholar]
- 9.Kim JG, Bae JH, Lee SY, Cho WT, Lim HC. The parameters affecting the success of irrigation and débridement with component retention in the treatment of acutely infected total knee arthroplasty. Clin Orthop Surg. 2015;7:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labruyère C, Zeller V, Lhotellier L, Desplaces N, Leonard P, Mamoudy P, Marmor S. Chronic infection of unicompartmental knee arthroplasty: one-stage conversion to total knee arthroplasty. Orthop Traumatol Surg Res. 2015;101:553–557. [DOI] [PubMed] [Google Scholar]
- 11.McPherson EJ, Woodson C, Holtom P, Roidis N, Shufelt C, Patzakis M. Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res. 2002;403:8–15. [PubMed] [Google Scholar]
- 12.Parvizi J. New definition for periprosthetic joint infection. Am J Orthop (Belle Mead NJ). 2011;40:614–615. [PubMed] [Google Scholar]
- 13.Price AJ, Dodd CA, Svard UG, Murray DW. Oxford medial unicompartmental knee arthroplasty in patients younger and older than 60 years of age. J Bone Joint Surg Br. 2005;87:1488–1492. [DOI] [PubMed] [Google Scholar]
- 14.Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;435:171–180. [DOI] [PubMed] [Google Scholar]
- 15.Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental knee arthroplasty in the United States. J Arthroplasty. 2008;23:408–412. [DOI] [PubMed] [Google Scholar]
- 16.Sherrell JC, Fehring TK, Odum S, Hansen E, Zmistowski B, Dennos A, Kalore N; Periprosthetic Infection Consortium. The Chitranjan Ranawat Award: fate of two-stage reimplantation after failed irrigation and débridement for periprosthetic knee infection. Clin Orthop Relat Res. 2011;469:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28:128–132. [DOI] [PubMed] [Google Scholar]
- 18.Singer J, Merz A, Frommelt L, Fink B. High rate of infection control with one-stage revision of septic knee prostheses excluding MRSA and MRSE. Clin Orthop Relat Res. 2012;470:1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214–220. [DOI] [PubMed] [Google Scholar]