Abstract
Background
Opioid prescription management is challenging for orthopaedic surgeons, and we lack evidence-based guidelines for responsible opioid prescribing. Our institution recently developed opioid prescription guidelines for patients undergoing several common orthopaedic procedures including TKA and THA in an effort to reduce and standardize prescribing patterns.
Questions/purposes
(1) How do opioid prescriptions at discharge and 30-day refill rates change in opioid-naïve patients undergoing primary TKA and THA before and after implementation of a novel prescribing guideline strategy? (2) What patient, surgical, and in-hospital factors influence opioid prescription quantity and refill rate?
Methods
New institutional guidelines for patients undergoing TKA and THA recommend a maximum postoperative prescription of 400 oral morphine equivalents (OME), comparable to 50 tablets of 5 mg oxycodone or 80 tablets of 50 mg tramadol. All opioid-naïve patients, defined as those who did not take any opioids within 90 days preceding surgery, undergoing primary TKA and THA at a single tertiary care institution were evaluated from program initiation on August 1, 2017, through December 31, 2017, as the postguideline era cohort. This group (n = 751 patients) was compared with all opioid-naïve patients undergoing TKA and THA from 2016 at the same institution (n = 1822 patients). Some providers were early adopters of the guidelines as they were being developed, which is why January to July 2017 was not evaluated. Patients in the preguideline and postguideline eras were not different in terms of age, sex, race, body mass index, education level, employment status, psychiatric illness, marital status, smoking history, outpatient use of benzodiazepines or gabapentinoids, or diagnoses of diabetes mellitus, peripheral neuropathy, or cancer. The primary outcome assessed was adherence to the new guidelines with a secondary outcome of opioid medication refills ordered within 30 days from any provider. Multivariable logistic regression analyses were performed with outcomes of guideline compliance and refills and adjusted for demographic, surgical, and patient care factors. Patients were followed for 30 days after surgery and no patients were lost to followup.
Results
Median opioid prescription and range of prescriptions decreased in the postguideline era compared with the preguideline era (750 OME, interquartile range [IQR] 575-900 OME versus 388 OME, IQR 350-389; difference of medians = 362 OME; p < 0.001). There was no difference among patients undergoing TKA before and after guideline implementation in terms of the 30-day refill rate (35% [349 of 1011] versus 35% [141 of 399]; p = 0.77); this relationship was similar among patient undergoing THA (16% [129 of 811] versus 17% [61 of 352]; p = 0.55). After controlling for relevant patient-level factors, we found that implementation of an institutional guideline was the strongest factor associated with a prescription of ≤ 400 OME (adjusted odds ratio, 36; 95% confidence interval, 25-52; p < 0.001); although a number of patient-level factors also were associated with prescription quantity, the effect sizes were much smaller.
Conclusions
This study provides a proof of concept that institutional guidelines to reduce postoperative opioid prescribing can improve aftercare in patients undergoing arthroplasty in a short period of time. The current report evaluates our experience with the first 5 months of this program; therefore, longer term data will be mandatory to determine longitudinal guideline adherence and whether the cutoffs established by this pilot initiative require further refinement for individual procedures.
Level of Evidence
Level II, therapeutic study.
Introduction
A recent report from our institution examined postoperative prescribing patterns for 25 common elective procedures across a wide spectrum of surgical disciplines in opioid-naïve patients [14]. TKA and THA resulted in the two highest median postoperative opioid prescriptions as measured by oral morphine equivalents (OME). Furthermore, these procedures also had the highest variability in prescription quantity. Finally, despite having the highest median postoperative prescription, patients undergoing TKA also displayed the highest refill rate at 49% (second highest of all 25 procedures was 20%) [14]. Orthopaedic surgeons are the third highest opioid prescribers with TKA and THA accounting for much of this utilization [4]. The American Academy of Orthopaedic Surgeons recently made efforts to improve opioid prescribing a primary goal, introducing widespread public service announcements and providing education materials to assist surgeons in more effectively managing pain with an online “Pain Relief Toolkit” in addition to other impactful measures [4]. Various strategies have been proposed for arriving at responsible and judicious use of opioids in orthopaedic patients. Multimodal perioperative pain management strategies have drastically improved postoperative pain after TKA and THA, enabling shorter hospital stays and greater participation in early rehabilitation [1, 8]. Nevertheless, prescriptions at the time of hospital discharge remain, as yet, unaffected by these initiatives [14]. Current postdischarge opioid-prescribing practices after surgery typically are not evidence-based and often involve prescribing arbitrary round numbers of pills at the discretion of the surgeon [14]. The Centers for Disease Control and Prevention (CDC) suggest that the treatment of acute pain should be limited to < 7 days [2], and the state of Massachusetts has gone as far as to restrict opioid prescriptions for acute pain to a 7-day supply [11]. A proposed Minnesota state guideline recommends no more than 7 days or 200 OME (25 tablets of 5 mg oxycodone) after a surgical procedure [12]. These guidelines are informed by data recently published by the CDC, which identified a sharp increase in the risk of long-term opioid use for patients prescribed > 5 days of opioids [13]. However, the amount of opioids appropriate for pain management after orthopaedic surgical procedures remains undefined. Therefore, it is incumbent on orthopaedic surgeons to provide procedure-specific data to inform the creation of guidelines and policy. Through consensus from a multidisciplinary team, our department recently developed and implemented novel postoperative opioid-prescribing guidelines for opioid-naïve patients undergoing a variety of common orthopaedic procedures including TKA and THA. We evaluate the process and initial results of this program, specifically in the primary TKA and THA populations, while also identifying targets for incremental refinement.
This study attempts to answer the following questions: (1) How do opioid prescriptions at discharge and 30-day refill rates change in opioid-naïve patients undergoing primary TKA and THA before and after implementation of a novel prescribing guideline strategy? (2) What patient, surgical, and in-hospital factors influence opioid prescription quantity and refill rate?
Materials and Methods
This retrospective study drew from a number of longitudinally maintained data sources at the Mayo Clinic (Rochester, MN, USA) from January 2016 to December 2017. The first group included all eligible patients who underwent primary TKA or THA in 2016 before the implementation of departmental procedure-specific opioid-prescribing guidelines. The second group included all eligible patients who underwent primary TKA or THA from August 1, 2017, to December 31, 2017, after formal initiation of the program. Some providers were early adopters from January to July 2017 while the guidelines were being developed; therefore, this time period was excluded from analysis. The program was initially conceived and evaluated as an institutional quality improvement project and granted an exemption from our institutional review board to allow publication of results because of the potential importance of the findings.
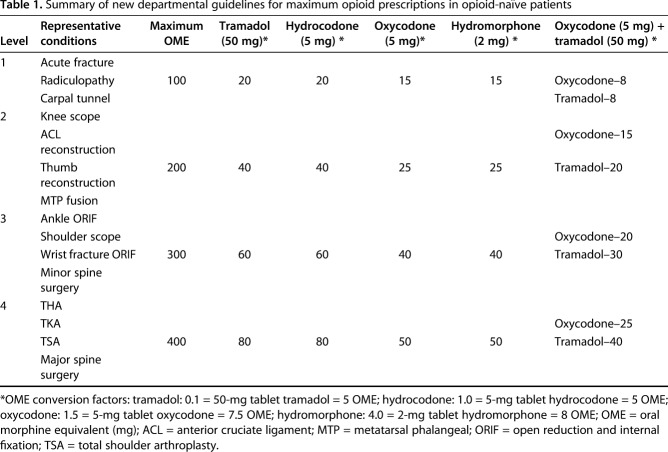
Our department designed guidelines for a variety of common orthopaedic procedures using multidisciplinary input from orthopaedic surgeons, anesthesiology pain management specialists, health policy experts, orthopaedic advanced practice providers, pharmacists, and nurses. The guidelines established four levels of magnitude for procedures (Table 1). These guidelines were driven primarily by expert opinion with attention toward evaluation of internal data showing prescription quantities that did not result in excess refill requests. Providers were recommended to prescribe a maximum OME for an opioid prescription based on the procedure level: Level 1 = 100 OME, Level 2 = 200 OME, Level 3 = 300 OME, and Level 4 = 400 OME. Both TKA and THA were designated Level 4 procedures along with total shoulder arthroplasty and major spine surgery. The guidelines also provide conversions to the number of tablets that may be prescribed for different opioids (Table 1). As a reference, 400 OME is approximately equivalent to 50 tablets of 5 mg oxycodone, 80 tablets of 50 mg tramadol, 50 tablets of 2 mg hydromorphone, and 80 tablets of 5 mg hydrocodone. Recommendations were provided for clinicians who prefer to discharge patients with a combination of milder versus more potent opioids. For example, Level 4 procedures allowed a maximum of 25 tablets of 5 mg oxycodone in conjunction with 40 tablets of 50 mg tramadol (Table 1). Guidelines are communicated clearly to patients at the preoperative visit and during the hospital stay. During the evaluated study period, there was no concurrent pain-management protocol standardization while patients remained in the hospital nor did the current iteration of the guidelines include recommendations for nonopioid pain medications.
Table 1.
Summary of new departmental guidelines for maximum opioid prescriptions in opioid-naïve patients
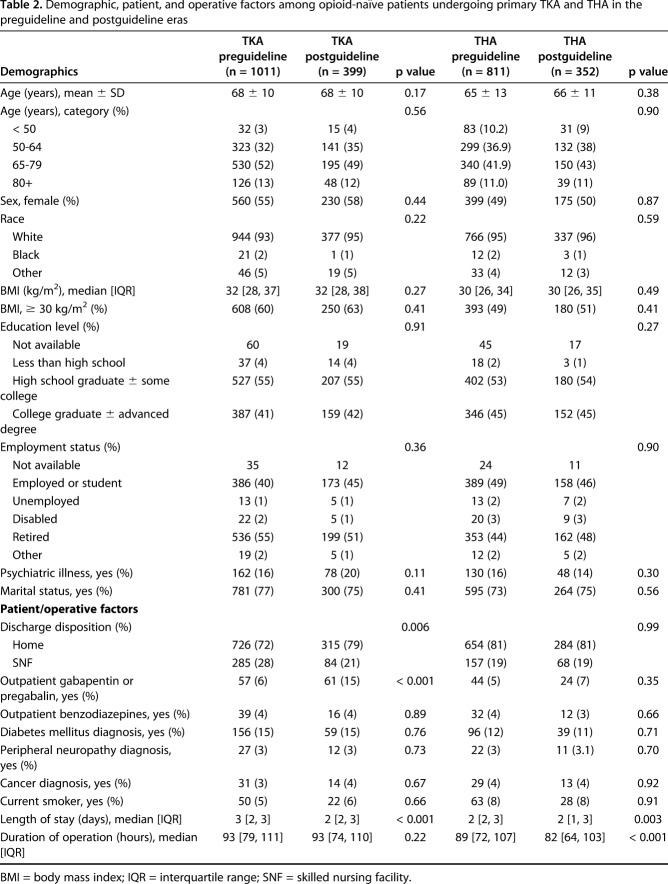
Study participants were deemed eligible if they underwent primary TKA or THA at our institution in the defined study period and were opioid-naïve at the time of surgery. Patients were considered opioid-naïve if they had not used opioids or obtained an opioid prescription within 90 days before surgery. Refills were defined as any opioid prescribed from 1 to 30 days after discharge regardless of where the refill originated, thus including those written by surgeons, emergency department providers, or primary care providers. In addition to demographics, a variety of factors were analyzed based on procedure type and pre- versus postguideline eras (Table 2).
Table 2.
Demographic, patient, and operative factors among opioid-naïve patients undergoing primary TKA and THA in the preguideline and postguideline eras
There were 1011 TKA preguideline patients and 399 TKA postguideline patients; there were 811 THA preguideline patients and 352 THA postguideline patients. In general, patients were not different before and after guideline implementation when stratified by procedure (Table 2). After stratifying by procedure, the following factors did not differ based on era: age, sex, body mass index, race, education level, employment status, psychiatric illness (defined as treated anxiety or depression), marital status, use of outpatient benzodiazepines, diabetes, peripheral neuropathy, cancer, and smoking status (Table 2). The following factors were found to differ by era: percentage of patients undergoing TKA discharged to skilled nursing facilities, percentage of patients undergoing TKA who used outpatient gabapentinoids, median operative duration for patients undergoing THA, and median length of hospital stay for both TKA and THA (Table 2).
All opioid-naïve patients undergoing TKA or THA at our institution within the study period were included and followed for 30 days after discharge; no patients were lost to followup. Observational studies of this nature also are potentially subject to selection bias and assessor bias. Selection bias was addressed by maintaining clear inclusion/exclusion criteria. Assessor bias was mitigated by separating the surgeons involved in patient care from data analysis as well as specifying our endpoints a priori and abstracting this information from longitudinally maintained institutional databases.
Statistical Analysis
No a priori power calculations or sample size determinations were performed for this study. It was undertaken as an institutional quality improvement project with monthly assessment of performance. After 5 months of data were acquired, the outcomes were deemed consistent and therefore worth sharing. A comparison group of all 2016 patients was chosen to achieve a two-to-one ratio of controls to cases. Univariate comparisons of demographics, surgical factors, in-hospital management, discharge prescriptions, and opioid refills used Wilcoxon rank-sum and t-tests for continuous variables and chi square and Fisher’s exact tests for categorical variables. Discharge opioid-prescribing practices in opioid-naïve patients were assessed for each surgical procedure before and after guideline implementation. Multivariable logistic regression was performed with outcomes of guideline compliance and postdischarge refills adjusted for demographic, surgical, and patient care factors. There were no data points that had missing information for at least 10 patients; therefore, these were not treated separately. Statistical analysis was performed using SAS Version 9.4 (SAS Institute Inc, Cary, NC, USA). Probability values < 0.05 were considered statistically significant.
Results
Reduction in Opioid Prescriptions After Guideline Implementation
Procedure-specific guideline implementation resulted in a reduction in both the amount of opioids prescribed and in variability of prescribing patterns (Fig. 1). Median TKA opioid prescriptions and range of prescriptions decreased in the postguideline era compared with the preguideline era (750 OME, IQR 600-900 OME versus 388 OME, IQR 338-463; difference of medians = 362 OME; p < 0.001); this relationship was similar for the THA cohort (750 OME, IQR 575-900 OME versus 388 OME, IQR 350-389; difference of medians = 362 OME; p < 0.001) (Table 3). Although initial prescriptions were lower in the postguideline era, there was no difference among patients undergoing TKA before and after guideline implementation in terms of the 30-day refill rate (35% [349 of 1011] versus 35% [141 of 399]; p = 0.77) (Table 3). Likewise, among patients undergoing THA, there was no difference in the 30-day refill rate (16% [129 of 811] versus 17% [61 of 352]; p = 0.55) (Table 3).
Fig. 1.
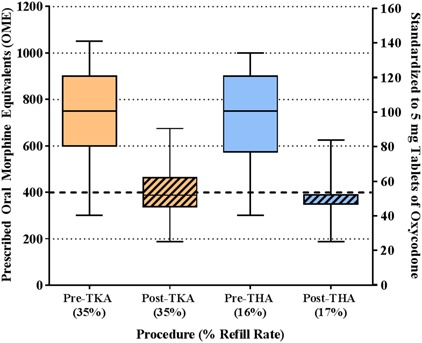
This box-and-whisker plot demonstrates the outpatient opioid prescriptions for both TKA and THA in the preguideline and postguideline eras. Orange boxes represent TKA and blue boxes represent THA; solid-colored boxes indicate the preguideline era and hashed colored boxes indicate the postguideline era. The box represents the 25th to 75th percentile IQR and the horizontal line within the box shows the median value. The tails from each box stretch from the 5th to 95th percentile. The left y-axis standardizes the outpatient opioid prescription to OME, whereas the right y-axis standardizes to 5-mg tablets of oxycodone. The dashed horizontal line at 400 OME/50 tablets of 5 mg oxycodone represents the new guideline maximum for primary TKA and THA in opioid-naïve patients. The plot demonstrates a reduction in the discharge opioid prescription for both TKA and THA as well as a much tighter distribution of prescription values in the postguideline era.
Table 3.
Summary of outpatient opioid prescription quantity and refill rate among opioid-naïve patients undergoing primary TKA and THA in the preguideline and postguideline eras
Factors Associated With Opioid Use
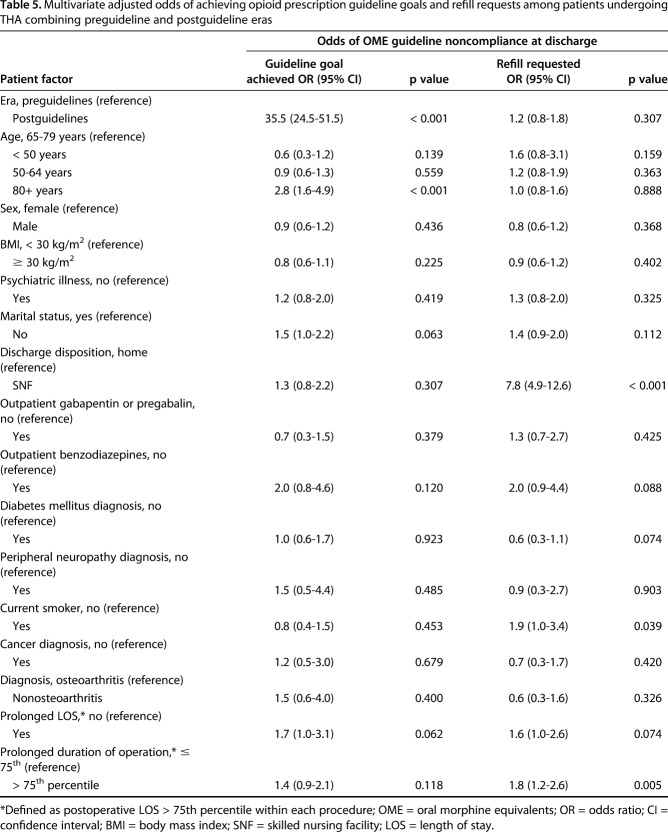
After adjusting for relevant patient-level factors, guideline implementation was the strongest factor in successfully discharging patients undergoing TKA and THA with opioid prescriptions ≤ 400 OME. Among patients undergoing TKA, the improved odds ratio (OR) of achieving opioid prescriptions ≤ 400 OME in the postguideline era compared with the preguideline era was 25 (95% confidence interval [CI], 18-34; p < 0.001) (Table 4). Similarly, among patients undergoing THA, the improved OR of achieving opioid prescriptions ≤ 400 OME in the postguideline era compared with the preguideline era was 36 (95% CI, 25-52; p < 0.001) (Table 5). Among patients undergoing TKA, other factors contributing to a lesser degree in achievement of opioid prescriptions ≤ 400 OME (either increased or decreased OR) included the following: age < 50 years (versus 65-79 years; OR, 0.3; 95% CI, 0.1-0.7; p = 0.011); age > 80 years (versus 65-79 years; OR, 2.9; 95% CI, 1.8-4.6; p < 0.001); use of outpatient gabapentinoids (OR, 2.8; 95% CI, 1.6-4.7; p < 0.001); use of outpatient benzodiazepines (OR, 0.4; 95% CI, 0.2-1.0; p = 0.040); and prolonged length of stay (OR, 2.2; 95% CI, 1.4-3.6; p < 0.001; Table 4). Among patients undergoing THA, the only evaluated factor contributing to achievement of opioid prescriptions ≤ 400 OME was age > 80 years (versus 65-79 years; OR, 2.8; 95% CI, 1.6-4.9; p < 0.001) (Table 5).
Table 4.
Multivariable adjusted odds of achieving opioid prescription guideline goals and refill requests among patients undergoing TKA combining preguideline and postguideline eras
Table 5.
Multivariate adjusted odds of achieving opioid prescription guideline goals and refill requests among patients undergoing THA combining preguideline and postguideline eras
After adjusting for relevant patient-level factors, guideline implementation did not impact opioid refills among patients undergoing TKA and THA. Among patients undergoing TKA, the following factors were associated with increasing or decreasing the odds of receiving an opioid prescription refill: discharge to a skilled nursing facility (OR, 3.6; 95% CI, 2.7-4.9; p < 0.001); age 50 to 64 years (versus 65-79 years; OR, 1.4; 95% CI, 1.1-1.8; p = 0.016); diagnosis of anxiety or depression (OR, 1.4; 95% CI, 1.0-1.9; p = 0.026); and use of outpatient gabapentinoids (OR, 0.6; 95% CI, 0.4-0.9; p = 0.026; Table 4). Among patients undergoing THA, the following factors were associated with increased odds of receiving an opioid prescription refill: discharge to a skilled nursing facility (OR, 7.8; 95% CI, 4.9-12.6; p < 0.001); current smokers (OR, 1.9; 95% CI, 1.0-3.4; p = 0.039); and prolonged operative time (OR, 1.8; 95% CI, 1.2-2.6; p = 0.005; Table 5).
Discussion
Responsible and evidence-based prescribing of opioids represents one of the most challenging contemporary issues for orthopaedic surgeons, particularly as it pertains to patients undergoing TKA and THA. The American Academy of Orthopaedic Surgeons has made addressing the opioid epidemic a top priority with a well-advertised campaign aspiring toward improved evidence-based opioid-prescribing strategies while still adequately addressing pain [4]. However, there is a paucity of evidence to identify cogent opioid-prescribing practices after orthopaedic surgical procedures [4]. Our institution recently implemented a series of opioid-prescribing procedure-specific guidelines with input from a multidisciplinary team. These guidelines have been disseminated to all members of the care team and, most importantly, are clearly communicated to patients at the preoperative visit and during hospitalization. After early implementation of the guidelines, we have demonstrated a roughly 50% decrease in median opioid prescription quantity and significantly tighter opioid prescription ranges without an increase in refill rate.
This study must be interpreted in light of important limitations. First, given the recent implementation of these novel guidelines, only the initial 5 months of results were reported here; postguideline data continue to be prospectively evaluated. However, we have shown that compliance with the program has steadily increased over the first 5 months of the program with 54% during the first month and 92% in the most recent month of data capture (Fig. 2). Nevertheless, longer term monitoring of guideline adherence will be mandatory. Another limitation is that although these guidelines have largely achieved their original intent of curbing opioid prescriptions after TKA and THA, the guideline threshold of 400 OME may not represent the final value as the program will undergo continued prospective evaluation and refinement through an iterative process. Although individual provider prescribing patterns have been quite uniform, often with no distinction between patients undergoing TKA and THA, it is well documented that postoperative pain tends to be more of a challenge for patients undergoing TKA than patients undergoing THA. We have preliminary data (not reported) to suggest that specific procedures yield differential yet well-correlated amounts of tablets actually consumed by patients who do not request a refill. Therefore, the guidelines proposed in this report may undergo refinement over time. Nevertheless, a proof of concept has shown that simple guidelines establishing prescription amounts much lower than traditional prescribing patterns, in conjunction with patient counseling on these expectations, can yield robust improvements. Another limitation is that this study only evaluated opioid-naïve patients. Previous work suggests that up to 25% of patients undergoing elective surgery are opioid-tolerant [7]. This is an important group that requires further study; however, given the wide variability of this patient population, current guidelines only apply to opioid-naïve patients.
Fig. 2.
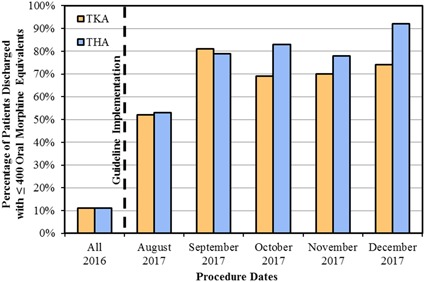
This bar graph shows the percentage of patients for whom successful adherence to the new opioid guideline maximum (≤ 400 OME at discharge) was achieved in both patients undergoing TKA (orange) and those undergoing THA (blue). The vertical dashed line indicates separation between the preguideline and postguideline eras. Since implementing the guidelines, there has been improvement from initiation until most recent data collection (p < 0.001).
Our study demonstrated that guideline implementation was the most impactful factor by a wide margin on prescription reduction and consistency without a concomitant increase in refill rate. To our knowledge, this is the first study documenting a change of this nature through practice guideline standardization in total joint arthroplasty. Guidelines have been suggested by a variety of governing bodies in response to the opioid epidemic. The CDC and the states of Massachusetts and Minnesota suggest that the treatment of acute pain should be limited to < 7 days after a surgical procedure [2, 11, 12]. Although well intentioned, these declarations lack critical procedure-specific distinctions that must be taken into account to create guidelines that maintain appropriate care for postoperative pain. Based on our recent experience creating specialty- and procedure-specific opioid-prescribing guidelines, the single most important element was involvement of a multidisciplinary team. Guidelines underwent several iterations as new perspectives were considered. Establishing this group effort not only enriched the final product, but was also critical to ensure buy-in from all parties who interact with patients to reinforce the guidelines during the early execution phase. Finally, we are unable to comment on whether guideline implementation affected clinical outcomes after TKA and THA. Longer term followup will be required to document any differences between groups. In particular, pain management is important after TKA for patients to participate effectively in ROM exercises and functional rehabilitation, and it will be important that future studies confirm that the new pain management protocol does not result in decreased ROM or poorer knee scores after TKA.
A recent report from our institution demonstrated that among 25 common elective surgical procedures, TKA and THA were the highest and second highest, respectively, in terms of median opioid prescription, highest prescription variability, and refill rates despite getting the largest initial prescriptions [14]. Since implementing guidelines, the median prescription has dropped 48% from 750 OME to 388 OME. This rapid and dramatic change highlights the power of patient counseling, expectations management, and the mind-body interaction with pain. Perception of pain and conceptions on how to treat it are largely culturally entrained. One recent series of investigations queried patients undergoing TKA and THA in America as well as part of Operation Walk in Guatemala and Nicaragua, asking patients to rate their level of concern about 56 issues on a Likert scale. For patients in America, “pain immediately after surgery” ranked as the most important concern, whereas this was not in the top half of concerns for both Central American cohorts [9, 10]. The push to recognize pain as the “fifth vital sign” since the early 1990s has certainly contributed to the problem in America. Providers are required to document pain level at every encounter and treatment of pain has become a benchmark for quality and reimbursement. The convenient answer often is more opioids; however, evidence suggests even short-term prescriptions increase addiction risk and alter neural pathways related to pain [13]. This can have important downstream effects; previous work has shown opioid-dependent patients have higher complication rates, increased length of stay, and higher costs [5].
Concerns were raised in our institution after implementation of the guidelines about the impact this would have on refill requests; however, we have found no differences in the frequency of refill requests after either TKA or THA. Recent work from an academic general surgery group has shown similarly that after implementing an education program and opioid prescription restrictions, there was not an appreciable increase in refills [6]. As stated previously, a limitation is that pill use remains unknown among patients who do not request a refill. This question must be elucidated to further refine the current effort. We have gathered preliminary data to suggest that the total use rate is lower among patients undergoing THA compared with patients undergoing TKA who do not request a refill; however, further study is needed to validate our early finding. If this proves to be the case, perhaps even shorter duration prescriptions may become a reality, yet this could also increase refill rates. One option for curbing any subsequent refill burden would be leveraging electronic prescribing of opioids. More than 80% of pharmacies nationwide maintain the ability to handle electronic opioid prescriptions; nevertheless, only 8% of physicians utilize this practice [3]. Our center currently does not participate in electronic prescribing, but adoption of this service may improve patient-specific titration of postoperative prescriptions.
In addition to guideline implementation as the driving factor, there were other elements shown to contribute, although to a lesser degree, to opioid prescription quantity and refill rate. For example, patients discharging to a skilled nursing facility were more likely to have compliant opioid prescriptions ≤ 400 OME, but also more likely to request a refill. This may be related to the fact that skilled nursing facilities more closely approximate the hospital environment than a home setting. Prescribers trust that a skilled nursing facility will be able to monitor how quickly the prescription is being used, enhancing confidence in writing a smaller prescription. However, nursing staff at skilled nursing facilities work on timed protocols similar to a hospital, offering medications on regular schedules. This may reinforce the perceived need for pain medication among patients and increase refill rates. Other factors that were shown to have an impact included outpatient use of gabapentinoids or benzodiazepines as well as a diagnosis of anxiety or depression. These patient populations have been well documented to experience more complex perceptions of pain that alter opioid consumption [4, 7].
It is incumbent on orthopaedic surgeons to help lead the effort to address the opioid epidemic. Data clearly support the increased risks for addiction and opioid diversion after overzealous postoperative prescriptions in addition to worse postoperative clinical outcomes. The status quo will not be solved with one singular measure, but rather through a collection of thoughtful innovations. One primary current deficiency is the lack of evidence-based guidelines for procedure-specific prescriptions in the orthopaedic opioid-naïve population. Perhaps the most impactful aspect of our established guidelines is the ability to provide patient counseling that sets appropriate expectations. Mind-body interactions for pain are some of the most powerful in all of medicine. Appropriate patient counseling and expectations management help surgeons lead a self-fulfilling prophecy toward success. This study provides a proof of concept that development and implementation of departmental guidelines are both possible and able to drive dramatic improvement in a short period of time. The process outlined in this report and the resulting successes should serve to inform the critical debate of establishing evidence-based practices for postoperative pain management after TKA and THA.
Acknowledgments
We thank the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery and the Mayo Clinic Total Joint Registry.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Amundson AW, Johnson RL, Abdel MP, Mantilla CB, Panchamia JK, Taunton MJ, Kralovec ME, Hebl JR, Schroeder DR, Pagnano MW, Kopp SL. A three-arm randomized clinical trial comparing continuous femoral plus single-injection sciatic peripheral nerve blocks versus periarticular injection with ropivacaine or liposomal bupivacaine for patients undergoing total knee arthroplasty. Anesthesiology. 2017;126:1139–1150. [DOI] [PubMed] [Google Scholar]
- 2.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA. 2016;315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gawande AA. It's time to adopt electronic prescriptions for opioids. Ann Surg. 2017;265:693–694. [DOI] [PubMed] [Google Scholar]
- 4.Hilibrand AS, Matzkin E. Combatting Opioid Misuse. 2017. Available at: https://www.aaos.org/AAOSNow/2017/Jun/YourAAOS/youraaos10/?ssopc=1. Accessed November 18, 2017.
- 5.Hill MV, McMahon ML, Stucke RS, Barth RJ., Jr Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265:709–714. [DOI] [PubMed] [Google Scholar]
- 6.Hill MV, Stucke RS, McMahon ML, Beeman JL, Barth RJ., Jr An educational intervention decreases opioid prescribing after general surgical operations. Ann Surg. 2017. Mar 6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Jiang X, Orton M, Feng R, Hossain E, Malhotra NR, Zager EL, Liu R. Chronic opioid usage in surgical patients in a large academic center. Ann Surg. 2017;265:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson RL, Amundson AW, Abdel MP, Sviggum HP, Mabry TM, Mantilla CB, Schroeder DR, Pagnano MW, Kopp SL. Continuous posterior lumbar plexus nerve block versus periarticular injection with ropivacaine or liposomal bupivacaine for total hip arthroplasty: a three-arm randomized clinical trial. J Bone Joint Surg Am. 2017;99:1836–1845. [DOI] [PubMed] [Google Scholar]
- 9.Kavolus JJ, Ritter MA, Claverie JG, Barfield WR, Lackland DT, Trousdale RT. Concerns of an itinerant surgeon: results of a Guatemalan surgical aid trip. J Arthroplasty. 2014;29:861–866. [DOI] [PubMed] [Google Scholar]
- 10.Kavolus JJ, Ritter MA, Claverie JG, Salas MD, Kavolus CH, Trousdale RT. Cultural nuance in orthopedic foreign aid: differences in patient concerns. J Arthroplasty. 2016;31:27–30. [DOI] [PubMed] [Google Scholar]
- 11.Meier B, Tavernise S. States move to control how painkillers are prescribed. 2016. Available at: https://www.nytimes.com/2016/03/12/business/states-move-to-control-how-painkillers-are-prescribed.html. Accessed November 18, 2017.
- 12.Opioid Prescribing Work Group. Acute Pain Prescribing Recommendations. 2016. Available at: https://mn.gov/dhs/assets/draft-acute-pain-recommendations_tcm1053-281603.pdf. Accessed November 18, 2017.
- 13.Shah A, Hayes CJ, Martin BC. Factors influencing long-term opioid use among opioid naive patients: an examination of initial prescription characteristics and pain etiologies. J Pain. 2017;18:1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiels CA, Anderson SS, Ubl DS, Hanson KT, Bergquist WJ, Gray RJ, Gazelka HM, Cima RR, Habermann EB. Wide variation and overprescription of opioids after elective surgery. Ann Surg. 2017;266:564. [DOI] [PubMed] [Google Scholar]