Abstract
The cell proliferation marker, Ki67 and the immature neuron marker, doublecortin are both expressed in the major human neurogenic niche, the subependymal zone (SEZ), but expression progressively decreases across the adult lifespan (PMID: 27932973). In contrast, transcript levels of several mitogens (transforming growth factor α, epidermal growth factor and fibroblast growth factor 2) do not decline with age in the human SEZ, suggesting that other growth factors may contribute to the reduced neurogenic potential. While insulin like growth factor 1 (IGF1) regulates neurogenesis throughout aging in the mouse brain, the extent to which IGF1 and IGF family members change with age and relate to adult neurogenesis markers in the human SEZ has not yet been determined. We used quantitative polymerase chain reaction to examine gene expression of seven IGF family members [IGF1, IGF1 receptor, insulin receptor and high-affinity IGF binding proteins (IGFBPs) 2, 3, 4 and 5] in the human SEZ across the adult lifespan (n=50, 21-103 years). We found that only IGF1 expression significantly decreased with increasing age. IGFBP2 and IGFBP4 expression positively correlated with Ki67 mRNA. IGF1 expression positively correlated with doublecortin mRNA, whereas IGFBP2 expression negatively correlated with doublecortin mRNA. Our results suggest IGF family members are local regulators of neurogenesis and indicate that the age-related reduction in IGF1 mRNA may limit new neuron production by restricting neuronal differentiation in the human SEZ.
Keywords: neurogenesis, subventricular zone, doublecortin, Ki67, IGF binding proteins
The human brain retains the ability to generate new neurons throughout postnatal life with the largest reservoir of newly-born cells in the subependymal zone (SEZ, also subventricular zone) [1]. Neurogenesis in the human SEZ after infancy has been called into question [2, 3] despite evidence supporting the existence of cells in different stages of neurogenesis throughout adulthood from both our laboratory and other groups [4-8]. Although the functional significance of neurogenesis in the human SEZ remains to be established, the putative integration of newly-generated interneurons into subcortical and cortical brain regions may contribute to synaptic plasticity and cognitive flexibility [9-12]. Cell culture experiments have advanced our understanding of the molecular control of neurogenesis by identifying growth factors as key regulators of proliferation and cell fate decision. However, the extent to which growth factors and their receptors are altered with age in the human SEZ in parallel with the decline in cell proliferation and neuronal differentiation markers is only partially characterized [5, 7, 13]. Transcript levels of transforming growth factor α, epidermal growth factor, fibroblast growth factor 2 and cognate receptors do not decrease during aging [7, 14] and point to the involvement of other mitogens such as insulin like growth factor (IGF) 1 and 2.
IGF1 and 2 transcripts decrease in most regions of the rodent nervous system during postnatal life but levels remain elevated in adult neurogenic regions [15, 16]. IGF1 is expressed by neurons [17], astrocytes [18] and oligodendrocytes [19], whereas IGF2 is produced by the leptomeninges and choroidal epithelial cells [20]. IGF1 signals predominantly through the IGF1 receptor (IGF1R) but can also bind with low affinity to the insulin receptor (INSR). IGF1R expression predominates in neuronal precursor cells, whereas INSR is abundantly expressed by neural stem cells in the adult SEZ [21]. IGF1 bioavailability is regulated by high- and low-affinity IGF binding proteins (IGFBPs), which are expressed by endothelial cells, neurons and glia [22, 23]. IGFBP3 and IGFBP4 modulate neural precursor proliferation, differentiation and survival [24, 25].
IGF1 and IGFBPs are expressed in the peripheral blood and cerebrospinal fluid and show distinct age-related alterations [26-29]. The entrance of peripheral IGF1 and insulin into the brain parenchyma is controlled by IGF1R, INSR, IGFBPs and low-density lipoprotein receptor-related proteins [30], and influences neurogenesis and cognition [31-33]. Werry and colleagues did not detect a change in IGF1 protein levels in the SEZ from adulthood into aging [14]; however, the amount of IGF1 available to signal could be impacted by age-related alterations in IGFBPs without a change in IGF1 itself. Since brain IGF1 protein levels can also be derived from the peripheral blood and cerebrospinal fluid, we hypothesized that local production of IGF1 and IGF family members would be reduced in the human SEZ and would correlate with expression of cell proliferation and neuronal differentiation markers.
MATERIALS AND METHODS
Human post-mortem brain samples
Tissue from the anterior caudate of 50 healthy individuals was obtained from the Stanley Medical Research Institute and New South Wales Brain Tissue Resource Centre (Sydney, Australia; HREC 12435, HC16442). Cases had no known history of psychiatric symptoms or substance abuse and showed no significant neuropathology on post-mortem examination. The brain cohort consisted of 9 females and 41 males, with an average age of 52 years (±16.76, range 21-103 years), average pH of 6.59 (±0.25, range 5.95-7.03) and average post-mortem interval (PMI) of 29 hours (±10.75, range 9-58 hours). Demographic details of each individual have been described previously [7].
Processing of brain tissue
Fresh-frozen caudate tissue was sectioned on a Leica CM3050 S cryostat, taking 20 x 60 µm sections interspersed with 10 x 14 µm sections. SEZ tissue was dissected from the caudate nucleus while frozen over dry ice from 60 µm thick sections, ~2 mm deep to the surface of the lateral ventricle. For each case, tissue was dissected from 3 sets of 3-4 adjacent 60 µm sections spaced ~1340 µm to give 10 sections per case (~40 mg tissue total).
RNA extraction and cDNA synthesis
Total RNA was extracted for all cases using Trizol (Life Technologies). RNA quality and concentration were assessed with Agilent Technologies 2100 Bioanalyzer and Nanodrop ND-1000 spectrophotometer. The average RNA integrity number (RIN) was 7. cDNA was synthesized from 3 µg total RNA per case using SuperScript® First-Strand Synthesis kit and random hexamers (Life Technologies).
Assessment of mRNA expression of IGF family members using quantitative reverse transcription polymerase chain reaction
mRNA levels were measured by TaqMan Gene Expression Assays (Applied Biosystems; IGF1, Hs01547656_m1; IGF1R, Hs00609566_m1; IGFBP2, Hs01040719_m1; IGFBP3, Hs00181211_m1; IGFBP4, Hs01057900_m1; IGFBP5, Hs00181213_m1; INSR, Hs00961557_m1) using an ABI Prism 7900HT fast real-time PCR system and a 384-well format. All measurements from each subject were performed in duplicate and relative quantities were determined from a seven-point standard curve of pooled cDNA. The no template controls did not produce a signal for any mRNA examined. Expression of two housekeeping genes, TATA-box binding protein (Hs00427620_m1) and ubiquitin C (Hs00824723_m1), was used to calculate the normalizing factor for gene expression (geometric mean), and neither of these mRNAs nor the geometric mean correlated significantly with age (all p>0.05, data not shown). Quantitative reverse transcription polymerase chain reaction data were captured with sequence detector software (SDS version 2.4, Applied Biosystems). SDS software plotted real-time fluorescence intensity and the threshold was set within the linear phase of the amplification profiles.
Statistics
Statistical analyses were performed using IBM SPSS Statistics Version 24 and GraphPad Prism Version 7.0 B. Results were considered as significant at an α level of p<0.05. Population outliers were defined as points lying outside of a 95% prediction interval from the linear regression line (1-3 individuals per target gene). Data were tested for normality using the Shapiro-Wilk test. Pearson’s product-moment correlations were used to investigate the relationships of brain cohort characteristics (age, pH, PMI and RIN) to each other and target gene expression. Pearson’s product-moment or semi-partial correlations were used to analyze age-related changes in target gene expression and their relationships to markers of cell proliferation and neuronal differentiation. When semi-partial correlations were performed, the semi-partial correlation coefficient sr is reported. Independent t-tests or analysis of variance co-varying for brain cohort characteristics were used as appropriate to detect sex-related differences in gene expression, and sex did not show a significant effect on gene expression (p>0.05).
RESULTS
Expression of IGF family members in the human SEZ from young adulthood into aging
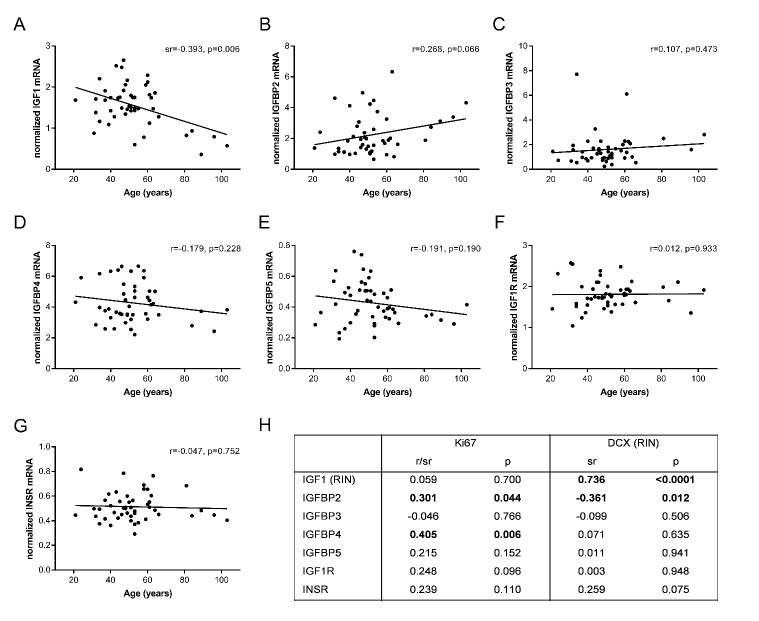
Gene expression of seven out of ten IGF family members was reliably detected by quantitative polymerase chain reaction in the adult human SEZ from 21-103 years. Transcript levels of IGFBP1, IGF2 and IGF2 receptor were below the level of detection. IGF1 mRNA decreased significantly with age (sr=-0.39, p=0.006; Fig. 1A). IGFBP2 mRNA showed a trend towards an increase with age (r=0.26, p=0.06; Fig. 1B), whereas IGFBP3, IGFBP4 and IGFBP5 mRNAs did not change across the adult lifespan (all p≥0.19; Fig. 1C-E). Transcript levels of IGFBPs did not correlate with IGF1 mRNA (all p≥0.09, data not shown). IGF1R and INSR mRNAs remained stable throughout adulthood (all p≥0.75; Figs. 1F, G).
Figure 1.
Gene expression of IGF family members and their relationships to neurogenesis markers in the human SEZ from young adulthood into aging. IGF1 mRNA significantly decreased in the aging SEZ (A). IGFBP2 mRNA showed a trend increase with age (B), while expression of IGFBPs 3-5, IGF1R and INSR remained stable throughout adulthood (C-G). Pearson’s product-moment and semi-partial correlations demonstrated different relationships between IGF family member expression and cell proliferation (Ki67) and immature neuron markers (DCX). Confounding brain cohort characteristics (in brackets) were considered as covariates in semi-partial correlation analyses (H). RIN, RNA integrity number; sr, semi-partial correlation coefficient. Bold type = p≤0.05.
Relationships of IGF family member transcripts to decreased expression of adult neurogenesis markers
We analyzed the relationships between expression of IGF family members and expression of the cell proliferation marker Ki67 and of the immature neuron marker doublecortin (DCX) (Fig. 1H), which we have previously shown to progressively decline with age in the SEZ [7]. Ki67 mRNA positively correlated with IGFBP2 (r=0.30, p=0.04) and IGFBP4 mRNAs (r=0.40, p=0.006). IGF1 mRNA was strongly positively correlated with DCX mRNA (sr=0.73, p<0.0001), whereas IGFBP2 mRNA showed a negative relationship with DCX mRNA (sr= -0.36, p=0.01).
Correlations between brain cohort characteristics and target gene expression
Detailed statistical data for the relationships between brain cohort characteristics and target gene expression are presented in Table 1. Brain pH correlated with IGF1 (r=0.51, p<0.0001), IGF1R (r=-0.32, p=0.02) and IGFBP2 mRNAs (r=-0.36, p=0.01). RIN positively correlated with IGF1 mRNA (r=0.33, p=0.02). No other significant relationships were detected between brain cohort characteristics and target gene expression. The relationships between age and other brain cohort characteristics (pH, PMI and RIN) have been described previously [7]. Briefly, age negatively correlated with pH (r=-0.43, p=0.002). No other significant relationships were detected between age, PMI, pH and RIN (all p>0.05, data not shown). Brain pH was excluded as a covariate during statistical analyses since aging is commonly associated with brain acidosis and thus dependent on the variable of interest.
Table 1.
Pearson’s product-moment correlations between gene expression of IGF family members and brain cohort characteristics in the human SEZ.
| pH
|
PMI
|
RIN
|
||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| IGF1 | 0.518 | <0.0001 | -0.072 | 0.628 | 0.332 | 0.021 |
| IGFBP2 | -0.361 | 0.012 | -0.021 | 0.890 | -0.137 | 0.354 |
| IGFBP3 | -0.206 | 0.165 | -0.229 | 0.122 | 0.185 | 0.214 |
| IGFBP4 | -0.180 | 0.226 | -0.031 | 0.837 | -0.218 | 0.141 |
| IGFBP5 | 0.213 | 0.141 | 0.120 | 0.412 | -0.085 | 0.561 |
| IGF1R | -0.325 | 0.023 | -0.198 | 0.172 | -0.177 | 0.224 |
| INSR | -0.046 | 0.756 | 0.263 | 0.071 | -0.014 | 0.923 |
PMI, post-mortem interval; r, Pearson’s product-moment correlation coefficient; RIN, RNA integrity number. Bold type = p≤0.05.
DISCUSSION
This study provides the first molecular evidence for an age-related reduction in local IGF1 expression in the human SEZ from adulthood into aging and lends translational support for the fundamental work in rodents. Our results suggest that subependymal cells remain responsive to IGF1 as indicated by stable expression of cognate receptors IGF1R and INSR. We found that IGFBP2 and IGFBP4 may be important in the regulation of cell proliferation, while IGF1 may promote neuronal differentiation. This study only allows evaluation of transcriptional alterations in the human SEZ at one time point; however, it is of high value considering the pronounced interspecies differences in neurogenesis and migration between rodents and humans [3, 9-11, 34].
The role of IGF1 signalling during aging remains unclear in many aspects [35]. Our results support studies in mice showing that IGF1 is important for neuronal differentiation in the hippocampus [36] and neuronal migration to the olfactory bulb [37]. Thus, the function of IGF1 in adult neurogenesis may be conserved across species and neurogenic regions. IGF1 restoration rescues the age-related decline in hippocampal neurogenesis and cognitive impairments in rodents [31, 32, 38] and represents a putative therapeutic target for neurodegenerative and neurodevelopmental disorders [39, 40]. In contrast, downregulation of peripheral IGF1 signalling by genetic mutations delays aging and increases longevity [41-43]. IGF1R suppression in neural stem cells in the rodent SEZ prevents the age-related decrease in neurogenesis and olfactory deficits. This proliferation-promoting effect of reduced IGF1 signalling may prematurely deplete the neural stem cell pool; however, in silico modelling predicts that the number of stem cells is preserved until late adulthood [44] and accords with findings in the aged human SEZ [6, 7]. Long-lived Ames dwarf mice deficient in peripheral IGF1 show increased levels of hippocampal IGF1, suggesting that peripheral levels may negatively feedback on local IGF1 synthesis. Ames dwarf mice have increased neurogenesis and maintain normal cognitive function until advanced age [45]. These opposing reports highlight the need for further studies to discern the complex role of IGF1 signalling during aging in the mammalian brain.
The age-related decrease in IGF1 mRNA in the SEZ was not unexpected, although important to document in humans; however, it was unexpected that none of the other IGF family members declined with age. Since IGF1 was the only transcript to change significantly throughout adulthood, there may be an age-related alteration in control of gene expression specific to IGF1, such as methylation, histone modifications or loss of transcriptional activators. Age-related changes in local and peripheral neurogenesis-regulating factors may act in concert to decrease Ki67 and DCX expression in the SEZ across the human lifespan [5, 7, 13, 46]. Ki67 relates to proliferation of cell types other than neural stem cells such as astrocytes and microglia but displays a more robust expression in canonical and non-canonical neurogenic niches in the human brain [47]. Significant correlations of Ki67 with IGFBP2 and IGFBP4 as well as DCX with IGF1 and IGFBP2 suggest that IGF family members may work together to regulate adult neurogenesis in the human SEZ. IGFBP4 impairs proliferation and enhances neuronal differentiation of progenitor cells [25], whereas our results indicate that IGFBP4 may promote cell proliferation in conjunction with IGFBP2. We suggest that other mitogen signalling pathways in addition to IGF1 signalling may act independently or synergistically to stimulate cell proliferation as fibroblast growth factor receptor 1 expression also positively correlates with Ki67 mRNA [7]. IGF1 may act in concert with brain-derived neurotrophic factor and neuregulin signalling to promote neuronal differentiation as full-length tyrosine kinase receptor B and Erb-B2 receptor tyrosine kinase 4 expression positively correlate with DCX mRNA [7, 13]. In contrast, IGFBP2 may negatively regulate neuronal differentiation. It is unlikely that IGFBP2 acts in isolation as epidermal growth factor and truncated tyrosine kinase receptor B expression also negatively correlate with DCX mRNA [7, 13].
In summary, our results support that IGF family members impact the age-related decline in cell proliferation and neuronal differentiation markers in the human SEZ, though specific factors involved may depend on the stage of neurogenesis. We suggest that IGF family members only partially contribute to the complex local milieu available to regulate neurogenesis in the adult brain. This study is limited by the homogenate-based experimental approach and further cell-type specific analysis would shed light on whether proliferating cells and immature neurons maintain responsiveness to IGF1 during human aging. Gene expression studies also only provide clues as to whether protein levels may be altered and cannot ascertain if differences in brain protein levels may be of functional significance; however, several studies demonstrate that transcription changes in IGF family members significantly contribute to protein levels and to biological function [48-50]. We suggest that loss of local IGF1 function may impair neuronal differentiation and future work needs to establish if IGF1 restoration could rescue deficits in neurogenesis in the adult SEZ in the human brain.
Acknowledgements
This work was funded by the NSW Ministry of Health, Office of Health and Medical Research and by the Australian Research Council Discovery Project grant (DP150104168). CSW is a recipient of a National Health and Medical Research Council (Australia) Principal Research Fellowship (1117079). Tissues were received from the New South Wales Brain Tissue Resource Centre at the University of Sydney and the Sydney Brain Bank at Neuroscience Research Australia which are supported by The University of New South Wales, Neuroscience Research Australia and Schizophrenia Research Institute. Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R28AA012725. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure Statement
Cynthia Shannon Weickert is on an advisory board for Lundbeck, Australia Pty Ltd and in collaboration with Astellas Pharma Inc., Japan. All other authors have no conflicts of interest to disclose.
References
- [1].Curtis MA, Low VF, Faull RL (2012). Neurogenesis and progenitor cells in the adult human brain: a comparison between hippocampal and subventricular progenitor proliferation. Dev Neurobiol., 72: 990-1005 [DOI] [PubMed] [Google Scholar]
- [2].Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT (2016). Human adult neurogenesis across the ages: An immunohistochemical study. Neuropathol Appl Neurobiol., 42: 621-638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, et al. (2011). Corridors of migrating neurons in the human brain and their decline during infancy. Nature, 478: 382-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tepavcevic V, Lazarini F, Alfaro-Cervello C, Kerninon C, Yoshikawa K, Garcia-Verdugo JM, et al. (2011). Inflammation-induced subventricular zone dysfunction leads to olfactory deficits in a targeted mouse model of multiple sclerosis. J Clin Invest, 121: 4722-4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Weickert CS, Webster MJ, Colvin SM, Herman MM, Hyde TM, Weinberger DR, et al. (2000). Localization of epidermal growth factor receptors and putative neuroblasts in human subependymal zone. J Comp Neurol., 423: 359-372 [DOI] [PubMed] [Google Scholar]
- [6].van den Berge SA, Middeldorp J, Zhang CE, Curtis MA, Leonard BW, Mastroeni D, et al. (2010). Longterm quiescent cells in the aged human subventricular neurogenic system specifically express GFAP-delta. Aging Cell., 9: 313-326 [DOI] [PubMed] [Google Scholar]
- [7].Weissleder C, Fung SJ, Wong MW, Barry G, Double KL, Halliday GM, et al. (2016). Decline in proliferation and immature neuron markers in the human subependymal zone during aging: Relationship to EGF- and FGF-related transcripts. Front Aging Neurosci., 8: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maheu ME, Devorak J, Freibauer A, Davoli MA, Turecki G, Mechawar N (2015). Increased doublecortin (DCX) expression and incidence of DCX-immunoreactive multipolar cells in the subventricular zone-olfactory bulb system of suicides. Front Neuroanat., 9: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Paredes MF, James D, Gil-Perotin S, Kim H, Cotter JA, Ng C, et al. (2016). Extensive migration of young neurons into the infant human frontal lobe. Science., 354: aaf7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, et al. (2014). Neurogenesis in the striatum of the adult human brain. Cell, 156: 1072-1083 [DOI] [PubMed] [Google Scholar]
- [11].Ernst A, Frisen J (2015). Adult neurogenesis in humans- common and unique traits in mammals. PLoS Biol., 13: e1002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gould E, Reeves AJ, Graziano MS, Gross CG (1999). Neurogenesis in the neocortex of adult primates. Science., 286. [DOI] [PubMed] [Google Scholar]
- [13].Weissleder C, Kondo MA, Yang C, Fung SJ, Rothmond DA, Wong MW, et al. (2017). Early-life decline in neurogenesis markers and age-related changes of TrkB splice variant expression in the human subependymal zone. Eur J Neurosci., 46: 1768-1778 [DOI] [PubMed] [Google Scholar]
- [14].Werry EL, Enjeti S, Halliday GM, Sachdev PS, Double KL (2010). Effect of age on proliferation-regulating factors in human adult neurogenic regions. J Neurochem., 115: 956-964 [DOI] [PubMed] [Google Scholar]
- [15].Rotwein P, Burgess SK, Milbrandt JD, Krause JE (1988). Differential expression of insulin-like growth factor genes in rat central nervous system. Proc Natl Acad Sci U S A., 85: 265-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bartlett WP, Li XS, Williams M, Benkovic S (1991). Localization of insulin-like growth factor-1 mRNA in murine central nervous system during postnatal development. Dev Biol., 147: 239-250 [DOI] [PubMed] [Google Scholar]
- [17].Bondy CA, Cheng CM (2004). Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol., 490: 25-31 [DOI] [PubMed] [Google Scholar]
- [18].Shetty AK, Hattiangady B, Shetty GA (2005). Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia, 51: 173-186 [DOI] [PubMed] [Google Scholar]
- [19].Drago J, Murphy M, Carroll SM, Harvey RP, Bartlett PF (1991). Fibroblast growth factor-mediated proliferation of central nervous system precursors depends on endogenous production of insulin-like growth factor I. Proc Natl Acad Sci U S A., 88: 2199-2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stylianopoulou F, Herbert J, Soares MB, Efstratiadis A (1988). Expression of the insulin-like growth factor II gene in the choroid plexus and the leptomeninges of the adult rat central nervous system. Proc Natl Acad Sci U S A., 85: 141-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ziegler AN, Schneider JS, Qin M, Tyler WA, Pintar JE, Fraidenraich D, et al. (2012). IGF-II promotes stemness of neural restricted precursors. Stem Cells, 30: 1265-1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee WH, Michels KM, Bondy CA (1993). Localization of insulin-like growth factor binding protein-2 messenger RNA during postnatal brain development: correlation with insulin-like growth factors I and II. Neuroscience, 53: 251-265 [DOI] [PubMed] [Google Scholar]
- [23].Bondy C, Lee WH (1993). Correlation between insulin-like growth factor (IGF)-binding protein 5 and IGF-I gene expression during brain development. J Neurosci., 13: 5092-5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kalluri HS, Dempsey RJ (2011). IGFBP-3 inhibits the proliferation of neural progenitor cells. Neurochem Res., 36: 406-411 [DOI] [PubMed] [Google Scholar]
- [25].Niu H, Gou R, Xu Q, Duan D (2017). Recombinant insulin-like growth factor binding protein-4 inhibits proliferation and promotes differentiation of neural progenitor cells. Neurosci Lett., 642: 71-76 [DOI] [PubMed] [Google Scholar]
- [26].Bartke A, Chandrashekar V, Dominici F, Turyn D, Kinney B, Steger R, et al. (2003). Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology, 4: 1-8 [DOI] [PubMed] [Google Scholar]
- [27].Bunn RC, King WD, Winkler MK, Fowlkes JL (2005). Early developmental changes in IGF-I, IGF-II, IGF binding protein-1, and IGF binding protein-3 concentration in the cerebrospinal fluid of children. Pediatr Res., 58: 89-93 [DOI] [PubMed] [Google Scholar]
- [28].Kelijman M (1991). Age-related alterations of the growth hormone/insulin-like-growth-factor I axis. J Am Geriatr Soc., 39: 295-307 [DOI] [PubMed] [Google Scholar]
- [29].van Dam PS, Aleman A (2004). Insulin-like growth factor-I, cognition and brain aging. Eur J Pharmacol., 490: 87-95 [DOI] [PubMed] [Google Scholar]
- [30].Fernandez AM, Torres-Aleman I (2012). The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci, 13: 225-239 [DOI] [PubMed] [Google Scholar]
- [31].Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS (2000). Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci., 20: 2896-2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Markowska AL, Mooney M, Sonntag WE (1998). Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience, 87: 559-569 [DOI] [PubMed] [Google Scholar]
- [33].Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA (2009). Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci., 29: 6734-6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dzaja D, Hladnik A, Bicanic I, Bakovic M, Petanjek Z (2014). Neocortical calretinin neurons in primates: increase in proportion and microcircuitry structure. Front Neuroanat., 8: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wrigley S, Arafa D, Tropea D (2017). Insulin-like growth factor 1: At the crossroads of brain development and aging. Front Cell Neurosci., 11: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nieto-Estevez V, Oueslati-Morales CO, Li L, Pickel J, Morales AV, Vicario-Abejon C (2016). Brain insulin-like growth factor-I directs the transition from stem cells to mature neurons during postnatal/adult hippocampal neurogenesis. Stem Cells, 34: 2194-2209 [DOI] [PubMed] [Google Scholar]
- [37].Hurtado-Chong A, Yusta-Boyo MJ, Vergano-Vera E, Bulfone A, de Pablo F, Vicario-Abejon C (2009). IGF-I promotes neuronal migration and positioning in the olfactory bulb and the exit of neuroblasts from the subventricular zone. Eur J Neurosci., 30: 742-755 [DOI] [PubMed] [Google Scholar]
- [38].Pardo J, Uriarte M, Console GM, Reggiani PC, Outeiro TF, Morel GR, et al. (2016). Insulin-like growth factor-I gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. Eur J Neurosci, 44: 2120-2128 [DOI] [PubMed] [Google Scholar]
- [39].Vahdatpour C, Dyer AH, Tropea D (2016). Insulin-Like Growth Factor 1 and Related Compounds in the Treatment of Childhood-Onset Neurodevelopmental Disorders. Front Neurosci., 10: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I (2002). Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med., 8: 1390-1397 [DOI] [PubMed] [Google Scholar]
- [41].Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, et al. (2008). FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A., 105: 13987-13992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Suh Y, Atzmon G, Cho M-O, Hwang D, Liu B, Leahy DJ, et al. (2008). Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A., 105: 3438-3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, et al. (2003). IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature, 421: 182-187 [DOI] [PubMed] [Google Scholar]
- [44].Chaker Z, Aid S, Berry H, Holzenberger M (2015). Suppression of IGF-I signals in neural stem cells enhances neurogenesis and olfactory function during aging. Aging Cell., 14: 847-856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A (2005). Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging., 26: 929-937 [DOI] [PubMed] [Google Scholar]
- [46].Chong VZ, Webster MJ, Rothmond DA, Weickert CS (2008). Specific developmental reductions in subventricular zone ErbB1 and ErbB4 mRNA in the human brain. Int J Dev Neurosci., 26: 791-803 [DOI] [PubMed] [Google Scholar]
- [47].Nogueira AB, Sogayar MC, Colquhoun A, Siqueira SA, Nogueira AB, Marchiori PE, et al. (2014). Existence of a potential neurogenic system in the adult human brain. J Transl Med., 12: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Damon SE, Plymate SR, Carroll JM, Sprenger CC, Dechsukhum C, Ware JL, et al. (2001). Transcriptional regulation of insulin-like growth factor-I receptor gene expression in prostate cancer cells. Endocrinology, 142: 21-27 [DOI] [PubMed] [Google Scholar]
- [49].Delafontaine P, Lou H, Alexander RW (1991). Regulation of insulin-like growth factor I messenger RNA levels in vascular smooth muscle cells. Hypertension., 18: 742-747 [DOI] [PubMed] [Google Scholar]
- [50].Delafontaine P, Song YH, Li Y (2004). Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol., 24: 435-444 [DOI] [PubMed] [Google Scholar]