Abstract
This retrospective cohort study investigated dementia risk associated with metformin use in type 2 diabetes patients by using the reimbursement database of the Taiwan’s National Health Insurance. The patients had new-onset diabetes during 1999-2005 and were followed up until December 31, 2011. An unmatched cohort of 147,729 ever users and 15,676 never users of metformin were identified, and a matched-pair cohort of 15,676 ever users and 15,676 never users was created by propensity score (PS). Hazard ratios were estimated by Cox regression incorporated with the inverse probability of treatment weighting using PS. Results showed that in the unmatched cohort, 713 never users and 3943 ever users developed dementia with respective incidence of 1029.20 and 570.03 per 100,000 person-years. The overall hazard ratio was 0.550 (95% confidence interval: 0.508-0.596). The hazard ratio for the first (<27.0 months), second (27.0-58.1 months) and third (>58.1 months) tertile of cumulative duration of metformin therapy was 0.975 (0.893-1.066), 0.554 (0.506-0.607) and 0.286 (0.259-0.315), respectively. Analyses in the matched cohort showed an overall hazard ratio of 0.707 (0.632-0.791) and the hazard ratio for the respective tertile was 1.279 (1.100-1.488), 0.704 (0.598-0.829) and 0.387 (0.320-0.468). In conclusion, metformin use is associated with a reduced dementia risk.
Keywords: dementia, diabetes mellitus, metformin, Taiwan
Dementia can be caused by vascular etiology or neurodegenerative disease (Alzheimer’s disease). It is a syndrome characterized by deterioration in memory and loss of daily self-care ability. It affects mainly the older people but may also happen in the younger generation. The World Health Organization (2017) has recognized the growing incidence of dementia in the world population and estimated that the number of people with dementia is currently around 47 million in the world and each year nearly 10 million new cases will add into the growing pool of patients (www.who.int/mediacentre/factsheets/fs362/en/). A call for actions and research priorities to reduce the global burden of dementia has been advocated following the First World Health Organization Ministerial Conference on Global Action Against Dementia summoned in March 2015 [1].
Elevated blood glucose may impair cerebral function and patients with diabetes have an increased risk of dementia [2]. The link between diabetes and dementia is probably multifactorial and mechanisms may involve inflammation, oxidative stress, atherosclerosis, amyloid-β deposition, brain insulin resistance with hyper-insulinemia, advanced glycation end-products (AGEs) and dysregulation of lipid metabolism [3,4].
Metformin is now considered the first-line therapy for type 2 diabetes mellitus. It reduces blood glucose level by reducing hepatic gluconeogenesis and increasing muscular glucose uptake through activation of the 5’-adenosine monophosphate-activated protein kinase (AMPK) [5]. In patients with type 2 diabetes mellitus, in addition to its glucose lowering effect, metformin has also been shown to reduce the risk of atherosclerotic events and cancers and have an anti-aging effect [6].
Studies evaluating the effect of metformin on the risk of dementia are still rare. Four population-based observational studies can be found in the literature, three from Taiwan using the administrative database of the National Health Insurance (NHI) and one from the UK using the General Practice Research Database. The first study by Hsu et al. from Taiwan showed that users of metformin only (n=1864, hazard ratio 0.76, 95% confidence interval 0.58-0.98) and users of metformin plus sulfonylureas (n=9257, hazard ratio 0.65, 95% confidence interval 0.56-0.74) had lower risk of dementia while compared to diabetes patients without taking any antidiabetic drugs (n=10519) [7]. The second study from Taiwan by Cheng et al. enrolled new-onset type 2 diabetes patients who had been using single oral antidiabetic drug of metformin, sulfonylureas and thiazolidinediones (TZDs), respectively [8]. When metformin users were treated as the referent group, the risk of dementia was significantly higher for users of TZDs but not for users of sulfonylureas [8]. The third study from Taiwan by Kuan et al. published recently compared 4651 metformin users and a comparable number of non-users matched on propensity score (PS) [9]. They showed a significantly higher risk in metformin users with an adjusted hazard ratio of 1.66 (95% confidence interval 1.35-2.04). The UK study by Imfeld et al. showed an increased risk of dementia associated with metformin use (odds ratio 1.71, 95% confidence interval 1.12-2.60) by using a matched case-control design including 7086 incident cases of Alzheimer’s disease and 7086 controls without dementia [10].
In a recent meta-analysis evaluating the impact of insulin sensitizers on the incidence of dementia, Ye et al. showed a statistical trend of risk reduction associated with the use of either TZDs (relative risk 0.75, 95% confidence interval 0.56-1.00, P=0.050) or metformin (relative risk 0.79, 95% confidence interval 0.62-1.01, P=0.064) [11].
Conflicting findings in the effect of metformin on cognitive function were also observed between a follow-up study conducted in Singapore and a small clinical study conducted in Australia. Ng et al. compared the cognitive function of 204 metformin users versus 161 non-users of diabetes patients recruited from the population-based Singapore Longitudinal Aging Study [12]. They showed that metformin use was associated with a lower risk of cognitive impairment (odds ratio 0.49, 95% confidence interval 0.25-0.95). In the Australian clinical study, Moore et al. showed that, among subgroup participants with diabetes (n=104, 35 metformin users and 91 non-users), worse cognitive performance was observed in metformin users (odds ratio 2.23, 95% confidence interval 1.05-4.75) [13].
Because metformin is widely used in a large number of diabetes patients, the conflicting findings of metformin on dementia risk and cognitive function warrant more in-depth research to clarify whether it can be beneficial or harmful. Therefore, the present study aimed at investigating the risk of dementia associated with metformin use in type 2 diabetes patients with careful consideration of potential bias and confounding commonly encountered in pharmacoepidemiological studies using existing administrative databases.
MATERIALS AND METHODS
The National Health Insurance (NHI) was implemented in Taiwan since March 1995. It is a unique healthcare system that covers 99.6% of the Taiwan’s population and has contracts with all in-hospitals and 93% of all medical settings [14]. All records including disease diagnoses, prescribed medications and performed procedures are kept as a database, which can be used for academic research after approval by ethics review. The present study was granted an approval number 99274.
Diabetes was coded 250.XX according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Dementia was coded as abridged codes of A210 or A222, or as ICD-9-CM codes of 290.0, 290.1, 290.2, 290.4, 294.1, 331.0-331.2, or 331.7-331.9.
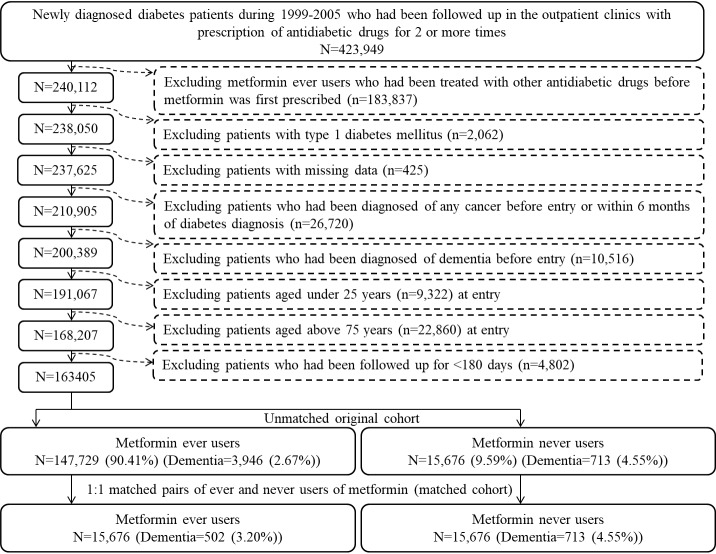
The database was described in more detail in previously published papers [15,16]. Figure 1 shows the procedures used to create the unmatched original cohort and the matched cohort from the database. A total of 423,949 patients diagnosed of new-onset diabetes during 1999-2005 in the outpatient clinics with prescription of antidiabetic drugs for 2 or more times were identified. Because longitudinal reimbursement data from 1996 to 2001 were available for each patient, to ensure a diagnosis of new-onset diabetes after 1999, patients with a diabetes diagnosis noted between 1996 and 1998 were not included. Ever users of metformin who had received any other antidiabetic drugs before metformin was initiated were first excluded (n=183,837). Other exclusion criteria included patients with 1) type 1 diabetes mellitus (n=2,062), 2) missing data (n=425), 3) diagnosis of any cancer before entry or within 6 months of diabetes diagnosis (n=26,720, cancer patients were excluded because of possible inclusion of distorted follow-up time due to shortened lifespan and possible misdiagnosis of dementia due to clinical presentations of malignancy), 4) diagnosis of dementia before entry (n=10,516), 5) age <25 years (n=9,322), 6) age >75 years (n=22,860) and 7) follow-up duration <180 days (n=4,802). As a result, 147,729 ever users and 15,676 never users of metformin were identified as the unmatched original cohort. The matched-pair cohort (the matched cohort) of ever and never users was created by matching the PS based on the Greedy 8 →1-digit match algorithm [17]. Logistic regression was used to create the PS from all characteristics (collected until the end of follow-up) listed in Table 1 plus the date of entry. This matching method has been used in our previous research and described in detail elsewhere [15,16].
Figure 1.
Flowchart showing the procedures followed in creating the unmatched original cohort and a cohort of 1:1 matched-pairs of metformin ever and never users from the reimbursement database of the National Health Insurance.
Table 1.
Characteristics of metformin never users and ever users in the unmatched original cohort and in the propensity, score matched cohort.
| Variable | Unmatched original cohort | Matched cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Never users | Ever users | Never users | Ever users | |||||||||
| (n=15676) | (n=147729) | P | SD | (n=15676) | (n=15676) | P | SD | |||||
| n | % | n | % | n | % | n | % | |||||
| Demographic data | ||||||||||||
| Age* (years) | 63.4±10.4 | 61.6±10.0 | <0.01 | -17.83 | 63.4±10.4 | 63.5±9.9 | 0.30 | 1.67 | ||||
| Sex (men) | 9009 | 57.47 | 80123 | 54.24 | <0.01 | -7.18 | 9009 | 57.47 | 8985 | 57.32 | 0.78 | -0.56 |
| Occupation | ||||||||||||
| I | 6142 | 39.18 | 58066 | 39.31 | <0.01 | 6142 | 39.18 | 6084 | 38.81 | 0.92 | ||
| II | 3122 | 19.92 | 34112 | 23.09 | 8.28 | 3122 | 19.92 | 3153 | 20.11 | 0.51 | ||
| III | 3301 | 21.06 | 30600 | 20.71 | -0.65 | 3301 | 21.06 | 3316 | 21.15 | 0.45 | ||
| IV | 3111 | 19.85 | 24951 | 16.89 | -8.58 | 3111 | 19.85 | 3123 | 19.92 | 0.02 | ||
| Living region | ||||||||||||
| Taipei | 5211 | 33.24 | 46512 | 31.48 | <0.01 | 5211 | 33.24 | 5296 | 33.78 | 0.31 | ||
| Northern | 1586 | 10.12 | 16577 | 11.22 | 3.71 | 1586 | 10.12 | 1499 | 9.56 | -1.86 | ||
| Central | 2769 | 17.66 | 27115 | 18.35 | 1.88 | 2769 | 17.66 | 2691 | 17.17 | -1.27 | ||
| Southern | 2746 | 17.52 | 25098 | 16.99 | -1.49 | 2746 | 17.52 | 2780 | 17.73 | 0.65 | ||
| Kao-Ping and Eastern | 3364 | 21.46 | 32427 | 21.95 | 1.35 | 3364 | 21.46 | 3410 | 21.75 | 0.81 | ||
| Major comorbidities | ||||||||||||
| Hypertension | 12804 | 81.68 | 120731 | 81.72 | 0.89 | 0.27 | 12804 | 81.68 | 12836 | 81.88 | 0.64 | 0.69 |
| Dyslipidemia | 11299 | 72.08 | 122549 | 82.96 | <0.01 | 28.63 | 11299 | 72.08 | 11290 | 72.02 | 0.91 | 0.26 |
| Obesity | 424 | 2.70 | 6676 | 4.52 | <0.01 | 9.96 | 424 | 2.70 | 389 | 2.48 | 0.21 | -1.29 |
| Nephropathy | 5356 | 34.17 | 40101 | 27.14 | <0.01 | -17.32 | 5356 | 34.17 | 5296 | 33.78 | 0.47 | -1.31 |
| Eye diseases | 2942 | 18.77 | 47803 | 32.36 | <0.01 | 31.61 | 2942 | 18.77 | 2662 | 16.98 | <0.01 | -4.94 |
| Stroke | 4996 | 31.87 | 42101 | 28.50 | <0.01 | -8.08 | 4996 | 31.87 | 4885 | 31.16 | 0.18 | -1.49 |
| Ischemic heart disease | 7384 | 47.10 | 67018 | 45.37 | <0.01 | -3.60 | 7384 | 47.10 | 7348 | 46.87 | 0.68 | -0.30 |
| Peripheral arterial disease | 3550 | 22.65 | 37742 | 25.55 | <0.01 | 6.98 | 3550 | 22.65 | 3550 | 22.65 | 1.00 | -0.12 |
| Antidiabetic drugs | ||||||||||||
| Insulin | 1282 | 8.18 | 3452 | 2.34 | <0.01 | -29.90 | 1282 | 8.18 | 1048 | 6.69 | <0.01 | -6.59 |
| Sulfonylurea | 11468 | 73.16 | 107896 | 73.04 | 0.75 | 5.13 | 11468 | 73.16 | 11768 | 75.07 | <0.01 | 5.32 |
| Meglitinide | 1283 | 8.18 | 5767 | 3.90 | <0.01 | -19.17 | 1283 | 8.18 | 1259 | 8.03 | 0.62 | -0.50 |
| Acarbose | 1743 | 11.12 | 8088 | 5.47 | <0.01 | -20.10 | 1743 | 11.12 | 1697 | 10.83 | 0.41 | -1.42 |
| Rosiglitazone | 464 | 2.96 | 7388 | 5.00 | <0.01 | 10.94 | 464 | 2.96 | 467 | 2.98 | 0.92 | -0.06 |
| Pioglitazone | 387 | 2.47 | 3943 | 2.67 | 0.14 | -20.10 | 387 | 2.47 | 398 | 2.54 | 0.69 | -1.42 |
| Commonly encountered comorbidities | ||||||||||||
| COPD | 7675 | 48.96 | 71044 | 48.09 | 0.03 | -2.12 | 7675 | 48.96 | 7807 | 49.80 | 0.14 | 1.86 |
| Tobacco abuse | 442 | 2.82 | 5943 | 4.02 | <0.01 | 6.83 | 442 | 2.82 | 469 | 2.99 | 0.36 | 1.06 |
| Alcohol-related diagnoses | 1231 | 7.85 | 10490 | 7.10 | <0.01 | -4.16 | 1231 | 7.85 | 1087 | 6.93 | <0.01 | -3.77 |
| Head injury | 538 | 3.43 | 5524 | 3.74 | 0.05 | 1.44 | 538 | 3.43 | 523 | 3.34 | 0.64 | -0.63 |
| Parkinson’s disease | 504 | 3.22 |
3349 |
2.27 | <0.01 | -6.26 |
504 |
3.22 |
504 |
3.22 | 1.00 | 0.10 |
| Commonly used medications in diabetes patients | ||||||||||||
| ACEI/ARB | 10854 | 69.24 | 107911 | 73.05 | <0.01 | 8.81 | 10854 | 69.24 | 10879 | 69.40 | 0.76 | -0.30 |
| Calcium channel blocker | 9771 | 62.33 | 88083 | 59.62 | <0.01 | -5.62 | 9771 | 62.33 | 9767 | 62.31 | 0.96 | 0.41 |
| Statin | 8428 | 53.76 | 97358 | 65.90 | <0.01 | 26.59 | 8428 | 53.76 | 8300 | 52.95 | 0.15 | 0.06 |
| Fibrate | 5338 | 34.05 | 63817 | 43.20 | <0.01 | 20.06 | 5338 | 34.05 | 5170 | 32.98 | <0.05 | -1.41 |
| Aspirin | 8871 | 56.59 | 90400 | 61.19 | <0.01 | 9.87 | 8871 | 56.59 | 8797 | 56.12 | 0.40 | -2.07 |
Age is expressed as mean ± standard deviation.
SD: standardized difference.
COPD: chronic obstructive pulmonary disease, ACEI/ARB: angiotensin converting enzyme inhibitor/angiotensin receptor blocker.
Refer to “Materials and Methods” for the classification of occupation
Cumulative duration of metformin therapy in months was calculated and categorized into tertiles for dose-response analyses. Potential confounders included demographic data (age, sex, occupation and living region), major comorbidities (hypertension, dyslipidemia and obesity), diabetes-related complications (nephropathy, eye disease, stroke, ischemic heart disease and peripheral arterial disease), antidiabetic drugs (insulin, sulfonylureas meglitinide, acarbose, rosiglitazone and pioglitazone), commonly encountered comorbidities (chronic obstructive pulmonary disease, tobacco abuse, alcohol-related diagnoses, head injury and Parkinson’s disease) and commonly used medications in diabetes patients (angiotensin converting enzyme inhibitor/angiotensin receptor blocker, calcium channel blocker, statin, fibrate and aspirin). The living region and occupation were classified as detailed elsewhere [18]. In brief, the living region was classified as Taipei, Northern, Central, Southern, and Kao-Ping/Eastern. Occupation was classified as class I (civil servants, teachers, employees of governmental or private businesses, professionals and technicians), class II (people without a specific employer, self-employed people or seamen), class III (farmers or fishermen) and class IV (low-income families supported by social welfare, or veterans). The ICD-9-CM codes for the related diagnoses are provided below: hypertension (401-405), dyslipidemia (272.0-272.4), obesity (278), nephropathy (580-589), eye diseases (250.5: diabetes with ophthalmic manifestations, 362.0: diabetic retinopathy, 369: blindness and low vision, 366.41: diabetic cataract, and 365.44: glaucoma associated with systemic syndromes), stroke (430-438), ischemic heart disease (410-414), peripheral arterial disease (250.7, 785.4, 443.81 and 440-448), chronic obstructive pulmonary disease (a surrogate for smoking; 490-496), tobacco abuse (305.1, 649.0 and 989.84), alcohol-related diagnoses (291, 303, 535.3, 571.0-571.3 and 980.0), head injury (959.01) and Parkinson’s disease (332).
Analyses were conducted in both the unmatched original cohort and the matched cohort to examine the consistency of the findings. Student’s t test compared the difference of age between never and ever users and Chi-square test was used for other variables. Standardized difference proposed by Austin and Stuart as a test for balance diagnostics was calculated for all covariates, and a value >10% might indicate potential confounding from the variable [19].
Incidence density of dementia was calculated with regards to the use of metformin in the following subgroups: never users, ever users and the tertiles of cumulative duration. The numerator was the case number of newly diagnosed dementia identified during follow-up. The denominator was the person-years of follow-up, which ended on December 31, 2011, at the time of a new diagnosis of dementia, or on the date of death or the last reimbursement record.
Hazard ratios and their 95% confidence intervals for ever users and for each tertile of cumulative duration in referent to never users were estimated by Cox regression incorporated with the inverse probability of treatment weighting (IPTW) using the PS. As proposed by Austin, this method reduces the potential confounding from the differences in characteristics [20].
Sensitivity analyses were conducted after excluding patients who received consecutive prescriptions of metformin spanning more than 4 months and 6 months, respectively. Because the Bureau of the NHI allows at most 3 months of drug prescriptions for the patients in each outpatient visit, these analyses might have excluded most of the patients with poor adherence and did not receive regular drug refill. Incretin-based therapies were not reimbursed by the NHI until after 2009 in Taiwan. Because a recent study suggested that sitagliptin use was associated with an improvement in cognitive function [21], to avoid the potential impact of incretin-based therapies, sensitivity analysis was also conducted after excluding patients who happened to receive an incretin-based therapy during follow-up.
In consideration that more antidiabetic drugs have been introduced into clinical practice during the last two decades and the guidelines for their use have evolved over these years, the PS-weighted hazard ratios were also estimated for patients enrolled in each specific year from 1999 to 2005 in the unmatched original cohort and the matched cohort, respectively.
Analyses were conducted using SAS statistical software, version 9.3 (SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
RESULTS
Table 1 compares the characteristics between never and ever users of metformin. In the unmatched original cohort, age and sex differed significantly. The mean age was older (63.4±10.4 vs. 61.6±10.0 years, P<0.01) and the proportion of men was higher (57.47% vs. 54.24%, P<0.01) in never users. All other variables, except hypertension, sulfonylureas, pioglitazone and head injury, also differed significantly in the original cohort. However, in the matched cohort, age and sex were similar and most variables were not different significantly (except for eye diseases, insulin, sulfonylureas, alcohol-related diagnoses and fibrate). While examining the standardized differences in the matched cohort, none had a value >10%.
The incidence of dementia and the hazard ratios by metformin exposure is shown in Table 2. The overall hazard ratios suggested a significantly lower risk of dementia associated with metformin use in either the unmatched cohort or the matched cohort. In tertile analyses, the hazard ratios suggested a reduced risk in a dose-response pattern. Patients who had used metformin for more than 2 years in the second and third tertiles consistently showed a significantly reduced risk. For the first tertile, the risk was neutral in the unmatched cohort analysis but was slightly higher with a significant p-value in the matched cohort.
Table 2.
Incidence rates of dementia and hazard ratios by metformin exposure
| Metformin use | n | N | Person-year | Incidence rate (per 100,000 person-years) | HR | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| Unmatched original cohort | |||||||
| Never users | 713 | 15676 | 69277.31 | 1029.20 | 1.000 | ||
| Ever users | 3943 | 147730 | 691712.02 | 570.03 | 0.550 | (0.508-0.596) | <0.0001 |
| Tertiles of cumulative duration of metformin therapy (months) | |||||||
| Never users | 713 | 15676 | 69277.31 | 1029.20 | 1.000 | ||
| <27.0 | 1657 | 48645 | 168899.36 | 981.06 | 0.975 | (0.893-1.066) | 0.5819 |
| 27.0-58.1 | 1363 | 48872 | 237111.30 | 574.84 | 0.554 | (0.506-0.607) | <0.0001 |
| >58.1 | 923 | 50213 | 285701.36 | 323.06 | 0.286 | (0.259-0.315) | <0.0001 |
| Matched cohort | |||||||
| Never users | 713 | 15676 | 69277.31 | 1029.20 | 1.000 | ||
| Ever users | 531 | 15676 | 72593.50 | 731.47 | 0.707 | (0.632-0.791) | <0.0001 |
| Tertiles of cumulative duration of metformin therapy (months) | |||||||
| Never users | 713 | 15676 | 69277.31 | 1029.20 | 1.000 | ||
| <26.6 | 226 | 5171 | 17707.20 | 1276.32 | 1.279 | (1.100-1.488) | 0.0014 |
| 26.6-57.8 | 180 | 5175 | 24707.24 | 728.53 | 0.704 | (0.598-0.829) | <0.0001 |
| >57.8 | 125 | 5330 | 30179.07 | 414.19 | 0.387 | (0.320-0.468) | <0.0001 |
n: incident case number of dementia, N: case number followed
HR: hazard ratio (weighted for propensity score), CI: confidence interval
Sensitivity analyses conducted in the unmatched cohort after excluding patients who had not received regular refill of metformin (i.e., periods between two consecutive prescriptions of metformin spanning >4 months and >6 months, respectively) or patients who happened to be treated with incretin-based therapies during follow-up did not change the conclusions of the study (Table 3).
Table 3.
Sensitivity analyses estimating hazard ratios for dementia for ever versus never users of metformin in the original cohort.
| Models | n | N | HR | 95% CI | P value |
|---|---|---|---|---|---|
| Excluding two consecutive prescriptions of metformin spanning more than 4 months | |||||
| Never users | 713 | 15676 | 1.000 | ||
| Ever users | 1046 | 49704 | 0.467 | (0.425-0.514) | <0.0001 |
| Tertiles of cumulative duration of metformin therapy (months) | |||||
| Never users | 713 | 15676 | 1.000 | ||
| <27.0 | 392 | 16043 | 0.937 | (0.826-1.064) | 0.3174 |
| 27.0-58.1 | 325 | 13665 | 0.528 | (0.463-0.603) | <0.0001 |
| >58.1 | 329 | 19996 | 0.261 | (0.229-0.298) | <0.0001 |
| Excluding two consecutive prescriptions of metformin spanning more than 6 months | |||||
| Never users | 713 | 15676 | 1.000 | ||
| Ever users | 1448 | 65976 | 0.469 | (0.429-0.513) | <0.0001 |
| Tertiles of cumulative duration of metformin therapy (months) | |||||
| Never users | 713 | 15676 | 1.000 | ||
| <27.0 | 512 | 19446 | 0.966 | (0.860-1.085) | 0.5606 |
| 27.0-58.1 | 482 | 19013 | 0.541 | (0.482-0.607) | <0.0001 |
| >58.1 | 454 | 27517 | 0.259 | (0.230-0.292) | <0.0001 |
| Excluding patients treated with incretin-based therapies during follow-up | |||||
| Never users | 692 | 14750 | 1.000 | ||
| Ever users | 3615 | 113090 | 0.655 | (0.604-0.711) | <0.0001 |
| Tertiles of cumulative duration of metformin therapy (months) | |||||
| Never users | 692 | 14750 | 1.000 | ||
| <27.0 | 1580 | 41031 | 1.072 | (0.979-1.173) | 0.1318 |
| 27.0-58.1 | 1241 | 37153 | 0.650 | (0.592-0.713) | <0.0001 |
| >58.1 | 794 | 34906 | 0.349 | (0.315-0.387) | <0.0001 |
n: incident case number of dementia, N: case number followed
HR: hazard ratio (weighted for propensity score), CI: confidence interval
Table 4 shows the hazard ratios for patients enrolled in each specific year from 1999 to 2005 in the unmatched cohort and the matched cohort, respectively. It is evident that the lower risk of dementia associated with metformin use was not affected by the year of enrollment.
Table 4.
Hazard ratios for dementia for ever versus never users of metformin estimated for each specific year from 1999 to 2005
| Year | Ever users
|
Never users
|
HR | 95% CI | P value | ||
|---|---|---|---|---|---|---|---|
| n | N | n | N | ||||
| Unmatched original cohort | |||||||
| 1999 | 793 | 21033 | 73 | 1335 | 0.562 | (0.442-0.714) | <0.0001 |
| 2000 | 704 | 21309 | 70 | 1473 | 0.588 | (0.460-0.752) | <0.0001 |
| 2001 | 639 | 22089 | 85 | 1726 | 0.514 | (0.410-0.645) | <0.0001 |
| 2002 | 535 | 21624 | 100 | 2110 | 0.485 | (0.392-0.600) | <0.0001 |
| 2003 | 502 | 21997 | 104 | 2411 | 0.511 | (0.414-0.632) | <0.0001 |
| 2004 | 397 | 20430 | 124 | 2873 | 0.456 | (0.373-0.558) | <0.0001 |
| 2005 | 376 | 19247 | 157 | 3748 | 0.507 | (0.420-0.611) | <0.0001 |
| Matched cohort | |||||||
| 1999 | 90 | 2304 | 73 | 1335 | 0.588 | (0.432-0.801) | 0.0008 |
| 2000 | 84 | 2329 | 70 | 1473 | 0.659 | (0.480-0.906) | 0.0101 |
| 2001 | 96 | 2420 | 85 | 1726 | 0.725 | (0.541-0.971) | 0.0309 |
| 2002 | 59 | 2231 | 100 | 2110 | 0.529 | (0.383-0.730) | 0.0001 |
| 2003 | 69 | 2223 | 104 | 2411 | 0.697 | (0.514-0.945) | 0.0200 |
| 2004 | 55 | 2142 | 124 | 2873 | 0.611 | (0.445-0.840) | 0.0024 |
| 2005 | 49 | 2027 | 157 | 3748 | 0.624 | (0.453-0.861) | 0.0041 |
n: incident case number of dementia, N: case number followed
HR: hazard ratio (weighted for propensity score), CI: confidence interval
DISCUSSION
The findings suggested that metformin use in type 2 diabetes patients was associated with a significantly lower risk of dementia, especially when it had been used for more than 2 years (Table 2). The risk reduction showed a dose-response pattern and was consistent in sensitivity analyses (Table 3). The lower risk of dementia associated with metformin use was not affected by the year of enrollment (Table 4).
Although the mechanisms of the reduced risk of dementia associated with metformin use have not been fully investigated, some biological actions of metformin could explain such a beneficial effect. Metformin inhibits gluconeogenesis in the liver and lowers blood glucose by activating the liver kinase B1 (LKB1)/AMPK pathway through inhibiting the mitochondrial respiratory-chain complex 1 [5]. Studies suggested that activation of AMPK-dependent pathway in the brain exerts neuroprotective effects [22]. Insulin resistance with impaired insulin signaling and decreased glucose metabolism is observed in patients with dementia [23]. Metformin improves insulin resistance by increasing insulin receptor expression and improving tyrosine kinase activity [24]. A pilot randomized placebo-controlled crossover trial showed that metformin was measurable in the cerebrospinal fluid with improvement in cognitive function [25]. Increased inflammation and oxidative stress are characteristic pathophysiological changes in the brain of patients with dementia [3]. Evidence suggested that metformin may protect the cardiac and vascular system from oxidative stress and inflammation via AMPK-dependent and -independent pathways [26]. In line with such findings, animal studies supported that treatment with metformin improved cognitive function in rats with a significant reduction in inflammation and oxidative stress in the brain [27,28]. Upregulation of the mammalian target of rapamycin (mTOR) pathway has also been implicated as a major pathological process leading to Alzheimer’s disease [29]. Metformin is well known for its inhibitory effect on mTOR via activation of LKB1/AMPK [24]. Although an early laboratory study suggested that metformin increased the biogenesis of amyloid-β in neuronal tissues, which might be potentially harmful to neuronal cells, this same study showed that metformin in combination with insulin reduced amyloid-β levels [30]. More recent studies, on the contrary, suggested that metformin was neuroprotective against amyloid-β-induced mitochondrial dysfunction in human neuronal stem cells via an AMPK-dependent pathway [31] and that metformin alleviated apoptosis induced by amyloid-β via suppressing the c-Jun N-terminal protein kinases/mitogen-activated protein kinase pathway in culture hippocampal neurons [32]. AGEs can be responsible for dementia in diabetes patients with poor glycemic control [33]. Metformin can reduce the formation of AGEs through improving glycemic control and additionally it has been shown that metformin may exert a scavenging effect on AGEs [34]. Dysregulation of lipid metabolism [4] and gut microbiota dysbiosis [35] have also been implicated as potential links between diabetes and dementia. Metformin may reverse insulin resistance, improve insulin signaling and correct lipid dysmetabolism [24]. Recent studies also suggested that metformin may change the composition of gut microbiota with an increase in Akkermansia species leading to improvement in insulin resistance and reduction in tissue inflammation [36]. The United Kingdom Prospective Diabetes Study supported that metformin might have a cardioprotective effect resulting in reduced atherosclerotic events in obese patients with type 2 diabetes mellitus [37]. It has been well recognized that atherosclerosis plays an important role in the development of vascular dementia. Therefore, metformin may also reduce the risk of dementia through its anti-atherogenic action on the vascular system. Taken together, metformin may exert its beneficial effect on dementia via either vascular protection or neuronal protection.
It is interesting that patients in the first tertile of short-term metformin use showed a significantly higher risk of dementia in the matched cohort analysis (Table 2). Because obesity is one of the major risk factors associated with an increased risk of dementia [38] and metformin is strongly indicated for diabetes patients with obesity [37], the increased risk in the first tertile might have been carried over from patients with obesity who were first initiated with metformin treatment.
Pharmacoepidemiological studies evaluating clinical outcomes related to medications using administrative databases may suffer from methodological limitations. These include prevalent user bias, immortal time bias and confounding by indication. Basically, these potential limitations have been carefully addressed in the present study.
The problem of prevalent user bias has been avoided by enrolling patients with newly diagnosed diabetes and new users of metformin. The potential impacts resulted from the use of other antidiabetic drugs before metformin was initiated were also avoided by including only patients who had been treated with metformin as the first antidiabetic drug in ever users (Figure 1). In consideration that the exclusion of these patients might introduce another selection bias, secondary analyses were conducted without excluding these patients. The overall hazard ratio for the unmatched cohort was 0.508 (0.471-0.549), and the hazard ratios for the respective tertiles of cumulative duration of metformin therapy were 0.894 (0.823-0.971), 0.511 (0.470-0.556) and 0.261 (0.239-0.285). For the matched cohort, the overall hazard ratio was 0.661 (0.590-0.742) and the hazard ratios for the respective tertiles were 1.210 (1.037-1.411), 0.717 (0.610-0.842) and 0.312 (0.254-0.385). Therefore, the results of the study were robust and would not be affected by the inclusion or exclusion of these patients.
Inappropriate assignment of treatment status and follow-up time may introduce immortal time bias by including the so-called immortal time (the follow-up period during which the outcome cannot happen) in the calculation of the follow-up period [39]. In the present study, it is unlikely to include ambiguous diagnosis of diabetes by enrolling only those who had been prescribed antidiabetic drugs for 2 or more times (Figure 1). The status of treatment was also less likely misclassified because the NHI is a universal healthcare system in Taiwan and all prescription information was kept for the whole period since the implementation of the NHI. Therefore, the approach used in the present study has avoided misdiagnosis of diabetes and misclassification of treatment status.
Furthermore, the exclusion of patients with a follow-up period of <180 days (Figure 1) has avoided the inappropriate assignment of follow-up time during the initial period of “immortal time”. The immortal time between diabetes diagnosis and the start of the use of antidiabetic drugs was actually not calculated in the follow-up person-years. Lévesque et al. [39] pointed out another potential source of immortal time that can be introduced during the waiting period between the prescription and dispense of medications when patients are discharged from the hospital. It is worthy to note that this would not happen in the present study because all patients were enrolled from the outpatient clinics. Even if the patients were enrolled from the hospitals, neither would this immortal time occur in Taiwan because all discharge medications can be obtained directly from the hospitals when the patients are discharged.
It is worthy to point out that immortal time might be introduced when the cumulative duration increased because the patients should have lived long enough without development of dementia up to the time of the cumulative duration. Lévesque et al. pointed out that there is a “direct relation between the immortal period and the magnitude of the bias” [39]. Therefore, the magnitude of the hazard ratios in the second and third tertiles (Table 2) should be interpreted more cautiously and the dose-response effect could not be fully clarified in the present study.
Confounding by indication could be much reduced by demonstrating the beneficial effects of metformin in both the unmatched original cohort and the PS-matched cohort (Table 2), by modeling with Cox regression incorporated with IPTW (Table 2), and by showing a lack of potential residual confounding by calculating the standardized differences and none of the covariates had a value >10% in the matched cohort (Table 1).
Small sample sizes, prevalent user bias, immortal time bias, confounding by indication, lack of dose-response analysis, and inadequate control group can be seen in earlier studies. For example, the study by Hsu et al. [7] compared the risk of dementia in subgroups of diabetes patients with the use of sulfonylureas only, metformin only and sulfonylurea plus metformin to a group of diabetes patients without ever use of any antidiabetic drugs might have included an inappropriate control group without the use of any antidiabetic drugs. Furthermore, prevalent user bias and immortal time bias were not well addressed. The study by Cheng et al. [8] included very small numbers of new-onset diabetes patients who had used solely metformin (n=1033), sulfonylureas (n=796) or TZDs (n=28) and compared users of sulfonylureas or TZDs to metformin users. This study has limitations of small sample sizes, lack of dose-response analysis and potential risk of immortal time bias and confounding by indication. Kuan et al. included new-onset diabetes patients identified from the cohort of the released LHID2000 database by the Bureau of NHI and defined users of metformin as any use of at least 90 days (n=4651) and non-users as never use of metformin (n=4651) during the baseline year of 2000 [9]. The LHID2000 database was formed by a cohort of 1 million insurants who joined the NHI in the year 2000 and does not include any one who was born or who joined the NHI after the year 2000. Therefore, the contamination of the use of other antidiabetic drugs for users and non-users of metformin at baseline was unavoidable during the long follow-up period to December 31, 2010. The matched case-control study by Imfeld et al. included 7086 incident cases of Alzheimer’s disease diagnosed between 1998 and 2008 and a comparable number of controls without dementia and matched on age, sex, general practice, calendar time and years of history in the UK General Practice Research Database [10]. Because of the cross-sectional nature of the case-control design, only odds ratios could be estimated, and it was not possible to completely exclude the potential risk of prevalent user bias, immortal time bias and confounding by indication in this study because these had not been well addressed. The Singaporean study by Ng et al. showing an improvement in cognitive function in users of metformin [12] and the Australian clinical study showing a significantly higher risk of dementia associated with metformin use [13] were not population-based studies. Furthermore, both enrolled very small sample sizes and evaluated cognitive function rather than dementia risk. They both certainly might suffer from the potential risk of bias and confounding commonly seen in large pharmacoepidemiological studies.
While compared to previous studies, the present study has a combined strength of including large samples of metformin users and patients with dementia, addressing most of the methodological limitations associated with pharmacoepidemiological studies and investigating the potential effect of dose-response in a follow-up design. The study has additional merits of using a nationwide database that covers >99% of the population. Therefore, the findings can be readily generalized to the whole population. The use of the medical records significantly reduced the potential biases related to self-reporting. Detection bias due to different socioeconomic status was less likely because the drug cost-sharing is low in the NHI of Taiwan and which can always be waived in patients with certain conditions like low-income household, veterans or receiving prescription refills for chronic disease.
The study limitations may include a lack of biochemical data and lack of measurement data of some confounders like anthropometric factors, smoking, alcohol drinking, lifestyle, nutritional status, dietary pattern, family history and genetic parameters (such as Apo E4 genotype). Furthermore, we did not have the data of AGEs for analyses.
In summary, the present study supports a beneficial effect of metformin on the prevention of dementia in type 2 diabetes patients. The findings give rationale for conducting clinical trials to prove such an effect. Given that metformin is safe and cheap and would not cause hypoglycemia when used as monotherapy, its usefulness for the prevention of dementia in both the diabetes patients and non-diabetes people is worthy of in-depth investigation.
Acknowledgments
The study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes. The study was supported by the Ministry of Science and Technology (MOST 103-2314-B-002-187-MY3) of Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- [1].Shah H, Albanese E, Duggan C, Rudan I, Langa KM, Carrillo MC, et al. (2016). Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol, 15: 1285-94. [DOI] [PubMed] [Google Scholar]
- [2].Li X, Song D, Leng SX (2015). Link between type 2 diabetes and Alzheimer’s disease: from epidemiology to mechanism and treatment. Clin Interv Aging, 10: 549-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rojas-Gutierrez E, Muñoz-Arenas G, Treviño S, Espinosa B, Chavez R, Rojas K, et al. (2017). Alzheimer’s disease and metabolic syndrome: A link from oxidative stress and inflammation to neurodegeneration. Synapse. [DOI] [PubMed] [Google Scholar]
- [4].Huynh K, Martins RN, Meikle PJ (2017). Lipidomic profiles in diabetes and dementia. J Alzheimers Dis, 59:433-44. [DOI] [PubMed] [Google Scholar]
- [5].Rena G, Pearson ER, Sakamoto K (2013). Molecular mechanism of action of metformin: old or new insights? Diabetologia, 56: 1898-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang YW, He SJ, Feng X, Cheng J, Luo YT, Tian L, et al. (2017). Metformin: a review of its potential indications. Drug Des Devel Ther, 11: 2421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hsu CC, Wahlqvist ML, Lee MS, Tsai HN (2011). Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis, 24: 485-93. [DOI] [PubMed] [Google Scholar]
- [8].Cheng C, Lin CH, Tsai YW, Tsai CJ, Chou PH, Lan TH (2014). Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J Gerontol A Biol Sci Med Sci, 69: 1299-305. [DOI] [PubMed] [Google Scholar]
- [9].Kuan YC, Huang KW, Lin CL, Hu CJ, Kao CH (2017). Effects of metformin exposure on neurodegenerative diseases in elderly patients with type 2 diabetes mellitus. Prog Neuropsychopharmacol Biol Psychiatry, 79(Pt B): 77-83. [DOI] [PubMed] [Google Scholar]
- [10].Imfeld P, Bodmer M, Jick SS, Meier CR (2012). Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc, 60: 916-21. [DOI] [PubMed] [Google Scholar]
- [11].Ye F, Luo YJ, Xiao J, Yu NW, Yi G (2016). Impact of insulin sensitizers on the incidence of dementia: a meta-analysis. Dement Geriatr Cogn Disord, 41: 251-60. [DOI] [PubMed] [Google Scholar]
- [12].Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B (2014). Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis, 41: 61-8. [DOI] [PubMed] [Google Scholar]
- [13].Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, et al. (2013). Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care, 36: 2981-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Introduction to the National Health Insurance (in Chinese). https://www.nhi.gov.tw/Content_List.aspx?n=5C56DAE8D685EA0E&topn=FB01D469347C76A7 (last accessed November 28, 2017). [Google Scholar]
- [15].Tseng CH (2017). Metformin and lung cancer risk in patients with type 2 diabetes mellitus. Oncotarget, 8: 41132-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tseng CH (2017). Metformin is associated with a lower risk of colorectal cancer in Taiwanese patients with type 2 diabetes: a retrospective cohort analysis. Diabetes Metab, 43: 438-45. [DOI] [PubMed] [Google Scholar]
- [17].D’Agostino RB Jr (1998) Tutorial in Biostatistics: Propensity Score Methods for Bias Reduction in the Comparison of a Treatment to a Non-Randomized Control Group, Statistics in Medicine, 17, 2265-2281 [DOI] [PubMed] [Google Scholar]
- [18].Tseng CH (2012). Diabetes, metformin use, and colon cancer: A population-based cohort study in Taiwan. Eur J Endocrinol, 167: 409-16. [DOI] [PubMed] [Google Scholar]
- [19].Austin PC, Stuart EA (2015). Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med, 34: 3661-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Austin PC (2013). The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med, 32: 2837-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Isik AT, Soysal P, Yay A, Usarel C (2017). The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer’s disease. Diabetes Res Clin Pract, 123: 192-8. [DOI] [PubMed] [Google Scholar]
- [22].Markowicz-Piasecka M, Sikora J, Szydłowska A, Skupień A, Mikiciuk-Olasik E, Huttunen KM (2017). Metformin - a future therapy for neurodegenerative diseases. Pharm Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen Y, Zhang J, Zhang B, Gong CX (2016). Targeting insulin signaling for the treatment of Alzheimer’s Disease. Curr Top Med Chem, 16: 485-92. [DOI] [PubMed] [Google Scholar]
- [24].Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F (2012). Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond), 122: 253-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koenig AM, Mechanic-Hamilton D, Xie SX, Combs MF, Cappola AR, Xie L, et al. (2017). Effects of the insulin sensitizer metformin in Alzheimer disease: pilot data from a randomized placebo-controlled crossover study. Alzheimer Dis Assoc Disord, 31: 107-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nesti L, Natali A (2017). Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data. Nutr Metab Cardiovasc Dis, 27: 657-69. [DOI] [PubMed] [Google Scholar]
- [27].Mostafa DK, Ismail CA, Ghareeb DA (2016). Differential metformin dose-dependent effects on cognition in rats: role of Akt. Psychopharmacology (Berl), 233: 2513-24. [DOI] [PubMed] [Google Scholar]
- [28].Pandey S, Garabadu D (2017). Piracetam facilitates the anti-amnesic but not anti-diabetic activity of metformin in experimentally induced type-2 diabetic encephalopathic rats. Cell Mol Neurobiol, 37: 791-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang C, Yu JT, Miao D, Wu ZC, Tan MS, Tan L (2014). Targeting the mTOR signaling network for Alzheimer’s disease therapy. Mol Neurobiol, 49: 120-35. [DOI] [PubMed] [Google Scholar]
- [30].Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, et al. (2009). Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A, 106: 3907-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chiang MC, Cheng YC, Chen SJ, Yen CH, Huang RN (2016). Metformin activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced mitochondrial dysfunction. Exp Cell Res, 347: 322-31. [DOI] [PubMed] [Google Scholar]
- [32].Chen B, Teng Y, Zhang X, Lv X, Yin Y (2016). Metformin alleviated Aβ-induced apoptosis via the suppression of JNK MAPK signaling pathway in cultured hippocampal neurons. Biomed Res Int, 2016: 1421430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Simó R, Ciudin A, Simó-Servat O, Hernández C (2017). Cognitive impairment and dementia: a new emerging complication of type 2 diabetes-The diabetologist’s perspective. Acta Diabetol, 54: 417-24. [DOI] [PubMed] [Google Scholar]
- [34].Bonnefont-Rousselot D (2001). Antioxidant and anti-AGE therapeutics: evaluation and perspectives. J Soc Biol, 195: 391-8. (Article in French with English abstract). [DOI] [PubMed] [Google Scholar]
- [35].Jiang C, Li G, Huang P, Liu Z, Zhao B (2017). The gut microbiota and Alzheimer’s disease. J Alzheimers Dis, 58: 1-15. [DOI] [PubMed] [Google Scholar]
- [36].Hur KY, Lee MS (2015). New mechanisms of metformin action: Focusing on mitochondria and the gut. J Diabetes Investig, 6: 600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].UK Prospective Diabetes Study (UKPDS) Group (1998). Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet, 352: 854-65. [PubMed] [Google Scholar]
- [38].Albanese E, Launer LJ, Egger M, Prince MJ, Giannakopoulos P, Wolters FJ, et al. (2017). Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst), 8: 165-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lévesque LE, Hanley JA, Kezouh A, Suissa S (2010). Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ, 340: b5087. [DOI] [PubMed] [Google Scholar]