Abstract
BACKGROUND AND PURPOSE:
It has been proposed that autism spectrums condition may represent a form of extreme male brain (EMB), a notion supported by psychometric, behavioral, and endocrine evidence. Yet, limited data are presently available evaluating this hypothesis in terms of neuroanatomy. Here, we investigated sex-related anatomic features in adults with AS, a “pure” form of autism not involving major developmental delay.
MATERIALS AND METHODS:
Males and females with AS and healthy controls (n = 28 and 30, respectively) were recruited. Structural MR imaging was performed to measure overall gray and white matter volume and to assess regional effects by means of VBM. DTI was used to investigate the integrity of the main white matter tracts.
RESULTS:
Significant interactions were found between sex and diagnosis in total white matter volume, regional gray matter volume in the right parietal operculum, and fractional anisotropy (FA) in the body of the CC, cingulum, and CR. Post hoc comparisons indicated that the typical sexual dimorphism found in controls, whereby males have larger FA and total white matter volume, was absent or attenuated in participants with AS.
CONCLUSIONS:
Our results point to a fundamental role of the factors that underlie sex-specific brain differentiation in the etiology of autism.
Autism spectrum conditions affect approximately 1% of the population and are characterized by relative deficits in social reciprocity and communication alongside restricted interests and resistance to change.1 Despite increasing recognition, our understanding of their neurobiologic bases remains minimal.
The behavioral manifestations of ASCs can be conceptualized within the framework of the “empathizing-systemizing” theory, which postulates that there are sex differences in the domains of empathy (the ability to identify another person's mental states and respond to these with an appropriate emotion) and “systemizing” (the ability to analyze and construct systems). At a population level, females on average have higher levels of empathy, whereas on average, males are better at systemizing.2 From this perspective, ASCs have been viewed as an extreme form of the typical male profile, having impaired empathy alongside intact or even enhanced ability to systemize.3,4 This hypothesis is termed the EMB theory and is substantiated by a growing body of psychometric, behavioral, and endocrine data.5 Among the biologic mechanisms that may be related to the EMB theory are the effects of fetal testosterone on behavioral and brain development (ie, evidence that, in typically developing children, amniotic testosterone levels positively correlate with autistic traits in later childhood).6 The theory is also relevant to observations of the higher prevalence of ASCs in males compared with females.7
Within the EMB literature, there is also a more subtle point about the lack of sexual dimorphism. For example, the typical sex differences on measures of empathizing, systemizing, and autistic traits are attenuated or absent in patients with ASCs.8,9 The finding of altered sexual dimorphism can have a substantial impact on current hypotheses of the etiology of ASCs, even in absence of overall “masculinization.” At present, it remains unclear whether the behavioral and neuroendocrine differences observed between patients with ASCs and neurotypical controls are reflected in terms of brain structure and function and whether, as the theory would predict, typical sexual dimorphism is affected and a general hypermasculinization is found.
There is a substantial body of literature highlighting significant differences between the typical male and female brains in terms of gross anatomy and distribution of dopamine, serotonine, and gamma-aminobutyric acid receptors.8,10 Absolute white matter volume is larger in males than females; albeit less consistently, a similar effect is also observed for gray matter.11,12 Differences in regional anatomy are more variable across studies and have been reported mainly for the temporal and parietal regions, amygdala, insula, and cerebellum.12–15 DTI studies measuring FA indicate higher white matter density in the CC, cingulum bundle, and thalamocortical projection fibers in males.16,17
On the other hand, subtle neuroanatomic abnormalities have been reported for ASCs across many brain regions, including frontal, parietal, limbic areas, basal ganglia, and the cerebellum.18–25 In some investigations, total brain volume26,27 and amygdala volume27,28 are identified as abnormal. The broad involvement of areas across the whole brain echoes another neurobiologic account, which is the “underconnectivity” theory, which holds that ASCs are related to reduced long-range connectivity between frontal and temporal-parietal association cortices and enhanced short-range local connectivity.29,30 It is proposed that this connectivity profile compromises global integration while favoring an over-reliance on local information processing.29,30 Some functional neuroimaging evidence is consistent with this hypothesis, but a coherent account of the neural underpinnings of ASCs has yet to emerge.31 FA has been found to be lower than that in controls in the CC,32,33 frontal white matter,34 superior temporal gyrus,35 anterior cingulum,36 and cerebellum,37 indicating reduced connectivity. Relatively small sample sizes, clinical variability, and methodologic differences may, in part, account for this heterogeneity of findings.
The investigation of sex-related effects in brain structure is obviously relevant to understanding the pathophysiology of ASCs. The EMB theory postulates a link between sex-related differences and the manifestations of ASCs, but to date, to our knowledge, very limited neuroimaging data are available to evaluate this view. Two recent studies considering young children with ASCs suggest that females have more widespread and pronounced structural abnormalities than males,38,39 but no information is available for adults.
Here, we use a factorial design to explore effects related to sex, diagnostic status, and their interaction on brain structure in adults with AS. AS can be considered a “pure” ASC, in that it does not involve a learning disability or major delay in the development of spoken language. We assessed whole-brain volume, regional anatomy through VBM, and the integrity of white matter tracts by using DTI.
Materials and Methods
Participants and Assessment
We recruited participants with a diagnosis of AS and healthy controls for the study, which was approved by the local ethics committee for patient research. Written informed consent was obtained after a complete description of the study, in line with the principles of the Declaration of Helsinki. Twenty patients were recruited from a local specialist clinic for diagnosis and evaluation of high-functioning adults with suspected neurodevelopmental conditions (Neurobehavioural Clinic, Sussex Partnership National Health Service Foundation Trust, Brighton, UK). This is a tertiary clinic that receives referrals from consultant community psychiatrists who undertake initial screening of general practitioner referrals. The remaining 8 patients, each of whom had validated diagnoses established as children, were recruited through advertisement from a local voluntary sector organization supporting people with AS. A total of 15 male (age 32 ± 10 years; 14 right-handed) and 13 female (age 32 ± 7 years; 12 right-handed) participants with a diagnosis of AS were recruited, alongside 15 male (age 28 ± 8 years; 14 right-handed) and 15 female healthy controls (age 32 ± 8 years; 13 right-handed). As determined by χ2 test, the proportion of left-handed participants was comparable across the 4 groups (P = .3). Participants with any history of neurologic pathology, general learning disability, or head injury were excluded.
The diagnosis of AS (characterized by life-long impairments in social and emotional communication with restricted interests and repetitive stereotyped behavior in the absence of language delay or general intellectual dysfunction) was established in the clinic by using the Diagnostic and Statistical Manual of Mental Disorders, 4th ed, Revised criteria by specialist clinical assessment and informant interviews conducted, through an experienced multidisciplinary team including a neuropsychiatrist, a clinical psychologist, and a speech and language therapist. Confirmatory evidence was obtained by using the Diagnostic Interview for Social and Communication Disorders.40 Concurrent expression of autistic traits was quantified by using AQ.41 All patient scores on the Adult Autism Assessment (combined AQ and Empathy Quotient) fell within the range for a likely diagnosis of AS. The NART was also administered as a proxy of overall intellectual function (all participants were UK-educated).42 All patients were high-functioning, graduating at high school level with 8 of the 28 patients achieving a university degree. Patients were not taking long-term medication at the time of scanning and had no comorbid psychiatric diagnoses, with the exception of 4 who were taking antidepressant medication. All patients were in contact with health and social services for support and advocacy.
Imaging Data Acquisition
Structural MR imaging was performed on a Magnetom Avanto 1.5T system (Siemens, Erlangen, Germany). During scanning, the head was gently restrained by surrounding it with foam cushions. Anatomic images were acquired by using a sagittal 3D T1-weighted magnetization-prepared rapid acquisition of gradient echo sequence with 192 sections (TR = 1160 ms, TE = 4 ms, TI = 600 ms, 0.9-mm isotropic voxels). To exclude gross brain pathology, T2-weighted images were also acquired by using a coronal turbo spin-echo sequence (TR = 6500 ms, TE = 120 ms, FOV = 260 × 183 mm, section thickness = 4.4 mm). Diffusion-weighted images were obtained with a spin-echo echo-planar sequence (TR = 8000 ms, TE = 95 ms, FOV = 240 × 180 mm, 110 × 82 matrix, section thickness = 2.6 mm, duration = 8 minutes) yielding 2.2 × 2.2 × 2.6 mm voxels, b = 1000 s/mm2, 64 directions; bipolar gradient pulses were used, yielding an eddy current−related distortion below 1 voxel.
Analysis of Structural Images
All T1- and T2-weighted images were reviewed by a neuroradiologist to exclude macroscopic pathology and white matter hyperintensities or ventricular/sulcal enlargement beyond the level expected for the age. All participants had normal imaging findings, and therefore none were excluded. The structural analysis was performed as follows: First, the anatomic volumetric scans were automatically segmented with SPM8 (Wellcome Department of Imaging Neuroscience, London, UK), yielding normalized images of gray and white matter. Second, to improve the accuracy of intersubject alignment, we applied a diffeomorphic registration algorithm, using a template created with the data from all participants. This nonlinear warping technique minimizes interindividual structural variance and thereby improves the sensitivity of VBM analysis.43 A Jacobian modulation step was performed using the flow fields (u_rc1*.nii) and rc1*.nii/rc2*.nii data files, preserving voxelwise information about local tissue volume. Third, resulting tissue-volume maps in MNI space (voxel size, 2 mm isotropic) were smoothed with an 8-mm Gaussian kernel and entered into voxelwise factorial analyses (see below). The individual total volumes of gray and white matter were calculated by summing over all voxels in the corresponding tissue maps.
Analysis of Diffusion-Weighted Images
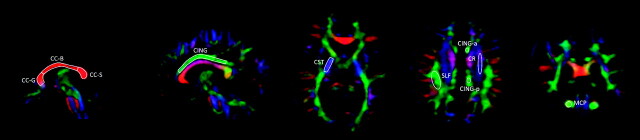
Diffusion-weighted images were fitted with rank-2 diffusion tensor fields, and FA and MD were calculated by using the Diffusion Toolkit software (Massachusetts General Hospital, Boston, Massachusetts). The non-diffusion-weighted images (ie, the b = 0 s/mm2 scans) were normalized to MNI space, by coregistering them to the corresponding individual volumetric T1 scans and iteratively segmenting and normalizing the T1 images. The resulting transformation matrices were applied to the FA and MD maps, which were interpolated to 1-mm isotropic resolution. ROIs for measurement of average regional FA and MD were drawn by experienced operators (L.M. and F.D.B.) blinded to sex and diagnostic status. The ROIs, shown in Fig 1, were traced on the midsagittal plane separately for the CC-genu (area, 96 ± 30 mm2), CC-body (104 ± 29 mm2), and CC-splenium (93 ± 29 mm2). We drew the principal ROIs for the cingulum (131 ± 32 mm2) on the sagittal plane, choosing the section on which the area of the bundle was maximal for each hemisphere and including its portion overlying the CC. Additional ROIs for the cingulum were drawn on the same plane chosen for the SLF and CR (below), separately for the anterior and posterior portions of the cingulum to the center of the body of the CC. The ROIs for the CST (105 ± 21 mm2) were drawn on the axial plane at the level of the posterior limb of the internal capsule. Those for the SLF (147 ± 30 mm2) were drawn on the axial section on which its area was maximal; at the same level, ROIs were also drawn on the ascending fibers of the CR (133 ± 25 mm2). The ROIs for the MCP (53 ± 11 mm2) were positioned on the coronal plane, on the section immediately posterior to the limit of the pons. All coordinates are given in MNI space.
Fig 1.
Example of positioning of ROIs for FA measurement on a representative participant.
Statistical Analysis
Before statistical analysis, for each parameter and group (control males, control females, AS males and AS females), a preliminary evaluation was performed comparing the left-handed participants with the rest of the group by means of a 2-sample t test. Because no significant differences were found, the left-handed participants were pooled together with the rest of the groups.
Group differences in AQ and NART scores were tested for in 2-way ANOVAs with sex and diagnosis as between-subject factors.
The diffusional data (FA and MD measurements) were analyzed by means of ANCOVA, which included sex and diagnosis as between-subject factors as well as the NART score as nuisance covariate, to control for significantly lower scores in AS compared with control participants. For those regions in which an ROI was drawn on each hemisphere (ie, all except the CC), an extra within-subjects factor for hemisphere (left or right) was included.
Similarly, the total gray and white matter volumes were entered as dependent variables in 2-way ANCOVAs, having between-subject factors for sex and diagnosis and NART scores as a nuisance covariate.
The VBM results were analyzed with voxel wise ANCOVAs, including sex and diagnosis as factors and total gray or white matter volume, age, and NART score as nuisance covariates. For this analysis, the voxel-level threshold was set to P < .0001, and the cluster-level threshold, to P < .05, family-wise error−corrected.
Where significant sex × diagnosis interactions were found, 4 post hoc contrasts were run: 1) control males versus control females, 2) AS males versus AS females, 3) control males versus AS males, and 4) control females versus AS females. A Bonferroni correction for multiple comparisons was applied.
Results
AQ and NART Scores
As predicted, AQ score was higher for AS participants than controls [36.8 ± 7.1 versus 13.7 ± 7.7; F(1,54) = 166, P < .001, ηp2 = 0.75] and for males than females [27.4 ± 14.1 versus 22.4 ± 13.2; F(1,54) = 10.8, P = .002, ηp2 = 0.17]. There was no significant sex × diagnosis interaction. Furthermore, AS participants had lower NART scores than controls [30.3 ± 7.7 versus 35.4 ± 6.3; F(1,54) = 7.4, P = .009, ηp2 = 0.12], without any main effect of sex or sex × diagnosis interaction.
Structural Measurements
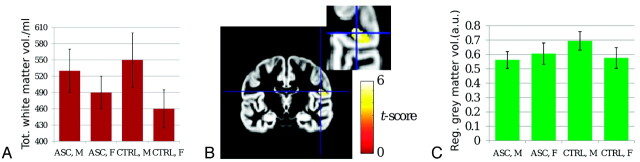
As reported in Table 1 and shown in Fig 2A, total gray matter volume (computed summing over the entire brain on corresponding tissue maps) was larger for males than females, with no main effect of diagnosis or sex × diagnosis interaction. Total white matter volume was also higher in males than females. Again, there was no main effect of diagnosis; however, there was a significant sex × diagnosis interaction. Post hoc comparisons revealed that the difference between males and females, present in both groups, was weaker in AS participants than controls. There was a trend (ie, an effect that did not survive correction for multiple comparisons) toward larger volume in AS females than control females, but no such effect was observed for males.
Table 1:
Statistics for structural measurementsa
| Statistics |
|---|
| Gross brain volume |
| Total gray matter volume |
| Sex: males 780 ± 65 mL vs females 700 ± 40 mL; F(1,53) = 33.4, P < .001, ηp2 = 0.40 |
| Diagnosis: n.s; sex × diagnosis: n.s. |
| Total white matter volume |
| Sex: males 540 ± 45 mL vs females 470 ± 35 mL; F(1,53) = 43.3, P < .001, ηp2 = 0.45 |
| Diagnosis: n.s.; sex × diagnosis: F(1,53) = 7.1, P = .01, ηp2 = 0.12 |
| 1) Control males 550 ± 50 mL vs control females 460 ± 35 mL; F(1,27) = 37.8, P < .001b |
| 2) AS males 530 ± 40 mL vs AS females 490 ± 30 mL; F(1,25) = 9.1, P = .006b |
| 3) Control males 550 ± 50 mL vs AS males 530 ± 40 mL; n.s. |
| 4) Control females 460 ± 35 mL vs AS females 490 ± 30 mL; F(1,25) = 6.8, P = .02 |
| Right inferior parietal lobe and rolandic operculum |
| Regional gray matter volume |
| Sex: males 0.63 ± 0.09 vs females 0.59 ± 0.07; F(1,53) = 4.6, P = .04, ηp2 = 0.08 |
| Diagnosis: controls 0.63 ± 0.09 vs AS 0.58 ± 0.07; F(1,53) = 4.8, P = .03, ηp2 = 0.08 |
| Sex × diagnosis: F(1,53) = 23.3, P < .001, ηp2 = 0.31 |
| 1) Control males 0.69 ± 0.06 vs control females 0.57 ± 0.07; F(1,27) = 24.2, P < .001b |
| 2) AS males 0.56 ± 0.06 vs AS females 0.61 ± 0.07; n.s. |
| 3) Control males 0.69 ± 0.06 vs AS males 0.56 ± 0.06; F(1,27) = 27.1, P < .001b |
| 4) Control females 0.57 ± 0.07 vs AS females 0.61 ± 0.07; n.s. |
Note:—n.s. indicates not significant.
All tests include the NART as a covariate. According to the Bonferroni correction, we set α = .013 for post hoc comparisons. All values are given as means ± standard deviation. The values reported after “Sex” correspond to males and females, irrespective of diagnosis; the values reported after “Diagnosis” correspond to control and AS participants, irrespective of sex. Separate values for each group are given only in the presence of a significant interaction.
Statistically significant post hoc comparison at the corrected threshold.
Fig 2.
A, Bar graph of total white matter volume. B, Gray matter VBM statistical map demonstrating a sex × diagnosis interaction in the right inferior parietal lobe and rolandic operculum (peak coordinates [49, −14, 19]). C, Bar graph of gray matter volume for the cluster shown in B demonstrates attenuated male-female difference in AS patients compared with controls. Please see Table for statistical results.
With VBM, larger regional gray matter volume for males was found in the left inferior parietal lobe and rolandic operculum (ke = 614 voxels, Pcorr = 0.008) and in the superior occipital lobe (ke = 389, Pcorr = 0.04); the converse was found in the cerebellar vermis (ke = 814, Pcorr = 0.002) and left cerebellar hemisphere (ke = 562, Pcorr = 0.011). As shown in Fig 2B, -C, a significant sex × diagnosis interaction emerged in the right inferior parietal lobe and rolandic operculum (peak t-score, 5.9; peak coordinates, [49, −14, 19]; ke = 495 corresponding to a cluster extent of 4 mL; Pcorr = 0.02). Post hoc comparisons for this cluster, reported in Table 1, indicated that regional gray matter volume was larger for males than females in controls but not in AS participants and that it was reduced in AS males with respect to control males. No significant effects were detected for white matter in any region.
Diffusional Measurements
For the thalamus, there was a main effect of diagnosis (F(1,54) = 6.5, P = .01, ηp2 = 0.11), with reduced MD in AS participants (0.72 ± 0.01 versus 0.75 ± 0.01 mm2/s); there was no main effect of sex or sex × diagnosis interaction. No other effects were detected for MD in any region.
The statistics for FA measurements are given in the On-line Table. For the body of the CC, there was no main effect of diagnosis or sex, but a significant sex × diagnosis interaction was found. Post hoc comparisons indicated that FA was higher for males than females in controls but not in AS participants; furthermore, there was a trend toward lower FA in AS males with respect to control males. No effects were observed for the genu and splenium of the CC.
For the right and left cingulum, there was no main effect of diagnosis; however, FA was higher in males than females and a significant sex × diagnosis interaction was found. As above, post hoc comparisons indicated that FA was larger for males than females in controls but not in AS participants, and there was a trend toward lower FA in AS males with respect to control males. The additional analyses conducted separately for the left and right anterior and posterior portions of the cingulum revealed a significant sex × diagnosis interaction in the anterior portion, and no effects in the posterior portion.
Similarly, for the left and right CR, there was no main effect of diagnosis; the FA was higher in males than in females and a significant sex × diagnosis interaction was found. Here, there was a side × sex × diagnosis interaction, but no lateralization effect was observed in the post hoc comparisons. As above, post hoc comparisons indicated that the FA was larger for males than females in controls but not in AS participants, and there was a trend toward lower FA in AS males with respect to control males.
For the right and left SLF, there was no main effect of diagnosis or sex. A borderline sex × diagnosis interaction was found, but none of the post hoc comparisons reached statistical significance.
No effects were found in the right and left CST and right and left MCP.
Discussion
Our study provides the first evidence of atypical sexual dimorphism in the brain structure of adults with AS. We found significant interactions between sex and diagnosis on measures of gross white matter volume, regional gray matter volume, and water diffusion anisotropy in white matter. These interactions indicate that the sex-related differences in neurotypical controls are attenuated or absent in AS participants. However, we did not find that individuals with AS have a more masculinized brain, as postulated in the core hypothesis of the EMB theory.5
Our results concerning differences between control males and females in white matter volume and FA are concordant with existing literature.8,10,15–17 No sex difference was found for total gray matter volume, which previous studies have inconsistently reported to be larger in males.11,12 We found a significant interaction in the anatomy of the right inferior parietal lobe, which had increased gray matter volume in males versus females for controls but not patients with AS. Sex-related differences in this region had been previously found, but with inconsistencies between studies using VBM and manual segmentation.14,16,44
Our findings regarding patients with AS substantiate the view that atypical expression of sex differences is linked to the etiology of AS. Because the key findings were interactions between sex and diagnosis, this indicates that investigating atypical sexual dimorphism in AS is essential to gain insight into the etiology of the condition. It is possible that to a certain extent, differential neurobiologic developmental mechanisms exist between the sexes in adults with AS.45
Two biologic theories propose specific mechanisms that might relate to our finding of atypical sexual dimorphism: first, the “fetal testosterone” theory, which postulates that ASCs are related to exposure to elevated levels of fetal testosterone. This hypothesis is supported by evidence that prenatal androgens are critically involved in sex differentiation of the human brain.6 Treating pregnant females with high levels of testosterone increases male-typical play behavior in female offspring, while prohibiting testosterone exposure has the opposite effect.46 Furthermore, follow-up studies of typically developing children show that testosterone levels in amniotic fluid relate positively to the presence of autistic traits and restricted interests6 and negatively with the frequency of eye contact, quality of social relationships, and empathy scores.47 A second theory focuses on the protective effects of the X chromosome.48 According to this view, the expression of maternally inherited X-loci may result in social-communication deficits specifically in males but not in females (because females also inherit a paternal X chromosome). While the paternal X may be “silenced” through X-linked inactivation, 10%–15% of paternally inherited X-loci are still expressed and, according to this theory, may act as a protective mechanism against the detrimental maternal imprinted X-loci. The genetic basis of ASCs is very complex and multifactorial, and the relative contribution of fetal testosterone and X-linked genes remains to be established.
The finding of sex-specific effects is not without precedence in autism research. For example, it has been recently shown that males and females with ASCs are characterized by sex-specific biomarker patterns in the expression of blood proteins.45 It is at present difficult to relate our finding of attenuated sex-related differences to the fetal testosterone and X chromosome theories. Most important, both theories could, in principle, explain attenuated sex differences even in the absence of significant overall masculinization; however, neither could account for an inversion of the typical pattern in patients. Because the present study investigated the brain anatomy in adults with AS, who have all undergone a long period of developmental changes from childhood, these theories are going to be hard to test. Given the complexity of brain plasticity and behavioral development, it is unsurprising that behavioral and endocrine findings may not map to anatomic features with a straightforward relationship.
Our DTI results demonstrate AS-related changes in microstructural organization, consistent with reduced axonal density or myelination,49 in the CC, cingulum, and CR, as inconsistently reported in previous literature,32–37 selectively in males but not in females. Studies of patients with callosal agenesis have demonstrated that reduced interhemispheric connectivity, in addition to impacting intelligence quotient intellect and information integration, may lead to specific emotional and social deficits resembling those typical of ASCs.50 The central role of the cingulate cortex in emotion and behavioral regulation is well-established.51 Most interesting, our results suggest that AS affects primarily its anterior portion, which is intimately linked to the prefrontal cortex and insula and is functionally related to emotion processing and response selection, with sparing of the posterior portion, which is more closely linked to the precuneus and parietal gyri and is mainly implicated in personal and visuospatial orientation.52 Involvement of the CR, generally associated with motor and sensory function, could be explained in light of recent evidence suggesting that corticothalamic connectivity may play a much broader role than previously thought in the integration of activity among distant cortical areas.53,54 The superior longitudinal fasciculus comprises 4 functionally distinct subpopulations, related to visuospatial attention, motor coordination, and language articulation.55 Because it was not possible to isolate these 4 components by tractography, it is difficult to draw definite conclusions; nevertheless, the observed borderline interaction could reflect differences in the development of language or attentional functions.
On the other hand, preserved connectivity in the superior longitudinal fasciculus may be expected, given that the AS participants were high-functioning in terms of intellect and language. These findings are only partially in line with the underconnectivity theory. While they confirm that long-range connectivity is altered, the observed differences were not in the form of main effects of the condition, as the theory would predict, but in the form of interactions with sex. Even though the post hoc comparisons between AS participants and controls for males and females did not reach the corrected statistical threshold, the FA appeared to be generally reduced in AS males with respect to control males, without a corresponding effect in females. If confirmed, this would signal that long-range connectivity is more severely affected in males.
The study has limitations. First, as with most neuroimaging investigations of ASCs, we used a cross-sectional design and are, therefore, unable to make direct inferences on brain development. Second, we restricted the study to participants with high-functioning ASCs, and our results may, therefore, not be immediately generalizable to more severe forms of autism. There was, nevertheless, a significant difference in NART scores between AS participants and controls, which was accounted for by entering this variable as a covariate in all tests. Third, our study, which was based on a larger cohort in comparison with previous literature, is still constrained by a relatively small sample size in each subgroup and is thus susceptible to type II error. Our inferences await substantiation by larger multicenter investigations focusing on predetermined sexual dimorphic regions. As important, these studies of the EMB theory at the neuroanatomic level require extension into the examination of toddlers and children with ASCs, who have undergone fewer developmental compensatory changes in the brain. Finally, the ROI set was drawn only once, and the inter-rater reproducibility was, therefore, not assessed; nevertheless, rater bias was intrinsically avoided by blinding, and all ROIs were cross-checked between the 2 operators.
Conclusions
Our results provide the first empiric test of the predictions of the EMB theory at the level of brain structure. No evidence of hypermasculinization was found, indicating the previously described behavioral and neuroendocrine effects do not map onto anatomic features in a straightforward way. However, we found multiple interactions between sex-specific brain differences and AS. This provides a novel perspective into the etiology of ASCs and may partly explain the inconsistency of existing literature. Further research is warranted to extend these observations to other ASCs subtypes and to explore in more detail the relationship between behavioral, endocrine, and anatomic findings.
Supplementary Material
Acknowledgments
The authors are grateful to M. Graham for operational support during collection of control data, to the participants for their generous involvement in the study, and to 2 anonymous reviewers for useful suggestions on an earlier draft.
ABBREVIATIONS
- ANCOVA
analysis of covariance
- AQ
autism quotient
- AS
Asperger syndrome
- ASC
autism spectrum conditions
- CC
corpus callosum
- CR
corona radiata
- CST
corticospinal tract
- EMB
extreme male brain
- FA
fractional anisotropy
- ke
cluster extent in isotropic 2 × 2 × 2 mm MNI space voxels
- MCP
middle cerebellar peduncle
- MD
mean diffusivity
- MNI
Montreal Neurological Institute
- NART
National Adult Reading Test
- ηp2
effect size expressed as partial-eta squared
- SLF
superior longitudinal fasciculus
- VBM
voxel-based morphometry
Footnotes
Disclosures: Ludovico Minati—UNRELATED: Grants/Grants Pending: Italian Ministry of Health; Patents: 1) Caldiroli D, Minati L. Method and Device to Detect the Breathing Pattern at a Distance, even in Presence of Radiation or Intense Electromagnetic Fields. Application no. MI2005A000106 of January 26, 2005; patent no. 0001364602 of July 31, 2009; 2) Minati L, Vitali P. Method and Device to Detect at a Distance Motor Acts Performed by a Person Undergoing Magnetic Resonance Imaging and Not Determined By Physiological Function. Application no. MI2005A001223 of June 29, 2005, patent no. 0001365705 of September 11, 2009. Hugo D. Critchley—UNRELATED: Board Membership: Wellcome Trust, Comments: I sit on the Wellcome Trust Clinical Interview Committee; Payment for Lectures (including service on Speakers Bureaus): I have spoken in industry-sponsored events on several occasions in the last 3 years on human behavioral neuroscience, clinical conditions (eg, attention deficit/hyperactivity disorder), and neuroimaging; the contents of my presentations were of my own choosing and were not determined by industry sponsors; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Autonomic/Neuroscience Conference, Comments: I have been a keynote speaker at a number of neuroscience conferences in the past 3 years; the funds to support my attendance and presentations at these conferences were through the conference organizations such as the Society of Psychophysiological Research, the International Organization for Psychophysiology, and the International Society for Autonomic Neuroscience.
Hugo D. Critchley, Neil A. Harrison, and Ludovico Minati were supported by a Wellcome Trust Programme grant to Hugo D. Critchley (no. 074333) during the period of this work. All data were acquired at the Clinical Imaging Sciences Centre, Brighton and Sussex Medical School. Simon Baron-Cohen was supported by University of Cambridge and by MRC UK. Michael V. Lombardo was supported by the Shirley Foundation, by a Research Fellowship at Jesus College (Cambridge, UK), and by the Wellcome Trust. Meng-Chuan Lai was supported by the Ministry of Education, Taiwan. Ludovico Minati was employed by the Fondazione IRCCS Istituto Neurologico Carlo Besta during the final period of the study.
References
- 1. Baron-Cohen S, Scott FJ, Allison C, et al. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry 2009;194:500–09 [DOI] [PubMed] [Google Scholar]
- 2. Baron-Cohen S. Autism: the empathizing-systemizing (E-S) theory. Ann N Y Acad Sci 2009;1156:68–80 [DOI] [PubMed] [Google Scholar]
- 3. Baron-Cohen S, Richler J, Bisarya D, et al. The systemizing quotient: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philos Trans R Soc Lond B Biol Sci 2003;358:361–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord 2004;34:163–75 [DOI] [PubMed] [Google Scholar]
- 5. Baron-Cohen S. Testing the extreme male brain (EMB) theory of autism: let the data speak for themselves. Cogn Neuropsychiatry 2005;10:77–81 [DOI] [PubMed] [Google Scholar]
- 6. Auyeung B, Baron-Cohen S, Ashwin E, et al. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychol Sci 2009;20:144–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baird G, Simonoff E, Pickles A, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet 2006;368:210–15 [DOI] [PubMed] [Google Scholar]
- 8. Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science 2005;310:819–23 [DOI] [PubMed] [Google Scholar]
- 9. Wheelwright S, Baron-Cohen S, Goldenfeld N, et al. Predicting Autism Spectrum Quotient (AQ) from the Systemizing Quotient-Revised (SQ-R) and Empathy Quotient (EQ). Brain Res 2006;1079:47–56 [DOI] [PubMed] [Google Scholar]
- 10. Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 2007;62:847–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allen JS, Damasio H, Grabowski TJ, et al. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage 2003;18:880–94 [DOI] [PubMed] [Google Scholar]
- 12. Luders E, Narr KL, Thompson PM, et al. Mapping cortical gray matter in the young adult brain: effects of gender. Neuroimage 2005;26:493–501 [DOI] [PubMed] [Google Scholar]
- 13. Good CD, Johnsrude I, Ashburner J, et al. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 2001;14:685–700 [DOI] [PubMed] [Google Scholar]
- 14. Chen X, Sachdev PS, Wen W, et al. Sex differences in regional gray matter in healthy individuals aged 44–48 years: a voxel-based morphometric study. Neuroimage 2007;36:691–9 [DOI] [PubMed] [Google Scholar]
- 15. Huster RJ, Westerhausen R, Kreuder F, et al. Hemispheric and gender related differences in the midcingulum bundle: a DTI study. Hum Brain Mapp 2009;30:383–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Menzler K, Belke M, Wehrmann E, et al. Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage 2011;54:2557–62 [DOI] [PubMed] [Google Scholar]
- 17. Westerhausen R, Kompus K, Dramsdahl M, et al. A critical re-examination of sexual dimorphism in the corpus callosum microstructure. Neuroimage 2011;56:874–80 [DOI] [PubMed] [Google Scholar]
- 18. Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci 2008;31:137–45 [DOI] [PubMed] [Google Scholar]
- 19. Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol 2005;15:225–30 [DOI] [PubMed] [Google Scholar]
- 20. Courchesne E, Pierce K, Schumann CM, et al. Mapping early brain development in autism. Neuron 2007;56:399–413 [DOI] [PubMed] [Google Scholar]
- 21. Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol 2007;17:103–11 [DOI] [PubMed] [Google Scholar]
- 22. McAlonan GM, Cheung V, Cheung C, et al. Mapping the brain in autism: a voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain 2005;128:268–76 [DOI] [PubMed] [Google Scholar]
- 23. Rojas DC, Peterson E, Winterrowd E, et al. Regional grey matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry 2006;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waiter GD, Williams JH, Murray AD, et al. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage 2004;22:619–25 [DOI] [PubMed] [Google Scholar]
- 25. Ecker C, Rocha-Rego V, Johnston P, et al. Investigating the predictive value of whole-brain structural MR scans in autism: a pattern classification approach. Neuroimage 2010;49:44–56 [DOI] [PubMed] [Google Scholar]
- 26. Sparks BF, Friedman SD, Shaw DW, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology 2002;59:184–92 [DOI] [PubMed] [Google Scholar]
- 27. Kates WR, Burnette CP, Eliez S, et al. Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. Am J Psychiatry 2004;161:539–46 [DOI] [PubMed] [Google Scholar]
- 28. Pierce K, Muller RA, Ambrose J, et al. Face processing occurs outside the fusiform ‘face area' in autism: evidence from functional MRI. Brain 2001;124:2059–73 [DOI] [PubMed] [Google Scholar]
- 29. Baron-Cohen S, Belmonte MK. Autism: a window onto the development of the social and the analytic brain. Annu Rev Neurosci 2005;28:109–26 [DOI] [PubMed] [Google Scholar]
- 30. Just MA, Cherkassky VL, Keller TA, et al. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain 2004;127:1811–21 [DOI] [PubMed] [Google Scholar]
- 31. Minshew NJ, Keller TA. The nature of brain dysfunction in autism: functional brain imaging studies. Curr Opin Neurol 2010;23:124–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the corpus callosum in autism. Neuroimage 2007;34:61–73 [DOI] [PubMed] [Google Scholar]
- 33. Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport 2007;18:23–27 [DOI] [PubMed] [Google Scholar]
- 34. Sundaram SK, Kumar A, Makki MI, et al. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex 2008;18:2659–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee JE, Bigler ED, Alexander AL, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett 2007;424:127–32 [DOI] [PubMed] [Google Scholar]
- 36. Thakkar KN, Polli FE, Joseph RM, et al. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD). Brain 2008;131:(pt 9):2464–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Catani M, Jones DK, Daly E, et al. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage 2008;41:1184–91 [DOI] [PubMed] [Google Scholar]
- 38. Bloss CS, Courchesne E. MRI neuroanatomy in young girls with autism: a preliminary study. J Am Acad Child Adolesc Psychiatry 2007;46:515–23 [DOI] [PubMed] [Google Scholar]
- 39. Schumann CM, Bloss CS, Barnes CC, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci 2010;30:4419–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wing L, Leekam SR, Libby SJ, et al. The diagnostic interview for social and communication disorders: background, inter-rater reliability and clinical use. J Child Psychol Psychiatry 2002;43:307–25 [DOI] [PubMed] [Google Scholar]
- 41. Baron-Cohen S, Wheelwright S, Skinner R, et al. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord 2001;31:5–17 [DOI] [PubMed] [Google Scholar]
- 42. Nelson HE. National Adult Reading Test: Test Manual. Windsor, Berkshire, UK: NFER-Nelson; 1982 [Google Scholar]
- 43. Ashburner J, Friston KJ. Computing average shaped tissue probability templates. Neuroimage 2009;45:333–41 [DOI] [PubMed] [Google Scholar]
- 44. Frederikse ME, Lu A, Aylward E, et al. Sex differences in the inferior parietal lobule. Cereb Cortex 1999;9:896–901 [DOI] [PubMed] [Google Scholar]
- 45. Schwarz E, Guest PC, Rahmoune H, et al. Sex-specific serum biomarker patterns in adults with Asperger's syndrome. Mol Psychiatry 2011;16:1213–20 [DOI] [PubMed] [Google Scholar]
- 46. Hines M, Golombok S, Rust J, et al. Testosterone during pregnancy and sex role behavior of preschool children: a longitudinal, population study. Child Dev 2002;73:1678–87 [DOI] [PubMed] [Google Scholar]
- 47. Whitehouse AJ, Maybery MT, Hart R, et al. Fetal androgen exposure and pragmatic language ability of girls in middle childhood: implications for the extreme male-brain theory of autism. Psychoneuroendocrinology 2010;35:1259–64 [DOI] [PubMed] [Google Scholar]
- 48. Skuse DH. X-linked genes and mental functioning. Hum Mol Genet 2005;14:27–32 [DOI] [PubMed] [Google Scholar]
- 49. Minati L, Weglarz WP. Physical foundations, models and methods of diffusion magnetic resonance imaging of the brain: a review. Conc Magn Reson A 2007;30:278–307 [Google Scholar]
- 50. Paul LK, Brown WS, Adolphs R, et al. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci 2007;8:287–99 [DOI] [PubMed] [Google Scholar]
- 51. Phillips ML, Drevets WC, Rauch SL, et al. Neurobiology of emotion perception. I. The neural basis of normal emotion perception. Biol Psychiatry 2003;54:504–14 [DOI] [PubMed] [Google Scholar]
- 52. Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005;6:533–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull 2009;78:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Briggs F, Usrey WM. Emerging views of corticothalamic function. Curr Opin Neurobiol 2008;18:403–07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 2005;15:854–69. Epub 2004 Dec 8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.