Abstract
The emission of isoprenoids (e.g. isoprene and monoterpenes) by plants plays an important defensive role against biotic and abiotic stresses. Little is known, however, about the functional traits linked to species-specific variability in the types and rates of isoprenoids emitted and about possible co-evolution of functional traits with isoprenoid emission type (isoprene emitter, monoterpene emitter or both).
We combined data for isoprene and monoterpene emission rates per dry mass with key functional traits (i.e., foliar nitrogen and phosphorus concentrations, leaf mass per area) and climate for 113 plant species, covering the boreal, wet temperate, Mediterranean and tropical biomes.
Foliar nitrogen was positively correlated with isoprene emission, and foliar phosphorus was negatively correlated with both isoprene and monoterpene emission rate. Non-emitting plants generally had the highest nutrient concentrations, and those storing monoterpenes had the lowest concentrations. Our phylogenetic analyses found that the type of isoprenoid emission followed an adaptive, rather than a random model of evolution.
Evolution of isoprenoids may be linked to nutrient availability and foliar nitrogen and phosphorus are good predictors of the type of isoprenoid emission and the rate at which monoterpenes, and to a lesser extent isoprene, are emitted.
Keywords: Volatile organic compounds, phylogeny, monoterpenes, nutrient availability, nitrogen, phosphorus
Introduction
Terrestrial ecosystems are responsible for the emission to the atmosphere of large amounts of biogenic volatile organic compounds (BVOCs). BVOCs play an important role in atmospheric chemistry (Carslaw et al., 2009) and even climate (Peñuelas & Llusià, 2003; Arneth et al., 2010). Isoprenoids, including isoprene and monoterpenes, are amongst the most important BVOCs emitted by plants, even though not all plant species emit them (Fineschi et al., 2013; Loreto & Fineschi, 2015). In those plants that do emit, isoprene and monoterpenes are both produced during photosynthetic metabolism and can represent up to 2–5% of total photosynthesis in healthy leaves, and a much higher share in stressed leaves (Loreto & Schnitzler, 2010), thus potentially contributing to the carbon (C) balance of ecosystems. They also have various functions in biotic and abiotic stresses such as defence from herbivores or thermal and oxidative stress (Singsaas et al., 1997; Llusia & Penuelas, 2000; Loreto et al., 2001; Peñuelas & Llusià, 2003; Penuelas & Llusia, 2004; Vickers et al., 2009; Niinemets, 2010).
Isoprenoids are important compounds for both plants and ecosystems, but why emissions differ amongst species is not yet clear. Previous studies have reported a trade-off between the constitutive emissions of isoprene and monoterpenes (i.e. plants emitting isoprene are less likely to emit monoterpenes, or emit less) (Harrison et al., 2013). This trade-off should be associated with plant life histories and functional traits. For example, isoprene emission is more common in woody than non-woody species (Vickers et al., 2009). Isoprene emission has also been suggested to be more common in species from mesic than from xeric habitats, and once emitted, mesic species emit at higher rates than corresponding emitting xeric species. Conversely, monoterpene emission has the opposite behaviour (Loreto et al., 2014a). However, a recent survey found that perennial plants of different biomes share a similar fraction (around 20%) of isoprene emitters, with a significantly higher emission only in deciduous plants with respect to evergreens, both in temperate and tropical environments (Loreto & Fineschi, 2015).
Nutrient availability plays a key role in plant ecophysiology and ecosystem functioning: photosynthetic rates are linked to foliar nitrogen (N) concentrations (Wright et al., 2004) and sometimes also to foliar phosphorus (P) (Domingues et al., 2010). Forest fruit production is linked to foliar P and zinc (Zn) concentrations (Fernández-Martínez et al., 2016b), whereas foliar potassium (K) is linked to drought resistance (Sardans & Peñuelas, 2015). Nutrient availability is generally linked to forest C sequestration (Fernández-Martínez et al., 2014) and to changes in the allocation of C into different plant compartments (Litton et al., 2007; Fernández-Martínez et al., 2016a). However, it remains poorly known whether or not BVOC emission types and rates in various species from different biomes correlate with foliar nutrient content and the stoichiometry among key nutrients. A previous study found no significant correlation between isoprenoid emissions and foliar N or P concentrations, leaf mass per area (LMA), or photosynthetic capacity for 70 plant species from Hawaii (Llusià et al., 2010). Many studies of single species, however, generally reported higher foliar N concentrations to be linked with higher rates of isoprene emission (Harley et al., 1994; Monson et al., 1994; Lerdau et al., 1995; Litvak et al., 1996; Staudt et al., 2001; Possell et al., 2004). This positive relationship between isoprene emission and foliar N is consistent with the observation that higher foliar N is associated to higher rates of photosynthesis (Wright et al., 2004), which, in turn, correlates with isoprene emission (Monson et al., 1994; Litvak et al., 1996). Surprisingly, phosphorus seems to present a negative relationship with isoprene emission, clearly uncoupling isoprene emission from photosynthesis in Phragmites australis (Fares et al., 2008). No direct relationship was found between phosphorus and foliar volatile monoterpenes and sesquiterpenes in Pinus pinaster (Sampedro et al., 2010). The role of nutrient availability in isoprenoid emission thus remains unclear.
Plant functional traits may account for some of the species-specific variability of isoprenoid emission, but phylogenetic relationships may also play an important role. Previous studies have reported a strong phylogenetic signal for isoprene but not for monoterpene emissions (Llusià et al., 2010; Loreto et al., 2014a). This strong and consistent phylogenetic signal in isoprene emission supports the hypothesis that isoprene emission may have evolved in the first terrestrial plants as a mechanism to cope with environmental stress and water deficit (Vickers et al., 2009; Loreto et al., 2014a). Although storage structures for terpenes are present in several phylogenetically old plant groups including gymnosperms, non-storage stress-dependent emissions might have evolved much more recently. Some studies have analysed the emission of isoprenoids in various taxa and discussed the emission type of their ancestors (Loreto et al., 1998; Loreto, 2002). Recently, a broad reconstruction of the ancestral emission type of a large array of phylogenetically distant species has been attempted (Li et al., 2017). However, the mode of evolution of isoprenoids (e.g. Brownian motion [random evolution] vs. adaptive mode linked to functional traits [evolution has pushed species towards optimal values for adaptation] see Lapiedra et al., (2013), Watson et al., (2014) and Sayol et al., (2016) for examples), has not yet been discerned.
Here we study the relationships of isoprenoid emissions with plant functional traits and climate, and analyse their model of evolution by performing comparative phylogenetic analyses. To do so, we gathered data from published literature on isoprenoid emission for 113 plant species and classified them as: i) non-emitters (NE); ii) only isoprene emitters (ISP); iii) only monoterpene emitters (MTP); iv) emitters of both isoprene and monoterpenes (TWO); v) MTP that also stored monoterpenes (MTPs); and vi) TWO that also stored monoterpenes (TWOs). Given the role that leaf functional traits have in plant ecophysiology (e.g. photosynthesis, C allocation), we hypothesised that different isoprenoid emission types were associated with differences in functional traits, especially nitrogen and phosphorus concentrations, and were strongly evolutionarily linked.
Materials and methods
Data set
Combining our previously published data with an extensive literature search, we compiled a data set (Table S1) of leaf isoprene and monoterpene emissions containing records for plants from four biomes (boreal, wet temperate, Mediterranean, and tropical). In addition to the emission rates, we have included the measurement conditions for emission rate estimation (e.g. PPFD [photosynthetic photon flux density], temperature, plant and leaf age, canopy position, growing conditions, measurement technique) and used these data to convert emission rates conducted at non-standard conditions into standardised values (µg g-1 h-1) at 30 °C and 1000 µmol m-2 s-1 PPFD following the Guenther et al., (1993) equations. Emission of monoterpenes included both de-novo emissions and emissions of monoterpenes from storage structures. The list of the 113 species included in the database and the references from where we extracted the information are shown in Table S2. For every species for which we had values of isoprene and/or monoterpene emission, we also compiled information about the species, such as geographical coordinates of sampling, and species traits, such as leaf habit, whether the species was woody or herbaceous, LMA, foliar N and P concentrations, and monoterpene storage. When data for LMA and/or foliar N and P concentrations were missing for a given species in the reviewed literature we used data derived from the TRY trait database (http://www.try-db.org) (Kattge et al., 2011). Foliar concentrations of N and P for those species present both in the database obtained from compilation of available data and in the TRY database were strongly correlated (Pearson’s R = 0.93 for N and R = 0.91 for P, P < 0.001 for both). Climatic data for each location (MAT and MAP) were extracted from the WorldClim database (Hijmans et al., 2005). That database contains long-term climate averages (1950-2000), calculated on a 30 arc-second grid.
We used the plant phylogeny provided by Qian & Jin, (2016) for the phylogenetic analyses. The names of the species in our database were matched with those in the phylogenetic tree using The Plant List database in the R package Taxonstand (Cayuela & Oksanen, 2016).
Data analyses
Relationships between plant functional traits and climate with emission type
We first categorised each species based on their emission type as: i) non-emitters (NE), considered only when isoprene and monoterpene emissions equalled zero; ii) only isoprene emitters (ISP); iii) only monoterpene emitters (MTP); iv) emitters of both isoprene and monoterpenes (TWO); v) MTP that also stored monoterpenes (MTPs); and vi) TWO that also stored monoterpenes (TWOs). MTP and TWO species produce only de-novo monoterpene emissions while MTPs and TWOs species produce both, de-novo emissions and emissions from monoterpene storing structures. We then determined whether foliar functional traits and the climate to which the plants were exposed were correlated with emission type. We performed a phylogenetic principle components analysis (PCA) following Revell (2009), using leaf habit (evergreens vs. deciduous, as a dummy variable), foliar concentrations of N and P, foliar N:P ratio, LMA, and climate (MAT and MAP). Phylogenetic PCA differs from standard PCA in that it incorporates phylogenetic information of the species, and allows extracting orthogonal axes, which are free from potential phylogenetic autocorrelation. We also included as a binomial trait whether or not the species was woody. We then performed one-way ANOVAs to determine whether the emission types affected the values of the axes extracted by the phylogenetic PCA analysis. Tukey HSD tests were performed for multiple comparisons.
Using functional traits and climate we further tried to differentiate ISP from MTP species, and MTPs from MTP species. We used binomial models including phylogenetic information, run via the function phyloglm in the R phylolm package (Tung Ho & Ané, 2014). Response variables were coded as 0 or 1; e.g., in the model for separating ISP from MTP emitters, we coded MTP emitter plants with 0 and ISP emitter plants with 1. In the model separating MTPs from MTP, we coded with 0 plants that do not store monoterpenes and with 1 those that store them. In both cases, the predictor variables were leaf habit, LMA, foliar N and P concentrations, foliar N:P ratio, plant woodiness, MAT, and MAP, in addition to all the numerical variables also included as ln-transformed to account for potential non-linearities. The final model was obtained using stepwise backwards model selection, beginning with the full model (the model containing all possible predictors). Models were further fitted using a standard general linear model to determine if including phylogenetic information modified our results. The results are presented as partial-residuals plots from the visreg (Breheny & Burchett, 2015) R package.
Relationship between plant functional traits and climate with emission rates
We explored whether foliar traits and climate could explain the amount of isoprene and monoterpene emissions while also incorporating phylogenetic information in the analysis. We used the phylolm function in the R phylolm package (Tung Ho & Ané, 2014). We fitted the models using isoprene and monoterpenes as response variables and, as predictors, LMA, leaf nutrients (foliar N and P concentrations and N:P ratio), MAT, MAP, the natural-logarithmic transformations of all previous covariates to account for non-linear relationships, leaf habit (evergreens vs. deciduous), and whether the species was woody. Phylogenetic models were fitted optimising lambda (i.e., the strength of phylogenetic signal). The final model was obtained using stepwise backwards model selection, beginning with the full model. Isoprene and monoterpene emissions were transformed to natural logarithms to normalise the residuals.
Ancestral reconstruction of emission type and their mode of evolution
We used stochastic character mapping (Nielsen, 2002; Huelsenbeck et al., 2003) to reconstruct ancestral transitions amongst the emission types across the phylogeny. This technique reconstructs the state of the ancestors of a phylogeny based on its structure and the observed traits of the current species. The ancestral reconstruction was achieved using the make.simmap function in the phytools R package (Revell, 2012), simulating 1000 stochastic ancestral reconstructions using the “mcmc” method and specifying equal rates of transition amongst the character states. This analysis also allowed us to distinguish between convergent and divergent evolution of type of isoprenoid emission.
Finally, we tested if the inferred evolutionary trajectories in foliar N and P concentrations, LMA, or their adaptation to climate were associated with BVOC emission type and whether an adaptive (Ornstein–Uhlenbeck: OU) or random (Brownian motion—BM) model of evolution (O’Meara et al., 2006; Thomas et al., 2006; Beaulieu et al., 2012) best fits the data. We fitted generalised OU-based Hansen models of continuous characters (e.g. foliar N concentration) evolving under discrete selective regimes (i.e. emission type) using the OUwie R package (Beaulieu & O’Meara, 2016). We fitted these models using 1000 randomly generated ancestral reconstructions for six types of underlying evolutionary processes: i) a single-state BM model (BM1), ii) a BM model with different evolutionary rates for each state (emission type) on a tree (BMS), iii) an OU model with a single optimal value of the continuous trait for all species (OU1), iv) an OU model with different optimal values but a single alpha (the strength of the pull towards the optimal values of the trait) and rate of phenotypic variation around the optimal value for all emission types (OUM), v) an OU model that assumed different optimal values with multiple rates of phenotypic variation per emission type (OUMV), and vi) an OU model that assumed different optimal values with multiple alphas (OUMA). We deleted all models containing negative eigenvalues when summarising our results. For OUMA models, 99% of the stochastic character maps provided models with negative eigenvalues and were therefore completely excluded from our results (non-sound models). We only present the results of the best types of models based on the average second-order Akaike information criterion (AICc) amongst all sound models. Emission types were considered significantly different when the 2.5 and 97.5% confidence intervals of two categories did not overlap. All analyses used the 113 species for which we had data for BVOC emissions, foliar nutrient concentrations, LMA, and climate.
Results
Correlations of plant functional traits and climate with emission types and rates
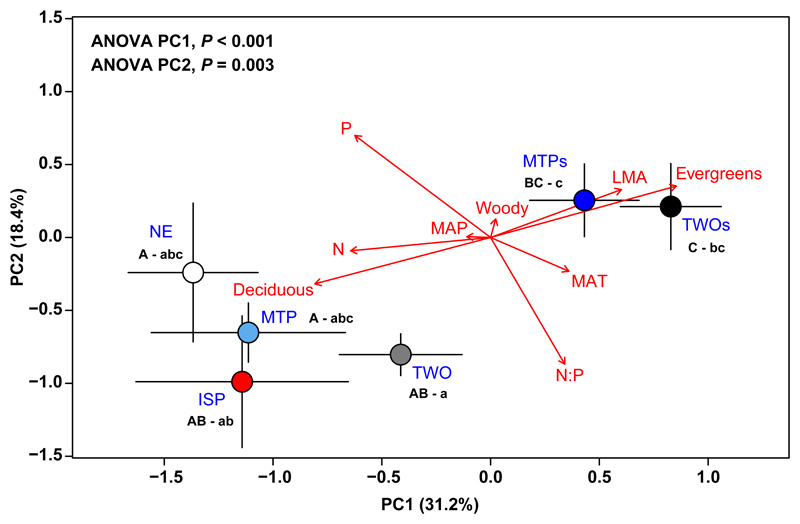
The first two axes extracted from the phylogenetic PCA identified significant differences amongst emission types (Figure 1). Together they explained 49.6% of the variance of the functional traits and climate. Variables most strongly aligned with PC1 (Table S3) were LMA (positively), foliar N and foliar P (both negatively), and whether a species was evergreen or deciduous (evergreens having higher LMA and lower foliar nutrient concentrations). Both mean annual temperature (MAT) and foliar N:P ratio were positively associated to PC1, but more weakly than these other traits. PC2 was most strongly correlated with foliar P concentration (positively) and N:P ratio (negatively). Additionally, PC2 was positively correlated with LMA (evergreens) and negatively with MAT.
Figure 1.
Average (± standard error) PC1 and PC2 scores per emission type: non-emitters (NE), monoterpene and isoprene emitters only (MTP and ISP, respectively), and emitters of monoterpenes and isoprene (TWO). MTPs and TWOs are the emission types that store monoterpenes. Different upper- and lowercase letters (e.g., AB – ab) indicate statistically significant differences at the 0.05 level amongst emission types for the PC1 and PC2 axes, respectively, following Tukey’s HSD test. Red arrows represent the loadings in the phylogenetic PCA. Factor loadings can be found in Table S3.
Both axes mainly separated the species that do (MTPs, TWOs) and do not store monoterpenes (NE, MTP, ISP, TWO) (ANOVA; PC1, P < 0.001; PC2, P = 0.003). The analysis also found that nutrient-rich plants belonged to the types that did not emit isoprenoids or emitted only either isoprene or monoterpenes (i.e. NE, ISP, and MTP). The third factor extracted did not show significant differences amongst emission types.
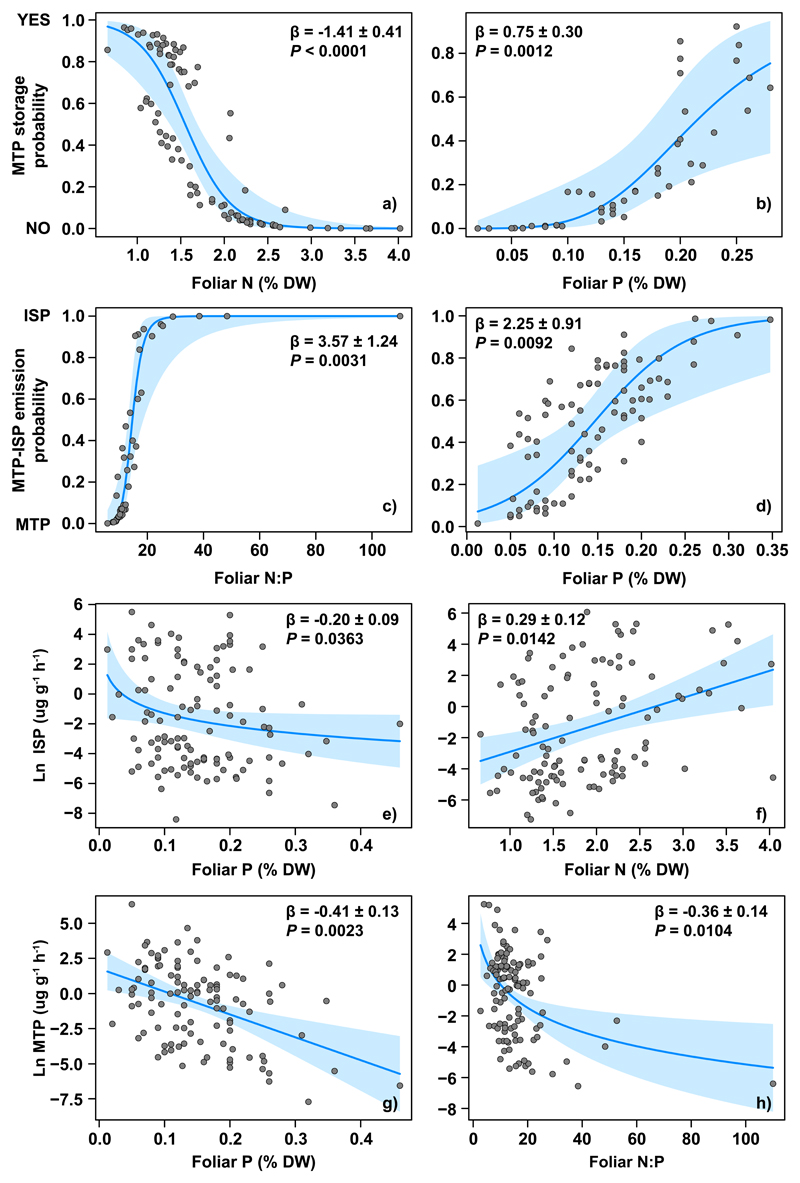
More detailed analyses for discriminating the emission types using phylogenetically-informed binomial regressions found that plants with high foliar P concentrations and low foliar N concentrations were more likely monoterpene storers (MTPs or TWOs; Figure 2). We also found that woody plants with higher foliar N:P and P concentrations were more likely ISP than MTP (Table 1). Models with and without phylogenetic “correction” provided the same results in both cases, although those including phylogenetic information better fitted our data based on ΔAICc (except for MTP emission rate, in which both models were undistinguishable, see Table 1). This fact indicates that emission type and rates present a certain degree of trait conservatism that could slightly bias model estimates when considering species as independent observations.
Figure 2.
Partial-residual plots showing the relationships between foliar nutrient concentrations of nitrogen (N) and phosphorus (P) and their stoichiometry (N:P ratio) with the probability that a species stores monoterpenes (1) or not (0) (a, b), or that it emits either monoterpenes (0) or isoprene (1) (c, d), and with emission rates of isoprene (e, f) and monoterpenes (g, h). Results of the models are presented in Table 1. Ln indicates that the variable was log-transformed. Partial-residual plots show variation in the dependent variable in relation to a given predictor (the fitted line), while simultaneously controlling for all other predictors in the model. The blue-shaded area indicated the 95% confidence bands of the slope around the fitted line. Results of the models are presented in Table 1. DW, dry weight.
Table 1.
Summary of the phylogenetically-corrected models correlating monoterpene and isoprene emissions with foliar nitrogen (N) and phosphorus (P) concentrations and N:P ratio, leaf mass per area (LMA), mean annual precipitation (MAP), and type of plant (woody or non-woody). See Methods for further information on how the models were adjusted. Estimates (β ± SE) are standardised coefficients ± standard error. For the factor woody, the estimate reflects the change from non-woody to woody plants. ΔAICc indicates the difference in AICc between the general linear model and the model controlling for phylogeny; positive values indicate a better adjustment of the phylogenetic model. λ and α indicate the phylogenetic corrections for Gaussian and binomial models, respectively. Continuous variables indicated with “Ln” were log-transformed, except for models indicated with †. MTP and ISP emission rate were log-transformed to fit the models.
| MTP vs. ISP | MTP storage | MTP emission rate | ISP emission rate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | P | Estimate | P | Estimate | P | Estimate | P | |||||
| Foliar N | -1.41 ± 0.41 | <0.0001 | 0.29 ± 0.12 | 0.0142 | ||||||||
| Ln Foliar P | 2.25 ± 0.91 | 0.0092 | 0.75 ± 0.30† | 0.0012 | -0.41 ± 0.13† | 0.0023 | -0.20 ± 0.09 | 0.0363 | ||||
| Ln Foliar N:P | 3.58 ± 1.24 | 0.0032 | -0.36 ± 0.14 | 0.0104 | ||||||||
| LMA | 0.17 ± 0.09 | 0.0773 | ||||||||||
| MAP | 0.18 ± 0.09 | 0.0345 | ||||||||||
| Woody | 6.75 ± 3.36 | 0.0417 | 0.87 ± 0.42 | 0.0382 | ||||||||
| ΔAICc | 5.13 | 5.13 | -0.94 | 16.28 | ||||||||
| λ | 0.14 | 0.51 | ||||||||||
| α | 2.85 | 1.22 | ||||||||||
Our phylogenetically-informed models for predicting isoprenoid emission indicated that foliar P was negatively correlated with the rates of monoterpene and isoprene emissions (Figure 2, Table 1). Foliar N was positively correlated with ISP emission rates, and a high N:P ratio was negatively correlated with monoterpene emissions. Plants with higher rates of isoprene emission were more typically woody, occurred at higher MAP and had (marginally) higher LMA (Table 1). However, when only de-novo monoterpene emission was considered (removing from the analyses species belonging to MTPs and TWOs emission types) only P (negatively) and MAP (positively) were marginally significantly related to monoterpene emission (P=0.065 and P=0.058 respectively). Again, models with and without phylogenetic correction led to the same conclusions. However, including phylogenetic information improved model fit of all variables except for monoterpene emission.
Evolutionary reconstruction and models of emission types
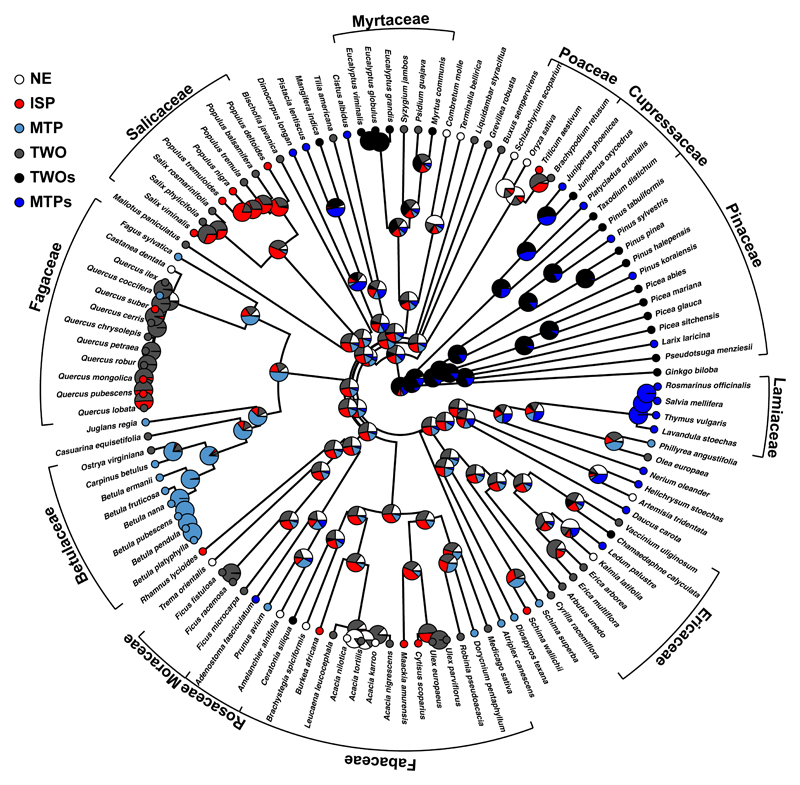
Evolutionary reconstructions calculated using stochastic mapping provided the probability that ancestral nodes in the phylogeny represented a specific emission type (Figure 3). Our reconstruction indicated that the oldest ancestor in our phylogeny was most likely to emit both isoprene and monoterpenes and also to store monoterpenes (emission type TWOs). Note, however, that our database did not contain bryophytes or ferns. Most of the nodes and species throughout the gymnosperm clade belonged to the TWOs emission type, despite a few transitions to emit (and store) only monoterpenes (emission type MTPs). Our analysis suggests that angiosperms lost their ability to store monoterpenes at some time during their evolution, but a few clades later reacquired it (e.g. family Lamiaceae, genus Eucalyptus). This suggests a clear case of divergent evolution (i.e., diversification of the trait through evolution) from the gymnosperms which was likely associated to the evolution of different storage organs (e.g., oil glands). Variability in emission type increased substantially during the diversification of angiosperms, which interfered with the reconstruction of several angiosperm nodes in our phylogeny. Our analysis nonetheless found that some clades had well-defined ancestors (in terms of isoprenoid emission types). Species of Salicaceae and Fagaceae were either in the ISP or TWO groups, and species of Betulaceae were mainly emitters of monoterpenes only. The complete loss of the ability to emit isoprene or monoterpenes was uncommon in our phylogeny, although the analysis indicated some NE nodes. Having a larger number of annual plants would have likely increased the number of NE nodes given that they have been suggested to be non-emitters (Loreto & Fineschi, 2015).
Figure 3.
Phylogenetic tree including the probability of emission type of ancestor nodes (large circles) as pie charts. Small circles indicate the emission type of the species. The ancestral reconstruction was performed using 1000 stochastic character mapped trees (see Methods for further information). NE, non-emitters; MTP, monoterpene emitters only; ISP, isoprene emitters only; TWO, emitters of both monoterpenes and isoprene. MTPs and TWOs are the emission types that store monoterpenes.
An adaptive model (OU, Ornstein–Uhlenbeck model (Beaulieu et al., 2012)), which assumed a different optimum for each emission type and a different phenotypic variability around each optimum, was the best evolutionary model explaining the link between emission type and foliar concentrations of N and P, LMA, and climate (MAP). For MAT, the best evolutionary model (OU) also assumed different optimal MATs for each emission type, but equal phenotypic variabilities. Brownian motion (BM) models always had a higher AICc (were less supported) than the OU models (Table S4). The fact that OU models fitted data better than BM models indicates that species with different emission types have most likely been pushed towards optimal values (i.e., average values for a specific trait) of the variables throughout evolutionary history.
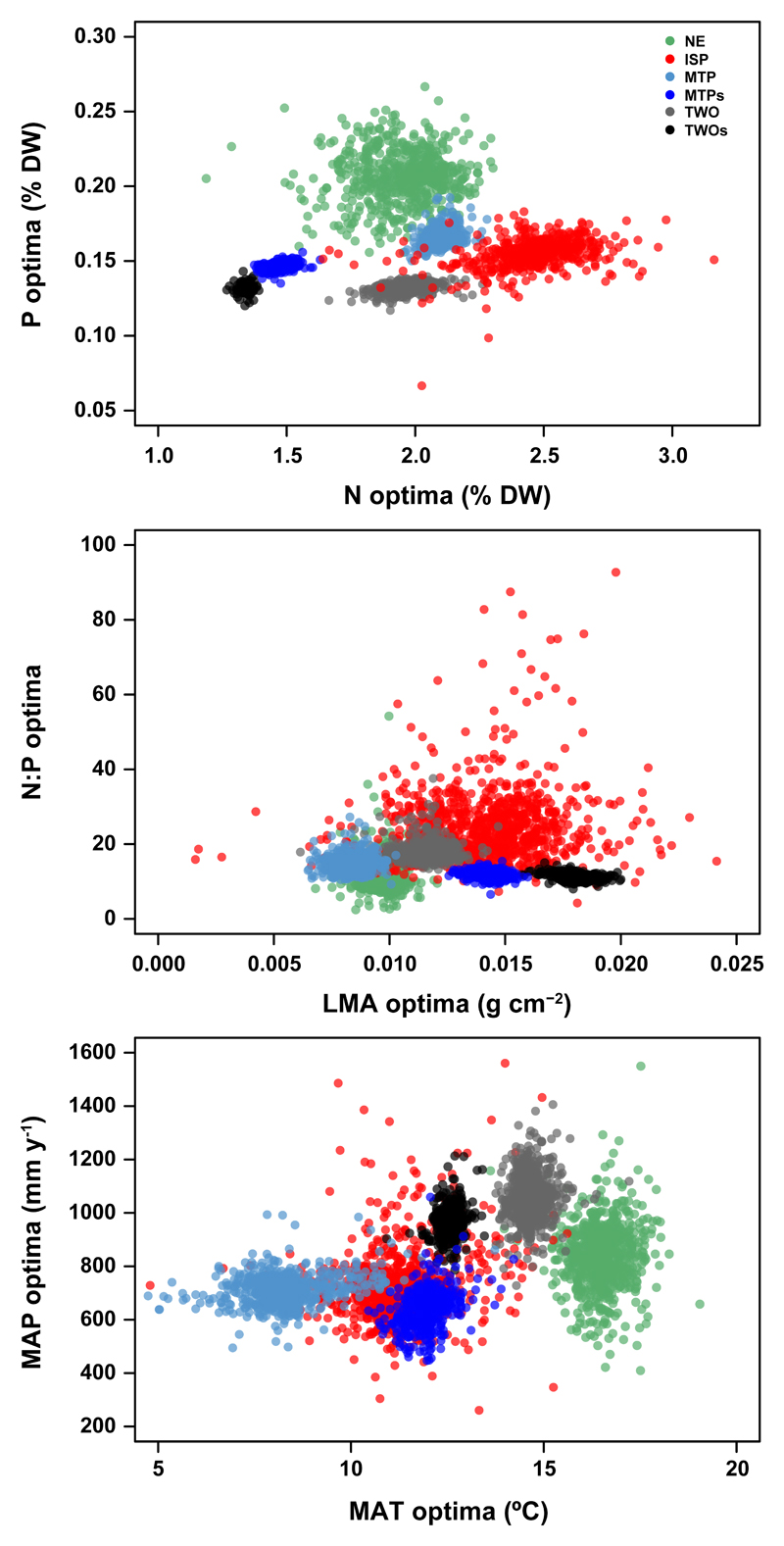
Optimal foliar N concentrations were highest for the emission types that did not store monoterpenes and were especially high for isoprene emitters only (Figure 4, Table 2). In contrast, foliar P concentration was highest for non-emitters and lowest for TWO and TWOs species, while ISP, MTP, and MTPs species had intermediate concentrations. Differences in the optimal N:P ratio per emission type, however, were not as clear; the optimal ratio was only significantly higher for TWO species compared to MTPs and TWOs species, and the other types could not be differentiated from any of these groups. Also, ISP species presented large variability for both N:P and LMA optimum values. LMA optimal values were higher for the species that stored monoterpenes (MTPs and TWOs) and lowest for MTP species. Non-emitter species had the highest optimal temperatures, followed by TWO, TWOs, and MTPs species. Again, ISP species presented very large variability in both MAT and MAP optimal values. MTP species had the lowest optimal temperature. Precipitation did not separate the different emission types as much as temperature, but optimal precipitation was higher for the TWO and TWOs than for the MTPs emission type, which showed the lowest average MAP optimum. In summary, our results suggest two main different strategies concerning leaf functional traits and isoprenoid emission type: on one side, species storing monoterpenes are located towards the lower range of N and P concentrations, and higher LMA. The opposite is found for non-emitters, ISP and MTP, all of which tend to have higher foliar N and P and lower LMA values. Although climate, especially temperature, also separated emission types, the separation was not as clear as for foliar functional traits.
Figure 4.
Optimal values of the predictor variables for the six emission types estimated with OUMV models (Ornstein-Uhlenbeck assuming different optimal values and phenotypic variability for each emission type) for the 1000 stochastic character maps (in the graphs there is a point for every emission type and model). The results for MAT were calculated with OUM models (assuming different state means but equal multiple rates of evolution) due to lower AICc (see Table S4). N, foliar nitrogen concentration; P, foliar phosphorus concentration; LMA, leaf mass per area; MAT, mean annual temperature; MAP, mean annual precipitation; NE, non-emitters; ISP, isoprene emitters only; MTP, monoterpene emitters only; MTPs, monoterpene emitters only that store monoterpenes; TWO, emitters of both isoprene and monoterpenes; TWOs, emitters of both isoprene and monoterpenes that also store monoterpenes; DW, dry weight. Medians are presented in Table 2.
Table 2.
Median optimal values, and their 2.5-97.5% confidence intervals, calculated with OUMV models (Ornstein-Uhlenbeck, assuming evolution has pushed species towards optimal values for each emission type and to different amounts of phenotypic variation around each optimum) for the six emission types. The number of sound models (i.e., without negative eigenvalues) is shown in column “N”. Different letters indicate significant differences between groups based on the overlap of the confidence intervals. The results for MAT were calculated with OUM models (assuming different state means but equal multiple rates of evolution) due to a lower AICc (see Table S4). N, foliar nitrogen concentration (% dry weight); P, foliar phosphorus concentration (% dry weight); LMA, leaf mass per area (g cm-2); MAT, mean annual temperature (°C); MAP, mean annual precipitation (mm y-1); NE, non-emitters; ISP, isoprene emitters only; MTP, monoterpene emitters only; MTPs, monoterpene emitters only that store monoterpenes; TWO, emitters of both isoprene and monoterpenes; TWOs, emitters of both isoprene and monoterpenes that also store monoterpenes.
| NE | ISP | MTP | MTPs | TWO | TWOs | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | CI | Median | CI | Median | CI | Median | CI | Median | CI | Median | CI | N | ||||||||||||
| N | 1.98 | 2.22 | c | 2.48 | 2.76 | c | 2.09 | 2.18 | c | 1.49 | 1.54 | b | 1.97 | 2.10 | c | 1.35 | 1.37 | a | 782 | |||||
| 1.64 | 2.06 | 2.02 | 1.41 | 1.83 | 1.32 | |||||||||||||||||||
| P | 0.206 | 0.240 | c | 0.156 | 0.172 | abc | 0.163 | 0.178 | c | 0.147 | 0.151 | b | 0.133 | 0.138 | a | 0.131 | 0.136 | a | 814 | |||||
| 0.170 | 0.132 | 0.157 | 0.142 | 0.126 | 0.129 | |||||||||||||||||||
| N:P | 10.7 | 18.8 | ab | 22.8 | 49.2 | ab | 14.8 | 18.9 | ab | 11.8 | 13.4 | a | 17.5 | 23.0 | b | 11.6 | 12.7 | a | 976 | |||||
| 7.0 | 12.9 | 11.4 | 10.3 | 15.1 | 9.9 | |||||||||||||||||||
| LMA | 0.0098 | 0.0107 | ab | 0.0144 | 0.0195 | ab | 0.0086 | 0.0094 | a | 0.0144 | 0.0151 | c | 0.0117 | 0.0128 | b | 0.0178 | 0.0191 | d | 979 | |||||
| 0.0086 | 0.0090 | 0.0072 | 0.0133 | 0.0101 | 0.0170 | |||||||||||||||||||
| MAT | 16.5 | 17.6 | d | 11.0 | 12.9 | ab | 8.3 | 9.8 | a | 12.0 | 12.6 | b | 14.6 | 15.3 | c | 12.7 | 13.0 | b | 954 | |||||
| 15.4 | 9.6 | 6.6 | 11.3 | 14.0 | 12.1 | |||||||||||||||||||
| MAP | 838.2 | 1060.7 | ab | 719.0 | 1081.1 | ab | 715.6 | 814.0 | a | 665.2 | 751.1 | a | 1059.2 | 1220.7 | b | 968.8 | 1057.5 | b | 875 | |||||
| 586.3 | 521.4 | 617.2 | 518.7 | 907.1 | 885.6 | |||||||||||||||||||
Discussion
The role of plant functional traits and climate in determining emission type and rate
Leaf functional traits (foliar N and P, N:P ratio and LMA) were the variables that better explained isoprenoid emission type, and the rate at which monoterpenes, and to a lesser extent, isoprene, are emitted. However, experimental studies should be carried out to further confirm that the results we found in this study did not appear because of the correlation between leaf functional traits and other non-considered variables. Annual climate played a clearly secondary role. This is a surprising result, considering the importance of certain environmental parameters (namely light and temperature) in setting the emission rates of isoprene and monoterpenes (Guenther et al., 1993; Jardine et al., 2014, 2015). Absence of climate effects is instead consistent with the finding that isoprene emitters are equally distributed among biomes of the world (Loreto & Fineschi, 2015). However, refining the manner in which climate is considered (e.g. using data for growing seasons rather than annual means) could further help in understanding the role of climate as a determinant of isoprenoid type and emission. Nonetheless, some studies for other foliar traits and ecosystem processes have shown that annual values usually provide the same results as extreme values or averages over the growing season (Niinemets, 2013, 2015; Fernández-Martínez et al., 2017). Different emission types had, on average, different foliar nutrient concentrations, and the relationships between foliar nutrient concentrations and the emission of volatile isoprenoids differed for isoprene and monoterpene emission. Based on our statistical models, species with N-rich leaves were less likely to have structures for storing monoterpenes but had higher rates of isoprene emission, supporting previous findings linking high leaf N contents to high rates of isoprenoid emission (Harley et al., 1994, 1999; Monson et al., 1994; Litvak et al., 1996; Staudt et al., 2001; Possell et al., 2004). Plants with higher foliar N:P ratios were more likely to emit isoprene but tended to emit less monoterpenes. Our results thus suggest that the isoprene-monoterpene emission trade-off (Harrison et al., 2013) might be associated to different strategies of N use and uptake. In other words, N seems to be important for emitting isoprene but not for emitting monoterpenes (Litvak et al., 2002), albeit the relationship between isoprene emission and foliar N is not very strong (Figure 2, Table 1). This relationship may also be due to the hygrophilous nature of isoprene emitters (Loreto et al., 2014a) that grow in soils in which N mineralisation is not likely limited by water. These favourable environmental conditions (water and nitrogen availability) enable high rates of photosynthesis (Wright et al., 2004) which, in turn, have been linked to high rates of isoprene emission (Monson et al., 1994; Litvak et al., 1996). However, it is not clear whether the positive correlation between foliar N and isoprene emission rate appears because of a direct effect of nitrogen on isoprene emission rate or because of an indirect effect through its positive effect on photosynthesis (Monson et al., 1994). Hence, further research on the mechanisms behind this observation is warranted.
Species with higher foliar P were more likely to emit isoprene and store monoterpenes, but tended to emit lower rates of isoprene and monoterpenes compared to those with lower foliar P concentrations (Table 1, Figures 2 and 3). These results fully support a previous study reporting a negative correlation between foliar P concentrations and isoprene emission in Phragmites australis, suggesting that isoprene emission may not only be limited by energetic (ATP) requirements (Fares et al., 2008). This negative correlation between isoprene emission and foliar P is puzzling because higher foliar P concentrations are also related to higher photosynthetic rates (Wright et al., 2004; Domingues et al., 2010), which are usually linked to higher isoprene emissions. One possible explanation for this observation would be that high pyruvate in P-rich plants allows mitochondrial respiration to more efficiently compete with the MEP pathway (Loreto et al., 2007), therefore inhibiting isoprene biosynthesis and emission. This is similar to what may happen in plants grown under elevated CO2 where isoprene emission is expected to increase concurrently with photosynthesis, but is instead limited by competition with cytosolic phosphoenolpyruvate (PEP) that is used to sustain mitochondrial respiration (Rosenstiel et al., 2003; Loreto et al., 2007). Perhaps under high P nutrition, P is mainly stored in PEP and is made unavailable for isoprene biosynthesis. Alternatively, both isoprene and monoterpene emission should be limited by factors other than P. Further research is clearly needed to validate these hypotheses and to better determine the role of P in isoprenoid emission, given the scarce literature available on this subject (Peñuelas & Staudt, 2010).
Plants that do not emit isoprenoids were generally within the lower range of observed LMAs and the higher range of foliar N and P concentrations (and lower N:P ratios), similar to what was found in previous studies on terpene content (Penuelas et al., 2011). These trait types suggest that the NE plants fall towards the ‘fast-return’ end of the leaf economic spectrum (Wright et al., 2004); i.e., they have potential to make faster photosynthetic returns on investments of dry mass and nutrients in leaves, than species with high LMA and low N and P concentrations. Conversely, MTPs and TWOs plants were within the lower range of foliar nutrient concentrations and higher range of LMAs (Table 2, Figures 1 and 4), and so group towards the ‘slow-return’ end of the leaf economic spectrum. The remaining groups of species (ISP, MTP and TWO) were generally found within intermediate ranges of LMA and foliar nutrient concentrations. Nutrient-rich plants may increase their aboveground production to the detriment of root exudates and secondary metabolites (Peñuelas & Estiarte, 1998; Vicca et al., 2012). As secondary compounds, BVOC production may be stimulated under stress conditions (e.g. stressful weather, pathogens, herbivores, or low nutrient availability), i.e. when plants must invest a larger proportion of resources into defence at the expense of reducing growth (Peñuelas & Estiarte, 1998; Loreto et al., 2014b). Our findings also support the hypothesis that nutrient-rich plants release less carbon to the atmosphere. Therefore, our results highlight the paramount role of nutrients in determining plant physiology and ecosystem functioning (Elser et al., 2010; Peñuelas et al., 2013).
Evolutionary history of isoprenoid emission type
Our ancestral reconstruction suggests that the evolution of isoprenoid emission type has been mainly divergent (i.e., starting from an initial trait, through evolution, species develop different phenotypes amongst them) and that the primary ancestor in our phylogeny was most likely to emit and store monoterpenes and emit isoprene (Figure 3). Non emitters appear to be a minority in our database. However, Loreto and Fineschi (2015) recently presented a survey of more than 1200 plant species showing that isoprene is emitted by around 20% of the species worldwide. Thus, our database is clearly skewed toward isoprenoid-emitting plants and the presence of non-emitters should be reconsidered. Emission of isoprene and monoterpenes in absence of storage appears to be a second evolutionary event. However, a previous study suggested that isoprene emission was developed as the most primitive method to cope with heat stress, which is not a problem in aquatic environments (Vickers et al., 2009). Isoprene emitters are more common in hygrophilous environments than in more xeric habitats, further supporting this hypothesis (Loreto et al., 2014a). If true, primitive terrestrial plants should belong mostly to the ISP emission type, which should therefore be much more common in bryophytes and pteridophytes than in vascular plants. In this sense, some available reports suggest that indeed emission of isoprene is a trait present in mosses (Hanson et al., 1999; Lantz et al., 2015) and ferns (Dani et al., 2014). Our phylogeny, however, contained neither bryophytes nor pteridophytes, and gymnosperms were the evolutionary more ancient plants in the analysis. Hornworts (Anthocerotophytes) and liverworts (Marchantiophytes), however, do not emit isoprene (Vickers et al., 2009). Hence, future research should include these older taxonomic groups, especially bryophytes, because they were the first plants to colonise land, and may provide a clearer picture of the early evolutionary history of isoprenoid emission type.
In many families of plants, species that emit isoprene can be found together with non-emitters and monoterpene emitters. Previous studies suggested that a single evolutionary event led to isoprene emission in early rosids, followed by multiple losses (Sharkey et al., 2013). For example, in the case of the oak genus, the original trait (isoprene emission) may have been lost, or may have evolved into the capacity to emit more complex isoprenoids (Loreto et al., 1998, 2009). On the other hand, several authors have embraced the hypothesis that the capacity to emit isoprene was gained and lost multiple times during evolution (Loreto et al., 2009; Sharkey et al., 2013). Li et al., (2017) recently discovered that isoprene synthase neo-functionalization occurs by active site mutation triggered by a single amino acid mutation, supporting evolution of isoprene synthase from the large class of monoterpene synthases (TPS-b). Isoprene emission may be conserved only in the narrow range of environmental conditions in which it clearly benefits plant fitness (Monson et al., 2013), or when plant genera undergo extensive speciation, more typically in perennial plants and in many trees (Dani et al., 2014).
Our models indicated that the rate of isoprene emission had a relatively strong phylogenetic signal (λ = 0.51) but the rate of monoterpene emission was poorly explained by phylogeny (λ = 0.14). These results fully support previous findings for phylogenetic signals in isoprene and monoterpene emission (Llusià et al., 2010; Loreto et al., 2014a). Whether a plant belonged to the ISP or MTP emission type per se or whether or not it stored monoterpenes, though, also had a clear phylogenetic signal (Table 1). These results indicate that emission type (ISP or MTP, monoterpene storage or not) was better preserved in the phylogeny than emission rate, which is likely because specific mutations are required for isoprene and monoterpene emission (and storage) and, once a species acquires them, rates of emission may vary depending on the environment. The stronger phylogenetic signal for isoprene than monoterpene emission, though, seems counterintuitive because of the previously reported trade-off that exist between them (Harrison et al., 2013; Li et al., 2017). Some authors have argued that the difference in phylogenetic signal of isoprene and monoterpene emission was due to the lack of ecological pressure in isoprene emission, while monoterpene emission developed as an adaptation to xeric environments (Loreto et al., 2014a). Our analyses, though, did not attribute higher optimal values of MAP for ISP than for MTP or MTPs (Figure 4, Table 1). However, ISP optimal values were more variable than for the rest of the emission types (specially for LMA and N:P ratio, see Figure 4), which might indicate larger variability in ISP plant traits compared to the other groups.
In contrast, our results indicated that isoprenoid emission type evolved together with foliar nutrient concentrations, LMA, and the climate they can tolerate, following an adaptive rather than a random model of evolution. This finding indicates that the different strategies of emission would have been selected under different specific environments together with functional traits. The inability to store monoterpenes for most of the angiosperms (Figure 3) might be due to an earlier adaptation to more fertile environments than for gymnosperms, which allowed angiosperms to better compete in these suitable cases. Instead, at some point of evolution, gymnosperms might have developed structures to store monoterpenes that were useful to tolerate stress, typically more severe in nutrient-limited environments. Angiosperms may have lost or not developed this ability until some clades began their adaptation to stressful environments. These clades might have developed or reacquired structures to store monoterpenes (e.g., Eucalyptus spp., family Lamiaceae) to better cope with stressful conditions.
Supplementary Material
Acknowledgements
This research was supported by the European Commission through the European Research Council Synergy grant ERC-2013-SyG 610028-IMBALANCE-P and Advanced grant 322603-SIP-VOL+, and through the European Regional Fund (the Center of Excellence EcolChange), the Spanish Government grant CGL2016-79835-P, the Catalan Government project SGR 2014-274 and the Estonian Ministry of Science and Education (institutional grant IUT-8-3). MFM is currently funded by a postdoc subsidy of the University of Antwerp. We thank Jutta Holst for her contribution building the database and Ferran Sayol for his advice on the phylogenetic analyses. The study has been supported by the TRY initiative on plant traits (http://www.try-db.org). The TRY initiative and database is hosted, developed and maintained by J. Kattge and G. Boenisch at the Max Planck Institute for Biogeochemistry, Jena, Germany. TRY is currently supported by DIVERSITAS/Future Earth and the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig.
Footnotes
Author contributions
M.F-M. and J.P. conceived, analysed and wrote the paper. J.L., I.F., U.N., A.A., I.J.W. and F.L. provided data and contributed substantially to the writing and discussion of the paper.
Competing interests
The authors declare no conflict of interests.
References
- Arneth A, Harrison SP, Zaehle S, Tsigaridis K, Menon S, Bartlein PJ, Feichter J, Korhola A, Kulmala M, O’Donnell D, et al. Terrestrial biogeochemical feedbacks in the climate system. Nature Geoscience. 2010;3:525–532. [Google Scholar]
- Beaulieu JM, Jhwueng DC, Boettiger C, O’Meara BC. Modeling stabilizing selection: Expanding the Ornstein-Uhlenbeck model of adaptive evolution. Evolution. 2012;66:2369–2383. doi: 10.1111/j.1558-5646.2012.01619.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, O’Meara B. OUwie: Analysis of Evolutionary Rates in an OU Framework. 2016.
- Breheny P, Burchett W. Visualization of Regression Models Using visreg, R package version 2.2-0. 2015 http://cran.r-project.org/package=visreg, 10/12/2016.
- Carslaw KS, Boucher O, Spracklen DV, Mann GW, Rae JGL, Woodward S, Kulmala M. Atmospheric aerosols in the earth system: a review of interactions and feedbacks. Atmospheric Chemistry and Physics Discussions. 2009;9:11087–11183. [Google Scholar]
- Cayuela L, Oksanen J. Taxonstand: Taxonomic Standardization of Plant Species Names. 2016.
- Dani KGS, Jamie IM, Prentice IC, Atwell BJ. Evolution of isoprene emission capacity in plants. Trends in Plant Science. 2014;19:439–446. doi: 10.1016/j.tplants.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Domingues TF, Meir P, Feldpausch TR, Saiz G, Veenendaal EM, Schrodt F, Bird M, Djagbletey G, Hien F, Compaore H, et al. Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant, Cell and Environment. 2010;33:959–980. doi: 10.1111/j.1365-3040.2010.02119.x. [DOI] [PubMed] [Google Scholar]
- Elser JJ, Fagan WF, Kerkhoff aJ, Swenson NG, Enquist BJ. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytologist. 2010;186:593–608. doi: 10.1111/j.1469-8137.2010.03214.x. [DOI] [PubMed] [Google Scholar]
- Fares S, Brilli F, Noguès I, Velikova V, Tsonev T, Dagli S, Loreto F. Isoprene emission and primary metabolism in Phragmites australis grown under different phosphorus levels. Plant Biology. 2008;10:38–43. doi: 10.1055/s-2007-965429. [DOI] [PubMed] [Google Scholar]
- Fernández-Martínez M, Vicca S, Janssens IA, Campioli M, Peñuelas J. Nutrient availability and climate as the main determinants of the ratio of biomass to NPP in woody and non-woody forest compartments. Trees, structure and function. 2016a;30:775–783. [Google Scholar]
- Fernández-Martínez M, Vicca S, Janssens IA, Ciais P, Obersteiner M, Bartrons M, Sardans J, Verger A, Canadell JG, Chevallier F, et al. Atmospheric deposition, CO2, and change in the land carbon sink. Scientific Reports. 2017;7:1–13. doi: 10.1038/s41598-017-08755-8. 9632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Martínez M, Vicca S, Janssens IA, Espelta JM, Peñuelas J. The role of nutrients, productivity and climate in determining tree fruit production in European forests. New Phytologist. 2016b;213:669–679. doi: 10.1111/nph.14193. [DOI] [PubMed] [Google Scholar]
- Fernández-Martínez M, Vicca S, Janssens IA, Sardans J, Luyssaert S, Campioli M, Chapin FS, III, Ciais P, Malhi Y, Obersteiner M, et al. Nutrient availability as the key regulator of global forest carbon balance. Nature Climate Change. 2014;4:471–476. [Google Scholar]
- Fineschi S, Loreto F, Staudt M, Peñuelas J. Diversification of volatile isoprenoid emissions from trees: evolutionary and ecological perspectives. In: Niinemets Ülo, Monson Russell K., editors. Biology, controls and models of tree volatile organic compound emissions, Tree Physiology. Berlin: Springer; 2013. pp. 1–20. [Google Scholar]
- Guenther AB, Zimmerman PR, Harley PC, Monson RK. Isoprene and Monoterpene Emission Rate Variability’ Model Evaluations and Sensitivity Analyses. Journal of Geophysical Research. 1993;98617:609–12. [Google Scholar]
- Hanson DT, Swanson S, Graham LE, Sharkey TD. Evolutionary significance of isoprene emission from mossess. American Journal of Botany. 1999;86:634–639. [PubMed] [Google Scholar]
- Harley PC, Litvak ME, Sharkey TD, Monson RK. lsoprene Emission from Velvet Bean Leaves ’. Plant Physiology. 1994;105:279–285. doi: 10.1104/pp.105.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley PC, Monson RK, Lerdau MT. Ecological and evolutionary aspects of isoprene emission from plants. Oecologia. 1999;118:109–123. doi: 10.1007/s004420050709. [DOI] [PubMed] [Google Scholar]
- Harrison SP, Morfopoulos C, Dani KGS, Prentice IC, Arneth A, Atwell BJ, Barkley MP, Leishman MR, Loreto F, Medlyn BE, et al. Volatile isoprenoid emissions from plastid to planet. New Phytologist. 2013;197:49–57. doi: 10.1111/nph.12021. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Huelsenbeck JP, Nielsen R, Bollback JP. Stochastic Mapping of Morphological Characters. Syst Biol. 2003;52:131–158. doi: 10.1080/10635150390192780. [DOI] [PubMed] [Google Scholar]
- Jardine K, Chambers J, Alves EG, Teixeira A, Garcia S, Holm J, Higuchi N, Manzi A, Abrell L, Fuentes JD, et al. Dynamic Balancing of Isoprene Carbon Sources Reflects Photosynthetic and Photorespiratory Responses to Temperature Stress. Plant Physiology. 2014;166:2051–2064. doi: 10.1104/pp.114.247494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine AB, Jardine KJ, Fuentes JD, Martin ST, Martins G, Durgante F, Carneiro V, Higuchi N, Manzi AO, Chambers JQ. Highly reactive light-dependent monoterpenes in the Amazon. Geophysical Research Letters. 2015;42:1576–1583. [Google Scholar]
- Kattge J, Díaz S, Lavorel S, Prentice IC, Leadley P, Bönisch G, Garnier E, Westoby M, Reich PB, Wright IJ, et al. TRY - a global database of plant traits. Global Change Biology. 2011;17:2905–2935. [Google Scholar]
- Lantz AT, Cardiello JF, Gee TA, Richards MG, Rosenstiel TN, Fisher AJ. Biochemical characterization of an isoprene synthase from Campylopus introflexus (heath star moss) Plant Physiology and Biochemistry. 2015;94:209–215. doi: 10.1016/j.plaphy.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Lapiedra O, Sol D, Carranza S, Beaulieu JM. Behavioural changes and the adaptive diversification of pigeons and doves. Proceedings of the Royal Society B. 2013;50 doi: 10.1098/rspb.2012.2893. 20122893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerdau M, Matson P, Fall R, Monson R. Ecological Controls over Monoterpene Emissions from Douglas-Fir (Pseudotsuga Menziesii) Ecology. 1995;76:2640–2647. [Google Scholar]
- Li M, Xu J, Algarra Alarcon A, Carlin S, Barbaro E, Cappellin L, Velikova V, Vrhovsek U, Loreto F, Varotto C. In Planta Recapitulation of Isoprene Synthase Evolution from Ocimene Synthases. Molecular Biology and Evolution. 2017:1–17. doi: 10.1093/molbev/msx178. msx178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litton CM, Raich JW, Ryan MG. Carbon allocation in forest ecosystems. Global Change Biology. 2007;13:2089–2109. [Google Scholar]
- Litvak ME, Constable JVH, Monson RK. Supply and demand processes as controls over needle monoterpene synthesis and concentration in Douglas fir [Pseudotsuga menziesii (Mirb.) Franco] Oecologia. 2002;132:382–391. doi: 10.1007/s00442-002-0964-y. [DOI] [PubMed] [Google Scholar]
- Litvak ME, Loreto F, Harley PC, Sharkey TD, Monson RK. The response of isoprene emission rate and photosynthetic rate to photon flux and nitrogen supply in aspen and white oak trees. Plant Cell and Environment. 1996;19:549–559. [Google Scholar]
- Llusia J, Penuelas J. Seasonal patterns of terpene content and emission from seven Mediterranean woody species in field conditions. American Journal of Botany. 2000;87:133–140. [PubMed] [Google Scholar]
- Llusià J, Peñuelas J, Sardans J, Owen SM, Niinemets Ü. Measurement of volatile terpene emissions in 70 dominant vascular plant species in Hawaii: Aliens emit more than natives. Global Ecology and Biogeography. 2010;19:863–874. [Google Scholar]
- Loreto F. Distribution of isoprenoid emitters in the Quercus genus around the world : chemo-taxonomical implications and evolutionary considerations based on the ecological function of the trait. Perspectives in Plant Ecology, Evolution and Systematics. 2002;5:185–192. [Google Scholar]
- Loreto F, Bagnoli F, Calfapietra C, Cafasso D, De Lillis M, Filibeck G, Fineschi S, Guidolotti G, Sramkó G, Tökölyi J, et al. Isoprenoid emission in hygrophyte and xerophyte European woody flora: Ecological and evolutionary implications. Global Ecology and Biogeography. 2014a;23:334–345. [Google Scholar]
- Loreto F, Bagnoli F, Fineschi S. One species, many terpenes: matching chemical and biological diversity. Trends in Plant Science. 2009;14:416–420. doi: 10.1016/j.tplants.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Loreto F, Centritto M, Barta C, Calfapietra C, Fares S, Monson RK. The relationship between isoprene emission rate and dark respiration rate in white poplar (Populus alba L.) leaves. Plant, Cell and Environment. 2007;30:662–669. doi: 10.1111/j.1365-3040.2007.01648.x. [DOI] [PubMed] [Google Scholar]
- Loreto F, Ciccioli P, Brancaleoni E, Valentini R, De Lillis M, Csiky O, Seufert G. A hypothesis on the evolution of isoprenoid emission by oaks based on the correlation between emission type and Quercus taxonomy. Oecologia. 1998;115:302–305. doi: 10.1007/s004420050520. [DOI] [PubMed] [Google Scholar]
- Loreto F, Dicke M, Schnitzler JP, Turlings TCJ. Plant volatiles and the environment. Plant, Cell and Environment. 2014b;37:1905–1908. doi: 10.1111/pce.12369. [DOI] [PubMed] [Google Scholar]
- Loreto F, Fineschi S. Reconciling functions and evolution of isoprene emission in higher plants. New Phytologist. 2015;206:578–582. doi: 10.1111/nph.13242. [DOI] [PubMed] [Google Scholar]
- Loreto F, Schnitzler JP. Abiotic stresses and induced BVOCs. Trends in Plant Science. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V, Nazionale C, Vegetali E, Km VS. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products, and Reduces Lipid Peroxidation of Cellular Membranes. 2001;127:1781–1787. 1. [PMC free article] [PubMed] [Google Scholar]
- Monson RK, Harley PC, Litvak ME, Wildermuth M, Guenther AB, Zimmerman PR, Fall R. Environmental and developmental controls over the seasonal pattern of isoprene emission from aspen leaves. Oecologia. 1994;99:260–270. doi: 10.1007/BF00627738. [DOI] [PubMed] [Google Scholar]
- Monson RK, Jones RT, Rosenstiel TN, Schnitzler JP. Why only some plants emit isoprene. Plant, Cell and Environment. 2013;36:503–516. doi: 10.1111/pce.12015. [DOI] [PubMed] [Google Scholar]
- Nielsen R. Mapping Mutations on Phylogenies. Systematic Biology. 2002;51:729–739. doi: 10.1080/10635150290102393. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. Mild versus severe stress and BVOCs: thresholds, priming and consequences. Trends in Plant Science. 2010;15:145–153. doi: 10.1016/j.tplants.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology. 2013;82:453–469. [Google Scholar]
- Niinemets Ü. Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytologist. 2015;205:79–96. doi: 10.1111/nph.13001. [DOI] [PubMed] [Google Scholar]
- O’Meara BC, Ané C, Sanderson MJ, Wainwright PC. Testing for different rates of continuous trait evolution using likelihood. Evolution. 2006;60:922–933. [PubMed] [Google Scholar]
- Penuelas J, Llusia J. Plant VOC emmisions: making use of the unavoidable. Trends in Ecology and Evolution. 2004;19:402–404. doi: 10.1016/j.tree.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Penuelas J, Sardans J, Llusia J, Owen SM, Niinemets U. Lower P contents and more widespread terpene presence in old Bornean than in young Hawaiian tropical plant species guilds. Ecosphere. 2011;2:1–19. [Google Scholar]
- Peñuelas J, Estiarte M. Can elevated CO2 affect secondary metabolism and ecosystem function? Trends in Ecology and Evolution. 1998;13:20–24. doi: 10.1016/s0169-5347(97)01235-4. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Llusià J. BVOCs: Plant defense against climate warming? Trends in Plant Science. 2003;8:105–109. doi: 10.1016/S1360-1385(03)00008-6. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Poulter B, Sardans J, Ciais P, van der Velde M, Bopp L, Boucher O, Godderis Y, Hinsinger P, Llusia J, et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nature communications. 2013;4 doi: 10.1038/ncomms3934. 2934. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Staudt M. BVOCs and global change. Trends in Plant Science. 2010;15:133–144. doi: 10.1016/j.tplants.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Possell M, Heath J, Hewitt CN, Ayres E, Kerstiens G. Interactive effects of elevated CO2 and soil fertility on isoprene emissions from Quercus robur. Global Change Biology. 2004;10:1835–1843. [Google Scholar]
- Qian H, Jin Y. An updated megaphylogeny of plants, a tool for generating plant phylogenies and an analysis of phylogenetic community structure. Journal of Plant Ecology. 2016;9:233–239. [Google Scholar]
- Revell LJ. Size-correction and principal components for interspecific comparative studies. Evolution. 2009;63:3258–3268. doi: 10.1111/j.1558-5646.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- Revell LJ. phytools: An R package for phylogenetic comparative biology (and other things) Methods in Ecology and Evolution. 2012;3:217–223. [Google Scholar]
- Rosenstiel TN, Potosnak MJ, Griffin KL, Fall R, Monson RK. Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature. 2003;421:256–259. doi: 10.1038/nature01312. [DOI] [PubMed] [Google Scholar]
- Sampedro L, Moreira X, Llusia J, Peñuelas J, Zas R. Genetics, phosphorus availability, and herbivore-derived induction as sources of phenotypic variation of leaf volatile terpenes in a pine species. Journal of Experimental Botany. 2010;61:4437–4447. doi: 10.1093/jxb/erq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardans J, Peñuelas J. Potassium: a neglected nutrient in global change. Global Ecology and Biogeography. 2015;24:261–275. [Google Scholar]
- Sayol F, Maspons J, Lapiedra O, Iwaniuk AN, Székely T, Sol D. Environmental variation and the evolution of large brains in birds. Nature Communications. 2016;7 doi: 10.1038/ncomms13971. 13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Gray DW, Pell HK, Breneman SR, Topper L. Isoprene synthase genes form a monophyletic clade of acyclic terpene synthases in the Tps-b terpene synthase family. Evolution. 2013;67:1026–1040. doi: 10.1111/evo.12013. [DOI] [PubMed] [Google Scholar]
- Singsaas L, Lerdau M, Winter K, Sharkey TD. lsoprene lncreases Thermotolerance of Isoprene-Emitting species. Plant physiology. 1997;115:1413–1420. doi: 10.1104/pp.115.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt M, Joffre R, Rambal S, Kesselmeier J. Effect of elevated CO2 on monoterpene emission of young Quercus ilex trees and its relation to structural and ecophysiological parameters. Tree physiology. 2001;21:437–45. doi: 10.1093/treephys/21.7.437. [DOI] [PubMed] [Google Scholar]
- Thomas GH, Freckleton RP, Székely T. Comparative analyses of the influence of developmental mode on phenotypic diversification rates in shorebirds. Proceedings of the Royal Society of London B. 2006;273:1619–24. doi: 10.1098/rspb.2006.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung Ho LS, Ané C. A linear-time algorithm for gaussian and non-gaussian trait evolution models. Systematic Biology. 2014;63:397–408. doi: 10.1093/sysbio/syu005. [DOI] [PubMed] [Google Scholar]
- Vicca S, Luyssaert S, Peñuelas J, Campioli M, Chapin FS, Ciais P, Heinemeyer A, Högberg P, Kutsch WL, Law BE, et al. Fertile forests produce biomass more efficiently. Ecology letters. 2012;15:520–6. doi: 10.1111/j.1461-0248.2012.01775.x. [DOI] [PubMed] [Google Scholar]
- Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nature chemical biology. 2009;5:283–291. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- Watson CM, Makowsky R, Bagley JC. Reproductive mode evolution in lizards revisited: Updated analyses examining geographic, climatic and phylogenetic effects support the cold-climate hypothesis. Journal of Evolutionary Biology. 2014;27:2767–2780. doi: 10.1111/jeb.12536. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–7. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.