Abstract
Background
Alopecia areata is a relatively common condition affecting patients seen in community dermatology clinics. A 2014 study implicated the JAK1/JAK2 inhibitor, ruxolitinib in short-term treatment of alopecia, however little information exists about the long-term use in otherwise healthy individuals in the community setting.
Methods
A patient with chronic alopecia areata and a patient with acute onset alopecia universalis were treated with oral ruxolitinib for over a year.
Results
Both patients experienced sustained, near-complete regrowth without hematologic or other complications after one year of treatment. Oral ruxolitinib effectively and safely treated alopecia in two women.
Conclusions
Ruxolitinib should be considered for cases of unresponsive alopecia in the community.
Report of cases
Case 1
A 59-year-old female with a 10-year history of chronic alopecia areata and 2 years of alopecia totalis was treated with ruxolitinib. At presentation, she had near total alopecia of the scalp, thinning of the eyebrows, and complete loss of hair on her body. Follicular ostia were visible in areas of alopecia, and her scalp was nonerythematous and nonscaly (Fig. 1a). Laboratory studies including a CBC, CMP, and TSH were within normal limits. Quantiferon gold was negative. She had previously undergone 5 years of treatment with intermittent topical steroids and minoxidil and 1.5 years of intralesional kenalog injections and twice weekly PUVA. None of the prior therapies led to sustained hair regrowth. She has no other autoimmune illness.
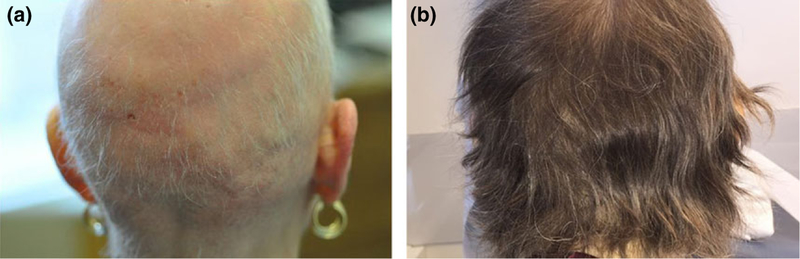
Figure 1.
Scalp hair regrowth following Ruxolitinib treatment for Patient 1. (a) Scalp prior to treatment. (b) Scalp following 1 year of treatment
Treatment was initiated with ruxolitinib 5 mg twice daily for 2 weeks and then slowly increased to a total daily dose of 30 mg by 3 months. Dosing schedule was based on dosing used in treating myelofibrosis.1,2 The patient began to notice regrowth of hair on her scalp, eyelashes, and genitals after 2 months, with more rapid regrowth when full dose was reached. After 8 months of therapy, she had complete regrowth of hair on the scalp and throughout her body (Fig. 1b). At 14 months, hair growth persisted, and dosing was decreased to 20 mg 3 days per week, then 30 mg 4 days per week without increased shedding. Further taper is planned.
Initially, a CBC was evaluated biweekly. Once stable dosing was achieved, it was evaluated bimonthly. CBC was notable for a mild decrease in hemoglobin and hematocrit after stable dosing was achieved but has remained within normal limits throughout treatment (Table 1). She noted a 5-pound weight gain over the first 6 months of therapy.
Table 1.
Hematologic parameters throughout ruxolitinib treatment
| 0 months | 1 month | 3 months | 6 months | 12 months | |
|---|---|---|---|---|---|
| Patient 1 | |||||
| Hemoglobin (g/dl)/Hematocrit (%) | 14.2/42.6 | 13.9/41.0 | 12.4/37.5 | 12.5/38.5 | 12.4/37.2 |
| Leukocytes (103/ul) | 9.1 | 5.4 | 6.6 | 5.6 | 5.9 |
| Platelets (103/ul) | 345 | 365 | 391 | 387 | 403 |
| Patient 2 | |||||
| Hemoglobin (g/dl)/Hematocrit (%) | 10.9/34.2 | 11.6/36.3 | 12.2/36.2 | 11.6/34.8 | 11.6/34.6 |
| Leukocytes (103/ul) | 6.3 | 6.4 | 4.6 | 5.5 | 6.2 |
| Platelets (103/ul) | 276 | 279 | 293 | 327 | 332 |
Case 2
A 45-year-old woman with a history of eczema and progressive alopecia areata was treated with ruxolitinib. She initially presented with patchy alopecia areata, which was unresponsive to intralesional kenalog, and combined methotrexate and PUVA. During treatment, her condition gradually progressed and was consistent with alopecia universalis at 14 months after initial presentation. At the time ruxolitinib was initiated, her scalp showed alopecia totalis with intact follicular ostia (Fig. 2a). Initial laboratory studies demonstrated a mild anemia, which was treated with iron supplementation. CBC, CMP, and LFTs were otherwise within normal limits, and quantiferon gold was negative.
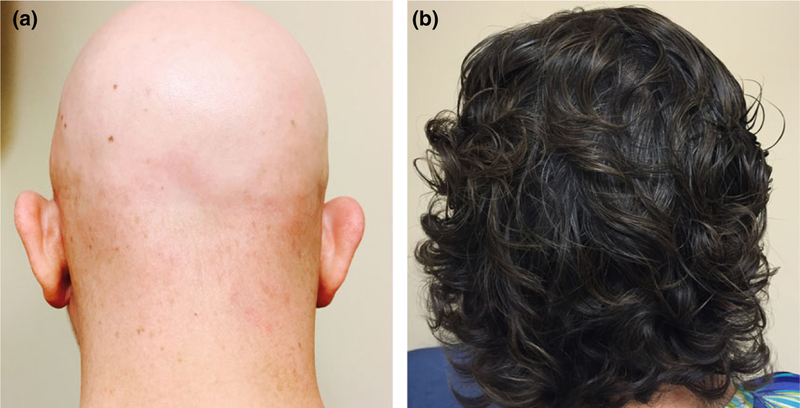
Figure 2.
Scalp hair regrowth following Ruxolitinib treatment for Patient 2. (a) Scalp prior to treatment. (b) Scalp following 1 year of treatment
Treatment was initiated with ruxolitinib 5 mg twice daily for 1 month, then up-titrated to a total daily dose of 30 mg by 3 months. The patient began to notice regrowth of hair on her scalp, armpits, eyebrows, and eyelashes after 2 months at full dose. After 6 months of therapy, she had near complete regrowth of hair on her scalp and body (Fig. 2b). At 13 months of drug therapy, the dose was tapered to 20 mg daily. When the dose was tapered below 20 mg, alopecia patches began to reappear. This was reversed by resuming long-term treatment with 20 mg daily.
Initially, a CBC and CMP were evaluated monthly. Once stable dosing was reached, it was spaced to bimonthly. The patient’s initial anemia resolved with iron supplementation. White blood cell count was noted to decline after 3 months of drug therapy but remained within normal limits (Table 1). The patient noted some bloating and bruising throughout drug treatment.
Discussion
Alopecia areata is a relatively common form of hair loss, with a prevalence of 0.1–0.2% in the United States.3 Patients most commonly present with loss of hair on the scalp, however it can affect any hair-bearing surface. Patterns of loss vary from patchy loss to complete loss of all body hair. For patients with chronic alopecia areata, there are few treatments with a strong evidence base.4
The exact pathogenesis of alopecia areata is not completely understood, however there is strong association with autoimmune disease. It is postulated that the disease initiates when hair follicle immune privilege is lost, resulting in presentation of follicular autoantigens to autoreactive CD8+ T-cells. Consistent with this idea, pathology of lesional skin shows a dense infiltration of antigen presenting cells around affected hair follicles.
A 2014 investigation identified a causative role for a subset of T-cells, NKG2D+ T cells, and identified a possible new therapy. In mice, these cells release IFNγ, which promotes IL-15 production in the hair follicle via JAK1/JAK2 signaling. IL-15, a known driver of cytotoxic T-cell activity, then stimulates increased IFNγ production by T-cells via JAK1/JAK3 signaling, amplifying the inflammatory response surrounding the hair follicle. Given the role of JAK signaling in this cascade, an FDA-approved protein kinase inhibitor specific to JAK1 and JAK2, ruxolitinib, was given to three patients with moderate to severe alopecia areata. While taking 40 mg daily, all three patients experienced near complete hair regrowth within 5 months.5
Ruxolitinib has been extensively studied in patients with intermediate and high-grade myelofibrosis (MF).1,2 In these trials, doses up to 50 mg daily for up to 24 months have been generally well tolerated. In patients with intermediate-grade MF, the most common Grade 3/4 adverse effects observed are anemia (33%), thrombocytopenia (12.5%), neutropenia (3.9%), and pneumonia (3.6%), however each of these led to discontinuation in less than 5% of participants. Though observed in less than 1% of patients in large trials, ruxolitinib has been anecdotally linked to new tuberculosis infection and reactivation of tuberculosis infection,6,7 likely due to drug effects on Th-1 immunity.
Following the 2014 study, multiple case reports have demonstrated ruxolitinib may be clinically useful for alopecia and other autoimmune conditions. A patient being treated for thrombocytopenia saw incidental improvement in alopecia,8 and a patient with alopecia and vitiligo saw improvement in both while taking 40 mg/day oral ruxolitinib for 20 weeks.9 Another report demonstrated hair regrowth in a patient with alopecia after 12 weeks of topical ruxolitinib application.10 While intriguing, the reported cases demonstrate only short-term use and do not address the possibility of serious complications.
The cases reported here offer evidence of the clinical utility of ruxolitinib for treating alopecia in otherwise healthy patients. These cases demonstrate the clinical course of prolonged treatment and provide evidence for the safety of ruxolitinib given over this course. Given the adverse effect profile observed in MF therapy, both patients were screened for tuberculosis prior to initiation of therapy, and CBCs were closely monitored throughout treatment. Both of the patients presented have demonstrated a sustained response to long-term treatment at the appropriate dose. Given the reappearance of alopecia patches in Case 2 when the dose was tapered, it may be necessary to maintain patients on the lowest possible dose necessary to maintain hair growth. The absence of major hematologic effects in the patients presented here provides evidence that ruxolitinib can be well tolerated for long-term treatment in otherwise healthy alopecia patients and should be considered for cases of unresponsive alopecia by clinicians in the community.
Acknowledgements
LAG was supported by RO1AR064297, RO1AR068280 from NIAMS, NIH.
Footnotes
COI Disclosures: None declared.
References
- 1.Al-Ali HK, Griesshammer M, le Coutre P, et al. Safety and efficacy of ruxolitinib in an open-label, multicenter, single-arm phase 3b expanded-access study in patients with myelofibrosis: a snapshot of 1144 patients in the JUMP trial. Haematologica 2016; 101: 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia 2017; 31: 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMichael AJ, Pearce DJ, Wasserman D, et al. Alopecia in the United States: outpatient utilization and common prescribing patterns. J Am Acad Dermatol 2007; 57(2 Suppl): S49–S51. [DOI] [PubMed] [Google Scholar]
- 4.Delamere FM, Sladden MM, Dobbins HM, et al. Interventions for alopecia areata. Cochrane Database Syst Rev 2008; CD004413. [DOI] [PubMed]
- 5.Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med 2014; 20: 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YH, Lee CH, Pei SN. Pulmonary tuberculosis reactivation following ruxolitinib treatment in a patient with primary myelofibrosis. Leuk Lymphoma 2015; 56: 1528–1529. [DOI] [PubMed] [Google Scholar]
- 7.Hopman RK, Lawrence SJ, Oh ST. Disseminated tuberculosis associated with ruxolitinib. Leukemia 2014; 28: 1750–1751. [DOI] [PubMed] [Google Scholar]
- 8.Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol 2015; 90: 82–83. [DOI] [PubMed] [Google Scholar]
- 9.Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol 2016; 74: 370–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craiglow BG, Tavares D, King BA. Topical ruxolitinib for the treatment of alopecia universalis. JAMA Dermatol 2016; 152: 490–491. [DOI] [PubMed] [Google Scholar]