Abstract
Purpose of Review
The challenges presented to the lung by the space environment are the effects of prolonged absence of gravity, the challenges of decompression stress associated with spacewalking, and the changes in the deposition of inhaled particulate matter.
Recent Findings
Although there are substantial changes in the function of the lung in partial gravity, the lung is largely unaffected by sustained exposure, returning rapidly to a normal state after return to 1G. Provided there is adequate denitrogenation prior to a spacewalk, avoiding the development of venous gas emboli, the lung copes well with the low pressure environment of the spacesuit. Particulate deposition is reduced in partial gravity, but where that deposition occurs is likely in the more peripheral airspaces, with associated longer retention times, potentially raising the toxicological potential of toxic dusts.
Summary
Despite its delicate structure the lung performs well in partial gravity, with the greatest threat likely arising from inhaled particulate matter (extra-terrestrial dusts).
Keywords: Respiratory, Aerosol transport, Deposition, Gas exchange
Introduction
The lung is potentially particularly vulnerable to the effects of partial gravity both by nature of its intrinsic structure, and because it presents a huge surface area to the environment. The structure of the lung, which is a direct result of the way in which the lung functions, is one of delicate, interrelated networks of airways and blood vessels. This structure is designed to bring inhaled gas into close apposition with the blood flowing from the right heart so that the exchange of O2 and CO2 can occur. Since the critical process of gas exchange occurs solely though passive diffusion, the gas exchange occurs across a membrane with a thickness of less than 0.3 pm and with a surface area of 50–100 m2 (1).
The anatomical structure utilized to accomplish this resembles a tightly packed “foam” where the bubbles of the foam are the alveoli (which number ~480,000,000 in the human lung, (2)), and the capillaries run through the walls of these bubbles. Since the majority of the lung volume is air, the intrinsic structure of the lung is very delicate and it comes as no surprise that the lung deforms under its own weight, resulting in significant differences in alveolar volume (3) and ventilation (4) between the top and bottom of the upright lung. Further, the low pressure of the pulmonary circulation (necessarily low because of the thinness of the pulmonary capillaries) means that blood flow is dependent on hydrostatic gradients within the vasculature (5). These hydrostatic effects combined with the gravitational deformation of the lung (above) make pulmonary perfusion even more uneven than pulmonary ventilation. As a consequence pulmonary gas exchange (which depends on the ventilation-perfusion ratio) is markedly different between the top and bottom of the lung (6).
Taking these structural aspects into consideration, lung function would be expected to be significantly altered in partial-, or in zero-gravity. But there are other aspects of space exploration that also pose a potential challenge to the lung. Extra-vehicular activity (EVA, Spacewalk) presents a direct challenge to the lung though the low-pressure environment of the spacesuits in use today, and indeed in the foreseeable future. Further, in the context of space exploration beyond low earth orbit, be it the Moon, a near-earth object (asteroid or comet), or Mars, surface activities bring with them the risk of exposure to inhaled particulate matter, as do incidents within a spacecraft itself (e.g. a fire). Since particulate deposition in the lung is dependent to some extent on gravity, this too is expected to change compared to the situation on the ground.
Although the title of this review refers to the lung in partial gravity, almost all of the studies performed to date have occurred in low earth orbit (and thus necessarily in zero-gravity), or in parabolic flight, where although partial gravity is feasible, the vast majority of the studies have been performed in zero-gravity. It seems unlikely that the presence of partial gravity (~1/6G on the Moon, ~3/8G on Mars) would serve to make conclusions drawn either from zero-gravity, or from interpolating between zero-gravity and normal Earth-gravity (1G), invalid.
Low gravity and lung function
The earliest measurements of pulmonary function in spaceflight date back to the early 1970s during the Skylab series of flights. The principal measurement was that of vital capacity (VC, the volume of the greatest possible inspiration or expiration). Data from Skylab 4, an 84-day period in zero-gravity showed an approximately 10% reduction in VC compared to before and after flight in the 3 crewmembers (7). However, because of the physical structure of Skylab (it was built in the fuel tank of a rocket upper stage) the absolute cabin pressure was only 258 mmHg and in order to avoid severe hypoxia, the fraction of oxygen (FIO2) was 0.74 (74%). Ground chamber tests with a somewhat similar atmosphere also showed a similar reduction in VC (8). It was not until 1991 when the Spacelab Life Sciences-1 (SLS-1) mission flew that measurements could be made in a normoxic, normobaric environment. Those measurement showed that VC was reduced by ~10% compared to that measured standing in 1G about 24 hours after the onset of zero-gravity (9). However subsequent measurements (after ~4 and 9 days) showed that VC had returned to its preflight value. The interpretation was that early in flight the headward translocation of circulating blood into the thorax essentially occupied space that was normally part of the VC, but that after a downward adjustment of circulating blood volume VC was unaltered.
SLS-1 and subsequent flights performed studies of numerous aspects of lung function as described in Table 1. As expected from the delicate structure of the lung, there were marked reductions in the heterogeneity of both ventilation (10) and perfusion (11) as inferred from variants of single breath washout tests. However, it was striking that while heterogeneity in both these was reduced in zero-gravity, it was far from absent, highlighting the combined effects of gravity and of heterogeneous lung structure in determining gas exchange. One of the more important results was that while both ventilation and perfusion became much more uniform in zero-gravity, the matching between them (the ventilation-perfusion ratio) did not become more uniform, with the heterogeneity of ventilation -perfusion ratio remaining comparable to that seen on the ground (12). While this may seem counterintuitive, it points to the intrinsic spatial interdependence of ventilation and perfusion: anatomical heterogeneity in one pathway (airways or vasculature) is likely matched in the other by the parallel nature of the airway and pulmonary arterial tree (1, 13), and similarly deformation of the lung due to gravity serves to affect both the airways and the vasculature. As such, from a gas exchange efficiency standpoint, to a large extent the normal lung behaves the same in zero-gravity as in 1G, a result that serves to refute a previous anecdotal report of significant gas exchange impairment inflight aboard Mir (14). (Table 1)
Table 1:
Lung function performed in zero-gravity.
| Topic | Key Result | Citation(s) |
|---|---|---|
| Lung volumes | Functional Residual Capacity intermediate between standing and supine | (9) |
| Forced spirometry | Modest changes possibly consistent with increased lung water | (15, 16) |
| Cardiac output | Increased initially with subsequent decline | (17, 18) |
| Diffusing capacity | Sustained increase Increases in both components | (17) |
| Heterogeneity of ventilation | Reduced in vital capacity breaths Minimal changes in tidal volume breaths | (10, 19–22) |
| Ventilation heterogeneity in lung periphery | Altered in sustained but not short term zero gravity | (23–26) |
| Heterogeneity of perfusion | Reduced but not absent | (11) |
| Gas exchange and ventilation- perfusion matching | Only slightly changed in zero gravity | (12, 27, 28) |
| Control of ventilation | Changes in hypoxic but not hypercapnic responses | (29, 30) |
| Sleep disordered breathing | Evidence for a reduction in zero gravity | (31, 32) |
| Heart rate variability | Altered sinus arrhythmia | (33) |
| Chest wall mechanics | Changes in abdominal contribution | (32, 34) |
| Extra-vehicular activity | No pulmonary disruption | (35) |
| Long duration zero gravity | Minimal changes postflight | (36, 37) |
Other important aspects relating to gas exchange were clearly altered by zero-gravity, but not in a deleterious manner. Cardiac output, which not strictly a pulmonary variable, is important since the lung receives virtually 100% of the right heart output. Early in flight there was a marked (~30%) rise in cardiac output above upright in 1G, rising to approximately that when subjects were acutely supine in 1G (17). However, after a few days, as circulating blood volume was adjusted downwards, reducing venous return, cardiac output fell to values nearer to that seen preflight. That of course set the scene for a marked reduction in cardiac output upon return to 1G. Diffusing capacity of the lung (measured using a single breath CO technique however rose by ~28% and remained elevated for the duration of exposure to zero-gravity. This was due to simultaneous and sustained increases in both the amount of blood in the pulmonary capillaries (Vc) and the membrane surface area available for gas exchange (Dm) (17). This was interpreted as being a result of the abolition of the hydrostatic gradients in the pulmonary vasculature with the result that, unlike the situation in 1G where the uppermost part of the lung is relatively poorly perfused (38), the entire pulmonary vasculature was fully recruited and participating in gas exchange.
In relation to the complete recruitment of the pulmonary vasculature, there was one curious and currently still unexplained result observed. At the time of the flights it had long been speculated that zero-gravity would result in an increase in transudation of fluid through the pulmonary capillaries with the possible consequence of the development of pulmonary edema (39). The observed increase in the diffusing capacity for carbon monoxide (DLCO) (17) argues against significant alveolar edema, as does the absence of an increase in lung tissue volume (40). However, on SLS-2 single breath washout tests were performed in which trace amounts of He and sulfur hexafluoride (SF6) were included in the inspirate. These two gases differ widely in their diffusivity (by a factor of ~6) and it had previously been shown that their concentration slopes during exhalation probe the lung structure in the vicinity of the acinar entrance. There was a marked change in these slopes in zero-gravity in a manner only seen before in lung pathologies associated with allograft rejection following lung transplantation. The speculation then was that the development of pulmonary interstitial edema near the acinar entrance was to blame (23). Buttressing this speculation was a subsequent study using the same techniques in the transient zero-gravity of parabolic flight (~25 seconds) (24). In that circumstance the changes in He and SF6 slopes seen in spaceflight were not seen (the study even included one of the spaceflight subjects), indicating that the effect was not simply one of lung distortion bought about by the removal of gravity, but rather was an effect that took some time (certainly longer than 25 seconds) to develop. Whether these studies indicate some reduced margin of safety for the development of pulmonary edema that might impair gas exchange in zero-gravity remains unclear.
Table 1 lists the major findings from the Shuttle era experiments. These are quite numerous and extensive, and there is insufficient room to discuss them all here. The reader is referred to the primary articles (cited in table 1) and to other more extensive reviews (41–43).
Long duration exposure to low gravity
All of the results that came from the Shuttle era pertain to relatively short exposure to zero- gravity, with the longest exposure being 17 days. To expand that exposure duration, a limited number of studies were performed in the first few year of the International Space Station (ISS). At the time the capability of the ISS were rather limited (it was still being built), but over 4 crew rotations, with exposure times of 130 to 196 days, a number of measurements could be made. These were limited by the inability (at that time) to carry compressed gases for experimental use, and so experiments had to be those that could be performed while only breathing air. Much more extensive measurements were made on multiple occasions before flight and following return to 1G.
The picture that emerged from the inflight measurements was little different to that seen in the Shuttle flights. Heterogeneity of ventilation-perfusion ratio was largely unchanged, gas exchange was preserved and overall the lung appeared to be able to function perfectly well in long duration zero-gravity (37). VC was unchanged over the course of the flight, again emphasizing that the changes seen in Skylab were likely atmosphere related, as opposed to a zero-gravity effect per se (36). Respiratory muscle strength as evidenced by maximum inspiratory and expiratory pressures, although slightly different to that in 1G, likely as a result of different biomechanics in the absence of gravity, was preserved throughout flight (36).
The preflight and postflight comparison showed that in the first week back in 1G following ~6 months in zero-gravity, there were a few changes that were statistically significant as shown in Table 2 (37). Interestingly one of these was the He-SF6 slope difference (discussed above) which persisted into the first postflight week, suggesting that the speculated excess lung hydration with associated interstitial edema was still present. (Table 2)
Table 2:
Percentage changes from preflight (standing control) in several variables measured numerous times preflight and, in the week, following return to 1G after between 130–196 days in zero gravity, and in subsequent periods (out to mission duration). Measurements were in 10 subjects except where otherwise noted. Changes that reached the level of statistical significance are highlighted. Of particular note is the absence of change in any variable after 1 week of re-exposure to 1G. Data from (37).
| First week after return | >1 week after return | |
|---|---|---|
| Total Lung Capacity | −1% | +2% |
| Functional Residual Capacity | +4% | −1% |
| Expiratory Reserve Volume | +25% | −4% |
| Residual Volume | −12% | +1% |
| Nitrogen Phase III slope | +6% | −5% |
| SF6-He Phase III slope | −27% | 0% |
| Heterogeneity of Specific Ventilation | −8% | +4% |
| Heart Rate | +16% | +7% |
| Stroke Volume | −12% | −5% |
| Cardiac Output | +3% | +2% |
| DLCO (n=3) | −21% | −17% |
However in many respects it was the postflight results from more than 1 week following flight that were the most interesting. In this context, the striking result was that despite the lung having been in the novel-environment of zero-gravity for ~6 months, after one week of re-exposure to 1G, there were virtually no changes that persisted (see Table 2). This is in striking contrast to many (perhaps most) other organ systems such as the cardiovascular system (44), muscles (45), and bones (46), all of which undergo considerable change with exposure to zero-gravity with those changes persisting well into the postflight period. While this might be considered a “negative result”, it is important from the aspect of future long duration exposure to zero-gravity that will be part of any “Exploration Class” mission. It suggests that provided the zero-gravity exposure is under normoxic and normobaric conditions, the lung can be expected to function well, and to not suffer deleterious consequences.
Spaceflight operational challenges to the lung
Spaceflight, and in particular “Exploration Class” missions bring with them significant operational challenges that extend beyond “just” being in partial gravity. In terms of the lung, the largest challenge is extra-vehicular activity (EVA, spacewalk). Mission models for a 6- month stay on the Moon call for surface activities (which implies an EVA) 5 days a week (weekends are “off”). All present EVA suits operate at a very low absolute pressure in order to maintain adequate mobility, and the engineering challenges of a high-pressure suit are sufficiently great that this low pressure design will likely persist. The US suits operate at a 220 mmHg absolute pressure and the Russian Orlan suits at 290 mmHg, both 100% O2. As such there is a significant risk of decompression illness (DCI) in going from the 760 mmHg, ~79% N2 atmosphere inside the ISS to the suit (47, 48). In order to avoid DCI, there is an extensive and time-consuming denitrogenation protocol undertake as the preparatory steps to an EVA. However despite this, ground studies in hypobaric chambers showed a ~50% incidence of venous gas emboli, and a ~24% incidence of clinical DCI (47). Venous gas emboli are filtered by the pulmonary vasculature and in studies of saturation divers have been shown to increase the heterogeneity of ventilation-perfusion ratio in the lung (49). Despite those ground results there are no recorded cases of DCI associated with EVA.
As part of the studies on the ISS in 2001–2003, a study was performed to address whether there was disruption to gas exchange in the lung following EVA. Such a disruption would be expected if the denitrogenation protocol was ineffective at preventing VGE. The experiment measured the heterogeneity of pulmonary perfusion and of ventilation-perfusion ratio before and on the day following EVA (50). In this respect it is one of the very few spaceflight experiments to be performed entirely in zero-gravity; both the control and test conditions were performed in orbit. The results showed that if there had been some disruption to perfusion or ventilation-perfusion heterogeneity immediately after the EVA, there were no persisting effects the following day. Although this is again a “negative result” study, the result is operationally important as it shows that current EVA denitrogenation protocols serve to protect the lung to an adequate degree.
The fact that the post-EVA measurements were performed on the day following EVA raises an important point for future “Exploration Class” missions. The delay in the measurements was driven by the long (~4 hour) denitrognation protocol required prior to EVA. Put simply the work day became too long and the measurements had to be postponed to the following day. But in the context of a mission model with 5 EVAs a week, a 4 hour denitrogenation protocol is a huge mission overhead. However, by appropriate choice of the habitat atmosphere, this can be reduced. The core target here is to reduce the N2 partial pressure in the habitat, all the while avoiding excessive hypoxia that would result from the reduction in absolute pressure, and avoiding excessive fire risk from raising the oxygen fraction too high in order to avoid hypoxia. A compromise habitat atmosphere of ~390 mmHg absolute pressure, FIO2 of 0.32, providing an equivalent altitude in terms of PIO2 of below 8000ft provides for an EVA denitrogenation time of only ~15 minutes. How the lung will respond to this nonstandard atmosphere is unknown, however the Fio2 of only 0.32 compared to the 0.74 seen in Skylab is a much smaller stress in terms of the development of atelectasis that was thought to be the genesis of the VC changes seen in those missions.
Inhaled aerosol in low gravity
The Apollo series of Lunar landings showed clearly that although surface exploration occurs within the confines of a sealed spacesuit, that does not imply that exposure to surface dust is not an issue. The dust sticks to the surface of the suit, is difficult to remove and is tracked into the cabin interior of the spacecraft. Upon doffing of the suit the crew are inevitably exposed to the dust, and the nuisance it imposed was a universal complaint from every Apollo landing mission crew debrief. Further the dust itself contains a significant fine fraction in the respirable range with ~10% being less than 10 μm in diameter (51). Since in 1G particles between ~8 and 0.5 μm in diameter deposit primarily by gravitational sedimentation, aerosol transport and deposition in partial-gravity is likely to be significantly altered. In terms of the risks associated with exposure to this dust, the amount and site of deposition is important, as is the nature of the dust itself in terms of potential toxicity (see the accompanying review by Loftus on the toxicity of celestial dusts).
Unlike the studies discussed above, all of the information on aerosol transport and deposition in the lung has been derived from studies performed in transient zero- or partial-gravity in parabolic flights aboard research aircraft. This platform provides short periods of zero-gravity (20–25 seconds) and slightly longer periods of partial gravity (~35 seconds in Lunar gravity) (52). Given the short time constants of the physical processes involved this platform is both less expensive than spaceflight and, relatively speaking, more accessible.
The first studies of total deposition by Hoffmann and Billingham (53) examined the deposition of 2μm particles in 1G, zero-gravity and in the periods of hypergravity afforded as part of parabolic flight (52). They showed a predictable linear relationship in deposition as a function of gravity, consistent with gravitational sedimentation being the dominant mechanism. Subsequent studies by Darquenne et al (54–56), which used different particle sizes showed similar results, but with the notable exception of an unexpectedly high deposition of 1μm particles in zero-gravity (54). The speculation was that these particles were showing the effects of another mechanism that was serving to raise deposition, although at the time there was no clear explanation of what that mechanism might be. A theoretical and in vitro study (57) provided a possible explanation by pointing out that because of the complicated nature of the flow streamlines within the airway tree, streamlines that were initially widely separated could be brought into close apposition with each other, permitted a very short mixing distance to have an enhanced effect of deposition. The effect was termed “stretch and fold” based on the repeated stretching and folding of a sheet of pastry that serves to bring normally widely separated points into close proximity. Attempts to verify this experimentally (58) were inconclusive and suggested that the stretch and fold mechanism occurred even within a single breath. Nevertheless, the studies indicate that compared to standard models, the deposition of particles of ~1μm in size in zero-gravity is higher than would be expected, an important point in terms of the risks this might pose in space exploration (59).
The other factor that is important in the context of potential toxicity is not only how much of an aerosol deposits, but also how long the deposited particles remain in the lung before being removed by the clearance mechanisms in the lung. Much of this depends on where in airway tree the deposition occurs. The more central airways are lined with a ciliated epithelium that works to effectively transport deposited material mouthward where it is ultimately eliminated. As such, residence time of particles in the ciliated airways is only on the order of hours to days (60). However, for particles that penetrate further into the airway tree, beyond the ciliated region, the residence time is vastly longer, with a timescale of months (61). In this context the results from a study in lunar-gravity are relevant. As shown in Figure 1, lunar gravity greatly reduces the amount of a 1μm aerosol that deposits, but considering a given amount of deposition (say 25%), the position at which this aerosol deposits (the penetration volume of the bolus) is much greater, indicating a more peripheral site of deposition (62). In essence gravity serves to protect the lung periphery by causing deposition to occur in the ciliated airways. In the absence of gravity, those particles that would otherwise have deposited by gravitational sedimentation remain in suspension and are able to be carried deeper into the airway tree where they eventually deposit. A study which examined the effects of both zero-gravity, and of the lower gas density that would be present in an Exploration habitat (simulated using carefully selected gas mixtures containing helium) showed that the effects of the gravity difference far outweighed any effect from the density change (63). (Figure 1)
Figure 1:
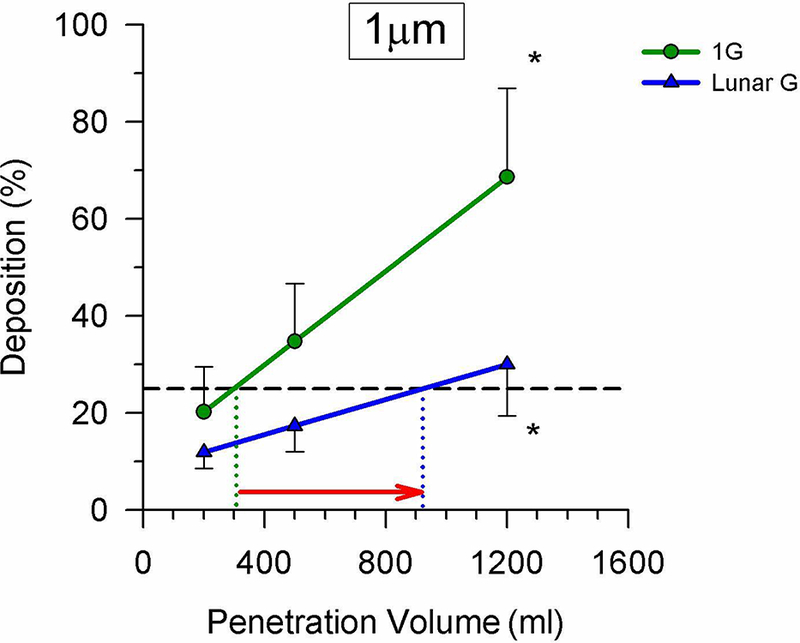
The deposition of aerosol boluses of 1μm particles inhaled to different penetration depths in the airways in both 1G (green line) and in lunar gravity (~1/6G, blue line). Data were collected during parabolic flight. Partial gravity served to reduce deposition, however, as indicated by the red arrow, for an equivalent amount of deposition (say 25%), this occurred more peripherally in partial gravity, raising the potential of increased residence time in the lung of the particles once deposited. Modified from (62), with permission.
Direct studies of clearance rates in partial- or zero-gravity have never been performed. The technical challenges to such studies is very large. There are however limited studies of the actual site of deposition in the lung, something that is not able to be measured using the aerosol bolus technique. In humans the only study used large particles (~5μm) labeled with 99mTc to look at regional deposition. By measuring the subsequent clearance of these particles, they were able to infer that for these large particles, relative deposition was more central in zero-gravity than in 1G, because of a decrease in alveolar deposition in the absence of gravity (particles remained in suspension and were therefore able to be subsequently exhaled) (64). The only other study used ~1μm ferric oxide particles deposited in rat lungs. These were administered during the zero- gravity portion of the parabolic flight to spontaneously breathing rats. The deposition was subsequently measured in the excised and fixed lungs using MRI measurement (65, 66). That study served to reinforce the aerosol deposition studies in humans as it showed that the relative deposition of these small particles occurred more peripherally in zero-gravity than in 1G. The implication of that study is that the residence time of those deposited particles would therefore be higher when the deposition occurred in zero-gravity, but this was not able to be directly measured.
Taken overall, these studies serve to suggest that deposition of inhaled aerosol in zero- or partial- gravity will be reduced, however for the fine size fraction (~ 1μm) that deposition will likely occur in more peripheral locations in the airway tree. As a direct consequence it is likely that the residence time of these deposited particles will be longer, potentially raising their toxicological potential. How great such an effect is will depend on the nature of those particles, a topic covered in the accompanying review by Loftus.
Conclusion and Future Perspectives
Despite the apparent fragility of the lung from a structural standpoint, studies show that while there are changes in lung function in partial- or zero-gravity, the lung continues to function well in this novel environment. There is limited evidence to suggest that the water content of the lung, presumably in the form of interstitial edema, may be slightly elevated in zero-gravity. This does not seem to produce any impairment in gas exchange, and while it might be hypothesized that this reduces the “safety margin” of the lung to an insult, there is no direct evidence to support or refute this concept. While both ventilation and perfusion become more uniform in the absence of gravity, the effects on ventilation-perfusion matching are minimal at best, perhaps not surprising given the coupled nature of the airway and vascular trees, which are necessarily similarly affected by changes in gravity.
From the standpoint of spaceflight operations, EVA (spacewalk) presents a potentially large challenge because of the change in absolute pressure going from the cabin environment to that of the suit. However, the limited evidence available suggests that on ISS, the current denitrogenation protocols are effective at avoiding negative consequences for the lung. Looking forward to an exploration habitat, the proposed atmosphere largely eliminates the risk of a significant decompression stress that would have the potential to affect the lung. Whether the increased fraction of oxygen (Fio2 ~0.32) in that atmosphere would have negative effects on the lung is an open question. Certainly, the very high oxygen fraction (~0.74) in the Skylab experience had some effects, but whether that applies in a more modest “exploration atmosphere” (Fio2 ~0.32) is unknown.
But perhaps the biggest challenge to the lung in the realm of exploration (where that includes activities on celestial bodies) is the most prosaic; dust. The Apollo experience showed that exposure to dust was inevitable and despite a diligent application of engineering to minimize this risk, dust exposure seems almost inevitable. The nature of these dusts will depend on where the exploration occurs, and it seems reasonable that the Moon (and perhaps near-Earth objects) will present the largest challenge since they lack an atmosphere that has the potential to chemically passivate the dust. However, what is universal is that the partial-gravity environments will serve to alter the location and amount of deposited aerosol, and quite possibly alter the residence time of deposited particles in the lung. Combined with the potential of a reactive dust species this may prove to be the largest routine challenge to the lung in future space exploration.
Acknowledgements
Many of the studies were funded by NASA or the National Space Bimbedical Research Institute under various contracts and grants. GK Prisk is currently funded by NIH under R01 HL119263.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Dr. Prisk has nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
References
Recently published papers of particular interest have been highlighted as:
• Of importance
•• Of major importance
- 1.Weibel ER. Morphometry of the Human Lung. New York: Academic Press; 1963. [Google Scholar]
- 2.Ochs M, Nyengaard JR, Jung A, Knudsen L, Voigt M, Wahlers T, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169(1):120–4. [DOI] [PubMed] [Google Scholar]
- 3.Glazier JB, Hughes JMB, Maloney JE, West JB. Vertical gradient of alveolar size in lungs of dogs frozen intact. Journal of Applied Physiology. 1967;23:694–705. [DOI] [PubMed] [Google Scholar]
- 4.Bryan AC, Milic-Emili J, Pengelly D. Effect of gravity on the distribution of pulmonary ventilation. Journal of Applied Physiology. 1966;21:778–84. [DOI] [PubMed] [Google Scholar]
- 5.West JB, Dollery CT, Naimark A. Distribution of bloodflow in isolated lung: Relation to vascular and alveolar pressures. Journal of Applied Physiology. 1964;19:713–24. [DOI] [PubMed] [Google Scholar]
- 6.West JB. Pulmonary Gas Flow and Gas Exchange In: West JB, editor. Respiratory Physiology: People and Ideas. New York: Oxford Press; 1966. p. 140–96. [Google Scholar]
- 7.Sawin CF, Nicogossian AE, Rummel JA, Michel EL. Pulmonary function evaluation during the Skylab and Apollo-Soyuz Missions. Aviation Space and Environmental Medicine. 1976;47:168–72. [PubMed] [Google Scholar]
- 8.Robertson WG, McRae GL. Study of man during a 56-day exposure to an oxygen-helium atmosphere at 258 mm Hg total Pressure. VII. Respiratory function. Aerospace Medicine. 1966;37:453–6. [PubMed] [Google Scholar]
- 9.Elliott AR, Prisk GK, Guy HJB, West JB. Lung volumes during sustained microgravity on spacelab SLS-1. Journal of Applied Physiology. 1994;77:2005–14. [DOI] [PubMed] [Google Scholar]
- 10.Guy HJB, Prisk GK, Elliott AR, Deutschman RA III, West JB Inhomogeneity of pulmonary ventilation during sustained microgravity as determined by single-breath washouts. Journal of Applied Physiology. 1994;76:1719–29. [DOI] [PubMed] [Google Scholar]
- 11.Prisk GK, Guy HJB, Elliott AR, West JB. Inhomogeneity of pulmonary perfusion during sustained microgravity on SLS-1. Journal of Applied Physiology. 1994;76:1730–8. [DOI] [PubMed] [Google Scholar]
- 12.Prisk GK, Elliott AR, Guy HJB, Kosonen JM, West JB. Pulmonary gas exchange and its determinants during sustained microgravity on Spacelabs SLS-1 and SLS-2. Journal of Applied Physiology. 1995;79:1290–8. This study shows that despite changes in the heterogeneity of both ventilation and perfusion in zerogravity, the important matching of these two remains comparable to that seen in 1G [DOI] [PubMed] [Google Scholar]
- 13.Haefeli-Bleuer B, Weibel ER. Morphometry of the human pulmonary acinus. Anatomical Record. 1988;220:401–14. [DOI] [PubMed] [Google Scholar]
- 14.Haase H, Baranov VM, Asyamolova NM, Polyakov VV, Avak yan YG, Dannenberg R, et al. First results of PO 2 examinations in the capillary blood of cosmonauts during a long-term flight in the space station “MIR”. 41st Congress of the International Astronautical Federation, Dresden, Germany: 1990:1–4. [Google Scholar]
- 15.Guy HJB, Prisk GK, Elliott AR, West JB. Maximum expiratory flow-volume curves during short periods of microgravity. Journal of Applied Physiology. 1991;70:2587–96. [DOI] [PubMed] [Google Scholar]
- 16.Elliott AR, Prisk GK, Guy HJB, Kosonen JM, West JB. Forced expirations and maximum expiratory flow-volume curves during sustained microgravity on SLS-1. Journal of Applied Physiology. 1996;81:33–43. [DOI] [PubMed] [Google Scholar]
- 17.Prisk GK, Guy HJB, Elliott AR, Deutschman RA III, West JB. Pulmonary diffusing capacity, capillary blood volume and cardiac output during sustained microgravity. Journal of Applied Physiology. 1993;75:15–26. [DOI] [PubMed] [Google Scholar]
- 18.Prisk GK, Fine JM, Elliott AR, West JB. Effect of 6-degree head-down tilt on cardiopulmonary function: Comparison with microgravity. Aviation Space and Environmental Medicine. 2002;73:8–16. [PubMed] [Google Scholar]
- 19.Prisk GK, Guy HJB, Elliott AR, Paiva M, West JB. Ventilatory inhomogeneity determined from multiple-breath washouts during sustained microgravity on Spacelab SLS-1. Journal of Applied Physiology. 1995;78:597–607.7759429 [Google Scholar]
- 20.Verbanck S, Linnarsson D, Prisk GK, Paiva M. Specific ventilation distribution in microgravity. Journal of Applied Physiology. 1996;80:1458–65. [DOI] [PubMed] [Google Scholar]
- 21.Prisk GK, Elliott AR, Guy HJB, Verbanck S, Paiva M, West JB. Multiple-breath washin of helium and sulfur hexafluoride in sustained microgravity. Journal of Applied Physiology. 1998;84:244–52. [DOI] [PubMed] [Google Scholar]
- 22.Dutrieue B, Verbanck S, Darquenne C, Prisk GK. Airway closure in microgravity. Respir Physiol Neurobiol. 2005;148(1–2):97–111. [DOI] [PubMed] [Google Scholar]
- 23.Prisk GK, Lauzon AM, Verbanck S, Elliott AR, Guy HJB, Paiva M, et al. Anomalous behavior of helium and sulfur hexafluoride during single-breath tests in sustained microgravity. Journal of Applied Physiology. 1996;80:1126–32. [DOI] [PubMed] [Google Scholar]
- 24.Lauzon AM, Prisk GK, Elliott AR, Verbanck S, Paiva M, West JB. Paradoxical helium and sulfur hexafluoride single-breath washouts in short-term vs. sustained microgravity. Journal of Applied Physiology. 1997;82:859–65. [DOI] [PubMed] [Google Scholar]
- 25.Dutrieue B, Lauzon AM, Verbanck S, Elliott AR, West JB, Paiva M, et al. Helium and sulfur hexafluoride bolus washin in short-term microgravity. Journal of Applied Physiology. 1999;86(5):1594–602. [DOI] [PubMed] [Google Scholar]
- 26.Dutrieue B, Paiva M, Verbanck S, LeGouic M, Darquenne C, Prisk GK. Tidal volume single breath washin of SF6 and CH4 in transient microgravity. Journal of Applied Physiology. 2003;94:75–82. [DOI] [PubMed] [Google Scholar]
- 27.Lauzon AM, Elliott AR, Paiva M, West JB, Prisk GK. Cardiogenic oscillation phase relationships during single-breath tests performed in microgravity. Journal of Applied Physiology. 1998;84:661–8. [DOI] [PubMed] [Google Scholar]
- 28.Verbandt Y, Wantier M, Prisk GK, Paiva M. Ventilation-perfusion matching in long-term microgravity. Journal of Applied Physiology. 2000;89:2407–12. [DOI] [PubMed] [Google Scholar]
- 29.Elliott AR, Prisk GK, Schîllman C, Hoffman U. Hypercapnic ventilatory response in humans before, during and after 23 days of low level CO 2 exposure. Aviation Space and Environmental Medicine. 1998;69:391–6. [PubMed] [Google Scholar]
- 30.Prisk GK, Elliott AR, West JB. Sustained microgravity reduces the human ventilatory response to hypoxia but not hypercapnia. Journal of Applied Physiology. 2000;88:1421–30. [DOI] [PubMed] [Google Scholar]
- 31.Dijk DJ, Neri DF, Wyatt JK, Ronda JM, Riel E, Ritz-de Cecco A, et al. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. AmJPhysiolRegulatory Integrative CompPhysiol. 2001;281:R1647-R64. [DOI] [PubMed] [Google Scholar]
- 32.Sa RC, Prisk GK, Paiva M. Microgravity alters respiratory abdominal and rib cage motion during sleep. J Appl Physiol. 2009;107(5):1406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Migeotte PF, Verbandt Y, Prisk GK, Paiva M. Microgravity alters respiratory sinus arrhythmia and short-term heart rate variability in humans. AmJPhysiolHeart CircPhysiol. 2003;284:H1195-H2006. [DOI] [PubMed] [Google Scholar]
- 34.Wantier M, Estenne M, Verbanck S, Prisk GK, Paiva M. Chest wall mechanics in sustained microgravity. Journal of Applied Physiology. 1998;84:2060–5. [DOI] [PubMed] [Google Scholar]
- 35.Prisk GK, Fine JM, Cooper TK, West JB. Pulmonary gas exchange is not impaired 24-hours following extra-vehicular activity. Journal of Applied Physiology. 2005;99:2233–8. This study shows that on the day following spacewalk, there are no disruptions to pulmonary gas exchange. [DOI] [PubMed] [Google Scholar]
- 36.Prisk GK, Fine JM, Cooper TK, West JB. Vital Capacity, respiratory muscle strength and pulmonary gas exchange during long-duration exposure to microgravity. Journal of Applied Physiology. 2006;101:439–47. [DOI] [PubMed] [Google Scholar]
- 37.Prisk G, Fine J, Cooper T, West J. Lung function is unchanged in the 1 G environment following 6-months exposure to microgravity. Eur J Appl Physiol. 2008;103(6):617–23. This study shows that long-duration exposure to zero gravity does not result in lasting deliterious consequence for the lung [DOI] [PubMed] [Google Scholar]
- 38.West JB, Dollery CT. Distribution of blood flow and ventilation-perfusion ratio in the lung, measured with radioactive CO 2. Journal of Applied Physiology. 1960;15:405–10. [DOI] [PubMed] [Google Scholar]
- 39.Permutt S Pulmonary circulation and the distribution of blood and gas in the lungs Physiology in the Space Environment. Washington: NAS NRC 1485B; 1967. p. 38–56. [Google Scholar]
- 40.Verbanck S, Larsson H, Linnarsson D, Prisk GK, West JB, Paiva M. Pulmonary tissue volume, cardiac output, and diffusing capacity in sustained microgravity. Journal of Applied Physiology. 1997;83:810–6. [DOI] [PubMed] [Google Scholar]
- 41.Prisk GK. Invited review: Microgravity and the lung. Journal of Applied Physiology. 2000;89(1):385–96. [DOI] [PubMed] [Google Scholar]
- 42.Prisk GK. The lung in space. Clinics in Chest Medicine. 2005;26:415–38. [DOI] [PubMed] [Google Scholar]
- 43.Prisk GK. Microgravity and the respiratory system. Eur Respir J. 2014;43(5):1459–71. [DOI] [PubMed] [Google Scholar]
- 44.Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, et al. Cardiac atrophy after bed rest and spaceflight. Journal of Applied Physiology. 2001;91:645–53. [DOI] [PubMed] [Google Scholar]
- 45.Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JLW, Riley DR, et al. Effects of a 17 day spaceflight on contractile properties of human soleus muscle fibres. JPhysiol(London). 1999;516:915–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang T, Van Loon J, Bloomfield S, Vico L, Chopard A, Rittweger J, et al. Towards human exploration of space: the THESEUS review series on muscle and bone research priorities. NPJ Microgravity. 2017;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waligora JM, Horrigan D Jr, Conkin J, Hadley AT III. Verification of an altitude decompression sickness prevention protocol for shuttle operations utilizing a 10.2-psi pressure stage NASA Technical Memorandum 58259. Houston: NASA; 1984. p. 1–44. [Google Scholar]
- 48.Conkin J, Klein JS, Acock KE. Description of 103 cases of hypobaric decompression sickness from NASA-sponsored research (1982–1999) Houston, Texas: Johnson Space Center; 2003. Report No.: NASA Technical Publication; 2003–212052. [Google Scholar]
- 49.Cotes JE. Assessment and Application in Medicine. Lung Function. 1993;5:251–62. [Google Scholar]
- 50.Prisk GK, Fine JM, Cooper TK, West JB. Pulmonary gas exchange is not impaired 24 h after extravehicular activity. J Appl Physiol. 2005;99(6):2233–8. [DOI] [PubMed] [Google Scholar]
- 51.Graf JC. Lunar soil size catalog. 1993. Report No.: NASA-RP-1265.
- 52.Karmali F, Shelhamer M. The dynamics of parabolic flight: Flight characteristics and passenger percepts. Acta Astronautica. 2008;63:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffman RA, Billingham J. Effect of altered G levels on deposition of particulates in the human respiratory tract. Journal of Applied Physiology. 1975;38:955–60. [DOI] [PubMed] [Google Scholar]
- 54.Darquenne C, Paiva M, West JB, Prisk GK. Effect of microgravity and hypergravity on deposition of 0.5- to 3-μm-diameter aerosol in the human lung. Journal of Applied Physiology. 1997;83:2029–36. [DOI] [PubMed] [Google Scholar]
- 55.Darquenne C, West JB, Prisk GK. Deposition and dispersion of 1 μm aerosol boluses in the human lung: Effect of micro- and hypergravity. Journal of Applied Physiology. 1998;85:1252–9. [DOI] [PubMed] [Google Scholar]
- 56.Darquenne C, West JB, Prisk GK. Dispersion of 0.5–2 Êm aerosol in micro- and hypergravity as a probe of convective inhomogeneity in the human lung. Journal of Applied Physiology. 1999;86:1402–9. [DOI] [PubMed] [Google Scholar]
- 57.Butler JP, Tsuda A. Effect of convective stretching and folding on aerosol mixing deep in the lung, assessed by approximate entropy. Journal of Applied Physiology. 1998;83:800–9. [DOI] [PubMed] [Google Scholar]
- 58.Darquenne C, Prisk GK. Effect of small flow reversals on aerosol mixing in the alveolar region of the human lung. Journal of Applied Physiology. 2004;97:2083–9. [DOI] [PubMed] [Google Scholar]
- 59.Linnarsson D, Carpenter J, Fubini B, Gerde P, Karlsson LL, Loftus DJ, et al. Toxicity of Lunar Dust. Planetary and Space Science. 2012;74:57–71. [Google Scholar]
- 60.Bennett WD, Chapman WF, Lay JC, Gerrity TR. Pulmonary clearance of inhaled particles 24 to 48 hours post deposition: Effect of beta-adrenergic stimulation. Journal of Aerosol Medicine. 1993;6:53–62. [Google Scholar]
- 61.Moller W, Haussinger K, Winkler-Heil R, Stahlhofen W, Meyer T, Hofmann W, et al. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. Journal of Applied Physiology. 2004;97:2200–6. [DOI] [PubMed] [Google Scholar]
- 62.Darquenne C, Prisk G. Deposition of inhaled particles in the human lung is more peripheral in lunar than in normal gravity. Eur J Appl Physiol. 2008;103(6):687–95. This study shows that while aerosol deposition in reduced in lunar gravity, those particles that re deposited do so in more peripheral locations in the airways, likely increasing their residence time. [DOI] [PubMed] [Google Scholar]
- 63.Darquenne C, Prisk GK. Particulate deposition in the human lung under lunar habitat conditions. Aviat Space Environ Med. 2013;84(3):190–5. [DOI] [PubMed] [Google Scholar]
- 64.Darquenne C, Zeman KL, Sa RC, Cooper TK, Fine JM, Bennett WD, et al. Removal of sedimentation decreases relative deposition of coarse particles in the lung periphery. J Appl Physiol (1985). 2013;115(4):546–55. [DOI] [PubMed] [Google Scholar]
- 65.Oakes JM, Scadeng M, Breen EC, Prisk GK, Darquenne C. Regional distribution of aerosol deposition in rat lungs using magnetic resonance imaging. Ann Biomed Eng. 2013;41(5):967–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Darquenne C, Borja MG, Oakes JM, Breen EC, Olfert IM, Scadeng M, et al. Increase in relative deposition of fine particles in the rat lung periphery in the absence of gravity. J Appl Physiol (1985). 2014;117(8):880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


