Abstract
The combination of intellectual, communicative, and motor deficits limit the use of standardized behavioral assessments of cognition in individuals with Angelman syndrome (AS). The current study is the first to objectively evaluate learning and memory in AS using auditory event-related potentials (ERP) during passive exposure to spoken stimuli. Fifteen nonverbal individuals with the deletion subtype of AS (age 4–45 years) completed the auditory incidental memory paradigm. Auditory ERPs were recorded in response to a sequence of unfamiliar nonwords, in which one randomly selected stimulus was repeated multiple times and the rest were presented once. Larger parietal responses within 200–500ms for the repeated nonword compared to novel distracters were associated with caregiver reports of more adaptive communication skills. These findings demonstrate good tolerability of ERP procedures (94% success rate) and indicate that persons with AS can acquire new information following repeated auditory exposure, even in the absence of explicit memorization instructions. Strong associations between the caregiver reports of adaptive functioning and neural indices of auditory learning and memory support the utility of brain-based measures for objectively evaluating higher-order information processing in nonverbal persons with neurodevelopmental disorders.
Keywords: Angelman syndrome, auditory, ERP, memory, nonverbal
1. Introduction
Angelman syndrome (AS) is a rare neurodevelopmental disorder with prevalence rates of 1 in 10,000 to 20,000 (Buckley et al., 1998; Clayton-Smith, 1992; Petersen et al., 1995; Steffenburg et al., 1996; Williams et al., 2010). It is caused by loss of function of the ubiquitin–protein ligase E3A (UBE3A) gene, typically expressed on the maternal chromosome 15 (Buiting, 2010; Kishino et al., 1997). The most common genetic mechanism identified in up to 75% of the cases is the deletion of the 15q11–13 region (Nicholls, 1994), while paternal uniparental disomy, imprinting defects, and UBE3A mutations are more rare (Williams et al., 2006). Behavioral characteristics of AS include generally happy demeanor, frequent laughter, hyperactivity, and motor stereotypies (Williams et al., 2006; Williams, 2010).
When evaluated using standardized measures of cognition and adaptive functioning, individuals with AS typically score in the range of severe functional impairment (Andersen et al., 2001; Peters et al., 2004a; Thompson & Bolton, 2003). Persons with the deletion subtype are the most severely affected (Clayton-Smith & Laan, 2003; Gentile et al., 2010). Communication is a major area of weakness in AS (Jolleff & Ryan, 1993), with expressive language abilities more impaired than receptive language (Trillingsgaard & Ostergaard, 2004). Most individuals with AS do not develop speech, although some may produce several single words (Clayton-Smith, 1993; Andersen et al., 2001), and very few speak in simple sentences (Nazlican et al., 2004). Receptive communication skills in Angelman syndrome are also significantly delayed (Andersen et al., 2001; Joleff & Ryan, 1993; Micheletti et al., 2016) but show some improvement with age (Mertz et al., 2014). In addition to communication and adaptive behavior difficulties (Peters et al., 2012), persons with AS may also exhibit social deficits (Penner et al., 1993), and many meet the diagnostic criteria for autism (Trillingsgaard & Ostergaard, 2004; Peters et al., 2004b; Hogart et al., 2010).
While treatments for medical and behavioral concerns of AS, such as seizures and motor skills, are available, currently, very few interventions target the cognitive features of the disorder (Hong et al., 2017). This could be due in part to the lack of effective treatment outcome measures. Standard developmental assessments (e.g., Bayley Scales of Infant Development) are challenging to administer in AS due to the combination of intellectual, communication, and motor deficits (Bird, 2014). As a result, raw scores often represent less than 25% of the possible points (Grieco et al., 2014), and scaled scores may not exceed the lowest possible values (i.e., a floor effect) (Grieco et al., 2016). This may underestimate the abilities of persons with AS and also make it difficult to detect improvements after an intervention.
Biological and neurophysiological markers of cognition in AS could offer a new path to the development of more effective treatment outcome measures. Novel assessments are beginning to emerge, such as the quantification of communicative behaviors from recorded play sessions (Grieco et al., 2016) or social eye tracking and pupillometry metrics (Hong et al., 2017). Brain-based data offer another promising way to document information processing in AS. To date, a number of neuroimaging studies reported mild cortical atrophy, delayed myelination, and white matter abnormalities (Castro-Cago et al., 2010; Harting et al., 2009), particularly in pathways supporting language and cognitive functioning (Peters et al., 2011; Tiwari et al., 2012; Wilson et al., 2011). However, this work was done primarily in sedated patients, limiting the ability to offer a functional interpretation of the observed structural differences. UBE3A deficiency impairs synapse formation and experience-dependent synapse remodeling (Buiting et al., 2016). This can become evident in neural responses associated with learning. Indeed, studies in several animal models of AS consistently demonstrated that maternal UBE3A is preferentially expressed in cortex and hippocampus (see Jana, 2012 for review), and its loss is associated with reduced experience-dependent neural plasticity and poor performance on learning and memory tasks (Jiang et al., 1998, 2010; Mardirossian et al., 2009; Miura et al., 2002; Yashiro et al., 2009).
Translating work from mouse models to humans can be challenging, but it is a critical step toward understanding the mechanisms underlying AS (Sell & Margolis, 2015). Recently, Sidorov and colleagues (2017) demonstrated that delta rhythmicity, a quantitative measure derived from clinical EEGs, is similarly altered both in animal models and in humans with AS. However, the connection between delta characteristics and behavioral or cognitive features of AS is currently unknown.
The goal of the current study was to examine auditory learning and memory in nonverbal persons with AS using event-related potentials (ERPs), or stimulus-locked EEG. ERPs can measure cortical activity in response to an external (e.g., sound) or internal (e.g., attention shift) event with millisecond-level precision, making it a promising approach to evaluating multiple stages of information processing in AS. The choice of the auditory modality was motivated by the ubiquitous presence of spoken language in daily life, and the importance of auditory processing for adaptive communication and social functioning. Additionally, auditory stimuli place minimal attentional demands on the participants (a sound will be heard even if the person is not actively attending to it), and passive listening paradigms avoid the confounds associated with comprehension of the task instructions.
Auditory ERPs during passive listening to a variety of spoken stimuli, from single syllables to complete words, have been previously used to document typical and atypical language functioning, make predictions regarding developmental outcomes, and to evaluate treatment effects in other neurodevelopmental disorders (Key et al., 2016; Maitre et al., 2013, 2014; Sandbank et al., 2017; Yoder et al., 2013). Recently, we successfully used auditory ERPs in response to known or familiarized through repetition stimuli compared to novel distracters to document learning and memory in nonverbal individuals with other neurogenetic disorders, such as MECP2 duplication and Rett syndromes (Peters et al., 2015, 2017, 2018), the latter sharing several phenotypic features with AS. In particular, our auditory incidental memory paradigm allows to evaluate the extent of participant’s spontaneous engagement with auditory information. When presented with novel spoken inputs (e.g., a series of nonwords), greater attention to the stimuli would result in detection of repetitions within the otherwise highly varied sequence even in the absence of explicit task instructions. Previously, event-related fMRI data in healthy adults during passive exposure revealed differential activation for repeated vs. novel stimuli in bilateral anterior hippocampus as well as in a number of frontal, parietal and temporal cortical regions (Jessen et al., 2002). While ERPs cannot resolve hippocampal signal specifically, they are sensitive to cortical differences associated with stimulus recognition. Similar to the results of studies examining recognition and recall of known vs. novel stimuli following active memorization (Curran, 2004; Rugg et al., 2000, Wilding, 2000), incidental memory for the repeated stimuli was reflected in more positive parietal amplitudes within 200–500ms after the stimulus onset. Previously, we demonstrated that during a passive paradigm, this response develops only for personally salient stimuli and can be observed both in typical and atypical populations (Key & Corbett, 2014; Key & Dykens, 2016, 2017). Significant correlations between the ERP marker of incidental memory for the repeated stimulus and the participants’ behavioral characteristics (e.g., Aberrant Behavior Checklist scores) further support the idea that ERPs can be a valid direct measure of the processes previously assessed using mainly indirect, informant-based tools (Peters et al., 2018; Key & Corbett, 2014).
In this study, we predicted that auditory ERPs will be feasible in unsedated persons with AS, because modern EEG acquisition systems allow for fast and noninvasive electrode application, and these data are more tolerant of movement compared to other neuroimaging techniques. We anticipated that participants with AS whose caregivers report higher levels of communicative functioning will develop stronger incidental memory following spontaneous recognition of stimulus repetition, one of the most basic forms of learning. Based on our prior findings, we hypothesized that incidental memory would be reflected by more positive parietal ERP amplitudes within 200–500ms after stimulus onset.
2. Method
2.1. Participants
Fifteen individuals with the deletion subtype of AS (four females; M age = 14.42 years, SD = 11.02 years, range 4–45 years) participated in the study. One additional participant was excluded because of insufficient amount of artifact-free EEG data. Due to the level of intellectual and motor disability, IQ or handedness could not be directly and reliably assessed using standardized behavioral measures.
A chronological-age comparison group included 15 typically developing individuals (8 females, M age = 15.11 years, SD = 11.52 years, range 4–46 years) selected as the closest age match to the AS group from a larger pool of participants who completed the same task as a part of other studies on auditory processing. The groups were not significantly different in age, t(28)=0.168, p=.867. Typical development status was confirmed through caregiver or self-report indicating absence of learning disabilities and no history of neurological disease or trauma.
Typical adults and parents/guardians of children and of participants with AS provided written informed consent, all typical children provided their assent for the study procedures. Parents/guardians of all participants with AS remained present throughout the test session to help identify any dissenting behaviors. The study was conducted with approval from the Institutional Review Board of Vanderbilt University Medical Center.
2.2. Behavioral and electrophysiological assessments
2.2.1. Measures of adaptive functioning.
Caregivers of participants with AS completed the Parent/Caregiver Rating Form of the Vineland Adaptive Behavior Scales-3 (VABS-3; Sparrow et al., 2016). The domains of Communication and Socialization were selected a priori because they measure the strongest areas of functioning in AS and are also most likely to be affected by the auditory memory ability. These domains yield standardized scores with a mean of 100 and a standard deviation of 15. The scores can also be interpreted in the terms of age equivalence.
2.2.2. Auditory Incidental Memory task.
Stimuli included 51 novel single-syllable nonwords (M duration = 694.50 ms, SD=127.78 ms) spoken by an adult female native English speaker, digitized at 44,100 Hz, and equalized in loudness. The nonwords were delivered in positive tone using child-directed speech. One of the nonwords was randomly selected to be repeated 50 times throughout the session. The remaining stimuli were presented once, yielding a unique set of 50 repeated and 50 single-presentation trials for each participant. The entire task included 100 trials and lasted approximately 5 minutes. All stimuli were presented in random order with a varied inter-stimulus interval of 1300–1600 ms to prevent habituation and development of trial onset expectations.
2.2.3. ERP Data Acquisition
All testing was completed during a single visit. A high-density array of 128 Ag/AgCl electrodes embedded in soft sponges (Geodesic Sensor Net; EGI, Inc., Eugene, OR) was used to record the ERPs. Electrode impedance levels were at or below 50 kOhm. During testing data were sampled at 250Hz with the filters set to .1–100 Hz. All electrodes were referred to vertex and then re-referenced offline to an average reference, the recommended reference for high-density arrays (Picton et al., 2000).
Stimulus presentation was controlled by E-prime (v.2.0, PST, Inc., Pittsburgh, PA). The auditory stimuli were delivered at 75 dB SPL from a speaker positioned behind the participant. In accordance with the passive listening design, the participants were informed that they would “hear the computer say some words”, but no explicit attention or behavioral responses were required. For typical children and individuals with AS, a parent/guardian and a researcher were present in the testing room to monitor participants’ behavior. Participants were tested while seated independently or in the parent’s lap. Two of the younger participants with AS were tested seated in their strollers with the safety belts on. Caregivers assisted with keeping the participants from touching the electrodes and minimizing motor artifacts due to gross body movements. Participants had continuous access to their preferred comfort or entertainment items (e.g., sippy cups, snacks, toys, etc.). A TV show presented without sound served as the visual distractor. If participants became excessively restless, stimulus presentation was suspended until the source of motor artifacts was addressed.
2.3. Data Analyses
Collected EEGs were filtered using a 30Hz low-pass filter, segmented on stimulus onset to include a 100ms prestimulus baseline and a 900ms post-stimulus interval, and screened for ocular and movement artifacts using an automated algorithm in NetStation followed by a manual review. The automated artifact detection criteria were set as follows: for the eye channels, voltage in excess of 140 μV was interpreted as an eye blink and voltage above 55 μV was considered to reflect eye movements. Any channel with voltage exceeding 200 μV was marked as bad. Data for electrodes with poor signal quality within a trial were reconstructed using spherical spline interpolation procedures (Perrin et al., 1989). Trials with ocular artifacts or containing more than 20 electrodes with poor signal quality (15% of the array) were excluded from analysis.
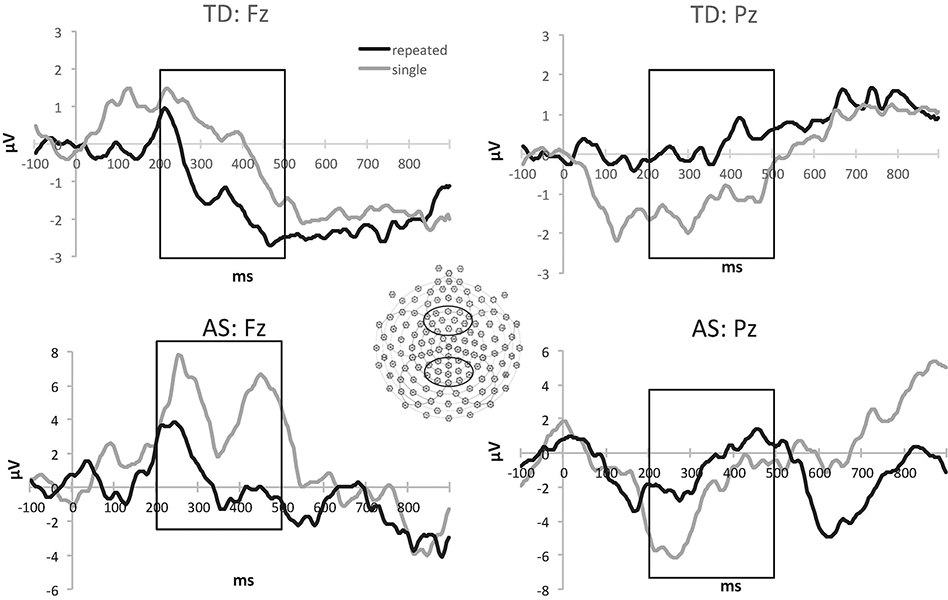
Following artifact screening, individual ERPs for each stimulus category were averaged, re-referenced to an average reference, and baseline-corrected by subtracting the average microvolt value across the 100ms prestimulus interval from the post-stimulus segment. Data from the electrodes corresponding to midline parietal and frontal regions (Figure 1) were used in the remaining statistical analyses. Averaging data over several spatially contiguous locations (frontal: 4, 5, 6, 11, 23, 13, 20, 21, 25, 113, 119, 124; parietal: 53, 54, 60, 61, 62, 67, 68, 73, 78, 79, 80, 86, 87) improved signal-to-noise ratio. The frontal electrode cluster was included for exploratory purposes because prior active memorization studies (e.g., Curran & Cleary, 2003) reported familiarity-related effects for the negative peak around 400 ms (FN400) in addition to the parietal effects associated with stimulus recall.
Figure 1.
Average ERP waveforms at frontal (Fz) and parietal (Pz) midline clusters in response to repeated and single presentations of novel nonwords in participants with Angelman syndrome (AS) and typical comparison (TD) group. Positive amplitudes are plotted up. The rectangular marquee identifies the time window used in the analysis.
Mean amplitudes within 200–500ms after stimulus onset served as the dependent variables in a repeated measures analysis of variance (rmANOVA) with Stimulus (2: single, repeated) x Electrode cluster (2: frontal, parietal) x Group (2: AS, typical) within-subject factors and Age as a covariate. Relative to the peak amplitude, mean amplitude metric is less susceptible to the effects of noise or variability in the number of artifact-free trials contributing to the averaged data (Luck, 2005). These spatial and temporal parameters were selected a priori based on our previous work examining incidental memory in persons with disabilities (Key & Corbett, 2014; Key & Dykens, 2016, 2017; Peters et al., 2018) and confirmed by visual inspection of the grand-averaged data. Significant stimulus-related effects were followed by two-tailed paired t-tests to test whether the repeated stimulus elicited more positive parietal amplitudes compared to the nonwords presented once. The association between the brain indices of incidental memory in AS (repeated-single stimulus amplitude difference) and caregiver reports of communicative and social functioning (standard scores on the corresponding domains of VABS-3), was evaluated using correlations.
3. Results
3.1. Caregiver reports of adaptive functioning
Families of three participants did not return the VABS-3 questionnaires. The remaining 12 participants achieved higher standard scores for the socialization than communication domain, 39.42 vs. 25.83, t(11)=4.388, p=.001, d=1.27, although on average, their scores fell in the severely affected range (see Table 1 for detailed sample categorization).
Table 1.
Caregiver categorization of communicative and social skills in participants with Angelman syndrome (n=12) according to Vineland Adaptive Behavior Scales-3.
| Domain/Subdomain | Score | M | SD | Range |
|---|---|---|---|---|
| Communication | Standard | 25.83 | 9.81 | 20–50 |
| Receptive | Raw | 37.17 | 17.69 | 13–68 |
| Expressive | Raw | 17.92 | 10.69 | 6–43 |
| Written | Raw | 5.75 | 5.34 | 0–16 |
| Socialization | Standard | 39.42 | 16.78 | 20–71 |
| Interpersonal Relationships | Raw | 30.58 | 8.31 | 17–45 |
| Play and Leisure | Raw | 18.58 | 8.51 | 4–30 |
| Coping skills | Raw | 17.17 | 7.06 | 8–28 |
3.2. Auditory Incidental Memory task
Fifteen of sixteen subjects with AS (94%) provided usable ERP data. Their trial retention rates were comparable across conditions (single nonwords: M=14.27, SD=3.81; repeated nonword: M=14.07, SD=4.04; p>.05). The number of artifact-free trials within the typical group was also comparable between the conditions (single nonwords: M=19.20, SD=6.33; repeated nonword: M=20.27, SD=6.90; p>.05), but higher than in the AS group (single nonword: F(1,28)=6.696, p=.015; repeated nonword: F(1,28)=9.011, p=.006). As would be expected, the number of retained trials was positively associated with age of the participants (single: r(29)=.386, p=.035; repeated: r(29)=.376, p=.040). Consistent with prior reports (Vendrame et al., 2012), qualitative review of the data noted strong presence of delta waves in the auditory ERPs recorded in the AS group, but not in the typical participants.
The ANOVA identified a Stimulus x Electrode interaction, F(1,27)=11.183, p=.002,ηp2=.293. No other main effects or interactions reached significance. Follow-up examination of stimulus discrimination effects identified the expected more positive parietal amplitudes in response to the repeated nonwords (M=−0.24 μV, SD=3.55) compared to the stimuli presented once (M=−2.05 μV, SD=3.39), t(29)=3.022, p=.005, d=.55. The pattern of condition differences was reversed at the frontal sites, t(29)=2.145, p=.04, d=.39 (Figure 1).
To confirm that these findings were not driven mainly by one of the participant groups, we examined condition differences within individuals with AS and typical peers separately. In the AS group, more positive amplitudes for the repeated (M=−0.66 μV, SD=4.66) than single (M=−2.80 μV, SD=4.44) stimuli were present at the parietal cluster, t(14)=2.115, p=.05, d=.55, but no significant differences at the frontal locations (p=.13). In the typical comparison group, repeated (M=.18 μV, SD=1.99) compared to single stimuli (M=−1.30 μV, SD=1.69) elicited more positive amplitudes at the parietal cluster, t(14)=2.217, p=.04, d=.57, while these differences were reversed at the frontal locations, t(14)=2.802, p=.014, d=.72 (−1.24 vs. 0.22 μV).
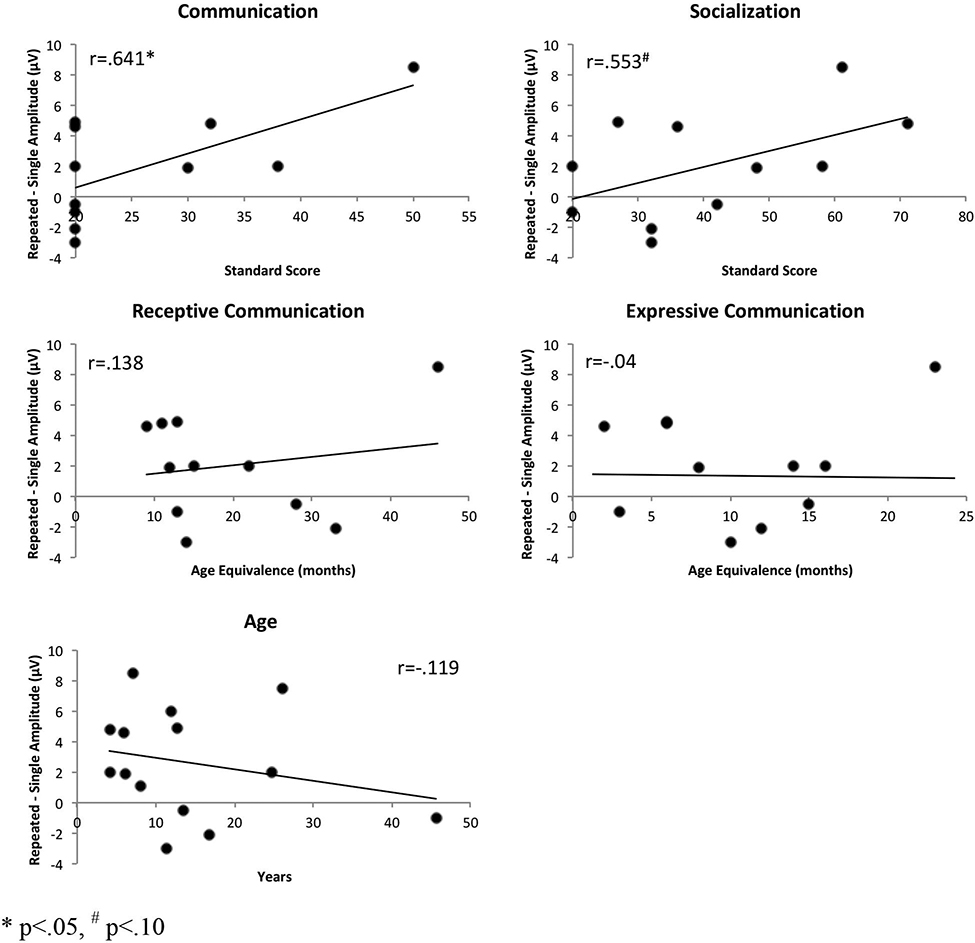
The correlational analysis examining brain-caregiver report associations within the AS group revealed that more positive parietal amplitudes to repeated than single nonwords (i.e., better incidental memory) were associated with higher standard scores on the Communication domain of VABS-3, r(11)=.641, p=.03 (Figure 2). A trend for a similar relationship with the standard scores for the Socialization domain, r(11)=.553, p=.06, was also observed. There were no significant correlations between the brain responses indexing incidental memory and chronological age or age equivalence scores for the receptive and expressive communication subdomains.
Figure 2.
Brain-behavior associations between the parietal ERP index of auditory incidental memory (amplitude difference between the repeated and single nonword) and caregiver reports of communication and socialization skills (VABS-3 standradr and age equivalence scores) and age in persons with Angelman syndrome.
* p<. 05, # p<. 10
4. Discussion
The purpose of this project was to evaluate auditory memory in nonverbal individuals with AS using auditory ERPs. Our results indicated that incidental memory for novel auditory speech stimuli following repeated exposure in a stream of continuously changing distracters can be detected in the ERPs of unsedated children and adults with the deletion subtype of AS. As hypothesized, the magnitude of the incidental memory response was positively associated with caregiver reports of communication abilities.
It is important to note that the passive auditory ERP paradigms in combination with a soft electrode array placed minimal demands on participants, and therefore we observed low attrition due to poor data quality: 94% (15/16) of subjects provided usable data for the 6-minute nonword learning task. For comparison, only 47% (8/17) yielded sufficient data in a 60-second social eye tracking and pupillometry paradigm (Hong et al., 2017). The difference in success rate is most likely due to the stimulus modality: visual tasks require higher levels of behavioral cooperation (e.g., looking at the screen) compared to the auditory paradigms, in which sound stimuli presented above the perceptual threshold will be processed in the brain even if attention is not directed toward them.
Analysis of the ERP responses indexing incidental memory following repeated exposure revealed that persons with AS actively engage with their auditory environment, attend to the spoken stimuli, and are capable of learning new information by hearing it during a brief period of time, even in the absence of concurrent visual cues or explicit instructions to remember the stimuli. The lack of significant group differences in ERP responses between participants with AS and typical development, with both groups demonstrating the expected pattern of more positive parietal amplitudes for repeated nonwords compared to the stimuli heard only once, further suggests that the basic neural process supporting experience-dependent learning and memory is functional in persons with AS.
The ERPs reflect neural activity in the cortex, which is among the brain areas where reductions of UBE3A are particularly noticeable in AS (Hayrapetyan et al., 2013; Jana, 2012). Previous auditory ERP studies in typical and atypical populations have reported that differences in cortical brain activity can be observed prior to and be predictive of differences in behavioral performance on standardized assessments (e.g., Kuhl et al., 2013; Maitre et al., 2013). Our ERP evidence of incidental auditory memory in AS and its associations with caregiver reports of communication (and socialization, albeit at trend level) skills, provide new support to the idea that measures of cortical activity during passive tasks targeting attention, learning, and memory have promise as an objective measure of neural functioning in nonverbal individuals. Additionally, unlike the standardized behavioral assessments, many of which were not designed for use in persons with intellectual and developmental disabilities, ERPs offer a direct way to capture individual differences without extensive demands for overt motor or verbal responses, positioning them to be a more sensitive marker of treatment outcomes. Furthermore, the passive nature of the task makes the results less susceptible to placebo effects, an important consideration for possible future use in intervention studies.
The results from this study also demonstrated the attention span and endurance limitations that should be taken into account when designing assessments for AS. Even though EEG/ERP measures are more tolerant of movement than other neuroimaging techniques, motor artifacts were a frequent concern in our study sample. The majority of the participants with AS, particularly the adolescents and adults, found the need to sit relatively still for 6 minutes to be the most challenging part of the testing experience. Thus, future studies need to carefully plan the length of the testing session and explore more effective management of motor artifacts through the use of seating options that offer upper body support (e.g., safety seats with a 5-point harness). Given the focus on cognitive processes, we believe that the use of more extensive restrains (e.g., inflatable papooses, etc.) may be counterproductive due to increased stress associated with the restriction of movement (Buynitsky & Mostofsky, 2009), which in turn may have a negative effect on cognitive performance (Kuhlmann et al., 2005; Schwabe & Wolf, 2010).
While reporting novel findings, this study has several limitations. Our sample included only individuals with the deletion subtype of AS, the most common as well as the most severely affected. Future studies will need to examine whether similar results would also be observed in persons with AS due to the uniparental disomy, imprinting deficits, or UBE3A mutations. Nevertheless, it is likely that the described procedures will be feasible regardless of the specific genetic subtype. Also, the number of participants, while average for a rare genetic disorder, was small, which limited statistical power to detect medium or small effect sizes and to examine potential effects of sex or epilepsy status. Based on our findings supporting the prediction that the parietal ERP response can serve as the index of incidental memory in AS, future studies may limit their analyses to a single electrode location (e.g., the parietal cluster) in order to reduce the number of variables and thus increase statistical power to detect additional effects (e.g., group differences). Related to the issue of a small sample size, the magnitude of the observed correlations should be interpreted with caution until further replication, because extreme values are more likely to be observed in smaller samples. Additionally, our participants represented a wide age range, from young children to adults. Previously published studies suggest that passive measures of incidental memory may be age-independent in children and young adults (Key & Corbett, 2014; Peters et al., 2017), however, spontaneous stimulus repetition detection is sensitive to aging-related cognitive decline in older participants (Key & Dykens, 2014). Little is known about the developmental trajectory over the lifespan in AS, and future studies in this population will need to examine possible age-related differences in auditory learning and memory.
In conclusion, our results demonstrate that measuring cortical brain responses associated with auditory learning and memory in nonverbal children and adults with AS is possible and can provide objective information complementary to the caregiver reports of adaptive functioning. Without the requirement of behavioral responses, the use of auditory stimuli during brief test sessions made the procedures suitable for a wide range of ages and ability levels. Thus, our findings support the utility of brain-based measures for evaluating information processing in nonverbal persons with neurodevelopmental disorders.
Highlights.
We used ERPs to investigate memory in nonverbal persons with Angelman syndrome.
Repeated nonwords evoked a more positive response in the 200–500 ms window.
ERP marker of incidental memory correlated with caregiver reports of functioning.
ERPs can help assess cognition in nonverbal persons with neurodevelopmental disorders.
Acknowledgments
We would like to thank the participants and their families for their support of the study. We are grateful to Dr. Evon Lee for guidance with interpretation of behavioral data. Requests for reprints should be sent to: Alexandra P. Key, Vanderbilt Kennedy Center, 230 Appleton Place, Peabody Box 74, Nashville, TN 37203. E-mail: sasha.key@vanderbilt.edu.
Funding
This research was supported in part by the pilot grant from the Angelman Syndrome Foundation and the Foundation for Angelman Syndrome Therapeutics, the International Rett Syndrome Foundation grant #3106, as well as by the EKS NICHD grant U54HD083211 (Vanderbilt Kennedy Center), NIDCD T35DC008763, and NCATS/NIH UL1TR000445. The content is solely the responsibility of the authors and does not necessarily represent the official views of funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
None.
References
- Andersen WH, Rasmussen RK, & Strømme P (2001). Levels of cognitive and linguistic development in Angelman syndrome: A study of 20 children. Logopedics Phoniatrics Vocology, 26(1), 2–9. [PubMed] [Google Scholar]
- Bird L (2014). Angelman syndrome: review of clinical and molecular aspects. The Application of Clinical Genetics, 93–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonati MT, Russo S, Finelli P, Valsecchi MR, Cogliati F, Cavalleri F, et al. (2007).Evaluation of autism traits in Angelman syndrome: a resource to unfold autism genes. Neurogenetics, 8(3), 169–178. [DOI] [PubMed] [Google Scholar]
- Buckley RH, Dinno N, Weber P. (1998). Angelman syndrome: are the estimates too low? American Journal of Medical Genetics, 80, 385–390. [DOI] [PubMed] [Google Scholar]
- Buiting K, Williams C, & Horsthemke B (2016). Angelman syndrome - insights into a rare neurogenetic disorder. Nature, 12(10), 584–593. [DOI] [PubMed] [Google Scholar]
- Buiting K (2010). Prader-Willi syndrome and Angelman syndrome. American Journal of Medical Genetics Part A, 154C(3), 365–376. [DOI] [PubMed] [Google Scholar]
- Buynitsky T, & Mostofsky DI (2009). Restraint stress in biobehavioral research: recent developments. Neuroscience & Biobehavioral Reviews, 33(7), 1089–1098. [DOI] [PubMed] [Google Scholar]
- Castro-Gago M, Gomez-Lado C, Eiris-Punal J, & Rodriguez-Mugico VM (2010). Abnormal myelination in Angelman syndrome. European Journal of Paediatric Neurology. 14(3):292. [DOI] [PubMed] [Google Scholar]
- Clayton-Smith J, & Pembrey ME (1992). Angelman syndrome. Journal of Medical Genetics, 29(6), 412–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton-Smith J (1993). Clinical research on Angelman syndrome in the United Kingdom: observations on 82 affected individuals. American Journal of Medical Genetics, 46, 12–15. [DOI] [PubMed] [Google Scholar]
- Clayton-Smith J, & Laan L (2003). Angelman syndrome: a review of the clinical and genetic aspects. Journal of Medical Genetics, 40(2), 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T (2004). Effects of attention and confidence on the hypothesized ERP correlates of recollection and familiarity. Neuropsychologia, 42(8), 1088–106 [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, & Brown E (1998). Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders, 28(6), 479–485. [DOI] [PubMed] [Google Scholar]
- Gentile JK, Tan WH, Horowitz LT, Bacino CA, Skinner SA, Barbieri-Welge R, … & Sahoo T (2010). A neurodevelopmental survey of Angelman syndrome with genotype-phenotype correlations. Journal of Developmental and Behavioral Pediatrics, 31, 592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco JC, Ciarlone SL, Gieron-Korthals M, Schoenberg MR, Smith AG, Philpot RM & Weeber EJ (2014) An open-label pilot trial of minocycline in children as a treatment for Angelman syndrome. BMC Neurology 14, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco JC, Bahr RH, Schoenberg MR, Conover L, Mackie LN, & Weeber EJ (2016). Quantitative measurement of communication ability in children with Angelman syndrome. Journal of Applied Research in Intellectual Disabilities, 17, 75–10. [DOI] [PubMed] [Google Scholar]
- Harting I, Seitz A, Rating D, Sartor K, Zschocke J, Janssen B, … & Wolf N (2009). Abnormal myelination in Angelman syndrome. European Journal of Paediatric Neurology, 13(3):271–6. [DOI] [PubMed] [Google Scholar]
- Hayrapetyan V, Castro S, Sukharnikova T, Yu C, Cao X, Jiang Y-H, & Yin HH (2013). Region-specific impairments in striatal synaptic transmission and impaired instrumental learning in a mouse model of Angelman syndrome. European Journal of Neuroscience, 39(6), 1018–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogart A, Wu D, LaSalle JM, & Schanen NC (2010). The comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13. Neurobiology of Disease, 38(2), 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong MP, Guilfoyle JL, Mooney LN, Wink LK, Pedapati EV, Shaffer RC, … & Erickson C (2017). Eye gaze and pupillary response in Angelman syndrome. Research in Developmental Disabilities, 68, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana NR (2012). Understanding the pathogenesis of Angelman syndrome through animal models. Neural Plasticity, 2012(2), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedele KB (2007). The overlapping spectrum of Rett and Angelman syndromes: A clinical review. Seminars in Pediatric Neurology, 14(3), 108–117. [DOI] [PubMed] [Google Scholar]
- Jessen F, Manka C, Scheef L, Granath D-O, Schild HH, & Heun R (2002). Novelty detection and repetition suppression in a passive picture viewing task: A possible approach for the evaluation of neuropsychiatric disorders. Human Brain Mapping, 17(4), 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, … & Beaudet A (1998). Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron, 21(4), 799–811. [DOI] [PubMed] [Google Scholar]
- Jiang Y-H, Pan Y, Zhu L, Landa L, Yoo J, Spencer C, … & Beaudet A (2010). Altered ultrasonic vocalization and impaired learning and memory in Angelman syndrome mouse model with a large maternal deletion from Ube3a to Gabrb3. PLoS ONE, 5(8), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolleff N, & Ryan MM (1993). Communication development in Angelman’s syndrome. Archives of Disease in Childhood, 69(1), 148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino T, Lalande M & Wagstaff J UBE3A/E6-AP mutations cause Angelman syndrome. Nature Genetics, 15, 70–73 (1997). [DOI] [PubMed] [Google Scholar]
- Key AP, & Dykens EM (2017). Incidental memory for faces in children with different genetic subtypes of Prader-Willi syndrome. Social Cognitive and Affective Neuroscience, 12(6), 918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, & Dykens EM (2014). Event-related potential index of age-related differences in memory processes in adults with Down syndrome. Neurobiology of Aging, 35(1), 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, & Dykens EM (2016). Face repetition detection and social interest: An ERP study in adults with and without Williams syndrome. Social Neuroscience, 11(6), 652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, & Corbett BA (2014). ERP responses to face repetition during passive viewing: A nonverbal measure of social motivation in children with autism and typical development. Developmental Neuropsychology, 39(6), 474–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, Jones D, & Peters SU (2016). Response to own name in children: ERP study of auditory social information processing. Biological Psychology, 119, 210–215. 10.1016/j.biopsycho.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, Yoder PJ, & Stone WL (2016). Consonant differentiation mediates the discrepancy between non-verbal and verbal abilities in children with ASD. Journal of Intellectual Disability Research, 60(5), 478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, Munson J, Estes A, & Dawson G (2013). Brain responses to words in 2-year-olds with autism predict developmental outcomes at age 6. PLoS ONE, 8(5), e64967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, & Wolf OT (2005). Impaired memory retrieval after psychosocial stress in healthy young men. Journal of Neuroscience, 25(11), 2977–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S (2005). An introduction to the event-related potential technique. MIT Press: Cambridge, MA. [Google Scholar]
- Maitre NL, Henderson G, Gogliotti S, Pearson J, Simmons A, Wang L, … & Key A (2014). Feasibility of event-related potential methodology to evaluate changes in cortical processing after rehabilitation in children with cerebral palsy: A pilot study. Journal of Clinical and Experimental Neuropsychology, 36(7), 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre NL, Lambert WE, Aschner JL, & Key AP (2013). Cortical speech sound differentiation in the neonatal intensive care unit predicts cognitive and language development in the first 2 years of life. Developmental Medicine & Child Neurology, 55(9), 834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardirossian S, Rampon C, Salvert D, Fort P & Sarda N (2009). Impaired hippocampal plasticity and altered neurogenesis in adult Ube3a maternal deficient mouse model for Angelman syndrome. Experimental Neurology, 220(2), 341–348. [DOI] [PubMed] [Google Scholar]
- Mertz LGB, Thaulov P, Trillingsgaard A, Christensen R, Vogel I, Hertz JM, & Østergaard JR (2014). Neurodevelopmental outcome in Angelman syndrome: Genotype–phenotype correlations. Research in Developmental Disabilities, 35(7), 1742–1747. [DOI] [PubMed] [Google Scholar]
- Micheletti S, Palestra F, Martelli P, Accorsi P, Galli J, Giordano L, et al. (2016). Neurodevelopmental profile in Angelman syndrome: more than low intelligence quotient. Italian Journal of Pediatrics, 42, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Kishino T, Li E, Webber H, Dikkes P, Holmes GL, & Wagstaff J (2002). Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal deficient mice. Neurobiology of Disease, 9(2), 149–159. [DOI] [PubMed] [Google Scholar]
- Nazlican H, Zeschnigk M, Claussen U, Michel S, Boehringer S, Gillessen-Kaesbach G, … & Horsthemke B (2004). Somatic mosaicism in patients with Angelman syndrome and an imprinting defect. Human Molecular Genetics, 13:2547–2555. [DOI] [PubMed] [Google Scholar]
- Nicholls RD (1994). Recombination model for generation of a submicroscopic deletion in familial Angelman syndrome. Human Molecular Genetics, 3: 9–11. [DOI] [PubMed] [Google Scholar]
- Penner KA, Johnston J, Faircloth BH, Irish P & Williams CA (1993). Communication, cognition, and social interaction in the Angelman syndrome. American Journal of Medical Genetics, 46, 34–39. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, & Echallier JF (1989). Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology, 72:184–187. [DOI] [PubMed] [Google Scholar]
- Peters S, Goddard-Finegold J, Beaudet AL, Madduri N, Turcich M, & Bacino CA (2004a) Cognitive and adaptive behavior profiles of children with Angelman syndrome. American Journal of Medical Genetics Part A.;128A(2):110–113. [DOI] [PubMed] [Google Scholar]
- Peters S, Beaudet AL, Madduri N, & Bacino CA (2004b). Autism in Angelman syndrome: implications for autism research. Clinical Genetics, 66(6): 530–536. [DOI] [PubMed] [Google Scholar]
- Peters S, Gordon RL, & Key AP (2015). Induced gamma oscillations differentiate familiar and novel voices in children with MECP2 duplication and Rett syndromes. Journal of Child Neurology, 30(2), 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Horowitz L, Barbieri-Welge R, Taylor JL, & Hundley RJ. (2012). Longitudinal follow-up of autism spectrum features and sensory behaviors in Angelman syndrome by deletion class. Journal of Child Psychology and Psychiatry, 53(2), 152–159. [DOI] [PubMed] [Google Scholar]
- Peters S, Jones D, & Key A (2018). 22. Non-Word Memory in Rett Syndrome and Rett-Related Disorders. Biological Psychiatry, 83(Supplement), S9. [Google Scholar]
- Peters S, Katzenstein A, Jones D, & Key AP (2017). Distinguishing response to names in Rett and MECP2 Duplication syndrome: An ERP study of auditory social information processing. Brain Research, 1675, 71–77. [DOI] [PubMed] [Google Scholar]
- Peters S, Kaufmann WE, Bacino CA, Anderson AW, Adapa P, Chu Z, … & Wilde E (2011). Alterations in white matter pathways in Angelman syndrome. Developmental Medicine & Child Neurology, 53(4), 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MB, Brondum-Nielsen K, Hansen LK, & Wulff K (1995). Clinical, cytogenetic, and molecular diagnosis of Angelman syndrome: estimated prevalence rate in a Danish county. American Journal of Medical Genetics, 60, 261–262. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, … & Taylor M (2000). Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology, 37(2), 127–152. [PubMed] [Google Scholar]
- Rugg MD, Allan K, & Birch CS (2000). Electrophysiological evidence for the modulation of retrieval orientation by depth of study processing. Journal of Cognitive Neuroscience,12(4), 664–678. [DOI] [PubMed] [Google Scholar]
- Sandbank M, Yoder P, & Key AP (2017). Word processing in children with autism spectrum disorders: Evidence from event-related potentials. Journal of Speech, Language, and Hearing Research, 60(12), 3441–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, & Wolf OT (2010). Learning under stress impairs memory formation. Neurobiology of Learning and Memory, 93(2), 183–188. [DOI] [PubMed] [Google Scholar]
- Sell GL, & Margolis SS (2015). From UBE3A to Angelman syndrome: a substrate perspective. Frontiers in Neuroscience, 9(18), 75–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorov MS, Deck GM, Dolatshahi M, Thibert RL, Bird LM, Chu CJ, & Philpot BD (2017). Delta rhythmicity is a reliable EEG biomarker in Angelman syndrome: A parallel mouse and human analysis, Frontiers in Neuroscience, 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S, Cicchetti D, & Saulnier C (2016). Vineland-3: Vineland adaptive behavior scales – Third Edition Pearson: Bloomington, MN. [Google Scholar]
- Steffenburg S, Gillberg CL, Steffenburg U, & Kyllerman M (1996). Autism in Angelman syndrome: a population-based study. Pediatric Neurology, 14(2), 131–136. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, & Bolton PF (2003). Angelman syndrome in an individual with a small SMC(15) and paternal uniparental disomy: A case report with reference to the assessment of cognitive functioning and autistic symptomatology. Journal of Autism and Developmental Disorders, 33, 171–176. [DOI] [PubMed] [Google Scholar]
- Tiwari VN, Jeong J-W, Wilson BJ, Behen ME, Chugani HT, & Sundaram SK (2012). Relationship between aberrant brain connectivity and clinical features in Angelman Syndrome: A new method using tract based spatial statistics of DTI color-coded orientation maps. NeuroImage, 59(1), 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillingsgaard A, & ØStergaard JR. (2004). Autism in Angelman syndrome: an exploration of comorbidity. Autism, 8(2), 163–174. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Loddenkemper T, Zarowski M, Gregas M, Shuhaiber H & Sarco DP (2012). Analysis of EEG patterns and genotypes in patients with Angelman syndrome. Epilepsy & Behavior, 23, 261–265. [DOI] [PubMed] [Google Scholar]
- Wilding EL (2000). In what way does the parietal ERP old/new effect index recollection? International Journal of Psychophysiology, 35(1), 81–87. [DOI] [PubMed] [Google Scholar]
- Williams CA, Beaudet AL, Clayton-Smith J, Knoll JH, Kyllerman M, Laan LA, … & Wagstaff L (2006). Angelman syndrome 2005: Updated consensus for diagnostic criteria. American Journal of Medical Genetics Part A, 140A(5), 413–418. [DOI] [PubMed] [Google Scholar]
- Williams CA Driscoll DJ, & Dagli AI (2010). Clinical and genetic aspects of Angelman syndrome. Genitourin. Medicine, 12, 385–395 [DOI] [PubMed] [Google Scholar]
- Wilson BJ, Sundaram SK, Huq AHM, Jeong J-W, Halverson SR, Behen ME, … & Chugani H (2011). Abnormal language pathway in children with Angelman syndrome. Pediatric Neurology, 44(5), 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R, … & Philpot B (2009). Ube3a is required for experience-dependent maturation of the neocortex. Nature Neuroscience, 12(6), 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder PJ, Molfese D, Murray MM, & Key AP (2013). Normative topographic ERP analyses of speed of speech processing and grammar before and after grammatical treatment. Developmental Neuropsychology, 38(8), 514–533. [DOI] [PMC free article] [PubMed] [Google Scholar]