Abstract
Objectives:
The ventrolateral prefrontal cortex (vlPFC) has been speculated to play an important role in complex processes that allow emotional factors to influence human cognition. Accumulating evidence from human neuroimaging studies, in conjunction with studies of patients with lesions and animal models shed light on the role of the vlPFC in emotion regulation (ER). This review aims to discuss and integrate recent findings related to vlPFC’s role in ER in the context of aging, drawing from diverse sources, and suggest future directions for research utilizing Transcranial Magnetic Stimulation (TMS).
Methods/Design:
We summarize findings from the existing literature investigating the neural basis of frontal-lobe mediated ER and then highlight major findings from recent studies directly comparing healthy younger and older adult groups. We conclude by pointing to unaddressed questions worth pursuing in future research.
Results & Discussion:
We propose future research directions utilizing TMS to answer key unaddressed questions. Moreover, we discuss the potential advantages, challenges, and limitations of using TMS as a complement to the existing neuroimaging methods in ER.
Keywords: Emotion Regulation, Ventral Prefrontal Cortex, Transcranial Magnetic Stimulation, Neuroimaging
This manuscript provides a brief overview of findings from the existing literature investigating the neural basis of frontal lobe-mediated emotion regulation (ER) and places these findings within the broader context of age-related differences in the behavioral and neural bases of ER. We first describe the adaptive nature of ER for mental health and well-being in the general population, as well as specifically in older adults (OA), and further discuss what is known about the ventrolateral prefrontal cortex (vlPFC)’s involvement in ER. We then highlight key findings from recent studies directly comparing healthy younger adult (YA) and OA groups and point to unaddressed questions for future research. We pay particular attention to the benefit of utilizing Transcranial Magnetic Stimulation (TMS) as a complement to the existing neuroimaging literature.
Emotion Regulation
ER is defined as processes that individuals utilize to change the trajectory (e.g., type, intensity, and time course) of their emotional experience.1, 2 These processes have been described as “multi-componential”, with a collection of dynamic progressions transpiring over time.3 The ER process begins with a specific psychologically relevant situation (e.g., a spider is on my arm). Then, individuals devote attentional resources to assess the situation (i.e., appraisal). Subsequently, initial emotional responses are engendered by one’s interpretation or appraisal of the situation the individual experiences. After appraising a situation, ER can be employed as a functionally adaptive process that individuals utilize for the purpose of changing the intensity or the trajectory of one’s emotional experience.
There are a variety of ER strategies that have been identified in the literature. As a method of ER that reframes the meaning of the situation, cognitive reappraisal is considered an antecedent-focused ER strategy.1, 4 This regulation strategy contrasts with response-focused strategies that rely on regulating outward expressions of emotional responses, such as via suppression or distraction. While distraction is thought to provide more relief from unpleasant emotion in the short-term5, reappraisal, through increased contextualizing of emotional events, provides longer-term adaptation.6
Cognitive reappraisal serves as an adaptive process for one’s affective functioning because it helps to regulate mood states that can be detrimental in the long-term to one’s mental health. In fact, faulty mood regulation and its resulting behavioral consequences are considered a hallmark feature of major psychiatric disorders such as depression and bipolar disorder.7 Critically, these psychiatric disorders commonly exhibit abnormal mood states, both in terms of intensity and duration.8 Furthermore, maintenance of anxiety disorders such as obsessive-compulsive disorder, post-traumatic stress disorder, and specific phobias are thought to include emotion dysregulation as a core component.9, 10 More broadly, poorly-controlled and intensified emotional distress is widely considered one of the main pillars of many, if not most, psychopathological conditions diagnosed by current clinical standards.11, 12 This common involvement of poor ER in multiple psychiatric disorders necessitates an effort to examine the core mechanism that underlies both normal and abnormal ER.
In the context of aging, ER may have even greater relevance in the maintenance of mental health and well-being. Aging is associated with loss and deteriorated physical and cognitive functioning. Thus, one’s ability to down-regulate negative affect and increase positivity arguably becomes even more relevant for well-being and mental health. It is speculated that ER skills serve a critical role in abnormal aging such as in the case of OA with neuropsychiatric and neurological disorders. Importantly, being diagnosed with a neuropsychiatric condition with known emotion dysregulation component later in life confers a greater risk of developing other neurological disorders.13 In this sense, identifying key neural components of ER and their age-related changes can help better elucidate the neurobiological mechanisms that underlie distressing mood problems and identify specific treatment targets for multiple psychopathological conditions across the life span.
Neural Bases of ER
Functional neuroimaging research has advanced our understanding of neural processes relevant to ER. Most of the neuroimaging studies of ER employ reappraisal-based ER. The vast majority of neuroimaging studies in ER have implicated ventral regions of the prefrontal cortex (PFC), and more specifically the lateral sub-region of the orbitofrontal cortex (OFC)/orbitalis portion of the inferior frontal gyrus, as a key area involved in regulatory control of affective states.14 These studies have reported that lateral OFC is heavily recruited when subjects try to engage in such reappraisal-based ER.15 Although this specific strategy has been most commonly used in studies of reappraisal, other studies that use other types of ER, such as suppression and distraction, have also shown similar ventral prefrontal involvement.16, 17 Correlational evidence gleaned from these neuroimaging studies are also consistent with our current knowledge of bidirectional structural connection between the amygdala and the caudolateral OFC18 as well as our knowledge of the existing cortical projection to the basolateral amygdala, specifically from the ventrolateral region of the prefrontal cortex.19
A comprehensive review of data from neuroimaging studies points to the vlPFC as a core neural substrate underlying ER processes regardless of valence or direction of regulation.14 Attempted regulation of either positive or negative emotions commonly recruit the left lateral OFC, regardless of the valence of affect being regulated16, providing more weight to the idea that attempt to regulate differently-valenced affect may rely on common neural underpinnings that include the lateral OFC for cognitive control of emotions14. This also suggests that while positive and negative valence systems have been traditionally theorized as orthogonal dimensions20, 21, they may still share a common regulatory control implemented by the vlPFC.
Moreover, it has been speculated that modulation of amygdala activity, which likely reflects changes in affective salience of sensory stimuli, occurs as a function of regulatory control via activating regions of the ventral PFC. Accumulating evidence from multiple neuroimaging studies has revealed that ER is accompanied by the modulation of amygdala activity regardless of the valence of the emotion being regulated. While amygdala activity is not strictly necessary for subjective experience of emotion22, 23, changes in the perceived salience of sensory stimuli has been associated with concomitant parametric modulation of amygdala activity.24 Neuroanatomically, a portion of the amygdala25 whereas more dorsal frontal areas such as dorsolateral prefrontal cortex lack a direct pathway. Moreover, studies have found that the degree of ER success as measured by self-report of changes in affect magnitude was correlated with lateral OFC activity during regulation strategy deployment26 and that tighter functional coupling between lateral OFC and amygdala activity predicted greater ER success. In other words, closer linkage between lateral OFC and amygdala activity predicted more successful ER.27
While greater vlPFC activity has been correlated positively with more successful ER, more recent neuroimaging data has focused on identifying a more mechanistic understanding of the regulatory process. For instance, Wager and colleagues28 have shown some evidence using mediation analysis that the vlPFC plays an important role in affect regulation through both positive and negative reappraisals via its functional linkage to the ventral striatum and the amygdala, respectively.28 A meta-analysis29 that integrated data from 23 fMRI and PET imaging studies of ER similarly concluded that vlPFC serves as a critical component of regulatory process. In their heuristic model of ER, vlPFC functions as a gatekeeper of ER such that activation of the region during ER serves a dual purpose of appraising the salience of the stimuli but also feeding forward this information to other frontal areas to allow effective reappraisal when deemed necessary. These data, in addition to a wealth of existing literature30, 31, 32, strongly suggest that vlPFC activity is critically involved in reappraisal-based ER.
Emotion Regulation in Aging
There has been growing interest in the aging research community to uncover the behavioral and neural bases of ER. Indeed, there are several studies indicating possible age-related differences in neural signatures associated not only with emotion processing but also the cognitive control of emotions. At the basic level of emotion processing, it has been shown that healthy OA tend to focus more on positively-valenced stimuli relative to negatively-valenced stimuli.33, 34, 35 Healthy OA are less accurate and slower in identifying facial emotions than are YA and demonstrate a bias toward categorizing and reacting quickly to emotions as positive.36 At the same time, studies have suggested decreased negativity and enhanced ER ability in older age37, 38, which is a potential explanation of the finding that increased life satisfaction is associated with healthy normal aging.39 Socioemotional selectivity theory40 postulates that OAs have a tendency to prefer positive stimuli, and avoid negative stimuli. This theory proposes that a changing perception of time that comes with aging increases motivation toward positive, and away from negative stimuli. Importantly, the positivity effect in OAs is observed more often with low- and medium- arousing stimuli, and less with high-arousing stimuli, which are processed more quickly and make greater demands on executive processes than less arousing stimuli.41, 42 Behavioral studies have also demonstrated a preference for distraction among OA, relative to YA, who report using more active strategies, such as confrontation and reflection.43,44 Importantly, choice of ER strategy is likely moderated by cognitive resources, as reappraisal requires greater resources than does distraction.45 Consistent with this, Scheibe et al. found that executive functioning (EF) measures negatively correlated with use of distraction in both YAs and OAs.43
In terms of neural level age-related differences in emotion processing, it has been shown that OA tend to over-recruit frontal control regions when processing positive stimuli.46, 47 It has been theorized that such biased shift towards positively-valenced information combined with enhanced recruitment of frontal control regions reflect efforts to increase one’s positive affect in older age by utilizing “enhanced elaboration and up-regulation of positively-valenced stimuli”.48 It has also been argued that due to frontal-lobe mediated up-regulation of positivity, individual differences in EF correlate with age-related positivity shifts. Importantly, studies have shown that individual differences in EF modulate the level of vlPFC engagement when inhibiting negative affect, and the intensity to which OA can down-regulate amygdala activity during reappraisal-based ER.49 This further provides weight to the hypothesis that the PFC and especially the vlPFC subregion serves a critical role in ER in general and in the OA population, more specifically.
Anatomically, the inferior frontal gyrus, which overlaps with the vlPFC, is known to be affected by age-related atrophy relative to inferior temporal and cingulate regions that are less impacted by age.52 This age-related preferential cortical thinning of this region also adds an additional burden on cognitive control of emotion in older age. Some have even suggested that normal EF decline seen in older age as evidence of anatomical deterioration of such frontal lobe regions. Indeed, anatomical deterioration of these frontal lobe regions may underlie age-related decline in EF skills, which have a disproportionate dependence on the fronto-striatal network.50, 51 Such selective vulnerability of prefrontal regions in aging has been suggested to fit the “last in, first out” hypothesis, which suggests that the brain regions such as the vlPFC that have been the newest to develop phylogenetically are likely the first to be adversely impacted by aging.52
While several studies have attempted to elucidate the neural bases of ER using neuroimaging methods such as functional Magnetic Resonance Imaging (fMRI), only a handful of studies have specifically included YA and OA samples for a direct comparison of the neural bases of ER. In a study by Winecoff and colleagues53, YA and OA groups performed a cognitive reappraisal task during an fMRI scan. The results indicated an age group-related activation difference, such that lower regulation-related activations in the left inferior frontal gyrus as well as left superior temporal gyrus were observed in OA relative to their younger counterpart. In this study, a separately collected measure of executive function predicted greater regulation-related reduction in amygdala activation above and beyond the effects of age alone, which suggested that the functional recruitment of the neural substrate important for ER may share the resources utilized by general cognitive control, an aspect of EF. In a subsequently published study by Opitz and colleagues54, YA and OA groups were compared in their neural response to reappraisal of negative emotions. In this study, OAs similarly under-recruited the left vlPFC region as well as the dorsomedial region relative to the YAs, and this activation level also tracked their worse performance on down-regulating negative affect behaviorally. More recently, Allard and Kensinger55 utilized a film-clip based ER task during an fMRI scan, which revealed a similar finding of under activation of the lateral PFC (including the vlPFC) in OA during regulation of negative emotions. Although studies comparing the two age-groups show consistent finding of OA’s showing under-recruitment of the vlPFC, this study demonstrated that the OA group show temporally-delayed recruitment of the vlPFC compared to their younger counterpart. which is speculated to be evidence of OA’s compensatory engagement of the region as adaptation to using a cognitively demanding reappraisal strategy.
Even though the aforementioned studies have indicated the potential age-related differences in the neural bases of ER between YA and OA, several unanswered questions remain. For instance, it is still unclear whether age-related frontal lobe anatomical changes directly mediate differences in recruitment of the vlPFC shown in older age, and also whether EF changes have a direct causal relationship with ER abilities. And if so, can ER be enhanced by improving one’s executive function? Elucidating the neural basis for ER in aging serves a critical role in: 1) Explicating how the aging brain works to cognitively control emotions; and 2) Helping to identify treatment targets for emotion dysregulation symptoms and syndromes seen in older age such as frontotemporal dementia, late life depression, and other neuropsychiatric or neurological disorders particularly vulnerable to being diagnosed in OAs.
Utilizing Neuromodulation to Study Emotion Regulation
While growing evidence provides weight to the hypothesized role of the vlPFC in ER, there are at least two unaddressed key issues requiring further examination. First, data reported to date have limited explanatory power to confidently claim a causal relationship between vlPFC activities and ER processes. Most current knowledge on the emotion-regulation related functions of the human vlPFC stems from neuroimaging studies that are inherently correlational in nature.56, 57 In other words, neuroimaging data reveal brain regions are activated during a task (or cognitive operation) of interest. While activation data may be misconstrued as sufficient evidence that the ventral prefrontal lobe is where reappraisal-based ER takes place, it is still unclear whether there is indeed a causal link between ventral prefrontal lobe activity and reappraisal.
Prior neuroimaging studies of ER thus far reviewed are, to a limited extent, supplemented by additional observational clues from studies of individuals with OFC damage. These studies suggest that individuals who sustain OFC damage have measurable deficits in regulating mood and engaging in culturally appropriate behavior in social contexts, despite their generally intact ability to experience a full spectrum of emotions to a similar level as healthy normal individuals would. For instance, individuals with OFC damage have been reported to show disinhibition and behavior that indicates the individual’s disregard of moral and social decorum.58, 59 It has also been reported that OFC lesion patients exhibit lower levels of self-reported as well as outwardly visible facial expressions of embarrassment.60 One possible interpretation of these data is that subjective experience of socially relevant emotions, such as embarrassment, guilt, shame, and pride, may be associated with the OFC. One problem with this speculation is that most human OFC lesion studies report results from an aggregate of heterogeneous lesion cases that include multiple subregions within the OFC. Importantly, studies with a more homogeneous sample of OFC lesion foci have generally restricted their samples to medial OFC lesion cases and rarely include ventrolateral OFC lesions. Furthermore, samples in such studies are commonly collected from neuropathological or clinically necessitated surgical lesions that are inherently heterogeneous sites within the larger OFC61, making generalizable inferences more difficult.
Non-human primate lesion studies can be valuable sources to gain more precision when making inference about specific sub-regions of the frontal lobe and their dissociable functions in the context of affective control. Conducting laboratory-controlled lesion studies of non-human primates may be considered a viable approach to address this issue, albeit to a limited extent. For example, in one study, researchers induced excitotoxic lesion to the anterior OFC and vlPFC in marmoset monkeys. This revealed that both groups of lesioned marmosets exhibited observably increased anxious behavior and fear responses to the presence of human intruders, which is generally regarded as only mildly threatening.62 Interestingly, lesions targeted specifically to the vlPFC, but not the anterior OFC, influenced the marmosets’ use of a coping mechanism of increasing proactive vocalization of alarm calls in response to the anxiety inducing stressor. However, an added layer of complication is the difficulty of ascribing to the behaviors of non-human primate subjects such a complex cognitive process as ER, especially since reappraisal-based ER is thought to require willful control of emotional experience.
An alternative method that combines the ability to probe ER process in humans while simultaneously allowing for more precise causal inferences about brain-behavior relationships is using a neurostimulation method called transcranial magnetic stimulation (TMS).63 TMS is a neurophysiological technique that allows the induction of a current in the brain using a magnetic field to pass the scalp and the skull safely.64 In TMS, depending on the parameters used such as frequency and pattern of pulses delivered one can depolarize or hyperpolarize the underlying cortex of the subject’s brain.65 TMS can be applied in trains of multiple pulses within a given period of time to a targeted cortical region, known as repetitive TMS (rTMS). Depending on the stimulation parameters, rTMS is capable of changing the activity in a brain area for a brief period of time beyond the duration of the rTMS application itself. In other words, it is possible to increase or decrease activity level in a given cortical region for extended period of time. Low-frequency rTMS (< 1 Hz) has been shown to suppress cortical excitability of the targeted region for several minutes following rTMS stimulation.65 This particular variant of TMS techniques has been used widely by researchers to transiently disrupt cortical activity within a targeted brain region, thus allowing examination of a focal cortical area’s hypothesized involvement in specific cognitive functions.66, 67, 68, 69
Neuromodulation techniques such as TMS methods and ER tasks can be conjunctively utilized to investigate the impact of modulated cortical excitability in specific brain regions and their impact on regulation of one’s emotional response. Using this approach, one can thus transiently disrupt brain excitability in a targeted cortical area and measure its effect on cognitive processes such as ER. Because the effects of rTMS-dependent cortical excitability modulation are transient, rTMS can safely test the hypothesis that vlPFC function contributes to effective ER in healthy individuals. Directly manipulating cortical excitability using this technique provides an invaluable window of opportunity to gather crucial causal evidence where only correlation evidence currently exists.63 More specifically, this approach could help answer questions about the causal nature of ventral prefrontal lobe activity in ER.
To date, there has not been any published TMS study that targets vlPFC to investigate its effect on ER. A handful of TMS studies that have targeted other regions of the PFC examined the effect of TMS in the broader context of mood and emotion, but not necessarily ER. One of the early TMS studies demonstrated that 5 Hz rTMS on dorsolateral regions of the frontal lobe could significantly shift mood states in healthy individuals.70 Another study reported that repeated sessions of rapid-rate (10 Hz) rTMS over the left dorsolateral PFC could significantly alleviate depressive symptoms in patients suffering from major depression.71 Moreover, meta-analysis studies has shown that high-frequency rTMS to the left dorsolateral PFC (DLPFC) has anti-depressant effects72, and the effect of low-frequency right DLPFC rTMS was comparable to that of left DLPFC rTMS.73, 74
In all of these studies, researchers have targeted the dorsal regions of the PFC, without additionally considering the effect of ventral PFC stimulation. Lack of ventral PFC rTMS studies is not surprising given that ventral prefrontal TMS stimulation may be logistically more difficult due to the region’s proximity to facial nerves and the anterior visual tracts. There are, however, a few exceptions. Among existing studies, Schutter and Van Honk75 reported that low-frequency rTMS specifically targeting the left frontopolar cortex facilitated study participants’ memory for happy faces but not fearful faces. Interestingly, a relatively recent study reported that low-frequency rTMS to the cerebellum prevented stimulated individuals from suppressing negative mood.76 However, there remains a dearth of TMS studies of ER that target the ventral frontal lobe. Even though previous functional neuroimaging data point to lateral regions of the ventral PFC as a prime candidate region associated with ER16, 28, there is a void of data in the area of neurostimulation research that could complement the existing neuroimaging data, perhaps due to technical difficulties associated with targeting ventral regions of the frontal cortex.
In summary, although neuroimaging research has reported a significant association between vlPFC activity and successful ER using cognitive reappraisal, little research has been done to causally link vlPFC activity and effectiveness of ER. In particular, to date, there has not been a TMS study that specifically examines whether disrupting activity in ventrolateral regions of the PFC influences individuals’ ability to exert conscious and effortful control over externally elicited emotions. Previous TMS studies targeting regions near the ventral PFC have rarely focused on processes relevant to ER. Therefore, TMS stimulation studies specifically designed to target the vlPFC to subsequently probe its effect on ER can greatly enhance our understanding of the frontal lobe’s involvement in affective processes.
Limitations & Anticipated Difficulties of Utilizing TMS
There are limitations to utilizing TMS that should be noted. First, TMS likely has restricted spatial coverage of the vlPFC. While the precise spatial resolution of the effect of rTMS is considered to vary somewhat by the equipment and parameter used during stimulation77, with currently available coil designs, it is generally believed to deliver stimulation over the target area in the order of approximately 0.5 to 1cm in diameter. vlPFC area however covers a wide region along the lateral portion of the OFC that cannot be captured within a 1cm diameter. Therefore, utilizing TMS-dependent focal stimulation will partially modulate the vlPFC. For a greater coverage, it is possible in the future to combine using multiple figure-8 coils.78 Second, the method and implementation of adapting the amplitude of cortical stimulation to each participant’s brain can be challenging. Motor-thresholding is a method that has been conventionally used to as a reference point for adjusting the amplitude of TMS stimulation, as this indicates the level of the individual’s excitatory cortical response of the primary motor cortex (M1). However, this method assumes that the excitability of M1 is comparable to the excitability of other regions being targeted by TMS, namely in our case, the vlPFC. However, further investigation is needed in order to conclude whether TMS-dependent cortical excitability can be generalized from one region to another. One way to directly address this issue is to utilize what is known as TMS-evoked potential (TEP), which is a measurement of cortical excitability directly from the TMS target. Consistent with idea of possible regional variability in cortical excitability, TEPs from different brain areas have been shown to produce different latencies and amplitudes.79 Thus, taking into account individual differences in cortical excitability in future neurostimulation studies of ER using TEP may help maximize the power to detect the impact of experimental manipulation.
Third, TMS studies with OA samples can be particularly challenging to apply the stimulation at equivalent amplitude across individuals. Specifically, the aging brain may show varying degrees of atrophy, which is accompanied by the dilation of sulci and ventricles of the brain. This increases distance between the skull and the brain, and also increases CSF density that populates the space between the skull and the brain.80 The distance from the TMS coil to the motor cortex is a significant predictor of motor-threshold81. Thus, when there is significant cortical atrophy, motor-threshold (MT) measured can be significantly increased, and considerably change the TMS stimulation amplitude delivered. Furthermore, studies suggest that increased CSF volume density occupying the space between the skull and the target cortex can have a significant influence on the direction and dosage of TMS on the target area by way of shunting the current delivered through the neuromodulation technique.82 This is known to be due to the fact that CSF has higher conductivity compared to the brain matter and skull.83 In order to address these issues, careful measurement of anatomical individual differences and subsequent prospective electrical field modeling of the neuromodulation technique will become necessary to demonstrate equitable methodological set up across samples, especially when compared the YA and OA groups known to have age-related confounding variables such as age-related brain atrophy.
Future Directions
Future studies should further aim to examine the impact of vlPFC structure and function on reappraisal of positive and negative emotions. Findings from such studies would provide further clarification of the role that intact vlPFC may play in successful positive and negative affect reappraisal, as well as elucidate the neural mechanisms involved in normative patterns of effective ER. Results from these studies can suggest possible differences in socio-affective experience of older individuals with frontal lobe insults, as observed in certain neurodegenerative conditions known to disproportionately affect OAs. Moreover, it will be important to not only probe the effectiveness of ER use but also examine how brain function and structure influence one’s preference for and deployment of ER strategies at one’s disposal. Recent studies have suggested that those who are successful at regulating negative emotions may be more adept at choosing the adaptive regulation strategy for the situation ER is needed.84 Thus, it will be important to learn how the vlPFC function and structure in older age contribute to one’s preference for and deployment of ER strategies that fit the situation being regulated.
Future studies should also further clarify the role of sub-regions of the vlPFC, as the current neuroimaging literature in ER suggest the critical role that lateral OFC may play in regulating the stability of positive and negative affect. Further investigations should also address whether and how differences in appraisal and regulation of emotional experience can translate to real-world differences in functional adaptiveness. A comparative examination of the commonalities and differences in neural mechanisms underlying successful and ineffective ER in the young and old in both healthy normal and pathological sample will also enhance our overall understanding of the neural bases of ER across the life span.
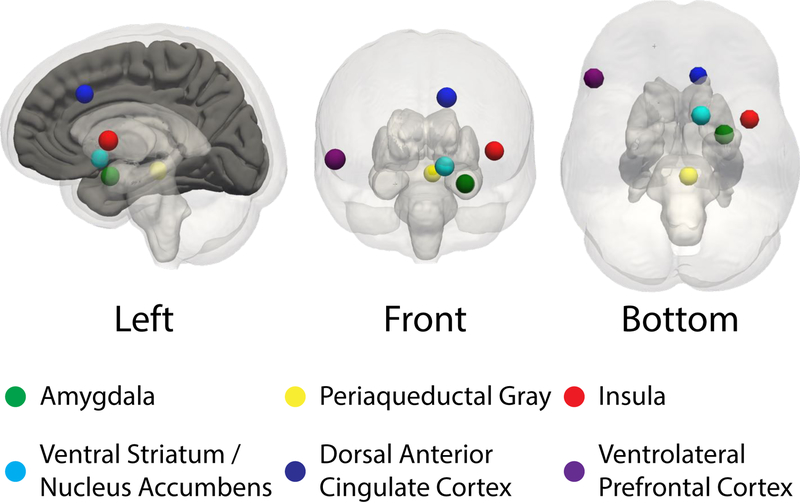
Figure 1.
The ventrolateral PFC and subcortical regions implicated in emotion regulation.
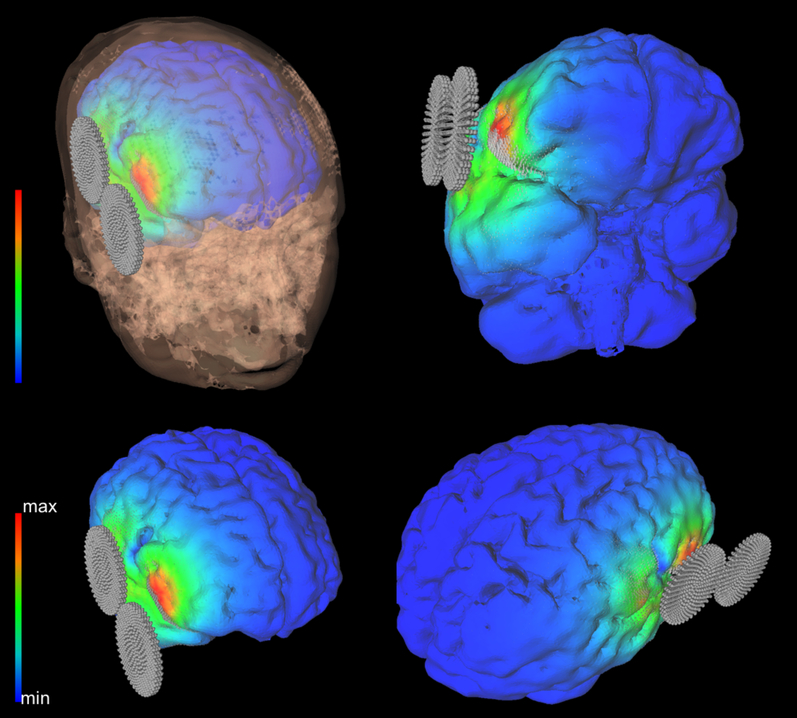
Figure 2.
An example of visualized electrical field modeling of the vlPFC rTMS.
Key Points:
Neuroimaging research suggests that vlPFC likely plays an important role in emotion regulation (ER).
FMRI studies comparing young adults and older adults, vlPFC has shown age-related recruitment differences during ER.
Further research utilizing neuromodulatory techniques such as TMS will allow testing the putative causal role of the vlPFC in reappraisal-based ER.
Acknowledgements
This work was supported by the National Institute on Mental Health (R01MH092751 to DHZ). Thanks to Dr. Vincent Koppelmans for assistance creating figures. Any opinion, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NIMH.
References
- 1.Gross JJ. The emerging field of emotion regulation: An integrative review. Review of general psychology. 1998. September;2(3):271. [Google Scholar]
- 2.Gross JJ. Thompson RA,(2007). Emotion regulation: Conceptual foundations. Handbook of emotion regulation.:3–24. [Google Scholar]
- 3.Thompson RA, editor. Socioemotional development. U of Nebraska Press; 1990. [Google Scholar]
- 4.Cutuli D Cognitive reappraisal and expressive suppression strategies role in the emotion regulation: an overview on their modulatory effects and neural correlates. Frontiers in Systems Neuroscience. 2014;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul S, Simon D, Kniesche R, Kathmann N, Endrass T. Timing effects of antecedent-and response-focused emotion regulation strategies. Biological Psychology. 2013. September 30;94(1):136–42. [DOI] [PubMed] [Google Scholar]
- 6.Wilson TD, Gilbert DT. Explaining away: A model of affective adaptation. Perspectives on Psychological Science. 2008. September;3(5):370–86. [DOI] [PubMed] [Google Scholar]
- 7.Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical psychology review. 2010. March 31;30(2):217–37. [DOI] [PubMed] [Google Scholar]
- 8.Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical psychology review. 2008. April 30;28(4):676–91. [DOI] [PubMed] [Google Scholar]
- 9.Suveg C, Zeman J. Emotion regulation in children with anxiety disorders. Journal of Clinical Child and Adolescent Psychology. 2004. November 1;33(4):750–9. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann SG, Sawyer AT, Fang A, Asnaani A. Emotion dysregulation model of mood and anxiety disorders. Depression and anxiety. 2012. May 1;29(5):409–16. [DOI] [PubMed] [Google Scholar]
- 11.DSM-5 American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 12.World Health Organization. ICD-10: international statistical classification of diseases and related health problems: tenth revision. 2004.
- 13.Potter GG, Wagner HR, Burke JR, Plassman BL, Welsh-Bohmer KA, Steffens DC. Neuropsychological predictors of dementia in late-life major depressive disorder. The American Journal of Geriatric Psychiatry. 2013. March 31;21(3):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012. March 1;1251(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage. 2004. October 31;23(2):483–99. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of cognitive neuroscience. 2007. May 8;19(5):776–98. [DOI] [PubMed] [Google Scholar]
- 17.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological psychiatry. 2008. March 15;63(6):577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002. December 16;115(4):1261–79. [DOI] [PubMed] [Google Scholar]
- 19.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007. February 1;34(3):905–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tellegen A Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. 1985.
- 21.Watson D, Tellegen A. Toward a consensual structure of mood. Psychological bulletin. 1985. September;98(2):219. [DOI] [PubMed] [Google Scholar]
- 22.Wiest G, Lehner-Baumgartner E, Baumgartner C. Panic attacks in an individual with bilateral selective lesions of the amygdala. Archives of neurology. 2006. December 1;63(12):1798–801. [DOI] [PubMed] [Google Scholar]
- 23.Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, Welsh MJ, Tranel D, Wemmie JA. Fear and panic in humans with bilateral amygdala damage. Nature neuroscience. 2013. March 1;16(3):270–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003. January 31;41(1):88–123. [DOI] [PubMed] [Google Scholar]
- 25.Ray RD, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience & Biobehavioral Reviews. 2012. January 31;36(1):479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urry HL, Van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006. April 19;26(16):4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala–frontal connectivity during emotion regulation. Social cognitive and affective neuroscience. 2007. December 1;2(4):303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008. September 25;59(6):1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. Neuroimage. 2014. February 15;87:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morawetz C, Bode S, Baudewig J, Jacobs AM, Heekeren HR. Neural representation of emotion regulation goals. Human brain mapping. 2016. February 1;37(2):600–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooker CI, Knight RT. in the inhibitory control of emotion. The orbitofrontal cortex. 2006. October 12:307. [Google Scholar]
- 32.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex. 2014. November 1;24(11):2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychology and aging. 2006. March;21(1):40. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy Q, Mather M, Carstensen LL. The role of motivation in the age-related positivity effect in autobiographical memory. Psychological science. 2004. March;15(3):208–14. [DOI] [PubMed] [Google Scholar]
- 35.Mather M, Knight M. Goal-directed memory: the role of cognitive control in older adults’ emotional memory. Psychology and aging. 2005. December;20(4):554. [DOI] [PubMed] [Google Scholar]
- 36.Calder AJ, Keane J, Manly T, Sprengelmeyer R, Scott S, Nimmo-Smith I, Young AW. Facial expression recognition across the adult life span. Neuropsychologia. 2003. December 31;41(2):195–202. [DOI] [PubMed] [Google Scholar]
- 37.Urry HL, Gross JJ. Emotion regulation in older age. Current Directions in Psychological Science. 2010. December;19(6):352–7. [Google Scholar]
- 38.Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Götestam Skorpen C, Hsu AY. Emotion and aging: experience, expression, and control. Psychology and aging. 1997. December;12(4):590. [DOI] [PubMed] [Google Scholar]
- 39.Scheibe S, Carstensen LL. Emotional aging: Recent findings and future trends. The Journals of Gerontology: Series B. 2010. March 1;65(2):135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carstensen LL. The influence of a sense of time on human development. Science. 2006. June 30;312(5782):1913–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: distraction reverses emotional biases. Emotion. 2007. November;7(4):705. [DOI] [PubMed] [Google Scholar]
- 42.Reed AE, Carstensen LL. The theory behind the age-related positivity effect. Frontiers in psychology. 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheibe S, Sheppes G, Staudinger UM. Distract or reappraise? Age-related differences in emotion-regulation choice. Emotion. 2015. December;15(6):677. [DOI] [PubMed] [Google Scholar]
- 44.Blanchard-Fields F, Stein R, Watson TL. Age differences in emotion-regulation strategies in handling everyday problems. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2004. November 1;59(6):P261–9. [DOI] [PubMed] [Google Scholar]
- 45.Domes G, Schulze L, Böttger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC. The neural correlates of sex differences in emotional reactivity and emotion regulation. Human brain mapping. 2010. May 1;31(5):758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leclerc CM, Kensinger EA. Effects of age on detection of emotional information. Psychology and aging. 2008. March;23(1):209. [DOI] [PubMed] [Google Scholar]
- 47.St. Jacques PL, Dolcos F, Cabeza R Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychological science. 2009. January;20(1):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in cognitive sciences. 2005. October 31;9(10):496–502. [DOI] [PubMed] [Google Scholar]
- 49.Ritchey M, Bessette-Symons B, Hayes SM, Cabeza R. Emotion processing in the aging brain is modulated by semantic elaboration. Neuropsychologia. 2011. March 31;49(4):p641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connelly SL, Hasher L, Zacks RT. Age and reading: the impact of distraction. Psychology and aging. 1991. December;6(4):533. [DOI] [PubMed] [Google Scholar]
- 51.Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P. Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. Journal of the International Neuropsychological Society. 2000. January;6(1):52–61. [DOI] [PubMed] [Google Scholar]
- 52.Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. Journal of Neuroscience. 2009. December 2;29(48):15223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winecoff A, LaBar KS, Madden DJ, Cabeza R, Huettel SA. Cognitive and neural contributors to emotion regulation in aging. Social cognitive and affective neuroscience. 2010. April 12;6(2):165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Opitz PC, Rauch LC, Terry DP, Urry HL. Prefrontal mediation of age differences in cognitive reappraisal. Neurobiology of aging. 2012. April 30;33(4):645–55. [DOI] [PubMed] [Google Scholar]
- 55.Allard ES, Kensinger EA. Age-related differences in neural recruitment during the use of cognitive reappraisal and selective attention as emotion regulation strategies. Frontiers in psychology. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu. Rev. Biomed. Eng. 2007. August 15;9:527–65. [DOI] [PubMed] [Google Scholar]
- 57.Iacoboni M Imitation, empathy, and mirror neurons. Annual review of psychology. 2009. January 10;60:653–70. [DOI] [PubMed] [Google Scholar]
- 58.Meyers CA, Berman SA, Scheibel RS, Hayman A. Case report: acquired antisocial personality disorder associated with unilateral left orbital frontal lobe damage. Journal of psychiatry and neuroscience. 1992. September;17(3):121. [PMC free article] [PubMed] [Google Scholar]
- 59.Burns JM, Swerdlow RH. Right orbitofrontal tumor with pedophilia symptom and constructional apraxia sign. Archives of Neurology. 2003. March 1;60(3):437–40. [DOI] [PubMed] [Google Scholar]
- 60.Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. Journal of personality and social psychology. 2003. October;85(4):594. [DOI] [PubMed] [Google Scholar]
- 61.Zald DH, Andreotti C. Neuropsychological assessment of the orbital and ventromedial prefrontal cortex. Neuropsychologia. 2010. October 31;48(12):3377–91. [DOI] [PubMed] [Google Scholar]
- 62.Agustín-Pavón C, Braesicke K, Shiba Y, Santangelo AM, Mikheenko Y, Cockroft G, Asma F, Clarke H, Man MS, Roberts AC. Lesions of ventrolateral prefrontal or anterior orbitofrontal cortex in primates heighten negative emotion. Biological psychiatry. 2012. August 15;72(4):266–272. [DOI] [PubMed] [Google Scholar]
- 63.Schutter DJ, Van Honk J, Panksepp J. Introducing transcranial magnetic stimulation (TMS) and its property of causal inference in investigating brain-function relationships. Synthese. 2004. August 1;141(2):155–73. [Google Scholar]
- 64.Hallett M Transcranial magnetic stimulation and the human brain. Nature. 2000. July 13;406(6792):147–50. [DOI] [PubMed] [Google Scholar]
- 65.Robertson EM, Theoret H, Pascual-Leone A. Studies in cognition: the problems solved and created by transcranial magnetic stimulation. Journal of Cognitive Neuroscience. 2003. October 1;15(7):948–60. [DOI] [PubMed] [Google Scholar]
- 66.Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, Brugger P. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. Journal of Neuroscience. 2006. June 14;26(24):6469–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. science. 2006. November 3;314(5800):829–32. [DOI] [PubMed] [Google Scholar]
- 68.van Honk J, Schutter DJ, d’Alfonso AA, Kessels RP, de Haan EH. 1 hz rTMS over the right prefrontal cortex reduces vigilant attention to unmasked but not to masked fearful faces. Biological psychiatry. 2002. August 15;52(4):312–7. [DOI] [PubMed] [Google Scholar]
- 69.Mottaghy FM, Gangitano M, Sparing R, Krause BJ, Pascual-Leone A. Segregation of areas related to visual working memory in the prefrontal cortex revealed by rTMS. Cerebral Cortex. 2002. April 1;12(4):369–75. [DOI] [PubMed] [Google Scholar]
- 70.George MS, Wassermann EM, Williams WA, Steppel J, Pascual-Leone A, Basser P, Hallett M, Post RM. Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. The Journal of Neuropsychiatry and Clinical Neurosciences. 1996. [DOI] [PubMed] [Google Scholar]
- 71.Pascual-Leone A, Rubio B, Pallardó F, Catalá MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. The Lancet. 1996. July 27;348(9022):233–7. [DOI] [PubMed] [Google Scholar]
- 72.Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychological medicine. 2009. January;39(1):65–75. [DOI] [PubMed] [Google Scholar]
- 73.Berlim MT, Van den Eynde F, Daskalakis ZJ. A systematic review and meta-analysis on the efficacy and acceptability of bilateral repetitive transcranial magnetic stimulation (rTMS) for treating major depression. Psychological medicine. 2013. November;43(11):2245–54. [DOI] [PubMed] [Google Scholar]
- 74.Chen J, Zhou C, Wu B, Wang Y, Li Q, Wei Y, Yang D, Mu J, Zhu D, Zou D, Xie P. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry research. 2013. December 30;210(3):1260–4. [DOI] [PubMed] [Google Scholar]
- 75.Schutter DJ, van Honk J. Increased positive emotional memory after repetitive transcranial magnetic stimulation over the orbitofrontal cortex. Journal of psychiatry & neuroscience. 2006. March;31(2):101. [PMC free article] [PubMed] [Google Scholar]
- 76.Schutter DJ, van Honk J. The cerebellum in emotion regulation: a repetitive transcranial magnetic stimulation study. The Cerebellum. 2009. March 1;8(1):28–34. [DOI] [PubMed] [Google Scholar]
- 77.Lockhart SN, DeCarli C. Structural imaging measures of brain aging. Neuropsychology review. 2014. September 1;24(3):271–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruohonen J, Ilmoniemi RJ. Focusing and targeting of magnetic brain stimulation using multiple coils. Medical and Biological Engineering and Computing. 1998. May 1;36(3):297–301. [DOI] [PubMed] [Google Scholar]
- 79.Chung SW, Rogasch NC, Hoy KE, Fitzgerald PB. Measuring brain stimulation induced changes in cortical properties using TMS-EEG. Brain stimulation. 2015. December 31;8(6):1010–20. [DOI] [PubMed] [Google Scholar]
- 80.Beauchamp MS, Beurlot MR, Fava E, Nath AR, Parikh NA, Saad ZS, Bortfeld H, Oghalai JS. The developmental trajectory of brain-scalp distance from birth through childhood: implications for functional neuroimaging. PLoS One. 2011. September 21;6(9):e24981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. Journal of neuroengineering and rehabilitation. 2009. March 17;6(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McConnell KA, Nahas Z, Shastri A, Lorberbaum JP, Kozel FA, Bohning DE, George MS. The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biological psychiatry. 2001. March 1;49(5):454–9. [DOI] [PubMed] [Google Scholar]
- 83.Li LM, Uehara K, Hanakawa T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Frontiers in cellular neuroscience. 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonanno GA, Burton CL. Regulatory flexibility: An individual differences perspective on coping and emotion regulation. Perspectives on Psychological Science. 2013. November;8(6):591–612. [DOI] [PubMed] [Google Scholar]