Abstract
Millions of people across the globe suffer from swallowing difficulties, known as dysphagia, which can lead to malnutrition, pneumonia, and even death. Swallowing cervical auscultation, which has been suggested as a noninvasive screening method for dysphagia, has not been associated yet with any physical events. In this paper, we have compared the hyoid bone displacement extracted from the videofluoroscopy images of 31 swallows to the signal features extracted from the cervical auscultation recordings captured with a tri-axial accelerometer and a microphone. First, the vertical displacement of the anterior part of the hyoid bone is related to the entropy rate of the superior–inferior swallowing vibrations and to the kurtosis of the swallowing sounds. Second, the vertical displacement of the posterior part of the hyoid bone is related to the bandwidth of the medial–lateral swallowing vibrations. Third, the horizontal displacements of the posterior and anterior parts of the hyoid bone are related to the spectral centroid of the superior–inferior swallowing vibrations and to the peak frequency of the medial–lateral swallowing vibrations, respectively. At last, the airway protection scores and the command characteristics were associated with the vertical and horizontal displacements, respectively, of the posterior part of the hyoid bone. Additional associations between the patients’ characteristics and auscultations’ signals were also observed. The hyoid bone maximal displacement is a cause of swallowing vibrations and sounds. High-resolution cervical auscultation may offer a noninvasive alternative for dysphagia screening and additional diagnostic information.
Keywords: Cervical auscultation, dysphagia, hyoid displacement, signal processing, swallowing
In this study, we analyze the relationships between hyoid bone displacement during swallowing and signal features extracted from the swallowing vibrations and sounds. The results are useful for screening and treatment of dysphagia.
I. Introduction
Swallowing is a vital biomechanical process that is necessary for nourishing and hydrating the human body [1]. It is a complex activity, recruiting more than 20 pairs of muscles during its one to two second duration. The precise timing of muscular contractions requires intricately coordinated neurological programming and execution to ensure the complete anterograde propulsion of swallowed material and closure of the upper airway to prevent aspiration into the respiratory system [1], [2]. In the oral preparatory stage, mastication reduces solid foods into a cohesive bolus that is then propelled toward the pharynx during the subsequent oral transit stage [1]. Then, a biological sensor in the pharynx detects the bolus and multiple sensorimotor feedback and feedforward signals between the brainstem central pattern generator, and cortical, subcortical and peripheral structures in the oropharynx, generate a pharyngeal response that begins typically at the time the bolus enters the pharynx [2], [3]. Displacement of the hyolaryngeal complex, a key component of the pharyngeal stage, repositions the laryngeal inlet, the entrance to the airway, anteriorly and superiorly and away from the path of the oncoming bolus while also applying traction forces to distend the upper esophageal sphincter which has momentarily relaxed from its tightly-closed resting posture due to vagal inhibition [4]. This hyolaryngeal displacement also repositions the epiglottis over the laryngeal inlet while the larynx collapses at the level of the vocal folds preventing the bolus from entering the airway which leads to the lungs [2]. These pharyngeal events ensure delivery of the bolus into the digestive system while preventing aspiration of material into the airway, an event that leads to immediate complications such as airway obstruction, and more insidious outcomes such as aspiration pneumonia, a significant contributor to morbidity and mortality in people with oropharyngeal swallowing disorders, also known as dysphagia [2], [5]–[7]. As many as 50% of both older adults and people with neurological conditions suffer from oropharyngeal dysphagia [8] and following conditions like stroke, those who develop pneumonia have a threefold higher adjusted mortality risk compared to stroke patients for whom pneumonia is avoided [9].
The diagnosis of dysphagia can take many forms depending on the resources available and the patient’s symptomatology. Both noninstrumental, clinical examinations and sophisticated, imaging studies using endoscopic or x-ray (i.e., videofluoroscopy) instrumentation are commonplace, with the latter methods serving as the gold standards for identification of oropharyngeal kinematic impairments, airway protection deficits, and disordered transfer of swallowed material to the digestive system [10]–[12]. Clinical evaluations rely on evaluation of sensorimotor functions of observable oropharyngeal structures by an experienced examiner but are blind to any activity occurring once the mouth is closed, which is the case during the majority of swallowing activity [13]. Videofluoroscopy (VF) records real-time radiographic images of the swallow of a patient at an optimal temporal resolution to enable human judgment of kinematic events in relation to one another, timing and completeness of airway closure, and consequences of impaired kinematics, among other important observations [10], [11]. VF is the most ubiquitous gold standard diagnostic method, but it is not always available at the time that dysphagia is first suspected, is not feasible in some settings, and is too expensive to use as a screening method [13]–[15]. Moreover, VF data needs to be analyzed by a clinical expert [16] and involves the use of invasive ionizing radiation, although the amount of radiation doses is minimal [17], [18].
During VF, the clinical expert can identify and quantify aspiration with the penetration aspiration scale (PAS) [19], [20]. The PAS identifies whether the bolus entered the airway, how deeply it penetrated the airway, and whether material entering the airway is ejected by reflexive airway protection [16]. PAS scores range from a score of 1, representing no penetration of swallowed material into the airway, to 8, indicating silent aspiration of swallowed material through the larynx and into the trachea without any reflexive effort by the patient to eject aspirated material [19], [21]. Various disease processes disrupt airway protection that are reflected in PAS scores, however other factors such as age, sex, consistency, and volume of material swallowed, have also been shown to influence various swallow kinematic patterns [22]–[27]. Moreover, many studies have shown that swallowing is altered by using a verbal command to initiate the swallow [28], [29]. Simply, a verbal command in swallowing means that the bolus is administered to the subject and held in mouth until instructed to swallow [28], [29].
The elusive “holy grail” of dysphagia diagnostics is a noninvasive method of detecting dysphagia before patients are placed at risk of aspiration. Less invasive methods to assess specific aspects of dysphagia have been investigated in previous studies such as ultrasonography to assess soft tissue function [30], manometry to assess pressure generation and propagation from the oral cavity through the esophagus [31], [32], and electromyography to assess the timing and sequence of muscle activation [33]. Cervical auscultation (CA) which uses stethoscopes to observe sounds emanating from the pharyngeal mechanism to infer about swallow physiology [16], [34]–[41], enables an examiner to “hear”a swallow, however it has been shown repeatedly to provide inconsistent information leading to subjective interpretations by the examiner due limitations of the human auditory system and to variations in instrument design since stethoscopes are not designed to transmit pharyngeal sounds [42], [43]. Despite its poor precision in clinical practice, CA has intrigued investigators including ourselves with interests in signal processing, who hypothesize that the acoustic-vibratory information arising from oropharyngeal physiology contains useful diagnostic clues as to the nature of dysphagia. High-resolution cervical auscultation (HRCA) based on recording of swallowing vibrations and sounds using accelerometers and microphones, has been proposed and investigated as an affordable, noninvasive and automated system that can screen patients suspected for dysphagia to identify those for whom oral intake may be dangerous. As a result, HRCA can expedite referral for gold-standard diagnostic testing with VF while identifying those who do not need diagnostic testing and who should not be unnecessarily deprived of oral intake. More recently, the value of HRCA has been investigated as a potential surrogate when VF is either unavailable, infeasible, or not desired by the patient [16], [41].
Previous study has suggested that swallowing kinematics, in particular hyoid bone movement, could be a physiological component of swallowing producing the change of several HRCA signal features [44]. They have found that the weak accelerometry signals recorded from a dual-axial accelerometer are linked to a small hyoid bone excursion [44]. Other studies have explored the possibility that the HRCA acoustic time features are associated with laryngeal vestibule closure and its re-opening, with the opening of the upper esophageal sphincter (UES) and with contact of the base of the tongue to the posterior pharyngeal wall during bolus propulsion [45]. The same study has also found associations between HRCA signals and the position of the hyoid bone at the beginning and end of a swallow, and the maximal excursion of the hyoid bone, and that both the closure and opening of the laryngeal vestibule and UES opening are correlated to acoustic swallowing sounds [45]. Another study has shown that HRCA signal features are affected by the head position commonly used to compensate for dysphagia, especially the chin-tuck maneuver [41].
However, which physical events cause the swallowing vibrations and sounds still remains as an unanswered question, though we hypothesize that the displacement of oropharyngeal structures during swallowing are the sources of perceived or recorded acoustic and vibratory swallowing signals. In particular, we wondered if tri-axial vibratory signals (superior-inferior, anterior-posterior and medial-lateral) from the pharynx might be surrogates for the three-dimensions of hyolaryngeal displacement occurring during swallowing. Therefore, we sought to compare HRCA signal features in the time, frequency, and time-frequency domains to the maximal vertical and horizontal hyoid bone displacement during swallowing as measured with VF. We hypothesized that both acoustic and tri-axial accelerometer HRCA signals would be strongly associated with vertical and horizontal displacement of both the anterior and posterior tips of the body of the hyoid bone. We further hypothesized that as in prior studies, our methods would confirm that other swallow and participant factors (e.g., sex, PAS score, command or not, and age) would be associated with hyoid bone maximal displacement, and associated HRCA signals.
II. Methodology
A. Data Acquisition
In this study, we evaluated 31 single swallows collected from 25 patients (13 females, 12 males, mean age: 60 ± 12 years) undergoing a VF exam at the University of Pittsburgh Medical Center. Video images were recorded at 60 frames per second and the resolution of images is clipped into 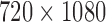 . Boluses consisted of thin liquid (50% of water at room temperature with 50% of barium sulfate) in 3mL–5mL volumes administered with a spoon, with participants assuming a neutral head position. All analyzed swallows were considered to be safe swallows, that is, they were rated to have a penetration-aspiration score of 1 or 2 [19].
. Boluses consisted of thin liquid (50% of water at room temperature with 50% of barium sulfate) in 3mL–5mL volumes administered with a spoon, with participants assuming a neutral head position. All analyzed swallows were considered to be safe swallows, that is, they were rated to have a penetration-aspiration score of 1 or 2 [19].
Cervical auscultation signals were recorded using two different sensors. A tri-axial accelerometer (ADXL 327, Analog Devices, Norwood, Massachusetts) was attached to the anterior of the patient’s neck [40]. The signals extracted from the tri-axial accelerometer sensors are recorded with a National Instrument 6210 DAQ at a sampling rate of 20 kHz by the LabVIEW Program Signal Express (National Instrument, Austin, Texas) [16], [40]. The tri-axial accelerometer was powered by a 3 Volt power supply (model 1504, BK Precision, Yorba Linda, California). The signal was then passed through a bandpass filter from 0.1 to 3000 Hz with an amplification gain of 10 (model P55, Grass Technologies, Warwick, Rhode Island) [16], [40]. A microphone (model C 411L, AKG, Vienna, Austria) was also placed below the accelerometer to record swallowing sounds. The microphone was powered by a B29L power supply (model B29L, AKG, Vienna, Austria) with its line impedance set to volume level 9 while the resulting voltage signal was sent to the previously mentioned DAQ.
B. Pre-Processing of Cervical Auscultation Recordings
First, to determine the start and end times of each swallow, the fluoroscopic videos associated to the cervical signals were analyzed by a speech language pathologist (SLP) trained in kinematic analysis of VF swallow data. Prior to the analysis, the inter- and intra- rater reliability in the judgment of these measures had been established between the primary rater and and the 2nd rater who also is an experienced SLP. The intraclass correlation coefficient both for intra- and inter-rater reliability was 1.0. The signals have been segmented in the same manner as in prior publications [16], [41], [46]–[48].
In order to reduce the multi-source noises inherent in both the accelerometer and microphone devices, each component of the segmented swallow signals was filtered by an axis-specific finite impulse response (FIR) filter. The FIR filters were generated by using the coefficients of an auto-regressive model fitted to the output of sensors without any input [47]. Then, low-frequency components associated with head movements were removed from acceleration signals only using a fourth-order splines approximation algorithm [16], [48], [49]. Finally, we used wavelet denoising with the tenth-order Meyer wavelet to remove any additional noise from all signals [50].
C. Feature Extraction From Cervical Auscultation Signals
In order to determine the relationship between cervical auscultation signals and vertical and horizontal hyolaryngeal displacements during swallowing, several features in different domains were extracted from four cervical auscultation signals (e.g., superior-inferior vibrations, anterior-posterior vibrations, mediolateral vibrations, and swallowing sounds). We carefully chose a set of features from literature that proved significance for swallowing kinematics and disorders [47], [51]. In the time domain, we extracted the standard deviation, that characterizes the fluctuation of the signal around the mean, the skewness, that quantifies the symmetry of the signal and the kurtosis that quantifies how the signal is peaked or flat compared to a normal distribution [51]. Also, information-theoretic features such as the normalized entropy rate, that quantifies the regularity of a signal [47], [52], and the Lempel-Ziv complexity [51], [53], [54], that determines the predictability of the signal are computed. In the frequency domain, we extracted the bandwidth, the spectral centroid that indicates the frequency of the mass of the spectrum and the peak frequency that shows the frequency at the maximum power have been calculated from cervical auscultation recordings [47]. Finally, in the time-frequency domain, we extracted the wavelet entropy to quantify whether the signal is disordered or not [51], [55]. Table 1 summaries the features we applied in this investigation.
TABLE 1. Summary of Features.
| Time Domain Features | |
|---|---|
| Standard deviation | Reflect the signal variance around its mean value. |
| Skewness | Describe the asymmetry of amplitude distribution about mean. |
| Kurtosis | Describe the peakness of the distribution relative to normal distribution. |
| Information-Theoretic Domain Features | |
| Lempel-Ziv Complexity | Describe the randomness of a signal. |
| Entropy rate | Evaluate the degree of regularity of the signal distribution. |
| Frequency Domain Features | |
| Peak frequency (Hz) | Describe the frequency of maximum power. |
| Spectral centroid (Hz) | Evaluate the median of the spectrum of the signal. |
| Bandwidth (Hz) | Describe the range of frequencies of a signal. |
| Time-Frequency Domain Features | |
| Wavelet entropy | Evalute the disorderly behavior for non-stationary signal. |
D. Analysis of Videofluoroscopy Images
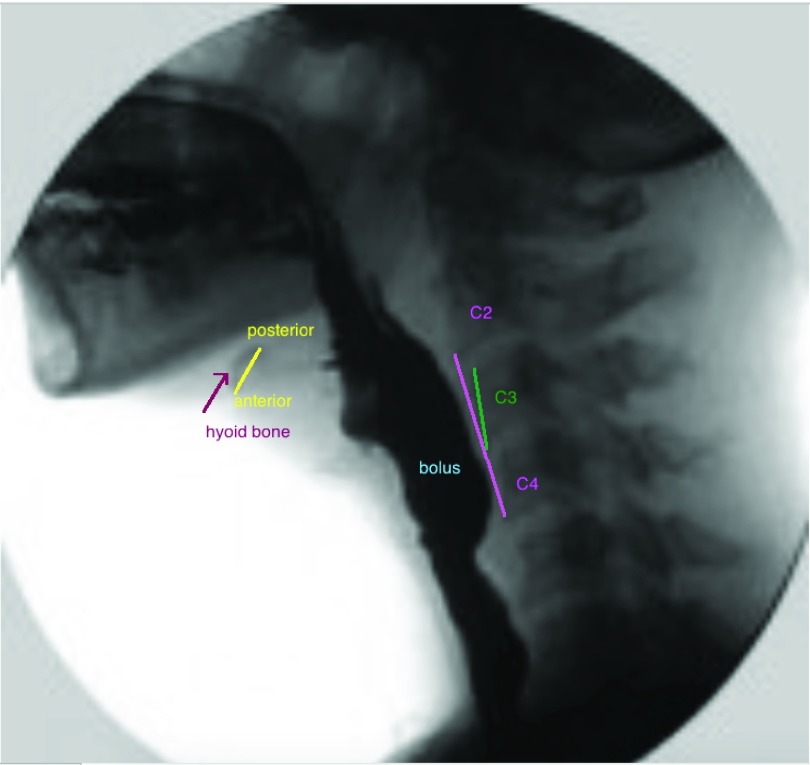
The coordinates of the hyoid bone were located in each VF image, as shown in Fig. 2. The hyoid bone is denoted in yellow (anterior and posterior part linked). The upper and lower extremity of the pink segment represents the linear distance between the inferior anterior corners of the bodies of the second (C2) and fourth (C4) cervical vertebrae respectively. This linear distance measure was performed using previously published methods in order to normalize each subject’s height to a criterion referenced anatomic referent [44], [56] and to provide the vertical axis of the coordinate system we deployed to hyoid displacements across subjects. Reliability in judgment of these measures has been also established. 30% of all measures were randomly selected and repeated by the same rater and a 2nd rater to determine the inter- and intra- rater reliability. The intraclass correlation coefficient for both was greater than 0.98.
FIGURE 1.
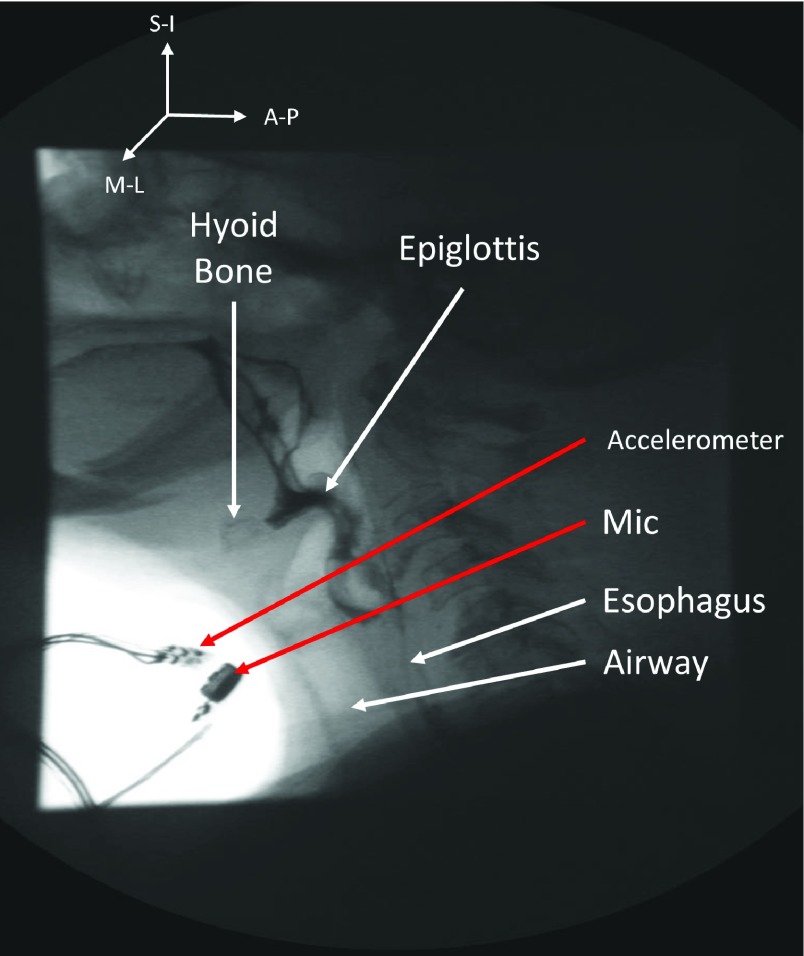
Placement of the tri-axial accelerometer and the associated microphone. Accelerometer is placed over the cricoid cartilage and microphone is mounted over suprasternal notch (right lateral side of the larynx).
FIGURE 2.
Position of the hyoid bone, C2–C4 and C3 on a videofluoroscopic image.
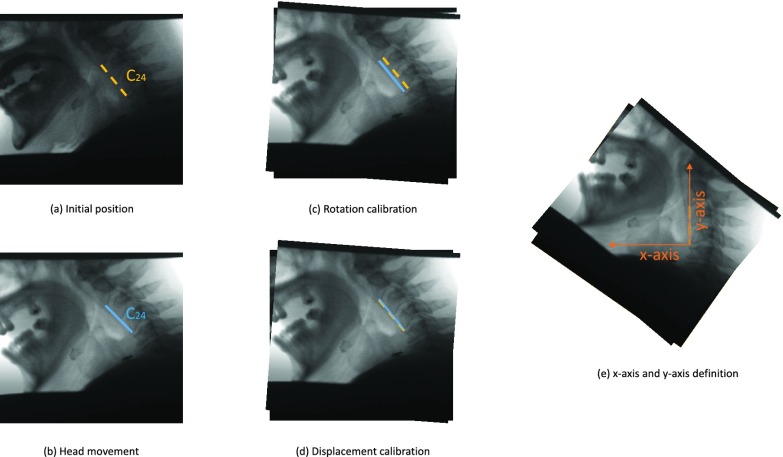
As some patients tend to move their head during the VF exam, a motion compensation procedure was applied to the hyoid bone at each frame in order to avoid over or underestimation of hyoid motion caused by head movement. In the first frame of each VF image, we located the C2–C4 segment (distance between the second and fourth cervical), as shown in Fig. 3 (a) and was taken as the reference structure (static, fixed length segment) to calibrate for head movement. For each subsequent frame, the same segment was located and compared to the reference position taken from the first frame as in Fig. 3(b-d). Through this comparison with the reference position of C2–C4 segment, the linear transformation parameters (translational distance 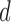 and rotational angle
and rotational angle 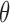 ) that compensate for head movement, can be calculated. The translational distance was defined as the displacement of C4 and the rotational angle is the angle between the current frame’s C2–C4 and the reference segment. This transformation was applied to the hyoid bone segment in order to compensate for head movement and avoid any biased measurements.
) that compensate for head movement, can be calculated. The translational distance was defined as the displacement of C4 and the rotational angle is the angle between the current frame’s C2–C4 and the reference segment. This transformation was applied to the hyoid bone segment in order to compensate for head movement and avoid any biased measurements.
FIGURE 3.
Head movement calibration. (a) First frame of the swallow. (b) One of the subsequent frames with head movement. (c) Rotation applied to the frame. (d) Translation applied to completely register the two frames.
Head movement was eliminated in each VF frame. As a result, the coordinates of hyoid bone were obtained independent of any possible head movement during VF procedure. The maximal horizontal displacement of the anterior part of the hyoid bone (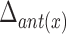 ) was defined as the difference between the maximum and minimum of the horizontal coordinates of the anterior part of the hyoid bone. The maximal vertical displacement of the anterior part of the hyoid bone (
) was defined as the difference between the maximum and minimum of the horizontal coordinates of the anterior part of the hyoid bone. The maximal vertical displacement of the anterior part of the hyoid bone (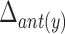 ) was defined as the difference between the maximum and minimum of the vertical coordinates of the anterior part of the hyoid bone. In the same way, we calculated the maximal horizontal and vertical displacements of the posterior part of the hyoid bone (
) was defined as the difference between the maximum and minimum of the vertical coordinates of the anterior part of the hyoid bone. In the same way, we calculated the maximal horizontal and vertical displacements of the posterior part of the hyoid bone (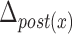 and
and 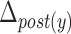 respectively).
respectively).
Lastly, sometimes, patients’ hyolaryngeal complex was enlarged on VF images because they stood closer to the x-ray tube of the VF machine. So, the displacement had to be normalized by a physical unit of measurement. We have chosen the length of C2–C4 as in previous studies [56] and all the hyoid bone displacements used in this study were divided by the length of C2–C4.
E. Statistical Analysis
First, to examine association between measures of displacement, participants’ characteristics, and swallowing conditions, we fit a series of linear mixed models with each of the displacement measures as the response variable; each of the participant characteristics and swallowing conditions, both individually and together as the independent variable(s); and a participant random effect to account for multiple measurements from the same participant. Then, we fit a series of similar models with each of the individual HRCA signal features, one at a time, as independent variable to examine the associations between displacement measures and HRCA signal features. Finally, we adjusted the said models for participant characteristics and swallowing conditions as covariates to examine independent associations between the same. SAS® version 9.3 (SAS Institute, Inc., Cary, North Carolina) was the main platform used for analysis.
III. Results
Table 2 depicts the considered feature values for cervical auscultation recordings. Table 3 depicts the values of horizontal and vertical displacements of the anterior part of the hyoid bone and the horizontal and vertical displacement of the posterior part of the hyoid bone, relative to the C2–C4 length.
TABLE 2. Mean and Standard Deviation of Considered Feature Values. AP Represents the Anterior-Posterior Axis, ML the Medial-Lateral Axis and SI the Superior-Inferior Axis of Swallowing Vibrations. SS Represents Swallowing Sounds.
| Extracted features | SI | ML | AP | SS |
|---|---|---|---|---|
| Time domain | ||||
| Standard deviation | 0.036 ± 0.018 | 0.011 ± 0.006 | 0.029 ± 0.014 | 0.032 ± 0.018 |
| Skewness | −0.417 ± 1.125 | 0.135 ± 0.903 | 0.614 ± 1.281 | −0.338 ± 1.316 |
| Kurtosis | 13.021 ± 13.529 | 12.951 ± 13.348 | 12.175 ± 10.060 | 11.919 ± 14.187 |
| Normalized entropy rate | 0.926 ± 0.026 | 0.944 ± 0.019 | 0.925 ± 0.029 | 0.904 ± 0.042 |
| Lempel-Ziv complexity | 0.233 ± 0.060 | 0.197 ± 0.057 | 0.220 ± 0.058 | 0.279 ± 0.071 |
| Frequency domain | ||||
| Peak frequency (Hz) | 9.694 ± 4.116 | 10.460 ± 7.568 | 11.330 ± 15.077 | 9.885 ± 12.703 |
| Bandwidth (Hz) | 61.643 ± 46.661 | 94.796 ± 89.831 | 84.707 ± 54.732 | 51.759 ± 22.390 |
| Spectral centroid (Hz) | 37.173 ± 23.724 | 43.369 ± 29.115 | 43.584 ± 33.284 | 36.498 ± 17.811 |
| Time-frequency domain | ||||
| Wavelet entropy | 1.025 ± 0.654 | 0.973 ± 0.654 | 0.877 ± 0.736 | 1.054 ± 0.821 |
TABLE 3. Mean and Standard Deviation of the Vertical and Horizontal Maximum Displacement of Both Anterior and Posterior Part of the Hyoid Bone Relative to the C2–C4 Length.
| Maximum displacement | Values | |
|---|---|---|
| anterior | horizontal | 0.357 ± 0.067 |
| vertical | 0.411 ± 0.172 | |
| posterior | horizontal | 0.380 ± 0.069 |
| vertical | 0.404 ± 0.162 | |
Table 4 depicts the relationship between each maximal displacement and different patients’ characteristics such as sex, age, and race. In addition, the command Yes/No characteristic represents whether a patient is asked to swallow or whether he/she swallows by him/herself instinctively. Finally, we have studied only two PAS scores (i.e., PAS = 1 and PAS = 2) [19]. All ‘+’ signs denote that there exists a relationship between the criterion and the displacements of the hyoid bone and all ‘−’ signs represent no dependency between the hyoid displacement and considered characteristics. Only the displacement of the posterior part of the hyoid bone was found to be affected by the PAS score or the command Yes/No characteristics. The vertical displacement of the posterior part of the hyoid bone (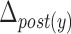 ) is 0.19 units more for a PAS score of 1 than for a PAS score of 2. The horizontal displacement of the posterior part of the hyoid bone (
) is 0.19 units more for a PAS score of 1 than for a PAS score of 2. The horizontal displacement of the posterior part of the hyoid bone (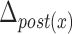 ) is 0.072 less for a command swallow than for a non-command swallow.
) is 0.072 less for a command swallow than for a non-command swallow.
TABLE 4. Relationships Between Displacement of the Hyoid Bone and Clinical Variables.
| Patient characteristics | 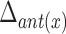 |
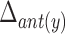 |
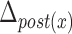 |
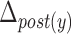 |
|---|---|---|---|---|
| Sex | − | − | − | − |
| Age | − | − | − | − |
| Race | − | − | − | − |
| Command Yes/No | − | − | + | − |
| PAS score | − | − | − | + |
Table 5 depicts the dependency between some of the features extracted from cervical auscultation signals and the maximal displacement of the hyoid bone in different directions. The horizontal maximal displacement of the anterior part increases by 0.004 units when the peak frequency of the ML axis increases. The vertical displacement of the anterior part increases when the entropy rate of the SI axis and the kurtosis of the swallowing sounds increases (2.231 units and 0.003 units, respectively). The horizontal displacement of the posterior part decreases by 0.002 units when the spectral centroid of the SI axis increases. The vertical displacement of the posterior part of the hyoid bone decreases by 0.0005 when the bandwidth of the ML axis increases.
TABLE 5. Maximum Displacement and its Dependent Features Extracted From the Tri-Axial Accelerometer.
| Signal | Extracted features | 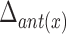 |
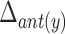 |
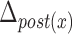 |
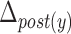 |
|---|---|---|---|---|---|
| ML | Bandwidth | − | − | − | + |
| Peak frequency | + | − | − | − | |
| SI | Spectral centroid | − | − | + | − |
| Entropy rate | − | + | − | − | |
| SS | Kurtosis | − | + | − | − |
Table 6 depicts the dependency between the features extracted from cervical auscultation recordings and patient/swallow characteristics: sex (i.e., females compared to males), age, race (i.e., white race versus other race), command Yes/No, and PAS scores (i.e., 1 versus 2). The values stand for a dependency between the patient’s characteristics and the extracted features. They represent the slope of the general trend between the two dependent variables. A negative value represents a decreasing general trend and a positive value stands for an increasing general trend. For example, the bandwidth and the spectral centroid of the ML axis are linked to the PAS score. The spectral centroid is lower by 31.60 Hz when the PAS score is 1 compared to the PAS score of 2, and the bandwidth is lower by 83.21 Hz when the PAS score is 1 compared to a PAS score of 2.
TABLE 6. Relationships Between Extracted Features From Cervical Auscultation Recordings and Clinical Variables.
| Signal | Extracted features | Sex | Age | Race | Command Yes/No | PAS score |
|---|---|---|---|---|---|---|
| AP | Wavelet entropy | − | − | − | −0.768 | − |
| ML | Kurtosis | − | − | 14.236 | − | − |
| Spectral centroid | − | − | − | − | 31.599 | |
| Bandwidth | − | − | − | − | 83.208 | |
| SS | Kurtosis | − | − | 19.533 | − | − |
| Spectral centroid | − | − | 19.773 | −17.825 | − | |
| Wavelet entropy | − | − | 1.179 | − | − |
IV. Discussion
A. Hyoid Maximal Displacement and the Patient’s Characteristics
In general, our results depict larger hyoid bone displacements in the vertical direction than in the horizontal direction. The maximal vertical displacements are similar to those found in a previous study and said to be a reference for young healthy swallows of an ultra thin liquid barium [56]. Our sample age is from 44 to 86 so we can extend the reference results for all ages and healthy swallows of a thin liquid barium. The maximal horizontal displacements that we found can be stated as a reference for healthy participants swallowing thin liquid barium since the hypotenuse values of the displacement have similar values compared to Molfenter’s study [56].
Our results highlight that the maximal displacement of the anterior and posterior part of the hyoid bone, vertically and horizontally, has no correlation with race, sex, or age. In previous papers, it has been found that the horizontal displacement of the hyoid bone depends on the age [56]–[58], however, our results did not show this dependency. However, the range of our patients’ age is 44–86 years old. Therefore, our lack of young patients from 18 to 43 may have affected our ability to detect the effects of age on the maximal hyoid bone displacement with our sensors and algorithms.
In concordance with previous studies, our results show that hyoid bone displacement does not depend on sex [56], [59]. Since men are usually taller than women, our method along with those of Molfenter had adjusted absolute linear measures of distance between anatomic structures using participants’ own anatomic referents [56]. This demonstrated that the difference in the hyoid displacement arises out of difference in height between participants and not of the patient’s sex [56], [59]. The effect of the difference of size between sexes on hyoid bone displacement is canceled by the normalization of C2–C4 length [56], [59]. However, whether another possible normalization that would also cancel the size effect on the hyoid bone displacement remains as an open question. We are currently investigating other anatomic scalars to determine if this is the case and the extent to which other, more convenient scalars might prove as accurate while being clinically expedient in order to judge hyoid displacement during VF rather than following sophisticated data analysis.
The horizontal maximal displacement of the posterior part of the hyoid bone only seems to depend on whether the patient has been commanded to swallow or not. Prior studies have documented that the “command swallow” condition alters several temporal aspects of swallow physiology when compared to a natural uncued swallowing condition in healthy participants [24], [28], [29]. However, our sample contains only five commanded swallows out of 31. Moreover, despite our patients’ producing PAS scores of 1 and 2 which are “healthy” PAS scores, all of our patients were referred because of suspected dysphagia. The use of healthy people without suspicion of dysphagia could produce a different result.
Vertical displacement of the posterior landmark of the hyoid was associated with PAS scores, but the range of PAS scores that we investigated was narrow (PAS scores of 1 and 2). Our conjecture is that because a score of 1 represents no laryngeal penetration and 2 represents shallow, transient laryngeal penetration, hyoid vertical movement may be associated with more timely laryngeal closure. However, conclusions regarding this result cannot be made.
B. Feature Extraction From Cervical Auscultation Recordings
According to the results shown in Table 2, the amplitude of the recorded signals is narrowly distributed around their mean values, as all four different recordings exhibited small standard deviation values. This is further demonstrated by high kurtosis values. The distribution of signal amplitude values is symmetric since the skewness values are low for all signals considered. Each signal has close peak and spectral centroid values, but all have different bandwidth values. As in a previous study, the wavelet entropies are almost the same and close to one [16]. We can conclude that all signals considered have approximately the same level of disorder. All the extracted features are in the same range as it has been found for safe swallows of thin bolus [16], [40].
C. Hyoid Bone Displacement and Cervical Auscultation Recordings
The maximal vertical displacement seems to be related to features from the superior-inferior, medial-lateral vibrations, and from swallowing sounds. First, the vertical displacement of the anterior part seems to affect the entropy rate of the SI axis vibrations and the kurtosis of the swallowing sounds. In other words, the vertical displacement of the anterior part may affect how the swallowing sounds are peaked or flat compared to normal distribution and how a signal is regular or random [16]. When the vertical displacement of the anterior part increases, the swallowing sounds tend to be less peaked, due to a 0.003 units increase in kurtosis. In addition, the signal from the SI axis seems to be more regular since the entropy rate increases by 2.231 units. Second, when the vertical displacement of the posterior part increases, the bandwidth of the ML axis vibrations seems to decrease by 0.002 units. In other words, a larger vertical displacement of the posterior part results in a signal spread on a smaller frequency range.
Moreover, the horizontal displacement is dependent on features from the SI and ML vibrations. The horizontal displacement of the anterior part of the hyoid bone seems to depend on the peak frequency ML vibrations. For a larger maximal horizontal displacement of the anterior part of the hyoid bone, the maximal frequency of ML vibrations decreases. In addition, when the maximal horizontal displacement of the posterior part of the hyoid bone increases, the median of the difference between the upper and lower frequencies of the SI vibrations decreases (by 0.002 units).
D. Extracted Cervical Auscultation Features and Patients’ Characteristics
In previous investigations, it has been found that sex slightly affects some features from the AP and SI vibrations and from the swallowing sounds [41], [46]. However, according to a previous study, age seems to not significantly affect swallowing vibrations [46]. In our study, we have found that sex also does not affect swallowing vibrations or sounds. This slight difference between results may be explained by the smaller sample of men and women we have (13 females and 12 males), compared to their study (28 females and 27 males) [46]. Hence, sex effects may still remain as an open question, and future studies should investigate sex effects on a larger sample of female and male participants.
Age seems to have no effect on swallowing vibrations or sounds. In a previous study, it was found that age affects some swallowing vibrations and sounds features but their sample were divided in four age groups (18–29, 30–41, 42–53, 54–65) [46]. However, our sample age range is 44–86 years old. Hence, we lack young participants from 18 to 40 years of age to examine similar age effects on swallowing sounds and vibrations.
Our results also showed that race impacted some extracted features from swallowing vibrations and sounds. On the other hand, previous studies have found no racial differentiation in swallow physiology. Yet, our sample is very small and not racially diverse, therefore it does not provide any evidence regarding race effects.
In our study, we focused on safe swallows (PAS score < 3). A PAS score of 1 represents a healthy swallow without material penetrating the airway [19], [20]. A PAS score of 2 represents the case when a part of the bolus entered the airway but remained above the vocal folds before being totally ejected from the airway into the pharynx [19], [20]. Nevertheless, even this narrow range of PAS score seems to demonstrate an influence of some features from the ML vibrations. For a PAS score of 2, the signal of the ML sensor has a significantly larger spectral centroid and bandwidth (31.59 and 83.21 units more) than for swallows with PAS scores of 1. This result may be explained by the ejection of material from the airway producing additional signal information in the ML vibrations and in the median of the range of those frequencies. Hence, future studies should investigate whether the other PAS scores from 3 to 8 could differently affect swallowing vibrations and sounds, and whether it would be possible to classify the different PAS scores according to the dependent features from the swallowing sounds and vibrations.
In this study, we investigated the relationship between HRCA signal features and hyoid bone maximum displacement including only single unimpaired swallows (PAS = 1 or 2). Further studies should investigate these relationships in other types of swallows, such as multiple and sequential swallows. In addition, we used swallowing barium in this study as water cannot be used alone for x-ray-based studies because it needs contrast agents to be visible enough in radiographic images. The goal of using HRCA signal is to get rid of the usage of the radiological examination, thus, further studies should also compare cervical auscultation between barium and water in order to establish a new diagnostic test with water rather than barium.
A higher value of wavelet entropy for a command swallow than for a non-command swallow indicates a more ordered signal for a command swallow than for a non-command swallow. Therefore, a command swallow seems to have more disordered AP vibrations than a non-command swallow. This is logical given that a command swallow entails waiting then swallowing after a verbal command. Additionally, the swallowing sounds of command swallows have lower spectral centroid values than non-command swallows. However, it should be stated that our sample has only five non-command swallows out of 31 swallows. Therefore, the difference between command and non-command swallows still remains an open question.
V. Conclusion
In this study, we have analyzed the relationships between the hyoid bone displacement during swallowing and signal features extracted from the swallowing vibrations and sounds. We have considered 31 swallows, and obtained the time, frequency, and time-frequency domain features. The results showed relationships between the extracted features and each displacement of the hyoid bone considered: vertical and horizontal for both anterior and posterior parts of the hyoid bone. We have also verified the dependency of all the different maximal displacements of the hyoid bone towards specified patients’ characteristics such as sex, age, race origin, and command swallows. Finally, we have found dependency between some of the different patients’ characteristics and the extracted features from cervical auscultation recordings. Further research is needed to fully elucidate the potential value of HRCA in screening, or as an adjunct to diagnosis of dysphagia.
Acknowledgment
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Statement
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Grant R01HD074819.
References
- [1].Miller A. J., “The neurobiology of swallowing and dysphagia,” Develop. Disabilities Res. Rev., vol. 14, no. 2, pp. 77–86, 2008. [DOI] [PubMed] [Google Scholar]
- [2].Logemann J. A., “The evaluation and treatment of swallowing disorders,” Current Opinion Otolaryngol. Head Neck Surg., vol. 6, no. 6, pp. 395–400, Dec. 1998. [Google Scholar]
- [3].Logemann J. A., Evaluation and Treatment of Swallowing Disorders, 2nd ed. Austin, TX, USA: PRO-ED, 1998. [Google Scholar]
- [4].Miller A. J., “Neurophysiological basis of swallowing,” Dysphagia, vol. 1, no. 2, p. 91, 1986. [Google Scholar]
- [5].Wiles C. M., “The neuroscientific principles of swallowing and dysphagia,” Brain, vol. 122, no. 4, pp. 788–789, Apr. 1999. [Google Scholar]
- [6].Sherman S. L.et al. , “Biomechanical analysis of the pectoralis major tendon and comparison of techniques for tendo-osseous repair,” Amer. J. Sports Med., vol. 40, no. 8, pp. 1887–1894, Aug. 2012. [DOI] [PubMed] [Google Scholar]
- [7].Gillespie M. B., Brodsky M. B., Day T. A., Sharma A. K., Lee F., and Martin-Harris B., “Laryngeal penetration and aspiration during swallowing after the treatment of advanced oropharyngeal cancer,” Arch. Otolaryngol. Head Neck Surg., vol. 131, no. 7, p. 615, Jul. 2005. [DOI] [PubMed] [Google Scholar]
- [8].Clavé P. and Shaker R., “Dysphagia: Current reality and scope of the problem,” Nature Rev. Gastroenterol. Hepatol., vol. 12, no. 5, pp. 259–270, Apr. 2015. [DOI] [PubMed] [Google Scholar]
- [9].Katzan I. L., Cebul R. D., Husak S. H., Dawson N. V., and Baker D. W., “The effect of pneumonia on mortality among patients hospitalized for acute stroke,” Neurology, vol. 60, no. 4, pp. 620–625, 2003. [DOI] [PubMed] [Google Scholar]
- [10].Cook I. J., “Diagnostic evaluation of dysphagia,” Nature Clin. Pract. Gastroenterol. Hepatol., vol. 5, no. 7, pp. 393–403, Jul. 2008. [DOI] [PubMed] [Google Scholar]
- [11].Coyle J. L. and Robbins J., “Assessment and behavioral management of oropharyngeal dysphagia,” Current Opinion Otolaryngol. Head Neck Surg., vol. 5, no. 3, pp. 147–152, Jun. 1997. [Google Scholar]
- [12].Langmore S. E., “Evaluation of oropharyngeal dysphagia: Which diagnostic tool is superior?” Current Opinion Otolaryngol. Head Neck Surg., vol. 11, no. 6, pp. 485–489, 2003. [DOI] [PubMed] [Google Scholar]
- [13].Nierengarten M. B., “Evaluating dysphagia: Current approaches,” Oncol. Times, vol. 31, no. 14, pp. 29–30, Jul. 2009. [Google Scholar]
- [14].Steele C.et al. , “Dysphagia service delivery by speech-language pathologists in Canada: Results of a national survey,” Can. J. Speech-Lang. Pathol. Audiol., vol. 31, no. 4, pp. 166–177, 2007. [Google Scholar]
- [15].Lee J., Sejdić E., Steele C. M., and Chau T., “Effects of liquid stimuli on dual-axis swallowing accelerometry signals in a healthy population,” Biomed. Eng. Online, vol. 9, no. 1, p. 7, Feb. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Movahedi F., Kurosu A., Coyle J. L., Perera S., and Sejdić E., “Anatomical directional dissimilarities in tri-axial swallowing accelerometry signals,” IEEE Trans. Neural Syst. Rehabil. Eng., vol. 25, no. 5, pp. 447–458, May 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bonilha H. S.et al. , “Radiation exposure time during MBSS: Influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use,” Dysphagia, vol. 28, no. 1, pp. 77–85, Mar. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zammit-Maempel I., Chapple C.-L., and Leslie P., “Radiation dose in videofluoroscopic swallow studies,” Dysphagia, vol. 22, no. 1, pp. 13–15, Jan. 2007. [DOI] [PubMed] [Google Scholar]
- [19].Rosenbek J. C., Robbins J. A., Roecker E. B., Coyle J. L., and Wood J. L., “A penetration-aspiration scale,” Dysphagia, vol. 11, no. 2, pp. 93–98, 1996. [DOI] [PubMed] [Google Scholar]
- [20].Robbins J., Coyle J., Rosenbek J., Roecker E., and Wood J., “Differentiation of normal and abnormal airway protection during swallowing using the penetration-aspiration scale,” Dysphagia, vol. 14, no. 4, pp. 228–232, Aug. 1999. [DOI] [PubMed] [Google Scholar]
- [21].Coyle J. L., “Scaling the swallow,” ASHA Leader, vol. 22, pp. 36–38, May 2017. [Online]. Available: https://doi.org/10.1044/leader.OTP.22052017.36 [Google Scholar]
- [22].Hiss S. G., Treole K., and Stuart A., “Effects of age, gender, bolus volume, and trial on swallowing apnea duration and swallow/respiratory phase relationships of normal adults,” Dysphagia, vol. 16, no. 2, pp. 128–135, 2001. [DOI] [PubMed] [Google Scholar]
- [23].Dantas R. O., de Aguiar Cassiani R., dos Santos C. M., Gonzaga G. C., Alves L. M. T., and Mazin S. C., “Effect of gender on swallow event duration assessed by videofluoroscopy,” Dysphagia, vol. 24, no. 3, pp. 280–284, 2009. [DOI] [PubMed] [Google Scholar]
- [24].Palmer J. B., Hiiemæ K. M., Matsuo K., and Haishima H., “Volitional control of food transport and bolus formation during feeding,” Physiol. Behav., vol. 91, no. 1, pp. 66–70, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kurosu A. and Logemann J. A., “Gender effects on airway closure in normal subjects,” Dysphagia, vol. 25, no. 4, pp. 284–290, Dec. 2010. [DOI] [PubMed] [Google Scholar]
- [26].Rademaker A. W., Pauloski B. R., Colangelo L. A., and Logemann J. A., “Age and volume effects on liquid swallowing function in normal women,” J. Speech, Lang., Hearing Res., vol. 41, no. 2, pp. 275–284, 1998. [DOI] [PubMed] [Google Scholar]
- [27].Logemann J. A., Pauloski B. R., Rademaker A. W., and Kahrilas P. J., “Oropharyngeal swallow in younger and older women: Videofluoroscopic analysis,” J. Speech, Lang., Hearing Res., vol. 45, no. 3, pp. 434–445, Jun. 2002. [DOI] [PubMed] [Google Scholar]
- [28].Daniels S. K., Schroeder M. F., DeGeorge P. C., Corey D. M., and Rosenbek J. C., “Effects of verbal cue on bolus flow during swallowing,” Amer. J. Speech, vol. 16, no. 2, pp. 140–147, May 2007. [DOI] [PubMed] [Google Scholar]
- [29].Nagy A., Leigh C., Hori S. F., Molfenter S. M., Shariff T., and Steele C. M., “Timing differences between cued and noncued swallows in healthy young adults,” Dysphagia, vol. 28, no. 3, pp. 428–434, 2013. [DOI] [PubMed] [Google Scholar]
- [30].Van Den Engel-Hoek L., Lagarde M., and Van Alfen N., “Ultrasound of oral and masticatory muscles: Why every neuromuscular swallow team should have an ultrasound machine,” Clin. Anatomy, vol. 30, no. 2, pp. 183–193, 2017. [DOI] [PubMed] [Google Scholar]
- [31].Oliveira L. A., Fontes L. H., and Cahali M. B., “Swallowing and pharyngo-esophageal manometry in obstructive sleep apnea,” Brazilian J. Otorhinolaryngol., vol. 81, no. 3, pp. 294–300, Jun. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mielens J. D., Hoffman M. R., Ciucci M. R., Jiang J. J., and McCulloch T. M., “Automated analysis of pharyngeal pressure data obtained with high-resolution manometry,” Dysphagia, vol. 26, no. 1, pp. 3–12, Mar. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schultheiss C., Schauer T., Nahrstaedt H., and Seidl R. O., “Automated detection and evaluation of swallowing using a combined EMG/bioimpedance measurement system,” Sci. World J., vol. 2014, Jul. 2014, Art. no. 405471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fukuike C.et al. , “A novel automated detection system for swallowing sounds during eating and speech under everyday conditions,” J. Oral Rehabil., vol. 42, no. 5, pp. 340–347, 2015. [DOI] [PubMed] [Google Scholar]
- [35].Steele C. M. and Sejdić E., and Chau T., “Noninvasive detection of thin-liquid aspiration using dual-axis swallowing accelerometry,” Dysphagia, vol. 28, no. 1, pp. 105–112, Mar. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Crary M. A., Sura L., and Carnaby G., “Validation and demonstration of an isolated acoustic recording technique to estimate spontaneous swallow frequency,” Dysphagia, vol. 28, no. 1, pp. 86–94, Mar. 2013. [DOI] [PubMed] [Google Scholar]
- [37].Golabbakhsh M., Rajaei A., Derakhshan M., Sadri S., Taheri M., and Adibi P., “Automated acoustic analysis in detection of spontaneous swallows in Parkinson’s disease,” Dysphagia, vol. 29, no. 5, pp. 572–577, Oct. 2014. [DOI] [PubMed] [Google Scholar]
- [38].Ferrucci J. L., Mangilli L. D., Sassi F. C., Limongi S. C. O., and de Andrade C. R. F., “Swallowing sounds in speech therapy practice: A critical analysis of the literature,” Einstein, vol. 11, no. 4, pp. 535–539, Dec. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Afkari S., “Measuring frequency of spontaneous swallowing,” Australas. Phys. Eng. Sci. Med., vol. 30, no. 4, pp. 313–317, Dec. 2007. [PubMed] [Google Scholar]
- [40].Dudik J. M., Coyle J. L., and Sejdić E., “Dysphagia screening: Contributions of cervical auscultation signals and modern signal-processing techniques,” IEEE Trans. Human-Mach. Syst., vol. 45, no. 4, pp. 465–477, Aug. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dudik J. M., Jestrović I., Luan B., Coyle J. L., and Sejdić E., “Characteristics of dry chin-tuck swallowing vibrations and sounds,” IEEE Trans. Biomed. Eng., vol. 62, no. 10, pp. 2456–2464, Oct. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Leslie P., Drinnan M. J., Zammit-Maempel I., Coyle J. L., Ford G. A., and Wilson J. A., “Cervical auscultation synchronized with images from endoscopy swallow evaluations,” Dysphagia, vol. 22, no. 4, pp. 290–298, Oct. 2007. [DOI] [PubMed] [Google Scholar]
- [43].Leslie P., Drinnan M. J., Finn P., Ford G. A., and Wilson J. A., “Reliability and validity of cervical auscultation: A controlled comparison using videofluoroscopy,” Dysphagia, vol. 19, no. 4, pp. 231–240, Sep. 2004. [PubMed] [Google Scholar]
- [44].Zoratto D. C., Chau T., and Steele C. M., “Hyolaryngeal excursion as the physiological source of swallowing accelerometry signals,” Physiol. Meas., vol. 31, no. 6, pp. 843–855, May 2010. [DOI] [PubMed] [Google Scholar]
- [45].Kurosu A., Coyle J. L., Dudik J. M., and Sejdić E., “Detection of swallow kinematic events from acoustic high-resolution cervical auscultation signals in patients with stroke,” Arch. Phys. Med. Rehabil., to be published. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0003999318308827 [DOI] [PMC free article] [PubMed]
- [46].Dudik J. M., Jestrović I., Luan B., Coyle J. L., and Sejdić E., “A comparative analysis of swallowing accelerometry and sounds during saliva swallows,” Biomed. Eng. Online, vol. 14, no. 1, p. 3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sejdić E., Komisar V., Steele C. M., and Chau T., “Baseline characteristics of dual-axis cervical accelerometry signals,” Ann. Biomed. Eng., vol. 38, no. 3, pp. 1048–1059, Mar. 2010. [DOI] [PubMed] [Google Scholar]
- [48].Sejdić E., Steele C. M., and Chau T., “The effects of head movement on dual-axis cervical accelerometry signals,” BMC Res. Notes, vol. 3, pp. 269-1–269-6, Dec. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sejdić E., Steele C. M., and Chau T., “A method for removal of low frequency components associated with head movements from dual-axis swallowing accelerometry signals,” PLoS ONE, vol. 7, no. 3, pp. e33464-1–e33464-8, Mar. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sejdić E., Steele C. M., and Chau T., “A procedure for denoising dual-axis swallowing accelerometry signals,” Physiol. Meas., vol. 31, no. 1, p. N1, 2010. [DOI] [PubMed] [Google Scholar]
- [51].Dudik J. M., Kurosu A., Coyle J. L., and Sejdić E., “A statistical analysis of cervical auscultation signals from adults with unsafe airway protection,” J. NeuroEng. Rehabil., vol. 13, no. 1, p. 7, Jan. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Porta A.et al. , “Entropy, entropy rate, and pattern classification as tools to typify complexity in short heart period variability series,” IEEE Trans. Biomed. Eng., vol. 48, no. 11, pp. 1282–1291, Nov. 2001. [DOI] [PubMed] [Google Scholar]
- [53].Aboy M., Hornero R., Abasolo D., and Alvarez D., “Interpretation of the Lempel–Ziv complexity measure in the context of biomedical signal analysis,” IEEE Trans. Biomed. Eng., vol. 53, no. 11, pp. 2282–2288, Nov. 2006. [DOI] [PubMed] [Google Scholar]
- [54].Hu J., Gao J., and Principe J. C., “Analysis of biomedical signals by the Lempel–Ziv complexity: The effect of finite data size,” IEEE Trans. Biomed. Eng., vol. 53, no. 12, pp. 2606–2609, Dec. 2006. [DOI] [PubMed] [Google Scholar]
- [55].Kumar Y., Dewal M. L., and Anand R. S., “Relative wavelet energy and wavelet entropy based epileptic brain signals classification,” Biomed. Eng. Lett., vol. 2, no. 3, pp. 147–157, 2012. [Google Scholar]
- [56].Molfenter S. M. and Steele C. M., “Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing,” J. Speech Lang. Hearing Res., vol. 57, no. 3, pp. 768–778, Jun. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kim Y. and McCullough G. H., “Maximum hyoid displacement in normal swallowing,” Dysphagia, vol. 23, no. 3, pp. 274–279, 2008. [DOI] [PubMed] [Google Scholar]
- [58].Kang B.-S., Oh B.-M., Kim I. S., Chung S. G., Kim S. J., and Han T. R., “Influence of aging on movement of the hyoid bone and epiglottis during normal swallowing: A motion analysis,” Gerontology, vol. 56, no. 5, pp. 474–482, 2010. [DOI] [PubMed] [Google Scholar]
- [59].Molfenter S. M. and Steele C. M., “Physiological variability in the deglutition literature: Hyoid and laryngeal kinematics,” Dysphagia, vol. 26, no. 1, pp. 67–74, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]