Abstract
Recent molecular data has strongly suggested that field-collected cysts of snow algae that are morphologically identifiable as the zygotes of Chloromonas nivalis are composed of multiple species. Motile vegetative cells, however, have not been directly obtained from these cysts because of the difficulties involved in inducing their germination. Recently, our comparative molecular analyses, using both field-collected and cultured materials, demonstrated that one Japanese lineage of “C. nivalis zygotes” belongs to C. miwae. Herein, we examined another Japanese lineage of field-collected “C. nivalis zygotes” and a new strain originating from Japan. Our molecular data demonstrated that these two different life cycle stages are conspecific, and that they represent a new species that we herein describe as C. muramotoi sp. nov., based on the vegetative and asexual morphological characteristics of the strain. Multigene phylogenetic analyses showed that this new species was sister to C. miwae. Scanning electron microscopy demonstrated that the cysts of C. muramotoi are different from those of C. miwae, based on the arrangement of the flanges developing on the cell wall.
Introduction
Snowfields and snowpacks are generally considered extreme environments due to e.g. low temperature, excessive irradiation, and minimal nutrients. However, many species of unicellular microalgae have adapted to such harsh environmental conditions and exclusively thrive within melting snow during spring to summer [1–5]. Blooms of snow-inhabiting microalgae can stain snow green, red, or other colors, and such phenomena are known as “colored snow.” Among snow algae, species belonging to the genus Chloromonas Gobi (Volvocales, Chlorophyceae) sensu Pröschold et al. [6] (i.e. Chloromonadinia clade [7]) are typical. Most of the species are phylogenetically close and form a robust monophyletic group (subclade 2 of clade A [8,9] or SA clade [10]) within Chloromonadinia clade. In snow-inhabiting Chloromonas spp., it is known that striking morphological changes from vegetative flagellates to nonmotile cysts/zygotes occur [9,11–15]. Generally, biflagellate vegetative cells are dominant in greenish snow, and nonmotile, dormant cysts or zygotes with a thick cell wall, which accumulate orange carotenoid pigments within the cell, dominate in reddish snow [2–5].
Nonmotile cells or cysts of spindle shape with approximately five to eight flanges on the cell wall are frequently observed within greenish or reddish snowpacks in mid-latitude mountainous areas, as well as in polar regions [1]. According to the species diagnoses from previous studies [12,13], such globally distributed cysts were identified as the zygote stage of Chloromonas nivalis (Chodat) Hoham & Mullet. Vegetative cells directly obtained from field-collected cysts identified as C. nivalis zygotes, however, have not been reported yet. This is possibly due to the difficulty of inducing germination of cysts or zygotes of snow-inhabiting Chloromonas species, under controlled laboratory conditions.
Based on short sequences of the large subunit of the RuBisCO (rbcL) gene, Muramoto et al. [16] suggested that Japanese “C. nivalis zygotes” contain at least two independent lineages or species. In addition, they demonstrated the presence of two types of flanges developing on the zygote wall (uniformly straight or sigmate) using scanning electron microscopy (SEM). Correspondence between the two lineages and the two types of flange forms, however, was not revealed. Recently, we established a method for obtaining long sequences of multiple DNA regions from field-collected cysts or zygotes of species of snow-inhabiting Chloromonas [17]. By comparing the genetic differences in multiple DNA regions, we showed that field-collected cysts morphologically assignable to C. nivalis zygotes contained at least four distinct lineages or species; one of which was considered conspecific with C. miwae (Fukushima) Muramoto et al. Motile vegetative cells corresponding to other lineages of field-collected “C. nivalis zygotes,” however, have not been observed. In addition, our recent study demonstrated that the North American strain, with vegetative cells morphologically identifiable as C. nivalis, was phylogenetically separated from the “C. nivalis zygotes” with molecular data originating from Europe and Japan [18].
Herein, we examined one Japanese lineage of field-collected “C. nivalis zygotes” and a new strain of snow-inhabiting Chloromonas originating from Japan. Our analyses of multiple DNA regions demonstrated that these two different life cycle stages are conspecific, and that they represent a new species that we describe as C. muramotoi Matsuzaki et al. sp. nov., based on vegetative and asexual characteristics. In addition, we compared the cysts of this new species with those of field-collected cysts of the sister species C. miwae, using field emission SEM (FE-SEM).
Materials and methods
Ethics statement
We collected colored snow from snowpacks in three mountainous areas of Japan: Mt. Gassan in Bandai-Asahi National Park, Mt. Hakkoda in Towada-Hachimantai National Park, and Mt. Tateyama in Chubusangaku National Park. Collection locations and details are shown in S1 Table. No specific permission was required for the present investigation, since collection of snowpacks containing microalgae or other protists from Natural Parks is not legally restricted in Japan. In addition, we confirmed that the field-collected material did not contain protected organisms.
Observation of field-collected and cultured materials
Colored snow with nonmotile cysts/zygotes of snow-inhabiting Chloromonas was collected from summer snowpacks on Mt. Hakkoda, Aomori, Japan, on May 18, 2016 and in Mt. Tateyama, Toyama, Japan, on Jun 12, 2016 (S1 Table). Collection and preservation methods for the colored snow samples were carried out as described previously [19]. Field-collected cysts were identified to species level based on the species descriptions from previous studies [12,13]. Light microscopy (LM) was used to examine the specimens with a BX51 microscope equipped with Nomarski differential interference optics or a CKX41 microscope (Olympus Corp., Tokyo, Japan). For FE-SEM, field-collected cysts were cleaned using a modified sterilizing method [17,20] at room temperature (22–25°C). The samples were subsequently mounted on aluminum stubs with carbon conductive double-faced adhesive tape (Nisshin EM Co. Ltd., Tokyo, Japan) and were air-dried for more than 12 h at 23°C, 25–30% relative humidity. After coating with osmium under the osmium coater HPC-15 (Vacuum Device Corp., Ibaraki, Japan), the cells were observed using a S-4800 field emission scanning electron microscope (Hitachi High-Technologies Corp., Tokyo, Japan). For comparison, we also carried out FE-SEM on C. miwae cysts within a single snow sample collected from Mt. Gassan, Yamagata, Japan, on Jun 30, 2013, as described above; cysts from the sample were used for the specimen “C. nivalis zygotes” (Gassan-C) in our previous study [17]. To determine the flange characteristics, we observed 300 cells of “C. nivalis zygotes” or C. miwae cysts per field-collected sample under FE-SEM.
Chloromonas muramotoi strain HkCl-57 was isolated using the spread plate method [21] from a green snow sample collected from a snowpack in the Hakkoda Botanical Garden of Tohoku University, Mt. Hakkoda, Aomori, Japan, on May 16, 2012 (S1 Table). This strain was deposited as NIES-4284 in the Microbial Culture Collection at the National Institute for Environmental Studies [22]. The culture was maintained on an AF-6 medium (liquid or 1.5% agar slants; see [22]) at 5°C, with a light:dark cycle of 14:10 h under cool-white light-emitting diodes (color temperature = 5000 K) at 35–90 μmol m−2 s−1. Light and epifluorescence microscopy and transmission electron microscopy (TEM) of the strain were performed as described previously [18]. The method for inducing sexual reproduction by nitrogen starvation was according to a previous study [23].
Molecular analysis
The method used for extraction of total DNA from the 50 field-collected cysts or the strain HkCl-57 follows that of previous studies [17,24]. The nucleotide sequences of the nuclear-encoded small and large subunits (SSU and LSU, respectively) of ribosomal DNA (rDNA), internal transcribed spacer 2 (ITS2) region of nuclear rDNA, ATP synthase beta subunit (atpB), P700 chlorophyll a apoprotein A2 (psaB), and rbcL genes were determined by direct sequencing of PCR products as described previously [17], but using newly designed specific primers (S2 Table) from the three specimens of field-collected cysts morphologically identifiable as the zygotes of C. nivalis (Hakkoda-Green, Tateyama-Green, and Tateyama-Orange; S1–S3 Figs) and the strain HkCl-57. Since the direct sequencing methodology for the DNA samples extracted from the Japanese cysts resulted in unambiguous data, we did not clone the PCR products.
For the multigene phylogenetic analysis, we used the 31 operational taxonomic units (OTUs) examined in a previous study [18], as well as three specimens of 50 isolated “C. nivalis zygotes” and C. muramotoi strain HkCl-57 (S3 Table); all belonging to the genus Chloromonas sensu Pröschold et al. [6] or Chloromonadinia clade [7]. Since the phylogenetic relationships within the monophyletic group composed entirely of snow species (corresponding to SA clade [10]) were analyzed, we treated the 13 strains that were representatives of the sister group of the SA clade [8–10,25] as the outgroup (S3 Table). The sequences of SSU and LSU rDNA, atpB, and psaB genes from the OTUs were aligned to the 5,497 base pairs as described previously [25–27]. The resulting data matrix was subjected to Bayesian inference (BI), maximum likelihood (ML), maximum parsimony (MP), and neighbor-joining (NJ) analyses, following methods described in a previous study [18]. Since unusual rbcL gene substitutions in Chloromonas are common and may result in artefacts [17,26,28], we did not concatenate the rbcL gene sequences with the data matrix.
For comparison of the previously published sequence data of field-collected cysts identified as C. nivalis zygotes, we performed single-gene phylogenetic analyses with wide taxon sampling using rbcL gene sequences as described above. Additional OTUs were selected according to previous studies [16,29,30] and are shown in S3 Table. The substitution models for each phylogenetic analysis are described in S4 Table. The data matrices used in this study are available from TreeBASE [31] (matrix accession number, S23486). Methods for annotation and prediction of the secondary structure of the nuclear rDNA ITS2 region follow those described in a previous study [27]. For detecting compensatory base changes (CBCs), the ITS2 sequences were aligned based on a sequence-structure analysis [32] using 4SALE [33,34].
Nomenclature
The electronic version of this article in Portable Document Format (PDF) in a work with an ISSN or ISBN will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) (https://www.iapt-taxon.org/nomen/pages/intro/title_page.html), and hence the new names contained in the electronic publication of a PLOS article are effectively published under that Code from the electronic edition alone, so there is no longer any need to provide printed copies.
Results
Morphological observation of field-collected cysts
The characteristics of the cysts collected from Mt. Hakkoda and Mt. Tateyama in Japan were observed to be almost identical. They also corresponded to those of the zygotes of C. nivalis [12,13]. Cells were spindle-shaped or ellipsoid, 9.1–13.4 μm wide and 15.6–22.4 μm long, with several flanges on the wall (Fig 1A and 1B). The cells from Mt. Hakkoda lacked visible accumulations of carotenoid pigments within the protoplast (specimen Hakkoda-Green; S1 Fig). Conversely, those from Mt. Tateyama were subdivided into two types based on the presence or absence of a large quantity of carotenoid pigments within the cell. Thus, cells of either type were selected and assigned to a single specimen or OTU for our molecular analyses [specimens Tateyama-Green (S2 Fig) and Tateyama-Orange (S3 Fig), respectively].
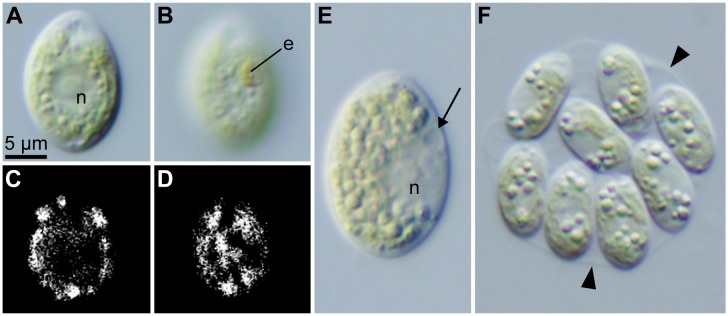
Fig 1. Morphological observation of field-collected cysts/zygotes of snow-inhabiting Chloromonas.
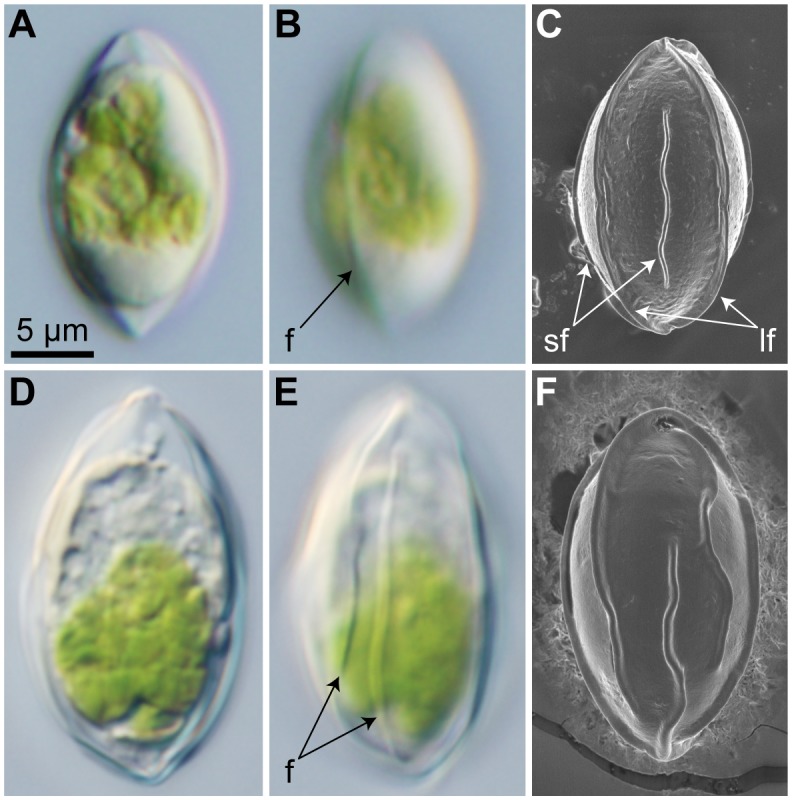
Identical magnification throughout. For detailed information of collection sites, see S1 Table. (A–C) “C. nivalis zygotes” from site 160518Hk2G1 in Mt. Hakkoda, Japan. (A, B) Light micrographs. (A) Optical section. (B) Surface view, showing a flange (f). (C) Field emission scanning electron micrograph. Abbreviations: lf, long flange (extending the entire cell length); sf, short flange (reaching neither pole of the cell). (D–F) C. miwae cysts from site 130630Gs4G in Mt. Gassan, Japan. (D, E) Light micrographs. (D) Optical section. (E) Surface view, showing flanges (f). (F) Field emission scanning electron micrograph.
The results of the FE-SEM revealed that the field-collected “C. nivalis zygotes” possessed approximately eight flanges at the equatorial plane. The flanges were straight or slightly undulant and could be classified into two types, either long or short (Fig 1C). In each cell, four long flanges (lf in Fig 1C) reached to both poles of the cell, whereas the remaining four short flanges (sf in Fig 1C) were medially located and extended to neither pole. Each short flange was positioned between two long flanges. Bifurcated flanges were not observed, while segmentation and overlapping of flanges was occasionally observed. The cells with those characteristics occupied 59.3% (178/300) and 82.3% (247/300) of “C. nivalis zygotes” within the field-collected materials from Mt. Hakkoda and Mt. Tateyama, respectively. Although the size and shape of the cells resembled those of the cysts of C. miwae (which are morphologically assignable to C. nivalis zygotes [17]) under LM (Fig 1D and 1E), the regular arrangement of the two types of flanges as shown in Fig 1C was hardly observed (1/300) in C. miwae cysts under FE-SEM (Fig 1F). The number of flanges was commonly 8–10 at the equatorial plane in the cysts of C. miwae.
Molecular phylogenetic analyses
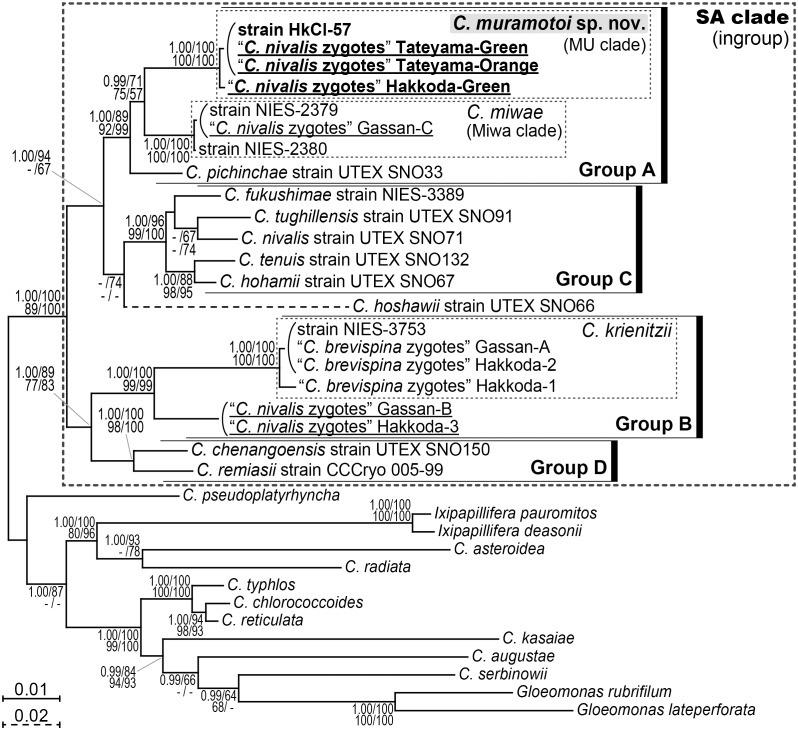
The results of our multigene phylogenetic analysis (Fig 2) essentially corresponded with previous results for species of Chloromonas living in snow [18], i.e., four robust monophyletic groups (A–D) and an independent lineage of C. hoshawii Matsuzaki et al. were resolved. Three Japanese specimens of “C. nivalis zygotes,” Hakkoda-Green, Tateyama-Green, and Tateyama-Orange, were included within group A, and they formed a small robust subclade (‘MU’ clade) with a Japanese strain HkCl-57, with 1.00 posterior probability (PP) in BI and 100% bootstrap values (BV) in ML, MP, and NJ analyses. Group A also contained another robust subclade (Miwa clade) which was composed of a previously examined Japanese specimen of “C. nivalis zygotes,” Gassan-C [17], and two Japanese strains of C. miwae (1.00 PP in BI and 100% BV in ML, MP, and NJ analyses). This subclade was considered as a single species in our recent study [17]. These subclades were sister to each other with moderate statistical support (0.99 PP in BI and 67–75% BV in ML, MP, and NJ analyses). C. pichinchae Wille strain UTEX SNO33 from North America was the most basal within group A. The other Japanese specimens of “C. nivalis zygotes” examined previously (Gassan-B and Hakkoda-3 [17]) were positioned within group B. In addition, the C. nivalis strain UTEX SNO71, originating from North America, was included within group C.
Fig 2. Bayesian phylogenetic tree of snow-inhabiting Chloromonas spp. based on 5,497 base pairs from four genes.
The small subunit and large subunit of rDNA, and the first and second codon positions of atpB and psaB genes were partitioned and unlinked (S4 Table). Specimens of field-collected “C. nivalis zygotes” are underlined. Corresponding posterior probabilities (0.95 or more) are shown at the top left. Numbers shown at the top right, bottom left, and bottom right indicate bootstrap values (50% or more) in the maximum likelihood, maximum parsimony, and neighbor-joining analyses, respectively.
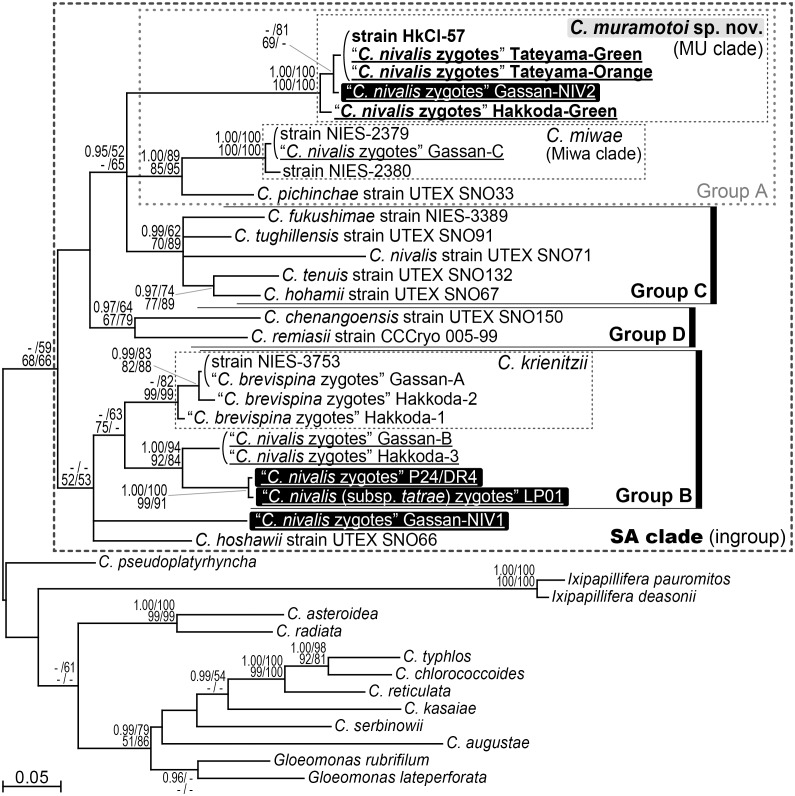
For the rbcL-based phylogenetic analysis with wide taxon sampling (Fig 3), groups B–D in the present multigene phylogenetic tree (Fig 2) were reconstructed with lower statistical support values. Conversely, group A in Fig 2 was not resolved in the rbcL tree, although the MU and Miwa clades were robustly resolved as shown in Fig 2. This was possibly due to the unusual rbcL gene substitution in Chloromonas reported by previous studies [17,26,28]. The MU clade, in the rbcL-based tree, contained a previously examined Japanese specimen of “C. nivalis zygotes,” Gassan-NIV2 [16], in addition to the three specimens of “C. nivalis zygotes” (Hakkoda-Green, Tateyama-Green, and Tateyama-Orange) and the strain HkCl-57 (with 1.00 PP in BI and 100% BV in ML, MP, and NJ analyses). This robust subclade was phylogenetically separated from other specimens of “C. nivalis zygotes” collected from Europe (LP01 [30] and P24/DR4 [29,30] within group B) as well as those from Japan (Gassan-B and Hakkoda-3 [17] within group B, Gassan-C [17] within the Miwa clade in group A, and Gassan-NIV1 [16] located just outside of group B).
Fig 3. Bayesian phylogenetic tree of snow-inhabiting Chloromonas spp. based on 340–1,128 base pairs of rbcL.
Each codon position was partitioned and unlinked (S4 Table). Specimens of field-collected “C. nivalis zygotes” are underlined. The operational taxonomic units not included in Fig 2 are highlighted in black. Corresponding posterior probabilities (0.95 or more) are shown at the top left. Numbers shown at the top right, bottom left, and bottom right indicate bootstrap values (50% or more) in the maximum likelihood, maximum parsimony, and neighbor-joining analyses, respectively.
Comparative analyses of internal transcribed spacer 2
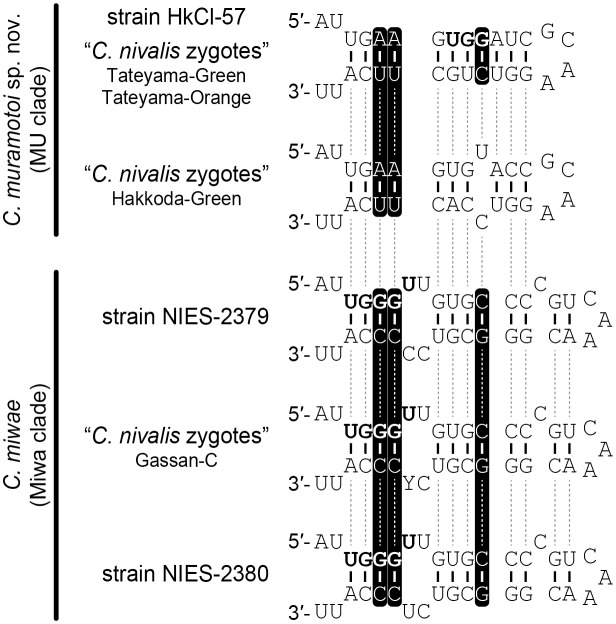
Within the MU clade (Fig 2), the number of nucleotide differences in the entire ITS2 region ranged from 0–10 and no CBCs were detected (S4 Fig). Conversely, at least two or three CBCs were detected between the MU and Miwa clades, even near the branch encompassing the YGGY motif in ITS2 helix III, the most conserved region of ITS2 [35] (Fig 4).
Fig 4. Comparison of the tip of internal transcribed spacer 2 (ITS2) helix III between MU and Miwa clades.
For the complete ITS2 secondary structures within the MU clade (= C. muramotoi sp. nov.), see S4 Fig. Secondary structures of ITS2 within the Miwa clade (= Chloromonas miwae) are based on previous results [17]. Black backgrounds indicate compensatory base changes between the two clades. Boldfaces marks the YGGY motif.
Morphological observation of the Chloromonas muramotoi sp. nov. strain HkCl-57
Under light and fluorescent microscopy, vegetative cells were solitary and ovoid or spindle-shaped with a rounded posterior end, 8.5–13.3 μm wide and 12.3–19.5 μm long (Figs 5 and 6A). Each cell had two equal flagella at the anterior end, a single chloroplast, two contractile vacuoles near the base of the flagella, and a single nucleus, without a prominent anterior papilla (Figs 5 and 6A). The chloroplast was cup-shaped with an eyespot and was lacking pyrenoids (Figs 5 and 6A–6C). The chloroplast was seemingly composed of angular discs, showing irregular incisions in the surface view (Figs 5, 6B and 6D). The eyespot was D-shaped to rod-shaped, positioned in the anterior half to one third of the cell (Figs 5 and 6B). The nucleus was almost spherical, located in the center of the protoplast (Figs 5 and 6A). The flagella were of equal length to the whole cell.
Fig 5. Vegetative cells of Chloromonas muramotoi sp. nov.: Line drawings.
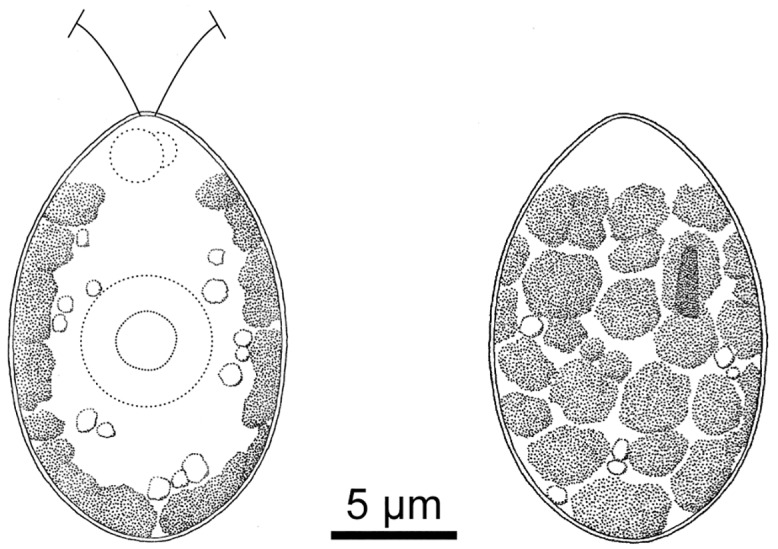
Anterior end of the cell is arranged upward. Left, optical section. Right, surface view.
Fig 6. Vegetative and asexual characteristics of Chloromonas muramotoi sp. nov. strain HkCl-57: Light micrographs.
Identical magnification throughout. Abbreviations: e, eyespot; n, nucleus. (A–D) Vegetative cell. Anterior end of the cell is arranged upward. (A) Optical section. (B) Surface view. (C) Epifluorescence image of (A). (D) Epifluorescence image of (B). (E, F) Asexual reproduction. (E) Just before the first transverse division, showing the position of a parental contractile vacuole (arrow). (F) Autosporangium with eight daughter cells within the parental cell wall (arrowheads).
Asexual reproduction was via zoospore formation. Just before the first cell division, the protoplast rotated, and the parental contractile vacuoles moved to the equator of the parent cell (Fig 6E). The protoplast then divided transversely. Generally, two, four, or eight daughter cells were produced within the parental cell wall (Fig 6F). Aggregates of 16 or more cells resulting in repeated division of daughter cells within the parental cell wall [15,18,27] were not produced even in the two-month-old cultures. Sexual reproduction was not observed in the culture even under nitrogen starvation; nor was the production of cysts. Cells of C. muramotoi did not grow at 20°C after cultivation for 2 weeks, as described in previous reports of other species of snow-inhabiting Chloromonas [17,18,27,36].
Examination by TEM showed that each cell had a cup-shaped chloroplast without pyrenoid matrices and a centrally located nucleus (Fig 7A). Mitochondria and Golgi bodies were located between the nucleus and chloroplast. In addition, several mitochondria were also recognized near the surface region of the cell, surrounded by chloroplast profiles. As in other snow Chloromonas species, small vacuoles with crystalline content were observed in the cytoplasm (e.g. [18]). In the tangential sections of the cell, the chloroplast profiles were almost angular (Fig 7B), corresponding with the LM results (Fig 6B and 6D). The eyespot was composed of a single layer of electron-dense globules (Fig 7C).
Fig 7. Vegetative cells of Chloromonas muramotoi sp. nov. strain HkCl-57: Transmission electron micrographs.
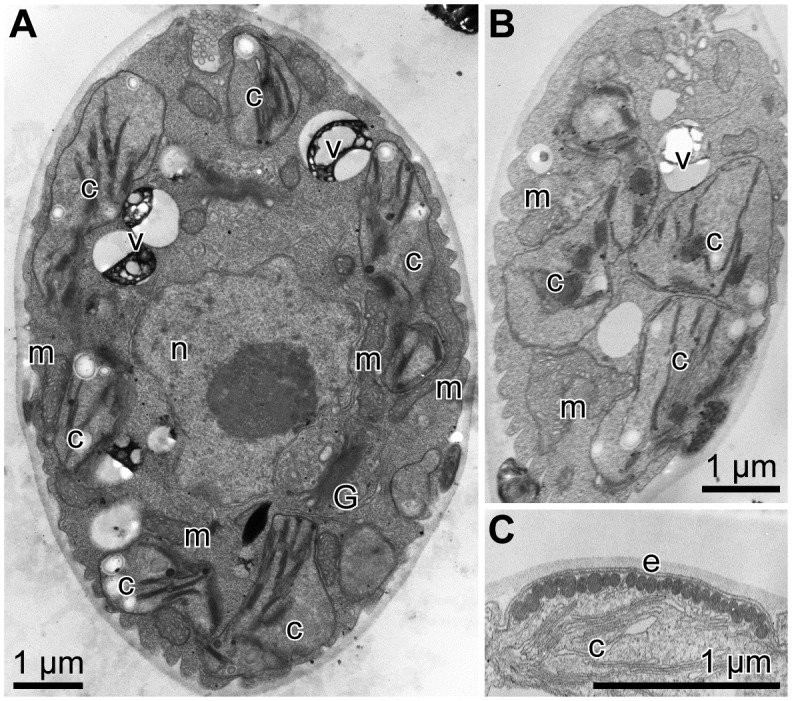
Abbreviations: c, chloroplast; e, eyespot; G, Golgi body; m, mitochondrion; n, nucleus; v, vacuole with crystalline content. (A) Longitudinal cell section. (B) Tangential cell section, showing angular chloroplast profiles. (C) Eyespot composed of a single layer of electron-dense globules.
Discussion
The multigene phylogenetic tree presented herein shows that the three Japanese specimens of “C. nivalis zygotes,” Hakkoda-Green, Tateyama-Green, and Tateyama-Orange, are closely related to the C. muramotoi strain HkCl-57. This species lacks pyrenoids within the chloroplast, even when examined under TEM, and phylogenetically belongs to Chloromonadinia clade, corresponding to both traditional [37,38] and phylogenetically revised [6] generic diagnoses of Chloromonas. Among snow-inhabiting Chloromonas species, vegetative cells of C. muramotoi resemble those of C. brevispina (F.E. Fritsch) Hoham et al. in having ovoid cell shapes without a prominent anterior papilla [14]. In addition, vegetative cells of C. alpina Wille, C. miwae, and C. pichinchae that lack a prominent anterior papilla are sometimes ovoid or elongate-ovoid [11,19,27,39] and are similar to those of C. muramotoi. C. muramotoi, however, is distinguished from those four snow-inhabiting species by vegetative cell size, chloroplast morphology, presence of an eyespot, and the lack of production of cell aggregates (resulting from repeated divisions of daughter cells retained within the parental cell wall [15,18,27]) in culture (Table 1).
Table 1. Vegetative morphological characteristics of five snow-inhabiting Chloromonas species.
| C. muramotoi sp. nov. | C. alpina | C. brevispina | C. miwae | C. pichinchae | |
|---|---|---|---|---|---|
| Strain(s) | HkCl-57 | − | − | NIES-2379, NIES-2380 | UTEX SNO33 |
| Cell shape | ovoid or spindle-shaped with a rounded posterior end | ellipsoidal to ovoid | ellipsoidal to ovoid or pyriform | spherical or ovoid | elongate-ovoid to ellipsoidal |
| Cell width × cell length (μm) | 8.5–13.3 × 12.3–19.5 | 4–7 × 9–12 | 5–13 × 10–19 | 10–13 × 9–15 | 8–15 × 18–26 |
| Chloroplast shape | cup-shaped, seemingly composed of angular discs | parietal, seemingly composed of numerous discoid lobes obviously separated from each other | cup-shaped | cup-shaped | cup-shaped, seemingly composed of angular discs |
| Eyespot | present | present | absent | Absent | absent |
| Cell aggregates in culture | not observed | − | − | − | observed |
| References | present study | [39] | [14] | [19] | [11,27,39] |
Although C. muramotoi was isolated from a green snow sample, a comparison of vegetative morphology with Chloromonas species not sampled from snow is still informative. Based on Ettl [37,38], C. muramotoi resembles two species collected from ponds, C. gutenbrunnensis Wawrik and C. hyperstigmata (H. Ettl) H. Ettl & Gerloff, in possessing ovoid vegetative cells and a cup-shaped chloroplast with irregular incisions and in lacking a prominent anterior papilla. C. muramotoi differs from C. gutenbrunnensis, however, in its smaller vegetative cells (8.5–13.3 μm wide and 12.3–19.5 μm long vs. up to 23 μm wide and up to 27 μm long) and eyespot positions [anterior half to one third (Figs 5 and 6B) vs. posterior half [40]]. Chloromonas hyperstigmata possesses quite a large disc-shaped eyespot (diameter up to one fourth of the cell length [41]), whereas, such a large eyespot has never been observed in C. muramotoi (Figs 5 and 6B). Thus, C. muramotoi represents a new morphological species of the genus Chloromonas, even after careful verification.
Chloromonas gutenbrunnensis was collected from an ice-covered pond in winter [40], and C. hyperstigmata was sampled from a small pond with many fallen leaves in autumn [41]. Therefore, the two species possibly adapt to low temperatures. Interestingly, the vegetative cells of most of the species within SA clade (which is composed entirely of snow algae; Fig 2) possess a chloroplast seemingly subdivided into several lobes or sections [9,18,27]. This indicates that such the chloroplast morphology might be a survival strategy for low temperatures. For instance, the ratio of surface to volume of the chloroplast is in this case better, and an exchange of metabolites with cytoplasm is probably facilitated. Therefore, the morphological similarity of the chloroplasts between C. muramotoi and the two species not collected from snow may relate to the physiological adaptation to low temperatures.
Within the MU clade (see Fig 2), the genetic differences in a rapidly evolving DNA region (nuclear rDNA ITS2) were observed to be 0.0–4.1%. These results range from being much smaller than to being almost identical to the intraspecific genetic differences in the same region of mesophilic Chloromonas reticulata (Goroschankin) Gobi or Chlamydomonas reinhardtii P.A. Dangeard (3.3–4.1%) (S5 Fig). In addition, genetic differences in the nuclear-encoded SSU and LSU rDNA and the chloroplast-encoded atpB and psaB genes within the MU clade were much smaller than those between snow-inhabiting Chloromonas hohamii H.U. Ling & Seppelt and C. tenuis Matsuzaki & Nozaki, or between mesophilic C. chlorococcoides (H. Ettl & K. Schwarz) Matsuzaki et al. and C. reticulata (sister species delineated by morphological and molecular data [27,42]) (S6 Fig). Since the comparison of p-distances in these regions did not show differences that were sufficient for distinguishing between species, we concluded that the MU clade should be treated as a single species, namely, C. muramotoi. In this case, visible carotenoid accumulation observed in the cysts from Mt. Tateyama (assigned to the specimen Tateyama-Orange; S3 Fig) is considered to be a protective reaction against excessive visible and ultraviolet irradiation (see S1 Table). Although C. muramotoi was sister to C. miwae (or the Miwa clade [17,19]) (Fig 2), the separation of the two is supported by the differences in vegetative morphology (Table 1), the presence of at least two or three CBCs in the most conserved region of the ITS2 secondary structures (corresponding with the separation of biological species [43]) (Fig 4), and genetic differences (S6 Fig).
Among the specimens of field-collected “C. nivalis zygotes” with available molecular data, the Japanese specimen Gassan-NIV2 [16] is robustly positioned within the MU clade (= C. muramotoi) (Fig 3). The available sequence for the specimen (433 base pairs of rbcL) is identical to the region of the C. muramotoi strain HkCl-57 and the two specimens of field-collected cysts (Tateyama-Green and Tateyama-Orange). In addition, a previous study that reported the molecular data of Gassan-NIV2 also exhibited two types of flange morphologies in Japanese “C. nivalis zygotes” [16], one of which (straight flanges; fig 3 in [16]) is morphologically similar to the cysts of C. muramotoi (specimens Hakkoda-Green, Tateyama-Green, and Tateyama-Orange; Fig 1C). Thus, we considered that the specimen Gassan-NIV2 may belong to C. muramotoi, although the morphological characteristics of the specimen at SEM level are unclear.
The present FE-SEM and molecular data indicate that the cysts of C. muramotoi (which resemble the zygotes of North American C. nivalis) possess eight regularly arranged flanges (Fig 1C). Among the field-collected C. nivalis zygotes previously examined by SEM (e.g. [12,16,29,30,44]), this flange arrangement has not been reported, excluding the Japanese C. nivalis zygotes (see above; S5 Table). Although the morphological variability of flanges has been treated as intraspecific variation [12,13], our FE-SEM results suggest that the flange arrangement of the cysts of C. muramotoi could be distinguished from that of its sister species, C. miwae (Fig 1C and 1F). The result indicates that ultrastructural characteristics of the cysts might be useful taxonomic traits at species level, in snow-inhabiting species of Chloromonas.
In addition to field-collected “C. nivalis zygotes,” recent molecular studies have also indicated the necessity for taxonomic re-examination of field-collected nonmotile cells that have been morphologically identified as the cysts or zygotes of snow-inhabiting Chloromonas spp. For instance, part of Japanese cysts assigned to the zygotes of C. brevispina are actually conspecific with C. krienitzii [17]. Scotiella cryophila Chodat (Chlorococcales, Chlorophyceae) is considered to be the asexual cyst of C. rosae var. psychrophila Hoham et al. [8]; however, field-collected cysts identifiable as S. cryophila originating from Austrian Alps were phylogenetically separated from the authentic strain of C. rosae var. psychrophila from North America [45]. Moreover, part of nonmotile spherical red cells morphologically identified as the cysts of Chlamydomonas nivalis (F.A. Bauer) Wille, one of the most famous snow-inhabiting green algae, also seem to belong to the genus Chloromonas [46]. Thus, further taxonomic studies of cultured materials as well as increasing molecular data of field-collected cysts/zygotes of snow algae will be required for better understanding of accurate taxonomy, actual diversity, and distribution of snow-inhabiting Chloromonas species.
Taxonomic treatment
Chloromonas muramotoi Matsuzaki, Nozaki & Kawachi sp. nov.
Vegetative cell solitary, with two flagella, without prominent anterior papilla. Cells ovoid or spindle-shaped with rounded posterior end, 8.5–13.3 μm wide and 12.3–19.5 μm long. Cells with central nucleus and single cup-shaped chloroplast. Chloroplast apparently composed of angular disks, with irregularly incised surface and eyespot, without pyrenoids. Eyespot D-shaped to rod-shaped, positioned in anterior half to one third of the cell. Asexual reproduction by formation of usually two, four, or eight zoospores; protoplast rotation prior to the first cell division. Cell aggregates not observed in culture. Sexual reproduction unknown. Zygotes or cysts spindle-shaped or ellipsoid, 9.1–13.4 μm wide and 15.6–22.4 μm long. Cells with commonly eight flanges attached to cell wall; flanges straight or slightly undulant, subdivided into two types; four long flanges reaching to both poles of the cell, four short flanges reaching neither pole of the cell. Each short flange positioned between two long flanges. Flanges not bifurcated generally but segmented and overlapped occasionally.
Holotype: Specimen TNS-AL-58957 deposited at TNS (National Museum of Nature and Science, Tsukuba, Japan); material consists of resin-embedded vegetative cells from strain HkCl-57.
Strain examined: HkCl-57 (Table 1). This strain was deposited as NIES-4284 at the Microbial Culture Collection at the National Institute for Environmental Studies [22].
Etymology: The species epithet ‘muramotoi’ is in honor of Mr. Kyohei Muramoto who was the first to indicate that the Japanese specimens of “C. nivalis zygotes,” Gassan-NIV2 were a separate species using molecular data [16] (Fig 3).
Type locality: 40°38.82′ N, 140°51.08′ E; the Hakkoda Botanical Garden of Tohoku University, Mt. Hakkoda, Aomori, Japan.
Distribution: 40°39.22′ N, 140°50.97′ E; Mt. Hakkoda, Aomori, Japan. 36°35.78′ N, 137°36.63′ E; Mt. Tateyama, Toyama, Japan. The Yamagata Prefectural Nature Conservation Park, Mt. Gassan, Yamagata, Japan [16].
Supporting information
Identical magnification throughout. Scale bar = 10 μm.
(JPG)
Identical magnification throughout. Scale bar = 10 μm.
(JPG)
Identical magnification throughout. Scale bar = 10 μm.
(JPG)
The 3' end of the 5.8S ribosomal RNA (rRNA) and the 5' end of the large subunit (LSU) rRNA are shown (DDBJ/ENA/GenBank accession number: LC438455). The sequences of this region are identical among the strain (HkCl-57) and the two specimens of field-collected “C. nivalis zygotes” (Tateyama-Green and Tateyama-Orange; DDBJ/ENA/GenBank accession number: LC438456 and LC438457, respectively). Differences between the strain and the specimen of field-collected “C. nivalis zygotes” (Hakkoda-Green; DDBJ/ENA/GenBank accession number: LC438458) are described just outside the structure. Note U-U mismatch in helix II (arrowheads) and the YGGY motif on the 5' side near the apex of helix III (boldface), common structural hallmarks of eukaryotic ITS2 secondary structures [47,48].
(TIF)
(1) Chloromonas muramotoi strain HkCl-57 vs. a specimen of “C. nivalis zygotes,” Tateyama-Green. (2) Strain HkCl-57 vs. a specimen of “C. nivalis zygotes,” Tateyama-Orange. (3) Strain HkCl-57 vs. a specimen of “C. nivalis zygotes,” Hakkoda-Green, (4) C. reticulata strains, UTEX 1970 (epitype strain proposed by Pröschold et al. [6]) vs. SAG 26.90. (5) C. reticulata strains, UTEX 1970 vs. SAG 32.86. (6) Chlamydomonas reinhardtii strains, SAG 11-32a (which can be crossed with the strain SAG 11-32b, the epitype strain of this species [49]) vs. NIES-2463.
(TIF)
Black: nuclear-encoded 1,748 bases of the small subunit (SSU) ribosomal DNA (rDNA). Green: nuclear-encoded 2,017 bases of the large subunit (LSU) rDNA. Red: chloroplast-encoded 1,128 bases of ATP synthase beta subunit gene (atpB). Blue: chloroplast-encoded P700 chlorophyll a apoprotein A2 gene (psaB). Note that the sequences from the two specimens of “Chloromonas nivalis zygotes,” Tateyama-Green and Tateyama-Orange, were identical in the regions examined. The nucleotide differences between snow-inhabiting and mesophilic sister species (C. hohamii vs. C. tenuis and C. chlorococcoides vs. C. reticulata) are according to a previous study [27]. (1) The C. muramotoi strain HkCl-57 vs. a specimen of “C. nivalis zygotes,” Tateyama-Green. (2) Strain HkCl-57 vs. a specimen of “C. nivalis zygotes,” Hakkoda-Green. (3) Strain HkCl-57 vs. the C. miwae strain NIES-2380. (4) Strain HkCl-57 vs. the C. miwae strain NIES-2379. (5) The C. hohamii strain UTEX SNO67 vs. the C. tenuis strain UTEX SNO132. (6) The C. chlorococcoides strain SAG 15.82 (authentic strain) vs. the C. reticulata strain UTEX 1970 (epitype strain proposed by Pröschold et al. [6]).
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We are grateful to Dr. Koji Yonekura (Tohoku University, Japan) for his kind help for collecting colored snow in Mt. Hakkoda. We also thank Ms. Yasuko Yoshikawa and Ms. Shizuko Kinoshita (National Institute for Environmental Studies, Japan) for their kind support in transmission electron microscopy.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
RM was supported by Grant-in-Aid for JSPS Research Fellow (No. 16J09828) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT)/Japan Society for the Promotion of Science (JSPS) KAKENHI (https://www.jsps.go.jp/english/e-grants/). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kol E. Kryobiologie. Biologie und Limnologie des Schnees und Eises Kryovegetation I. In: Elster H-J, Ohle W, editors. Die Binnengewässer 24. Stuttgart: E. Schweizerbart’sche Verlagsbuchhandlung (Nägele u. Obermiller); 1968. p. 1–216 with 16 pls. German. [Google Scholar]
- 2.Hoham RW. Unicellular chlorophytes—snow algae In: Cox ER, editor. Phytoflagellates. New York: Elsevier-North Holland; 1980. p. 61–84. [Google Scholar]
- 3.Hoham RW, Duval B. Microbial ecology of snow and freshwater ice with emphasis on snow algae In: Jones HG, Pomeroy JW, Walker DA, Hoham RW, editors. Snow ecology. An interdisciplinary examination of snow-covered ecosystems. Cambridge: Cambridge University Press; 2001. p. 168–228. [Google Scholar]
- 4.Komárek J, Nedbalová L. Green cryosestic algae In: Seckbach J, editor. Algae and cyanobacteria in extreme environments. Dordrecht: Springer; 2007. p. 321–342. [Google Scholar]
- 5.Remias D. Cell structure and physiology of alpine snow and ice algae In: Lütz C, editor. Plants in alpine regions. Vienna: Springer; 2012. p. 175–185. [Google Scholar]
- 6.Pröschold T, Marin B, Schlösser UG, Melkonian M. Molecular phylogeny and taxonomic revision of Chlamydomonas (Chlorophyta). I. Emendation of Chlamydomonas Ehrenberg and Chloromonas Gobi, and description of Oogamochlamys gen. nov. and Lobochlamys gen. nov. Protist. 2001;152: 265–300. 10.1078/1434-4610-00068 . [DOI] [PubMed] [Google Scholar]
- 7.Nakada T, Misawa K, Nozaki H. Molecular systematics of Volvocales (Chlorophyceae, Chlorophyta) based on exhaustive 18S rRNA phylogenetic analyses. Mol Phylogenet Evol. 2008;48: 281–291. 10.1016/j.ympev.2008.03.016 . [DOI] [PubMed] [Google Scholar]
- 8.Hoham RW, Bonome TA, Martin CW, Leebens‐Mack JH. A combined 18S rDNA and rbcL phylogenetic analysis of Chloromonas and Chlamydomonas (Chlorophyceae, Volvocales) emphasizing snow and other cold-temperature habitats. J Phycol. 2002;38: 1051–1064. 10.1046/j.1529-8817.2002.t01-1-01227.x [DOI] [Google Scholar]
- 9.Hoham RW, Berman JD, Rogers HS, Felio JH, Ryba JB, Miller PR. Two new species of green snow algae from Upstate New York, Chloromonas chenangoensis sp. nov. and Chloromonas tughillensis sp. nov. (Volvocales, Chlorophyceae) and the effects of light on their life cycle development. Phycologia. 2006;45: 319–330. 10.2216/04-103.1 [DOI] [Google Scholar]
- 10.Matsuzaki R, Nakada T, Hara Y, Nozaki H. Description of Chloromonas kasaiae sp. nov. (Volvocales, Chlorophyceae), based on comparative electron microscopy and molecular data. Phycologia. 2013;52: 239–245. 10.2216/12-083.1 [DOI] [Google Scholar]
- 11.Hoham RW. The life history and ecology of the snow alga Chloromonas pichinchae (Chlorophyta, Volvocales). Phycologia. 1975;14: 213–226. 10.2216/i0031-8884-14-4-213.1 [DOI] [Google Scholar]
- 12.Hoham RW, Mullet JE. The life history and ecology of the snow alga Chloromonas cryophila sp. nov. (Chlorophyta, Volvocales). Phycologia. 1977;16: 53–68. 10.2216/i0031-8884-16-1-53.1 [DOI] [Google Scholar]
- 13.Hoham RW, Mullet JE. Chloromonas nivalis (Chod.) Hoh. & Mull. comb. nov., and additional comments on the snow alga, Scotiella. Phycologia. 1978;17: 106–107. 10.2216/i0031-8884-17-1-106.1 [DOI] [Google Scholar]
- 14.Hoham RW, Roemer SC, Mullet JE. The life history and ecology of the snow alga Chloromonas brevispina comb. nov. (Chlorophyta, Volvocales). Phycologia. 1979;18: 55–70. 10.2216/i0031-8884-18-1-55.1 [DOI] [Google Scholar]
- 15.Ling HU, Seppelt RD. Snow algae of the Windmill Islands, continental Antarctica. 3. Chloromonas polyptera (Volvocales, Chlorophyta). Polar Biol. 1998;20: 320–324. 10.1007/s003000050309 [DOI] [Google Scholar]
- 16.Muramoto K, Kato S, Shitara T, Hara Y, Nozaki H. Morphological and genetic variation in the cosmopolitan snow alga Chloromonas nivalis (Volvocales, Chlorophyta) from Japanese mountainous area. Cytologia. 2008;73: 91–96. 10.1508/cytologia.73.91 [DOI] [Google Scholar]
- 17.Matsuzaki R, Kawai-Toyooka H, Hara Y, Nozaki H. Revisiting the taxonomic significance of aplanozygote morphologies of two cosmopolitan snow species of the genus Chloromonas (Volvocales, Chlorophyceae). Phycologia. 2015;54: 491–502. 10.2216/15-33.1 [DOI] [Google Scholar]
- 18.Matsuzaki R, Nozaki H, Kawachi M. Taxonomic revision of Chloromonas nivalis (Volvocales, Chlorophyceae) strains, with the new description of two snow-inhabiting Chloromonas species. PLOS ONE. 2018;13: e0193603 10.1371/journal.pone.0193603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muramoto K, Nakada T, Shitara T, Hara Y, Nozaki H. Re-examination of the snow algal species Chloromonas miwae (Fukushima) Muramoto et al., comb. nov. (Volvocales, Chlorophyceae) from Japan, based on molecular phylogeny and cultured material. Eur J Phycol. 2010;45: 27–37. 10.1080/09670260903272607 [DOI] [Google Scholar]
- 20.Kawai-Toyooka H, Kuramoto C, Orui K, Motoyama K, Kikuchi K, Kanegae T et al. DNA interference: a simple and efficient gene-silencing system for high-throughput functional analysis in the fern adiantum. Plant Cell Physiol. 2004;45: 1648–1657. 10.1093/pcp/pch186 . [DOI] [PubMed] [Google Scholar]
- 21.Segawa T, Yoshimura Y, Watanabe K, Kanda H, Kohshima S. Community structure of culturable bacteria on surface of Gulkana Glacier, Alaska. Polar Sci. 2011;5: 41–51. 10.1016/j.polar.2010.12.002 [DOI] [Google Scholar]
- 22.Kawachi M, Ishimoto M, Mori F, Yumoto K, Sato M, Noël M-H. MCC-NIES. List of Strains, 9th Edition [DVD]. Tsukuba: National Institute for Environmental Studies; 2013.
- 23.Nakada T, Shinkawa H, Ito T, Tomita M. Recharacterization of Chlamydomonas reinhardtii and its relatives with new isolates from Japan. J Plant Res. 2010;123: 67–78. 10.1007/s10265-009-0266-0 . [DOI] [PubMed] [Google Scholar]
- 24.Nakada T, Nozaki H, Pröschold T. Molecular phylogeny, ultrastructure, and taxonomic revision of Chlorogonium (Chlorophyta): emendation of Chlorogonium and description of Gungnir gen. nov. and Rusalka gen. nov. J Phycol. 2008;44: 751–760. 10.1111/j.1529-8817.2008.00525.x . [DOI] [PubMed] [Google Scholar]
- 25.Nakada T, Tomita M, Wu J-T, Nozaki H. Taxonomic revision of Chlamydomonas subg. Amphichloris (Volvocales, Chlorophyceae), with resurrection of the genus Dangeardinia and descriptions of Ixipapillifera gen. nov. and Rhysamphichloris gen. nov. J Phycol. 2016;52: 283–304. 10.1111/jpy.12397 . [DOI] [PubMed] [Google Scholar]
- 26.Nozaki H, Nakada T, Watanabe S. Evolutionary origin of Gloeomonas (Volvocales, Chlorophyceae), based on ultrastructure of chloroplasts and molecular phylogeny. J Phycol. 2010;46: 195–201. 10.1111/j.1529-8817.2009.00773.x [DOI] [Google Scholar]
- 27.Matsuzaki R, Hara Y, Nozaki H. A taxonomic study of snow Chloromonas species (Volvocales, Chlorophyceae) based on light and electron microscopy and molecular analysis of cultured material. Phycologia. 2014;53: 293–304. 10.2216/14-3.1 [DOI] [Google Scholar]
- 28.Nozaki H, Onishi K, Morita E. Differences in pyrenoid morphology are correlated with differences in the rbcL genes of members of the Chloromonas lineage (Volvocales, Chlorophyceae). J Mol Evol. 2002;55: 414–430. 10.1007/s00239-002-2338-9 . [DOI] [PubMed] [Google Scholar]
- 29.Remias D, Karsten U, Lütz C, Leya T. Physiological and morphological processes in the Alpine snow alga Chloromonas nivalis (Chlorophyceae) during cyst formation. Protoplasma. 2010;243: 73–86. 10.1007/s00709-010-0123-y . [DOI] [PubMed] [Google Scholar]
- 30.Procházková L, Remias D, Řezanka T, Nedbalová L. Chloromonas nivalis subsp. tatrae, subsp. nov. (Chlamydomonadales, Chlorophyta): re-examination of a snow alga from the High Tatra Mountains (Slovakia). Fottea. 2018;18: 1–18. 10.5507/fot.2017.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.TreeBASE [Internet]. Durham (NC): 2010 Mar—[cited 2018 Oct 30]. https://treebase.org/treebase-web/home.html.
- 32.Schultz J, Wolf M. ITS2 sequence–structure analysis in phylogenetics: a how-to manual for molecular systematics. Mol Phylogenet Evol. 2009;52: 520–523. 10.1016/j.ympev.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 33.Seibel PN, Müller T, Dandekar T, Schultz J, Wolf M. 4SALE—A tool for synchronous RNA sequence and secondary structure alignment and editing. BMC Bioinformatics. 2006;7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seibel PN, Müller T, Dandekar T, Wolf M. Synchronous visual analysis and editing of RNA sequence and secondary structure alignments using 4SALE. BMC Res Notes. 2008;1: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coleman AW. Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acids Res. 2007;35: 3322–3329. 10.1093/nar/gkm233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoham RW, Frey FM, Mohn WW, Felio JH, Todd S, Duncan JE, et al. Optimum growth temperatures of three species of green Chloromonas snow algae from Upstate New York and the White Mountains, Arizona. Arct Antarct Alp Res. 2008;40: 355–363. [Google Scholar]
- 37.Ettl H. Die gattung Chloromonas Gobi emend. Wille (Chlamydomonas und die nächstverwandten gattungen I) Nova Hedwigia Beih. 1970;34: 1–283. German. [Google Scholar]
- 38.Ettl H. Chlorophyta I. Phytomonadina In: Ettl H, Gerloff J, Heynig H, Molenhauer D, editors. Süßwasserflora von Mitteleuropa 9. Stuttgart: G. Fischer Verlag; 1983. p. 1–807. German. [Google Scholar]
- 39.Wille N. Algologische notizen IX–XIV Nyt Magazin for Naturvidenskaberne. 1903;41: 89–185, with 3 pls. German. [Google Scholar]
- 40.Wawrik F. Drei neue Flagellaten aus Streckteichen des Waldviertels Nova Hedwigia 1974;25: 665–671. German. [Google Scholar]
- 41.Ettl H. Zwei neue Chlamydomonaden Arch Protistenkunde 1961;105: 273–280. German. [Google Scholar]
- 42.Matsuzaki R, Hara Y, Nozaki H. A taxonomic revision of Chloromonas reticulata (Volvocales, Chlorophyceae), the type species of the genus Chloromonas, based on multigene phylogeny and comparative light and electron microscopy. Phycologia. 2012;51: 74–85. 10.2216/11-18.1 [DOI] [Google Scholar]
- 43.Coleman AW. Is there a molecular key to the level of “biological species” in eukaryotes? A DNA guide. Mol Phylogenet Evol. 2009;50: 197–203. 10.1016/j.ympev.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 44.Müller T, Bleiß W, Martin C-D, Rogaschewski S, Fuhr G. Snow algae from northwest Svalbard: their identification, distribution, pigment and nutrient content. Polar Biol. 1998; 20: 14–32. 10.1007/s003000050272 [DOI] [Google Scholar]
- 45.Remias D, Procházková L, Holzinger A, Nedbalová L. Ecology, cytology and phylogeny of the snow alga Scotiella cryophila K-1 (Chlamydomonadales, Chlorophyta) from the Austrian Alps. Phycologia. 2018;57: 581–592. 10.2216/18-45.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segawa T, Matsuzaki R, Takeuchi N, Akiyoshi A, Navarro F, Sugiyama S, Yonezawa T, et al. Bipolar dispersal of red-snow algae. Nat Commun. 2018;9: 3094 10.1038/s41467-018-05521-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coleman AW. ITS2 is a double-edged tool for eukaryote evolutionary comparisons. Trends Genet. 2003;19: 370–375. 10.1016/S0168-9525(03)00118-5 . [DOI] [PubMed] [Google Scholar]
- 48.Schultz J, Maisel S, Gerlach D, Müller T, Wolf M. A common core of secondary structure of the internal transcribed spacer 2 (ITS2) throughout the Eukaryota. RNA. 2005;11: 361–364. 10.1261/rna.7204505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pröschold T, Silva PC. Proposal to change the listed type of Chlamydomonas Ehrenb., nom. cons. (Chlorophyta). Taxon. 2007;56: 595–596. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identical magnification throughout. Scale bar = 10 μm.
(JPG)
Identical magnification throughout. Scale bar = 10 μm.
(JPG)
Identical magnification throughout. Scale bar = 10 μm.
(JPG)
The 3' end of the 5.8S ribosomal RNA (rRNA) and the 5' end of the large subunit (LSU) rRNA are shown (DDBJ/ENA/GenBank accession number: LC438455). The sequences of this region are identical among the strain (HkCl-57) and the two specimens of field-collected “C. nivalis zygotes” (Tateyama-Green and Tateyama-Orange; DDBJ/ENA/GenBank accession number: LC438456 and LC438457, respectively). Differences between the strain and the specimen of field-collected “C. nivalis zygotes” (Hakkoda-Green; DDBJ/ENA/GenBank accession number: LC438458) are described just outside the structure. Note U-U mismatch in helix II (arrowheads) and the YGGY motif on the 5' side near the apex of helix III (boldface), common structural hallmarks of eukaryotic ITS2 secondary structures [47,48].
(TIF)
(1) Chloromonas muramotoi strain HkCl-57 vs. a specimen of “C. nivalis zygotes,” Tateyama-Green. (2) Strain HkCl-57 vs. a specimen of “C. nivalis zygotes,” Tateyama-Orange. (3) Strain HkCl-57 vs. a specimen of “C. nivalis zygotes,” Hakkoda-Green, (4) C. reticulata strains, UTEX 1970 (epitype strain proposed by Pröschold et al. [6]) vs. SAG 26.90. (5) C. reticulata strains, UTEX 1970 vs. SAG 32.86. (6) Chlamydomonas reinhardtii strains, SAG 11-32a (which can be crossed with the strain SAG 11-32b, the epitype strain of this species [49]) vs. NIES-2463.
(TIF)
Black: nuclear-encoded 1,748 bases of the small subunit (SSU) ribosomal DNA (rDNA). Green: nuclear-encoded 2,017 bases of the large subunit (LSU) rDNA. Red: chloroplast-encoded 1,128 bases of ATP synthase beta subunit gene (atpB). Blue: chloroplast-encoded P700 chlorophyll a apoprotein A2 gene (psaB). Note that the sequences from the two specimens of “Chloromonas nivalis zygotes,” Tateyama-Green and Tateyama-Orange, were identical in the regions examined. The nucleotide differences between snow-inhabiting and mesophilic sister species (C. hohamii vs. C. tenuis and C. chlorococcoides vs. C. reticulata) are according to a previous study [27]. (1) The C. muramotoi strain HkCl-57 vs. a specimen of “C. nivalis zygotes,” Tateyama-Green. (2) Strain HkCl-57 vs. a specimen of “C. nivalis zygotes,” Hakkoda-Green. (3) Strain HkCl-57 vs. the C. miwae strain NIES-2380. (4) Strain HkCl-57 vs. the C. miwae strain NIES-2379. (5) The C. hohamii strain UTEX SNO67 vs. the C. tenuis strain UTEX SNO132. (6) The C. chlorococcoides strain SAG 15.82 (authentic strain) vs. the C. reticulata strain UTEX 1970 (epitype strain proposed by Pröschold et al. [6]).
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.