Abstract
Both inhibitory and stimulatory (known as hormesis) effects of the sublethal flupyradifurone, a butenolide insecticide, on Myzus persicae Sulzer (Hemiptera: Aphididae) were investigated for incorporating it into integrated pest management (IPM). A leaf-dip bioassay showed that flupyradifurone was very toxic against adult M. persicae with a 48 h LC50 of 8.491 mg/L. Using the age-stage two-sex life table approach, we assessed the effects of LC25 of flupyradifurone on adult M. persicae and its progeny (F1 and F2). On the one hand, aphids exposed to flupyradifurone had significantly negative effects on the life history traits acrossing the generations, such as reduced the adult longevity and fecundity of F0, shortened the duration of third instar and fourth instar nymphs, preadult period and the pre-reproductive period of F1, and decreased the reproductive days and adult longevity of F2. On the other hand, stimulatory effects on the duration of pre-adult, adult reproductive days, and reproduction of F1 were observed in the flupyradifurone-treated aphids. Consistently with the stimulation on individual traits, a higher net reproductive rate (R0) of F1 and a shorter mean generation time (T) of F2 were observed in the flupyradifurone-treated aphids, although the other population parameters including the intrinsic rate of increase (r), finite rate of increase (λ) and T of F1 and R0, r and λ of F2 were not significantly affected. These results revealed that adult M. persicae exposed to sublethal concentration of flupyradifurone can induce hormetic effects on F1, and also cause negative effects on F2. Our results would be useful for assessing the overall effects of flupyradifurone on M. persicae and the hormetic effects should take into consideration when use flupyradifurone for control M. persicae.
Introduction
The green peach aphid, Myzus persicae Sulzer (Hemiptera: Aphididae), is one of the most destructive and cosmopolitan insect pest of economical crops [1]. The green peach aphid feeding can cause direct damage and may cause indirect damage through the transmission over 100 plant pathogenic viruses [2]. Control of M. persicae has been dependent the use of chemical insecticides which frequently resulted in development of resistance to various classes of insecticides, including organophosphates, carbamates, pyrethroids, and neonicotinoids [3,4]. Therefore, the insecticides with environment safety and different modes of action remain critical for control of M. persicae.
The novel butenolide insecticide flupyradifurone was discovered and developed by Bayer CropScience in 2012 [5]. Flupyradifurone acts as a partial agonist on insect nicotinic acetylcholine receptors (nAChRs) and reversibly binds to acetylcholine (ACh) [6,7], but is structurally distinct from the class of neonicotinoid insecticides [7]. It was introduced as an effective insecticide to control a broad range of sucking pests and lacks significant cross resistance to both imidacloprid and pymetrozine in CYP6CM1-mediated resistance of whiteflies [7,8]. Especially, it shows an excellent safety profile for honey bees [5]. Insects are exposed to both lethal and low or sublethal concentrations of insecticide residues under field conditions due to misapplication, pesticide drift, shielding by vegetation, or residual levels after dissipation in the environment [9], thus they may experience directly mortality and certain related sublethal effects [10,11]. Sublethal effects are defined as physiological and/or behavioral effects on individuals that survived from exposure to a pesticide at sublethal concentration [10,12]. On the one hand, sublethal effects of insecticides could affect population dynamics through impaired behaviors and physiological traits, such as reduce insect longevity and fecundity [10,13]. On the other hand, sublethal effects could increase fecundity after exposure to an insecticide and have been documented in several insect pests, such as M. persicae [14–17], Rhopalosiphum padi L. (Hemiptera: Aphididae) [18], and Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) [19]. This stimulatory effects induced by insecticides is called “insecticide-induced hormesis”. Hormesis is a biphasic dose–response phenomenon characterized by a low-dose stimulation and a high-dose inhibition and this hormetic response is a modest overcompensation to a disruption in homeostasis or of a direct stimulatory nature [20–24]. It was an adaptive response of generally similar quantitative features with respect to amplitude and range of stimulatory response [21,25–27]. For example, the sublethal doses of imidacloprid and precocene can induce stimulation of reproduction of M. persicae, however, inhibition at high doses [14,16]. Flonicamid and thiamethoxan can prolong the duration of phloem ingestion of M. persicae at sublethal doses, whereas induce starvation or contact toxicity at high doses [28]. Further, some survivors with hormesis may develop resistance to the insecticide subsequently [18,19,29,30] and induce pest resurgence and outbreak [24,31,32].
Therefore, it is important to understand the sublethal effects on non-targeted arthropods and overall effects on targeted pests caused by pesticides. The flupyradifurone was recently introduced in markets and has proved to be especially effective against a wide range of homopterous insect pests, including M. persicae [7]. However, to date, the potential sublethal or hormetic effects of flupyradifurone on M. persicae are still unknown. To obtain a comprehensive understanding of the overall effects of flupyradifurone on M. persicae, we assessed the effects of a sublethal concentration (LC25) of flupyradifurone on biological traits and demographic parameters of M. persicae using the age-stage, two-sex life table. This information would be important to enable a more effective use of this insecticide in management programs for M. persicae through improved understanding of its activity profiles.
Materials and methods
Insects
The colony of M. persicae was established from apterous adults collected from Chinese cabbage (Brassica oleracea var. capitata L.) field in Fujian Province, China (Site: 26.90° N, 119.46° E) in February 2017. The insects were reared on vermiculite-cultured radish (Raphanus sativus L.) seedlings and maintained at 23 ± 1°C, 65–75% relative humidity (RH), with a photoperiod of 16: 8 (L: D) h in the laboratory.
Insecticide and solutions
Flupyradifurone (CAS number: 951659-40-8) at the 96% of active ingredient was obtained from Bayer CropScience Co. Ltd (Monheim, Germany). Triton X-100 was purchased from Sigma-Aldrich Co. Ltd (Saint Louis, USA). All other chemicals and solvents used were technique grade reagents. A stock solution of flupyradifurone was prepared in acetone and diluted to appropriate concentrations with 0.05% (v/v) aqueous Triton X-100. In our bioassays, the acetone was controlled less than 1% in all final used insecticide solutions. The control was performed with distilled water containing 0.05% (v/v) Triton X-100.
Toxicity of flupyradifurone against M. persicae
A leaf-dip bioassay procedure initially developed by Moores et al. [33] and slightly modified by Tang et al. [17] was used to evaluate the acute toxicity of flupyradifurone to M. persicae. Briefly, wells of 12-well tissue-culture plates purchased from Corning (NY, USA) were filled with 2 mL of 2% (w/v) agar to keep the leaves turgid. Leaf discs (20 mm in diameter) were excised from fresh Chinese cabbage using a stainless steel cork borer. Leaf discs were individually dipped to insecticide-free (control) or serial insecticide solutions for 15 s and allowed to air dry for 1 h on disposable plastic gloves (Haimen Yangzi Medical Equipment Co., Ltd, Jiangsu Province, China). After drying, discs were placed upside down on agar in a 12-well tissue-culture plate. Each concentration was conducted three replicates, and 20 apterous adult aphids (≤ 24 h old) were transferred to each well with a soft paintbrush; each bioassay consisted of six concentrations. Each well with aphids was covered with Chinese art paper, also called Xuan paper, purchased from China Xuan Paper Co., Ltd (Anhui Province, China) to prevent aphid escaping. Then the plates with aphids were maintained at the laboratory conditions as described above. Mortality was examined after 48 h. Aphids that unable to move when carefully touched with a soft brush were considered dead. The LC25 for subsequent experiments and LC50 were calculated using PoloPlus 2.0 software (LeOra Software Inc., Berkeley, CA). The concentration-mortality relationship was considered valid (i.e., they fitted the observed data) when there was absence of significant deviation between the observed and the expected mortality (P > 0.05) [34].
Sublethal and transgenerational effects of leaf-dip exposure to flupyradifurone
A concentration (LC25) of 2 mg/L approximate to 25% mortality level determined in preliminary bioassays was used in leaf-dip exposure experiments to evaluate the sublethal and transgenerational effects of flupyradifurone in M. persicae.
Chinese cabbage leaf discs (30 mm in diameter) were dipped in LC25 of flupyradifurone or control solution for 15 s, air-dried for 1 h, and then placed in a plastic Petri dish (35 mm diameter) contained 2 mL of 2% (w/v) agar. Single apterous adult (≤ 24 h old) was randomly introduced to each treated leaf disc and more than 150 adults were treated in each group. Then, the Petri dishes were held in the laboratory conditions as described above. Mortality of adults was calculated after 48 h. The survivors were transferred to untreated leaf-discs individually for further study of sublethal effects of flupyradifurone in M. persicae.
In the experiments, the longevity and fecundity of F0 adult aphids were recorded daily and newly laid nymphs were removed until the adult aphid died. In the succeeding progeny generations (F1 and F2), neonate nymphs with less than one-day of age (105 and 107 of F1 and 64 and 110 of F2 for the control and the flupyradifurone treatment, respectively) were randomly selected from the former generation and placed individually on the untreated Chinese cabbage leaf discs of Petri dishes for evaluating the transgenerational effects of flupyradifurone. Nymphs were observed throughout their development and total duration until adult emergence and survival were record. After the final ecdysis, adult survival and the number of F1 or F2 progeny were recorded daily until death. Leaf discs were replaced in each dish every five days with freshly untreated leaf discs during the experiments. All experiments were conducted at 23 ± 1°C, 65–75% RH, and a photoperiod of 16: 8 (L: D) h. Age-stage, two-sex life tables were constructed from the data obtained in these experiments.
Statistical analysis
The raw data for each M. persicae individual collected in the life table study were analyzed using the age-stage two-sex life table theory [35,36]. The population parameters, including the intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0), the mean generation time (T), age-stage specific survival rates (sxj, where x is age and j is stage), age-specific survival rate (lx), age-specific fecundity (mx), adult pre-reproductive period (APRP), total pre-reproductive period (TPRP), reproductive days (Rd) (i.e., the number of days that adult produced offspring), age-specific maternity (lxmx), age-stage specific life expectancy (exj), reproductive value (vxj), were calculated using the computer program TWOSEX-MSChart [35–37]. The variances and standard errors of the population parameters were estimated using the bootstrap procedure [38] with 100,000 random resampling and difference of population parameters between control and insecticide treatment groups and between generations within each treatment group were compared by using the paired bootstrap test based on the confidence intervals of differences implemented in TWOSEX-MSChart [37,39,40]. All graphics were created using SigmaPlot 12.0 (Systat Software Inc., San Jose, CA, USA).
Results
Toxicity of flupyradifurone on M. persicae adults
The toxicity of flupyradifurone to adult M. persicae was investigated at 48 h after leaf-dip exposure (Table 1). The LC50 value of flupyradifurone was estimated as 8.49 mg L-1 with a confidence interval of 5.33–12.39 mg L-1 and the LC25 value was estimated as 2.10 mg L-1 with a confidence interval of 0.99–3.55 mg L-1, respectively. The LC25 value of 2 mg L-1 flupyradifurone was used as the sublethal concentration for the subsequent experiments.
Table 1. Toxicity of flupyradifurone to adult Myzus persicae determined by using leaf-dip bioassay.
| Insecticide | N | Slope ± SEa | LC25 (95%CI)b mg L-1 | LC50 (95%CI)b mg L-1 | χ2 (df)c | P |
|---|---|---|---|---|---|---|
| Flupyradifurone | 665 | 1.11 ± 0.12 | 2.10 (0.99–3.55) | 8.49 (5.33–12.39) | 17.97 (27) | 0.91 |
a Standard error.
b 95% confidence intervals.
c Chi-square value (χ2) and degrees of freedom (df) as calculated by PoloPlus 2.0.
Sublethal effects of flupyradifurone on the longevity and fecundity of parental (F0) M. persicae
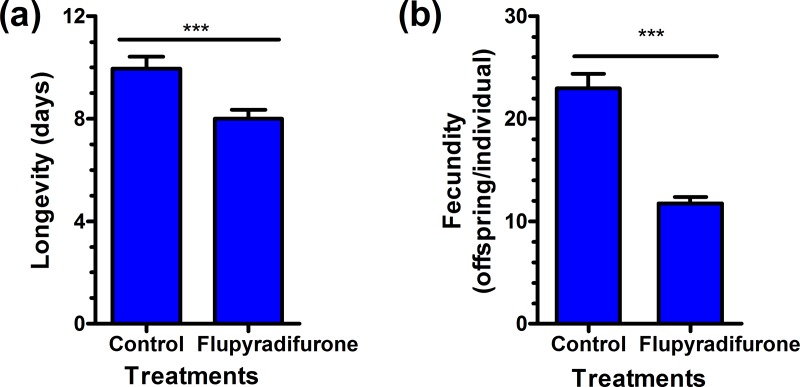
Short-term exposure (48 h) of adult M. persicae to LC25 of flupyradifurone on leaf discs had a significant effect on the longevity and fecundity of the exposed individuals (F0 generation) (Fig 1). As compared to the control group, the adult longevity of the F0 was significantly reduced from 9.96 d to 8.01 d by flupyradifurone treatment (P < 0.001; Fig 1). The fecundity of F0 adults were also significantly reduced from 22.96 to 11.75 offspring/female after exposure to LC25 of flupyradifurone (P < 0.001; Fig 1).
Fig 1. The longevity and fecundity of initial (F0) adult Myzus persicae treated with LC25 of flupyradifurone for 48 h.
The *** above bars indicate means are significant difference between the treatment and the control based on paired bootstrap test (P < 0.001).
Transgenerational effects of flupyradifurone on development, longevity and fecundity of M. persicae
The development time, longevity, fecundity and total preadult survival rate of the succeeding progeny generations (F1 and F2) were evaluated (Table 2). The sublethal concentration of flupyradifurone had significant effects on the development time and fecundity of F1 generation M. persicae (Table 2). The duration of first instar nymph (P = 0.0075) and reproductive days (P = 0.0006) of F1 individuals were significantly prolonged by flupyradifurone treatment, as well as the fecundity of F1 individuals was significantly stimulated by the sublethal flupyradifurone from 26.60 ± 1.32 to 35.25 ± 2.19 offspring/female (P = 0.0006; Table 2). Whereas, when compared to those of the control, the 48-h exposure of F0 adult M. persicae to flupyradifurone significantly decreased the duration of third instar and fourth instar nymph, preadult duration, adult pre-reproductive period (APRP) and total pre-reproductive period (TPRP) in F1 individuals (P < 0.01; Table 2) and significantly decreased the adult longevity, reproductive days and fecundity in F2 individuals (P < 0.01; Table 2). However, no significant differences of F1 generations were observed for the duration time of the second instar nymph, adult longevity, total longevity or total preadult survival rate between the control and flupyradifurone treatments. The total longevity, total preadult survival rate and the duration time of each instar nymph stage, preadult, APRP and TPRP of F2 individuals in flupyradifurone treatment were not significantly different from the control (P > 0.05; Table 2).
Table 2. Transgenerational effects on developmental time, longevity, adult pre-reproductive period (APRP), total pre-reproductive period (TPRP), total preadult survival rate, and mean fecundity of the succeeding generations after initial adult Myzus persicae 48-h exposed to LC25 of flupyradifurone.
| Biological parameters | Generation | Control | Flupyradifurone LC25 | ||
|---|---|---|---|---|---|
| N | Mean ± SEa, b | N | Mean ± SEa, b | ||
| First instar (d) | F1 | 94 | 1.99 ± 0.06bA | 91 | 2.23 ± 0.07aA |
| F2 | 60 | 1.52 ± 0.07aB | 107 | 1.67 ± 0.06aB | |
| Second instar (d) | F1 | 91 | 1.75 ± 0.07aA | 87 | 1.75 ± 0.08aA |
| F2 | 58 | 1.48 ± 0.07aB | 104 | 1.56 ± 0.06aA | |
| Third instar (d) | F1 | 89 | 1.99 ± 0.09aA | 86 | 1.60 ± 0.07bA |
| F2 | 58 | 1.48 ± 0.07aB | 102 | 1.47 ± 0.05aA | |
| Fourth instar (d) | F1 | 87 | 2.24 ± 0.09aA | 85 | 1.56 ± 0.07bA |
| F2 | 56 | 1.77 ± 0.07aB | 99 | 1.68 ± 0.05aA | |
| Pre-adult (d) | F1 | 87 | 7.87 ± 0.19aA | 85 | 7.11 ± 0.10bA |
| F2 | 56 | 6.29 ± 0.11aB | 99 | 6.34 ± 0.07aB | |
| Adult longevity (d) | F1 | 87 | 12.53 ± 0.43aB | 85 | 13.75 ± 0.67aA |
| F2 | 56 | 14.69 ± 0.78aA | 99 | 12.07 ± 0.45bB | |
| Total longevity (d) | F1 | 105 | 17.56 ± 0.72aA | 107 | 17.19 ± 0.88aA |
| F2 | 64 | 18.72 ± 1.02aA | 110 | 17.00 ± 0.57aA | |
| APRP (d) | F1 | 85 | 1.27 ± 0.09aA | 79 | 0.87 ± 0.07bB |
| F2 | 55 | 1.02 ± 0.09aB | 99 | 1.08 ± 0.07aA | |
| TPRP (d) | F1 | 85 | 9.14 ± 0.24aA | 79 | 7.94 ± 0.11bA |
| F2 | 55 | 7.29 ± 0.10aB | 99 | 7.42 ± 0.09aB | |
| Reproductive days (d) | F1 | 87 | 10.02 ± 0.29bB | 85 | 12.08 ± 0.51aA |
| F2 | 56 | 12.29 ± 0.66aA | 99 | 9.98 ± 0.39bB | |
| Total preadult survival | F1 | 105 | 0.83 ± 0.04aA | 107 | 0.79 ± 0.04aB |
| F2 | 64 | 0.87 ± 0.04aA | 110 | 0.90 ± 0.03aA | |
| Fecundity (offspring/female) | F1 | 87 | 26.60 ± 1.32bB | 85 | 35.25 ± 2.19aA |
| F2 | 56 | 39.52 ± 2.73aA | 99 | 32.77 ± 1.63bA | |
a Standard errors (SE) were estimated by using the bootstrap technique with 100,000 re-samplings.
b Significant difference at P < 0.05 between two different treatments and generations were compared with paired bootstrap test implemented in TWOSEX-MSChart. The lower-case letters show significant differences between control and flupyradifurone treatments in the same generation, while the capital letters indicate the significant differences between generations within the same treatment (P < 0.05).
In addition, the duration of first instar nymph, pre-adult period, adult longevity, TPRP, reproductive days and fecundity of F2 individuals were significantly lower than that of F1 individuals in the flupyradifurone treatment (P < 0.05; Table 2); By contrast, the APRP and total preadult survival rate of F2 individuals were significant higher than that of F1 individuals and no significant difference in duration time of second instar and third instar nymph and total longevity were observed between F1 and F2 generations in the flupyradifurone treatment group (P < 0.05; Table 2). Similarly, the duration time of each juvenile developmental stage (including 1st, 2nd, 3rd and 4th instar nymph stage and preadult period) and pre-reproductive period (including APRP and TPRP) of F2 individuals were significantly lower than that of F1 individuals in the control group (P < 0.05; Table 2); Whereas, the adult longevity, reproductive days and fecundity of F2 individuals were significantly higher than that of F1 individuals in the control (P < 0.05; Table 2).
Transgenerational effects of flupyradifurone on population parameters of M. persicae
The transgenerational effects of flupyradifurone (LC25) on the population parameters of F1 and F2 generations were evaluated with bootstrap technique based on life tables (Table 3). When compared to the control group, the net reproductive (R0) of F1 generation M. persicae was significantly increased after the sublethal flupyradifurone treatment (P = 0.0245; Table 3), and the intrinsic rate of increase (r) and finite rate of increase (λ) of F1 were not significantly affected by flupyradifurone, although a stimulation tendency was observed in these two parameters (P = 0.0628; Table 3).The mean generation time (T) was not significantly affected in F1 generation (P = 0.893; Table 3). However, compared with the control, the T of F2 individuals was significantly decreased in the flupyradifurone treatment (P = 0.0084; Table 3). The R0 of F2 individuals was also showed a decline tendency from 34.56 in the control to 29.50 offspring/female in the flupyradifurone treatment, while the difference was not significant (P = 0.1313; Table 3). In addition, the r and λ of F2 generation were not significantly affected by flupyradifurone as compared to that of the control (P = 0.9420 for r, P = 0.9407 for λ; Table 3). In addition, except no significant difference was observed in R0 of the flupyradifurone treatment group, the r, λ and T were significantly increased from F1 to F2 generation within the control or the flupyradifurone treatment, as well as R0 of F2 was higher than R0 of F1 in the control (P < 0.001; Table 3).
Table 3. Population parameters of the succeeding generations after initial adult Myzus persicae exposed to LC25 of flupyradifurone for 48 h.
| Population parametera | Generation | Bootstrap (mean ± SEb, c) | |
|---|---|---|---|
| Control | Flupyradifurone | ||
| r (d−1) | F1 | 0.2392 ± 0.0069aB | 0.2570 ± 0.0067aB |
| F2 | 0.2956 ± 0.0008aA | 0.2949 ± 0.0053aA | |
| λ (d−1) | F1 | 1.270 ± 0.009aB | 1.293 ± 0.009aB |
| F2 | 1.344 ± 0.010aA | 1.343 ± 0.007aA | |
| R0 (offspring/female) | F1 | 22.03 ± 1.46bB | 28.00 ± 2.22aA |
| F2 | 34.55 ± 2.86aA | 29.50 ± 1.74aA | |
| T (d) | F1 | 12.92 ± 0.18aA | 12.96 ± 0.15aA |
| F2 | 11.97 ± 0.15aB | 11.47 ± 0.12bB | |
a r, intrinsic rate of increase; λ, finite rate of increase; R0, net reproductive rate; T, mean generation time.
b Standard errors (SE) were estimated by using the bootstrap technique with 100,000 re-samplings.
c Significant difference at P < 0.05 between two different treatments and generations were compared with paired bootstrap test implemented in TWOSEX-MSChart. The small letters show significant differences between control and flupyradifurone treatments in each generation, while the capital letters indicate the significant differences between F1 and F2 generations within each treatment groups (P < 0.05).
Transgenerational effects of flupyradifurone on age-stage specific survival rate and fecundity of M. persicae
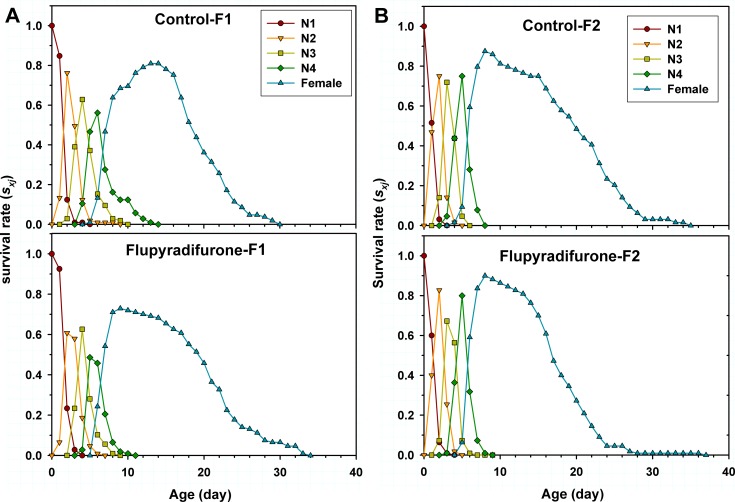
Age-stage survival rate curves (sxj) show the probability that a newborn nymph will survive to age x and stage j. Obvious overlaps between different stages occurred in both flupyradifurone-treated and the control groups as a result of the variable developmental rates among individuals (Fig 2). Declined survival rates of each developmental stage of F1 individuals were observed in the flupyradifurone treatment group as compared to the control group (Fig 2), however, the survival rates of the 2nd- (0.83) and 4th- (0.80) instar nymph of F2 individuals in the flupyradifurone treatment were higher than that of the control (0.75 and 0.44 for 2nd and 4th instar nymph, respectively; Fig 2). The fourth instar nymph peak and the female adult peak of F1 generation appeared at five days of age and nine days of age in the flupyradifurone treatment, while in the control at six days of age and at thirteen days of age, respectively (Fig 2). These showed the faster development in the flupyradifurone treatment group. Therefore, total development time of F1 generational nymph in the flupyradifurone treatment (10 days) was shorter than that of the control (13 days; Fig 2). For F2 generation, a similar trend of sxj curves was observed between the control and flupyradifurone treatment groups (Fig 2). In addition, between generations, the adult survival rate of F1 individuals was lower than F2 individuals in each treatment group (Fig 2).
Fig 2. Age-stage-specific survival rates (sxj) of Myzus persicae treated with LC25 of flupyradifurone in F1 and F2 generations.
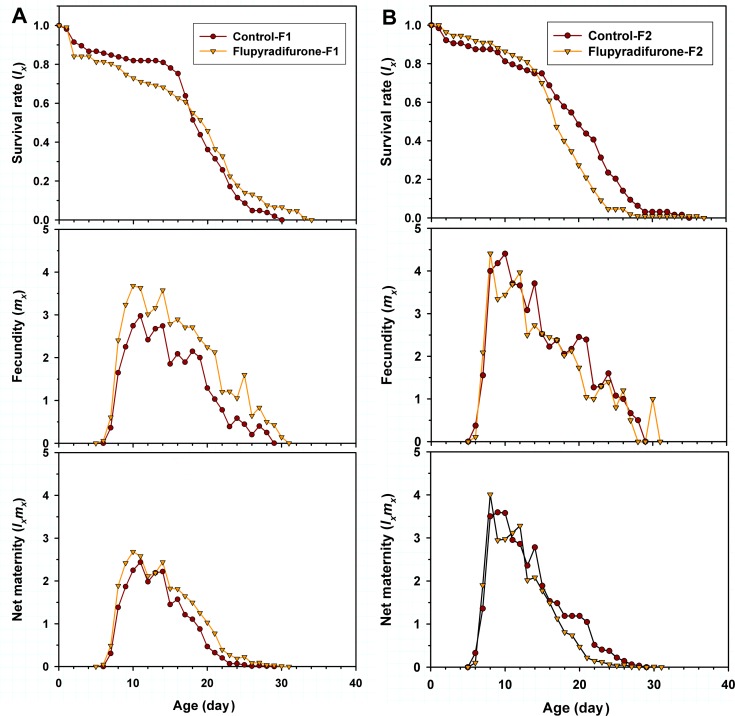
The age-specific survival rate (lx) demonstrates a simplified overview of the survival rate without accounting for the stage differentiation (Fig 3). The lx curves significantly declined on day 15 or 14 in F1 or F2 generation in both control and flupyradifurone treatment groups (Fig 3). Interesting, a higher lx of F1 generation were observed in flupyradifurone treated group from age 18 to 33 day (Fig 3), whereas a lower lx of F2 generation in the flupyradifurone treatment was observed from age 15 to 36 day (Fig 3). The age-specific fecundity of the total population (mx) and age-specific maternity (lxmx) of the flupyradifurone treated M. persicae were higher than that of the control group in F1 generation (Fig 3), while a similar trend of mx and lxmx of F2 generation M. persicae were observed both in the treatment and the control groups (Fig 3).
Fig 3. Age-specific survival rate (lx), age-specific fecundity of total population (mx), and age-specific maternity (lxmx) of initial Myzus persicae exposed to LC25 of flupyradifurone in F1 and F2 generations.
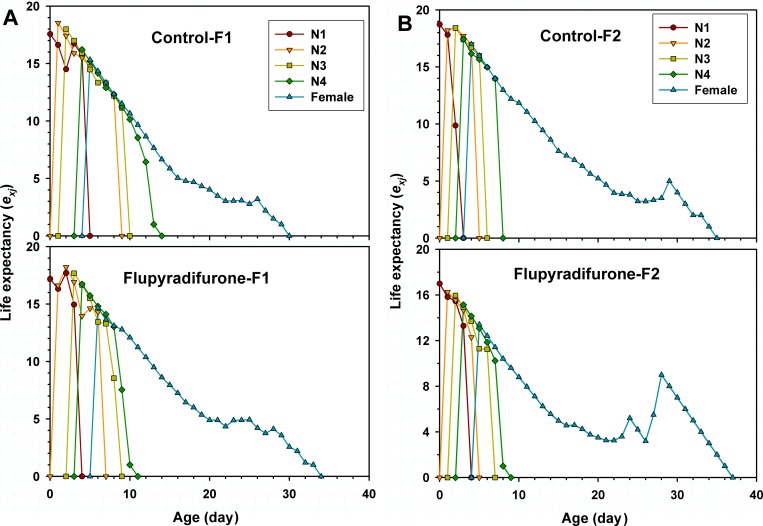
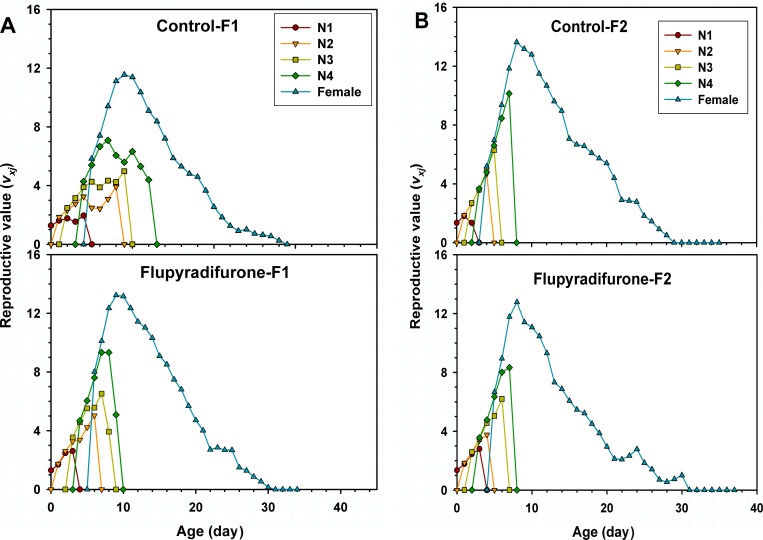
The age-stage-specific life expectancy (exj) is the length of time that an individual of age x and stage j is expected to survive after age x (Fig 4). The life expectancy (exj) curves indicated that offspring (F1 and F2) of adult M. persicae with one-time flupyradifurone exposure could to survive longer than the control (Fig 4). In addition, the age-stage-specific reproductive value (vxj) represents the devotion to future offspring of individuals from age x to stage j. A higher maximum reproductive value of each stage and a shorter preadult period of F1 generation M. persicae were observed in the treatment group than those of the control (Fig 5), while in F2 generation, except the 1st instar nymph, a lower vxj of each stage in the treatment group was observed than that of the control (Fig 5).
Fig 4. Age-stage-specific life expectancy (exj) of Myzus persicae treated with LC25 of flupyradifurone in F1 and F2 generations.
Fig 5. Age-stage reproductive value (vxj) of Myzus persicae treated with LC25 of flupyradifurone in F1 and F2 generations.
Discussion
The high toxicity of flupyradifurone has been reported for several sucking pests, including M. persicae, Aphis gossypii Glover (Hemiptera: Aphididae) and Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) [7]. In this study, flupyradifurone also showed a highly acute toxicity against adult M. persicae after 48 h leaf-dip exposure and the LC50 was 8.491 mg/L and this LC50 value is similar to that previously reported for Diaphorina citri Kuwayama (Hemiptera: Psyllidae), after 48 h leaf-dip exposure to flupyradifurone with 10.43 mg/L [41]. In addition to the lethal effects of insecticides, insect populations are frequently exposed to low concentrations of insecticides in the field due to the variable distribution and continuous degradation of insecticides [42,43]. Thus, sublethal effects of insecticides may increase or decrease insect population [10], assessing the development, survival, reproduction and behavioral response are important for overall understanding of the effects of flupyradifurone for IPM. In this study, an attempt was made to assess sublethal effects on life table characteristics of M. persicae over subsequent generations after exposed M. persicae adult to sublethal flupyradifurone for 48 h.
Sublethal effects that reduced fecundity and longevity, and altered behavior were usually observed in most insect pests after exposure to sublethal concentration of insecticides [10,44–46]. For example, endosulfan can significantly reduce fecundity of Apolygus lucorum Meyer-Dür (Hemiptera: Miridae) after treated with sublethal concentrations [47] and buprofezin reduce adult longevity of B. tabaci by sublethal doses [48]. Similarly, in the present study, when initial M. persicae adult was exposed to leaf discs treated with sublethal concentrations of flupyradifurone, significant reductions in the fecundity and adult longevity of F0 generation M. persicae were observed and the reproductive days, adult longevity, and fecundity were significantly shortened or reduced in the subsequent F2 generation, although no significant effects on the development time of each nymph stage and survival rates were found in F2. Moreover, these adverse effects on F2 individual aphids were translated to their population parameters including a lower R0 and mean generation time (T) and showed that sublethal concentrations of flupyradifurone suppressed the population growth of F2 generation M. persicae. Similar sublethal effects of insecticides on population growth have been reported in several insect pests, such as A. gossypii [49], A. lucorum [50], B. tabaci [51], Brevicoryne brassicae L. (Hemiptera: Aphididae) [52], Bradysia odoriphaga Yang et Zhang (Diptera: Sciaridae) [53] and M. persicae [54,55]. The negative sublethal effects may be due to increased biological fitness cost and also provide evidence that sublethal concentrations of flupyradifurone did have significantly sublethal and transgenerational effects on M. persicae.
Insecticide-induced hormesis that increased fecundity or change insect behavior have been reported in A. gossypii with bifenthrin [56], citrus thrip, Scirtothrips citri Moulton (Thysanoptera: Thripidae), with dicofol or malathion [57], mite, Tetranychus urticae Koch (Acari: Tetranychidae), with imidacloprid [58], and the brown planthopper, Nilaparvata lugens Stäl (Hemiptera: Delphacidae) [59,60] after exposure to low or sublethal concentrations of insecticides. In the present study, significantly increased fecundity and more reproductive days were observed in F1 adults, indicating that sublethal concentration of flupyradifuron has a stimulatory effect (i.e., hormesis) on reproduction of F1 generation M. persicae following parental adults 48-h exposure. Similar stimulatory effects on reproduction of M. persicae in offspring generations have been documented in response to sublethal concentration of several insecticides, including azinphosmethyl [61], azadirachtin [55], imidacloprid [55,62], and sulfoxaflor [17]. Additionally, in the present study, the sublethal concentration of flupyradifuron significantly affected the F1 generation population growth, notably through a significantly shortened duration of third instar and fourth instar nymph, preadult period and pre-reproductive periods (APRP and TPRP), a significantly prolonged reproductive days and an increased fecundity. The sum of these effects on F1 individual aphids translated to higher population parameters, including net reproductive rate (R0), intrinsic rate of increase (r) and finite rate of increase (λ). These increased population parameters and stimulated reproduction indicated that sublethal concentration of flupyradifuron could to stimulate population growth of M. persicae in F1 generation via maternal effects. Similarly, the reproduction of Daphnia carinata King (Diplostraca: Daphnidae) was negatively affected in the parental generation following exposure to low concentrations of chlorpyrifos, while a hormesis effect was observed for all reproductive parameters in the second generation [63] and the females of Chironomus riparius Kieffer (Diptera: Chironomidae) exposed to low tributylin concentrations for multiple generations significantly laid more eggs in the subsequent generations and acquired a higher tolerance towards the stressor [64]. These results suggested that sublethal effects on biological fitness may increase by exposure to low concentrations of insecticides.
Interestingly, in the present study, 48-h exposures of adult M. persicae (F0) to sublethal concentration of flupyradifuron can significantly stimulate fecundity in the following F1 generation, whereas reduced fecundity and adult longevity were observed in F0 and F2 generations, and no significant effects on development time of each instar nymph and population growth (R0) were found between the treatment and control in F2 generations. These results suggested that biological tradeoffs in resource allocation occurred across generations. Similarly, when M. persicae adults exposed to low/sublethal concentrations of azinphos-methyl [65], imidacloprid [15,62] and sulfoxaflor [17], this delayed stimulation of reproduction phenomenon was also observed in the subsequent generation of M. persicae, while not observed in initial adults. We hypothesized that the exposure of adult M. persicae to sublethal concentration of insecticides may: remove individuals of low fitness, cost biological fitness to cope with the stress of flupyradifurone in F0; and result in stimulating reproduction (i.e. hormesis) and higher reproduction of F1 progeny to overcompensate physiologically for their disrupted homeostasis via maternal effects, to optimize resource allocation between self-maintenance and reproductive output; and possibly restore homeostasis in F2 generation. This no long-term fitness cost for the stimulatory response in early generations was also been demonstrated for imidacloprid in M. persicae [23,66]. Therefore, hormesis induced by sublethal concentrations of flupyradifuron may lead to secondary population outbreaks of M. persicae.
In conclusion, our results indicated that LC25 concentration of flupyradifurone has transgenerational hormesis on M. persicae across three generations. The sublethal exposure of parental aphids resulted in significantly increase of duration of 1st instar nymph, reproductive days, fecundity and population parameters R0 in F1 generation and recovery R0 to control level and reduced T in F2 generation. These suggested that short-term exposure of the sublethal concentration of flupyradifurone might induce hormesis of M. persicae and this hormesis may lead to pest resurgence [57,67]. Because hormetic effects can be taken in form of shortened development, higher survival rate, or higher fecundity, these factors are not independent from each other and should not be analyzed separately [68,69]. Since life table analysis integrated all these factors in population parameters, it is the most important tool for an overall evaluation of population fitness and hormesis [17,70]. Nevertheless, given that the genetic variation in field populations is naturally greater than that of laboratory strains, the situation in the field may be more complex and further investigations on sublethal effects of this insecticide on M. persicae in the field are advisable. Consequently, flupyradifurone was very toxic against M. persicae M. persicae at the present study, but insecticide-induced hormesis should be taken into consideration, which may potentially occur after application of flupyradifurone for control M. persicae in the field.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
This study was supported by the National Key Research and Development Program (Contract No. 2016YFD0200506)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by the National Key Research and Development Program (Contract No. 2016YFD0200506) to XG.
References
- 1.Van Emden HF, Harrington R (2007) Aphids as Crop Pests, Cabi Publishing, Wallingford, Oxon, UK, 717p. [Google Scholar]
- 2.Blackman RL, Eastop VF (2000) Aphids on the world's crops: an identification and information guide. Second edition London, United Kingdom: John Wiley & Sons, Ltd; London, UK, 1–466 p. [Google Scholar]
- 3.Bass C, Puinean AM, Zimmer CT, Denholm I, Field LM, Foster SP, et al. (2014) The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem Mol Biol 51: 41–51. 10.1016/j.ibmb.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 4.Tang Q-L, Ma K-S, Hou Y-M, Gao X-W (2017) Monitoring insecticide resistance and diagnostics of resistance mechanisms in the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae) in China. Pestic Biochem Physiol 143: 39–47. 10.1016/j.pestbp.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 5.European Food Safety Authority (2015) Conclusion on the peer review of the pesticide risk assessment of the active substance flupyradifurone. EFSA Journal 13: 4020–4020. [Google Scholar]
- 6.Jeschke P, Nauen R, Gutbrod O, Beck ME, Matthiesen S, Haas M, et al. (2015) Flupyradifurone (Sivanto (TM)) and its novel butenolide pharmacophore: Structural considerations. Pestic Biochem Physiol 121: 31–38. 10.1016/j.pestbp.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 7.Nauen R, Jeschke P, Velten R, Beck ME, Ebbinghaus-Kintscher U, Thielert W, et al. (2015) Flupyradifurone: a brief profile of a new butenolide insecticide. Pest Manag Sci 71: 850–862. 10.1002/ps.3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith HA, Nagle CA, MacVean CA, McKenzie CL (2016) Susceptibility of Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae) to imidacloprid, thiamethoxam, dinotefuran and flupyradifurone in South Florida. Insects 7: 57–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duke SO (2014) Hormesis with pesticides. Pest Manag Sci 70: 689 10.1002/ps.3756 [DOI] [PubMed] [Google Scholar]
- 10.Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol. 52:81–106. 10.1146/annurev.ento.52.110405.091440 [DOI] [PubMed] [Google Scholar]
- 11.Biondi A, Zappala L, Stark JD, Desneux N (2013) Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS One 8:e76548 10.1371/journal.pone.0076548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CY (2000) Sublethal effects of insecticide on longevity, fecundity, and behaviour of insect pests: a review. J Bioscience 11:107–112. [Google Scholar]
- 13.Haynes KF (1988) Sublethal effects of neurotoxic insecticides on insect behavior. Annu Rev Entomol 33:149–168. 10.1146/annurev.en.33.010188.001053 [DOI] [PubMed] [Google Scholar]
- 14.Yu YS, Shen GQ, Zhu HL, Lu YT (2010) Imidacloprid-induced hormesis on the fecundity and juvenile hormone levels of the green peach aphid Myzus persicae (Sulzer). Pestic Biochem Physiol 98: 238–242. [Google Scholar]
- 15.Ayyanath MM, Cutler GC, Scottdupree CD, Sibley PK (2013) Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid. PLoS One 8: e74532 10.1371/journal.pone.0074532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayyanath MM, Scott-Dupree CD, Cutler GC (2015) Effect of low doses of precocene on reproduction and gene expression in green peach aphid. Chemosphere 128: 245–251. 10.1016/j.chemosphere.2015.01.061 [DOI] [PubMed] [Google Scholar]
- 17.Tang Q, Xiang M, Hu H, An C, Gao X (2015) Evaluation of sublethal effects of sulfoxaflor on the green peach aphid (Hemiptera: Aphididae) using life table parameters. J Econ Entomol 108: 2720–2728. 10.1093/jee/tov221 [DOI] [PubMed] [Google Scholar]
- 18.Lu YH, Zheng XS, Gao XW (2016) Sublethal effects of imidacloprid on the fecundity, longevity, and enzyme activity of Sitobion avenae (Fabricius) and Rhopalosiphum padi (Linnaeus). Bull Entomol Res 106:551–559. 10.1017/S0007485316000286 [DOI] [PubMed] [Google Scholar]
- 19.Gong Y, Xu B, Zhang Y, Gao X, Wu Q (2015) Demonstration of an adaptive response to preconditioning Frankliniella occidentalis (Pergande) to sublethal doses of spinosad: a hormetic-dose response. Ecotoxicology 24:1141–1151. 10.1007/s10646-015-1461-5 [DOI] [PubMed] [Google Scholar]
- 20.Calabrese EJ, Baldwin LA (1997) The dose determines the stimulation (and poison): Development of a chemical hormesis database. Int J Toxicol 16: 545–559. [Google Scholar]
- 21.Calabrese EJ, Baldwin LA (2002) Defining hormesis. Hum Exp Toxicol 21: 91–97. 10.1191/0960327102ht217oa [DOI] [PubMed] [Google Scholar]
- 22.Calabrese EJ, Baldwin LA (2003) Toxicology rethinks its central belief—Hormesis demands a reappraisal of the way risks are assessed. Nature 421: 691–692. 10.1038/421691a [DOI] [PubMed] [Google Scholar]
- 23.Calabrese EJ (2005) Paradigm lost, paradigm found: the re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Environ Pollut 138: 378–411. [DOI] [PubMed] [Google Scholar]
- 24.Cutler GC (2013) Insects, insecticides and hormesis: evidence and considerations for study. Dose-Response 11: 154–177. 10.2203/dose-response.12-008.Cutler [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carelli G, Iavicoli I (2002) Defining hormesis: the necessary tool to clarify experimentally the low dose-response relationship. Hum Exp Toxicol 21: 113–114. [DOI] [PubMed] [Google Scholar]
- 26.Guedes RNC, Cutler GC (2014) Insecticide-induced hormesis and arthropod pest management. Pest Manag Sci 70: 690–697. 10.1002/ps.3669 [DOI] [PubMed] [Google Scholar]
- 27.Guedes RNC, Walse SS, Throne JE (2017) Sublethal exposure, insecticide resistance, and community stress. Curr Opin Insect Sci 21: 47–53. 10.1016/j.cois.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 28.Cho SR, Koo HN, Yoon C, Kim GH (2011) Sublethal effects of flonicamid and thiamethoxam on green peach aphid, Myzus persicae and feeding behavior analysis. J Korean Soc Appl Bi 54: 889–898. [Google Scholar]
- 29.Guedes NMP, Tolledo J, Corrêa AS, Guedes RNC (2010) Insecticide-induced hormesis in an insecticide-resistant strain of the maize weevil, Sitophilus zeamais. J Appl Entomol 134: 142–148. [Google Scholar]
- 30.Liu X, Tang Q, Li Y, Campos MR, Liang P, Gao X (2017) Widespread resistance of the aphid Myzus persicae to pirimicarb across China, and insights on ace2 mutation frequency in this species. Entomol Gen 36: 285–299. [Google Scholar]
- 31.Cohen E (2006) Pesticide-mediated homeostatic modulation in arthropods. Pestic Biochem Physiol 85: 21–27. [Google Scholar]
- 32.Guedes RNC, Smagghe G, Stark JD, Desneux N (2015) Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu Rev Entomol 61:43–62. 10.1146/annurev-ento-010715-023646 [DOI] [PubMed] [Google Scholar]
- 33.Moores GD, Gao XW, Denholm I, Devonshire AL (1996) Characterisation of insensitive acetylcholinesterase in insecticide-resistant cotton aphids, Aphis gossypii Glover (Homoptera: Aphididae). Pestic Biochem Physiol 56: 102–110. [Google Scholar]
- 34.Robertson JL, Preisler HK (1992) Pesticide bioassays with arthropods. CRC Press, Boca Raton, USA. [Google Scholar]
- 35.Chi H, Liu H (1985) Two new methods for the study of insect population ecology. Bull Inst Zool Acad Sin (Taipei) 24: 225–240. [Google Scholar]
- 36.Chi H (1988) Life-table analysis incorporating both sexes and variable development rates among individuals. Environ Entomol 17: 26–34. [Google Scholar]
- 37.Chi H (2018) TWOSEX-MS Chart: A computer program for the age-stage, two-sex life table analysis. http://140.120.197.173/Ecology/Download/Twosex-MSChart-exe-B200000.rar. (accessed 30 June 2018).
- 38.Efron B, Tibshirani RJ (1993) An introduction to the bootstrap Monographs on Statistics and Applied Probability, Chapman and Hall//CRC Press, London, UK, 436 p. [Google Scholar]
- 39.Huang YB, Chi H (2013) Life tables of Bactrocera cucurbitae (Diptera: Tephritidae): with an invalidation of the jackknife technique. J App Entomol 137: 327–339. [Google Scholar]
- 40.Huang HW, Chi H, Smith CL (2018) Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) fed on Solanum photeinocarpum (Solanales: Solanaceae): with a new method to project the uncertainty of population growth and consumption. J Econ Entomol 111: 1–9. 10.1093/jee/tox330 [DOI] [PubMed] [Google Scholar]
- 41.Chen XD, Seo M, Stelinski LL (2017) Behavioral and hormetic effects of the butenolide insecticide, flupyradifurone, on Asian citrus psyllid, Diaphorina citri. Crop Prot 98: 102–107. [Google Scholar]
- 42.Desneux N, Fauvergue X, Dechaume-Moncharmont FX, Kerhoas L, Ballanger Y, Kaiser L. (2005) Diaeretiella rapae limits Myzus persicae populations after applications of deltamethrin in oilseed rape. J Econ Entomol 98: 9–17. [DOI] [PubMed] [Google Scholar]
- 43.Bonmatin JM, Marchand PA, Charvet R, Moineau I, Bengsch ER, Colin ME. (2005) Quantification of imidacloprid uptake in maize crops. J Agric Food Chem 53: 5336–5341. 10.1021/jf0479362 [DOI] [PubMed] [Google Scholar]
- 44.Han W, Zhang S, Shen F, Liu M, Ren C, Gao X. (2012) Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag Sci 68: 1184–1190. 10.1002/ps.3282 [DOI] [PubMed] [Google Scholar]
- 45.Guo L, Desneux N, Sonoda S, Liang P, Han P, Gao X-W. (2013) Sublethal and transgenerational effects of chlorantraniliprole on biological traits of the diamondback moth, Plutella xylostella L. Crop Prot 48: 29–34. [Google Scholar]
- 46.Zeng X, He Y, Wu J, Tang Y, Gu J, Ding W, et al. (2016) Sublethal effects of cyantraniliprole and imidacloprid on feeding behavior and life table parameters of Myzus persicae (Hemiptera: Aphididae). J Econ Entomol 109:1595–1602. 10.1093/jee/tow104 [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Lu Y, Wu K, Wyckhuys KAG, Xue F (2008) Lethal and sublethal effects of endosulfan on Apolygus lucorum (Hemiptera: Miridae). J Econ Entomol 101: 1805–1810. [DOI] [PubMed] [Google Scholar]
- 48.Sohrabi F, Shishehbor P, Saber M, Mosaddegh MS (2011) Lethal and sublethal effects of buprofezin and imidacloprid on Bemisia tabaci (Hemiptera: Aleyrodidae). Crop Prot 30: 1190–1195. [Google Scholar]
- 49.Chen X, Ma K, Li F, Liang P, Liu Y, Guo T, et al. (2016) Sublethal and transgenerational effects of sulfoxaflor on the biological traits of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). Ecotoxicology 25:1841–1848. 10.1007/s10646-016-1732-9 [DOI] [PubMed] [Google Scholar]
- 50.Tan Y, Biondi A, Desneux N, Gao XW (2012) Assessment of physiological sublethal effects of imidacloprid on the mirid bug Apolygus lucorum (Meyer-Dur). Ecotoxicology 21: 1989–1997. 10.1007/s10646-012-0933-0 [DOI] [PubMed] [Google Scholar]
- 51.He Y, Zhao J, Zheng Y, Weng Q, Biondi A, Desneux N, et al. (2013) Assessment of potential sublethal effects of various insecticides on key biological traits of the tobacco whitefly, Bemisia tabaci. Int J Biol Sci 9: 246–255. 10.7150/ijbs.5762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lashkari MR, Sahragard A, Ghadamyari M (2007) Sublethal effects of imidacloprid and pymetrozine on population growth parameters of cabbage aphid, Brevicoryne brassicae on rapeseed, Brassica napus L. Insect Sci 14: 207–212. [Google Scholar]
- 53.Zhang P, Liu F, Mu W, Wang Q, Li H, Chen C. (2014) Life table study of the effects of sublethal concentrations of thiamethoxam on Bradysia odoriphaga Yang and Zhang. Pestic Biochem Physiol 111: 31–37. 10.1016/j.pestbp.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 54.Devine GJ, Harling ZK, Scarr AW, Devonshire AL (1996) Lethal and sublethal effects of imidacloprid on nicotine-tolerant Myzus nicotianae and Myzus persicae. Pestic Sci 48: 57–62. [Google Scholar]
- 55.Wang XY, Yang ZQ, Shen ZR, Lu J, Xu WB (2008) Sublethal effects of selected insecticides on fecundity and wing dimorphism of green peach aphid (Hom., Aphididae). J Appl Entomol 132: 135–142. [Google Scholar]
- 56.Kerns DL, Stewart SD (2000) Sublethal effects of insecticides on the intrinsic rate of increase of cotton aphid. Entomol Exp Appl 94: 41–49. [Google Scholar]
- 57.Morse JG, Zareh N (1991) Pesticide-induced hormoligosis of citrus thrips (Thysanoptera, Thripidae) fecundity. J Econ Entomol 84: 1169–1174. [Google Scholar]
- 58.Maggi VL, Leigh TF (1983) Fecundity response of the twospotted spider mite to cotton treated with methyl parathion or phosphoric acid. J Econ Entomol 76: 20–25. [Google Scholar]
- 59.Chelliah S, Heinrichs EA (1980) Factors affecting insecticide-induced resurgence of the brown planthopper, Nilaparvata lugens (Homoptera, Delphacidae) on rice. Environ Entomol 9: 773–777. [Google Scholar]
- 60.Bao H, Liu S, Gu J, Wang X, Liang X, Liu Z. (2009) Sublethal effects of four insecticides on the reproduction and wing formation of brown planthopper, Nilaparvata lugens. Pest Manag Sci 65: 170–174. 10.1002/ps.1664 [DOI] [PubMed] [Google Scholar]
- 61.Lowery DT, Sears MK (1986) Stimulation of reproduction of the green peach aphid (Homoptera, Aphididae) by azinphosmethyl applied to potatoes. J Econ Entomol 79: 1530–1533. [Google Scholar]
- 62.Cutler GC, Ramanaidu K, Astatkie T, Isman MB (2009) Green peach aphid, Myzus persicae (Hemiptera: Aphididae), reproduction during exposure to sublethal concentrations of imidacloprid and azadirachtin. Pest Manag Sci 65: 205–209. 10.1002/ps.1669 [DOI] [PubMed] [Google Scholar]
- 63.Zalizniak L, Nugegoda D (2006) Effect of sublethal concentrations of chlorpyrifos on three successive generations of Daphnia carinata. Ecotox Environ Safe 64: 207–214. [DOI] [PubMed] [Google Scholar]
- 64.Vogt C, Nowak C, Diogo JB, Oetken M, Schwenk K, Oehlmann J. (2007) Multi-generation studies with Chironomus riparius—effects of low tributyltin concentrations on life history parameters and genetic diversity. Chemosphere 67: 2192–2200. 10.1016/j.chemosphere.2006.12.025 [DOI] [PubMed] [Google Scholar]
- 65.Gordon PL, Mcewen FL (1984) Insecticide-stimulated reproduction of Myzus persicae, the green peach aphid (Homoptera, Aphididae). Can Entomol 116: 783–784. [Google Scholar]
- 66.Stebbing ARD (1982) Hormesis—the stimulation of growth by low-levels of inhibitors. Sci Total Environ 22: 213–234. [DOI] [PubMed] [Google Scholar]
- 67.Morse JG (1998) Agricultural implications of pesticide-induced hormesis of insects and mites. Hum Exp Toxicol 17: 266–269. 10.1177/096032719801700510 [DOI] [PubMed] [Google Scholar]
- 68.Yokoyama VY, Pritchard J, 1984. Effect of pesticides on mortality, fecundity and egg viability of Geocoris pallens (Hemiptera: Lygaeidae). J Econ Entomol 77: 876–879. [Google Scholar]
- 69.Luckey TD, 1968. Insecticide hormoligosis. J Econ Entomol 61: 7–12. [DOI] [PubMed] [Google Scholar]
- 70.Akca I, Ayvaz T, Yazici E, Smith CL, Chi H (2015) Demography and population projection of Aphis fabae (Hemiptera: Aphididae): with additional comments on life table research criteria. J Econ Entomol 108: 1466–1478. 10.1093/jee/tov187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.